TiO2 Catalysts Co-Modified with Bi, F, SnO2, and SiO2 for Photocatalytic Degradation of Rhodamine B Under Simulated Sunlight
Abstract
1. Introduction
2. Results
2.1. Impact of Bi Addition and Compositing with SnO2 on Catalyst Properties
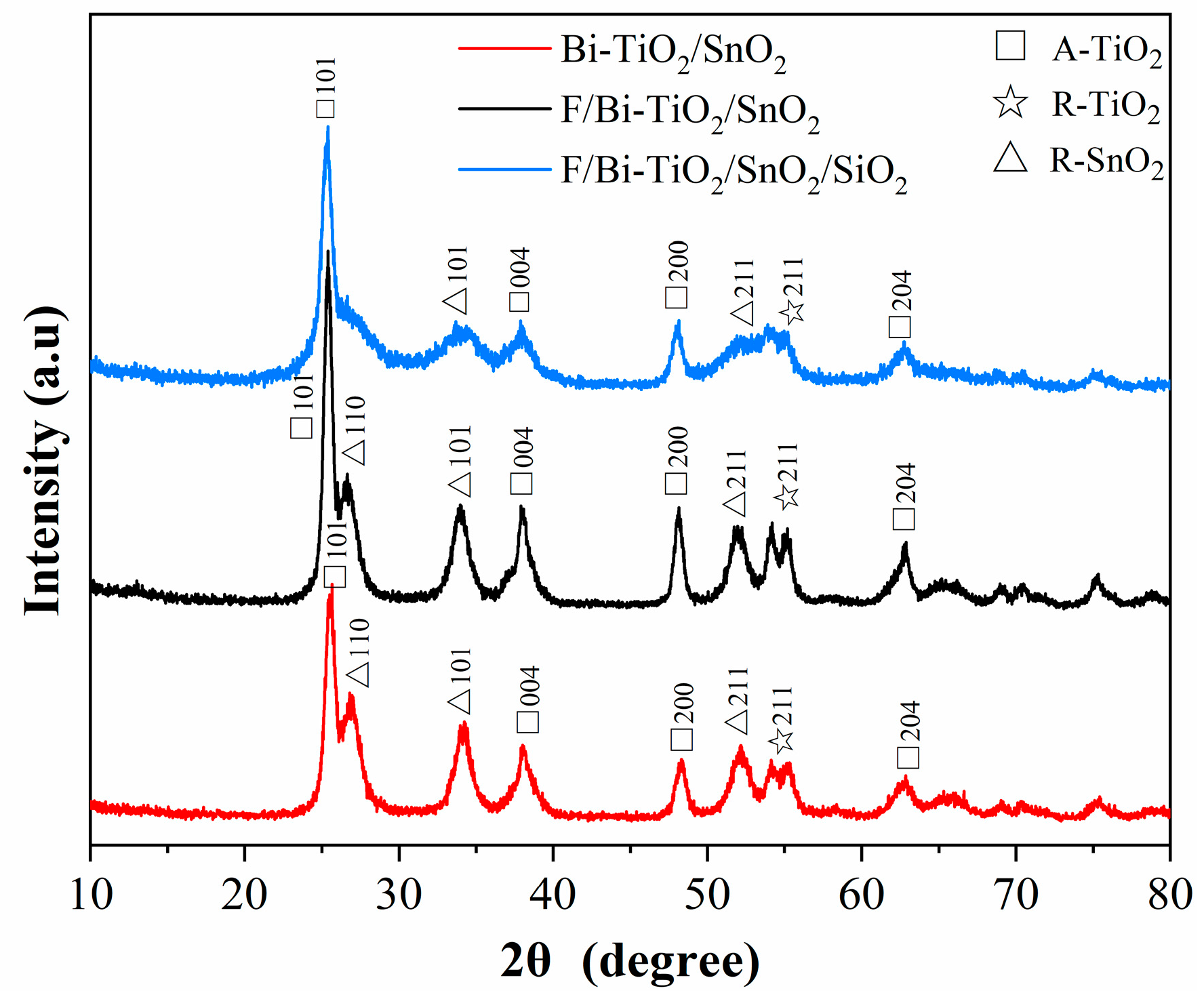
2.1.1. Impact on Crystal Phase
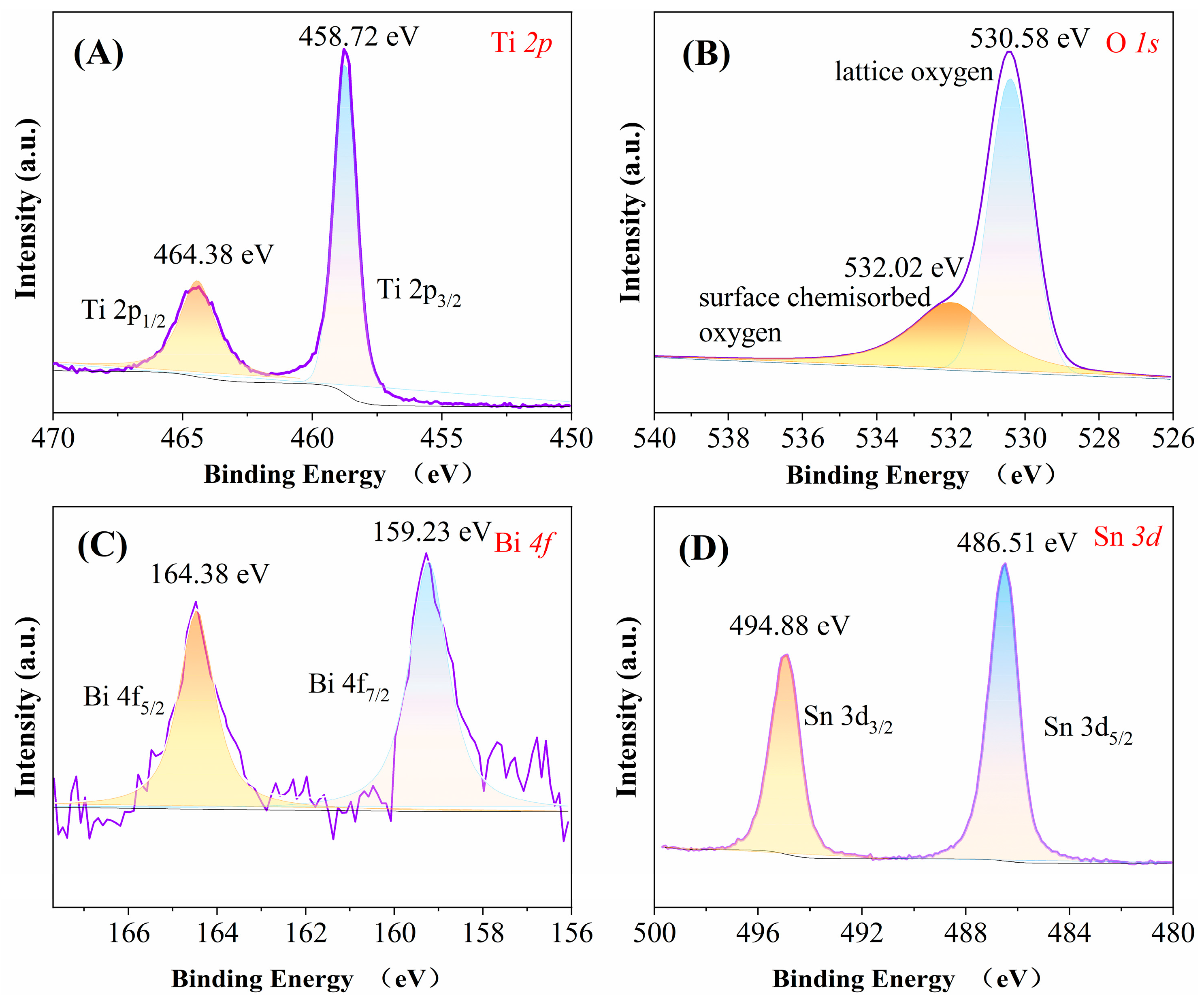
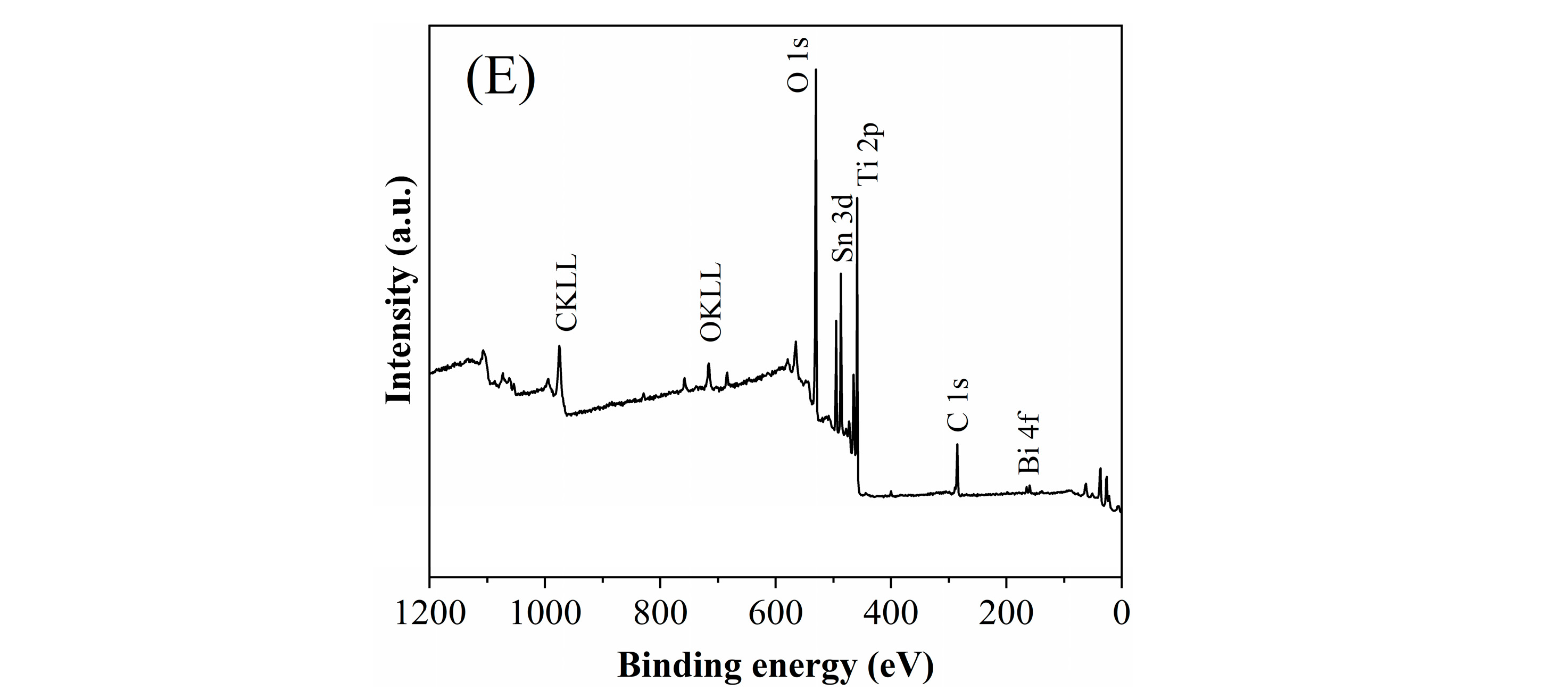
2.1.2. X-Ray Photoelectron Spectroscopy Analysis
2.1.3. Impact on Surface Morphology
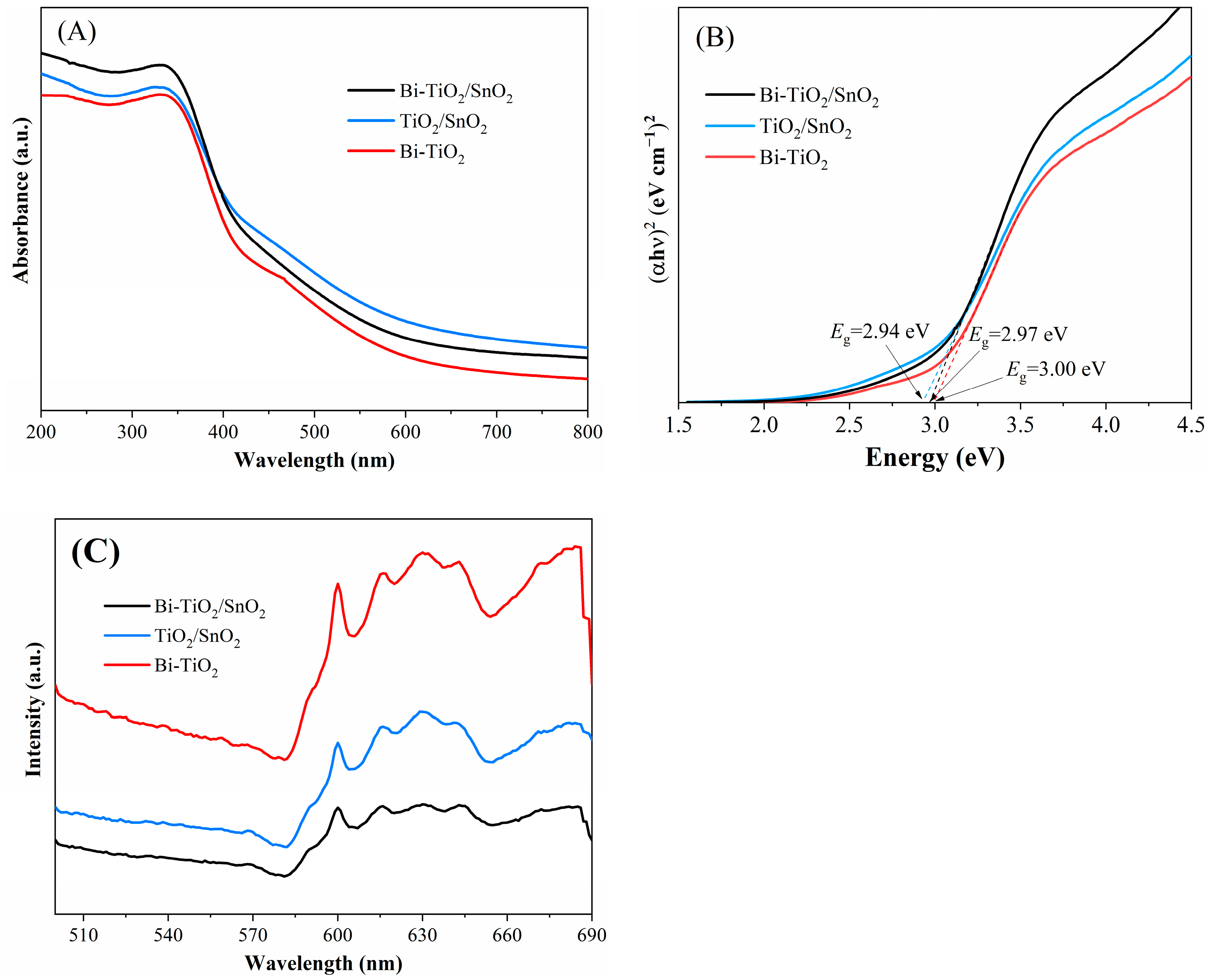
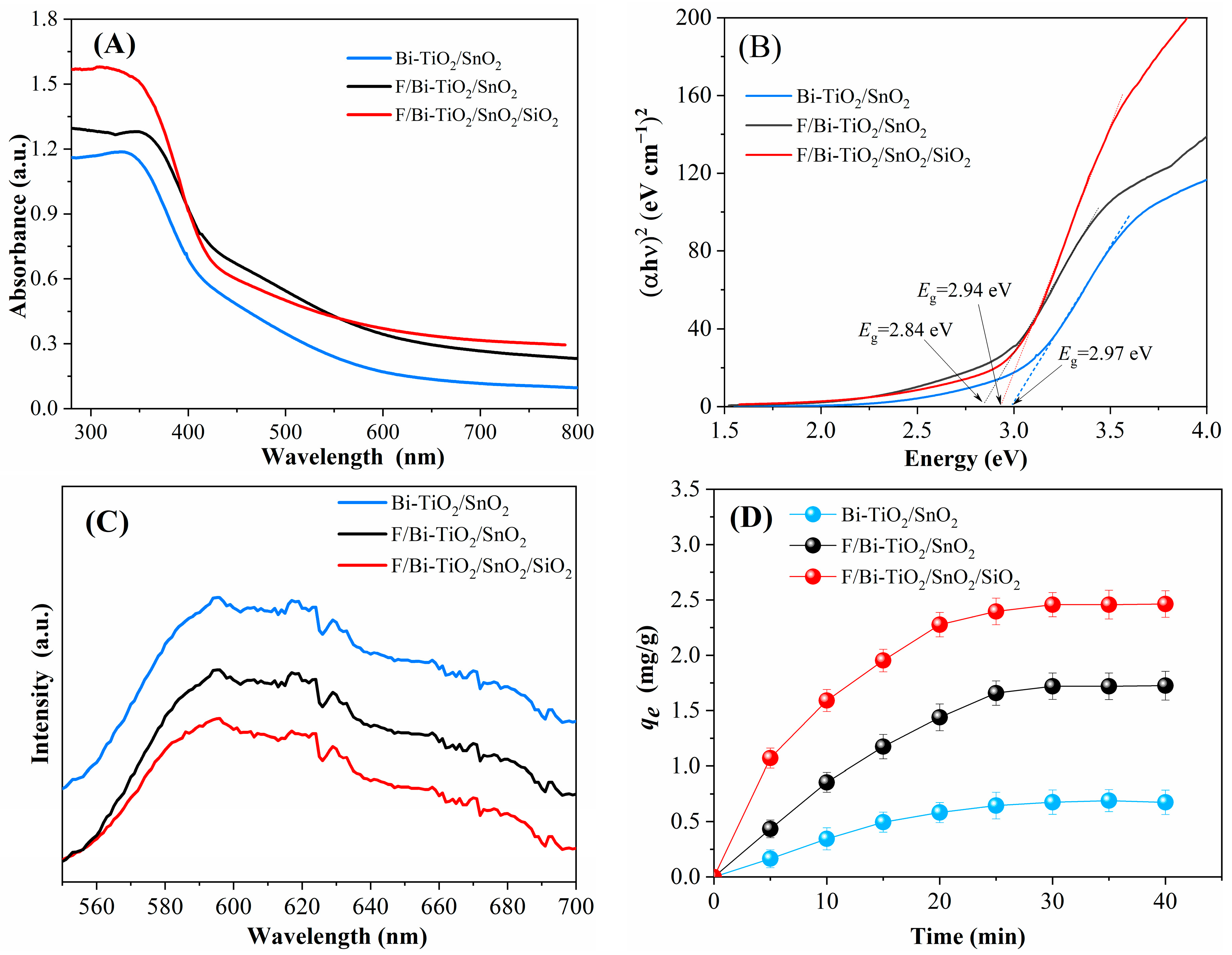
2.1.4. Impact on Optical Properties
2.2. Impact of Bi and SnO2 Addition on RhB Photocatalytic Degradation
2.3. Impact of F Addition and Compositing with SiO2 on Catalyst Properties
2.3.1. Surface Morphology
2.3.2. Crystal Phase
2.3.3. Nitrogen Adsorption Desorption Analysis
2.3.4. Optical Properties
2.3.5. Adsorption Performance
2.4. Impact of F and SiO2 Addition on RhB Photocatalytic Degradation
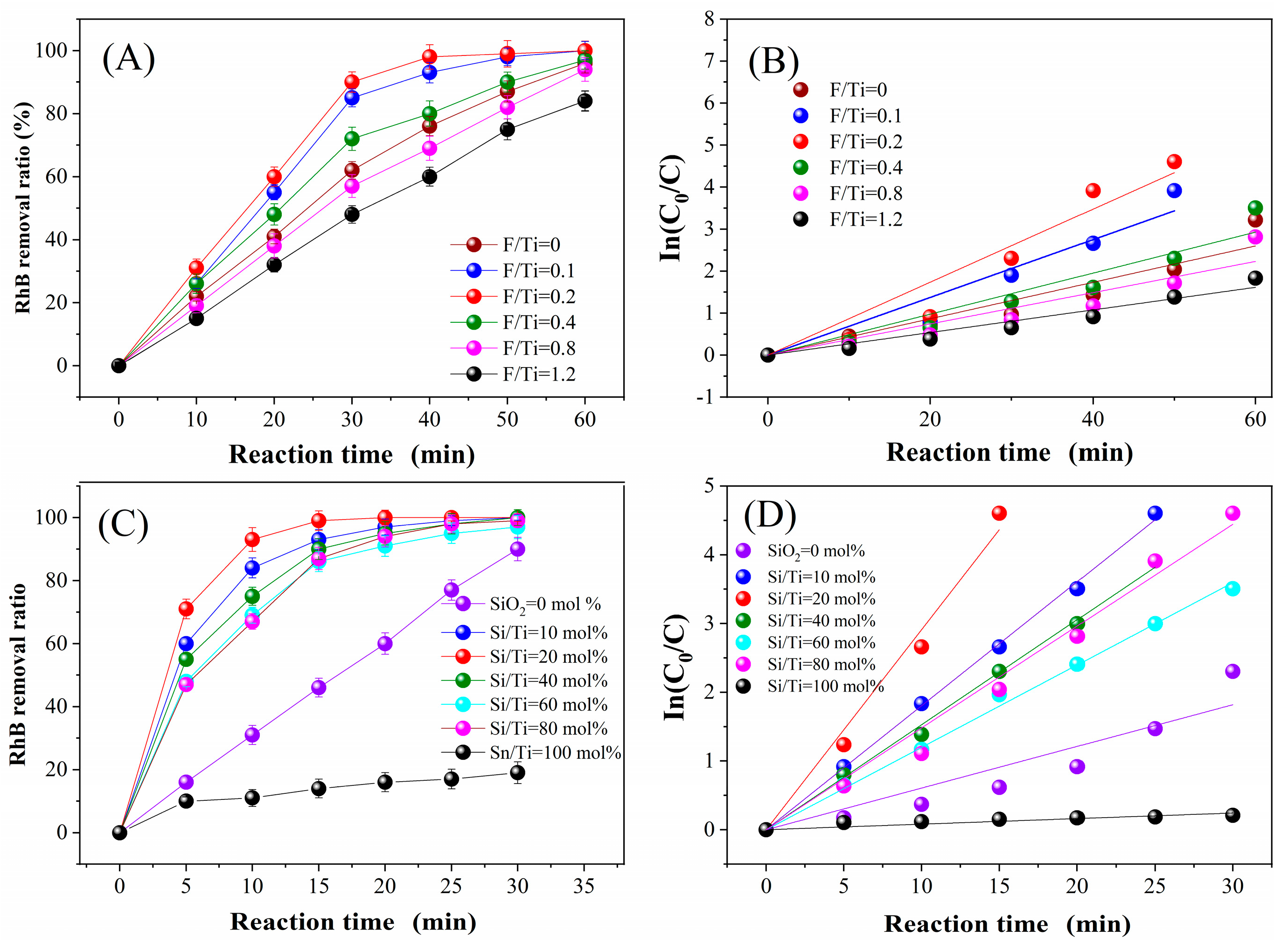
2.4.1. Impact of F Doping on RhB Degradation
2.4.2. Impact of SiO2/SnO2 Molar Ratio on RhB Degradation
2.4.3. Cyclic Use of the Photocatalyst
2.4.4. Effect of pH Value on Catalytic Activity
2.4.5. Investigation of Active Species
3. Discussion
4. Materials and Methods
4.1. Material and Reagents
4.2. Preparation of Photocatalysts
4.2.1. Synthesis of Bi-TiO2/SnO2
4.2.2. Synthesis of F-Bi-TiO2/SnO2
4.2.3. Synthesis of F-Bi-TiO2/SnO2/SiO2
4.3. Catalyst Evaluation
4.4. Catalyst Characterizations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Raizada, P.; Sudhaik, A.; Singh, P.; Shandilya, P.; Gupta, V.K.; Hosseini-Bandegharaei, A.; Agrawal, S. Ag3PO4 modified phosphorus and sulphur co-doped graphitic carbon nitride as a direct Z-scheme photocatalyst for 2, 4-dimethyl phenol degradation. J. Photochem. Photobiol. A 2019, 374, 22–35. [Google Scholar] [CrossRef]
- Mendoza-Diaz, M.-I.; Lecestre, A.; Salvagnac, L.; Bounor, B.; Pech, D.; Djafari-Rouhani, M.; Esteve, A.; Rossi, C. High surface area TiO2 photocatalyst for H2 production through silicon micromachining. Appl. Surf. Sci. 2022, 588, 152919. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.; Yantara, N.; Boix, P.P.; Kulkarni, S.A.; Mhaisalkar, S.; Grätzel, M.; Park, N.G. High Efficiency Solid-State Sensitized Solar Cell-Based on Submicrometer Rutile TiO2 Nanorod and CH3NH3PbI3 Perovskite Sensitizer. Nano Lett. 2013, 13, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Li, H.; Yang, Z.; Li, Y.; Zhang, P.; Zheng, Z.; Wang, Y.; Li, J.; Zhang, X. A novel CaIn2S4/TiO2NTAs heterojunction photoanode for highly efficient photocathodic protection performance of 316 SS under visible light. Nanotechnology 2021, 32, 395702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, Y.C.; Nadagouda, M.; Han, C.; O′Shea, K.; El-Sheikh, S.M.; Ismail, A.A.; Dionysiou, D.D. Visible light-sensitized S, N and C co-doped polymorphic TiO2 for photocatalytic destruction of microcystin-LR. Appl. Catal. B Environ. 2014, 144, 614–621. [Google Scholar] [CrossRef]
- Isari, A.A.; Hayati, F.; Kakavandi, B.; Rostami, M.; Motevassel, M.; Dehghanifard, E. N, Cu co-doped TiO2@functionalized SWCNT photocatalyst coupled with ultrasound and visible-light: An effective sono-photocatalysis process for pharmaceutical wastewaters treatment. Chem. Eng. J. 2020, 392, 123685. [Google Scholar] [CrossRef]
- Ismael, M. Enhanced photocatalytic hydrogen production and degradation of organic pollutants from Fe (III) doped TiO2 nanoparticles. J. Environ. Chem. Eng. 2020, 8, 103676. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. Dual Cocatalysts in TiO2 Photocatalysis. Adv. Mater. 2019, 31, 1807660. [Google Scholar] [CrossRef]
- Moon, H.S.; Hsiao, K.; Wu, M.; Yun, Y.; Hsu, Y.; Yong, K. Spatial separation of cocatalysts on Z-Scheme organic/inorganic heterostructure hollow spheres for enhanced photocatalytic H2 evolution and in-depth analysis of the charge-transfer mechanism. Adv. Mater. 2022, 35, 2200172. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, M.; He, J.; Lo, I.M. Recent developments and challenges in practical application of visible-light-driven TiO2-based heterojunctions for PPCP degradation: A critical review. Water Res. 2020, 170, 115356. [Google Scholar] [CrossRef]
- Meng, A.; Cheng, B.; Tan, H.; Fan, J.; Su, C.; Yu, J. TiO2/polydopamine S-scheme heterojunction photocatalyst with enhanced CO2-reduction selectivity. Appl. Catal. B Environ. 2021, 289, 120039. [Google Scholar] [CrossRef]
- Liang, Z.; Bai, X.; Hao, P.; Guo, Y.; Xue, Y.; Tian, J.; Cui, H. Full solar spectrum photocatalytic oxygen evolution by carbon-coated TiO2 hierarchical nanotubes. Appl. Catal. B Environ. 2019, 243, 711–720. [Google Scholar] [CrossRef]
- Singh, J.; Juneja, S.; Soni, R.K.; Bhattacharya, J. Sunlight mediated enhanced photocatalytic activity of TiO2 nanoparticles functionalized CuO-Cu2O nanorods for removal of methylene blue and oxytetracycline hydrochloride. J. Colloid Interf. Sci. 2021, 590, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhou, W.; Yang, K.; Rong, G. Hydrothermal synthesis of hemisphere-like F-doped anatase TiO2 with visible light photocatalytic activity. J. Mater. Sci. 2010, 45, 5756–5761. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zhu, X.; Liu, Y.; Wu, Z. Fluorine-induced oxygen vacancies on TiO2 nanosheets for photocatalytic indoor VOCs degradation. Appl. Catal. B Environ. 2022, 316, 121610. [Google Scholar] [CrossRef]
- Tosoni, S.; Lamiel-Garcia, O.; Fernandez, H.; Doña, J.M.; Illas, F. Electronic Structure of F-Doped Bulk Rutile, Anatase, and Brookite Polymorphs of TiO2. J. Phys. Chem. C 2012, 116, 12738–12746. [Google Scholar] [CrossRef]
- Pang, D.; Wang, Y.; Ma, X.; Ouyang, F. Fluorine promoted and silica supported TiO2 for photocatalytic decomposition of acrylonitrile under simulant solar light irradiation. Chem. Eng. J. 2014, 258, 43–50. [Google Scholar] [CrossRef]
- Wen, J.; Li, X.; Liu, W.; Fang, Y.; Xie, J.; Xu, Y. Photocatalysis fundamentals and surface modification of TiO2 nanomaterials. Chin. J. Catal. 2015, 36, 2049–2070. [Google Scholar] [CrossRef]
- Chakinala, N.; Gogate, P.R.; Chakinala, A.G. Highly efficient bi-metallic bismuth-silver doped TiO2 photocatalyst for dye degradation. Korean J. Chem. Eng. 2021, 38, 2468–2478. [Google Scholar] [CrossRef]
- Liang, Z.; Cao, Y.; Li, Y.; Xie, J.; Guo, N.; Jia, D. Solid-state chemical synthesis of rod-like fluorine-doped β-Bi2O3 and their enhanced photocatalytic property under visible light. Appl. Surf. Sci. 2016, 390, 78–85. [Google Scholar] [CrossRef]
- Cao, W.; Wang, W.; Yang, Z.; Wang, W.; Chen, W.; Wu, K. Enhancing photocathodic protection performance by controlled synthesis of Bi/BiOBr/TiO2 NTAs Z-scheme heterojunction films. J. Alloys Compd. 2023, 960, 170675. [Google Scholar] [CrossRef]
- Reddy, P.A.K.; Srinivas, B.; Kala, P.; Kumari, V.D.; Subrahmanyam, M. Preparation and characterization of Bi-doped TiO2 and its solar photocatalytic activity for the degradation of isoproturon herbicide. Mater. Res. Bull. 2011, 46, 1766–1771. [Google Scholar] [CrossRef]
- Huang, J.; Cheuk, W.; Wu, Y.; Lee, F.S.; Ho, W. Fabrication of Bi-Doped TiO2 spheres with ultrasonic spray pyrolysis and investigation of their visible-light photocatalytic properties. J. Nanotechnol. 2012, 2012, 214783. [Google Scholar] [CrossRef]
- Althamthami, M.; Guettaf Temam, E.; Temam, H.B.; Saâd, R. Improved photocatalytic activity under the sunlight of high transparent hydrophilic Bi-doped TiO2 thin-films. J. Photochem. Photobiol. A 2023, 443, 114818. [Google Scholar] [CrossRef]
- Murcia-López, S.; Hidalgo, M.C.; Navío, J.A. Synthesis, characterization and photocatalytic activity of Bi-doped TiO2 photocatalysts under simulated solar irradiation. Appl. Catal. A Gen. 2011, 404, 59–67. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Zhang, Y.; Zhou, X.; Guo, S. Fabrication of bidirectionally doped β-Bi2O3/TiO2-NTs with enhanced photocatalysis under visible light irradiation. J. Hazard. Mater. 2013, 258–259, 42–49. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Q.; Tan, X.; Zheng, S.; Zhang, H.; Wang, Y.; Gao, S. Solvothermal preparation of Bi/Bi2O3 nanoparticles on TiO2 NTs for the enhanced photoelectrocatalytic degradation of pollutants. J. Alloys Compd. 2020, 815, 152478. [Google Scholar] [CrossRef]
- Mohamed, I.M.A.; Dao, V.; Yasin, A.S.; Mousa, H.M. Nitrogen-doped&SnO2-incoportaed TiO2 nanofibers as novel and effective photoanode for enhanced efficiency dye-sensitized solar cells. Chem. Eng. J. 2016, 304, 48–60. [Google Scholar]
- Chawla, A.; Sudhaik, A.; Raizada, P.; Khan, A.A.P.; Singh, A.; Van Le, Q.; Nguyen, V.H.; Ahamad, T.; Alshehri, S.M.; Asiri, A.M.; et al. An overview of SnO2 based Z scheme heterojuctions: Fabrication, mechanism and advanced photocatalytic applications. J. Ind. Eng. Chem. 2022, 116, 515–542. [Google Scholar] [CrossRef]
- Roy, H.; Rahman, T.U.; Khan, M.A.J.R.; Al-Mamun, M.R.; Islam, S.Z.; Khaleque, M.A.; Hossain, M.I.; Khan, M.Z.H.; Islam, M.S.; Marwani, H.M.; et al. Toxic dye removal, remediation, and mechanism with doped SnO2-based nanocomposite photocatalysts: A critical review. J. Water Process Eng. 2023, 54, 104069. [Google Scholar] [CrossRef]
- Pesci, F.M.; Wang, G.; Klug, D.R.; Li, Y.; Cowan, A.J. Efficient suppression of electron-hole recombination in oxygen-deficient hydrogen-treated TiO2 nanowires for photoelectrochemical water splitting. J. Phys. Chem. C Nanomater. Interfaces 2013, 117, 25837–25844. [Google Scholar] [CrossRef] [PubMed]
- Kusior, A.; Zych, L.; Zakrzewska, K.; Radecka, M. Photocatalytic activity of TiO2/SnO2 nanostructures with controlled dimensionality/complexity. Appl. Surf. Sci. 2019, 471, 973–985. [Google Scholar] [CrossRef]
- Alagarasi, A.; Rajalakshmi, P.U.; Shanthi, K.; Selvam, P. Solar-light driven photocatalytic activity of mesoporous nanocrystalline TiO2, SnO2, and TiO2-SnO2 composites. Mater. Today Sustain. 2019, 5, 100016. [Google Scholar] [CrossRef]
- Magdalane, C.M.; Kanimozhi, K.; Arularasu, M.V.; Ramalingam, G.; Kaviyarasu, K. Self-cleaning mechanism of synthesized SnO2/TiO2 nanostructure for photocatalytic activity application for waste water treatment. Surf. Interfaces 2019, 17, 100346. [Google Scholar] [CrossRef]
- De Mendonça, V.R.; Avansi, W.; Arenal, R.; Ribeiro, C. A building blocks strategy for preparing photocatalytically active anatase TiO2/rutile SnO2 heterostructures by hydrothermal annealing. J. Colloid Interface Sci. 2017, 505, 454–459. [Google Scholar] [CrossRef]
- Angel, R.D.; Durán-Álvarez, J.; Zanella, R. TiO2-low band gap semiconductor heterostructures for water treatment using sunlight-Driven photocatalysis. Titan. Dioxide Mater. Sustain. Environ. 2018, 76501, 305–329. [Google Scholar]
- Albornoz, L.L.; Da Silva, S.W.; Bortolozzi, J.P.; Banús, E.D.; Brussino, P.; Ulla, M.A.; Bernardes, A.M. Degradation and mineralization of erythromycin by heterogeneous photocatalysis using SnO2-doped TiO2 structured catalysts: Activity and stability. Chemosphere 2021, 268, 128858. [Google Scholar] [CrossRef]
- Rajput, R.B.; Jamble, S.N.; Kale, R.B. A review on TiO2/SnO2 heterostructures as a photocatalyst for the degradation of dyes and organic pollutants. J. Environ. Manag. 2022, 307, 114533. [Google Scholar] [CrossRef]
- Hassan, S.M.; Ahmed, A.I.; Mannaa, M.A. Structural, photocatalytic, biological and catalytic properties of SnO2/TiO2 nanoparticles. Ceram. Int. 2018, 44, 6201–6211. [Google Scholar] [CrossRef]
- Hassan, S.M.; Ahmed, A.I.; Mannaa, M.A. Preparation and characterization of SnO2 doped TiO2 nanoparticles: Effect of phase changes on the photocatalytic and catalytic activity. J. Sci. Adv. Mater. Dev. 2019, 4, 400–412. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Zou, B.; Zhang, H.; Wang, J.; Schubert, U.; Rui, Y. Black SnO2-TiO2 Nanocomposites with High Dispersion for Photocatalytic and Photovoltalic Applications. ACS Appl. Nano Mater. 2020, 3, 4265–4273. [Google Scholar] [CrossRef]
- Lin, C.; Gao, Y.; Zhang, J.; Xue, D.; Fang, H.; Tian, J.; Zhou, C.; Zhang, C.; Li, Y.; Li, H. GO/TiO2 composites as a highly active photocatalyst for the degradation of methyl orange. J. Mater. Res. 2020, 35, 1307–1315. [Google Scholar] [CrossRef]
- Akti, F.; Balci, S. Synthesis of APTES and alcohol modified Sn/SBA-15 in presence of competitive ion: Test in degradation of Remazol Yellow. Mater. Res. Bull. 2022, 145, 111496. [Google Scholar] [CrossRef]
- Soni, S.S.; Henderson, M.J.; Bardeau, J.F.; Gibaud, A. Visible-light photocatalysis in titania-based mesoporous thin films. Adv. Mater. 2008, 20, 1493–1498. [Google Scholar] [CrossRef]
- Liao, Y.; Huang, C.; Liao, C.; Wu, J.; Wu, K. Synthesis of mesoporous titania thin films (MTTFs) with two different structures as photocatalysts for generating hydrogen from water splitting. Appl. Energ. 2012, 100, 75–80. [Google Scholar] [CrossRef]
- Li, L.; Yan, J.; Wang, T.; Zhao, Z.-J.; Zhang, J.; Gong, J.; Guan, N. Sub-10 nm rutile titanium dioxide nanoparticles for efficient visible-light-driven photocatalytic hydrogen production. Nat. Commun. 2015, 6, 5881. [Google Scholar] [CrossRef]
- Wang, Q.; Rhimi, B.; Wang, H.; Wang, C. Efficient photocatalytic degradation of gaseous toluene over F-doped TiO2/exfoliated bentonite. Appl. Surf. Sci. 2020, 530, 147286. [Google Scholar] [CrossRef]
- Preeti; Mishra, S.; Chakinala, N.; Chakinala, A.G. Bimetallic Bi/Zn decorated hydrothermally synthesized TiO2 for efficient photocatalytic degradation of nitrobenzene. Catal. Commun. 2022, 172, 106538. [Google Scholar] [CrossRef]
- Yan, B.; Chen, G.; Ma, B.; Guo, Y.; Zha, Y.; Li, J.; Wang, S.; Liu, J.; Zhao, B.; Xie, H. Construction of surface plasmonic Bi nanoparticles and α-Bi2O3 co-modified TiO2 nanotube arrays for enhanced photocatalytic degradation of ciprofloxacin: Performance, DFT calculation and mechanism. Sep. Purif. Technol. 2024, 330, 125180. [Google Scholar] [CrossRef]
- Bagwasi, S.; Tian, B.; Zhang, J.; Nasir, M. Synthesis, characterization and application of bismuth and boron Co-doped TiO2: A visible light active photocatalyst. Chem. Eng. J. 2013, 217, 108–118. [Google Scholar] [CrossRef]
- Huy, T.H.; Phata, B.D.; Kang, F.; Wang, Y.F.; Liu, S.H.; Thi, C.M.; You, S.J.; Chang, G.M.; Pham, V.V. SnO2/TiO2 nanotube heterojunction: The first investigation of NO degradation by visible light-driven photocatalysis. Chemosphere 2019, 215, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhu, H.; Jin, T.; Chen, L.; Zhang, J.; Qiao, K.; Chen, Z. Construction of Bi/Polyoxometalate doped TiO2 composite with efficient visible-light photocatalytic performance: Mechanism insight, degradation pathway and toxicity evaluation. Appl. Surf. Sci. 2023, 615, 156310. [Google Scholar] [CrossRef]
- Li, G.; Li, J.; Li, G.; Jiang, G. N and Ti3+ co-doped 3D anatase TiO2 superstructures composed of ultrathin nanosheets with enhanced visible light photocatalytic activity. J. Mater. Chem. A 2015, 3, 22073. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.; Bai, H.; Liu, J.; Hu, D.; Fan, J.; Shen, H. Enhanced visible-light photocatalytic performance of black TiO2/SnO2 nanoparticles. J. Alloy. Compd. 2023, 960, 170672. [Google Scholar] [CrossRef]
- Song, Y.; Long, A.; Ge, X.; Bao, Z.; Meng, M.; Hu, S.; Gu, Y. Construction of floatable flower-like plasmonic Bi/BiOCl-loaded hollow kapok fiber photocatalyst for efficient degradation of RhB and antibiotics. Chemosphere 2023, 343, 140240. [Google Scholar] [CrossRef]
- Li, J.; Jin, Z.; Zhang, Y.; Liu, D.; Ma, A.; Sun, Y.; Li, X.; Cai, Q.; Gui, J. Ag-induced anatase-rutile TiO2-x heterojunction facilitating the photogenerated carrier separation in visible-light irradiation. J. Alloy. Compd. 2022, 909, 164815. [Google Scholar] [CrossRef]
- Li, H.; Qiu, L.; Bharti, B.; Dai, F.; Zhu, M.; Ouyang, F.; Lin, L. Efficient photocatalytic degradation of acrylonitrile by Sulfur-Bismuth co-doped F-TiO2/SiO2 nanopowder. Chemosphere 2020, 249, 126135. [Google Scholar] [CrossRef]
- Choukaife, A.E.; Aljerf, L. A descriptive study-in vitro: New validated method for checking Hap and Fap Behaviours. Int. Med. J. 2017, 24, 394–397. [Google Scholar]
- Donohue, M.D.; Aranovich, G.L. A new classification of isotherms for Gibbs adsorption of gases on solids. Fluid Phase Equilibr. 1999, 158–160, 557–563. [Google Scholar] [CrossRef]
- Liu, G.; Yang, H.G.; Wang, X.; Cheng, L.; Lu, H.; Wang, L.; Lu, G.; Cheng, H.M. Enhanced Photoactivity of Oxygen-Deficient Anatase TiO2 Sheets with Dominant {001} Facets. J. Phys. Chem. C 2009, 113, 21784–21788. [Google Scholar] [CrossRef]
- Lin, Z.; Orlov, A.; Lambert, R.M.; Payne, M.C. New insights into the origin of visible light photocatalytic activity of nitrogen-doped and oxygen-deficient anatase TiO2. J. Phys. Chem. B 2005, 109, 20948–20952. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Greene, L.E.; Radenovic, A.; Kuykendall, T.; Liphardt, J.; Yang, P. ZnO-Al2O3 and ZnO-TiO2 core-shell nanowire dye-sensitized solar cells. J. Phys. Chem. B 2006, 110, 22652–22663. [Google Scholar] [CrossRef] [PubMed]
- Parayil, S.K.; Kibombo, H.S.; Wu, C.; Peng, R.; Baltrusaitis, J.; Koodali, R.T. Enhanced photocatalytic water splitting activity of carbon-modified TiO2 composite materials synthesized by a green synthetic approach. Int. J. Hydrogen Energ. 2012, 37, 8257–8267. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.C.; Liu, P.; Wang, X.; Su, W.; Fu, X. Probing of photocatalytic surface sites on SO42−/TiO2 solid acids by in situ FT-IR spectroscopy and pyridine adsorption. J. Photochem. Photobiol. A 2006, 179, 339–347. [Google Scholar] [CrossRef]
- Hong, W.; Li, C.; Tang, T.; Xu, H.; Yu, Y.; Liu, G.; Wang, F.; Lie, C.; Zhu, H. The photocatalytic activity of the SnO2/TiO2/PVDF composite membrane in rhodamine B degradation. New J. Chem. 2021, 45, 2631–2642. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, R.; Li, Y.; Hong, X.; Liang, B. Glucose-tailored SnO2/TiO2/RGO ternary composite for degradation of organic pollutants. J. Phys. Chem. Solids 2022, 161, 110442. [Google Scholar] [CrossRef]
- Stefan, M.; Leostean, C.; Pana, O.; Popa, A.; Toloman, D.; Macavei, S.; Perhaita, I.; Barbu-Tudoran, L.; Silipas, D. Interface tailoring of SnO2-TiO2 photocatalysts modified with anionic/cationic surfactants. J. Mater. Sci. 2019, 55, 3279–3298. [Google Scholar] [CrossRef]
- Wang, Q.; Fang, Y.; Meng, H.; Wu, W.; Chu, G.; Zou, H.; Cheng, D.; Chen, J. Enhanced simulated sunlight induced photocatalytic activity by pomegranate-like S doped SnO2@TiO2 spheres. Colloids Surf. A 2015, 482, 529–535. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, M.; Song, D.; Pan, X.; Fu, J.; Zhou, J.; Ma, S.; Wang, T. Highly porous SnO2/TiO2 electrospun nanofibers with high photocatalytic activities. Ceram. Int. 2014, 40, 10383–10393. [Google Scholar] [CrossRef]
- Gao, Q.; Wu, X.; Fan, Y.; Zhou, X. Low temperature fabrication of nanoflower arrays of rutile TiO2 on mica particles with enhanced photocatalytic activity. J. Alloys Compd. 2013, 579, 322–329. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, Y.; Chen, B.; Tang, H.; Li, L.; Ding, Y.; Duan, H.; Wu, D. Insights into the enhanced photocatalytic activity of O-g-C3N4 coupled with SnO2 composites under visible light irradiation. J. Alloys Compd. 2022, 903, 163739. [Google Scholar] [CrossRef]
- Ma, H.; Wang, Y.; Zhang, Z.; Liu, J.; Yu, Y.; Zuo, S.; Li, B. A superior ternary Z-scheme photocatalyst of Bi/Black Phosphorus nanosheets/P-doped BiOCl containing interfacial P–P bond and metallic mediator for H2O2 production and RhB degradation. Chemosphere 2023, 330, 138717. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yu, S.; Liu, J.; Zhang, Y.; Wang, Y.; Yu, J.; Yuan, M.; Zhang, P.; Liu, W.; Zhang, J. C, F co-doping Ag/TiO2 with visible light photocatalytic performance toward degrading Rhodamine B. Environ. Res. 2023, 232, 116311. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, A.; Fu, X.; Peng, Z. Novel F-doped carbon nanotube@(N,F)-co-doped TiO2-δ nanocomposite: Highly active visible-light-driven photocatalysts for water decontamination. Appl. Surf. Sci. 2023, 609, 155460. [Google Scholar] [CrossRef]
- Khandekar, D.C.; Bhattacharyya, A.R.; Bandyopadhyaya, R. Role of impregnated nano-photocatalyst (SnxTi(1−x)O2) inside mesoporous silica (SBA-15) for degradation of organic pollutant (Rhodamine B) under UV light. J. Environ. Chem. Eng. 2019, 7, 103433. [Google Scholar] [CrossRef]
- Wu, Y.M.; Xing, M.Y.; Tian, B.Z.; Zhang, J.L.; Chen, F. Preparation of nitrogen and fluorine co-doped mesoporous TiO2 microsphere and photodegradation of acid orange 7 under visible light. Chem. Eng. J. 2010, 162, 710–717. [Google Scholar] [CrossRef]
- Yang, G.D.; Jiang, Z.; Shi, H.H.; Jones, M.O.; Xiao, T.C.; Edwards, P.P.; Yan, Z.F. Study on the photocatalysis of F-S co-doped TiO2 prepared using solvothermal method. Appl. Catal. B Environ. 2010, 96, 458–465. [Google Scholar] [CrossRef]





| Composite | Light | Concentration | D (%) | Time (min) |
|---|---|---|---|---|
| SnO2/TiO2/PVDF [65] | UV | 10 mg/L | 91.84 | 270 |
| SnO2/TiO2/RGO [66] | UV | 10 mg/L | 97.60 | 40 |
| SnO2–TiO2 [67] | Visible | 10−5 mol/L | 76.00 | 180 |
| S doped SnO2 @TiO2 [68] | Sunlight | 10 mg/L | 97.00 | 200 |
| SnO2/TiO2 [69] | UV–vis | 10 mg/L | 99.00 | 30 |
| SnO2/TiO2 [70] | UV–vis | 20 mg/L | 94.00 | 180 |
| SnO2/TiO2 [39] | UV–vis | 10 mg/L | 98.00 | 120 |
| B-TiO2/SnO2 [54] | VisibleUV–vis | 10 mg/L | 96.62100 | 9070 |
| O-g-C3N4/SnO2 [71] | Visible | 10 mg/L | 98.10 | 30 |
| Bi/Bi2O3/TNAs [24] | Sunlight | 2 mg/L | 35 | 240 |
| Bi/BPNs/P–BiOCl [72] | Xenon lamp | 20 mg/L | 100 | 30 |
| KF/Bi/BC [55] | Xenon lamp | 30 mg/L | 100 | 10 |
| C/F–Ag–TiO2 [73] | Xenon lamp | 10 mg/L | 84.2 | 240 |
| N,F-TiO2-δ [74] | LED lights | 10 mg/L | 77.2 | 60 |
| F/Bi-TiO2/SnO2/SiO2 (this study) | Xenon lamp | 10 mg/L | 100 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, L.; Li, H.; Xu, W.; Zhu, R.; Ouyang, F. TiO2 Catalysts Co-Modified with Bi, F, SnO2, and SiO2 for Photocatalytic Degradation of Rhodamine B Under Simulated Sunlight. Catalysts 2024, 14, 735. https://doi.org/10.3390/catal14100735
Qiu L, Li H, Xu W, Zhu R, Ouyang F. TiO2 Catalysts Co-Modified with Bi, F, SnO2, and SiO2 for Photocatalytic Degradation of Rhodamine B Under Simulated Sunlight. Catalysts. 2024; 14(10):735. https://doi.org/10.3390/catal14100735
Chicago/Turabian StyleQiu, Lu, Hanliang Li, Wenyi Xu, Rongshu Zhu, and Feng Ouyang. 2024. "TiO2 Catalysts Co-Modified with Bi, F, SnO2, and SiO2 for Photocatalytic Degradation of Rhodamine B Under Simulated Sunlight" Catalysts 14, no. 10: 735. https://doi.org/10.3390/catal14100735
APA StyleQiu, L., Li, H., Xu, W., Zhu, R., & Ouyang, F. (2024). TiO2 Catalysts Co-Modified with Bi, F, SnO2, and SiO2 for Photocatalytic Degradation of Rhodamine B Under Simulated Sunlight. Catalysts, 14(10), 735. https://doi.org/10.3390/catal14100735







