Enhancing RNA-Based Technologies Using Enzyme-Derived Lipoamino Acids
Abstract
1. Introduction
2. Results and Discussion
2.1. Enzymatic Production of LAAs
2.2. Self-Assembly Behavior of Surfactants
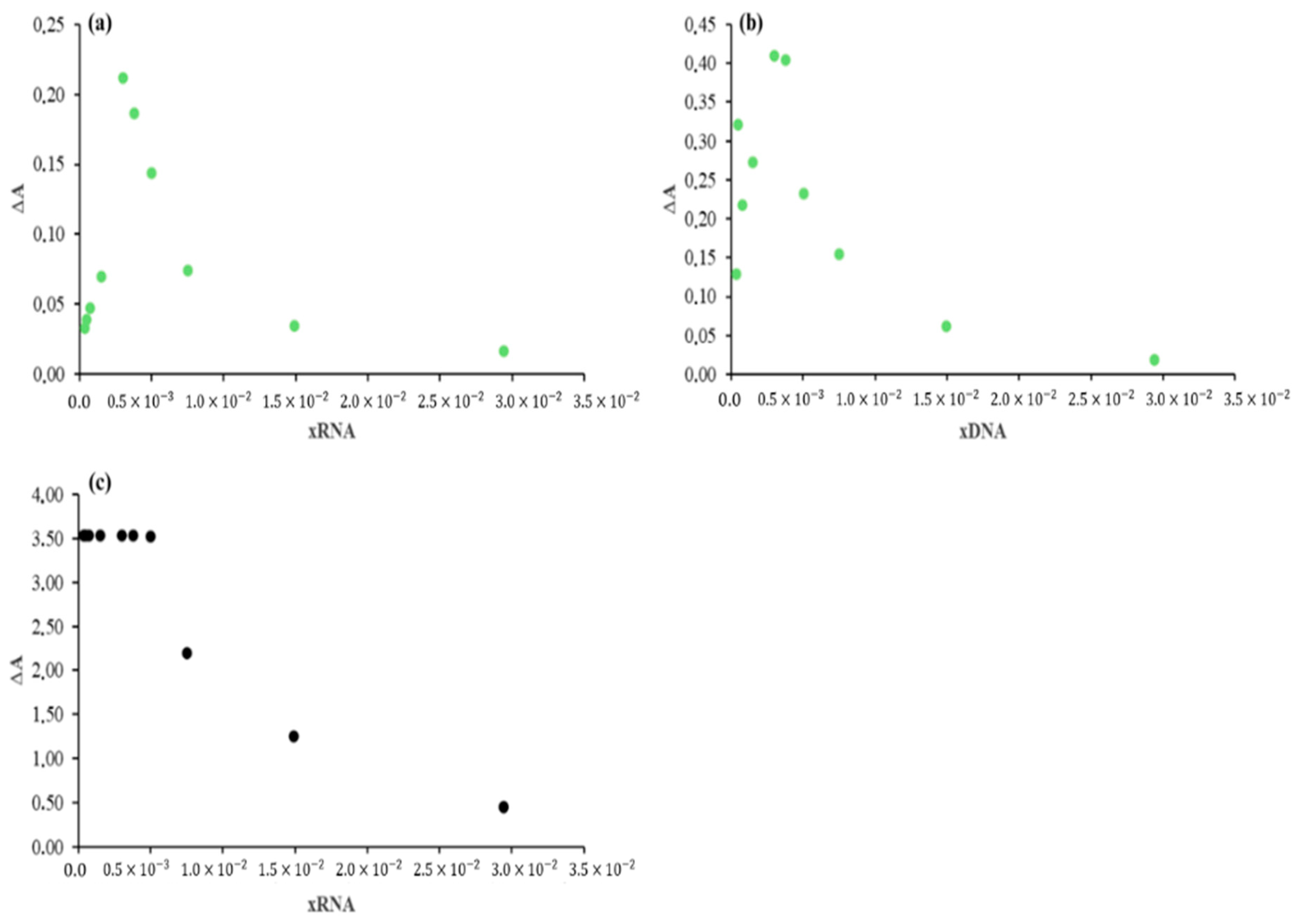
2.3. Surfactant–Nucleic Acid Interactions
2.3.1. Surfactant Effect
2.3.2. Effect of RNA Concentrations
2.3.3. Ethidium Bromide Fluorescence Displacement Assays
2.4. Viscosity
3. Experimental Section
3.1. Enzymes
3.2. Substrates and Nucleic Acids
3.3. Other Chemicals
3.4. Analytic Methods
3.5. Software
3.6. Magnetized Sol-Gel Lenses for Enzyme Encapsulation
3.7. Biosynthesis of LAAs
3.7.1. Synthesis in Tris Buffer 10 mM, pH 9
3.7.2. Solvent-Free Systems
3.8. Nucleic Acid Assays
3.8.1. Conductivity Measurements
3.8.2. Absorbance Assays at 260 nm
3.8.3. Viscosity Assays
Pre-CMC and Post-CMC Assays
Ethidium Bromide Exclusion Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rodrigues, L.; Banat, I.M.; Teixeira, J.; Oliveira, R. Biosurfactants: Potential applications in medicine. J. Antimicrob. Chemother. 2006, 57, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Faustino, C.; Martins, T.; Duarte, N.; Ribeiro, M.H. Self-Assembly of Lipoaminoacids-DNA Based on Thermodynamic and Aggregation Properties. J. Surfactants Deterg. 2020, 23, 581–593. [Google Scholar] [CrossRef]
- Yokouchi, Y.; Suzuki, S.; Ohtsuki, N.; Yamamoto, K.; Noguchi, S.; Soejima, Y.; Goto, M.; Ishioka, K.; Nakamura, I.; Suzuki, S.; et al. Rapid repair of human disease-specific single-nucleotide variants by One-SHOT genome editing. Sci. Rep. 2020, 10, 13927. [Google Scholar] [CrossRef]
- Scheller, E.L.; Krebsbach, P.H. Gene Therapy. J. Dent. Res. 2009, 88, 585–596. [Google Scholar] [CrossRef]
- Ramamoorth, M.; Narvekar, A. Non Viral Vectors in Gene Therapy—An Overview. J. Clin. Diagn. Res. JCDR 2015, 9, GE01–GE06. [Google Scholar] [CrossRef] [PubMed]
- Escoffre, J.M.; Teissié, J.; Rols, M.P. Gene Transfer: How Can the Biological Barriers Be Overcome? J. Membr. Biol. 2010, 236, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Wettig, S.D.; Verrall, R.E.; Foldvari, M. Gemini surfactants: A new family of building blocks for non-viral gene delivery systems. Curr. Gene Ther. 2008, 8, 9–23. [Google Scholar] [CrossRef]
- Friedmann, T. Overcoming the Obstacles to Gene Therapy. Sci. Am. 1997, 276, 96–101. [Google Scholar] [CrossRef]
- Anson, D.S. The use of retroviral vectors for gene therapy-what are the risks? A review of retroviral pathogenesis and its relevance to retroviral vector-mediated gene delivery. Genet. Vaccines Ther. 2004, 2, 9. [Google Scholar] [CrossRef]
- Yin, H.; Kanasty, R.; Eltoukhy, A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef]
- Niidome, T.; Huang, L. Gene Therapy Progress and Prospects: Nonviral vectors. Gene Ther. 2002, 9, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Carvalho, P.; Martins, T.; Faustino, C. Lipoaminoacids Enzyme-Based Production and Application as Gene Delivery Vectors. Catalysts 2019, 9, 977. [Google Scholar] [CrossRef]
- Wu, P.; Chen, H.; Jin, R.; Weng, T.; Ho, J.K.; You, C.; Zhang, L.; Wang, X.; Han, C. Non-viral gene delivery systems for tissue repair and regeneration. J. Transl. Med. 2018, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Morán, M.C.; Pinazo, A.; Pérez, L.; Clapés, P.; Angelet, M.; García, M.T.; Vinardell, M.P.; Infante, M.R. “Green” amino acid-based surfactants. Green Chem. 2004, 6, 233–240. [Google Scholar] [CrossRef]
- Gonçalves, R.A.; Holmberg, K.; Lindman, B. Cationic surfactants: A review. J. Mol. Liq. 2023, 375, 121335. [Google Scholar] [CrossRef]
- Dias, R.S.; Magno, L.M.; Valente, A.J.M.; Das, D.; Das, P.K.; Maiti, S.; Miguel, M.G.; Lindman, B. Interaction between DNA and Cationic Surfactants: Effect of DNA Conformation and Surfactant Headgroup. J. Phys. Chem. B 2008, 112, 14446–14452. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Tao, W.; Zhu, Y.; Yao, S. Comparative study on the interaction of DNA with three different kinds of surfactants and the formation of multilayer films. Bioelectrochemistry 2007, 70, 301–307. [Google Scholar] [CrossRef]
- Peetla, C.; Labhasetwar, V. Effect of Molecular Structure of Cationic Surfactants on Biophysical Interactions of Surfactant-Modified Nanoparticles with a Model Membrane and Cellular Uptake. Langmuir 2009, 25, 2369–2377. [Google Scholar] [CrossRef]
- Rajput, S.M.; Kumar, S.; Aswal, V.K.; El Seoud, O.A.; Malek, N.I.; Kailasa, S.K. Drug-Induced Micelle-to-Vesicle Transition of a Cationic Gemini Surfactant: Potential Applications in Drug Delivery. ChemPhysChem 2018, 19, 865–872. [Google Scholar] [CrossRef]
- Branco, M.A.; Pinheiro, L.; Faustino, C. Amino acid-based cationic gemini surfactant-protein interactions. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 105–112. [Google Scholar] [CrossRef]
- Elsabahy, M.; Badea, I.; Verrall, R.; Donkuru, M.; Foldvari, M. Dicationic gemini nanoparticle design for gene therapy. In Organic Nanomaterials; Torres, T., Bottari, G., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2013; Chapter 23. [Google Scholar]
- Yousefpour Marzbali, M.; Yari Khosroushahi, A. Polymeric micelles as mighty nanocarriers for cancer gene therapy: A review. Cancer Chemother. Pharmacol. 2017, 79, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kamal, A.; Abdinejad, M.; Mahajan, R.K.; Kraatz, H.-B. Advances in the synthesis, molecular architectures and potential applications of gemini surfactants. Adv. Colloid Interface Sci. 2017, 248, 35–68. [Google Scholar] [CrossRef] [PubMed]
- Mulinari, J.; Oliveira, J.V.; Hotza, D. Lipase immobilization on ceramic supports: An overview on techniques and materials. Biotechnol. Adv. 2020, 42, 107581. [Google Scholar] [CrossRef] [PubMed]
- Nickpour, M. Synthesis and Characteristics of Mesoporous Sol-gels for Lipase Immobilization. Int. J. Eng. 2014, 27, 1495–1502. [Google Scholar]
- Kandimalla, V.B.; Tripathi, V.S.; Ju, H. Immobilization of Biomolecules in Sol–Gels: Biological and Analytical Applications. Crit. Rev. Anal. Chem. 2006, 36, 73–106. [Google Scholar] [CrossRef]
- Copeland, R.A. Methods for Protein Quantitation. In Methods for Protein Analysis; Springer: Boston, MA, USA, 1994; pp. 39–58. [Google Scholar]
- Chan, J.K.C. The Wonderful Colors of the Hematoxylin–Eosin Stain in Diagnostic Surgical Pathology. Int. J. Surg. Pathol. 2014, 22, 12–32. [Google Scholar] [CrossRef]
- Nagaraj, K.; Senthil Murugan, K.; Thangamuniyandi, P.; Sakthinathan, S. Synthesis, Micellization Behaviour, DNA/RNA Binding and Biological Studies of a Surfactant Cobalt(III) Complex With Dipyrido[3,2-a:2′,4′-c](6,7,8,9-tetrahydro)phenazine. J. Fluoresc. 2014, 24, 1701–1714. [Google Scholar] [CrossRef]
- Ali, A.; Uzair, S.; Malik, N.A.; Ali, M. Study of interaction between cationic surfactants and cresol red dye by electrical conductivity and spectroscopy methods. J. Mol. Liq. 2014, 196, 395–403. [Google Scholar] [CrossRef]
- Nusselder, J.J.H.; Engberts, J.B.F.N. Toward a better understanding of the driving force for micelle formation and micellar growth. J. Colloid Interface Sci. 1992, 148, 353–361. [Google Scholar] [CrossRef]
- Bhadani, A.; Singh, S. Novel gemini pyridinium surfactants: Synthesis and study of their surface activity, DNA binding, and cytotoxicity. Langmuir 2009, 25, 11703–11712. [Google Scholar] [CrossRef]
- Durand-Vidal, S.; Bernard, O.; Medoš, Ž.; Bešter-Rogač, M. Theoretical interpretation of conductivity data below and above the CMC: The case of alkaline ion decanoate solutions. J. Mol. Liq. 2020, 309, 112968. [Google Scholar] [CrossRef]
- Faustino, C.M.C.; Calado, A.R.T.; Garcia-Rio, L. Gemini surfactant-protein interactions: Effect of pH, temperature, and surfactant stereochemistry. Biomacromolecules 2009, 10, 2508–2514. [Google Scholar] [CrossRef] [PubMed]
- Vila-Real, H.; Alfaia, A.J.; Rosa, M.E.; Calado, A.R.; Ribeiro, M.H.L. An innovative sol–gel naringinase bioencapsulation process for glycosides hydrolysis. Process Biochem. 2010, 45, 841–850. [Google Scholar] [CrossRef]
- Zana, R. Critical Micellization Concentration of Surfactants in Aqueous Solution and Free Energy of Micellization. Langmuir 1996, 12, 1208–1211. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Y.; Huang, X.; Cao, M.; Wang, Y. Aggregation behaviors of a series of anionic sulfonate gemini surfactants and their corresponding monomeric surfactant. J. Colloid Interface Sci. 2008, 319, 534–541. [Google Scholar] [CrossRef]
- Wang, F.; Huang, W.; Wang, Y.; Tang, B. Determination of nucleic acid by its enhancement effect on the fluorescence of Ellagic acid–Cationic surfactant system. J. Mol. Struct. 2010, 967, 183–188. [Google Scholar] [CrossRef]
- Veeralakshmi, S.; Sabapathi, G.; Nehru, S.; Venuvanalingam, P.; Arunachalam, S. Surfactant–cobalt(III) complexes: The impact of hydrophobicity on interaction with HSA and DNA–insights from experimental and theoretical approach. Colloids Surf. B Biointerfaces 2017, 153, 85–94. [Google Scholar] [CrossRef]
- Vashishat, R.; Chabba, S.; Kumar Mahajan, R. Surface active ionic liquid induced conformational transition in aqueous medium of hemoglobin. RSC Adv. 2017, 7, 13041–13052. [Google Scholar] [CrossRef]
- Hayakawa, K.; Santerre, J.P.; Kwak, J.C.T. The binding of cationic surfactants by DNA. Biophys. Chem. 1983, 17, 175–181. [Google Scholar] [CrossRef]
- Wang, M.; Yang, J.; Wu, X.; Huang, F. Study of the interaction of nucleic acids with acridine red and CTMAB by a resonance light scattering technique and determination of nucleic acids at nanogram levels. Anal. Chim. Acta 2000, 422, 151–158. [Google Scholar] [CrossRef]
- Ciotta, E.; Prosposito, P.; Pizzoferrato, R. Positive curvature in Stern-Volmer plot described by a generalized model for static quenching. J. Lumin. 2019, 206, 518–522. [Google Scholar] [CrossRef]
- Nilsson, S.; Thuresson, K.; Hansson, P.; Lindman, B. Mixed Solutions of Surfactant and Hydrophobically Modified Polymer. Controlling Viscosity with Micellar Size. J. Phys. Chem. B 1998, 102, 7099–7105. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford Method For Protein Quantitation. In The Protein Protocols Handbook. Springer Protocols Handbooks; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2009. [Google Scholar]
- Georgiou, C.D.; Grintzalis, K.; Zervoudakis, G.; Papapostolou, I. Mechanism of Coomassie brilliant blue G-250 binding to proteins: A hydrophobic assay for nanogram quantities of proteins. Anal. Bioanal. Chem. 2008, 391, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Chaudhury, N.K. Entrapment of biomolecules in sol–gel matrix for applications in biosensors: Problems and future prospects. Biosens. Bioelectron. 2007, 22, 2387–2399. [Google Scholar] [CrossRef] [PubMed]
- Erdinc, N.; Göktürk, S. Spectrophotometric and Conductometric Studies on the Interaction of Anionic Dye Eosin-Y with Cationic Micelles. Anal. Chem. Lett. 2014, 146–157. [Google Scholar] [CrossRef]
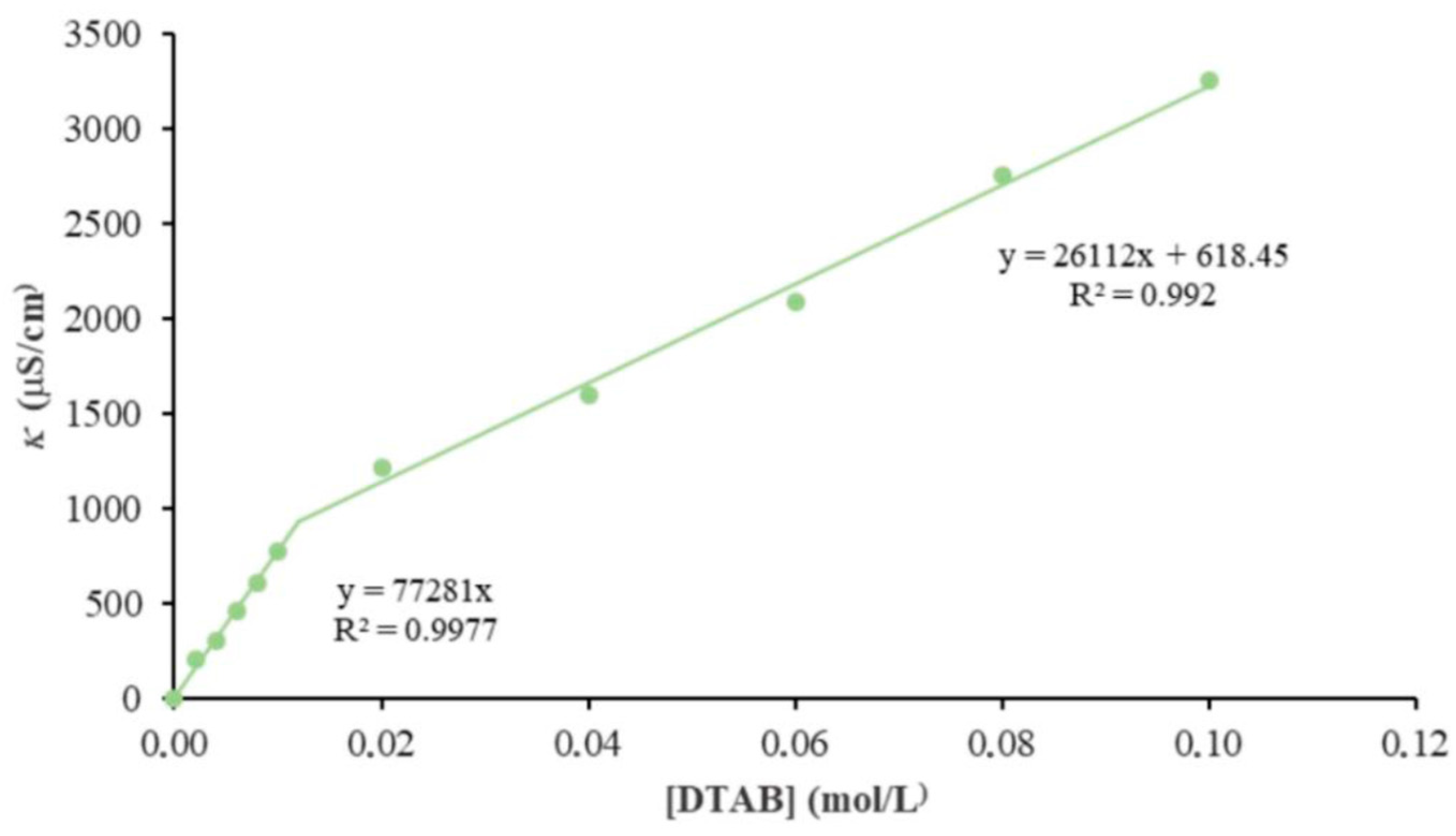
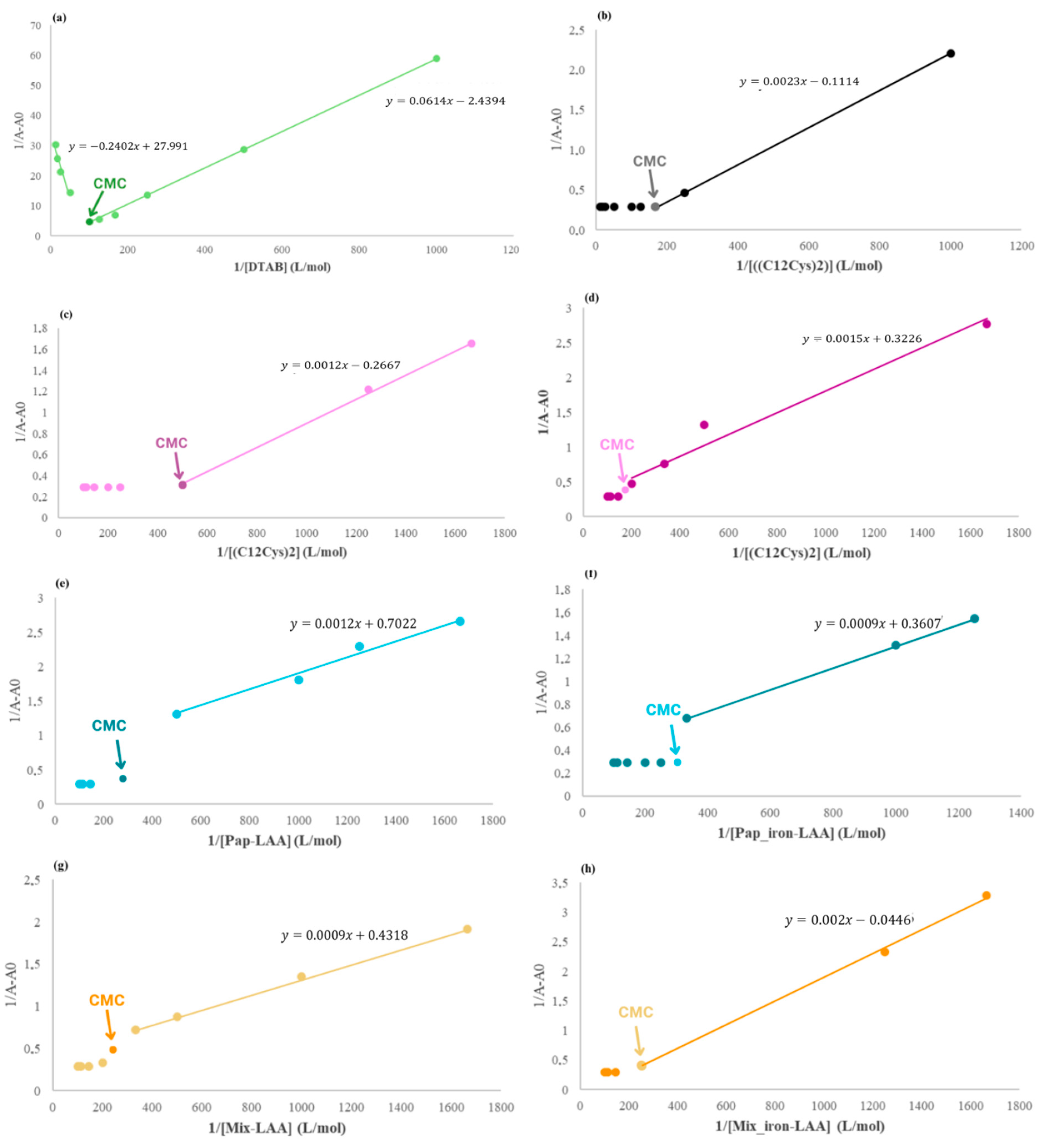
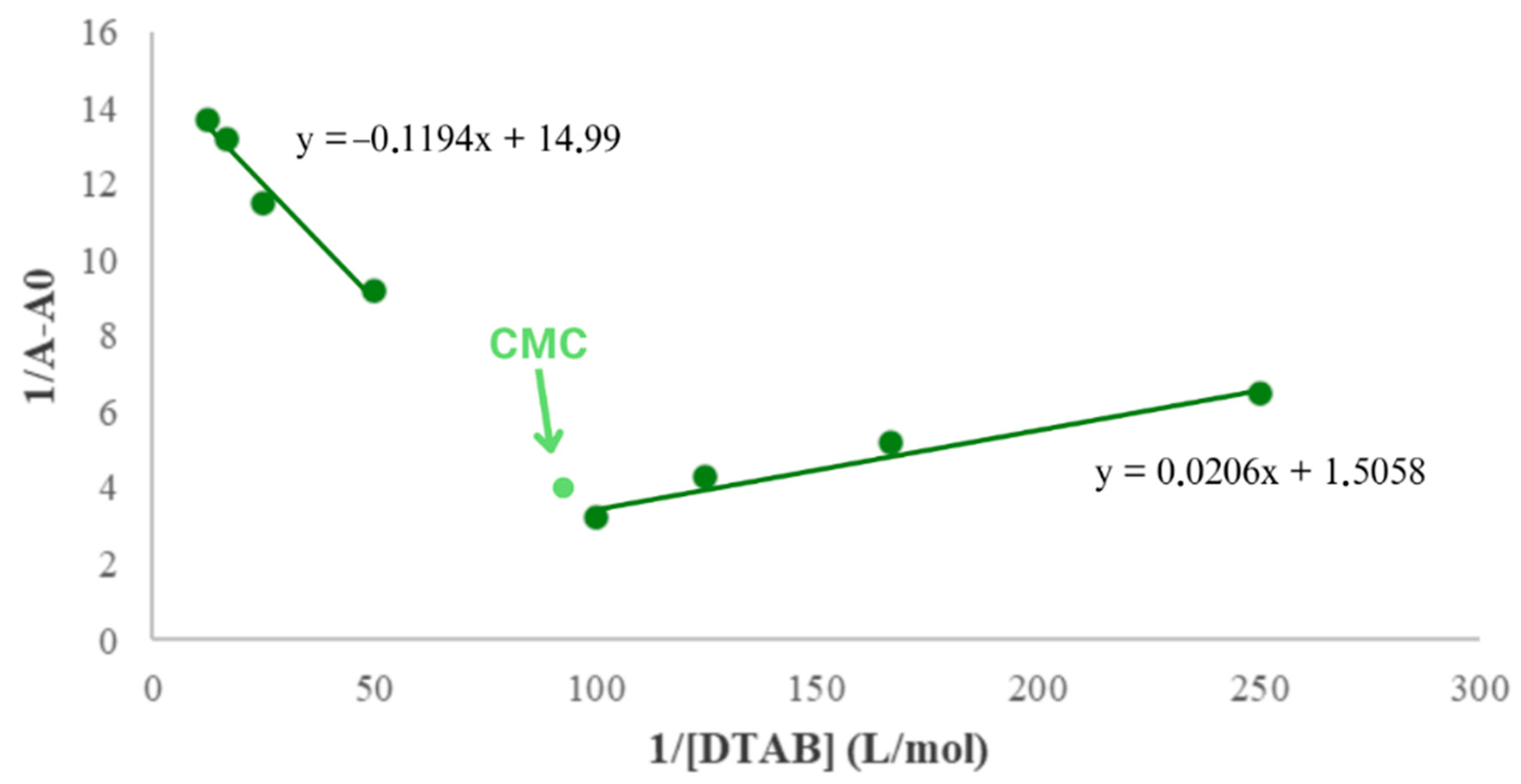

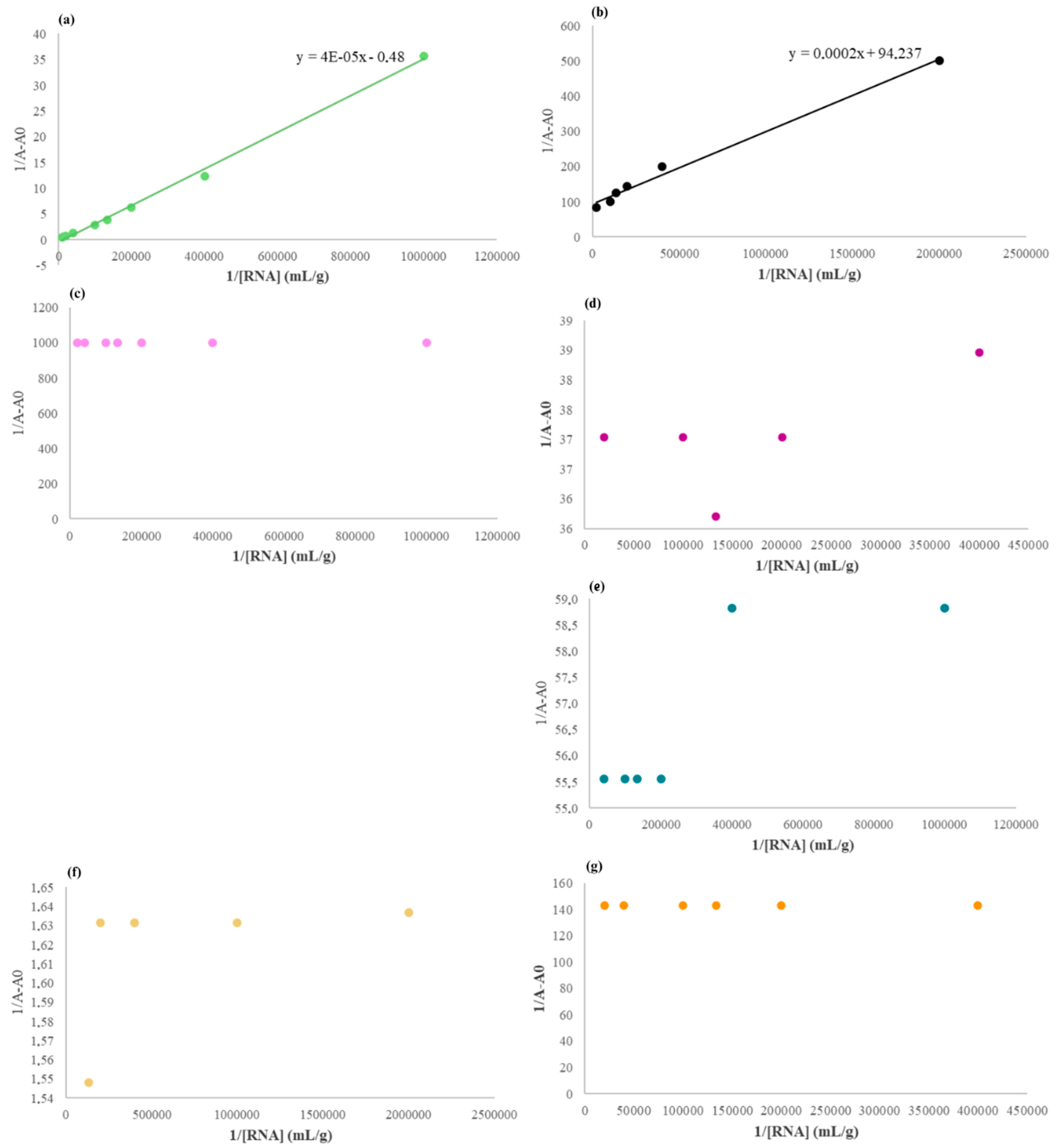
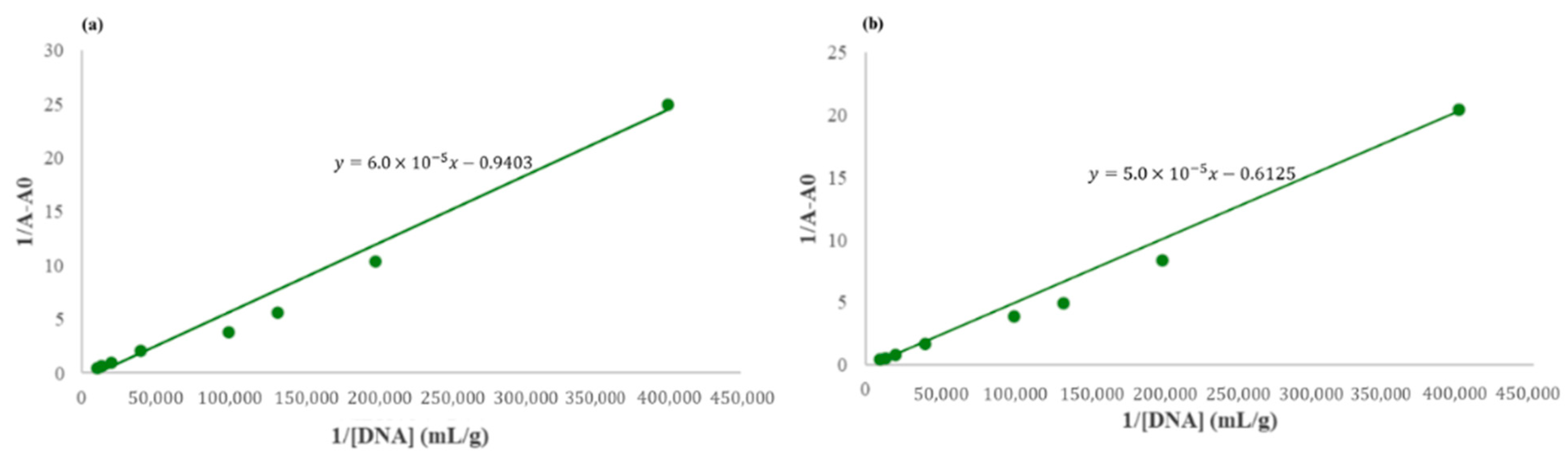
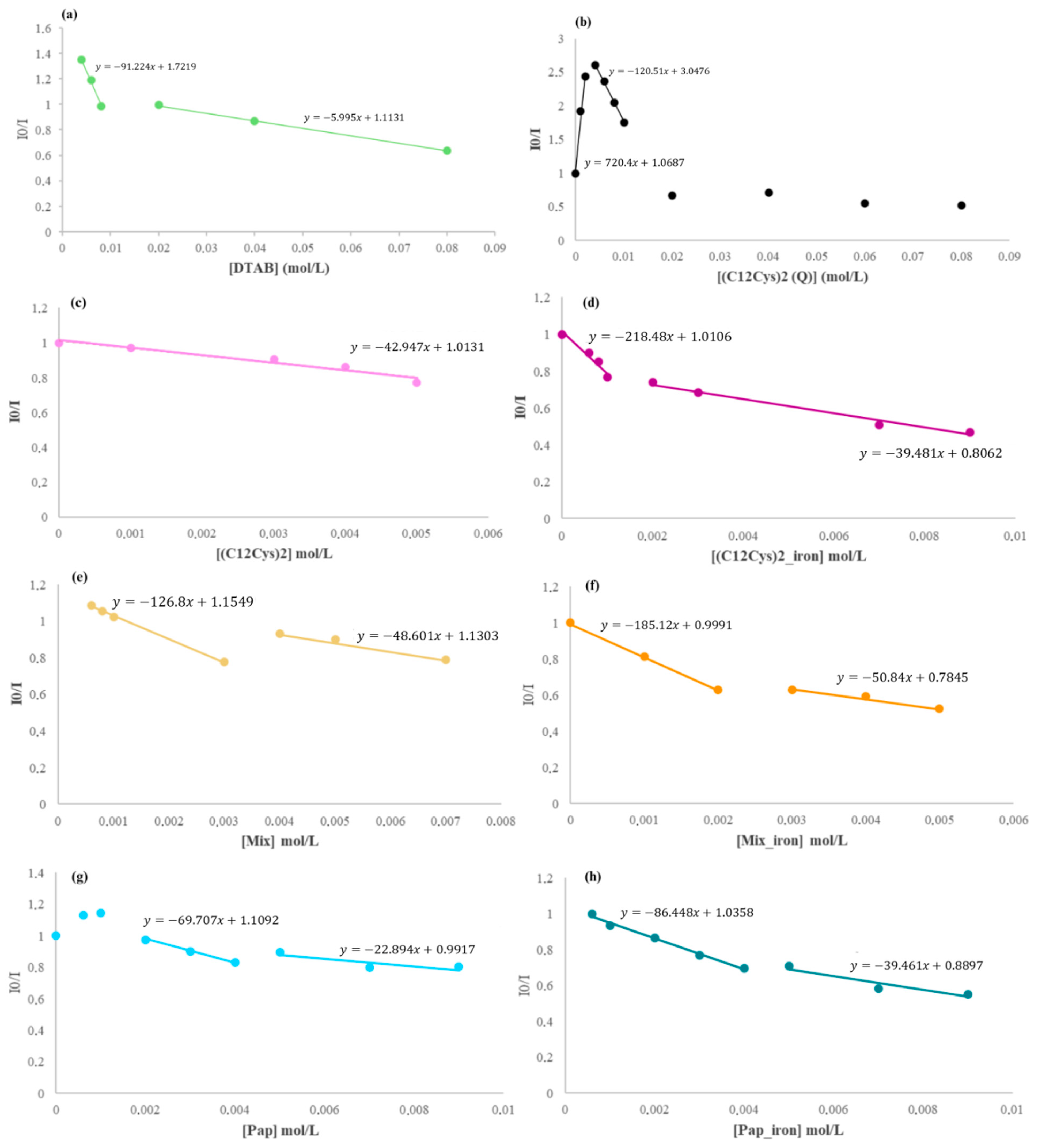
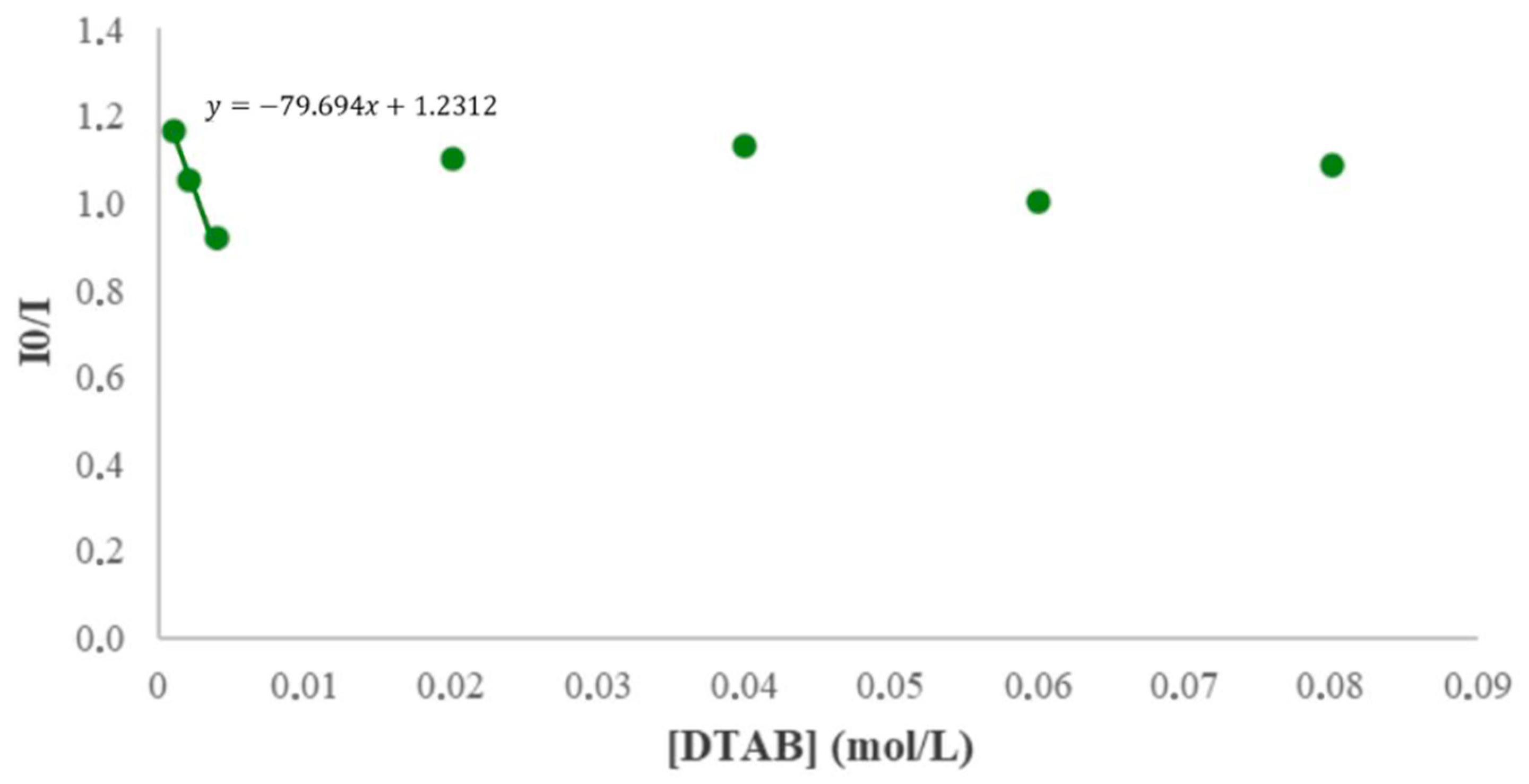
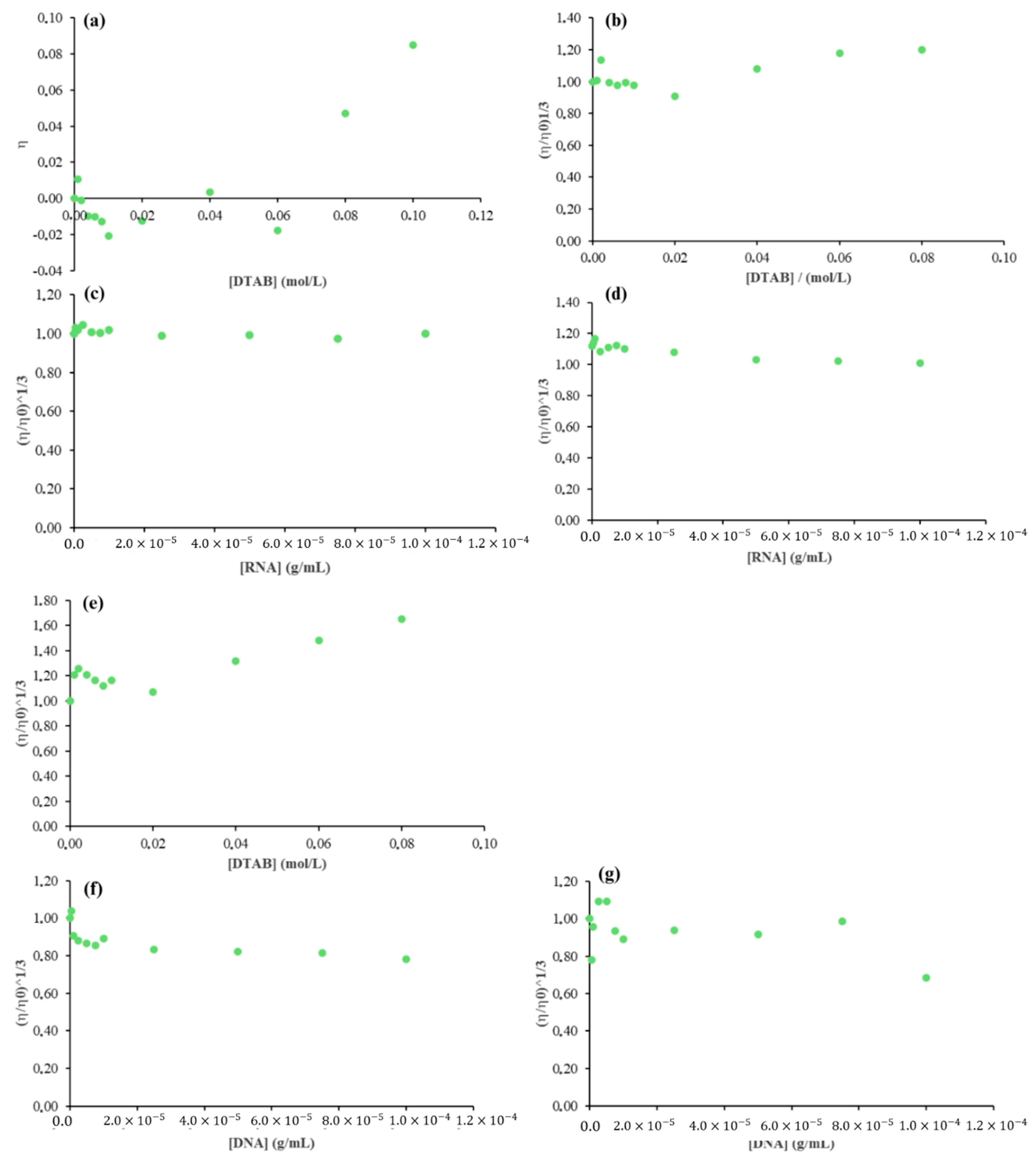

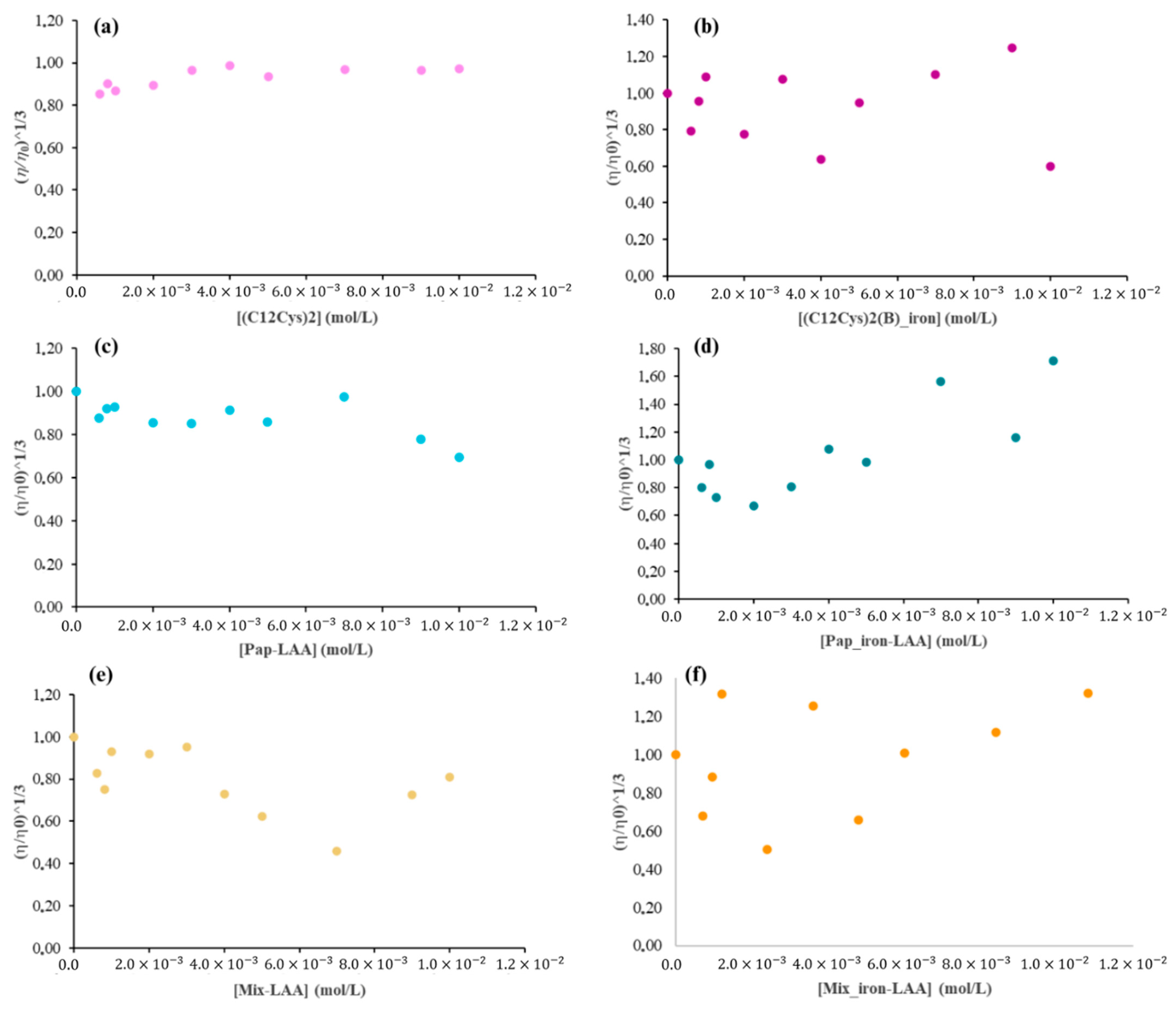
| Surfactant | CMC (mol/L) | β | (kJ/mol) | |
|---|---|---|---|---|
| DTAB | 0.01600 | 0.35 | 0.65 | −16.6 |
| 0.0025 | 0.63 | 0.37 | −27.7 | |
| 0.00820 | 0.5 | 0.5 | −25.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, S.F.; Faustino, C.M.; Ribeiro, M.H.L. Enhancing RNA-Based Technologies Using Enzyme-Derived Lipoamino Acids. Catalysts 2024, 14, 877. https://doi.org/10.3390/catal14120877
Azevedo SF, Faustino CM, Ribeiro MHL. Enhancing RNA-Based Technologies Using Enzyme-Derived Lipoamino Acids. Catalysts. 2024; 14(12):877. https://doi.org/10.3390/catal14120877
Chicago/Turabian StyleAzevedo, Sofia F., Célia M. Faustino, and Maria H. L. Ribeiro. 2024. "Enhancing RNA-Based Technologies Using Enzyme-Derived Lipoamino Acids" Catalysts 14, no. 12: 877. https://doi.org/10.3390/catal14120877
APA StyleAzevedo, S. F., Faustino, C. M., & Ribeiro, M. H. L. (2024). Enhancing RNA-Based Technologies Using Enzyme-Derived Lipoamino Acids. Catalysts, 14(12), 877. https://doi.org/10.3390/catal14120877







