Recent Advances in Photocatalytic Degradation of Imidacloprid in Aqueous Solutions Using Solid Catalysts
Abstract
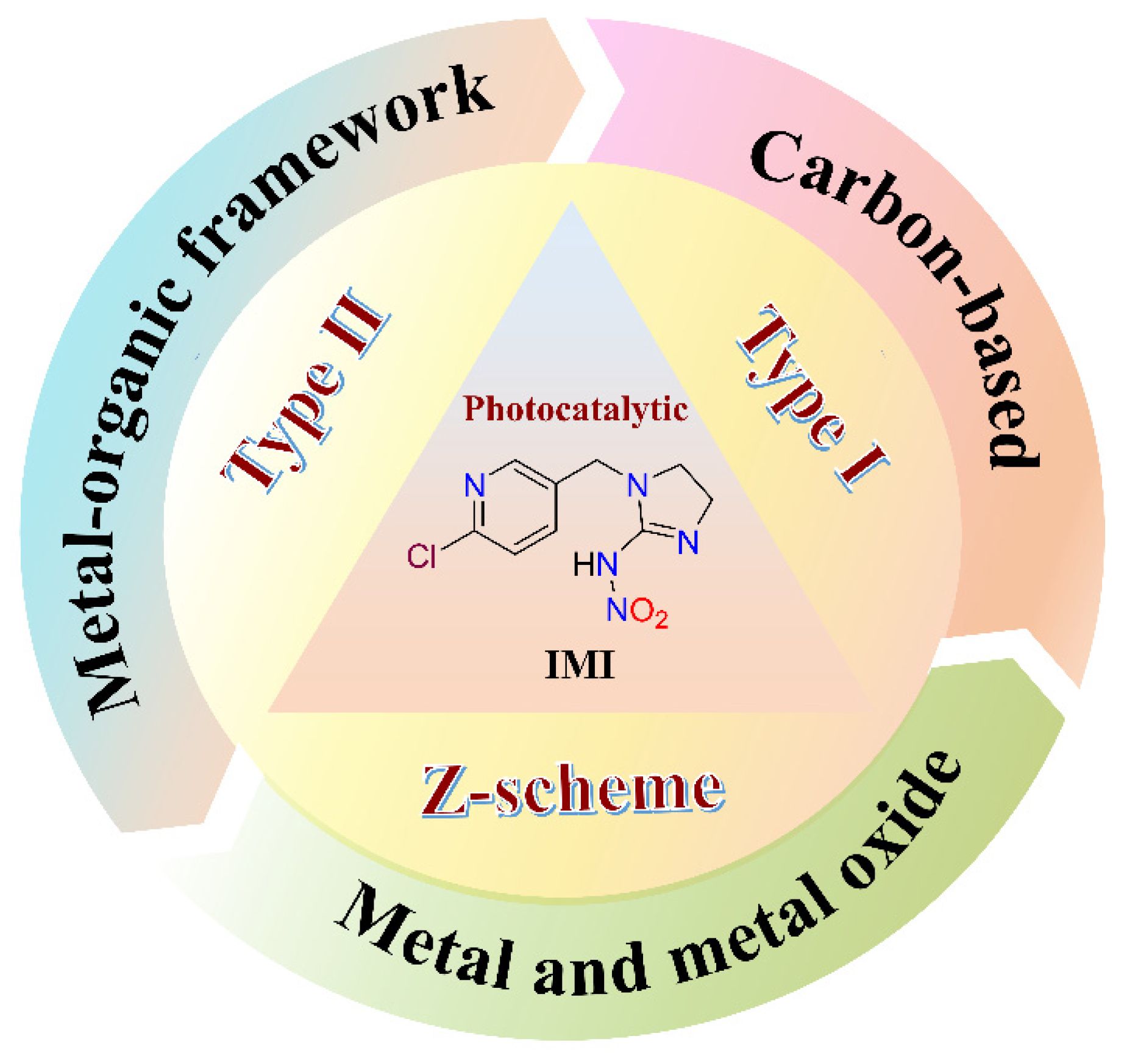
1. Introduction
2. Photocatalytic Degradation of Imidacloprid Using Metal Oxide Photocatalysts
2.1. Titanium Dioxide (TiO2)-Based Solid Photocatalysts
2.2. Zinc Oxide (ZnO)-Based Solid Photocatalysts
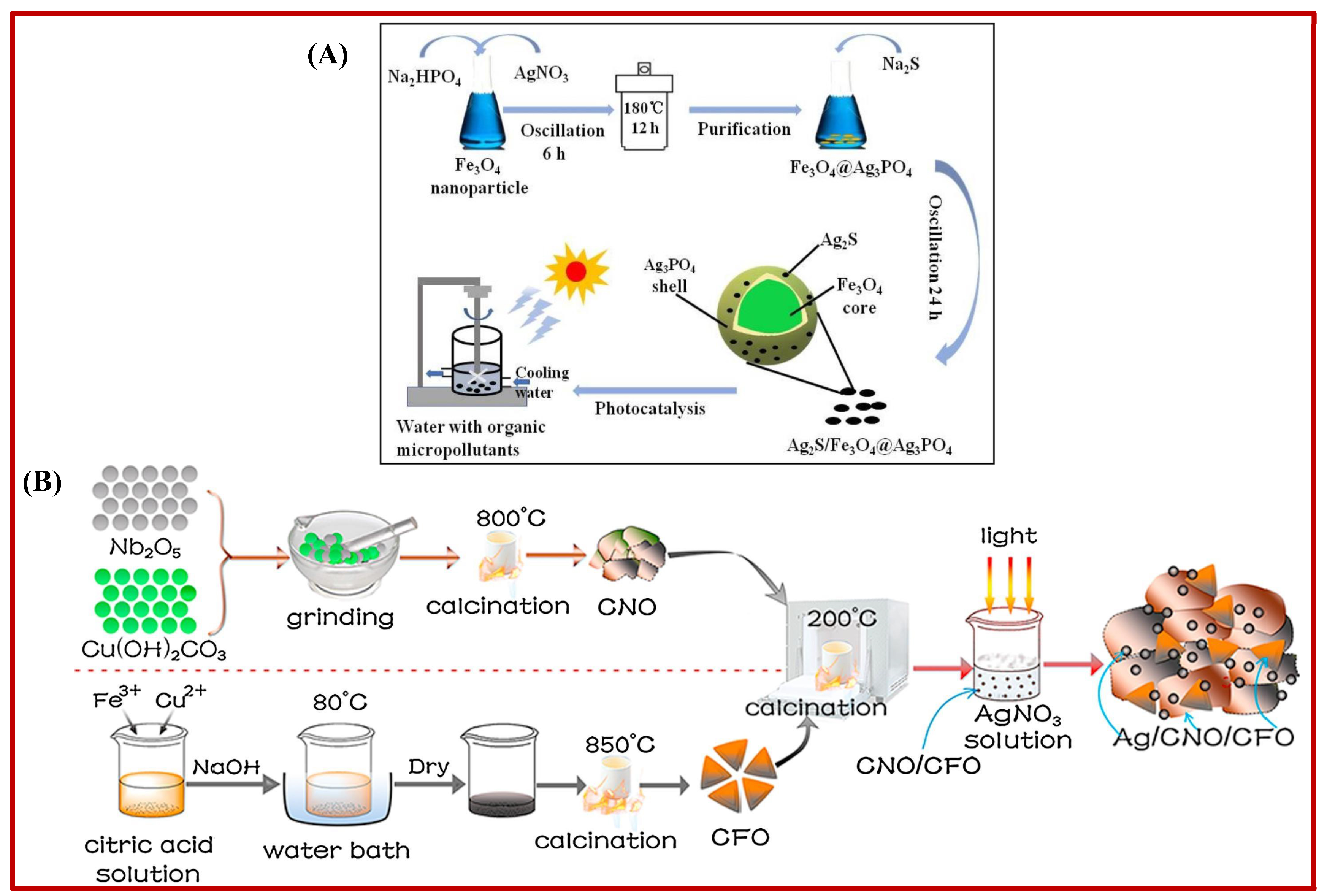
2.3. Magnetic Solid Photocatalysts
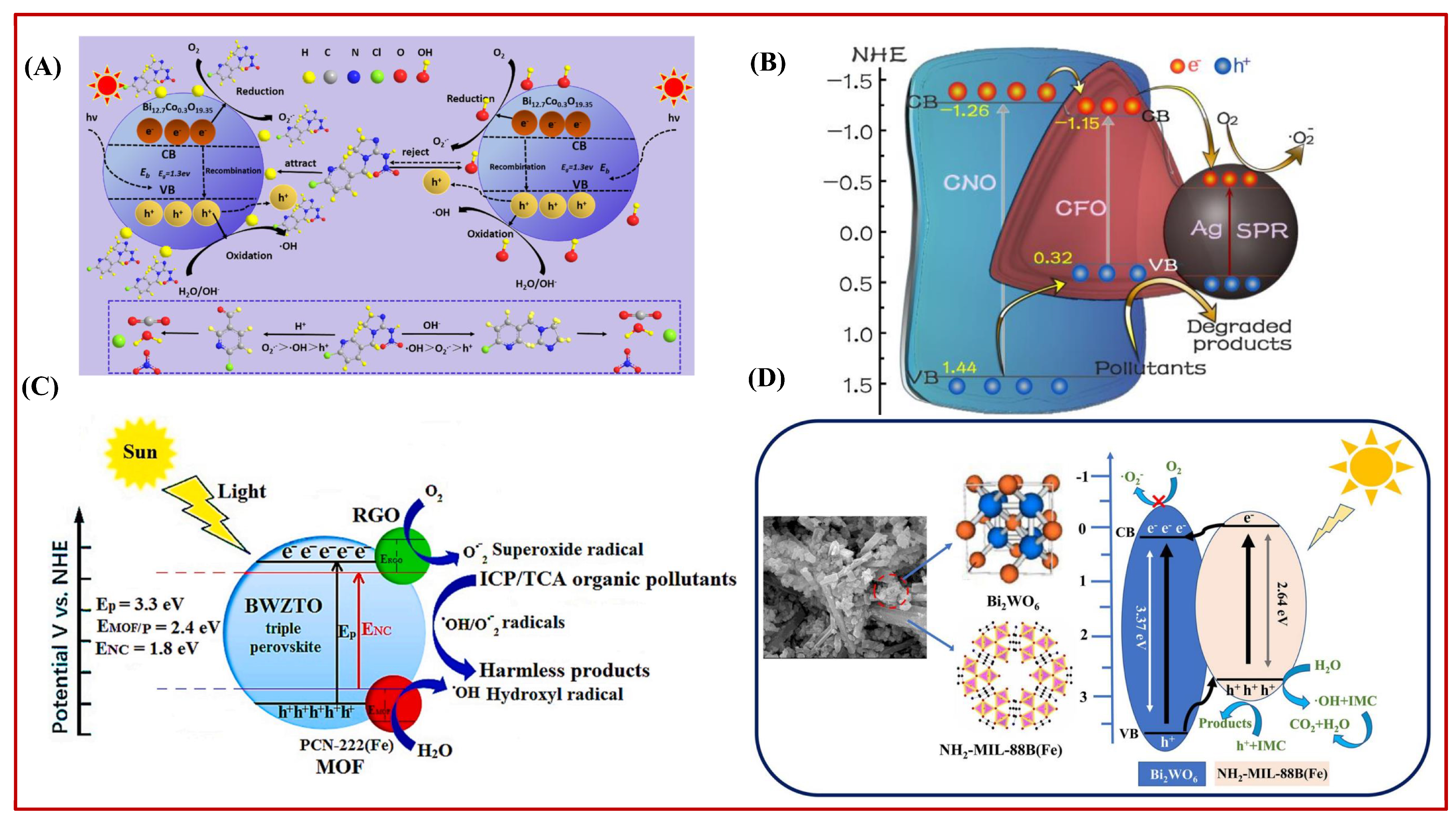
2.4. Other Metal Oxide Solid Photocatalysts
3. Photocatalytic Degradation of IMI Using Carbon-Based Photocatalysts
3.1. Graphitic Carbon Nitride (g-C3N4)-Based Solid Photocatalysts
3.2. Graphene-Oxide-Based (RGO) and Carbon Quantum Dots (CQD)-Based Solid Photocatalysts
4. Photocatalytic Degradation of IMI Using Metal–Organic-Framework-Based Solid Photocatalysts
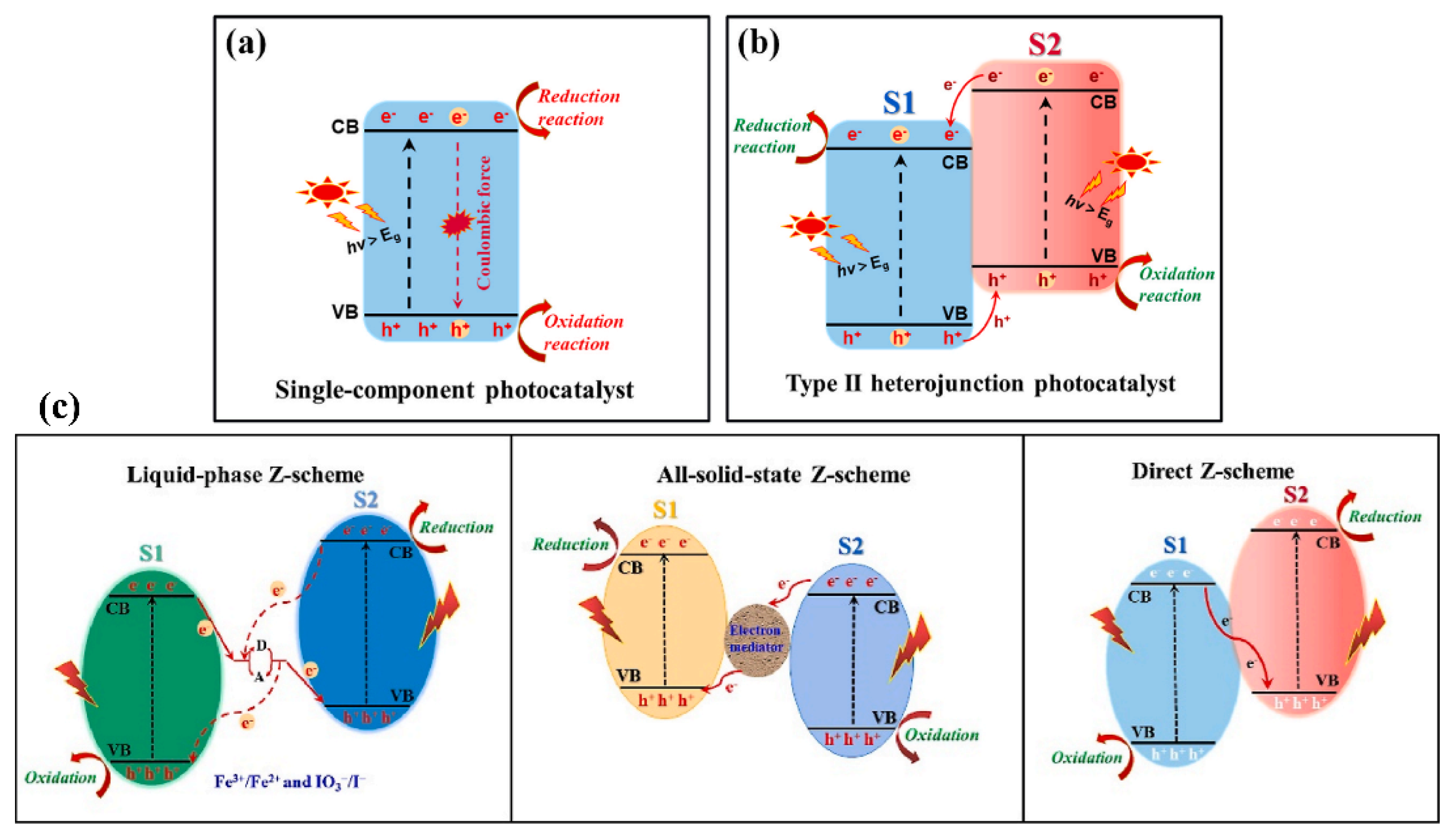
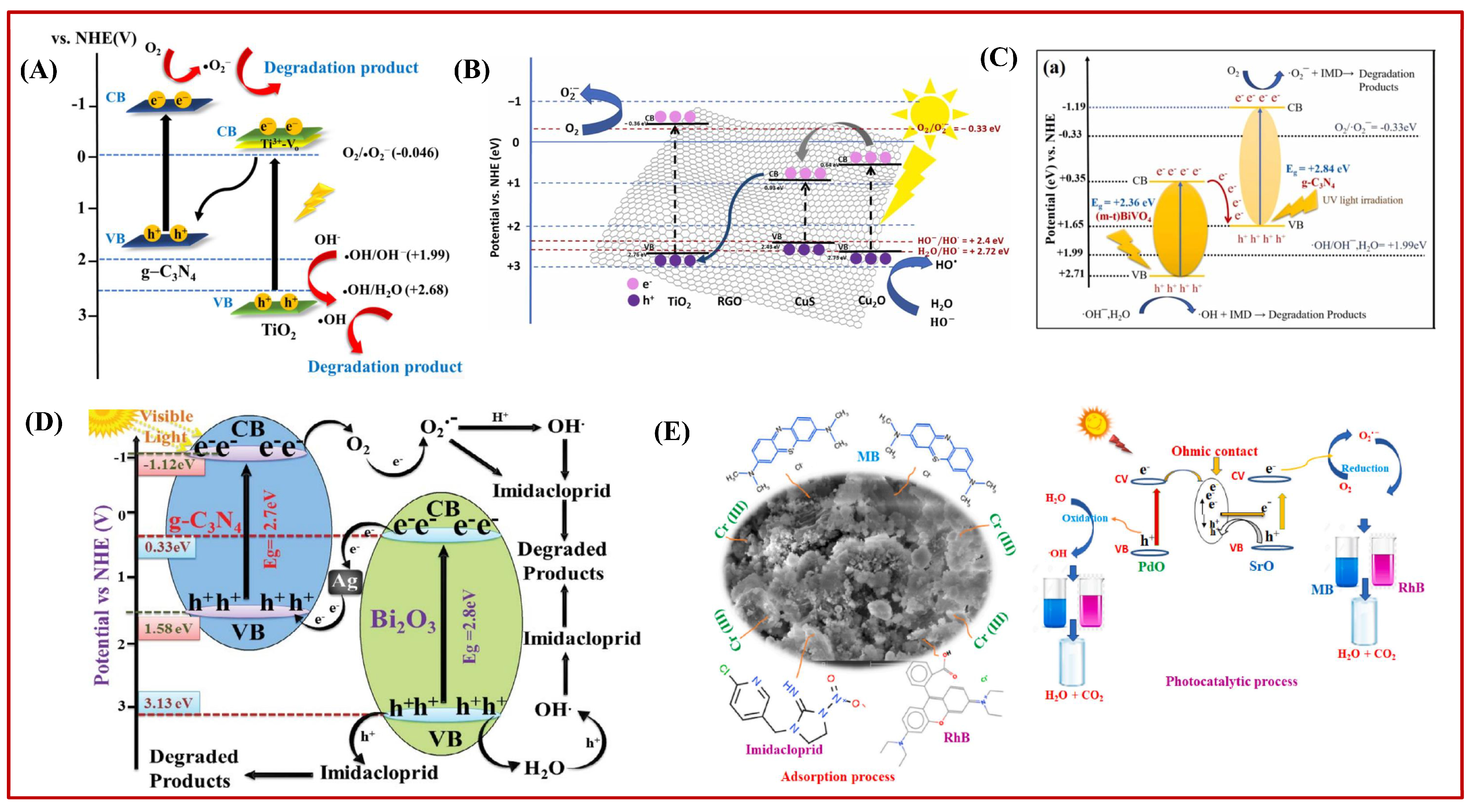
5. Possible Mechanism of Photocatalytic Degradation of IMI
5.1. Type I and Type Ⅱ Heterojunction
5.2. Z-Scheme Photocatalytic Mechanism
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| List of Abbreviations | |
| ACN | acidified carbon nitride |
| AIBN | azobisisobutyronitrile |
| AOPs | advanced oxidation processes |
| CB | conduction band |
| CNT | carbon nanotubes |
| CQD | carbon quantum dots |
| e– | electrons |
| Eg | bandgap |
| EGDMA | ethylene glycol dimethacrylate |
| g-CN | graphitic carbon nitride |
| GO | graphene oxide |
| h+ | holes |
| HPW | H3PW12O40 |
| IIhv | incident photons |
| IMI | Imidacloprid |
| KPW | potassium phosphotungstate |
| MCN | modified carbon nitride |
| MIPs | molecularly imprinted polymer |
| MOFs | metal–organic frameworks |
| MPW | phosphotungstic melamine |
| NPs | nanoparticles |
| OCN | oxygen-doped graphite carbon nitride |
| PANI | polyaniline |
| PCN | phosphorus-doped g-CN |
| PI | polyimide |
| PMS | peroxymonosulfate |
| PWO | phosphorous tungsten trioxide |
| RGO | reduced graphene oxide |
| ROS | reactive oxygen species |
| TCN | carbonitride/tungstophosphate acid |
| TOC | total organic carbon |
| VB | valence band |
| ZIF | zeolitic imidazolate framework |
References
- Paleolog, J.; Wilde, J.; Gancarz, M.; Wiącek, D.; Nawrocka, A.; Strachecka, A. Imidacloprid Pesticide Causes Unexpectedly Severe Bioelement Deficiencies and Imbalance in Honey Bees Even at Sublethal Doses. Animals 2023, 12, 615. [Google Scholar] [CrossRef] [PubMed]
- Keshvardoostchokami, M.; Bigverdi, P.; Zamani, A.; Parizanganeh, A.; Piri, F. Silver@ graphene oxide nanocomposite: Synthesize and application in removal of imidacloprid from contaminated waters. Environ. Sci. Pollut. Res. 2017, 25, 6751–6761. [Google Scholar] [CrossRef] [PubMed]
- Singla, A.; Barmota, H.; Kumar Sahoo, S.; Kaur Kang, B. Influence of neonicotinoids on pollinators: A review. J. Apic. Res. 2020, 60, 19–32. [Google Scholar] [CrossRef]
- Kalhor, M.M.; Rafati, A.A.; Rafati, L.; Rafati, A.A. Synthesis, characterization and adsorption studies of amino functionalized silica nano hollow sphere as an efficient adsorbent for removal of imidacloprid pesticide. J. Mol. Liq. 2018, 266, 453–459. [Google Scholar] [CrossRef]
- Bhende, R.S.; Jhariya, U.; Srivastava, S.; Bombaywala, S.; Das, S.; Dafale, N.A. Environmental Distribution, Metabolic Fate, and Degradation Mechanism of Chlorpyrifos: Recent and Future Perspectives. Biotechnol. Appl. Biochem. 2022, 194, 2301–2335. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, T.; Lu, J.; Li, Z. Seawater quality criteria derivation and ecological risk assessment for the neonicotinoid insecticide imidacloprid in China. Mar. Pollut. Bull. 2023, 190, 114871. [Google Scholar] [CrossRef] [PubMed]
- Bhende, R.S.; Dafale, N.A. Insights into the ubiquity, persistence and microbial intervention of imidacloprid. Arch. Microbiol. 2023, 205, 215. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.L.; Harmon-Threatt, A.N. Chronic contact with imidacloprid during development may decrease female solitary bee foraging ability and increase male competitive ability for mates. Chemosphere 2021, 283, 131177. [Google Scholar] [CrossRef]
- Baihetiyaer, B.; Jiang, N.; Li, X.; Song, J.; Wang, J.; Fan, X.; Zuo, Y.; Yin, X. Exploring the toxicity of biodegradable microplastics and imidacloprid to earthworms (Eisenia fetida) from morphological and gut microbial perspectives. Environ. Pollut. 2023, 337, 122547. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.D.; Nguyen, D.Q.; Do, P.T.; Tran, U.N.P. Kinetics of photocatalytic degradation of organic compounds: A mini-review and new approach. RSC Adv. 2023, 13, 16915–16925. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Wang, Y.; Jiang, Z.; Deng, B.; Jiang, Z.-J. Advances in the Removal of Organic Pollutants from Water by Photocatalytic Activation of Persulfate: Photocatalyst Modification Strategy and Reaction Mechanism. ChemSusChem 2024, 17, e202400254. [Google Scholar] [CrossRef]
- Mirhosseini, H.; Mostafavi, A.; Shamspur, T.; Sargazi, G. Preparation of novel ternary g-C3N4/WO3/ZnO nanocomposite adsorbent with highly effective imidacloprid removal: Optimization design and a controllable systematic study. J. Mater. Sci. Mater. Electron. 2020, 31, 17903–17920. [Google Scholar] [CrossRef]
- Palariya, D.; Mehtab, S.; Aziz, M.; Zaidi, M.G.H. Exploring Rare Earth Element Doped Nanocomposites as Promising Photocatalysts for Dyes Degradation in Water. Water Air Soil Pollut. 2024, 235, 286. [Google Scholar] [CrossRef]
- Nikbakht Fini, M.; Madsen, H.T.; Muff, J. The effect of water matrix, feed concentration and recovery on the rejection of pesticides using NF/RO membranes in water treatment. Sep. Purif. Technol. 2019, 215, 521–527. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, C.; Ren, X.; Tan, X.; Song, G.; Chen, D.; Alsaedi, A.; Hayat, T. Coupling g-C3N4 nanosheets with metal-organic frameworks as 2D/3D composite for the synergetic removal of uranyl ions from aqueous solution. J. Colloid Interface Sci. 2019, 550, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2019, 27, 2522–2565. [Google Scholar] [CrossRef]
- Faka, V.; Griniezaki, M.; Kiriakidis, G.; Grilla, E.; Mantzavinos, D.; Mao, S.; Shen, S.; Frontistis, Z.; Binas, V. Solar light induced photocatalytic degradation of sulfamethoxazole by ZnWO4/CNNs nanocomposites. J. Photochem. Photobiol. A 2022, 432, 114108. [Google Scholar] [CrossRef]
- Senthil Rathi, B.; Ewe, L.S.; Sanjay, S.; Sujatha, S.; Yew, W.K.; Baskaran, R.; Tiong, S.K. Recent trends and advancement in metal oxide nanoparticles for the degradation of dyes: Synthesis, mechanism, types and its application. Nanotoxicology 2024, 18, 272–298. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, J.K.; Paikra, S.K.; Mishra, M.; Sahoo, H. Amine functionalized magnetic iron oxide nanoparticles: Synthesis, antibacterial activity and rapid removal of Congo red dye. J. Mol. Liq. 2019, 282, 428–440. [Google Scholar] [CrossRef]
- Andronic, L.; Vladescu, A.; Enesca, A. Synthesis, Characterisation, Photocatalytic Activity, and Aquatic Toxicity Evaluation of TiO2 Nanoparticles. Nanomaterials 2021, 11, 3197. [Google Scholar] [CrossRef]
- Andronic, L.; Lelis, M.; Enesca, A.; Karazhanov, S. Photocatalytic activity of defective black-titanium oxide photocatalysts towards pesticide degradation under UV/VIS irradiation. Surf. Interfaces 2022, 32, 102123. [Google Scholar] [CrossRef]
- Tihana, Č.; Ivana, P.; Ivana, C.; Andreja, G. Nanostructured TiO2 photocatalyst modified with Cu for improved imidacloprid degradation. Appl. Surf. Sci. 2021, 569, 151026. [Google Scholar]
- Heng, H.; Gan, Q.; Meng, P.; Liu, X. H3PW12O40/TiO2-In2O3: A visible light driven type-II heterojunction photocatalyst for the photocatalytic degradation of imidacloprid. RSC Adv. 2016, 6, 73301–73307. [Google Scholar] [CrossRef]
- Bogatu, C.; Covei, M.; Polo-López, M.I.; Duta, A.; Malato, S. Novel ZnO Photocatalysts for Pollutants’ Abatement under Solar Radiation at Pilot Plant Scale. Catal. Today 2022, 413, 113947. [Google Scholar] [CrossRef]
- Kaur, A.; Mehta, V.S.; Kaur, G.; Sud, D. Biopolymer templated strategized greener protocols for fabrication of ZnO nanostructures and their application in photocatalytic technology for phasing out priority pollutants. Environ. Sci. Pollut. Res. 2023, 30, 25663–25681. [Google Scholar] [CrossRef]
- Iqbal, A.; ul Haq, A.; Rios-Aspajo, L.; Iturriaga-Chavez, A. Bio-inspired synthesis of CuO and ZnO nanoparticles by hydrothermal method: Characterization and evaluation as photocatalytic degradation of imidacloprid pesticide. Glob. Nest J. 2023, 25, 150–158. [Google Scholar]
- Kanwal, M.; Tariq, S.R.; Chotana, G.A. Photocatalytic degradation of imidacloprid by Ag-ZnO composite. Environ. Sci. Pollut. Res. 2018, 25, 27307–27320. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Gul, T.; Ali, M.; Khan, I.; Khan, W.; Asghar, H.; Saeed, K. Preparation, Analysis and UV-Accelerated Photocatalytic Degradation of Pesticide Over Mg Doped ZnO/Nylon 6,6/PMMA Ternary Blend. J. Inorg. Organomet. Polym. Mater. 2023, 33, 3441–3453. [Google Scholar] [CrossRef]
- Akbari, S.; Moussavi, G.; Decker, J.; Marin, M.L.; Bosca, F.; Giannakis, S. Superior visible light-mediated catalytic activity of a novel N-doped, Fe3O4-incorporating MgO nanosheet in presence of PMS: Imidacloprid degradation and implications on simultaneous bacterial inactivation. Appl. Catal. B Environ. 2022, 317, 12137. [Google Scholar] [CrossRef]
- Shi, E.; Xu, Z.; Wang, W.; Xu, Y.; Zhang, Y.; Yang, X.; Liu, Q.; Zeng, T.; Song, S.; Jiang, Y.; et al. Ag2S-doped core-shell nanostructures of Fe3O4@Ag3PO4 ultrathin film: Major role of hole in rapid degradation of pollutants under visible light irradiation. Chem. Eng. J. 2019, 366, 123–132. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, D.; Li, Y.; Huang, B.; Li, H.; Pu, X.; Geng, Y. Fabrication of magnetically recoverable Ag/CuNb2O6/CuFe2O4 ternary heterojunction composite for highly efficient photocatalytic degradation of pollutants. Sep. Purif. Technol. 2019, 220, 78–88. [Google Scholar] [CrossRef]
- Silva, R.R.M.; Valenzuela, L.; Rosal, R.; Ruotolo, L.A.M.; Nogueira, F.G.E.; Bahamonde, A. Peroxymonosulfate activation by Co3O4 coatings for imidacloprid degradation in a continuous flow-cell reactor under simulated solar irradiation. J. Environ. Chem. Eng. 2023, 11, 109265. [Google Scholar] [CrossRef]
- Andronic, L.; Mamedov, D.; Cazan, C.; Popa, M.; Chifiriuc, M.C.; Allaniyazov, A.; Palencsar, S.; Karazhanov, S.Z. Cerium oxide thin films: Synthesis, characterization, photocatalytic activity and influence on microbial growth. Biofouling 2022, 38, 865–875. [Google Scholar] [CrossRef]
- Meng, P.; Heng, H.; Sun, Y.; Liu, X. In situ polymerization synthesis of Z-scheme tungsten trioxide/polyimide photocatalyst with enhanced visible-light photocatalytic activity. Appl. Surf. Sci. 2018, 428, 1130–1140. [Google Scholar] [CrossRef]
- Mohanta, D.; Ahmaruzzaman, M. Au-SnO2-CdS ternary nanoheterojunction composite for enhanced visible light-induced photodegradation of imidacloprid. Environ. Res. 2021, 201, 111586. [Google Scholar] [CrossRef]
- Weng, J.; Chen, J.; Xu, Y.; Hu, X.; Guo, C.; Yang, Y.; Sun, J.; Fu, L.; Wang, Q.; Wei, J.; et al. Engineering highly dispersed AgI nanoparticles on hierarchical In2S3 hollow nanotube to construct Z-scheme heterojunction for efficient photodegradation of insecticide imidacloprid. J. Colloid Interface Sci. 2023, 652, 1367–1380. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S. Highly reusable visible light active hierarchical porous WO3/SiO2 monolith in centimeter length scale for enhanced photocatalytic degradation of toxic pollutants. Sep. Purif. Technol. 2019, 231, 115916. [Google Scholar] [CrossRef]
- Fang, L.; Yu-Hang, Z.; Bing, H.; Jiao, Y.; Qi, S.; Fa-Nian, S. Enhanced photocatalytic degradation of imidacloprid and RhB by the precursor derived Bi12.7Co0.3O19.35 under different pH value. J. Phys. Chem. Solids 2022, 164, 110638. [Google Scholar]
- Nyankson, E.; Agyei-Tuffour, B.; Adjasoo, J.; Ebenezer, A.; Dodoo-Arhin, D.; Yaya, A.; Mensah, B.; Efavi, J.K. Synthesis and Application of Fe-Doped TiO2-Halloysite Nanotubes Composite and Their Potential Application in Water Treatment. Adv. Mater. Sci. Eng. 2019, 2019, 4270310. [Google Scholar] [CrossRef]
- Lee, K.; Mazare, A.; Schmuki, P. One-Dimensional Titanium Dioxide Nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Inde, R.; Nishikawa, M.; Qiu, X.; Atarashi, D.; Sakai, E.; Nosaka, Y.; Hashimoto, K.; Miyauchi, M. Enhanced Photoactivity with Nanocluster-Grafted Titanium Dioxide Photocatalysts. ACS Nano 2014, 8, 7229–7238. [Google Scholar] [CrossRef] [PubMed]
- Eley, C.; Li, T.; Liao, F.; Fairclough, S.M.; Smith, J.M.; Smith, G.; Tsang, S.C.E. Nanojunction-Mediated Photocatalytic Enhancement in Heterostructured CdS/ZnO, CdSe/ZnO, and CdTe/ZnO Nanocrystals. Angew. Chem. Int. Ed. 2014, 53, 7838–7842. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, H.; Wu, H.; Yang, Y.; Wang, C.; Wang, Q.; Jia, B.; Zheng, J. Research progress on zinc oxide-based heterojunction photocatalysts. J. Mater. Chem. A 2024, 12, 20838–20867. [Google Scholar] [CrossRef]
- Chen, M.-L.; Li, S.-S.; Wen, L.; Xu, Z.; Li, H.-H.; Ding, L.; Cheng, Y.-H. Exploration of double Z-type ternary composite long-afterglow/graphitic carbon nitride@metal–organic framework for photocatalytic degradation of methylene blue. J. Colloid Interface Sci. 2023, 629, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yi, J.; She, X.; Liu, Q.; Song, L.; Chen, S.; Yang, Y.; Song, Y.; Vajtai, R.; Lou, J.; et al. 2D heterostructure comprised of metallic 1T-MoS2 Monolayer O-g-C3N4 towards efficient photocatalytic hydrogen evolution. Appl. Catal. B 2018, 220, 379–385. [Google Scholar] [CrossRef]
- Thaweesak, S.; Lyu, M.; Peerakiatkhajohn, P.; Butburee, T.; Luo, B.; Chen, H.; Wang, L. Two-dimensional g-C3N4/Ca2Nb2TaO10 nanosheet composites for efficient visible light photocatalytic hydrogen evolution. Appl. Catal. B 2017, 202, 184–190. [Google Scholar] [CrossRef]
- Stefa, S.; Skliri, E.; Gagaoudakis, E.; Kiriakidis, G.; Kotzias, D.; Papagiannakopoulos, P.; Konsolakis, M.; Mao, S.; Binas, V. Visible light photocatalytic oxidation of NO using g-C3N4 nanosheets: Stability, kinetics, and effect of humidity. Appl. Phys. A 2024, 130, 451. [Google Scholar] [CrossRef]
- Sh, X.; Wu, J.; Zhong, J.; Xu, H.; Yang, Y.; Vajtai, R.; Lou, J.; Liu, Y.; Du, D.; Li, H.; et al. Oxygenated monolayer carbon nitride for excellent photocatalytic hydrogen evolution and external quantum efficiency. Nano Energy 2016, 27, 138–146. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2017, 8, 1701503. [Google Scholar] [CrossRef]
- Stefa, S.; Zografaki, M.; Dimitropoulos, M.; Paterakis, G.; Galiotis, C.; Sangeetha, P.; Kiriakidis, G.; Konsolakis, M.; Binas, V. High surface area g-C3N4 nanosheets as superior solar-light photocatalyst for the degradation of parabens. Appl. Phys. A 2023, 129, 754. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.; Zhang, B.; Yuan, M.; Ma, Y.; Kong, F. A facile strategy for photocatalytic degradation of seven neonicotinoids over sulfur and oxygen co-doped carbon nitride. Chemosphere 2020, 253, 126672. [Google Scholar] [CrossRef] [PubMed]
- Sudhaik, A.; Raizada, P.; Thakur, S.; Saini, R.V.; Saini, A.K.; Singh, P.; Kumar Thakur, V.; Nguyen, V.-H.; Khan, A.A.P.; Asiri, A.M. Synergistic photocatalytic mitigation of imidacloprid pesticide and antibacterial activity using carbon nanotube decorated phosphorus doped graphitic carbon nitride photocatalyst. J. Taiwan Inst. Chem. Eng. 2020, 113, 142–154. [Google Scholar] [CrossRef]
- Faisal, M.; Rashed, M.A.; Ahmed, J.; Alsaiari, M.; Jalalah, M.; Alsareii, S.A.; Harraz, F.A. Au nanoparticles decorated polypyrrole-carbon black/g-C3N4 nanocomposite as ultrafast and efficient visible light photocatalys. Chemosphere 2021, 287, 131984. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Gupta, R.; Bansal, A. Photocatalytic degradation of imidacloprid using semiconductor hybrid nano-catalyst: Kinetics, surface reactions and degradation pathways. Int. J. Environ. Sci. Te 2020, 18, 1425–1442. [Google Scholar] [CrossRef]
- Kobkeatthawin, T.; Trakulmututa, J.; Amornsakchai, T.; Kajitvichyanukul, P.; Smith, S.M. Identification of Active Species in Photodegradation of Aqueous Imidacloprid over g-C3N4/TiO2 Nanocomposites. Catalysis 2022, 12, 120. [Google Scholar] [CrossRef]
- Liang, S.; Zhang, D.; Pu, X.; Yao, X.; Han, R.; Yin, J.; Ren, X. A novel Ag2O/g-C3N4 p-n heterojunction photocatalysts with enhanced visible and near-infrared light activity. Sep. Purif. Technol. 2019, 210, 786–797. [Google Scholar] [CrossRef]
- Raizada, P.; Sudhaik, A.; Singh, P.; Hosseini-Bandegharaei, A.; Gupta, V.K.; Agarwal, S. Silver-mediated Bi2O3 and graphitic carbon nitride nanocomposite as all solid state Z scheme photocatalyst for imidacloprid pesticide abatement from water. Desalination Water Treat. 2019, 171, 344–355. [Google Scholar] [CrossRef]
- Kundu, A.; Sharma, S.; Basu, S. ModulModulated BiOCl nanoplates with porous g-C3N4 nanosheets for photocatalytic degradation of color/colorless pollutants in natural sunlight. J. Phys. Chem. Solids 2021, 154, 100064. [Google Scholar] [CrossRef]
- Patial, B.; Bansal, A.; Gupta, R.; Mittal, S.K. Hydrothermal synthesis of (m-t)BiVO4/g-C3N4 heterojunction for enhancement in photocatalytic degradation of imidacloprid. J. Environ. Chem. Eng. 2023, 11, 111138. [Google Scholar] [CrossRef]
- Tan, J.; Li, Z.; Li, J.; Meng, Y.; Yao, X.; Wang, Y.; Lu, Y.; Zhang, T. Visible-light-assisted peroxymonosulfate activation by metal-free bifunctional oxygen-doped graphitic carbon nitride for enhanced degradation of imidacloprid: Role of non-photochemical and photocatalytic activation pathway. J. Hazard. Mater. 2021, 423, 127048. [Google Scholar] [CrossRef]
- Sun, Y.; Meng, P.; Liu, X. Self-assembly of tungstophosphoric acid/acidified carbon nitride hybrids with enhanced visible-light-driven photocatalytic activity for the degradation of imidacloprid and acetamiprid. Appl. Surf. Sci. 2018, 456, 259–269. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, X. Efficient visible-light photocatalytic degradation of imidacloprid and acetamiprid using a modified carbon nitride/tungstophosphoric acid composite induced by a nucleophilic addition reaction. Appl. Surf. Sci. 2019, 485, 423–431. [Google Scholar] [CrossRef]
- Xiao, Z.; Huang, X.; Tang, H.; Liu, X. Modified carbon nitride incorporating Keggin-type potassium phosphotungstate enhances photocatalytic degradation of imidacloprid under visible light. Mater. Sci. Semicond. Process 2024, 171, 108001. [Google Scholar] [CrossRef]
- Meng, P.; Huang, J.; Liu, X. In-situ solid phase thermal transformation of self-assembled melamine phosphotungstates produce efficient visible light photocatalysts. J. Colloid Interface Sci. 2019, 551, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, V.; Ramalingam, M.; Sekar, K.; Chuaicham, C.; Sasaki, K. Fabrication and characterization of carbon quantum dots decorated hollow porous graphitic carbon nitride through polyaniline for photocatalysis. Chem. Eng. J. 2021, 426, 131739. [Google Scholar] [CrossRef]
- Sudhaik, A.; Raizada, P.; Singh, P.; Hosseini-Bandegharaei, A.; Thakur, V.K.; Nguyen, V.-H. Highly effective degradation of imidacloprid by H2O2/fullerene decorated P-doped g-C3N4 photocatalyst. J. Environ. Chem. Eng. 2020, 8, 104483. [Google Scholar] [CrossRef]
- Inoue, T.; Chuaicham, C.; Saito, N.; Ohtani, B.; Sasaki, K. Z-scheme heterojunction of graphitic carbon nitride and calcium ferrite in converter slag for the photocatalytic imidacloprid degradation and hydrogen evolution. J. Photochem. Photobiol. A 2023, 440, 114644. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, D.; Wang, Y.; Chang, Y.; Zhang, D.; Tang, Y.; Pu, X.; Shao, X. Facial synthesis of a novel Ag4V2O7/g-C3N4 heterostructure with highly efficient photoactivity. J. Am. Ceram. 2019, 102, 3897–3907. [Google Scholar] [CrossRef]
- Huang, X.; Wang, W.; Liu, X. H3PW12O40-doped pyromellitic diimide prepared via thermal transformation as an efficient visible-light photocatalyst. J. Mater. Sci. 2020, 55, 8502–8512. [Google Scholar] [CrossRef]
- Abuzeyad, O.H.; El-Khawaga, A.M.; Tantawy, H.; Elsayed, M.A. An evaluation of the improved catalytic performance of rGO/GO-hybrid-nanomaterials in photocatalytic degradation and antibacterial activity processes for wastewater treatment: A review. J. Mol. Struct. 2023, 1288, 135787. [Google Scholar] [CrossRef]
- Xia, X.; Song, M.; Wang, H.; Zhang, X.; Sui, N.; Zhang, Q.; Colvin, V.L.; Yu, W.W. Latest progress in constructing solid-state Z scheme photocatalysts for water splitting. Nanoscale 2019, 11, 11071–11082. [Google Scholar] [CrossRef] [PubMed]
- Gul, M.M.; Ahmad, K.S. Review elucidating graphene derivatives (GO/rGO) supported metal sulfides based hybrid nanocomposites for efficient photocatalytic dye degradation. Rev. Inorg. Chem. 2021, 42, 337–354. [Google Scholar] [CrossRef]
- Feng, J.; Ye, Y.; Xiao, M.; Wu, G.; Ke, Y. Synthetic routes of the reduced graphene oxide. Chem. Pap. 2020, 74, 3767–3783. [Google Scholar] [CrossRef]
- Wang, Z.-M.; Hirotsu, T.; Wu, H.; Kanoh, H. Advantaging Synergy Photocatalysis with Graphene-Related Carbon as a Counterpart Player of Titania. Chem. Rec. 2018, 19, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- El-Shafai, N.M.; El-Khouly, M.E.; El-Kemary, M.; Ramadan, M.S.; Derbalah, A.S.; Masoud, M.S. Fabrication and characterization of graphene oxide-titanium dioxide nanocomposite for degradation of some toxic insecticides. J. Ind. Eng. Chem. 2019, 69, 315–323. [Google Scholar] [CrossRef]
- Piao, M.; Sun, Y.; Wang, Y.; Teng, H. Preparation of BiVO4/RGO-TNT Nanomaterials for Efficient and Recyclable Photocatalysis of Imidacloprid Insecticide. Chem. Sel. 2022, 7, e202200182. [Google Scholar] [CrossRef]
- El-Shafai, N.M.; El-Shaer, A.; Eraky, M.R.; Ibrahim, M.M.; Ramadan, M.S.; El-Mehasseb, I.M. Enhancing electron density, electrochemical, and dielectric properties of nanohybrid materials for advanced photocatalytic antifouling and energy storage. Diam. Relat. Mater. 2021, 119, 108543. [Google Scholar] [CrossRef]
- Behera, L.; Barik, B.; Mohapatra, S. Improved photodegradation and antimicrobial activity of hydrothermally synthesized 0.2Ce-TiO2/RGO under visible light. Colloid Surf. A 2021, 620, 126553. [Google Scholar] [CrossRef]
- Andronic, L.; Abreu-Jaureguí, C.; Silvestre-Albero, J. Construction of TiO2@Cu2O-CuS heterostructures integrating RGO for enhanced full-spectrum photocatalytic degradation of organic pollutants. J. Alloys Compd. 2024, 994, 174628. [Google Scholar] [CrossRef]
- El-Gohary, R.M.; El-Shafai, N.M.; El-Mehasseb, I.M.; Mostafa, Y.S.; Alamri, S.A.; Beltagi, A.M. Removal of pollutants through photocatalysis, adsorption, and electrochemical sensing by a unique plasmonic structure of palladium and strontium oxide nanoparticles sandwiched between 2D nanolayers. J. Environ. Manag. 2024, 363, 121257. [Google Scholar] [CrossRef]
- Soltani-nezhad, F.; Saljooqi, A.; Shamspur, T.; Mostafavi, A. Photocatalytic degradation of imidacloprid using GO/Fe3O4/TiO2-NiO under visible radiation: Optimization by response level method. Polyhedron 2019, 165, 188–196. [Google Scholar] [CrossRef]
- Malik, A.Q.; Tabasum, S.; Rani, S.; Lokhande, P.; Singh, P.P.; Mooney, J.; Singh, J.; Alberto, H.-A.C.; Sharma, A.; Aepuru, R.; et al. Fluorescent CdS QDs Modified With Molecular Imprinted Polymer for the Photodegradation of Imidacloprid and Buprofezin Pesticides Under Visible Light. J. Inorg. Organomet. Polym. Mater. 2023, 33, 3468–3484. [Google Scholar] [CrossRef]
- Bressi, V.; Balu, A.M.; Iannazzo, D.; Espro, C. Recent advances in the synthesis of carbon dots from renewable biomass by high-efficient hydrothermal and microwave green approaches. Curr. Opin. Green Sustain. Chem. 2022, 40, 100742. [Google Scholar] [CrossRef]
- Wang, L.; Wang, T.; Hao, R.; Wang, Y. Synthesis and applications of biomass-derived porous carbon materials in energy utilization and environmental remediation. Chemosphere 2023, 339, 139635. [Google Scholar] [CrossRef]
- Roopan, S.M.; Prakash, S.H.; Manjupriya, R.; Afridha, M.S.H.F.; Rajesh, A.; Sneha, R.; Kumar, P.V.; Shobika, M. Biomass-derived carbon quantum dots-supported metal oxide composite for the photocatalytic degradation of toxic pollutants. Biomass Convers. Biorefinery 2024, 054227. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, W.; Wei, C.; Li, R.; Guo, J.; Liu, B. Titanium incorporated and g-C3N4-coated NH2-UiO-66 for enhanced photocatalytic hydrogen evolution. Appl. Phys. A 2020, 126, 594. [Google Scholar] [CrossRef]
- Guo, S.; Kong, L.-H.; Wang, P.; Yao, S.; Lu, T.-B.; Zhang, Z.-M. Switching Excited State Distribution of Metal–Organic Framework for Dramatically Boosting Photocatalysis. Angew. Chem. Int. Ed. 2022, 64, e202206193. [Google Scholar]
- Bogdan, L.; Palčić, A.; Duplančić, M.; Leskovac, M.; Tomašić, V. Eco-Friendly Synthesis of TiO2/ZIF-8 Composites: Characterization and Application for the Removal of Imidacloprid from Wastewater. Processes 2023, 11, 963. [Google Scholar] [CrossRef]
- Gecgel, C.; Simsek, U.B.; Gozmen, B.; Turabik, M. Comparison of MIL-101(Fe) and amine-functionalized MIL-101(Fe) as photocatalysts for the removal of imidacloprid in aqueous solution. J. Iran. Chem. Soc. 2019, 16, 1735–1748. [Google Scholar] [CrossRef]
- Kadkhodayan, H.; Alizadeh, T. Manufacturing visible-light-driven heterojunction photocatalyst based on MOFs/Bi2WZnTiO9 triple perovskite/carbonous materials for efficient removal of poisons, antibiotics, and inorganic pollutants. J. Phys. Chem. Solids 2023, 183, 111620. [Google Scholar] [CrossRef]
- Chen, M.-L.; Lu, T.-H.; Li, S.-S.; Wen, L.; Xu, Z.; Cheng, Y.-H. Photocatalytic degradation of imidacloprid by optimized Bi2WO6/NH2-MIL-88B(Fe) composite under visible light. Environ. Sci. Pollut. 2022, 29, 19583–19593. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-S.; Wen, L.; He, S.-W.; Xu, Z.; Ding, L.; Cheng, Y.-H.; Chen, M.-L. Enhanced photocatalytic degradation of imidacloprid by a simple Z-type binary heterojunction composite of long afterglow with metal-organic framework. Catal. Commun. 2023, 183, 106775. [Google Scholar] [CrossRef]
- Chen, M.-L.; Lu, T.-H.; Long, L.-L.; Xu, Z.; Ding, L.; Cheng, Y.-H. NH2-Fe-MILs for effective adsorption and Fenton-like degradation of imidacloprid: Removal performance and mechanism investigation. Environ. Eng. Res. 2022, 27, 200702. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, X.; Lu, J.; Zhang, J. Fine Tuning the Redox Potentials of Carbazolic Porous Organic Frameworks for Visible-Light Photoredox Catalytic Degradation of Lignin β-O-4 Models. ACS Catal. 2017, 7, 5062–5070. [Google Scholar] [CrossRef]
- Deng, F.; Peng, J.; Li, X.; Luo, X.; Ganguly, P.; Pillai, S.C.; Ren, B.; Ding, L.; Dionysiou, D.D. Metal sulfide-based Z-scheme heterojunctions in photocatalytic removal of contaminants, H2 evolution and CO2 reduction: Current status and future perspectives. J. Clean. Prod. 2023, 416, 137957. [Google Scholar] [CrossRef]
- Yuan, Y.; Guo, R.T.; Hong, L.F.; Ji, X.Y.; Lin, Z.D.; Li, Z.S.; Pan, W.G. A review of metal oxide-based Z-scheme heterojunction photocatalysts: Actualities and developments. Mater. Today Energy 2021, 21, 100829. [Google Scholar] [CrossRef]
- Li, H.; Tu, W.; Zhou, Y.; Zou, Z. Z-Scheme Photocatalytic Systems for Promoting Photocatalytic Performance: Recent Progress and Future Challenges. Adv. Sci. 2016, 3, 1500389. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, L.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Jaroniec, M. Direct Z-scheme photocatalysts: Principles, synthesis, and applications. Mater. Today 2018, 21, 1042–1063. [Google Scholar] [CrossRef]




| Photocatalyst | Light Source | Catalysis Time (min) | Catalyst Loading (g/L) | IMI Concentration (mg/L) | Efficiency (%) | Refs. |
|---|---|---|---|---|---|---|
| TiO2 | UV light | 360 | 0.6 | 20 | 90.0% | [20] |
| Black TiO2 | visible light | 360 | 1.0 | 20 | 90.0% | [21] |
| Cu-TiO2 | fluorescent bulb | 60 | 0.5 | 25 | 45.0% | [22] |
| HPW/TiO2-In2O3 | Xenon lamp (225 W) | 300 | 3.6 | 8 | 83.0% | [23] |
| ZnO | Xenon lamp | 120 | 0.2 | 5 | 92.0% | [24] |
| nano-ZnO | visible light | 30 | 2.0 | 50 | 96.6% | [25] |
| CuO | UV light | 50 | 0.5 | 30 | 99.0% | [26] |
| Ag-ZnO | UV light | 80 | 0.6 | 25 | 65.0% | [27] |
| Mg-ZnO/Nylon,6/PMMA | UV light | 240 | 2.5 | 10 | 78.0% | [28] |
| N-MgO@Fe3O4 | Xenon lamps | 60 | 0.15 | 10 | 94.7% | [29] |
| Ag2S/Fe3O4@Ag3PO4 | Xenon lamp (300 W) | 90 | 0.5 | 2 | 73.0% | [30] |
| Ag/CuNb2O6/CuFe2O4 | halogen lamp | 240 | 0.5 | 10 | 96.0% | [31] |
| Co3O4/PMS | solar irradiation | 120 | 0.4 | 2.5 | 99.0% | [32] |
| CeO2 | light tubes (18 W) | 360 | 0.15 | 20 | 30.0% | [33] |
| PWO/PI | Xenon lamp (225 W) | 180 | 2.5 | 20 | 73.0% | [34] |
| Au-SnO2-CdS | LED bulb | 180 | 0.03 | 1.5 | 95.0% | [35] |
| In2S3/AgI-300 | Xenon lamp (300 W) | 60 | 0.5 | 10 | 76.2% | [36] |
| WO3/SiO2 | UV light | 60 | 0.5 | 5 | 59.0% | [37] |
| Bi12.7Co0.3O19.35 | visible light | 240 | 1.0 | 10 | 96.0% | [38] |
| TiO2-Fe-HNT | UV light | 300 | 0.5 | 8 | 41.0% | [39] |
| Photocatalyst | Light Source | Catalysis Time (min) | Catalyst Loading (g/L) | IMI Concentration (mg/L) | Highest Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| SOCN8 | xenon lamp (300 W) | 300 | 1.0 | 20 | 91.4 | [51] |
| CNT/PCN | LED light | 540 | 0.6 | 10 | 93.0 | [52] |
| Au @PPy-C/g-C3N4 | visible light (250 W) | 25 | 3.0 | 20 | 96.0 | [53] |
| g-C3N4/ZnO | UV light | 35 | 0.6 | 20 | 95.6 | [54] |
| g-C3N4/TiO2 | W lamp (300 W) | 150 | 1.0 | 10 | 93.0 | [55] |
| Ag2O/g-C3N4 | halogen lamp (1000 W) | 120 | 1.0 | 10 | 80.0 | [56] |
| Ag-BO/GCN | LED light | 600 | 0.5 | 10 | 93.0 | [57] |
| g-C3N4@BiOCl | sunlight | 180 | 0.3 | 10 | 73.4 | [58] |
| g-C3N4/BiVO4 | UV light | 30 | 0.06 | 20 | 94.2 | [59] |
| OCN | xenon lamp (500 W) | 120 | 0.5 | 3.0 | 94.5 | [60] |
| HPW/ACN | CEL-LAX500 xenon lamp | 350 | 0.6 | 10 | 90.0 | [61] |
| MCN450/HPW | CEL-LAX500 xenon lamp | 180 | 0.7 | 10 | 96.0 | [62] |
| g-C3N4/KPW-0.2 | xenon lamp (300 W) | 180 | 2.0 | 20 | 91.7 | [63] |
| 13TCN-390 | xenon lamp (225 W) | 180 | 2.0 | 20 | ~90.0 | [64] |
| CN-PANI-CQDs | xenon lamp (500 W) | 70 | 1.0 | 10 | 80.1 | [65] |
| Catalyst | Light Source | Catalysis Time (min) | Catalyst Loading (g/L) | IMI Concentration (mg/L) | Highest Efficiency (%) | Refs. |
|---|---|---|---|---|---|---|
| GO@TiO2 | UV light | 30 | 0.5 | 100 | 92.6 | [75] |
| BiVO4/RGO-TNT | UV lamp (40 W) | 30 | 1.4 | 80 | 73.0 | [76] |
| GO@TiO2·ZnO·Ag HNM | visible light | 115 | 0.5 | 10 | 50.0 | [77] |
| Ce-TiO2/RGO | UV light | 480 | 0.5 | 10 | 85.0 | [78] |
| TiO2/RGO/Cu2O-CuS | Full-spectrum fluorescent bulb solar (160 W) | 360 | 1.0 | 20 | >95.0 | [79] |
| GO@PdO@rGO.SrO | visible light | 210 | 3.5 | 30 | 86.0 | [80] |
| GO/Fe3O4/TiO2-NiO | visible light | 30 | 2.5 | 10 | 97.3 | [81] |
| CdS/MIPs | Hg light (400 W) | 90 | 1.0 | 10 | 84.0 | [82] |
| Catalyst | Light Source | Catalysis Time (min) | Catalyst Loading (g/L) | IMI Concentration (mg/L) | Highest Efficiency (%) | Refs. |
|---|---|---|---|---|---|---|
| TiO2/ZIF-8 | UV light | 240 | 1.0 | 10 | 55.0 | [88] |
| MIL-101(Fe) | Blue LED light | 30 | - | 40 | 100.0 | [89] |
| MOF/BWZTO/RGO | UV visible | 300 | 0.6 | 15 | 90.0 | [90] |
| Bi2WO6/NH2-MIL-88B(Fe) | xenon lamp | 180 | 0.4 | 10 | 84.5 | [91] |
| SAO/NH2-UiO-66 | xenon lamp | 120 | 0.6 | 20 | 97.0 | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Li, S.; Sun, S.; Chen, M. Recent Advances in Photocatalytic Degradation of Imidacloprid in Aqueous Solutions Using Solid Catalysts. Catalysts 2024, 14, 878. https://doi.org/10.3390/catal14120878
Gao S, Li S, Sun S, Chen M. Recent Advances in Photocatalytic Degradation of Imidacloprid in Aqueous Solutions Using Solid Catalysts. Catalysts. 2024; 14(12):878. https://doi.org/10.3390/catal14120878
Chicago/Turabian StyleGao, Song, Shanshan Li, Shaofan Sun, and Maolong Chen. 2024. "Recent Advances in Photocatalytic Degradation of Imidacloprid in Aqueous Solutions Using Solid Catalysts" Catalysts 14, no. 12: 878. https://doi.org/10.3390/catal14120878
APA StyleGao, S., Li, S., Sun, S., & Chen, M. (2024). Recent Advances in Photocatalytic Degradation of Imidacloprid in Aqueous Solutions Using Solid Catalysts. Catalysts, 14(12), 878. https://doi.org/10.3390/catal14120878






