Carbon-Based Catalysts from H3PO4 Activation of Olive Stones for Sustainable Solketal and γ-Valerolactone Production
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Catalysts
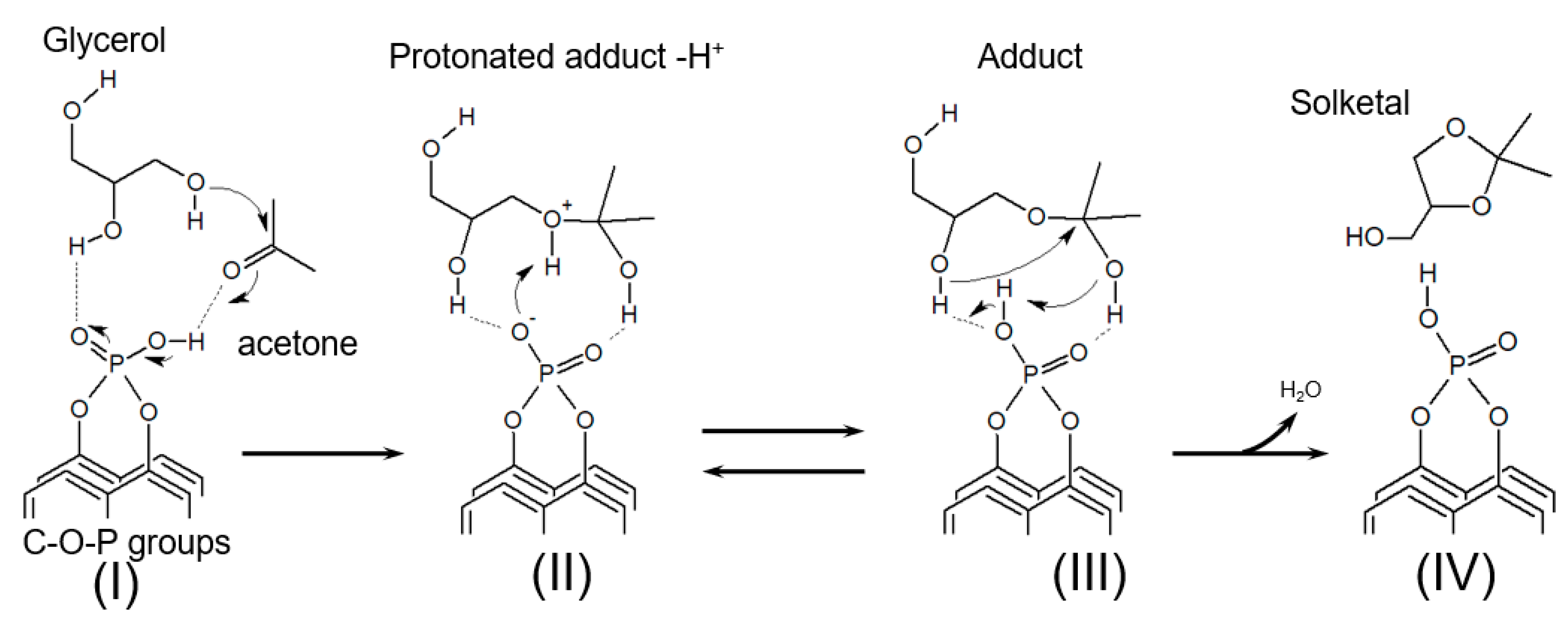
2.2. Catalytic Glycerol Acetalization
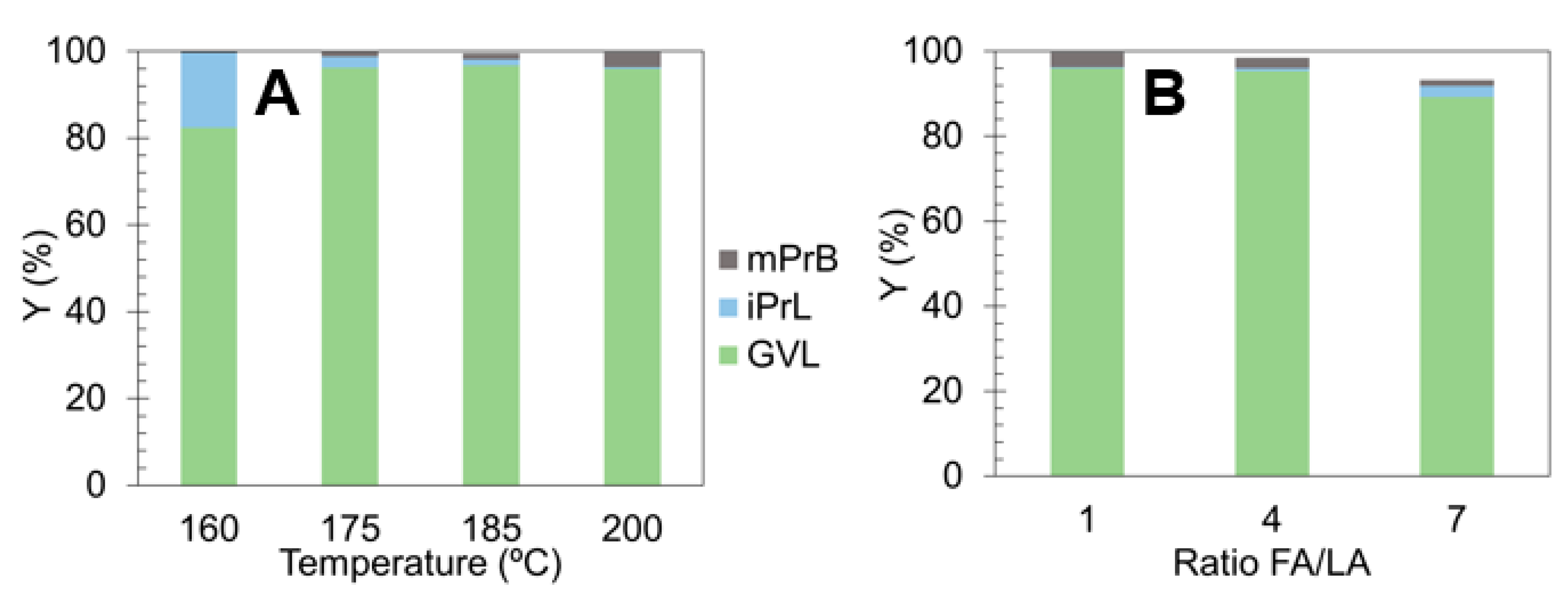
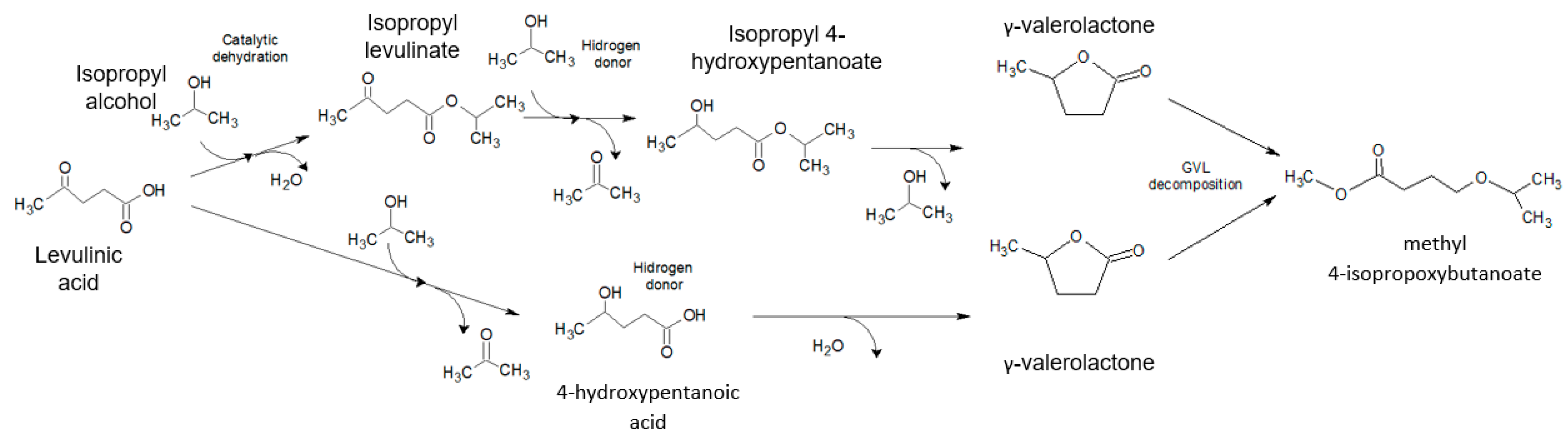
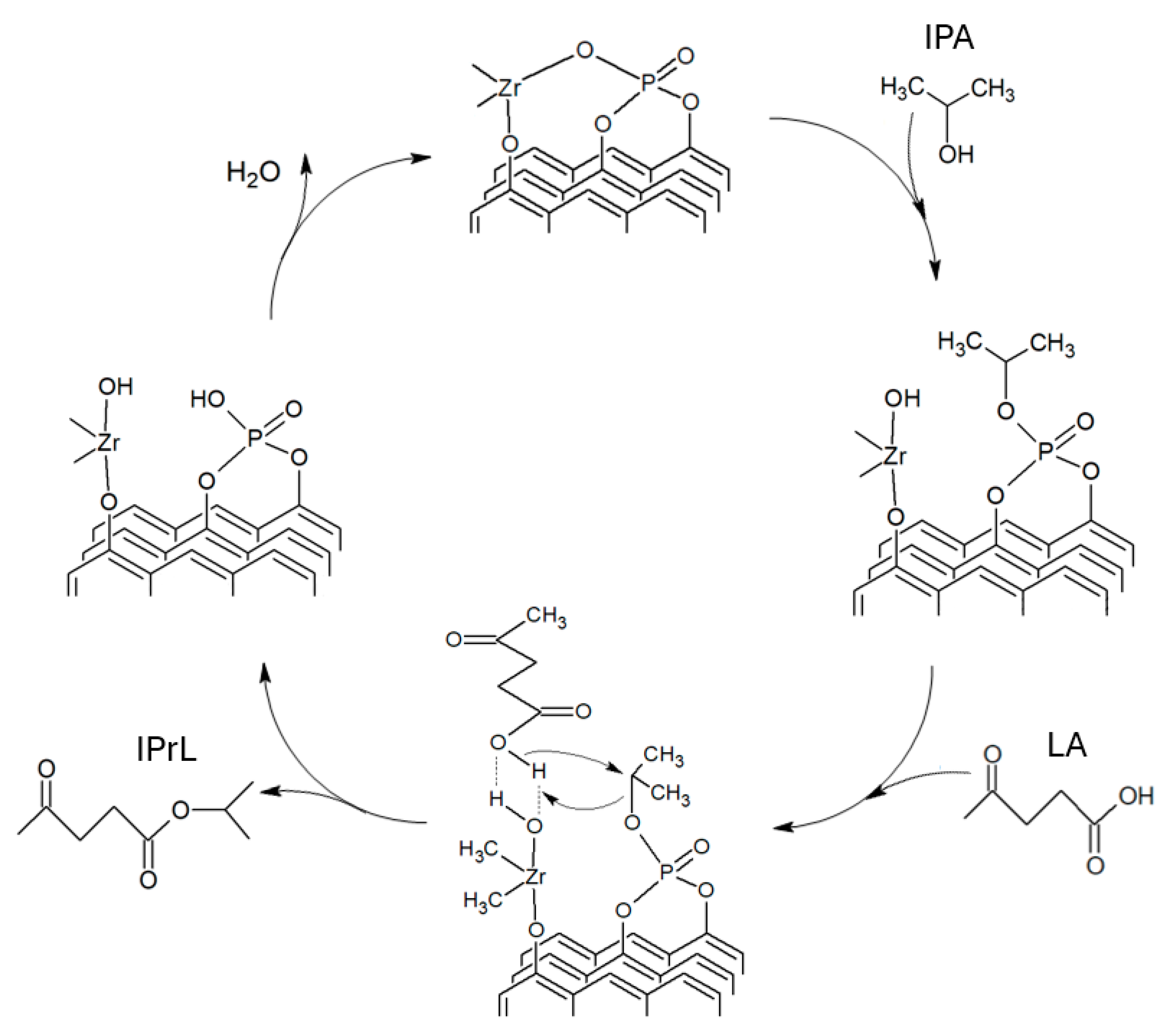
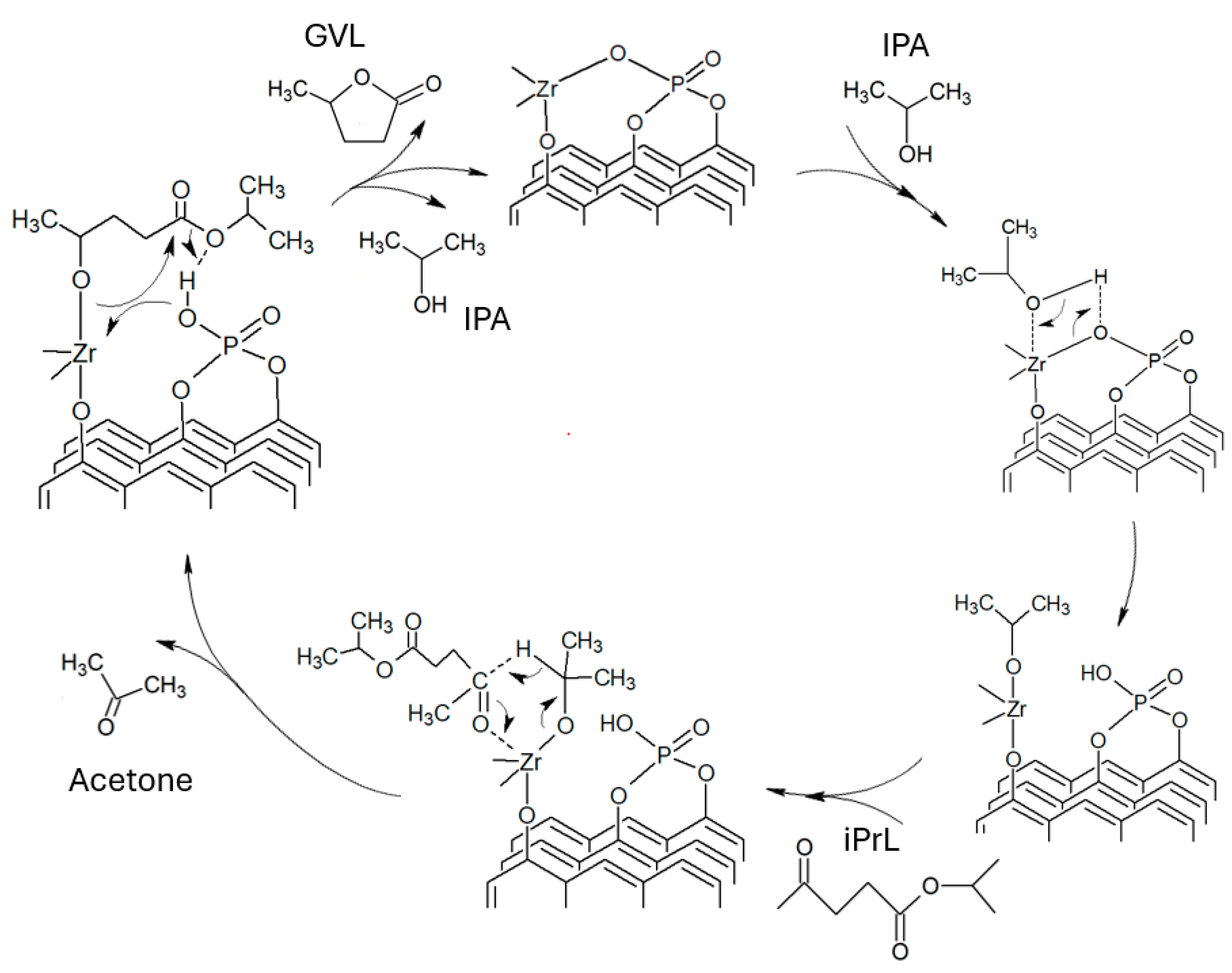
2.3. Catalytic Levulinic Acid Hydrogenation
2.4. GVL Production Kinetic Study
3. Materials and Methods
3.1. Carbon-Based Catalysts Preparation
3.2. Carbon-Based Catalysts’ Characterization
3.3. Catalytic Acetalization of Glycerol
3.4. Catalytic Hydrogenation of Levulinic Acid
3.5. Catalyst Regeneration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gutiérrez, M.C.; Rosas, J.M.; Rodríguez-Cano, M.A.; López-Luque, I.; Rodríguez-Mirasol, J.; Cordero, T. Strategic Situation, Design and Simulation of a Biorefinery in Andalusia. Energy Convers. Manag. 2019, 182, 201–214. [Google Scholar] [CrossRef]
- Wagh, M.S.; Sowjanya, S.; Nath, P.C.; Chakraborty, A.; Amrit, R.; Mishra, B.; Mishra, A.K.; Mohanta, Y.K. Valorisation of Agro-Industrial Wastes: Circular Bioeconomy and Biorefinery Process—A Sustainable Symphony. Process Saf. Environ. Prot. 2024, 183, 708–725. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F. The Role of Carbon Materials in Heterogeneous Catalysis. Carbon 1998, 36, 159–175. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Ania, C.O. Chapter 4 Surface Chemistry of Activated Carbons and Its Characterization. Interface Sci. Technol. 2006, 7, 159–229. [Google Scholar] [CrossRef]
- Rosas, J.M.; Berenguer, R.; Valero-Romero, M.J.; Rodríguez-Mirasol, J.; Cordero, T. Preparation of Different Carbon Materials by Thermochemical Conversion of Lignin. Front. Mater. 2014, 1, 29. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Gawdzik, B.; Tascón, J.M.D. Phosphorus-Containing Carbons: Preparation, Properties and Utilization. Carbon 2020, 157, 796–846. [Google Scholar] [CrossRef]
- Rosas, J.M.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T. Kinetic Study of the Oxidation Resistance of Phosphorus-Containing Activated Carbons. Carbon 2012, 50, 1523–1537. [Google Scholar] [CrossRef]
- Berenguer, R.; Ruiz-Rosas, R.; Gallardo, A.; Cazorla-Amorós, D.; Morallón, E.; Nishihara, H.; Kyotani, T.; Rodríguez-Mirasol, J.; Cordero, T. Enhanced Electro-Oxidation Resistance of Carbon Electrodes Induced by Phosphorus Surface Groups. Carbon 2015, 95, 681–689. [Google Scholar] [CrossRef]
- Sych, N.V.; Trofymenko, S.I.; Poddubnaya, O.I.; Tsyba, M.M.; Sapsay, V.I.; Klymchuk, D.O.; Puziy, A.M. Porous Structure and Surface Chemistry of Phosphoric Acid Activated Carbon from Corncob. Appl. Surf. Sci. 2012, 261, 75–82. [Google Scholar] [CrossRef]
- Valero-Romero, M.J.; García-Mateos, F.J.; Rodríguez-Mirasol, J.; Cordero, T. Role of Surface Phosphorus Complexes on the Oxidation of Porous Carbons. Fuel Process. Technol. 2017, 157, 116–126. [Google Scholar] [CrossRef]
- Bedia, J.; Rosas, J.M.; Márquez, J.; Rodríguez-Mirasol, J.; Cordero, T. Preparation and Characterization of Carbon Based Acid Catalysts for the Dehydration of 2-Propanol. Carbon 2009, 47, 286–294. [Google Scholar] [CrossRef]
- Valero-Romero, M.J.; Calvo-Muñoz, E.M.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T. Phosphorus-Containing Mesoporous Carbon Acid Catalyst for Methanol Dehydration to Dimethyl Ether. Ind. Eng. Chem. Res. 2019, 58, 4042–4053. [Google Scholar] [CrossRef]
- Cordero-Lanzac, T.; Palos, R.; Arandes, J.M.; Castaño, P.; Rodríguez-Mirasol, J.; Cordero, T.; Bilbao, J. Stability of an Acid Activated Carbon Based Bifunctional Catalyst for the Raw Bio-Oil Hydrodeoxygenation. Appl. Catal. B 2017, 203, 389–399. [Google Scholar] [CrossRef]
- Matos, I.; Silva, M.F.; Ruiz-Rosas, R.; Vital, J.; Rodríguez-Mirasol, J.; Cordero, T.; Castanheiro, J.E.; Fonseca, I.M. Methoxylation of α-Pinene over Mesoporous Carbons and Microporous Carbons: A Comparative Study. Microporous Mesoporous Mater. 2014, 199, 66–73. [Google Scholar] [CrossRef]
- Torres-Liñán, J.; García-Rollán, M.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Deactivation of a Biomass-Derived Zirconium-Doped Phosphorus-Containing Carbon Catalyst in the Production of Dimethyl Ether from Methanol Dehydration. Energy Fuels 2021, 35, 17225–17240. [Google Scholar] [CrossRef]
- Hirani, A.H.; Javed, N.; Asif, M.; Basu, S.K.; Kumar, A. A Review on First- and Second-Generation Biofuel Productions. In Biofuels: Greenhouse Gas Mitigation and Global Warming: Next Generation Biofuels and Role of Biotechnology; Springer Nature: Berlin/Heidelberg, Germany, 2018; pp. 141–154. [Google Scholar] [CrossRef]
- Monteiro, M.R.; Kugelmeier, C.L.; Pinheiro, R.S.; Batalha, M.O.; da Silva César, A. Glycerol from Biodiesel Production: Technological Paths for Sustainability. Renew. Sustain. Energy Rev. 2018, 88, 109–122. [Google Scholar] [CrossRef]
- González-Arias, J.; Zhang, Z.; Reina, T.R.; Odriozola, J.A. Hydrogen Production by Catalytic Aqueous-Phase Reforming of Waste Biomass: A Review. Environ. Chem. Lett. 2023, 21, 3089–3104. [Google Scholar] [CrossRef]
- Sahani, S.; Jaiswal, S.; Mishra, S.; Sharma, Y.C.; Han, S.S. Recent Advances in Bio-Glycerol Valorization to Glycerol Carbonate by Heterogenous Base-Catalyzed Transesterification. Mol. Catal. 2023, 550, 113508. [Google Scholar] [CrossRef]
- Coccia, F.; d’Alessandro, N.; Mascitti, A.; Colacino, E.; Tonucci, L. Transition Metal Catalysts for the Glycerol Reduction: Recent Advances. ChemCatChem 2024, 16, e202301672. [Google Scholar] [CrossRef]
- Rahmat, N.; Abdullah, A.Z.; Mohamed, A.R. Recent Progress on Innovative and Potential Technologies for Glycerol Transformation into Fuel Additives: A Critical Review. Renew. Sustain. Energy Rev. 2010, 14, 987–1000. [Google Scholar] [CrossRef]
- Dias, C.N.; Viana, A.M.; Cunha-Silva, L.; Balula, S.S. The Role of the Heterogeneous Catalyst to Produce Solketal from Biodiesel Waste: The Key to Achieve Efficiency. Nanomaterials 2024, 14, 828. [Google Scholar] [CrossRef] [PubMed]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Najaafi, N.; Tarighi, S. A Review on the Catalytic Acetalization of Bio-Renewable Glycerol to Fuel Additives. Front. Chem. 2018, 6, 573. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Hosseinpour, S.; Tabatabaei, M.; Rastegari, H.; Ghaziaskar, H.S. Multi-Objective Exergoeconomic and Exergoenvironmental Optimization of Continuous Synthesis of Solketal through Glycerol Ketalization with Acetone in the Presence of Ethanol as Co-Solvent. Renew. Energy 2019, 130, 735–748. [Google Scholar] [CrossRef]
- Aguado-Deblas, L.; Estevez, R.; Russo, M.; La Parola, V.; Bautista, F.M.; Testa, M.L. Sustainable Microwave-Assisted Solketal Synthesis over Sulfonic Silica-Based Catalysts. J. Environ. Chem. Eng. 2022, 10, 108628. [Google Scholar] [CrossRef]
- Raja, B.K.; Mohindra, N.; Goswami, U.; Modhera, B. Conversion of Glycerol to Solketal Using Heterogeneous Catalysts. J. Inst. Eng. Ser. E 2022, 103, 145–148. [Google Scholar] [CrossRef]
- Chen, L.; Nohair, B.; Zhao, D.; Kaliaguine, S. Highly Efficient Glycerol Acetalization over Supported Heteropoly Acid Catalysts. ChemCatChem 2018, 10, 1918–1925. [Google Scholar] [CrossRef]
- Nanda, M.R.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Poirier, M.A.; Xu, C.C. Thermodynamic and Kinetic Studies of a Catalytic Process to Convert Glycerol into Solketal as an Oxygenated Fuel Additive. Fuel 2014, 117, 470–477. [Google Scholar] [CrossRef]
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. γ-Valerolactone—A Sustainable Liquid for Energy and Carbon-Based Chemicals. Green. Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Bozell, J.J.; Moens, L.; Elliott, D.C.; Wang, Y.; Neuenscwander, G.G.; Fitzpatrick, S.W.; Bilski, R.J.; Jarnefeld, J.L. Production of Levulinic Acid and Use as a Platform Chemical for Derived Products. Resour. Conserv. Recycl. 2000, 28, 227–239. [Google Scholar] [CrossRef]
- Chia, M.; Dumesic, J.A. Liquid-Phase Catalytic Transfer Hydrogenation and Cyclization of Levulinic Acid and Its Esters to γ-Valerolactone over Metal Oxide Catalysts. Chem. Commun. 2011, 47, 12233–12235. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Li, J.; Lai, D.M.; Fu, Y.; Guo, Q.X. Catalytic Conversion of Biomass-Derived Carbohydrates into Gamma-Valerolactone without Using an External H2 Supply. Angew. Chem. Int. Ed. Engl. 2009, 48, 6529–6532. [Google Scholar] [CrossRef] [PubMed]
- Geilen, F.M.A.; Engendahl, B.; Harwardt, A.; Marquardt, W.; Klankermayer, J.; Leitner, W. Selective and Flexible Transformation of Biomass-Derived Platform Chemicals by a Multifunctional Catalytic System. Angew. Chem. 2010, 32, 5642–5646. [Google Scholar] [CrossRef]
- Yan, K.; Yang, Y.; Chai, J.; Lu, Y. Catalytic Reactions of Gamma-Valerolactone: A Platform to Fuels and Value-Added Chemicals. Appl. Catal. B 2015, 179, 292–304. [Google Scholar] [CrossRef]
- Jagtoyen, M.; Derbyshire, F. Activated Carbons from Yellow Poplar and White Oak by H3PO4 Activation. Carbon 1998, 36, 1085–1097. [Google Scholar] [CrossRef]
- Hosseinzaei, B.; Hadianfard, M.J.; Ruiz-Rosas, R.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Effect of Heating Rate and H3PO4 as Catalyst on the Pyrolysis of Agricultural Residues. J. Anal. Appl. Pyrolysis 2022, 168, 105724. [Google Scholar] [CrossRef]
- Molina-Sabio, M.; Caturla, F.; Rodriguez-Reinoso, F. Influence of the Atmosphere Used in the Carbonization of Phosphoric Acid Impregnated Peach Stones. Carbon 1995, 33, 1180–1182. [Google Scholar] [CrossRef]
- Benaddi, H.; Legras, D.; Rouzaud, J.N.; Beguin, F. Influence of the Atmosphere in the Chemical Activation of Wood by Phosphoric Acid. Carbon 1998, 36, 306–309. [Google Scholar] [CrossRef]
- Cazorla-Amorós, D.; Alcañiz-Monge, J.; de la Casa-Lillo, M.A.; Linares-Solano, A. CO2 As an Adsorptive To Characterize Carbon Molecular Sieves and Activated Carbons. Langmuir 1998, 14, 4589–4596. [Google Scholar] [CrossRef]
- Otake, Y.; Jenkins, R.G. Characterization of Oxygen-Containing Surface Complexes Created on a Microporous Carbon by Air and Nitric Acid Treatment. Carbon 1993, 31, 109–121. [Google Scholar] [CrossRef]
- Barbosa, F.F.; Loiola, A.R.; Pergher, S.B.C.; Braga, T.P. Challenges, Prospects and Comprehensive Evolution of Zeolite-Based Materials for the Catalytic Conversion of Glycerol: A Review. Catal. Today 2025, 444, 114998. [Google Scholar] [CrossRef]
- Nanda, M.R.; Zhang, Y.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Xu, C. Catalytic Conversion of Glycerol for Sustainable Production of Solketal as a Fuel Additive: A Review. Renew. Sustain. Energy Rev. 2016, 56, 1022–1031. [Google Scholar] [CrossRef]
- Agirre, I.; García, I.; Requies, J.; Barrio, V.L.; Güemez, M.B.; Cambra, J.F.; Arias, P.L. Glycerol Acetals, Kinetic Study of the Reaction between Glycerol and Formaldehyde. Biomass Bioenergy 2011, 35, 3636–3642. [Google Scholar] [CrossRef]
- Moreira, M.N.; Faria, R.P.V.; Ribeiro, A.M.; Rodrigues, A.E. Solketal Production from Glycerol Ketalization with Acetone: Catalyst Selection and Thermodynamic and Kinetic Reaction Study. Ind. Eng. Chem. Res. 2019, 58, 17746–17759. [Google Scholar] [CrossRef]
- Khayoon, M.S.; Hameed, B.H. Solventless Acetalization of Glycerol with Acetone to Fuel Oxygenates over Ni–Zr Supported on Mesoporous Activated Carbon Catalyst. Appl. Catal. A Gen. 2013, 464–465, 191–199. [Google Scholar] [CrossRef]
- Esteban, J.; Ladero, M.; García-Ochoa, F. Kinetic Modelling of the Solventless Synthesis of Solketal with a Sulphonic Ion Exchange Resin. Chem. Eng. J. 2015, 269, 194–202. [Google Scholar] [CrossRef]
- Jeeru, L.R.; Pradhan, N.C.; Naveen, P.; Guduru, R.K.; Praveen, B. Sustainable Synthesis of Automobile Fuel Additive from Glycerol and Acetone and Catalyst Reusability Studies. Chem. Pap. 2024, 78, 321–329. [Google Scholar] [CrossRef]
- Rossa, V.; Pessanha, Y.D.S.P.; Díaz, G.C.; Câmara, L.D.T.; Pergher, S.B.C.; Aranda, D.A.G. Reaction Kinetic Study of Solketal Production from Glycerol Ketalization with Acetone. Ind. Eng. Chem. Res. 2017, 56, 479–488. [Google Scholar] [CrossRef]
- Cheruvathoor Poulose, A.; Medveď, M.; Bakuru, V.R.; Sharma, A.; Singh, D.; Kalidindi, S.B.; Bares, H.; Otyepka, M.; Jayaramulu, K.; Bakandritsos, A.; et al. Acidic Graphene Organocatalyst for the Superior Transformation of Wastes into High-Added-Value Chemicals. Nat. Commun. 2023, 14, 1373. [Google Scholar] [CrossRef]
- Guarinos, J.M.; Cirujano, F.G.; Rapeyko, A.; Llabrés i Xamena, F.X. Conversion of Levulinic Acid to γ-Valerolactone over Zr-Containing Metal-Organic Frameworks: Evidencing the Role of Lewis and Brønsted Acid Sites. Mol. Catal. 2021, 515, 111925. [Google Scholar] [CrossRef]
- De Bruycker, R.; Carstensen, H.H.; Simmie, J.M.; Van Geem, K.M.; Marin, G.B. Experimental and Computational Study of the Initial Decomposition of Gamma-Valerolactone. Proc. Combust. Inst. 2015, 35, 515–523. [Google Scholar] [CrossRef]
- Chen, X.; Deng, L.; Zhou, S.; Qiao, C.; Tian, Y. Alkaline Modified Zr-Loading on Nanosheet Zeolite towards Production of γ-Valerolactone from Levulinic Acid through Catalytic Transfer Hydrogenation. Appl. Catal. A Gen. 2024, 682, 119816. [Google Scholar] [CrossRef]
- Palomo, J.; Rodríguez-Cano, M.A.; Rodríguez-Mirasol, J.; Cordero, T. On the Kinetics of Methanol Dehydration to Dimethyl Ether on Zr-Loaded P-Containing Mesoporous Activated Carbon Catalyst. Chem. Eng. J. 2019, 378, 122198. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Kaburagi, W.; Osada, Y.; Fujitani, T.; Yamashita, H. Catalytic Transfer Hydrogenation of Biomass-Derived Levulinic Acid and Its Esters to γ-Valerolactone over ZrO2 Catalyst Supported on SBA-15 Silica. Catal. Today 2017, 281, 418–428. [Google Scholar] [CrossRef]
- Valekar, A.H.; Cho, K.H.; Chitale, S.K.; Hong, D.Y.; Cha, G.Y.; Lee, U.H.; Hwang, D.W.; Serre, C.; Chang, J.S.; Hwang, Y.K. Catalytic Transfer Hydrogenation of Ethyl Levulinate to γ-Valerolactone over Zirconium-Based Metal–Organic Frameworks. Green. Chem. 2016, 18, 4542–4552. [Google Scholar] [CrossRef]
- Lázaro, N.; Franco, A.; Ouyang, W.; Balu, A.M.; Romero, A.A.; Luque, R.; Pineda, A. Continuous-Flow Hydrogenation of Methyl Levulinate Promoted by Zr-Based Mesoporous Materials. Catalysts 2019, 9, 142. [Google Scholar] [CrossRef]
- Jagiello, J.; Olivier, J.P. 2D-NLDFT Adsorption Models for Carbon Slit-Shaped Pores with Surface Energetical Heterogeneity and Geometrical Corrugation. Carbon 2013, 55, 70–80. [Google Scholar] [CrossRef]
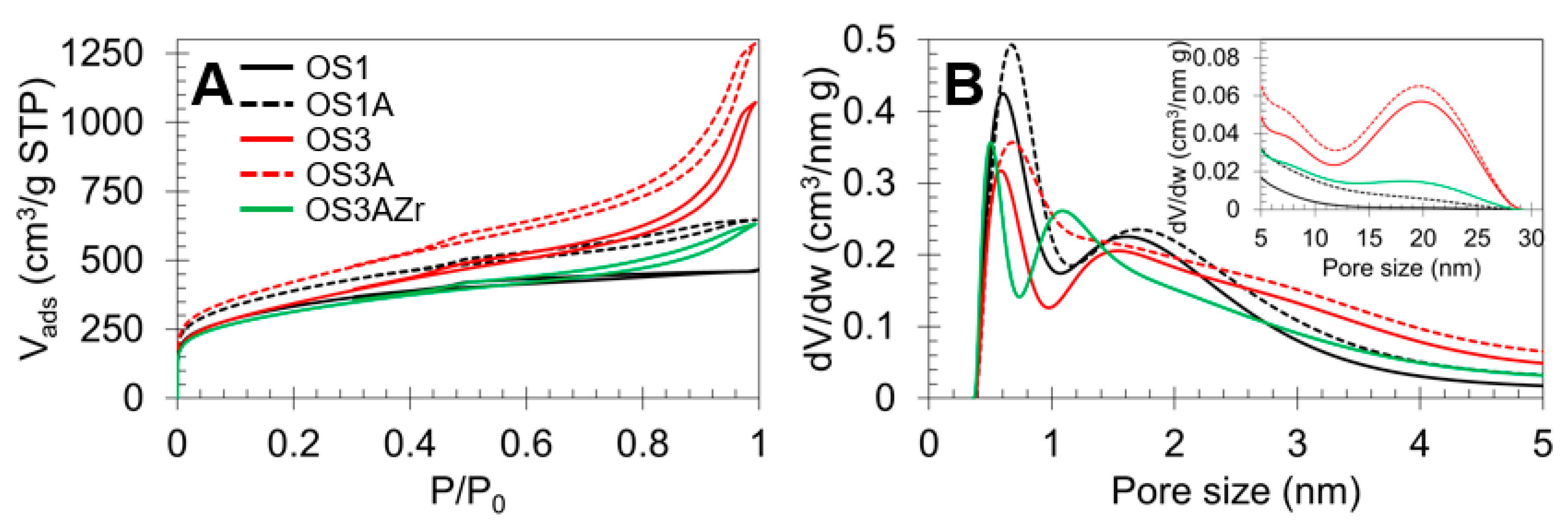
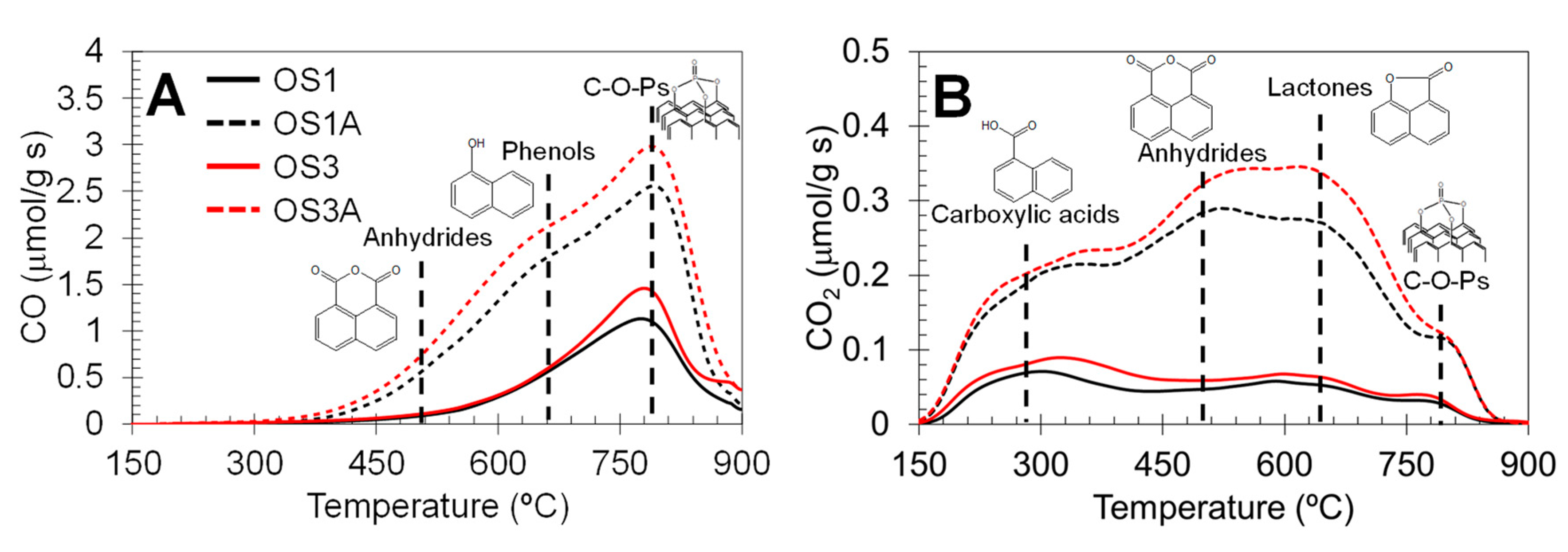
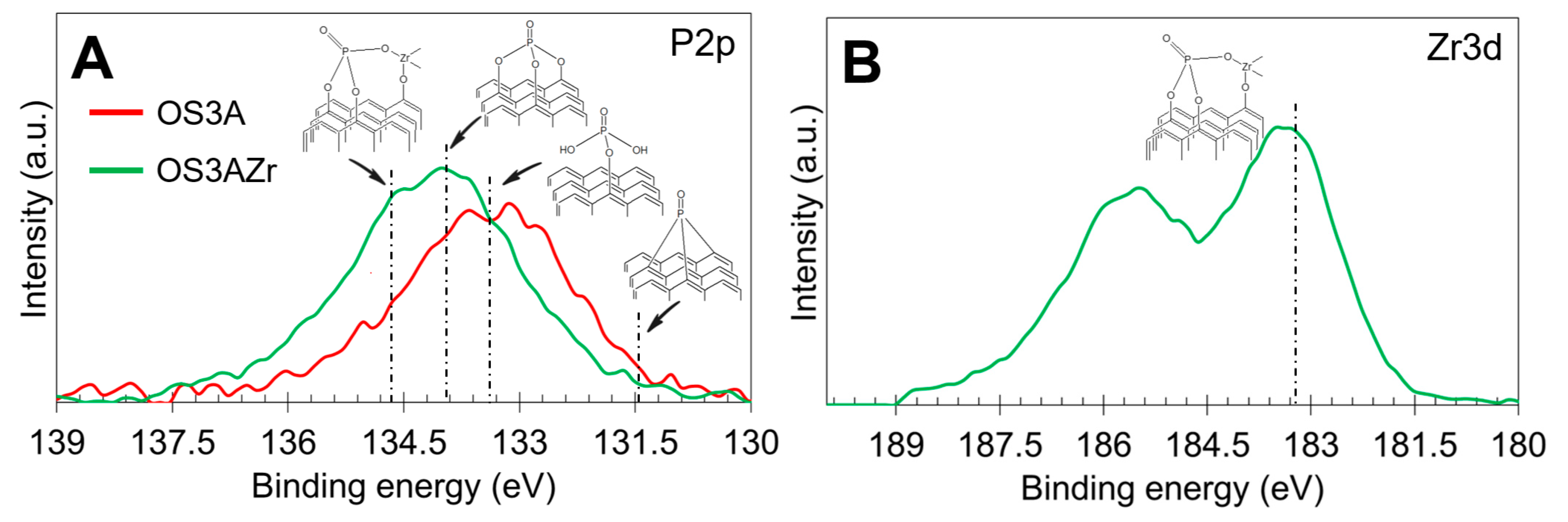




| Sample | Preparation | N2 Adsorption–Desorption | CO2 Adsorption | |||
|---|---|---|---|---|---|---|
| Yield (%) | ABET (m2/g) | Vs (cm3/g) | Vmeso (cm3/g) | ADR (m2/g) | VDR (cm3/g) | |
| OS1 | 42.0 | 1205 | 0.55 | 0.16 | 535 | 0.21 |
| OS1A | 43.4 | 1405 | 0.60 | 0.40 | 560 | 0.22 |
| OS3 | 42.9 | 1245 | 0.47 | 1.12 | 435 | 0.17 |
| OS3A | 42.8 | 1515 | 0.55 | 1.37 | 530 | 0.21 |
| OS3AZr | 42.9 | 1130 | 0.46 | 0.50 | 460 | 0.19 |
| Sample | TPD (mmol/g) | XPS (wt%) | |||||
|---|---|---|---|---|---|---|---|
| CO | CO2 | O | C | O | P | Zr | |
| OS1 | 1.51 | 0.19 | 1.88 | 87.7 | 9.1 | 3.2 | - |
| OS1A | 4.02 | 0.81 | 5.64 | 77.6 | 20.2 | 2.2 | - |
| OS3 | 1.82 | 0.24 | 2.30 | 87.9 | 9.6 | 2.5 | - |
| OS3A | 4.85 | 0.95 | 6.76 | 80.5 | 16.8 | 2.7 | - |
| OS3AZr | 1.76 | 0.42 | 2.61 | 76.7 | 13.5 | 2.7 | 6.7 |
| Ea (kJ·mol−1) | k0 (h−1) | |
|---|---|---|
| −r1 | 39 | 7.0 × 103 |
| −r2 | 42 | 1.1 × 104 |
| −r4 | 63 | 4.9 × 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Liñán, J.; García-Rollán, M.; Ruiz-Rosas, R.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Carbon-Based Catalysts from H3PO4 Activation of Olive Stones for Sustainable Solketal and γ-Valerolactone Production. Catalysts 2024, 14, 869. https://doi.org/10.3390/catal14120869
Torres-Liñán J, García-Rollán M, Ruiz-Rosas R, Rosas JM, Rodríguez-Mirasol J, Cordero T. Carbon-Based Catalysts from H3PO4 Activation of Olive Stones for Sustainable Solketal and γ-Valerolactone Production. Catalysts. 2024; 14(12):869. https://doi.org/10.3390/catal14120869
Chicago/Turabian StyleTorres-Liñán, Javier, Miguel García-Rollán, Ramiro Ruiz-Rosas, Juana María Rosas, José Rodríguez-Mirasol, and Tomás Cordero. 2024. "Carbon-Based Catalysts from H3PO4 Activation of Olive Stones for Sustainable Solketal and γ-Valerolactone Production" Catalysts 14, no. 12: 869. https://doi.org/10.3390/catal14120869
APA StyleTorres-Liñán, J., García-Rollán, M., Ruiz-Rosas, R., Rosas, J. M., Rodríguez-Mirasol, J., & Cordero, T. (2024). Carbon-Based Catalysts from H3PO4 Activation of Olive Stones for Sustainable Solketal and γ-Valerolactone Production. Catalysts, 14(12), 869. https://doi.org/10.3390/catal14120869










