Zirconium Phosphates and Phosphonates: Applications in Catalysis
Abstract
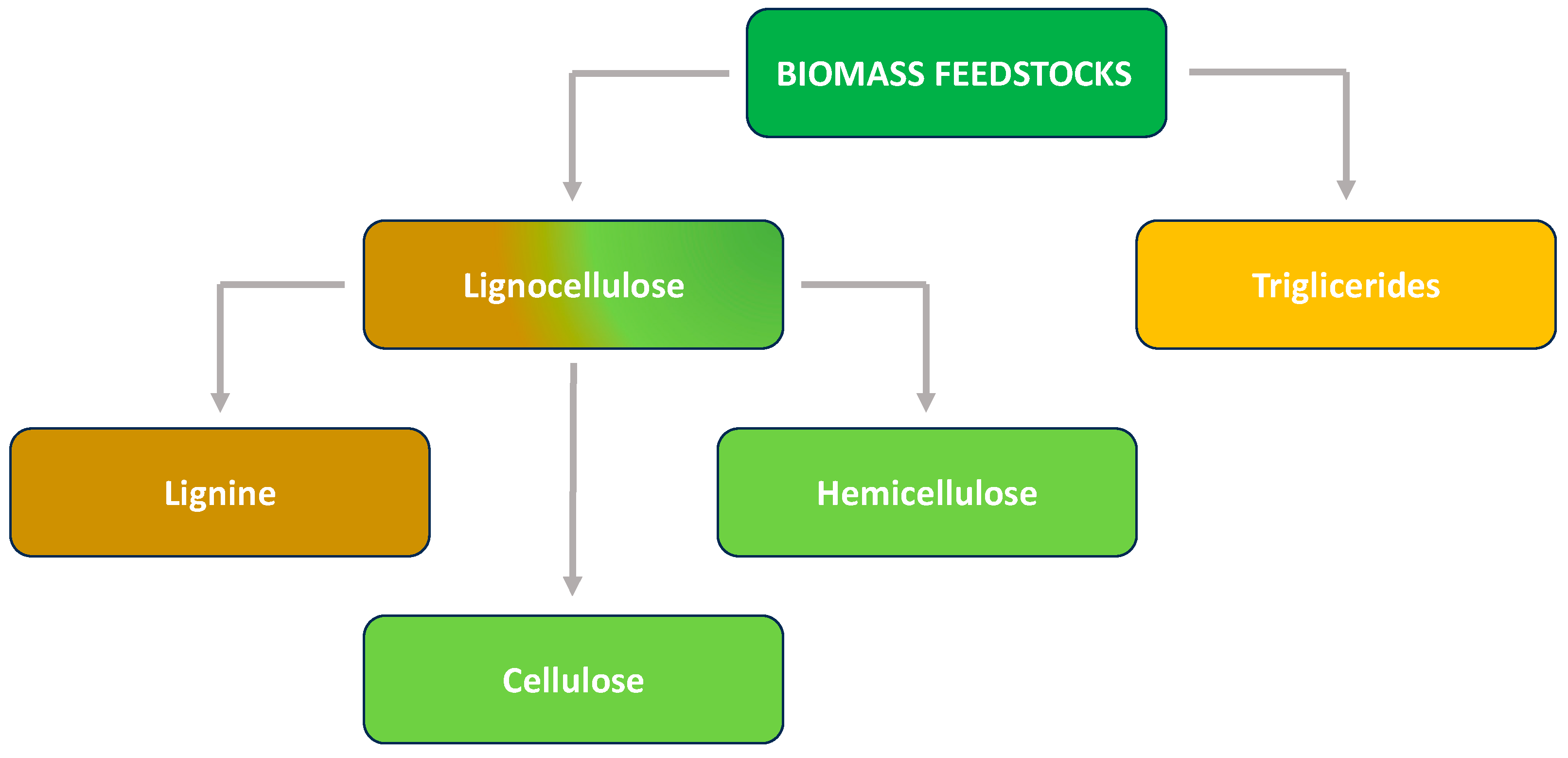
1. Introduction
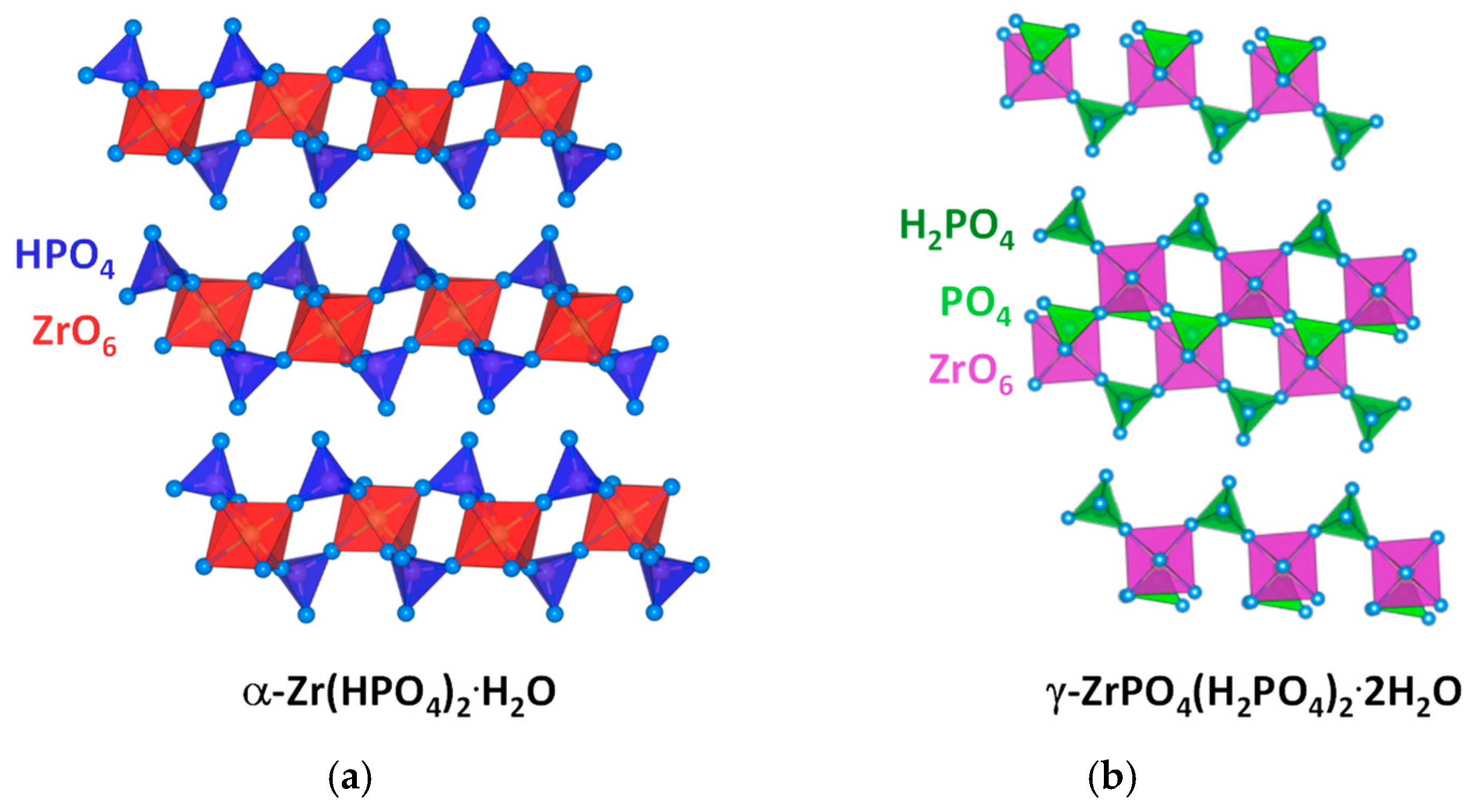
2. Zirconium Phosphates and Phosphonates (ZrPs): Structural Features and Synthetic Approaches
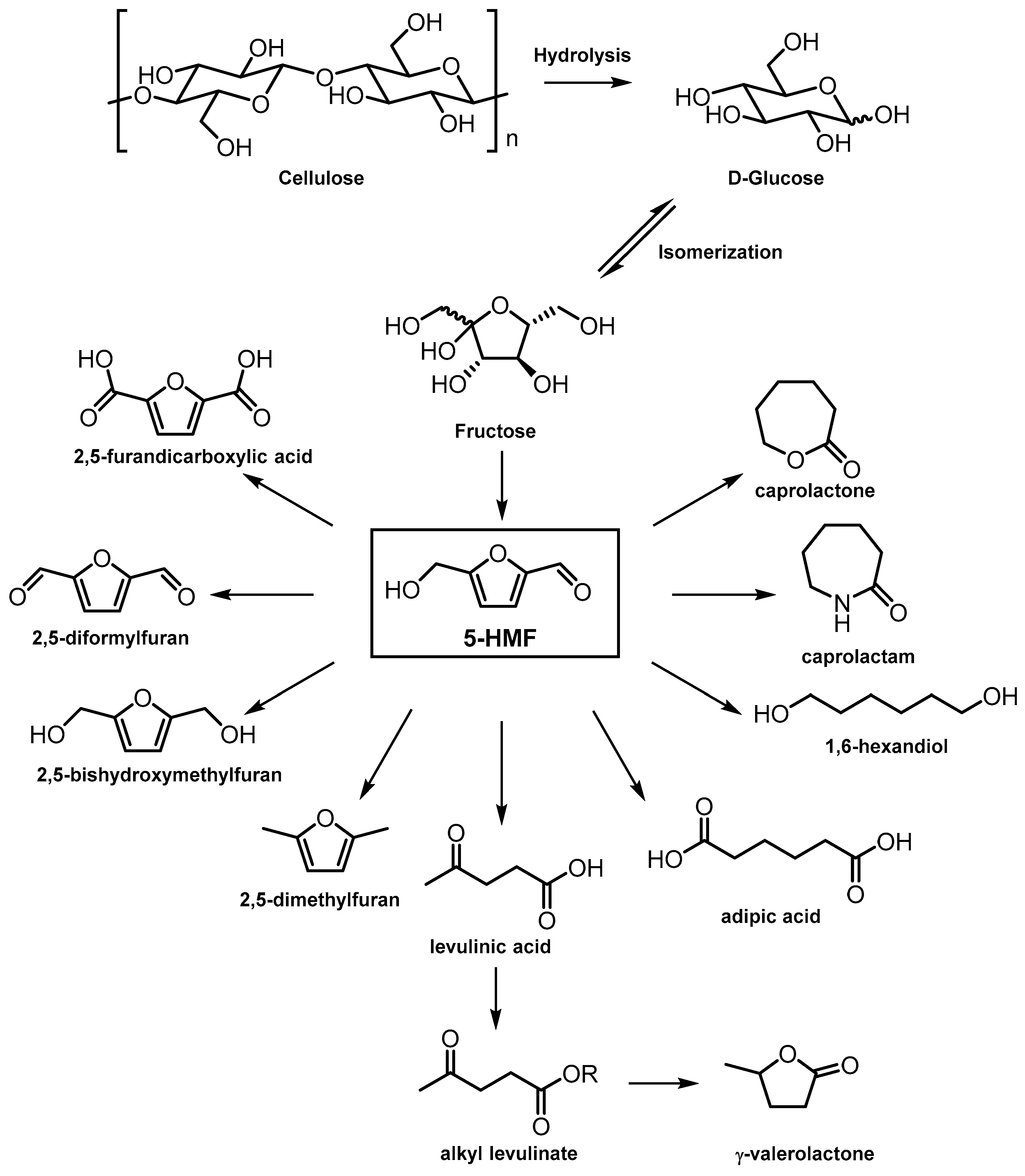
3. ZrPs as Solid Acid Catalysts
| Catalyst | Preparation Methods | BET Surface Area m2/g | Catalyzation Process | Ref. |
|---|---|---|---|---|
| ZrP mesoporous | Hydrothermal method (CTAB as template), calcination at 550 °C | 407 | Dehydration of sugar to 5-HMF | [38] |
| ZrP-x mesoporous P/Zr = 0.25–1.25 | Evaporation-induced self-assembly (F-127 as template), calcination at 500 °C | 118.5–163.8 | [39] | |
| ZrP amorphous | Precipitation, calcination at 400 °C | 108 | [40] | |
| ZrP mesoporous | Hydrothermal method (P-123 as template), calcination temp. 500–800 °C | 213–114.5 | [41] | |
| ZrP-x amorphous P/Zr = 0.5–2 | Precipitation, calcination at 400 °C | 104.6–160.5 | [42] | |
| ZrP-S amorphous | Acid-modified ZrP with oleum SO3 | 73.6 | [43] | |
| ZrP-S amorphous | Acid-modified ZrP with oleum SO3 | 73.6 | Dehydration of sorbitol to isosorbide | [43] |
| ZrP porous | Hydrothermal method (Pluronic P123 as template), calcination at 450 °C | 148 | [44] | |
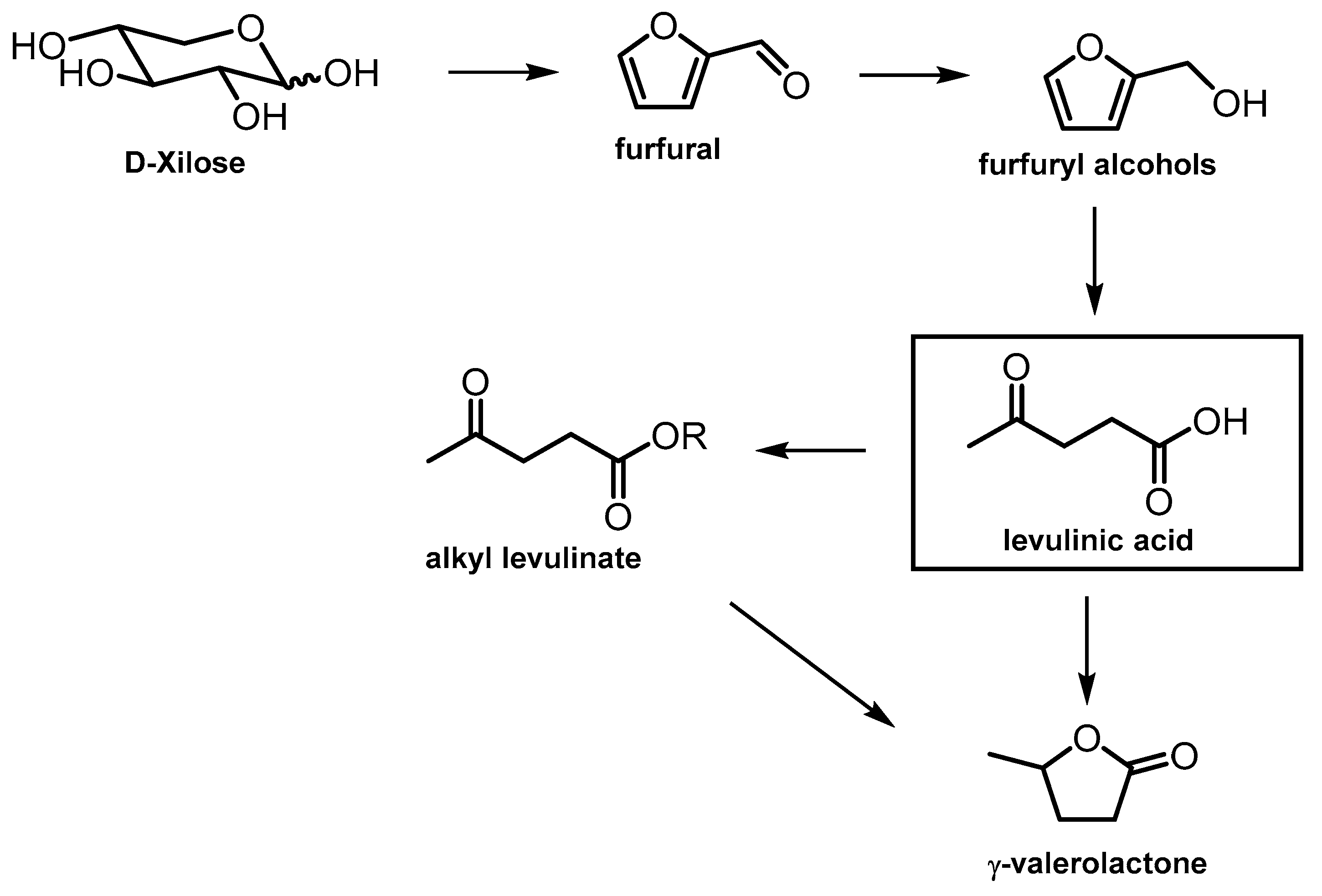
| ZrP porous | Sol–gel | 600 | Dehydration of xylose to furfural | [45] |
| ZrP amorphous | Hydrothermal method (P-123 as template), calcination at 400 °C | 232 | Hydrogenation of furfural to FA | [46] |
| ZrP-S | Acid-modified ZrP with H2SO4 S/Zr = 0–0.19 | 14–67 | Hydrogenation of FA to ethyl levulinate | [47] |
| ZrP-SAPO-34 P/Zr = 0.5–2 | Precipitation in presence of SAPO-34 zeolite powder, calcination at 400 °C | 424–290 | Hydrogenation of FA to GVL | [48] |
| ZrP_HT, ZrP_CT | Hydrothermal or coprecipitation method, calcination at 550 °C | 195.0, 192.6 | [49] | |
| ZrP-x amorphous P/Zr = 0.5–2 | Precipitation and calcination at 400 °C | 143.5–279.6 | Hydrogenation of alkyl levulinate to GVL | [50] |
| ZrP-PrSO3H | ZrP modified with propylsulfonic acid groups via post-grafting | 142.7 | Hydrogenation of xylose to alkyl levulinate | [51] |
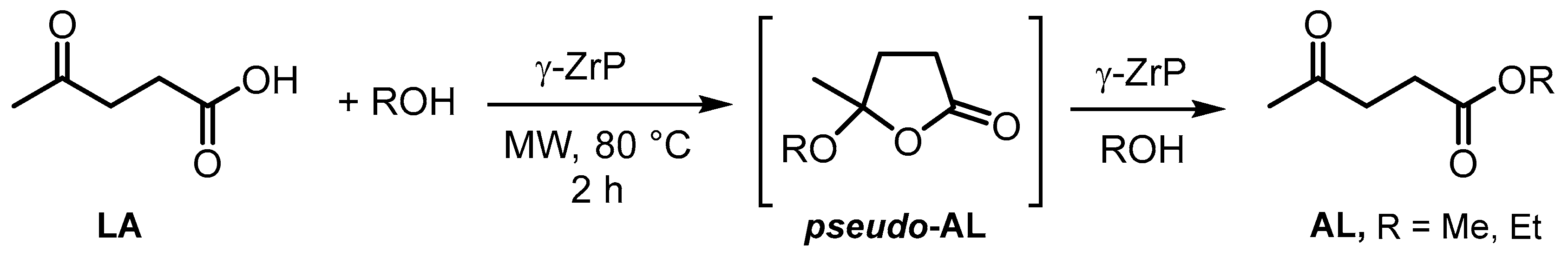
| ZrP-x mesoporous P/Zr = 1.5–2.5 | Hydrothermal method (CTAB as template), calcination at 550 °C | 366.1–262 | Esterification of levulinic acid | [52] |
| α-ZrP and γ-ZrP | Precipitation and reflux | - | [53] | |
| ZrPx-KIT, ZrPx-SBA P/Zr= 0–2 | ZrP grafted mesoporous silicas | 583.0–446.6 | p-Xylene production from biomass-derived 2,5-dimethylfuran | [54] |
| ZrPx P/Zr = 1–3 | Hydrothermal method, calcination at 550 °C | 196.5–410.9 | [55] | |
| SO3H-ZrP single layer nanosheets | Surface modification of exfoliated α-ZrP with propylsulfonic acid groups via post-grafting | - | Biodiesel production | [56] |
| ZP-P[SIH]-x composite, x = 1, 2, 3 | Hybridization of poly(ionic liquid) (P[SIH]) with mesoporous zirconium phenylvinyl phosphonate | 180–103 | [57] | |
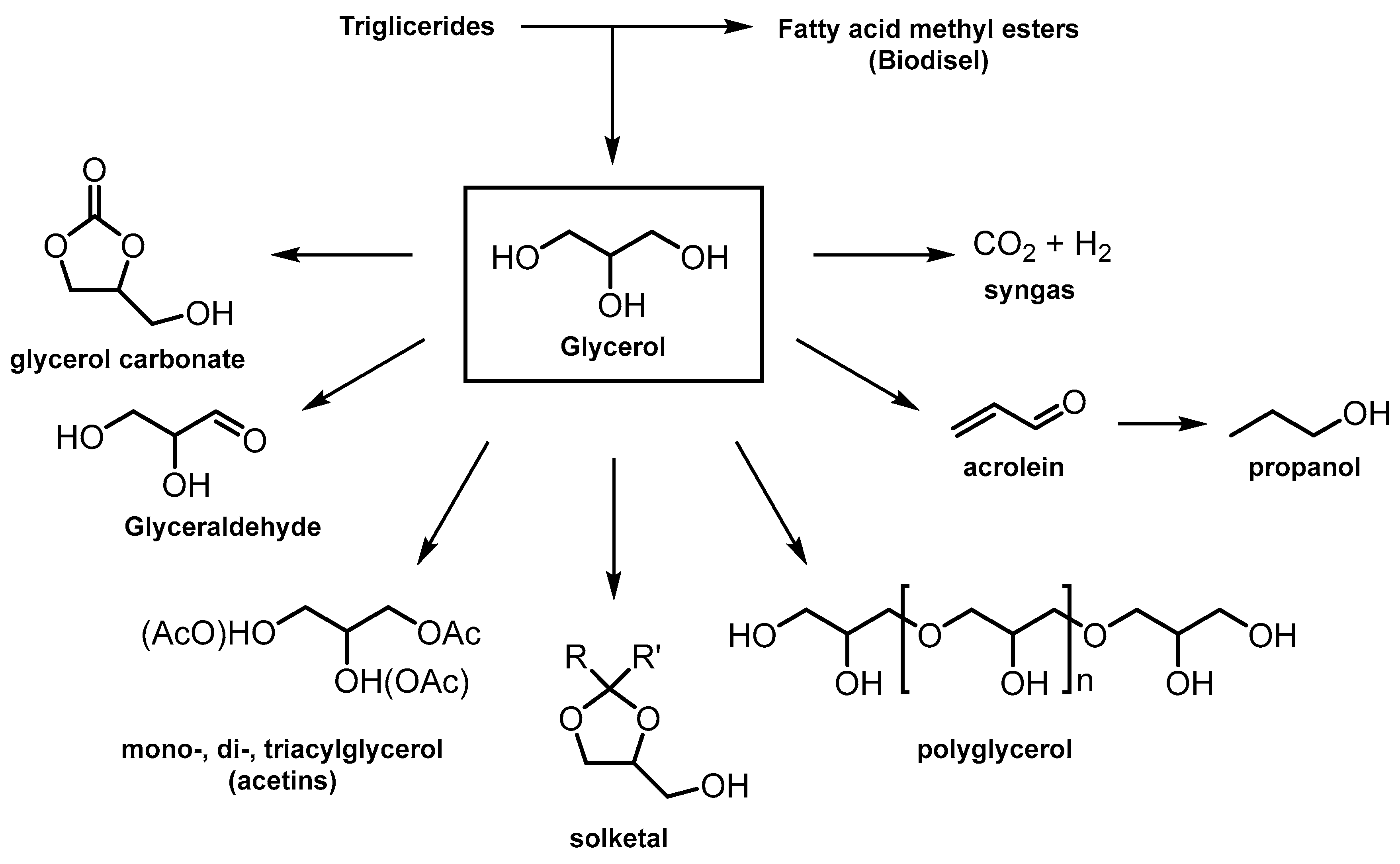
| ZrP amorphous | Precipitation, calcination at 400 °C | 101.3 | Dehydration of glycerol to acrolein | [58] |
| ZrP-x porous P/Zr = 0.33–3 | Sol–gel | 442.3–107.0 | [59] | |
| Zrx(PO4)y(SO4)z | Co-precipitation | 11–15 | Acetylation glycerol | [60] |
| PSA/K-ZrP-x x = 0.2–3 | p-Phenolsulfonic acid grafted onto the surface of KH560-modified α-ZrP | 6.3–1.7 | [61] | |
| ZrP | Hydrothermal method, calcination at temp. of 200–600 °C | 43.6–47.0 | Acetalization of glycerol (solketal) | [62] |
| ZrPPx flower-like x = 0–100% phenyl phosphonic acid | Hydrothermal method, calcination at 200 °C | 43.6–8.1 | [63] | |
| ZPS-PVPA-SO3H | Sulfonic acid-functionalized zirconium poly(styrene-phenylvinyl-phosphonate)-phosphate | - | Epoxidation of soybean oil | [64] |
| Catalyst | Preparation Methods | BET Surface Area m2/g | Catalyzed Process | Ref. |
|---|---|---|---|---|
| ZrHEDP, ZrATMP, ZrEDTMPS porous | Hydrothermal (CTAB as template) | 310–749 | Hydrolysis/esterification | [66] |
| ZrHEDP, ZrATMP, ZrEDTMPS porous | Hydrothermal (CTAB as template) | 310–749 | CO2 fixation | [67] |
| Zr(H4L) framework | Reflux (HF), H8L = tetraphenylsilane tetrakis-4-phosphonic acid | - | [68] | |
| CrZrP, CrZr2P porous | Sol–gel | 127.9, 149.3 | Oxidation alkanes and alkenes | [69] |
| ZrP/MCM-41 3–10% ZrP loading | Precipitation in presence of MCM-41, calcination at 400 °C | 671–642 | Oxidative desulfurization of benzothiophene | [70] |
| ZrP nanoparticles | Combustion method (550 °C) | Particle size 66 nm | Synthesis of nitriles | [71] |
| ZrP nanoparticles | Combustion method (550 °C) | Particle size 66 nm | Photocatalytic degradation of dyes | [71] |
| α-ZrP nanoparticles | Minimal solvent synthesis in polypropylene | Particle size 30 nm | [72] | |
| ZrP amorphous | Reflux and calcination at 600 °C | 23.11 | Isomerization α-pinene oxide to trans-carveol | [73] |
| ZrP-t-Bu xerogel | Non-hydrolytic sol–gel synthesis | 720 | Aminolysis styrene oxide | [74] |
| ZrPPAZOSO3H | Direct precipitation | 47.74 | Multi-component reactions | [75] |
| ZrP mesoporous | Surfactant template EISA (Pluronic P123) | 137 | [76] | |
| SAxAZP x = 2–20% SA | Sulfamic acid dispersed in micropores of Al-pillared α-ZrP | 80–120 | [77] | |
| BSA@α-ZrP nanoparticles | Butanesulfonic acid-modified α-ZrP | Particle size 12.61 nm | [78] | |
| ZrP | Reflux, calcination at temp. of 200–600 °C | 129.5–79 | [79] | |
| α-ZrP | Reflux | 33 | [80] |
4. ZrPs as Solid Base Catalysts
5. ZrPs as Support for Metal Ions and Metal Complex Immobilization
6. ZrPs as Supports for Metal NPs and Metal Oxide NPs Immobilization
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thomas, J.M.; Thomas, W.J. (Eds.) Principles and Practice of Heterogeneous Catalysis; Wiley-VCH: Weinheim, Germany, 1996. [Google Scholar]
- Schlögl, R. Heterogeneous catalysis. Angew. Chem. Int. Ed. 2015, 54, 3465–3520. [Google Scholar] [CrossRef] [PubMed]
- Hagen, J. Industrial Catalysis: A Practical Approach, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Mallesham, B.; Raikwar, D.; Shee, D. The role of catalysis in green synthesis of chemicals for sustainable future. In Advanced Functional Solid Catalysts for Biomass Valorization; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 1, pp. 1–37. [Google Scholar] [CrossRef]
- Pálinkó, I. Heterogeneous catalysis: A fundamental pillar of sustainable synthesis. In Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 3.12, pp. 415–447. [Google Scholar] [CrossRef]
- Vaccaro, L.; Curini, M.; Ferlin, F.; Lanari, D.; Marrocchi, A.; Piermatti, O.; Trombettoni, V. Definition of green synthetic tools based on safer reaction media, heterogeneous catalysis, and flow technology. Pure Appl. Chem. 2018, 90, 21–33. [Google Scholar] [CrossRef]
- Friend, C.M.; Xu, B. Heterogeneous catalysis: A central science for a sustainable future. Acc. Chem. Res. 2017, 50, 517–521. [Google Scholar] [CrossRef]
- Chatterjee, R.; Bhanja, P.; Bhaumik, A. The design and synthesis of heterogeneous catalysts for environmental applications. Dalton Trans. 2021, 50, 4765–4771. [Google Scholar] [CrossRef]
- Torok, B.; Schaefer, C.; Kokel, A. Heterogeneous Catalysis in Sustainable Synthesis; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Zhu, Y.P.; Ren, T.Z.; Yuan, Z.Y. Insights into mesoporous metal phosphonate hybrid materials for catalysis. Catal. Sci. Technol. 2015, 5, 4258–4279. [Google Scholar] [CrossRef]
- Pica, M. Zirconium phosphate catalysts in the XXI century: State of the art from 2010 to date. Catalysts 2017, 7, 190. [Google Scholar] [CrossRef]
- Li, D.; Ni, W.; Hou, Z. Conversion of biomass to chemicals over zirconium phosphate-based catalysts. Chin. J. Catal. 2017, 38, 1784–1793. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, S. Zirconium phosphate (ZrP)-based functional materials: Synthesis, properties and applications. Mater. Des. 2018, 155, 19–35. [Google Scholar] [CrossRef]
- Ramos-Garcés, M.V.; Colón, J.L. Preparation of zirconium phosphate nanomaterials and their applications as inorganic supports for the oxygen evolution reaction. Nanomaterials 2020, 10, 822. [Google Scholar] [CrossRef]
- Lv, X.W.; Weng, C.C.; Zhu, Y.P.; Yuan, Z.Y. Nanoporous Metal Phosphonate Hybrid Materials as a Novel Platform for Emerging Applications: A Critical Review. Small 2021, 17, 2005304. [Google Scholar] [CrossRef]
- Yadav, S.; Beniwal, N.; Rekha, P.; Singh, L. Recent advances in the synthesis and applications of porous zirconium phosphate. J. Porous Mater. 2022, 29, 1707–1725. [Google Scholar] [CrossRef]
- Amghouz, Z.; García, J.R.; Adawy, A. A Review on the Synthesis and Current and Prospective Applications of Zirconium and Titanium Phosphates. Eng 2022, 3, 161–174. [Google Scholar] [CrossRef]
- Rathore, K.; Jangir, R. Insight into Synthesis, properties and applications of metal Phosphonates: Emphasis on catalytic activities. Inorg. Chim. Acta 2024, 559, 121804. [Google Scholar] [CrossRef]
- Vivani, R.; Costantino, F.; Taddei, M. Zirconium phosphonates. In Metal Phosphonate Chemistry; Clearfield, A., Demadis, K.D., Eds.; The Royal Society of Chemistry: London, UK, 2011; Chapter 2, pp. 45–86. [Google Scholar] [CrossRef]
- Bashir, A.; Ahad, S.; Malik, L.A.; Qureashi, A.; Manzoor, T.; Dar, G.N.; Pandith, A.H. Revisiting the old and golden inorganic material, zirconium phosphate: Synthesis, intercalation, surface functionalization, and metal ion uptake. Ind. Eng. Chem. Res. 2020, 59, 22353–22397. [Google Scholar] [CrossRef]
- Pica, M.; Donnadio, A.; Casciola, M. From microcrystalline to nanosized α-zirconium phosphate: Synthetic approaches and applications of an old material with a bright future. Coord. Chem. Rev. 2018, 374, 218–235. [Google Scholar] [CrossRef]
- Bisio, C.; Brendlé, J.; Cahen, S.; Feng, Y.; Hwang, S.J.; Melanova, K.; O’Hare, D.; Rabu, P.; Leroux, F. Recent Advances and Perspectives for Intercalation Layered Compounds Part 1: Design and applications in the field of energy. Dalton Trans. 2024, 53, 14525–14550. [Google Scholar] [CrossRef]
- Bisio, C.; Brendle, J.; Cahen, S.; Feng, Y.; Hwang, S.J.; Nocchetti, M.; O’Hare, D.; Rabu, P.; Melanova, K.; Leroux, F. Recent Advances and Perspectives for Layered Intercalation Compounds Part 2: Applications in the field of catalysis, environment and health. Dalton Trans. 2024, 53, 14551–14581. [Google Scholar] [CrossRef]
- Ramos-Garcés, M.V.; Sanchez, J.; La Luz-Rivera, K.; Del Toro-Pedrosa, D.E.; Jaramillo, T.F.; Colón, J. LMorphology control of metal-modified zirconium phosphate support structures for the oxygen evolution reaction. Dalton Trans. 2020, 49, 3892–3900. [Google Scholar] [CrossRef] [PubMed]
- Alberti, G.; Casciola, M.; Vivani, R.; Biswas, R.K. Preparation and characterization of zirconium phosphate phosphonates, ZrPO4(H2PO4)1-x(RPO2OH)x·nH2O, with γ-layer structure (R = CH3, C3H7, C6H11). Inorg. Chem. 1993, 21, 4600–4604. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, X.; Jaenicke, S.; Chuah, G.K. Minimalistic Liquid-Assisted Route to Highly Crystalline α-Zirconium Phosphate. ChemSusChem 2017, 10, 3235–3242. [Google Scholar] [CrossRef]
- Cheng, Y.; Chui, S.S.Y.; Wang, X.D.T.; Jaenicke, S.; Chuah, G.K. One-pot synthesis of layered disodium zirconium phosphate: Crystal structure and application in the remediation of heavy-metal-contaminated wastewater. Inorg. Chem. 2019, 58, 13020–13029. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, H.; Jaenicke, J.A.; Tan, E.C.; Chuah, G.K. Minimalistic synthesis of α-zirconium diammonium phosphate and zirconia for applications in ion exchange and catalysis. ACS Sustain. Chem. Eng. 2019, 7, 895–904. [Google Scholar] [CrossRef]
- Bevara, S.; Giri, P.; Patwe, S.J.; Achary, S.N.; Mishra, R.K.; Kumar, A.; Sinha, A.K.; Kaushik, C.P.; Tyagi, A.K. Separation of 90Sr from nuclear waste by crystalline complex phosphates of Ce (IV) and Zr (IV). J. Environ. Chem. Eng. 2018, 6, 2248–2261. [Google Scholar] [CrossRef]
- Tarafdar, A.; Panda, A.B.; Pradhan, N.C.; Pramanik, P. Synthesis of spherical mesostructured zirconium phosphate with acidic properties. Microporous Mesoporous. Mater. 2006, 95, 360–365. [Google Scholar] [CrossRef]
- Sun, Y.; Afanasiev, P.; Vrinat, M.; Coudurier, G. Porous zirconium phosphates prepared by surfactant-assisted precipitation. J. Mater. Chem. 2000, 10, 2320–2324. [Google Scholar] [CrossRef]
- Chakraborty, R.; Bhattacharaya, K.; Chattopadhyay, P. Nanostructured zirconium phosphate as ion exchanger: Synthesis, size dependent property and analytical application in radiochemical separation. Appl. Radiat. Isot. 2014, 85, 34–38. [Google Scholar] [CrossRef]
- Zhu, Y.; Kanamori, K.; Moitra, N.; Kadono, K.; Ohi, S.; Shimobayashi, N.; Nakanishi, K. Metal zirconium phosphate macroporous monoliths: Versatile synthesis, thermal expansion and mechanical properties. Microporous Mesoporous Mater. 2016, 225, 122–127. [Google Scholar] [CrossRef]
- Alberti, G.; Casciola, M.; Marmottini, F.; Vivani, R. Preparation of mesoporous zirconium phosphate-pyrophosphate with a large amount of thermally stable acid groups on the pore surface. J. Porous Mater. 1999, 6, 299–305. [Google Scholar] [CrossRef]
- Lanari, D.; Montanari, F.; Marmottini, F.; Piermatti, O.; Orrù, M.; Vaccaro, L. New zirconium hydrogen phosphate alkyl and/or aryl phosphonates with high surface area as heterogeneous Brønsted acid catalysts for aza-Diels–Alder reaction in aqueous medium. J. Catal. 2011, 277, 80–87. [Google Scholar] [CrossRef]
- Angeloni, M.; Piermatti, O.; Pizzo, F.; Vaccaro, L. Synthesis of Zirconium Phosphonate Supported L-Proline as an Effective Organocatalyst for Direct Asymmetric Aldol Addition. Eur. J. Org. Chem. 2014, 2014, 1716–1726. [Google Scholar] [CrossRef]
- Ding, H.; Ahmed, A.; Shen, K.; Sun, L. Assembly of exfoliated α-zirconium phosphate nanosheets: Mechanisms and versatile applications: Nanoscience: Special Issue Dedicated to Professor Paul S. Weiss. Aggregate 2022, 3, e174. [Google Scholar] [CrossRef]
- Jain, A.; Shore, A.M.; Jonnalagadda, S.C.; Ramanujachary, K.V.; Mugweru, A. Conversion of fructose, glucose and sucrose to 5-hydroxymethyl-2-furfural over mesoporous zirconium phosphate catalyst. Appl. Catal. A Gen. 2015, 489, 72–76. [Google Scholar] [CrossRef]
- Xu, H.; Miao, Z.; Zhao, H.; Yang, J.; Zhao, J.; Song, H.; Liang, N.; Chou, L. Dehydration of fructose into 5-hydroxymethylfurfural by high stable ordered mesoporous zirconium phosphate. Fuel 2015, 145, 234–240. [Google Scholar] [CrossRef]
- Antonetti, C.; Melloni, M.; Licursi, D.; Fulignati, S.; Ribechini, E.; Rivas, S.; Parajó, J.C.; Cavani, F.; Raspolli Galletti, A.M. Microwave-assisted dehydration of fructose and inulin to HMF catalyzed by niobium and zirconium phosphate catalysts. Appl. Catal. B Environ. 2017, 206, 364–377. [Google Scholar] [CrossRef]
- Saravanan, K.; Park, K.S.; Jeon, S.; Bae, J.W. Aqueous phase synthesis of 5-hydroxymethylfurfural from glucose over large pore mesoporous zirconium phosphates: Effect of calcination temperature. ACS Omega 2018, 3, 808–820. [Google Scholar] [CrossRef]
- Zhu, C.; Cai, C.; Liu, Q.; Li, W.; Tan, J.; Wang, C.; Chen, L.; Zhang, Q.; Ma, L. Continuous Production of 5-Hydroxymethylfurfural from Monosaccharide over Zirconium Phosphates. ChemistrySelect 2018, 3, 10983–10990. [Google Scholar] [CrossRef]
- Ni, W.; Li, D.; Zhao, X.; Ma, W.; Kong, K.; Gu, Q.; Chen, M.; Hou, Z. Catalytic dehydration of sorbitol and fructose by acid-modified zirconium phosphate. Catal. Today 2019, 319, 66–75. [Google Scholar] [CrossRef]
- Cao, D.; Yu, B.; Zhang, S.; Cui, L.; Zhang, J.; Cai, W. Isosorbide production from sorbitol over porous zirconium phosphate catalyst. Appl. Catal. A Gen. 2016, 528, 59–66. [Google Scholar] [CrossRef]
- Zhu, Y.; Kanamori, K.; Brun, N.; Pélisson, C.H.; Moitra, N.; Fajula, F.; Hulea, V.; Galarneau, A.; Takeda, K.; Nakanishi, K. Monolithic acidic catalysts for the dehydration of xylose into furfural. Catal. Commun. 2016, 87, 112–115. [Google Scholar] [CrossRef]
- Kumar, A.; Srivastava, R. Zirconium Phosphate Catalyzed Transformations of Biomass-Derived Furfural to Renewable Chemicals. ACS Sust. Chem. Eng. 2020, 8, 9497–9506. [Google Scholar] [CrossRef]
- Zhai, P.; Lv, G.; Cai, Z.; Zhu, Y.; Li, H.; Zhang, X.; Wang, F. Efficient Production of Ethyl Levulinate from Furfuryl Alcohol Catalyzed by Modified Zirconium Phosphate. ChemistrySelect 2019, 4, 3940–3947. [Google Scholar] [CrossRef]
- Li, W.; Li, M.; Liu, H.; Jia, W.; Yu, X.; Wang, S.; Zeng, X.; Sun, Y.; Wei, J.; Tang, X.; et al. Domino transformation of furfural to γ-valerolactone over SAPO-34 zeolite supported zirconium phosphate catalysts with tunable Lewis and Brønsted acid sites. Mol. Catal. 2021, 506, 11538. [Google Scholar] [CrossRef]
- He, J.; Liu, X.; Bai, L.; Liu, S.; Song, K.; Zhou, X.; Guo, J.; Meng, X.; Li, C. Effective one-pot tandem synthesis of γ-valerolactone from biogenic furfural over zirconium phosphate catalyst. Biofuels Bioprod. Biorefin. 2022, 16, 1613–1626. [Google Scholar] [CrossRef]
- Li, F.; France, L.J.; Cai, Z.; Li, Y.; Liu, S.; Lou, H.; Long, J.; Li, X. Catalytic transfer hydrogenation of butyl levulinate to γ-valerolactone over zirconium phosphates with adjustable Lewis and Brønsted acid sites. Appl. Catal. B Environ. 2017, 214, 67–77. [Google Scholar] [CrossRef]
- Dookheh, M.; Najafi Chermahini, A.; Saraji, M. Preparation of Alkyl Levulinates from Xylose Over Modified Bifunctional Mesoporous Zirconium Phosphate Catalysts. Catal. Lett. 2022, 152, 2141–2154. [Google Scholar] [CrossRef]
- Jamali, F.; Chermahini, A.N.; Ayashi, N. Conversion of Levulinic Acid to n-Butyl Levulinate over Mesoporous Zirconium Phosphate Catalysts. Inorg. Chem. Res. 2021, 5, 149–162. [Google Scholar] [CrossRef]
- Rocha, G.O.; Lopes, F.S. Esterification of levulinic acid over tetravalent metal phosphates: An efficient route to fuel additives. Catal. Today 2024, 442, 114905. [Google Scholar] [CrossRef]
- Kasipandi, S.; Cho, J.M.; Park, K.S.; Shin, C.H.; Wook Bae, J. Unprecedented activity and stability on zirconium phosphates grafted mesoporous silicas for renewable aromatics production from furans. J. Catal. 2020, 385, 10–20. [Google Scholar] [CrossRef]
- Wu, C.; Wu, T.; Li, J.; Liu, C.L.; Dong, W.S. Highly efficient catalytic conversion of biomass-derived 2,5-dimethylfuran into renewable p-xylene over zirconium phosphate catalysts. Appl. Catal. A Gen. 2023, 663, 119323. [Google Scholar] [CrossRef]
- Zhou, Y.; Noshadi, I.; Ding, H.; Liu, J.; Parnas, R.S.; Clearfield, A.; Xiao, M.; Meng, Y.; Sun, L. Solid acid catalyst based on single-layer α-Zirconium phosphate nanosheets for biodiesel production via esterification. Catalysts 2018, 8, 17. [Google Scholar] [CrossRef]
- Pan, H.; Xia, Q.; Li, H.; Wang, Y.; Shen, Z.; Wang, Y.; Li, L.; Li, X.; Xu, H.; Zhou, Z.; et al. Direct production of biodiesel from crude Euphorbia lathyris L. Oil catalyzed by multifunctional mesoporous composite materials. Fuel 2022, 309, 122172. [Google Scholar] [CrossRef]
- Wang, M.; Yang, H.; Xie, Y.; Wu, X.; Chen, C.; Ma, W.; Dong, Q.; Hou, Z. Catalytic transformation of glycerol to 1-propanol by combining zirconium phosphate and supported Ru catalysts†. RSC Adv. 2016, 6, 29769–29778. [Google Scholar] [CrossRef]
- Srinivasa Rao, G.; Hussain, S.; Chary, K.V.R. Porous zirconium phosphate solid acid catalysts with variable Zr/P ratio for gas phase glycerol dehydration to acrolein. Mater. Today Proc. 2018, 5, 25773–25781. [Google Scholar] [CrossRef]
- Testa, M.L.; La Parola, V.; Mesrar, F.; Ouanji, F.; Kacimi, M.; Ziyad, M.; Liotta, L.F. Use of zirconium phosphate-sulphate as acid catalyst for synthesis of glycerol-based fuel additives. Catalysts 2019, 9, 148. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, R.; Zhao, H.; Ye, B.; Long, Y.; Wang, Z.; Hou, Z. A highly active and stable organic-inorganic combined solid acid for the transesterification of glycerol under mild conditions. Chin. J. Catal. 2021, 42, 1772–1781. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Y.; Zhou, R.; Hou, Z. Layered α-zirconium phosphate: An efficient catalyst for the synthesis of solketal from glycerol. Appl. Clay Sci. 2019, 174, 120–126. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Y.; Zhou, R.; Hou, Z. Acetalization of glycerol with acetone over appropriately-hydrophobic zirconium organophosphonates. Appl. Clay Sci. 2020, 189, 105555. [Google Scholar] [CrossRef]
- Zou, X.; Nie, X.; Tan, Z.; Shi, K.; Wang, C.; Wang, Y.; Zhao, X. Synthesis of sulfonic acid-functionalized zirconium poly(Styrene-phenylvinyl-phosphonate)-phosphate for heterogeneous epoxidation of soybean oil. Catalysts 2019, 9, 710. [Google Scholar] [CrossRef]
- Agarwal, B.; Kailasam, K.; Sangwan, R.S.; Elumalai, S. Traversing the history of solid catalysts for heterogeneous synthesis of 5-hydroxymethylfurfural from carbohydrate sugars: A review. Renew. Sust. Energy Rev. 2018, 82, 2408–2425. [Google Scholar] [CrossRef]
- Lin, X.Z.; Ren, T.Z.; Yuan, Z.Y. Mesoporous zirconium phosphonate materials as efficient water-tolerable solid acid catalysts. Catal. Sci. Technol. 2015, 5, 1485–1494. [Google Scholar] [CrossRef]
- Lin, X.Z.; Yang, Z.Z.; He, L.N.; Yuan, Z.Y. Mesoporous zirconium phosphonates as efficient catalysts for chemical CO2 fixation. Green Chem. 2015, 17, 795–798. [Google Scholar] [CrossRef]
- Gao, C.Y.; Ai, J.; Tian, H.R.; Wu, D.; Sun, Z.M. An ultrastable zirconium-phosphonate framework as bifunctional catalyst for highly active CO2 chemical transformation. Chem. Commun. 2017, 53, 1293–1296. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Kong, D.; Wang, F.; Luo, W.; Chen, Y.; Zhouzhou; Liu, J. Amorphous Porous Chromium-Zirconium Bimetallic Phosphate: Synthesis, Characterization and Application in Liquid Phase Oxidation of Hydrocarbons by Different Oxygen Sources. ChemistrySelect 2020, 5, 1552–1559. [Google Scholar] [CrossRef]
- Zhang, P.; Kang, L.; Zhu, M.; Dai, B. Oxidative desulfurization catalyzed by a novel ZrP/MCM-41 catalyst with high performance. Sust. Energy Fuels 2020, 4, 4293–4300. [Google Scholar] [CrossRef]
- Shashank, M.; Bhojya Naik, H.S.; Shashikanth, J.; Nizam, A.; Nagaraju, G. Green synthesis of zirconium phosphate by combustion method: Photocatalytic application and microwave-assisted catalytic conversion of aldehyde to nitriles. Bull. Mater. Sci. 2021, 44, 267. [Google Scholar] [CrossRef]
- Ye, Z.; Chen, L.; Chen, H.; Han, L.; Chen, Q.; Wang, D. α-Zirconium phosphate nanocrystals with various morphology for photocatalysis. Chem. Phys. Lett. 2018, 709, 96–102. [Google Scholar] [CrossRef]
- Singh, A.S.; Naikwadi, D.R.; Ravi, K.; Biradar, A.V. Chemoselective isomerization of α-Pinene oxide to trans-Carveol by robust and mild Brønsted acidic zirconium phosphate catalyst. Mol. Catal. 2022, 521, 112189. [Google Scholar] [CrossRef]
- Machac, P.; Styskalik, A.; Moravec, Z.; Pinkas, J. Non-hydrolytic sol-gel synthesis of zirconium phosphonates with controlled mesoporosity. Microp. Mesop. Mater. 2023, 362, 112787. [Google Scholar] [CrossRef]
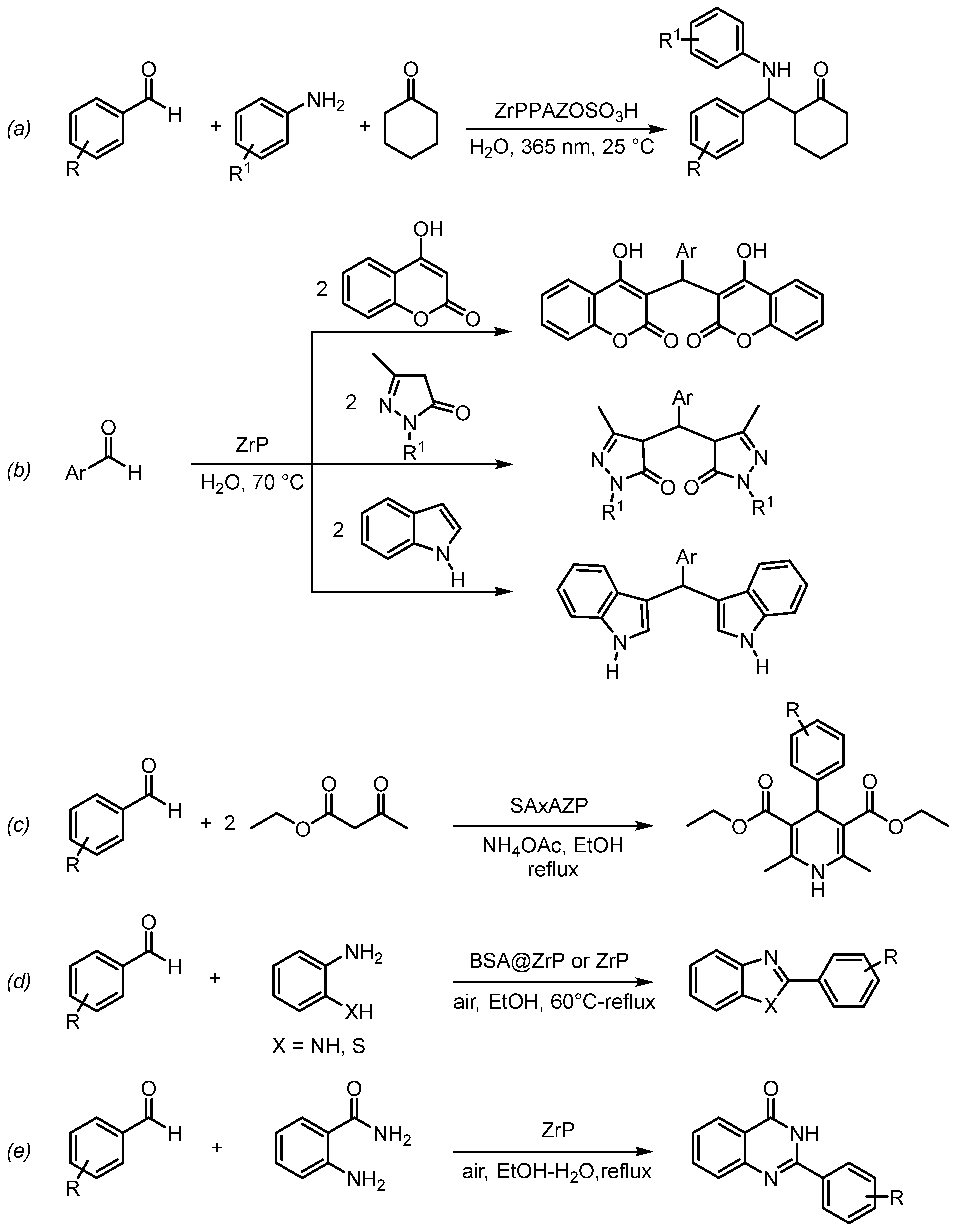
- Tang, Q.; Quan, H.J.; Liu, S.; Liu, L.T.; Chow, C.F.; Gong, C.B. An environmentally friendly, photocontrollable and highly recyclable catalyst for use in a one-pot three-component Mannich reaction. J. Mol. Catal. A Chem. 2016, 421, 37–44. [Google Scholar] [CrossRef]
- Karmakar, B.; Saha, A. A Convenient, Clean and Expeditious Synthesis of bis(heterocyclyl)methanes Over High Surface Area Zirconium Phosphate Catalyst in Water: A Green Approach. Curr. Green Chem. 2018, 5, 40–46. [Google Scholar] [CrossRef]
- Majhi, D.; Bhoi, Y.P.; Das, K.; Pradhan, S.; Mishra, B.G. Sulfamic acid well dispersed in the micropores of Al-pillared α-ZrP as efficient heterogeneous catalyst for synthesis of structurally diverse 1,4-dihydropyridines under mild conditions. J. Porous Mater. 2019, 26, 1391–1405. [Google Scholar] [CrossRef]
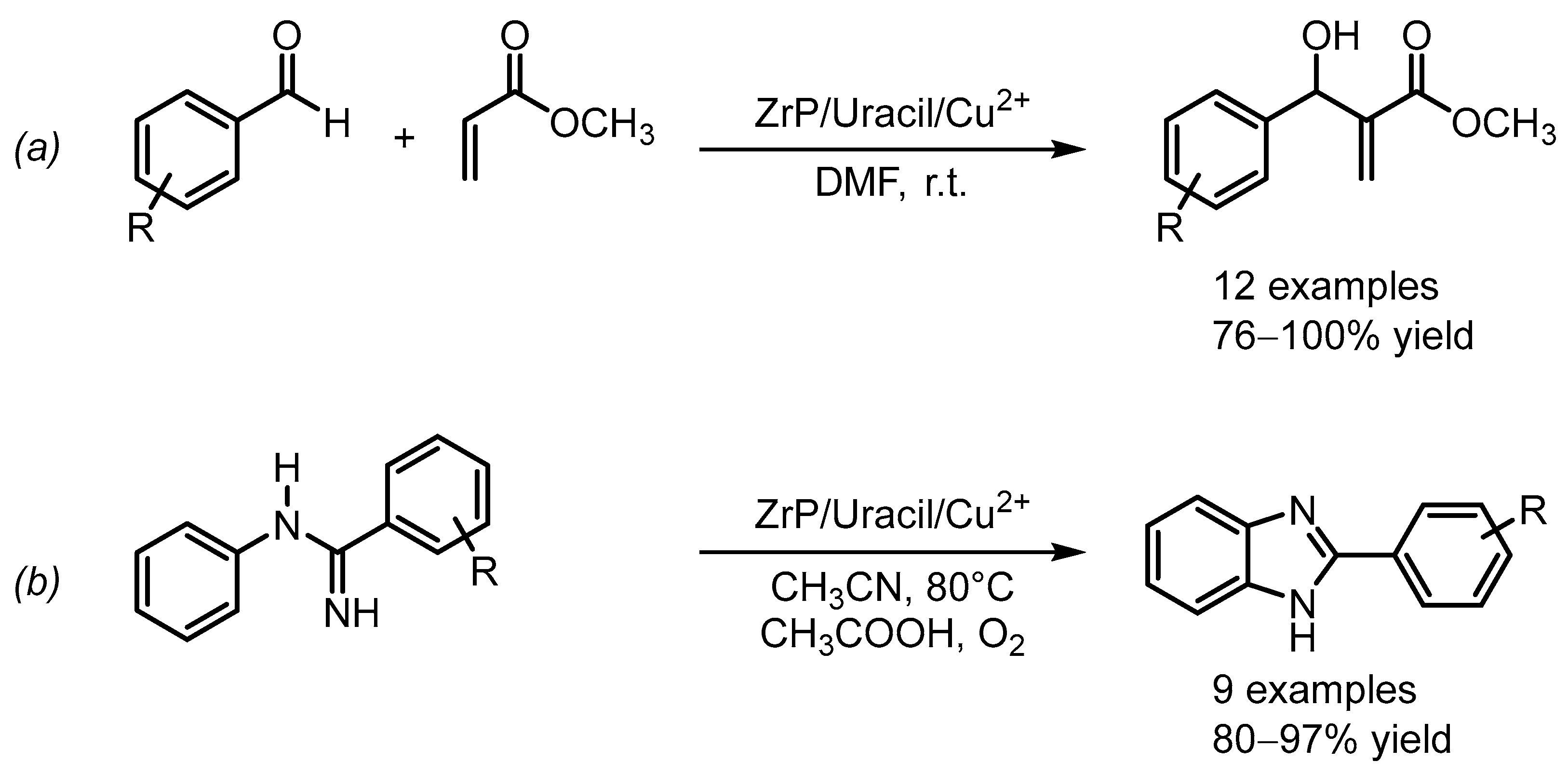
- Hajipour, A.R.; Zakery, S.; Khorsandi, Z. Synthesis of benzimidazoles by two methods (C–H functionalization and condensation reaction) catalyzed by α-zirconium hydrogen phosphate-based nanocatalyst. J. Iran. Chem. Soc. 2020, 17, 1919–1931. [Google Scholar] [CrossRef]
- Sadraoui, K.; Ahl el haj, T.; el Mejdoubi, K.; Benzekri, Z.; el Hezzat, M.; Boukhris, S.; Sallek, B. An Efficient and Practical Process for the Synthesis of Benzimidazole and Benzothiazole Derivatives Catalyzed by Layered Zirconium Phosphate: Effect of Calcinations Temperature. Kinet. Catal. 2023, 64, 616–626. [Google Scholar] [CrossRef]
- Ahl el haj, T.; el Mejdoubi, K.; Sadraoui, K.; El idrissi, B.C.; Sallek, B. Phosphate of Zirconium as a Reusable Efficient Catalyst for the Synthesis of 2-Arylquinazolin-4(3H)-ones. Kinet. Catal. 2022, 63, 707–715. [Google Scholar] [CrossRef]
- John, S.E.; Gulati, S.; Shankaraiah, N. Recent advances in multi-component reactions and their mechanistic insights: A triennium review. Org. Chem. Front. 2021, 8, 4237–4287. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Zi, H.; Wang, H.; Xia, Y.; Liu, X. Porous Organic Zirconium Phosphonate as Efficient Catalysts for the Catalytic Transfer Hydrogenation of Ethyl Levulinate to γ-Valerolactone without External Hydrogen. J. Chin. Chem. Soc. 2018, 65, 750–759. [Google Scholar] [CrossRef]
- Manal, A.K.; Advani, J.H.; Srivastava, R. Bifunctional Acid-Base Zirconium Phosphonate for Catalytic Transfer Hydrogenation of Levulinic Acid and Cascade Transformation of Furfural to Biofuel Molecules. ChemCatChem 2022, 14, e202200576. [Google Scholar] [CrossRef]
- Song, J.; Zhou, B.; Zhou, H.; Wu, L.; Meng, Q.; Liu, Z.; Han, B. Porous Zirconium–Phytic Acid Hybrid: A Highly Efficient Catalyst for Meerwein–Ponndorf–Verley Reductions. Angew. Chem. Int. Ed. 2015, 54, 9399–9403. [Google Scholar] [CrossRef]
- Rosati, O.; Pelosi, A.; Temperini, A.; Pace, V.; Curini, M. Potassium-Exchanged Zirconium Hydrogen Phosphate [α-Zr(KPO4)2]-Catalyzed Synthesis of 2-Amino-4 H -pyran Derivatives under Solvent-Free Conditions. Synthesis 2016, 48, 1533–1540. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.; Huang, R.; Zhang, M.; Xiao, M.; Meng, Y.; Sun, L. Covalently immobilized ionic liquids on single layer nanosheets for heterogeneous catalysis applications. Dalton Trans. 2017, 46, 13126–13134. [Google Scholar] [CrossRef]
- Rosati, O.; Lanari, D.; Scavo, R.; Persia, D.; Marmottini, F.; Nocchetti, M.; Curini, M.; Piermatti, O. Zirconium potassium phosphate methyl and/or phenyl phosphonates as heterogeneous catalysts for Knoevenagel condensation under solvent free conditions. Microp. Mesop. Mater. 2018, 268, 251–259. [Google Scholar] [CrossRef]
- Liu, B.; Ba, C.; Jin, M.; Zhang, Z. Effective conversion of carbohydrates into biofuel precursor 5-hydroxymethylfurfural (HMF) over Cr-incorporated mesoporous zirconium phosphate. Ind. Crops Prod. 2015, 76, 781–786. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, Z.; Liu, B.; Chen, S.; Zhang, Z. Catalytic oxidation of biomass derived 5-hydroxymethylfurfural (HMF) over RuIII-incorporated zirconium phosphate catalyst. J. Ind. Eng. Chem. 2016, 38, 181–185. [Google Scholar] [CrossRef]
- Chen, M.; Xia, J.; Li, H.; Zhao, X.; Peng, Q.; Wang, J.; Gong, H.; Dai, S.; An, P.; Wang, H.; et al. A Cationic Ru(II) Complex Intercalated into Zirconium Phosphate Layers Catalyzes Selective Hydrogenation via Heterolytic Hydrogen Activation. ChemCatChem 2021, 13, 3801–3814. [Google Scholar] [CrossRef]
- Lai, R.; Hou, Q.; Yu, G.; Xie, C.; Qian, H.; Xia, T.; Bai, X.; Tang, Y.; Rehman, M.L.U.; Ju, M. Incorporation of tin into zirconium phosphate to boost efficient conversion of trioses to lactic acid. Catal. Commun. 2023, 185, 106803. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, L.; Zeng, S.; Liu, J.; Xiao, M.; Wang, S.; Meng, Y.; Sun, L. Synthesis of Polylactide Nanocomposites Using an α-Zirconium Phosphate Nanosheet-Supported Zinc Catalyst via in Situ Polymerization. ACS Appl. Polym. Mater. 2019, 1, 1382–1389. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Karimi, H. Zinc zirconium phosphate as an efficient catalyst for chemoselective synthesis of 1,1-diacetates under solvent-free conditions. J. Chem. Sci. 2015, 127, 1945–1955. [Google Scholar] [CrossRef]
- Karimi, H. An Efficient Selective Oxidation of Alcohols with Zinc Zirconium Phosphate under Solvent-free Conditions. J. Chin. Chem. Soc. 2015, 62, 604–613. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Karimi, H.; Kohi, A. Highly efficient and recyclable acetylation of phenols and alcohols by nickel zirconium phosphate under solvent-free conditions. J. Iran. Chem. Soc. 2016, 13, 55–64. [Google Scholar] [CrossRef]
- Gong, H.; Zhao, X.; Qin, Y.; Xu, W.; Wei, X.; Peng, Q.; Ma, Y.; Dai, S.; An, P.; Hou, Z. Hydroformylation of olefins catalyzed by single-atom Co(II) sites in zirconium phosphate. J. Catal. 2022, 408, 245–260. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Zakery, S. α-ZrP/Uracil/Cu2+ nanoparticles as an efficient catalyst in the Morita-Baylis-Hillman reaction. Appl. Organomet. Chem. 2018, 32, e4487. [Google Scholar] [CrossRef]
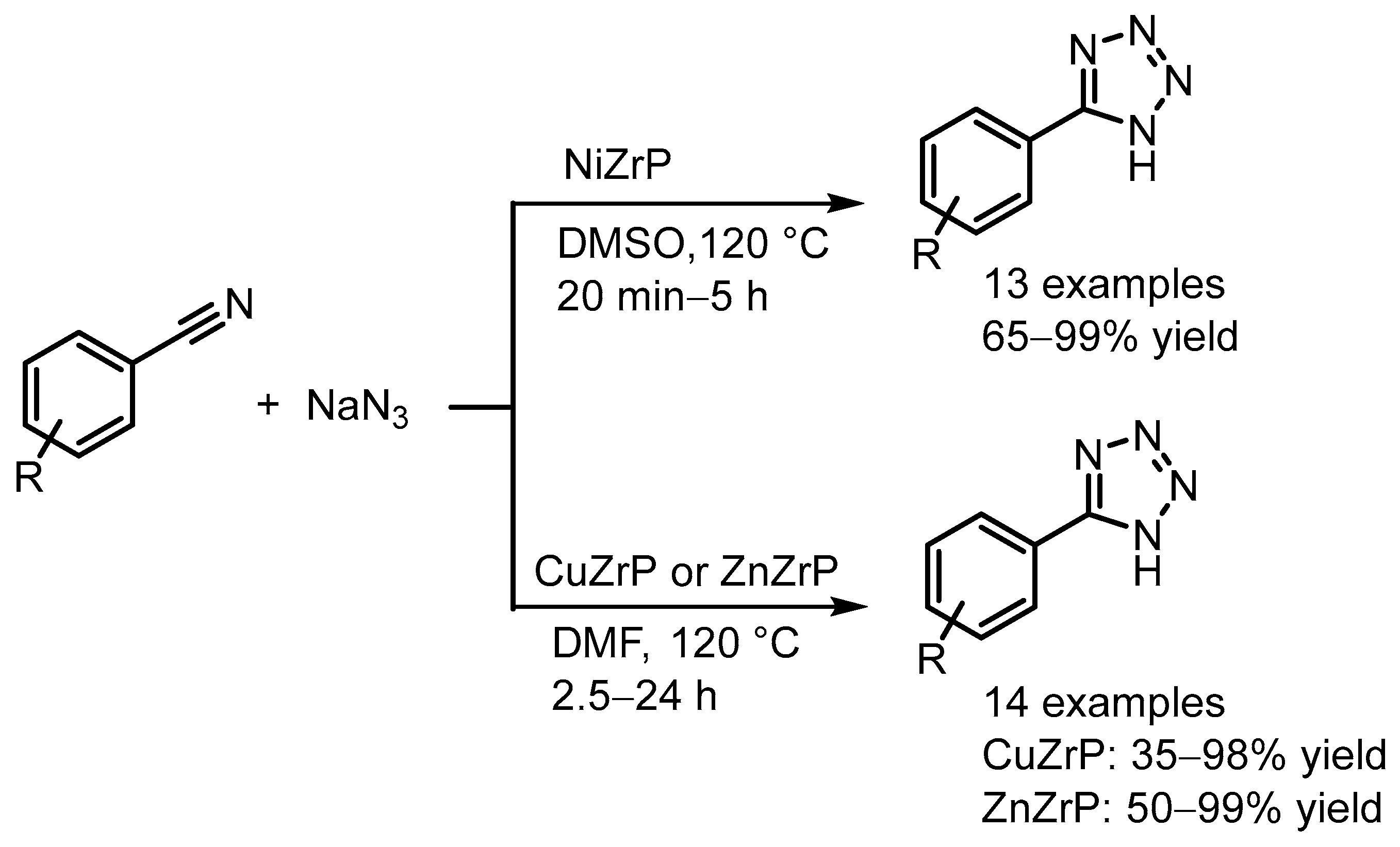
- Abrishami, F.; Ebrahimikia, M.; Rafiee, F. Facile synthesis of 5-substituted-1H-tetrazoles catalyzed by reusable nickel zirconium phosphate nanocatalyst. Iran. J. Catal. 2016, 6, 245–251. [Google Scholar]
- Abrishami, F.; Daryanavard, M.; Nakhaei, F. Synthesis of 5-substituted-1H-tetrazoles using zinc zirconium phosphate and copper zirconium phosphate as reusable heterogeneous catalysts. J. Iran. Chem. Soc. 2023, 20, 1821–1829. [Google Scholar] [CrossRef]
- Pica, M.; Nocchetti, M.; Ridolfi, B.; Donnadio, A.; Costantino, F.; Gentili, P.L.; Casciola, M. Nanosized zirconium phosphate/AgCl composite materials: A new synergy for efficient photocatalytic degradation of organic dye pollutants. J. Mater. Chem. A 2015, 3, 5525–5534. [Google Scholar] [CrossRef]
- Kassem, M.; Harmalani, H. Effects of Structural and Textural Aspects on the Photocatalytic Performance of Zirconium Hydrogen Phosphate Doped with Tin Metal. Kinet. Catal. 2021, 62, 264–269. [Google Scholar] [CrossRef]
- Khare, S.; Chokhare, R.; Shrivastava, P.; Kirar, J.S. Solvent-free liquid phase oxidation of styrene over iron zirconium phosphate using tert-butylhydroperoxide as an oxidant. Indian J. Chem. 2015, 54, 1032–1038. [Google Scholar]
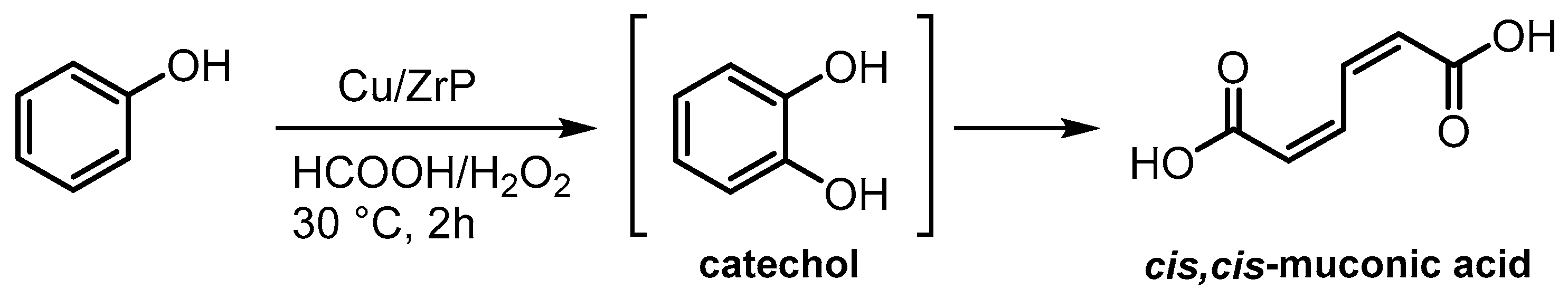
- He, J.; Jiang, Y.; Ding, B.; Wang, Y.; Qiu, H.; Dai, S.; Zhao, X.; Hou, Z. Zirconium phosphate supported copper catalyst for selective oxidation of phenol to cis, cis-muconic acid. Appl. Catal. A Gen. 2023, 664, 119351. [Google Scholar] [CrossRef]
- Fu, S.; Tian, Y.; Long, X.; Shao, Q.; Wang, K.; Lei, J.; Hao, H.; Xu, Q. A new kind of hierarchical porous zirconium phosphonate: Preparation and application on oxidation catalysis. J. Porous Mater. 2024. [Google Scholar] [CrossRef]
- He, S.; Liu, X.; Zhao, H.; Zhu, Y.; Zhang, F. Zirconium phenylphosphonate-anchored methyltrioxorhenium as novel heterogeneous catalyst for epoxidation of cyclohexene. J. Colloid Interface. Sci. 2015, 437, 58–64. [Google Scholar] [CrossRef]
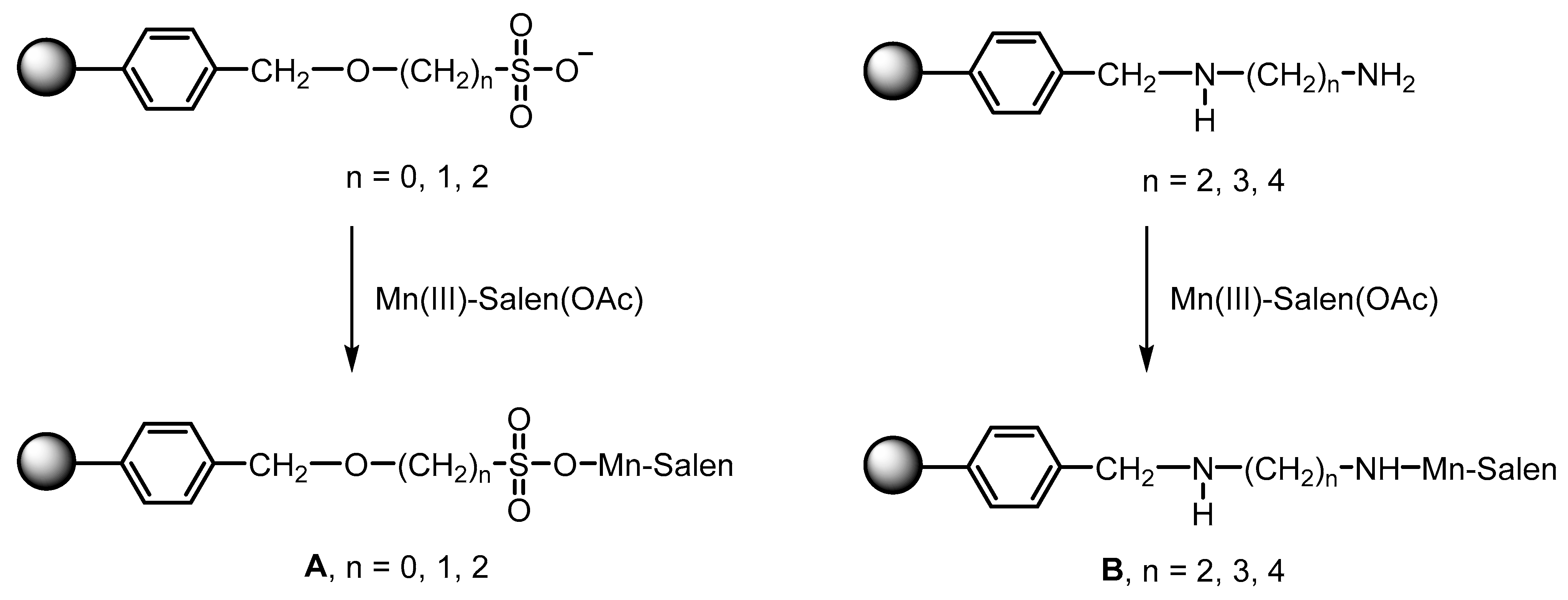
- Zou, X.; Wang, C.; Wang, Y.; Shi, K.; Wang, Z.; Li, D.; Fu, X. Chiral MnIII (Salen) covalently bonded on modified ZPS-PVPA and ZPS-IPPA as efficient catalysts for enantioselective epoxidation of unfunctionalized olefins. Polymers 2017, 9, 108. [Google Scholar] [CrossRef]
- Khare, S.; Chokhare, R.; Shrivastava, P.; Kirar, J.S.; Parashar, S. Catalytic oxidation of cyclohexene by α-zirconium phosphate intercalated Mn(Salen) using 70% tert-butylhydroperoxide as an oxidant. Indian J. Chem. 2016, 55A, 1449–1457. [Google Scholar]
- Khare, S.; Shrivastava, P.; Chokhare, R.; Kirar, J.S.; Parashar, S. α-Zirconium phosphate supported metal–salen complex: Synthesis, characterization and catalytic activity for cyclohexane oxidation. J. Porous Mater. 2017, 24, 855–866. [Google Scholar] [CrossRef]
- Khare, S.; Shrivastava, P. Solvent-free oxidation of cyclohexane over covalently anchored transition-metal salicylaldimine complexes to α-zirconium phosphate using tert-butylhydroperoxide. J. Mol. Catal. A Chem. 2016, 411, 279–289. [Google Scholar] [CrossRef]
- Zhou, L.; Tu, X.B.; Yang, Z.Y.; Wei, D.J.; Lu, W. Efficient and recyclable performance of organic-inorganic zirconium phosphonates supported salen-Mn(III) as catalysts for CO2 cycloaddition. IOP Conf. Ser. Mater. Sci. Eng. 2019, 479, 012020. [Google Scholar] [CrossRef]
- Zhou, L.; Gong, B.; Yang, Z.; Wei, D.; Lu, W. Application of Modified Organic-Inorganic Hybrid Zirconium Phosphate Material ZAMPS-PVPA-Salen-Mn(III) in Chemical Fixation of CO2. IOP Conf. Ser. Mater. Sci. Eng. 2019, 472, 012076. [Google Scholar] [CrossRef]
- Liao, H.; Jiang, Y.; Wei, X.; Zhao, X.; Lai, W.; An, N.; Ma, Y.; Dai, S.; Hou, Z. Intercalated Zirconium Phosphate Promotes Reductive Amination of Carbon Dioxide. ACS Sust. Chem. Eng. 2024, 12, 2632–2645. [Google Scholar] [CrossRef]
- Kanchi, S.; Ahmed, S. (Eds.) Green Metal Nanoparticles: Synthesis, Characterization and Their Applications; Wiley-Scrivener: Hoboken, NJ, USA, 2018. [Google Scholar] [CrossRef]
- El Shafey, A.M. Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review. Green Process. Synth. 2020, 9, 304–339. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, P.; Kumar, A.; Camargo, P.H.; Krishnan, V. Recent advances in plasmonic photocatalysis based on TiO2 and noble metal nanoparticles for energy conversion, environmental remediation, and organic synthesis. Small 2022, 18, 2101638. [Google Scholar] [CrossRef]
- Nocchetti, M.; Donnadio, A.; Vischini, E.; Posati, T.; Albonetti, C.; Campoccia, D.; Arciola, C.R.; Ravaioli, S.; Mariani, V.; Montanaro, L.; et al. Synthesis, Crystal Structure, and Antibacterial Properties of Silver-Functionalized Low-Dimensional Layered Zirconium Phosphonates. Inorg. Chem. 2022, 61, 2251–2264. [Google Scholar] [CrossRef]
- Piermatti, O. Green Synthesis of Pd Nanoparticles for Sustainable and Environmentally Benign Processes. Catalysts 2021, 11, 1258. [Google Scholar] [CrossRef]
- Valentini, V.; Piermatti, O.; Vaccaro, L. Metal and Metal Oxide Nanoparticles Catalyzed C–H Activation for C–O and C–X (X = Halogen, B, P, S, Se) Bond Formation. Catalysts 2023, 13, 16. [Google Scholar] [CrossRef]
- Sápi, A.; Rajkumar, T.; Kiss, J.; Kukovecz, Á.; Kónya, Z.; Somorjai, G.A. Metallic nanoparticles in heterogeneous catalysis. Catal. Lett. 2021, 151, 2153–2175. [Google Scholar] [CrossRef]
- Ohtaka, A. Recent Progress of Metal Nanoparticle Catalysts for C–C Bond Forming Reactions. Catalysts 2021, 11, 1266. [Google Scholar] [CrossRef]
- Ndolomingo, M.J.; Bingwa, N.; Meijboom, R. Review of supported metal nanoparticles: Synthesis methodologies, advantages and application as catalysts. J. Mater. Sci. 2020, 55, 6195–6241. [Google Scholar] [CrossRef]
- Kobayashi, S. (Ed.) Nanoparticles in Catalysis; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Hu, H.; Xin, J.H.; Hu, H.; Wang, X.; Miao, D.; Liu, Y. Synthesis and stabilization of metal nanocatalysts for reduction reactions–a review. J. Mater. Chem. A 2015, 3, 11157–11182. [Google Scholar] [CrossRef]
- Tao, F.F. Metal Nanoparticles for Catalysis: Advances and Applications, 1st ed.; Royal Society of Chemistry: Cambridge, UK, 2014. [Google Scholar]
- Zhu, C.; Liu, Q.; Li, D.; Wang, H.; Zhang, C.; Cui, C.; Chen, L.; Cai, C.; Ma, L. Selective Hydrodeoxygenation of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran over Ni Supported on Zirconium Phosphate Catalysts. ACS Omega 2018, 3, 7407–7417. [Google Scholar] [CrossRef]
- Ma, H.; Li, H.; Zhao, W.; Li, L.; Liu, S.; Long, J.; Li, X. Selective depolymerization of lignin catalyzed by nickel supported on zirconium phosphate. Green Chem. 2019, 21, 658–668. [Google Scholar] [CrossRef]
- Choudhary, H.; Das, L.; Pelton, J.G.; Sheps, L.; Simmons, B.A.; Gladden, J.M.; Singh, S. Funneled Depolymerization of Ionic Liquid-Based Biorefinery “Heterogeneous” Lignin into Guaiacols over Reusable Palladium Catalyst. Chem. Europ. J. 2023, 29, 330. [Google Scholar] [CrossRef]
- Gong, H.; Zhou, C.; Cui, Y.; Dai, S.; Zhao, X.; Luo, R.; An, P.; Li, H.; Wang, H.; Hou, Z. Direct Transformation of Glycerol to Propanal using Zirconium Phosphate-Supported Bimetallic Catalysts. ChemSusChem 2020, 13, 4954–4966. [Google Scholar] [CrossRef]
- Luo, R.; Zhao, X.; Gong, H.; Qian, W.; Li, D.; Chen, M.; Cui, K.; Wang, J.; Hou, Z. Effect of Tungsten Modification on Zirconium Phosphate-Supported Pt Catalyst for Selective Hydrogenolysis of Glycerol to 1-Propanol. Energy Fuels 2020, 34, 8707–8717. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, H.; Zhang, Y.; Zhao, H.; Du, W.; Chen, P.; Hou, Z. Catalytic recycling of polylactic acid over zirconium phosphate supported WOx active sites. Appl. Catal. A Gen. 2024, 686, 119917. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.; Li, N.; Ma, P.; Zhang, Y. Direct synthesis of hexitols from microcrystalline cellulose and birch over zirconium(iv) phosphate supported nickel catalysts and the mechanism study. Green Chem. 2021, 23, 1353–1360. [Google Scholar] [CrossRef]
- Han, G.H.; Lee, M.W.; Park, S.; Kim, H.J.; Ahn, J.P.; Seo, M.-G.; Lee, K.Y. Revealing the factors determining the selectivity of guaiacol HDO reaction pathways using ZrP-supported Co and Ni catalysts. J. Catal. 2019, 377, 343–357. [Google Scholar] [CrossRef]
- Gao, J.; Cao, Y.; Luo, G.; Fan, J.; Clark, J.H.; Zhang, S. High-efficiency catalytic hydrodeoxygenation of lignin-derived vanillin with nickel-supported metal phosphate catalysts. Chem. Eng. J. 2022, 448, 137723. [Google Scholar] [CrossRef]
- Li, D.; Gong, H.; Lin, L.; Ma, W.; Zhou, Q.; Kong, K.; Huang, R.; Hou, Z. Selective aerobic oxidation of glycerol over zirconium phosphate-supported vanadium catalyst. Mol. Catal. 2019, 474, 110404. [Google Scholar] [CrossRef]
- Li, X.; Ding, G.; Thompson, B.L.; Hao, L.; Deming, D.A.; Heiden, Z.M.; Zhang, Q. Microwave-Assisted Synthesis of Zirconium Phosphate Nanoplatelet-Supported Ru-Anadem Nanostructures and Their Catalytic Study for the Hydrogenation of Acetophenone. ACS Appl. Mater. Interfaces 2020, 12, 30670–30679. [Google Scholar] [CrossRef]
- Borah, G.; Borborah, A.; Gogoi, N. α-ZrP-supported CdS quantum dot composite material: An efficient and recyclable photocatalyst for selective oxidation of benzyl alcohol. Bull. Mater. Sci. 2022, 45, 136. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, A.; Wang, Z.; Chen, M.; Wang, W.; Sun, L.; Liu, X. Titanium functionalized α-zirconium phosphate single layer nanosheets for photocatalyst applications. RSC Adv. 2015, 5, 93969–93978. [Google Scholar] [CrossRef]
- Costantino, F.; Vivani, R.; Bastianini, M.; Ortolani, L.; Piermatti, O.; Nocchetti, M.; Vaccaro, L. Accessing stable zirconium carboxy-aminophosphonate nanosheets as support for highly active Pd nanoparticles. Chem. Commun. 2015, 51, 15990–15993. [Google Scholar] [CrossRef]
- Kozell, V.; Giannoni, T.; Nocchetti, M.; Vivani, R.; Piermatti, O.; Vaccaro, L. Immobilized palladium nanoparticles on zirconium carboxy-aminophosphonates nanosheets as an efficient recoverable heterogeneous catalyst for Suzuki–Miyaura and Heck coupling. Catalysts 2017, 7, 186. [Google Scholar] [CrossRef]
- Borah, S.; Mishra, S.; Cardenas, L.; Gogoi, N. Pd Nanoparticles Dispersed on ZrIV Organophosphonate: A Robust and Reusable Catalyst for Suzuki–Miyaura Cross-Coupling Reactions. Eur. J. Inorg. Chem. 2018, 2018, 751–758. [Google Scholar] [CrossRef]
- Bhattacharyya, B.; Biswas, J.P.; Mishra, S.; Gogoi, N. Rapid Suzuki-Miyaura cross-coupling reaction catalyzed by zirconium carboxyphosphonate supported mixed valent Pd(0)/Pd(II) catalyst. Appl. Organomet. Chem. 2019, 33, 5017. [Google Scholar] [CrossRef]
- Petrucci, C.; Cappelletti, M.; Piermatti, O.; Nocchetti, M.; Pica, M.; Pizzo, F.; Vaccaro, L. Immobilized palladium nanoparticles on potassium zirconium phosphate as an efficient recoverable heterogeneous catalyst for a clean Heck reaction in flow. J. Mol. Catal. A Chem. 2015, 401, 27–34. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, H.; Xu, C.; Wang, Z.; Zhang, H.; Guo, D.; Zhang, J.; Ji, X.; Liu, L.; Ma, J.; et al. Palladium nanoparticles supported on α-zirconium phosphate nanosheets as a highly efficient heterogeneous catalyst for the Heck reaction. J. Taiwan Inst. Chem. Eng. 2022, 138, 104478. [Google Scholar] [CrossRef]
- Costantino, F.; Nocchetti, M.; Bastianini, M.; Lavacchi, A.; Caporali, M.; Liguori, F. Robust Zirconium Phosphate-Phosphonate Nanosheets Containing Palladium Nanoparticles as Efficient Catalyst for Alkynes and Nitroarenes Hydrogenation Reactions. ACS Appl. Nano Mat. 2018, 1, 1750–1757. [Google Scholar] [CrossRef]
- Ferlin, F.; Cappelletti, M.; Vivani, R.; Pica, M.; Piermatti, O.; Vaccaro, L. Au@zirconium-phosphonate nanoparticles as an effective catalytic system for the chemoselective and switchable reduction of nitroarenes. Green Chem. 2019, 21, 614–626. [Google Scholar] [CrossRef]
- Zhou, Y.; Ding, H.; Liu, J.; LaChance, A.M.; Xiao, M.; Meng, Y.; Sun, L. Gold nanoparticles immobilized on single-layer α-zirconium phosphate nanosheets as a highly effective heterogeneous catalyst. Adv. Compos. Hybrid Mater. 2019, 2, 520–529. [Google Scholar] [CrossRef]
- Lai, G.H.; Huang, T.C.; Pai, Y.H.; Huang, B.S.; Tsai, M.H.; Yang, T.I.; Chung, Y.H. Preparation of highly-stable and recyclable novel Au/ZrP composite catalyst for 4-nitrophenol reduction. J. Taiwan Inst. Chem. Eng. 2019, 95, 525–531. [Google Scholar] [CrossRef]
- Gong, H.; Lin, L.; Zhao, X.; Li, H.; Li, D.; Xu, Z.; Chen, M.; Huang, R.; Hou, Z. Atomically precise Ag nanoclusters intercalated in zirconium pyrophosphate for efficient hydrogenation of nitroaromatics. Appl. Catal. A Gen. 2019, 574, 1–9. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, F.; Chen, M.; Hu, H.; Lin, L.; Wu, J.; Zhang, M. Facile assembly of 2D α-zirconium phosphate supported silver nanoparticles: Superior and recyclable catalysis. New J. Chem. 2020, 44, 9793–9801. [Google Scholar] [CrossRef]
- Lin, L.; Wen, Y.; Li, L.; Tan, Y.; Yang, P.; Liang, Y.; Xu, Y.; Hu, H.; Xu, Y. Mussel-Inspired Surface Modification of α-Zirconium Phosphate Nanosheets for Anchoring Efficient and Reusable Ultrasmall Au Nanocatalysts. Nanomaterials 2022, 12, 3339. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Jiang, Y.; Wei, X.; Ma, Y.; Liao, H.; Peng, Q.; Dai, S.; Wang, Z.; Zhao, X.; Hou, Z. Zirconium phosphate supported-silver nanoparticles for selective hydrogenation of nitrobenzene into azoxybenzene compounds. New J. Chem. 2023, 47, 14380–14394. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, Y.; Dai, S.; Wei, X.; Ma, Y.; Liao, H.; Qin, Y.; Peng, Q.; Zhao, X.; Hou, Z. Selective Hydrogenation of Nitrobenzene to para-Aminophenol on a Zirconium-Phosphate-Supported Platinum Catalyst. Ind. Eng. Chem. Res. 2023, 62, 5814–5825. [Google Scholar] [CrossRef]
- Awaya, K.; Sato, Y.; Miyazaki, A.; Furukubo, M.; Nishiyama, K.; Tsushida, M.; Ida, S.; Ohyama, J.; Machida, M. Selective catalytic NOx reduction by H2 in excess O2 over Pt/zirconium phosphate nanosheets. Catal. Sci. Technol. 2024, 14, 6055–6064. [Google Scholar] [CrossRef]
- Lawrence, S.A. Amines: Synthesis, Properties and Applications; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Rosenblatt, D.H.; Burrows, E.P. The Chemistry of Amino, Nitroso, and Nitro Compounds and Their Derivatives; Patai, S., Ed.; John Wiley &Sons: Chichester, UK, 1982; p. 1085. [Google Scholar]
- Nishimura, S. Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis; John Wiley & Sons: New York, NY, USA, 2001; pp. 315–387. [Google Scholar]
- Mitchell, S.C.; Waring, R.H. Ullmanns Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Hu, Z.N.; Liang, J.; Ding, K.; Ai, Y.; Liang, Q.; Sun, H.B. Insight into the selectivity of nano-catalytic nitroarenes reduction over other active groups by exploring hydrogen sources and metal components. Appl. Catal. A Gen. 2021, 626, 118339. [Google Scholar] [CrossRef]
- Blaser, H.U.; Steiner, H.; Studer, M. Selective catalytic hydrogenation of functionalized nitroarenes: An update. ChemCatChem 2009, 1, 210–221. [Google Scholar] [CrossRef]
- Kadam, H.K.; Tilve, S.G. Advancement in methodologies for reduction of nitroarenes. RSC Adv. 2015, 5, 83391–83407. [Google Scholar] [CrossRef]
- Begum, R.; Rehan, R.; Farooqi, Z.H.; Butt, Z.; Ashraf, S. Physical chemistry of catalytic reduction of nitroarenes using various nanocatalytic systems: Past, present, and future. J. Nanopart. Res. 2016, 18, 231. [Google Scholar] [CrossRef]
- Sedghi, R.; M Heravi, M.; Asadi, S.; Nazari, N.; R Nabid, M. Recently used nanocatalysts in reduction of nitroarenes. Curr. Org. Chem. 2016, 20, 696–734. [Google Scholar] [CrossRef]


| Catalyst | Preparation Methods | BET Surface Area m2/g | Catalyzed Process | Ref. |
|---|---|---|---|---|
| ZrHEDP ZrEDTMPS ZrATMP ZrDTPMPA | Coprecipitation of phosphonic acid with ZrOCl2 in NaOH | 231–108 | Levulinic acid and its esters to GVL | [82] |
| ZrNPO3 | Coprecipitation of nitrilotris(methylene)triphophonic acid with ZrCl4 | 356 | [83] | |
| Zr-PhyA porous | Precipitation of phytic acid (PhyA) with ZrCl4 | 215 | [84] | |
| Zr-PhyA porous | Precipitation of phytic acid (PhyA) with ZrCl4 | 215 | MPV reduction in ketones | [84] |
| α-ZrPK | Potassium-exchanged layered zirconium hydrogen phosphate | 17 | Multi-component reactions | [85] |
| ZrP(SIL) | Covalently immobilized ILs on ZrP nanosheet by post-grafting methods | - | Knoevenagel reaction | [86] |
| ZrPK-Me, ZrPK-Ph, ZrPK-MePh | Potassium-exchanged amorphous zirconium phosphate methyl and/or phenyl phosphonates | 42–147 | [87] |
| Catalyst | Preparation Methods | BET Surface Area m2/g | Catalyzed Process | Ref. |
|---|---|---|---|---|
| ZrP-Cr(III) | Mesoporous zirconium phosphate ion exchange with CrCl3 | 386 | Dehydration of sugar to 5-HMF | [88] |
| ZrP-Ru(III) | Mesoporous zirconium phosphate ion exchange with RuCl3 | 378 | Oxidation of 5-HMF | [89] |
| Ru-ZrP | Intercalation of Ru(II) complex into ZrP layers | 29 | Hydrogenation of furfural to FA Hydrogenation of aldehydes and ketones | [90] |
| Sn/ZrP | Ultrasonic-assisted impregnation of amorphous ZrP with SnCl4 and calcination at 400 °C | 89.1 | Isomerization of dihydroxyacetone to lactic acid | [91] |
| ZrP-Zn | Immobilization of Schiff base Zn(II) complex by covalent grafting on ZrP nanosheet | - | L-lactide polymerization | [92] |
| ZrP-Zn(II) | Zirconium phosphate ion exchange with Zn(OAc)2 | 102.4 | 1,1-diacetate synthesis Oxidation of alcohols | [93] [94] |
| ZrP-Ni(II) | Zirconium phosphate ion exchange with Ni(OAc)2 | 103.1 | Acetylation of alcohols and phenols | [95] |
| CoZrP | Ultrasonic-assisted impregnation of Co(II) | 146 | Hydroformylation of olefins | [96] |
| α-ZrP/Uracil/Cu(II) | Immobilization of uracil Cu(II) complex by covalent grafting on ZrP nanoparticles | 120.1 | Morita–Baylis–Hillman C-H functionalization | [97] [78] |
| NiZrP | Zirconium phosphate ion exchange with Ni(OAc)2 | - | Synthesis of tetrazoles | [98] |
| CuZrP and ZnZrP | Zirconium phosphate ion exchange with Cu(OAc)2 and Zn(OAc)2 | - | [99] | |
| ZP/xAgCl composite x = 0.28, 0.56, 1.16 | Precipitation of AgCl particles by using silver-exchanged nanosized ZrP | AgCl particle size 0.5–2 μm | Photodegradation of dyes | [100] |
| ZrP-Sn(IV) Sn/Zr = 0.25–1 | Zirconium phosphate impregnation with SnCl4 | 18.3–47.8 | [101] | |
| ZrP-Fe(III) | Zirconium phosphate ion exchange with FeCl3 | 18.56 | Oxidation of styrene | [102] |
| Cu/ZrP | Zirconium phosphate ion exchange with Cu(OAc)2 and calcination at 200 °C | 53 | Oxidation of phenol | [103] |
| Cu@ZrDP | Hierarchical porous zirconium phosphonate impregnation with Cu(NO3)2 | 408.6 | Benzyl alcohol oxidation | [104] |
| MTO/ZrPP | Impregnation of zirconium phenylphosphonate with methyltrioxyrhenium | - | Epoxidation of alkenes | [105] |
| ZPS-PVPA·Mn (Salen) ZPS-IPPA·Mn (Salen) | Covalent grafting method | 120.3, 100.3 | [106] | |
| α-ZrP·Mn (salen) | Flexible ligand method | 24.97 | Oxidation of cyclohexene | [107] |
| α-ZrP·M (salen) M = Fe, Mn | Flexible ligand method | 18.51, 19.57 | Oxidation of cyclohexane | [108] |
| α-ZrP·M(salicylaldimine) M = Co, Mn, Cu | Covalent bond immobilization of M(salicylaldimine) complex by grafting | 5.15–5.18 | [109] | |
| ZSPS-PVPA-Mn (Salen) | Zirconium polystyrene phosphonate-supported Salen Mn(III) complex | - | Fixation of CO2 | [110] |
| ZAMPS-PVPA-Mn (Salen) | Zirconium polystyrene phosphonate-supported Salen Mn(III) complex | - | [111] | |
| 10BMIMOAc_Ru-ZrP | Ru(II) complex intercalated into exfoliated α-ZrP modified with BMINOAc | - | Reductive amination of CO2 | [112] |
| Catalyst | Preparation Methods | MNPs Size | Catalyzed Process | Ref. |
|---|---|---|---|---|
| Ni/ZrP | Exfoliated ZrP ion exchange with Ni(NO3)2 and reduction with H2 flow at 400 °C | 30.3 nm | Hydrodeoxygenation of 5-HMF | [125] |
| Ni/ZrP | Impregnation with Ni(NO3)2, calcination, and reduction with H2 flow at 550 °C | - | Depolymerization of lignin | [126] |
| Pd@ZrP | Impregnation with Pd(NO3)2 and calcination | 11.9 nm | [127] | |
| Ru/CoO/ZrP | Impregnation with Co(NO3)2 and calcination followed by impregnation with RuCl3, calcination, and reduction | - | Hydrogenolysis of glycerol | [128] |
| Pt/7WOx-ZrP | Impregnation of tungsten modified ZrP with H2PtCl6·, calcination, and reduction | 3–6 nm | [129] | |
| WOx/ZrP | Impregnation with Na2WO4 and calcination at 300 °C | - | Alcoholysis of polylactic acid | [130] |
| NiZrP2 | Impregnation with Ni(NO3)2 and reduction with H2 flow at 400 °C | 20 nm | Hydrolysis/hydrogenation of cellulose | [131] |
| Co/ZrP and Ni/ZrP | Impregnation, calcination, and reduction with H2 flow at 500 °C | 5 nm, 29 nm | Hydrodeoxygenation of lignin derivatives | [132] |
| Ni/ZrP | Impregnation with Ni(NO3)2, calcination, and reduction with H2 flow at 550 °C | 20.74 nm | [133] | |
| 2V/ZrP-m | Mechanochemical synthesis and calcination at 550 °C | <4 nm | Glycerol oxidation to formic acid | [134] |
| Ru-ZrP | Ion exchange with RuCl3 and microwave-assisted reduction | 3.14 nm | Hydrogenation of acetophenone | [135] |
| CdS QD@ZrP | Ion exchange with Cd(OAc)2 followed by treatment with Na2S | 2–6 nm | Photocatalytic oxidation of benzyl alcohol | [136] |
| ZrP-Ti | TiO2−x cluster grafted on ZrP nanosheet via chemical bonding with P element | 2–5 nm | Photodegradation of dyes | [137] |
| Pd@ZPGly | Impregnation with Pd(OAc)2 | 2–5 nm | Suzuki reactions | [138,139] |
| Pd@MZrP | Impregnation with Pd(OAc)2 and reduction with NaBH4 | 7–8 nm | [140] | |
| Pd@ZrCP | Impregnation with Pd(OAc)2 and reduction with NaBH4 | 5 nm | [141] | |
| PdNP/α-ZrPK | Impregnation with Pd(OAc)2 in EtOH | 5–15 nm | Heck reactions | [142] |
| Pd@ZPGly | Impregnation with Pd(OAc)2 | 2–5 nm | [139] | |
| Pd@ZrP | Adsorption of Pd(NH3)4Cl2 and reduction with NaBH4 | 3–7 nm | [143] | |
| Pd@ZPGly | Impregnation with Pd(OAc)2 | 2–5 nm | Nitroarenes reduction | [144] |
| Au@ZP(AEP) | Impregnation with HAuCl4 and reduction with NaBH4 | 7.8 ± 2.4 nm | [145] | |
| ZrP-SH(Au) | Impregnation with HAuCl4 and reduction with NaBH4 | 2 ± 1 nm | [146] | |
| Au/ZrP composite | Impregnation with HAuCl4 and reduction with Na-citrate | 15 nm | [147] | |
| Ag@C/ZrPP | Ion exchange with [Ag74(C≡CPh)44](NO3)2 cluster, carbonization, calcination, and reduction | 1–2 nm | [148] | |
| ZrP@PDA/Ag | Ag+ impregnation and in situ reduction on ZrP nanosheets coated with polydopamine | 29.6 nm | [149] | |
| ZrP@PDA/Au | Au3+ impregnation and in situ reduction on ZrP nanosheets coated with polydopamine | 6.5 nm | [150] | |
| Ag/ZrP | Impregnation with AgNO3, calcination, and reduction with NaBH4 | 6.5 nm | [151] | |
| Pt/ZrP | Impregnation with H2PtCl2 and reduction with H2 flow at 200 °C | 8–11 nm | [152] | |
| Pt/ZrP | Adsorption of Pt(NH3)4Cl2 on ZrP nanosheet and annealing at 400 °C under air | 1.4 ± 0.3 nm | NOx reduction | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donnadio, A.; Pica, M.; Nocchetti, M.; Piermatti, O. Zirconium Phosphates and Phosphonates: Applications in Catalysis. Catalysts 2024, 14, 733. https://doi.org/10.3390/catal14100733
Donnadio A, Pica M, Nocchetti M, Piermatti O. Zirconium Phosphates and Phosphonates: Applications in Catalysis. Catalysts. 2024; 14(10):733. https://doi.org/10.3390/catal14100733
Chicago/Turabian StyleDonnadio, Anna, Monica Pica, Morena Nocchetti, and Oriana Piermatti. 2024. "Zirconium Phosphates and Phosphonates: Applications in Catalysis" Catalysts 14, no. 10: 733. https://doi.org/10.3390/catal14100733
APA StyleDonnadio, A., Pica, M., Nocchetti, M., & Piermatti, O. (2024). Zirconium Phosphates and Phosphonates: Applications in Catalysis. Catalysts, 14(10), 733. https://doi.org/10.3390/catal14100733










