Synthesis of ZnPc/BiVO4 Z-Scheme Heterojunction for Enhanced Photocatalytic Degradation of Tetracycline Under Visible Light Irradiation
Abstract
1. Introduction
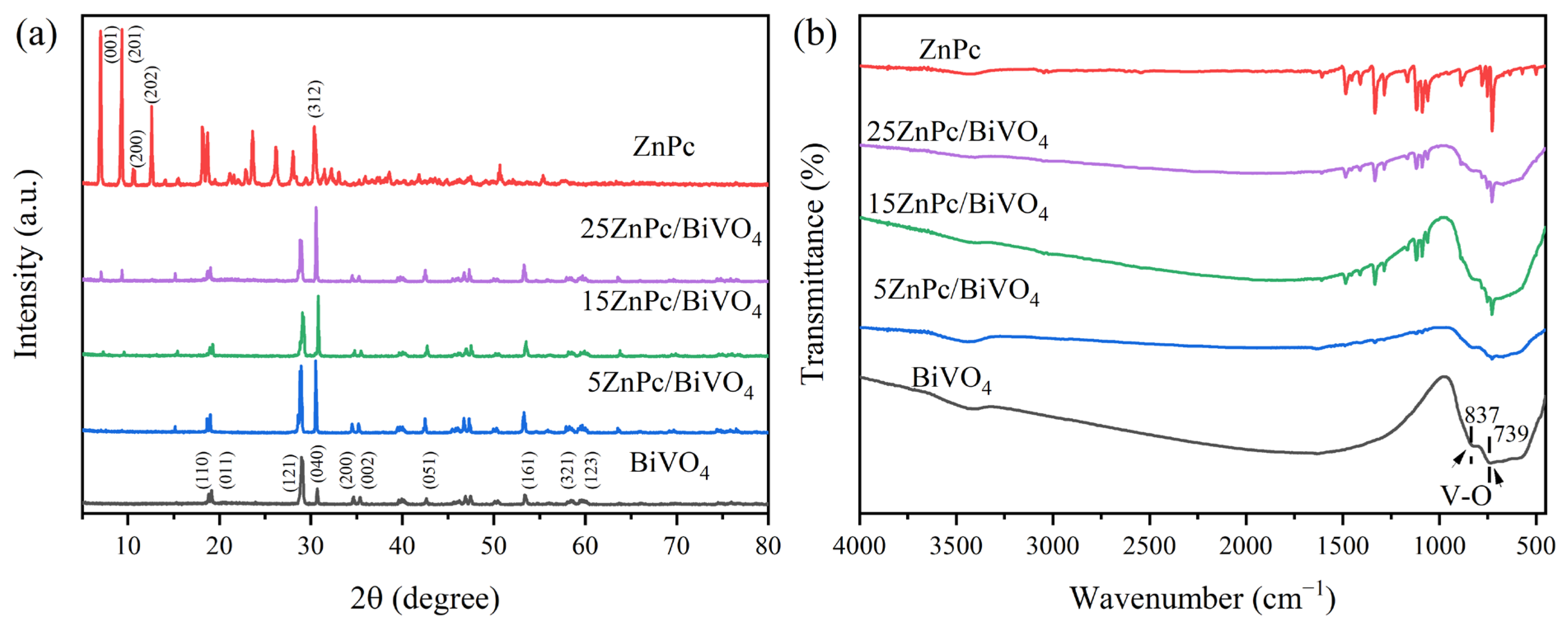
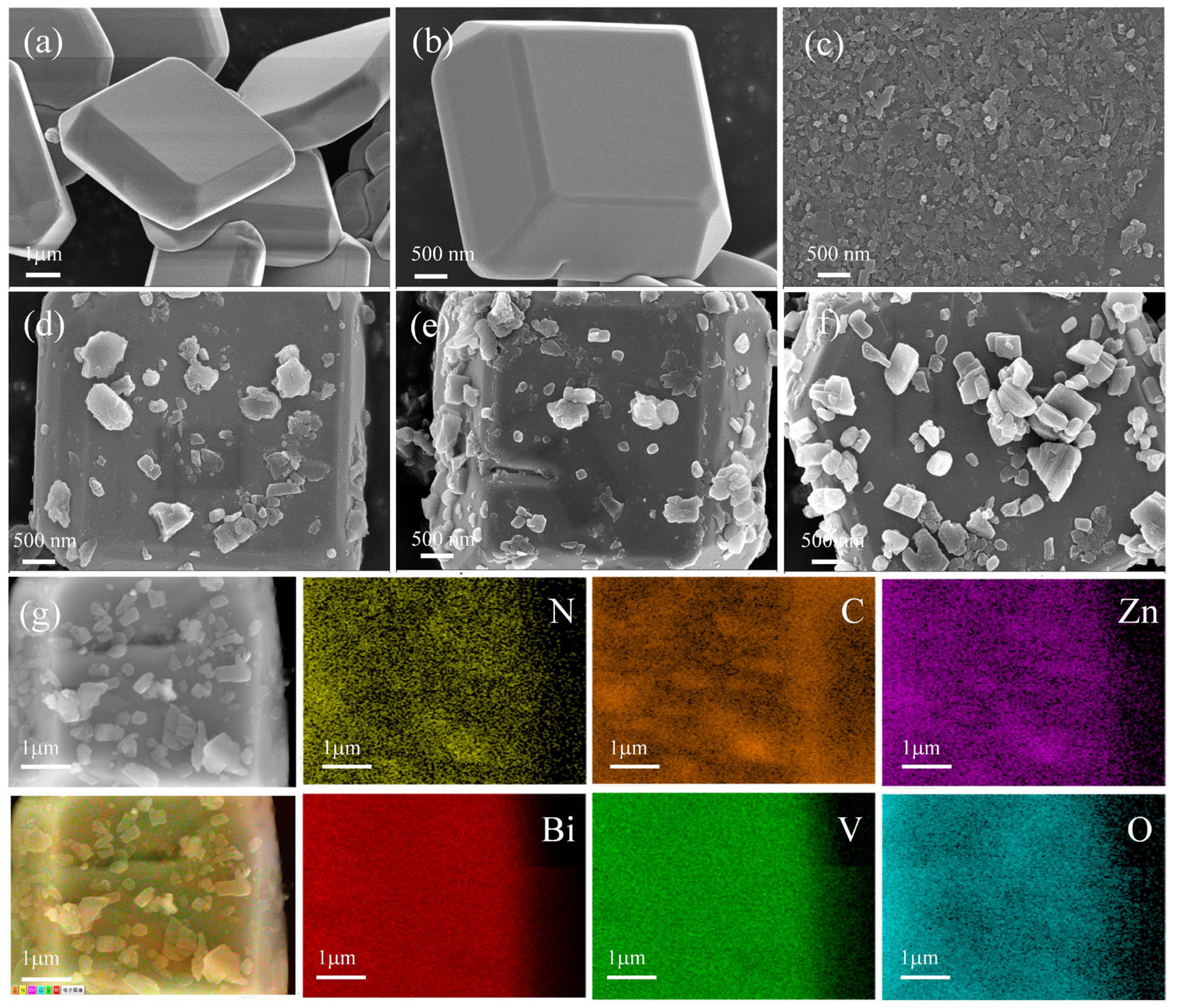
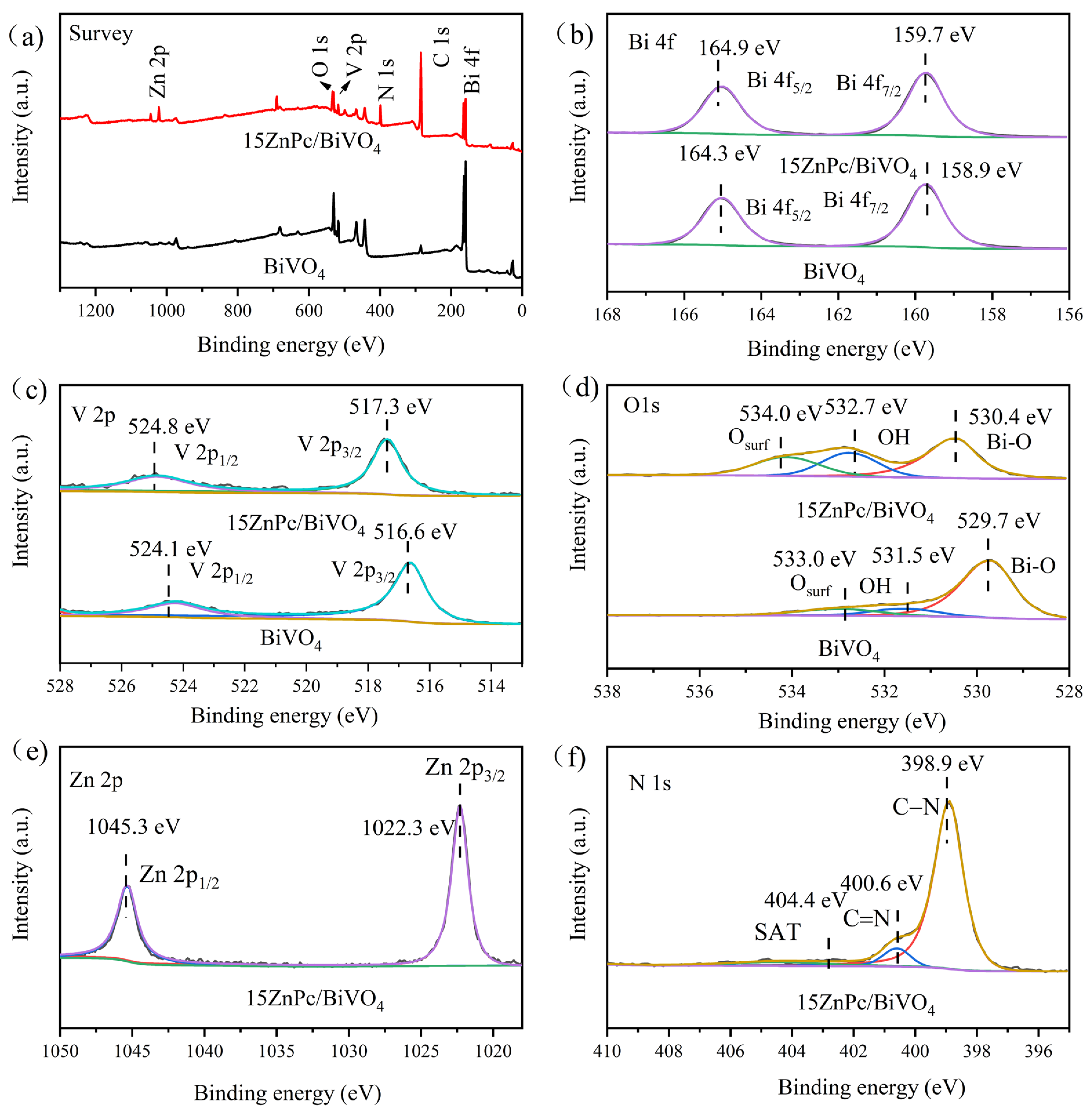
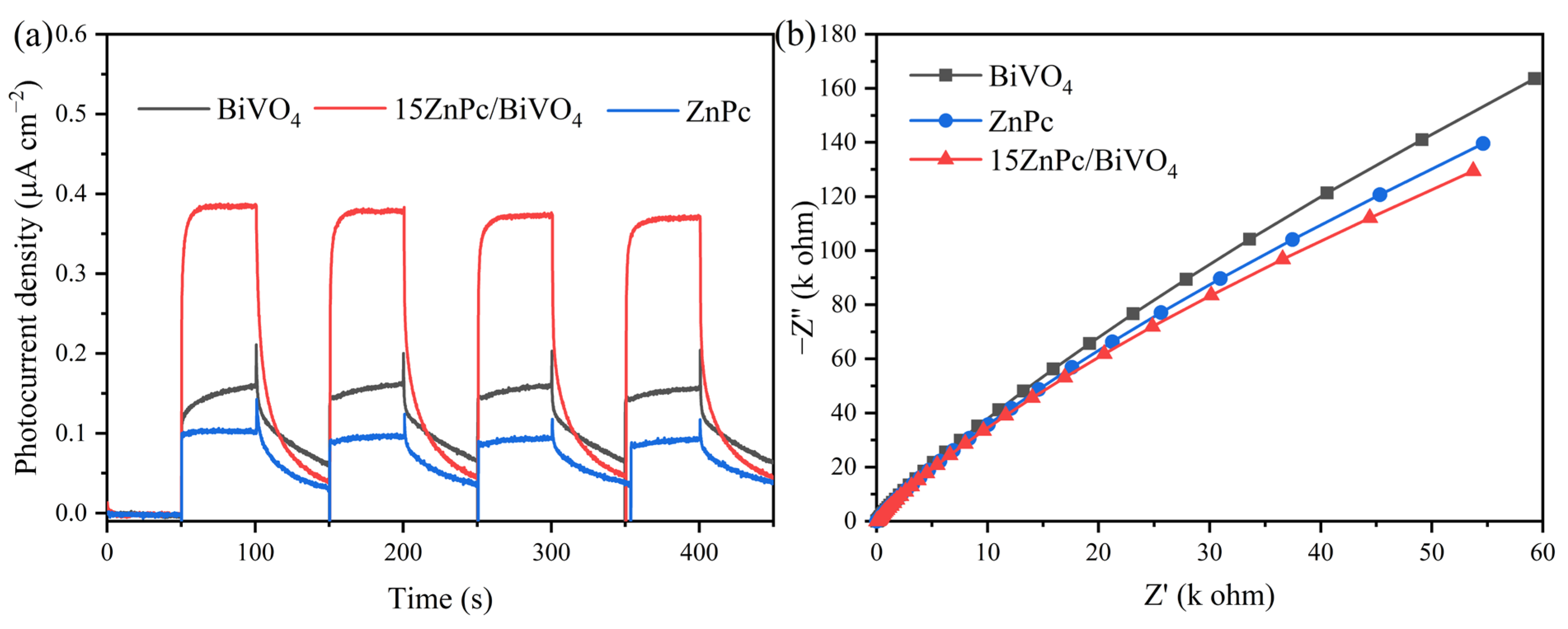
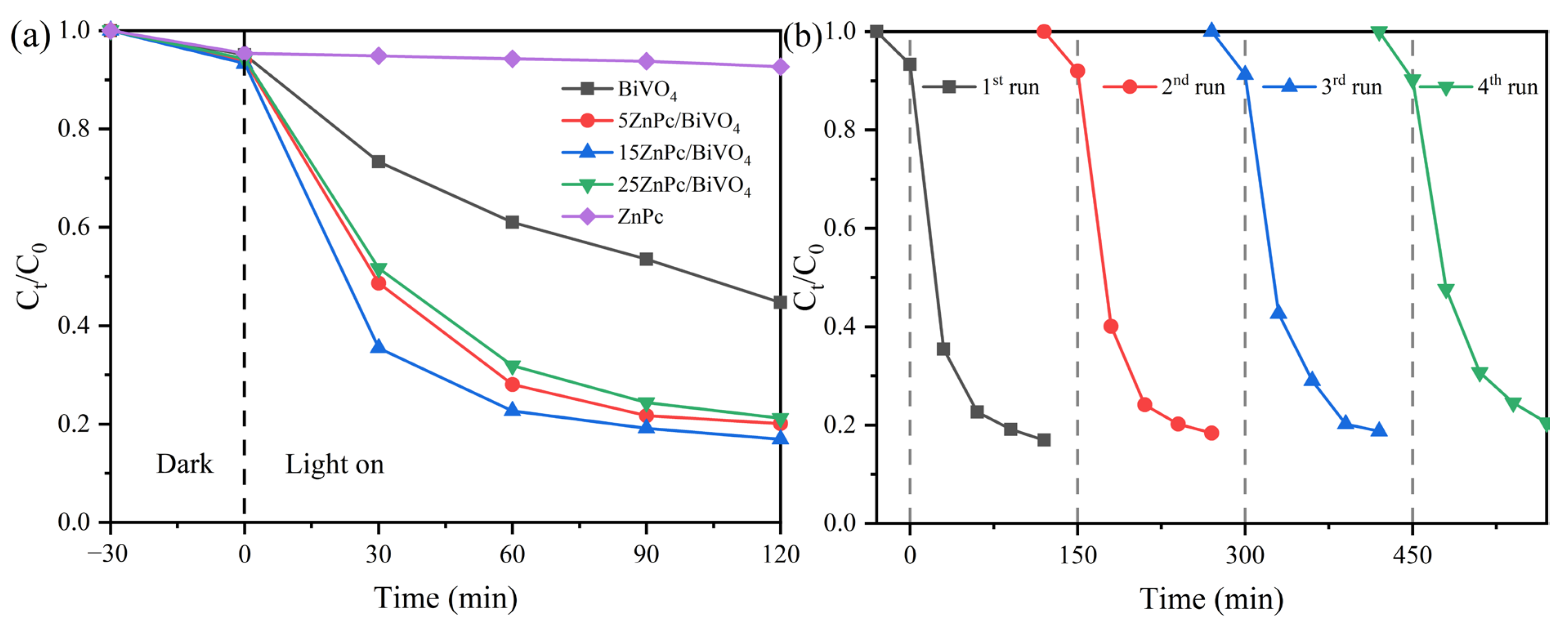
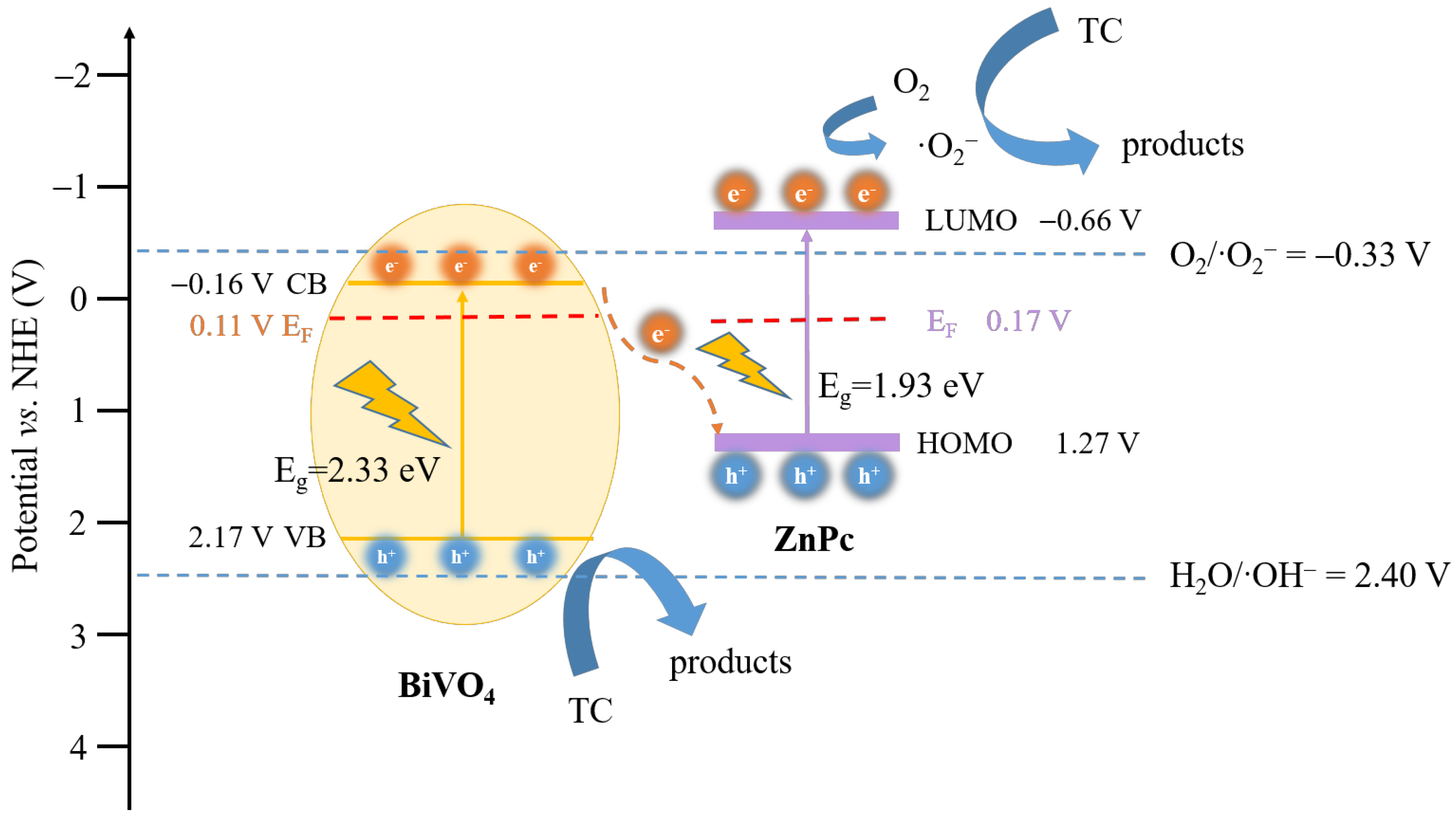
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Preparation of BiVO4 and ZnPc/BiVO4
3.3. Characterization of the Catalysts
3.4. Photoelectrochemical Measurements
3.5. Photocatalytic Activity Measurements
3.6. Stability Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jia, K.; Liu, G.; Lang, D.N.; Chen, S.F.; Yang, C.; Wu, R.L.; Wang, W.; Wang, J.D. Fast photodegradation of antibiotics and dyes by an anionic surfactant-aided CdS/ZnO nanodispersion. New J. Chem. 2022, 46, 11303–11314. [Google Scholar] [CrossRef]
- Miao, X.; Liu, X.; Wang, Y.; Chen, P. Enhanced photocatalytic degradation properties of a g-C3N4/BiVO4 heterostructure system induced by surface and interface heterojunctions. J. Mater. Sci. 2024, 59, 6285–6304. [Google Scholar] [CrossRef]
- Monfort, O.; Plesch, G. Bismuth vanadate-based semiconductor photocatalysts: A short critical review on the efficiency and the mechanism of photodegradation of organic pollutants. Environ. Sci. Pollut. Res. 2018, 25, 19362–19379. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, H.; Xiong, J.; Xu, X.; Cheng, J.; Liu, X.; Liu, G. A Mini Review on Bismuth-Based Z-Scheme Photocatalysts. Materials 2020, 13, 5057. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhang, Q.; Li, K.; Zhang, Y.; Liu, E.; Li, X. Recent advances on g-C3N4-based Z-scheme photocatalysts: Structural design and photocatalytic applications. Int. J. Hydrogen Energy 2023, 48, 196–231. [Google Scholar] [CrossRef]
- Gamelas, S.R.D.; Tomé, J.P.C.; Tomé, A.C.; Lourenço, L.M.O. Advances in photocatalytic degradation of organic pollutants in wastewaters: Harnessing the power of phthalocyanines and phthalocyanine-containing materials. RSC Adv. 2023, 13, 33957–33993. [Google Scholar] [CrossRef]
- Chee, M.K.T.; Ng, B.-J.; Chong, W.-K.; Tan, L.-L.; Chang, W.S.; Chai, S.-P. Unravelling the Charge Dynamics of a Molecular Photosensitizer of ZnPc on Polymeric g-C3N4 for Superior Photocatalytic Hydrogen Evolution from Seawater Splitting. ACS Appl. Energy Mater. 2023, 6, 7935–7943. [Google Scholar] [CrossRef]
- Chen, F.; Wu, C.; Wang, J.; François-Xavier, C.P.; Wintgens, T. Highly efficient Z-scheme structured visible-light photocatalyst constructed by selective doping of Ag@AgBr and Co3O4 separately on {010} and {110} facets of BiVO4: Pre-separation channel and hole-sink effects. Appl. Catal. B Environ. 2019, 250, 31–41. [Google Scholar] [CrossRef]
- Petala, A.; Noe, A.; Frontistis, Z.; Drivas, C.; Kennou, S.; Mantzavinos, D.; Kondarides, D.I. Synthesis and characterization of CoOx/BiVO4 photocatalysts for the degradation of propyl paraben. J. Hazard. Mater. 2019, 372, 52–60. [Google Scholar] [CrossRef]
- Atta-Eyison, A.A.; Anukwah, G.D.; Zugle, R. Photocatalysis using zinc oxide-zinc phthalocyanine composite for effective mineralization of organic pollutants. Catal. Commun. 2021, 160, 106357. [Google Scholar] [CrossRef]
- Li, N.; Wang, J.; Zhao, G.; Du, J.; Li, Y.; Bai, Y.; Li, Z.; Xiong, Y. Enabling CsPbBr3 Perovskites for Photocatalytic CO2 Methanation by Rationalizing a Z-Scheme Heterojunction with Zinc Phthalocyanine. ACS Mater. Lett. 2024, 6, 999–1006. [Google Scholar] [CrossRef]
- Klyamer, D.D.; Sukhikh, A.S.; Gromilov, S.A.; Kruchinin, V.N.; Spesivtsev, E.V.; Hassan, A.K.; Basova, T.V. Influence of Fluorosubstitution on the Structure of Zinc Phthalocyanine Thin Films. Macroheterocycles 2018, 11, 304–311. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, Y.; Liu, F.; Tian, Y.; Wang, Q.; Zeng, D.; Shen, T.; Song, J.; Guan, R.; Yuan, H. The {010} and {110} facets of BiVO4 were selectively modified by Cu and g-C3N4 to enhance its visible light photocatalytic performance. Sep. Purif. Technol. 2023, 323, 124471. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, H.; Han, J.; Liu, Y.; Sun, J.; Shen, T.; Wang, X.; Wang, Z.; Zhang, W.; Yao, X. Construction of a Bi2WO6/BiVO4 photocatalytic system for efficient visible light degradation of tetracycline drugs. RSC Adv. 2023, 13, 3612–3622. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Zheng, X.; Shi, H. Construction of BiVO4/CoPc S-scheme heterojunctions with enhanced photothermal-assisted photocatalytic activity. Sci. China Mater. 2024, 67, 550–561. [Google Scholar] [CrossRef]
- Ali, S.; Ali, S.; Khan, I.; Zahid, M.; Muhammad Ismail, P.; Ismail, A.; Zada, A.; Ullah, R.; Hayat, S.; Ali, H.; et al. Molecular modulation of interfaces in a Z-scheme van der Waals heterojunction for highly efficient photocatalytic CO2 reduction. J. Colloid Interface Sci. 2024, 663, 31–42. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, B.; Zhang, M.; Mu, J.; Shao, C.; Liu, Y. Zinc phthalocyanine hierarchical nanostructure with hollow interior space: Solvent-thermal synthesis and high visible photocatalytic property. J. Colloid Interface Sci. 2010, 348, 37–42. [Google Scholar] [CrossRef]
- Qin, N.; Zhang, S.; He, J.; Long, F.; Wang, L. In situ synthesis of BiVO4/BiOBr microsphere heterojunction with enhanced photocatalytic performance. J. Alloys Compd. 2022, 927, 166661. [Google Scholar] [CrossRef]
- Huang, H.; He, Y.; Du, X.; Chu, P.K.; Zhang, Y. A General and Facile Approach to Heterostructured Core/Shell BiVO4/BiOI p–n Junction: Room-Temperature in Situ Assembly and Highly Boosted Visible-Light Photocatalysis. ACS Sustain. Chem. Eng. 2015, 3, 3262–3273. [Google Scholar] [CrossRef]
- Liu, Y.; Kong, J.; Yuan, J.; Zhao, W.; Zhu, X.; Sun, C.; Xie, J. Enhanced photocatalytic activity over flower-like sphere Ag/Ag2CO3/BiVO4 plasmonic heterojunction photocatalyst for tetracycline degradation. Chem. Eng. J. 2018, 331, 242–254. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, Z.; Wu, Z.; Tian, F.; Yu, B. Hierarchical construction of a new Z-scheme Bi/BiVO4-CdS heterojunction for enhanced visible-light photocatalytic degradation of tetracycline hydrochloride. Sep. Purif. Technol. 2021, 275, 119152. [Google Scholar] [CrossRef]
- Ouedraogo, S.; Chouchene, B.; Gries, T.; Desmarets, C.; Balan, L.; Gaumet, J.-J.; Medjahdi, G.; Bayo, K.; Schneider, R. Bismuth oxybromide/reduced graphene oxide heterostructure sensitized with Zn-tetracarboxyphthalocyanine as a highly efficient photocatalyst for the degradation of Orange II and phenol. J. Environ. Chem. Eng. 2022, 10, 107332. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, J.; Tao, F.; Dong, Y.; Wang, L.; Hong, T. Facile construction of Z-scheme g-C3N4/BiVO4 heterojunctions for boosting visible-light photocatalytic activity. Mater. Sci. Eng. B 2022, 279, 115676. [Google Scholar] [CrossRef]
- Hasnain Bakhtiar, S.U.; Zada, A.; Raziq, F.; Ali, S.; Ali Shah, M.I.; Ateeq, M.; Khan, M.; Alei, D.; Fazil, P.; Naeem, M.; et al. Zinc phthalocyanine sensitized g-C3N4 photocatalyst for exceptional photocatalytic hydrogen evolution and pollutant degradation. Int. J. Hydrogen Energy 2023, 48, 16320–16329. [Google Scholar] [CrossRef]
- Du, S.; Chen, L.; Men, C.; Ji, H.; Su, T.; Qin, Z. Effect of surface defect states on Zn(1−x)CdxS for enhanced photocatalytic hydrogen evolution. J. Alloys Compd. 2023, 955, 170265. [Google Scholar] [CrossRef]
- Chai, H.; Nan, J.; Jin, W.; Wu, F.; Liu, B.; Guo, Y. Zinc phthalocyanine/polymer carbon nitride S-scheme heterojunction with internal electric field and near-infrared absorption for photocatalytic H2O2 production. Chem. Eng. J. 2024, 489, 151293. [Google Scholar] [CrossRef]
- Xu, T.; Wang, D.; Dong, L.; Shen, H.; Lu, W.; Chen, W. Graphitic carbon nitride co-modified by zinc phthalocyanine and graphene quantum dots for the efficient photocatalytic degradation of refractory contaminants. Appl. Catal. B Environ. 2019, 244, 96–106. [Google Scholar] [CrossRef]
- Qin, T.; Wei, J.; Zhou, C.; Zeng, X.; Zhou, J.; Li, Y.-Y. Directional crystal facets deposition constructed BiVO4/Ag/MnO2 with plasmon resonance for enhanced photocatalytic degradation of antibiotics in water. Sep. Purif. Technol. 2023, 317, 123793. [Google Scholar] [CrossRef]
- Vallejo, W.; Diaz-Uribe, C.; Cantillo, Á. Methylene blue photocatalytic degradation under visible irradiation on TiO2 thin films sensitized with Cu and Zn tetracarboxy-phthalocyanines. J. Photochem. Photobiol. A Chem. 2015, 299, 80–86. [Google Scholar] [CrossRef]
- Tang, Q.-Y.; Luo, X.-L.; Yang, S.-Y.; Xu, Y.-H. Novel Z-scheme In2S3/BiVO4 composites with improved visible-light photocatalytic performance and stability for glyphosate degradation. Sep. Purif. Technol. 2020, 248, 117039. [Google Scholar] [CrossRef]
- Luo, X.-L.; Yang, S.-Y.; Wang, Z.-L.; Xu, Y.-H. Synthesis of Z-scheme Bi2S3/RGO/BiVO4 photocatalysts with superior visible light photocatalytic effectiveness for pollutant degradation. Sep. Purif. Technol. 2023, 318, 123966. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, X.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. Noble-metal-free ultrathin MXene coupled with In2S3 nanoflakes for ultrafast photocatalytic reduction of hexavalent chromium. Appl. Catal. B Environ. 2021, 284, 119754. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, Z.; Liang, J.; Liang, B.; Huang, H.; Huang, G.; Junaid, M.; Wang, J.; Huang, K. Simultaneous photocatalytic removal of tetracycline and hexavalent chromium by BiVO4/0.6CdS photocatalyst: Insights into the performance, evaluation, calculation and mechanism. J. Colloid Interface Sci. 2024, 667, 650–662. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Tian, F.; Chen, X.; Wu, Z. Synthesis of BiVO4/g-C3N4 S-scheme heterojunction via a rapid and green microwave route for efficient removal of glyphosate. Sep. Purif. Technol. 2022, 287, 120507. [Google Scholar] [CrossRef]
- Mirzaei, A.; Seck, A.; Ma, D.; Chaker, M. Black TiO2 Nanotube Array/BiVO4 Heterojunction Photocatalysts for Tetracycline Removal with High Solution Detoxification Efficiency. ACS Appl. Nano Mater. 2022, 5, 7161–7174. [Google Scholar] [CrossRef]
- Li, M.; Lai, C.; Yi, H.; Huang, D.; Qin, L.; Liu, X.; Li, B.; Liu, S.; Zhang, M.; Fu, Y.; et al. Multiple charge-carrier transfer channels of Z-scheme bismuth tungstate-based photocatalyst for tetracycline degradation: Transformation pathways and mechanism. J. Colloid Interface Sci. 2019, 555, 770–782. [Google Scholar] [CrossRef]
- Wang, J.; Zhi, D.; Zhou, H.; He, X.; Zhang, D. Evaluating tetracycline degradation pathway and intermediate toxicity during the electrochemical oxidation over a Ti/Ti4O7 anode. Water Res. 2018, 137, 324–334. [Google Scholar] [CrossRef]
- Li, J.; Zhao, W.; Guo, Y.; Wei, Z.; Han, M.; He, H.; Yang, S.; Sun, C. Facile synthesis and high activity of novel BiVO4/FeVO4 heterojunction photocatalyst for degradation of metronidazole. Appl. Surf. Sci. 2015, 351, 270–279. [Google Scholar] [CrossRef]
- Truong, H.B.; Huy, B.T.; Ray, S.K.; Gyawali, G.; Lee, Y.-I.; Cho, J.; Hur, J. Magnetic visible-light activated photocatalyst ZnFe2O4/BiVO4/g-C3N4 for decomposition of antibiotic lomefloxacin: Photocatalytic mechanism, degradation pathway, and toxicity assessment. Chemosphere 2022, 299, 134320. [Google Scholar] [CrossRef]
- Koutavarapu, R.; Tamtam, M.R.; Lee, S.-G.; Rao, M.C.; Lee, D.-Y.; Shim, J. Synthesis of 2D NiFe2O4 nanoplates/2D Bi2WO6 nanoflakes heterostructure: An enhanced Z-scheme charge transfer and separation for visible-light-driven photocatalytic degradation of toxic pollutants. J. Environ. Chem. Eng. 2021, 9, 105893. [Google Scholar] [CrossRef]
- Li, X.; Chen, T.; Qiu, Y.; Zhu, Z.; Zhang, H.; Yin, D. Magnetic dual Z-scheme g-C3N4/BiVO4/CuFe2O4 heterojunction as an efficient visible-light-driven peroxymonosulfate activator for levofloxacin degradation. Chem. Eng. J. 2023, 452, 139659. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Zeng, G.; Liang, J.; Wu, Z.; Yu, H.; Mo, D.; Wang, H.; Xiao, Z.; Zhou, C. Nitrogen self-doped g-C3N4 nanosheets with tunable band structures for enhanced photocatalytic tetracycline degradation. J. Colloid Interface Sci. 2019, 536, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, J.; Shi, J.; Deng, H. Bi2Fe4O9/rGO nanocomposite with visible light photocatalytic performance for tetracycline degradation. Environ. Res. 2024, 249, 118361. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhou, Y.; Fei, L.; Cheng, X.; Zhu, X.; Deng, L.; Ma, X. Z-scheme CuO/Fe3O4/GO heterojunction photocatalyst: Enhanced photocatalytic performance for elimination of tetracycline. Chemosphere 2022, 309, 136721. [Google Scholar] [CrossRef]
- Li, X.; Shen, X.; Qiu, Y.; Zhu, Z.; Zhang, H.; Yin, D. Fe3O4 quantum dots mediated P-g-C3N4/BiOI as an efficient and recyclable Z-scheme photo-Fenton catalyst for tetracycline degradation and bacterial inactivation. J. Hazard. Mater. 2023, 456, 131677. [Google Scholar] [CrossRef]
- Ou, M.; Wan, S.; Zhong, Q.; Zhang, S.; Song, Y.; Guo, L.; Cai, W.; Xu, Y. Hierarchical Z-scheme photocatalyst of g-C3N4@Ag/BiVO4 (040) with enhanced visible-light-induced photocatalytic oxidation performance. Appl. Catal. B Environ. 2018, 221, 97–107. [Google Scholar] [CrossRef]
- Xue, Y.; Wu, Z.; He, X.; Li, Q.; Yang, X.; Li, L. Hierarchical fabrication Z-scheme photocatalyst of BiVO4(040)-Ag@CdS for enhanced photocatalytic properties under simulated sunlight irradiation. J. Colloid Interface Sci. 2019, 548, 293–302. [Google Scholar] [CrossRef]
- Wang, G.; Cheng, H. Boosting catalytic efficiency via BiVO4 surface heterojunction-induced interfacial Z-scheme NiFe2O4/{010}BiVO4 composite. Sep. Purif. Technol. 2023, 318, 123949. [Google Scholar] [CrossRef]
- Singh, P.; Devi, P. Deciphering the photoelectrochemical behaviour of MoSe2-decorated BiVO4 as a dual system for concurrent degradation of methylene blue and hydrogen generation. J. Clean. Prod. 2024, 448, 141502. [Google Scholar] [CrossRef]
- Ma, D.; Wang, W.; Wang, Q.; Dai, Y.; Zhu, K.; Xu, H.; Yuan, C.; Dong, P.; Xi, X. A novel visible-light-driven Z-scheme C3N5/BiVO4 heterostructure with enhanced photocatalytic degradation performance. Environ. Sci. Pollut. Res. 2024, 31, 19687–19698. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Jiang, H.; Ma, Y.; Zhu, R.; Zhang, G.; Ren, Z. Highly efficient visible-light driven dye degradation via 0D BiVO4 nanoparticles/2D BiOCl nanosheets p-n heterojunctions. Chemosphere 2024, 354, 141658. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Li, H.; Yu, H.; Chen, S.; Quan, X. Constructing BiVO4-Au@CdS photocatalyst with energic charge-carrier-separation capacity derived from facet induction and Z-scheme bridge for degradation of organic pollutants. Appl. Catal. B Environ. 2018, 227, 258–265. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Zhou, G.; Tang, L.; Xu, Y.; Ma, C.; Chen, Z.; Han, S.; Yan, M.; Lu, Z. Construction of a Direct Z-Type Heterojunction Relying on MoS2 Electronic Transfer Platform Towards Enhanced Photodegradation Activity of Tetracycline. Water Air Soil Pollut. 2022, 233, 516. [Google Scholar] [CrossRef]
- Yan, X.; Wang, B.; Zhao, J.; Liu, G.; Ji, M.; Zhang, X.; Chu, P.K.; Li, H.; Xia, J. Hierarchical columnar ZnIn2S4/BiVO4 Z-scheme heterojunctions with carrier highway boost photocatalytic mineralization of antibiotics. Chem. Eng. J. 2023, 452, 139271. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Liu, T.; Jia, Y.; Deng, H.; Wang, W.; Zhang, F. Alkali-mediated dissolution-recrystallization strategy for in situ construction of a BiVO4/Bi25VO40 heterojunction with promoted interfacial charge transfer: Formation mechanism and photocatalytic tetracycline degradation studies. Chem. Eng. J. 2022, 431, 134181. [Google Scholar] [CrossRef]
- Liaqat, M.; Khalid, N.R. Fabrication of Novel BiVO4 Homostructure with Superior Visible Light Induced Photocatalytic Properties Using Directing Agents. Water Air Soil Pollut. 2023, 234, 297. [Google Scholar] [CrossRef]
- Jiang, D.; Xiao, P.; Shao, L.; Li, D.; Chen, M. RGO-Promoted All-Solid-State g-C3N4/BiVO4 Z-Scheme Heterostructure with Enhanced Photocatalytic Activity toward the Degradation of Antibiotics. Ind. Eng. Chem. Res. 2017, 56, 8823–8832. [Google Scholar] [CrossRef]
- Ma, C.; Lee, J.; Kim, Y.; Cheol Seo, W.; Jung, H.; Yang, W. Rational design of α-Fe2O3 nanocubes supported BiVO4 Z-scheme photocatalyst for photocatalytic degradation of antibiotic under visible light. J. Colloid Interface Sci. 2021, 581, 514–522. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, L.; Chen, L.; Xie, X.; Qin, Z.; Su, T. Synthesis of ZnPc/BiVO4 Z-Scheme Heterojunction for Enhanced Photocatalytic Degradation of Tetracycline Under Visible Light Irradiation. Catalysts 2024, 14, 722. https://doi.org/10.3390/catal14100722
Zhong L, Chen L, Xie X, Qin Z, Su T. Synthesis of ZnPc/BiVO4 Z-Scheme Heterojunction for Enhanced Photocatalytic Degradation of Tetracycline Under Visible Light Irradiation. Catalysts. 2024; 14(10):722. https://doi.org/10.3390/catal14100722
Chicago/Turabian StyleZhong, Lulu, Liuyun Chen, Xinling Xie, Zuzeng Qin, and Tongming Su. 2024. "Synthesis of ZnPc/BiVO4 Z-Scheme Heterojunction for Enhanced Photocatalytic Degradation of Tetracycline Under Visible Light Irradiation" Catalysts 14, no. 10: 722. https://doi.org/10.3390/catal14100722
APA StyleZhong, L., Chen, L., Xie, X., Qin, Z., & Su, T. (2024). Synthesis of ZnPc/BiVO4 Z-Scheme Heterojunction for Enhanced Photocatalytic Degradation of Tetracycline Under Visible Light Irradiation. Catalysts, 14(10), 722. https://doi.org/10.3390/catal14100722







