Thermodynamic and Kinetic Investigation on Aspergillus ficuum Tannase Immobilized in Calcium Alginate Beads and Magnetic Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
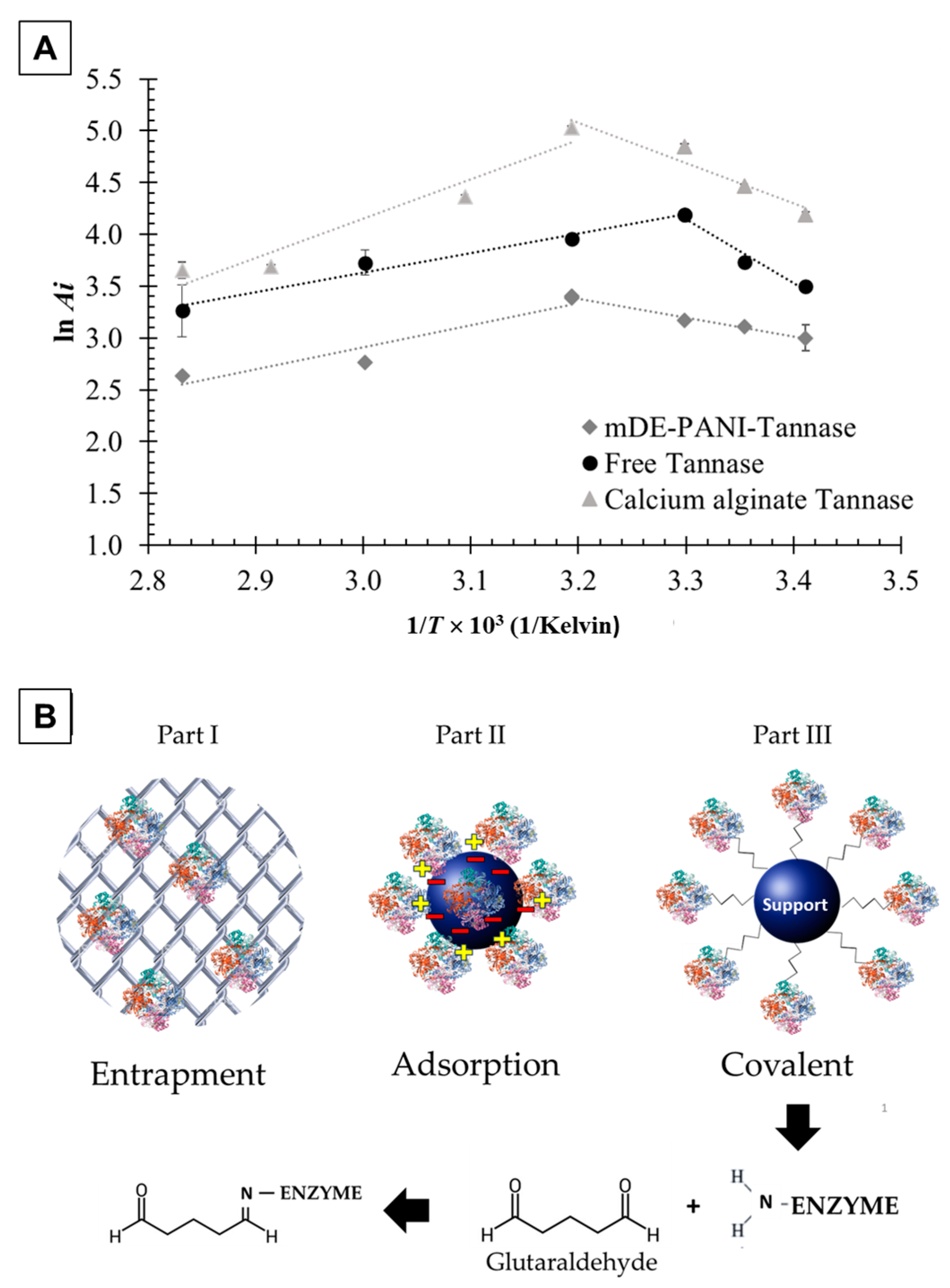
2.1. Thermodynamic Parameters of Tannase-Catalyzed Reaction
2.2. Thermodynamics of Tannase Thermal Inactivation
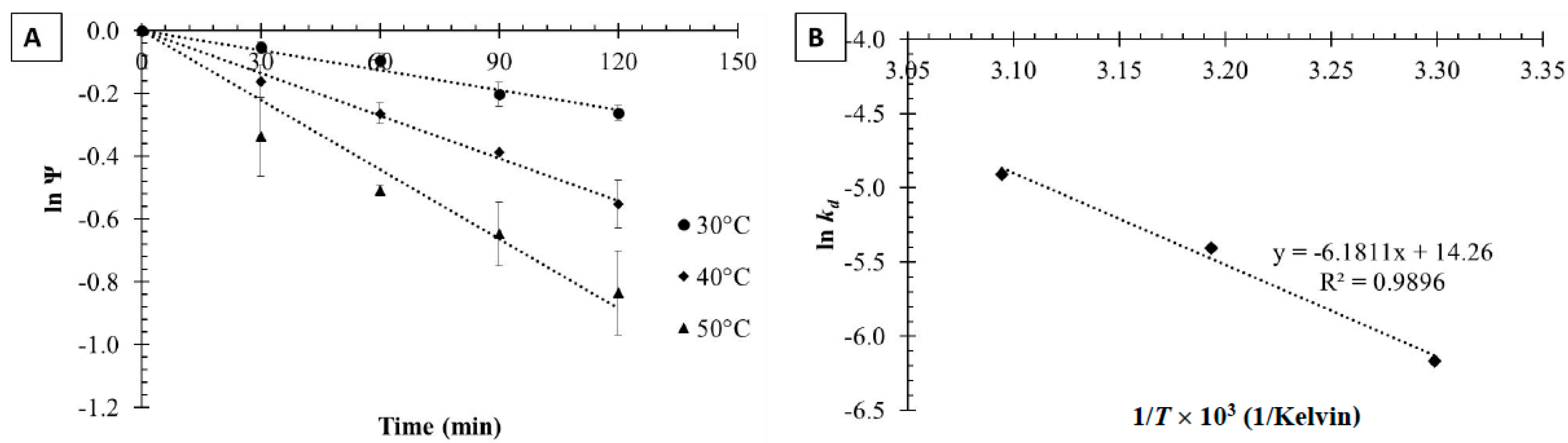
2.2.1. Free Tannase Thermal Inactivation
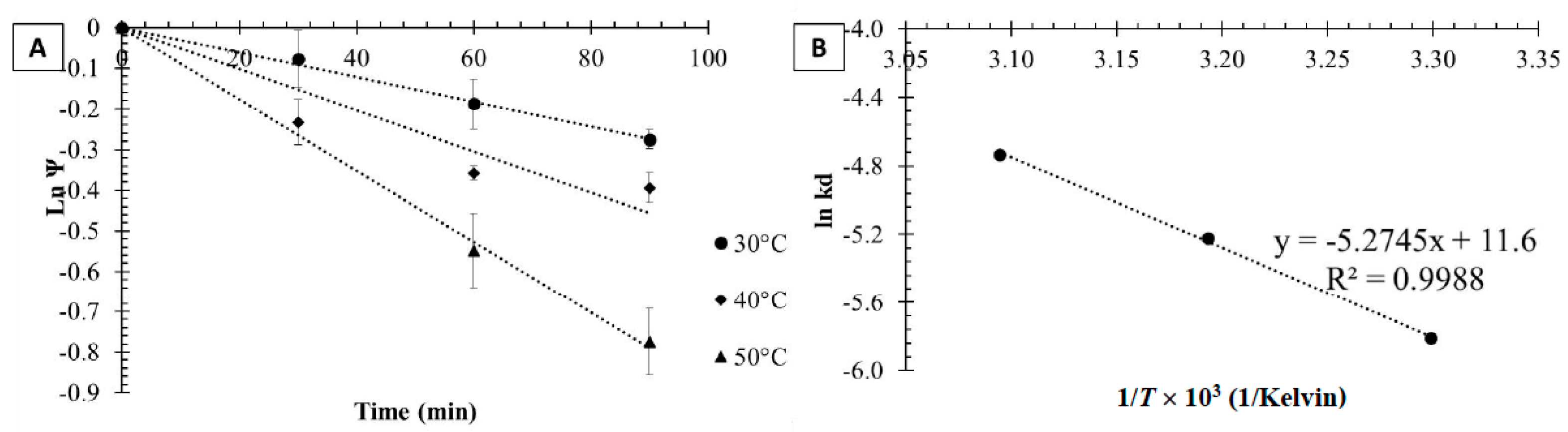
2.2.2. Thermal Inactivation of Tannase Immobilized on Magnetic Nanoparticles
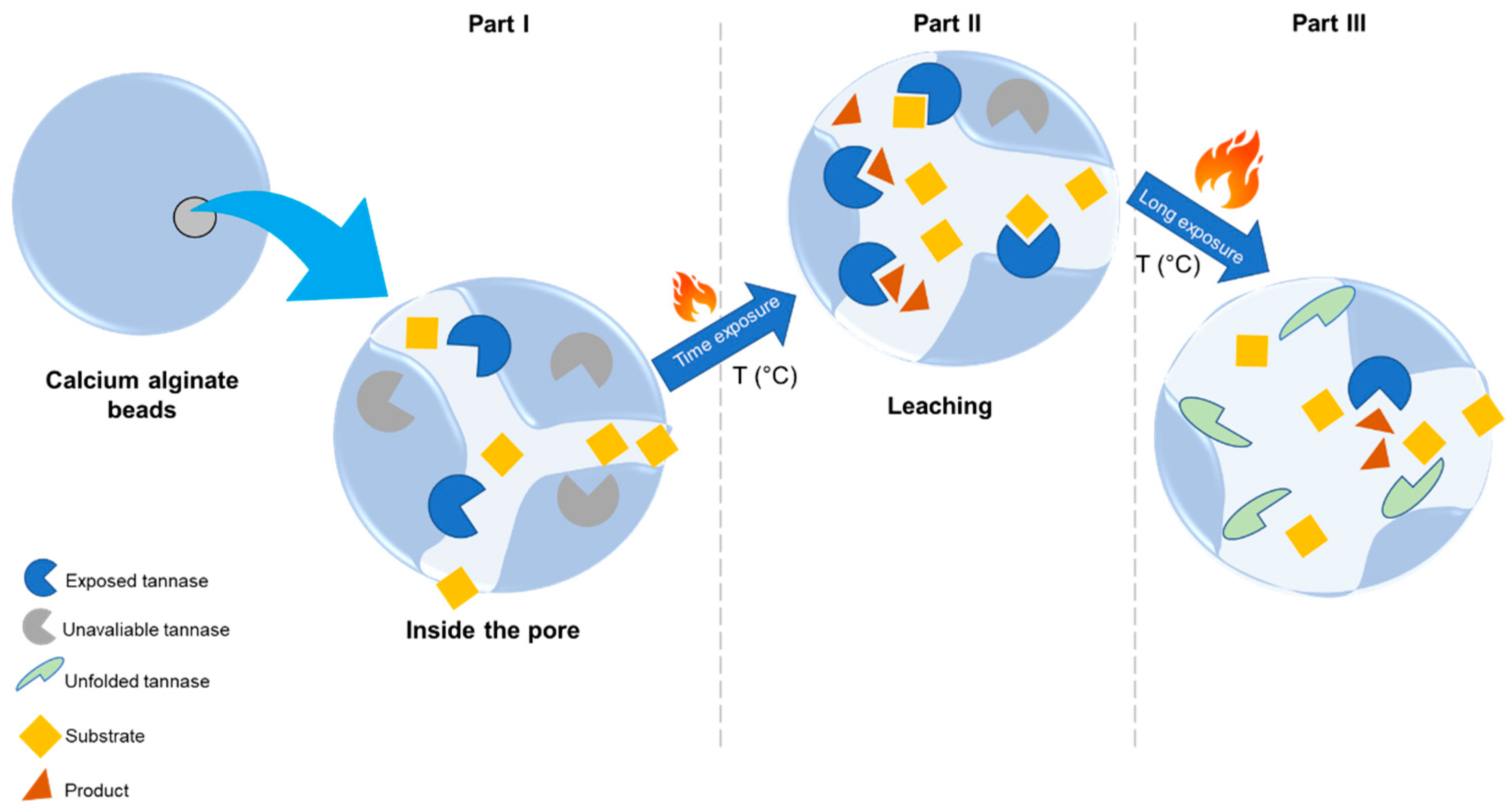
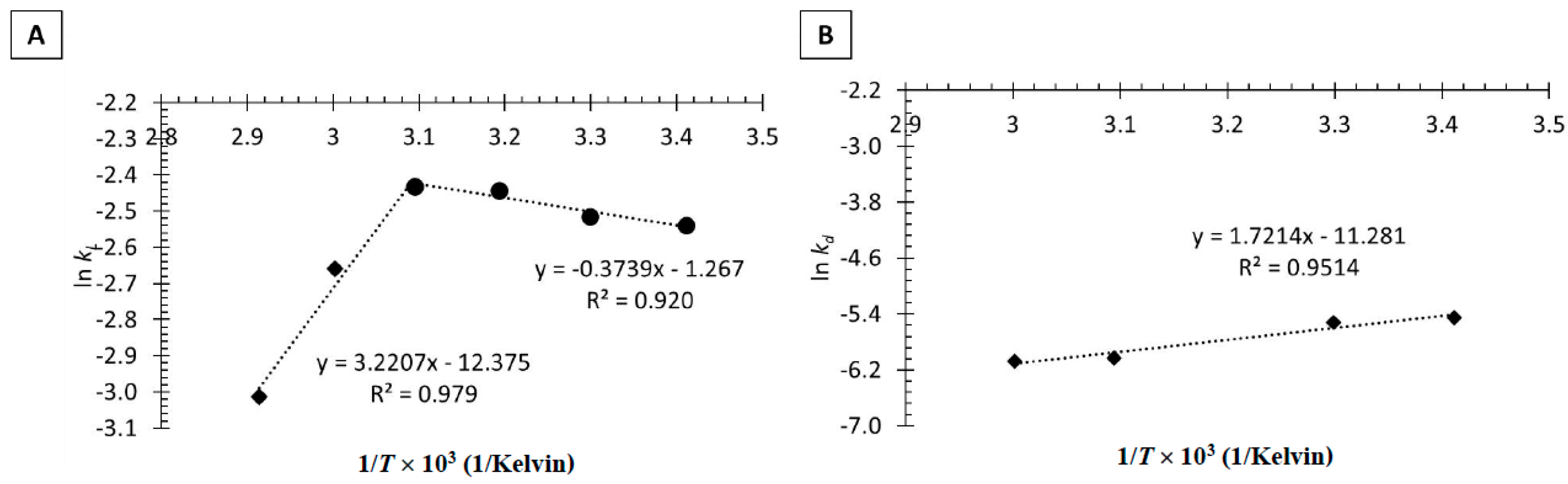
2.2.3. Thermal Inactivation of Tannase Immobilized in Calcium Alginate Beads
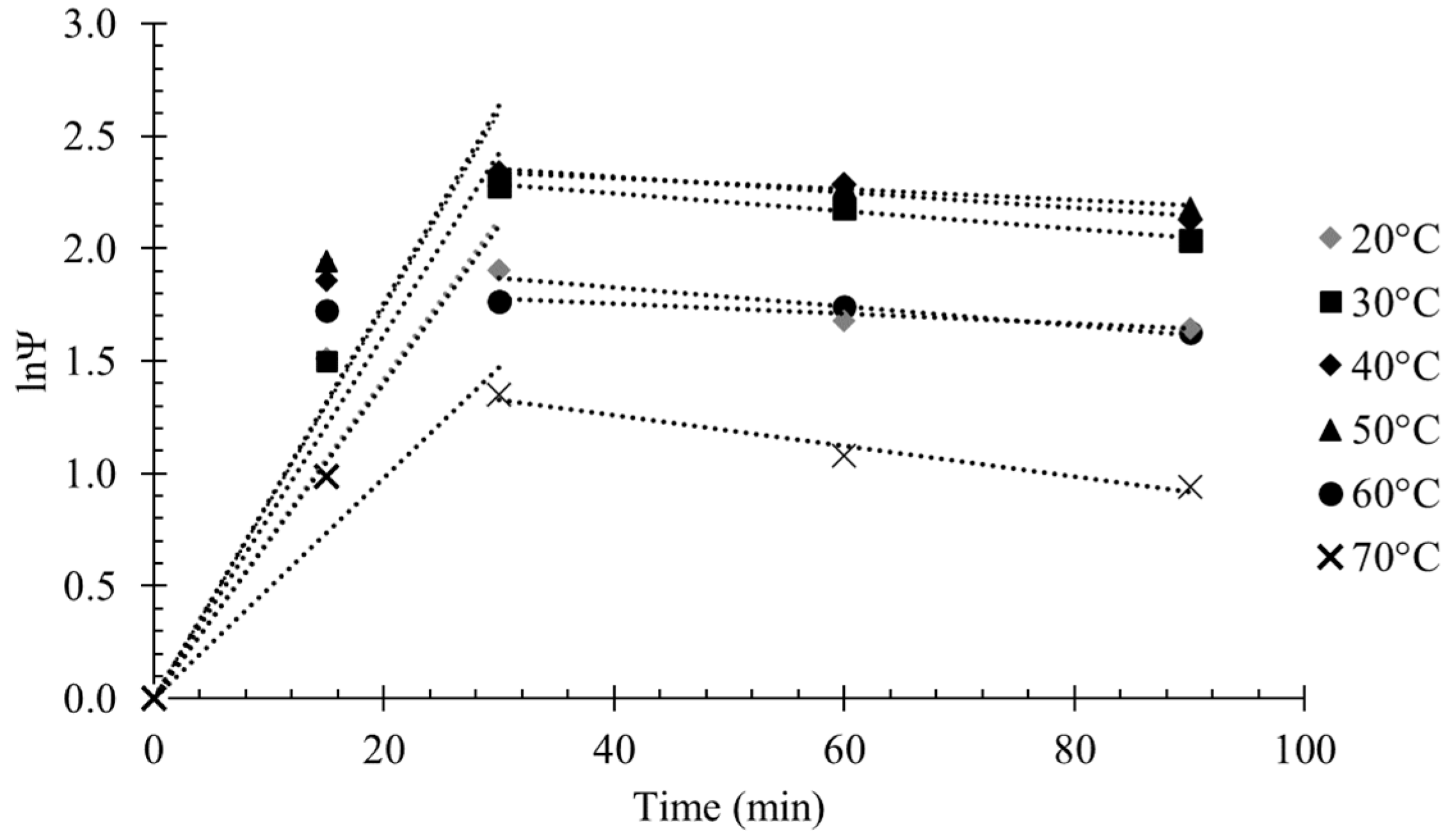
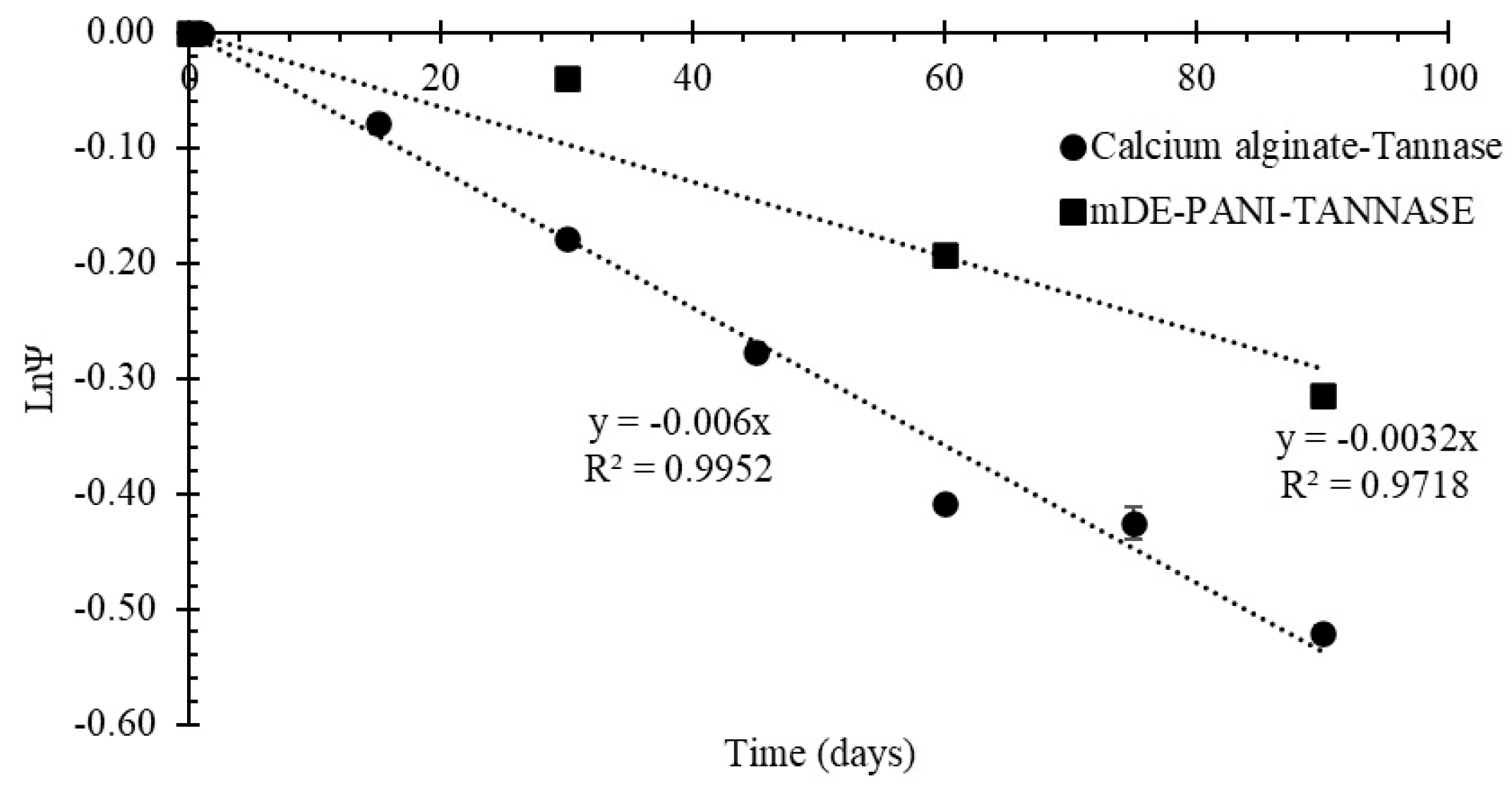
2.2.4. Inactivation of Immobilized Enzyme under Storage Conditions
3. Materials and Methods
3.1. Tannase Immobilization
3.1.1. Immobilization in Calcium Alginate Beads
3.1.2. Immobilization on mDE-PANI Nanoparticles
3.2. Determination of Free and Immobilized Tannase Activity
3.3. Thermodynamic Modeling
3.4. Shelf Life Stability of the Immobilized Enzyme Preparations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eixenberger, D.; Kumar, A.; Klinger, S.; Scharnagl, N.; Dawood, A.W.H.; Liese, A. Polymer-grafted 3D-printed material for enzyme immobilization—designing a smart enzyme carrier. Catalysts 2023, 13, 1130. [Google Scholar] [CrossRef]
- de Oliveira, R.L.; dos Santos, V.L.V.; da Silva, M.F.; Porto, T.S. Kinetic/thermodynamic study of immobilized β-fructofuranosidase from Aspergillus tamarii URM4634 in chitosan beads and application on invert sugar production in packed bed reactor. Food Res. Int. 2020, 137, 109730. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.D.C.; de França, P.R.L.; Converti, A.; Porto, T.S. Pectin hydrolysis in cashew apple juice by Aspergillus aculeatus URM4953 polygalacturonase covalently-immobilized on calcium alginate beads: A kinetic and thermodynamic study. Int. J. Biol. Macromol. 2019, 126, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, O.S.; de Oliveira, R.L.; Silva, J.D.C.; Converti, A.; Porto, T.S. Thermodynamic investigation of an alkaline protease from Aspergillus tamarii URM4634: A comparative approach between crude extract and purified enzyme. Int. J. Biol. Macromol. 2018, 109, 1039–1044. [Google Scholar] [CrossRef]
- Sayed Mostafa, H. Production of low-tannin Hibiscus sabdariffa tea through D-optimal design optimization of the preparation conditions and the catalytic action of new tannase. Food Chem. X. 2023, 17, 100562. [Google Scholar] [CrossRef] [PubMed]
- Mansor, A.; Samat, N.; Mat Amin, N.; Syaril Ramli, M.; Siva, R. Chapter. Microbial tannase production from agro-industrial byproducts for industrial applications. In Microbial Bioprocessing of Agri-Food Wastes. Industrial Enzymes, 1st ed.; Molina, G., Usmani, Z., Sharma, M., Benhida, R., Kuhad, R.C., Gupta, V.K., Eds.; CRC Press: Boca Raton, FL, USA, 2023; p. 40. ISBN 9781003341017. [Google Scholar]
- Benucci, I.; Lombardelli, C.; Esti, M. Innovative continuous biocatalytic system based on immobilized tannase: Possible prospects for the haze-active phenols hydrolysis in brewing industry. Eur. Food Res. Technol. 2023. [Google Scholar] [CrossRef]
- Kumar, M.; Mehra, R.; Yogi, R.; Singh, N.; Salar, R.K.; Saxena, G.; Rustagi, S. A novel tannase from Klebsiella pneumoniae KP715242 reduces haze and improves the quality of fruit juice and beverages through detannification. Front. Sustain. Food Syst. 2023, 7, 1173611. [Google Scholar] [CrossRef]
- Song, L.; Wang, X.-C.; Feng, Z.-Q.; Guo, Y.-F.; Meng, G.-Q.; Wang, H.-Y. Biotransformation of gallate esters by a pH-stable tannase of mangrove-derived yeast Debaryomyces hansenii. Front. Mol. Biosci. 2023, 10, 1211621. [Google Scholar] [CrossRef] [PubMed]
- Ristinmaa, A.S.; Coleman, T.; Cesar, L.; Weinmann, A.L.; Mazurkewich, S.; Branden, G.; Hasani, M.; Larsbrink, J. Structural diversity and substrate preferences of three tannase enzymes encoded by the anaerobic bacterium Clostridium butyricum. J. Biol. Chem. 2022, 298, 101758. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Naby, M.A.; El-Tanash, A.B.; Sherief, A.D.A. Structural characterization, catalytic, kinetic and thermodynamic properties of Aspergillus oryzae tannase. Int. J. Biol. Macromol. 2016, 92, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, Y.; Simpson, B. Food enzymes immobilization: Novel carriers, techniques and applications. Curr. Opin. Food Sci. 2022, 43, 27–35. [Google Scholar] [CrossRef]
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; El-Said Azzazy, H.M. Enzyme immobilization technologies and industrial applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.D.C.; de França, P.R.L.; Converti, A.; Porto, T.S. Kinetic and thermodynamic characterization of a novel Aspergillus aculeatus URM4953 polygalacturonase. Comparison of free and calcium alginate-immobilized enzyme. Process Biochem. 2018, 74, 61–70. [Google Scholar] [CrossRef]
- Miguel Júnior, J.; Mattos, F.R.; Costa, G.R.; Zurlo, A.B.R.; Fernandez-Lafuente, R.; Mendes, A.A. Improved catalytic performance of Lipase Eversa® Transform 2.0 via immobilization for the sustainable production of flavor esters—Adsorption process and environmental assessment studies. Catalysts 2022, 12, 1412. [Google Scholar] [CrossRef]
- Valls-Chivas, A.; Gómez, J.; Garcia-Peiro, J.I.; Hornos, F.; Hueso, J.L. Enzyme–iron oxide nanoassemblies: A review of immobilization and biocatalytic applications. Catalysts 2023, 13, 980. [Google Scholar] [CrossRef]
- Larosa, C.; Salerno, M.; de Lima, J.S.; Meri, R.M.; da Silva, M.F.; de Carvalho, L.B.; Converti, A. Characterisation of bare and tannase-loaded calcium alginate beads by microscopic, thermogravimetric, FTIR and XRD analyses. Int. J. Biol. Macromol. 2018, 115, 900–906. [Google Scholar] [CrossRef]
- Intisar, A.; Hedar, H.; Sharif, A.; Ahmed, E.; Hussain, N.; Hadibarata, H.; Ali Shariati, M.; Smaoui, S. Chapter 21–Enzyme immobilization on alginate biopolymer for biotechnological applications. In Microbial Biomolecules. Emerging Approach in Agriculture, Pharmaceuticals and Environment Management. Developments in Applied Microbiology and Biotechnology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 471–488. ISBN 978-0-323-99476-7. [Google Scholar] [CrossRef]
- de Lima, J.S.; Cabrera, M.P.; Casazza, A.A.; da Silva, M.F.; Perego, P.; de Carvalho, L.B., Jr.; Converti, A. Immobilization of Aspergillus ficuum tannase in calcium alginate beads and its application in the treatment of boldo (Peumus boldus) tea. Int. J. Biol. Macromol. 2018, 118, 1989–1994. [Google Scholar] [CrossRef]
- Cabrera, M.P.; da Fonseca, T.F.; de Souza, R.V.B.; de Assis, C.R.D.; Marcatoma, J.Q.; Maciel, J.D.C.; Neri, D.F.M.; Soria, F.; de Carvalho, L.B., Jr. Polyaniline-coated magnetic diatomite nanoparticles as a matrix for immobilizing enzymes. Appl. Surf. Sci. 2018, 457, 21–29. [Google Scholar] [CrossRef]
- de Lima, J.S.; Cabrera, M.P.; Motta, C.M.D.S.; Converti, A.; Carvalho, L.B., Jr. Hydrolysis of tannins by tannase immobilized onto magnetic diatomaceous earth nanoparticles coated with polyaniline. Food Res. Int. 2018, 107, 470–476. [Google Scholar] [CrossRef]
- Gao, X.; Pan, H.; Tian, S.; Su, L.; Hu, Z.; Qiao, C.; Liu, Q.; Zhou, C. Co-immobilization of bienzyme HRP/GOx on highly stable hierarchically porous MOF with enhanced catalytic activity and stability: Kinetic and thermodynamic studies. J. Environ. Chem. Eng. 2023, 11, 110684. [Google Scholar] [CrossRef]
- Wahba, M.I.; Saleh, S.A.A.; Mostafa, F.A.; Abdel Wahab, W.A. Immobilization impact of GEG-Alg-SPI as a carrier for Aspergillus niger MK981235 inulinase: Kinetics, thermodynamics, and application. Bioresour. Technol. Rep. 2022, 18, 101099. [Google Scholar] [CrossRef]
- De Oliveira, R.L.; da Silva, M.F.; da Silva, S.P.; Cavalcanti, J.V.F.L.; Converti, A.; Porto, T.S. Immobilization of a commercial Aspergillus aculeatus enzyme preparation with fructosyltransferase activity in chitosan beads: A kinetic/thermodynamic study and fructo-oligosaccharides continuous production in enzymatic reactor. Food Bioprod. Process. 2020, 122, 169–182. [Google Scholar] [CrossRef]
- De Oliveira, R.L.; da Silva, S.P.; Converti, A.; Porto, T.S. Production, biochemical characterization, and kinetic/thermodynamic study of inulinase from Aspergillus terreus URM4658. Molecules 2022, 27, 6418. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.L.; Claudino, E.S.; Converti, A.; Porto, T.S. Use of a sequential fermentation method for the production of Aspergillus tamarii URM4634 protease and a kinetic/thermodynamic study of the enzyme. Catalysts 2021, 11, 963. [Google Scholar] [CrossRef]
- Fernandes, L.M.G.; Carneiro-da-Cunha, M.N.; Silva, J.D.C.; Porto, A.L.F.; Porto, T.S. Purification and characterization of a novel Aspergillus heteromorphus URM 0269 protease extracted by aqueous two-phase systems PEG/citrate. J. Mol. Liq. 2020, 317, 113957. [Google Scholar] [CrossRef]
- Boudrant, J.; Woodley, J.M.; Fernandez-Lafuente, R. Parameters necessary to define an immobilized enzyme preparation. Process Biochem. 2020, 90, 66–80. [Google Scholar] [CrossRef]
- Abellanas-Perez, P.; Carballares, D.; Fernandez-Lafuente, R.; Rocha-Martin, J. Glutaraldehyde modification of lipases immobilized on octyl agarose beads: Roles of the support enzyme loading and chemical amination of the enzyme on the final enzyme features. Int. J. Biol. Macromol. 2023, 248, 125853. [Google Scholar] [CrossRef] [PubMed]
- Heidtmann, R.B.; Duarte, S.H.; de Pereira, L.P.; Braga, A.R.C.; Kalil, S.J. Caracterização cinética e termodinâmica de β-galactosidase de Kluyveromyces marxianus CCT 7082 fracionada com sulfato de amônio. Braz. J. Food Technol. 2012, 15, 41–49. [Google Scholar] [CrossRef]
- Khatun, S.; Riyazuddeen; Yasmeen, S. Unraveling the thermodynamics, enzyme activity and denaturation studies of triprolidine hydrochloride binding with model transport protein. J. Mol. Liq. 2021, 337, 116569. [Google Scholar] [CrossRef]
- Rodríguez-López, J.N.; Fenoll, L.G.; Tudela, J.; Devece, C.; Sánchez-Hernández, D.; de los Reyes, E.; García-Cánovas, F. Thermal Inactivation of Mushroom Polyphenoloxidase Employing 2450 MHz Microwave Radiation. J. Agric. Food Chem. 1999, 47, 3028–3035. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Pinto, G.A.S.; Couri, S.; Gonçalves, E.B. Replacement of methanol by ethanol on gallic acid determination by rhodanine and its impacts on the tannase assay. Electron. J. Environ. Agric. Food Chem. 2006, 5, 1560–1568. [Google Scholar]
- Da Silva, O.S.; Silva, J.D.C.; de Almeida, E.M.; Sousa, F.; Gonçalves, O.S.L.; Sarmento, B.; Neves-Petersen, M.T.; Porto, T.S. Biophysical, photochemical and biochemical characterization of a protease from Aspergillus tamarii URM4634. Int. J. Biol. Macromol. 2018, 118, 1655–1666. [Google Scholar] [CrossRef]
- Michel, D. Simply conceiving the Arrhenius law and absolute kinetic constants using the geometric distribution. Phys. A Stat. Mech. Appl. 2013, 392, 4258–4264. [Google Scholar] [CrossRef][Green Version]
- Converti, A.; Pessoa, A., Jr.; Silva, J.D.C.; de Oliveira, R.L.; Porto, T.S. Thermodynamics applied to biomolecules. In Pharmaceutical Biotechnology: A Focus on Industrial Application; Pessoa, A., Jr., Vitolo, M., Long, P.F., Eds.; CRC Press: Boca Raton, FL, USA, 2021; Volume 1, pp. 29–42. ISBN 9781000399844. [Google Scholar]
- Ortega, N.; de Diego, S.; Perez-Mateos, M.; Busto, M.D. Kinetic properties and thermal behaviour of polygalacturonase used in fruit juice clarification. Food Chem. 2004, 88, 209–217. [Google Scholar] [CrossRef]
| Temperature (°C) | kd 1 (min−1) | R2 | ΔHd 2 (kJ/mol) | ΔGd 3 (kJ/mol) | ΔSd 4 (J/(K·mol)) | t1/2 5 (min) | D-Value 6 (min) |
|---|---|---|---|---|---|---|---|
| 30 | 0.0021 | 0.98 | 50.99 | 100.15 | −162.15 | 330.07 | 1096.67 |
| 40 | 0.0045 | 0.99 | 50.91 | 101.55 | −161.72 | 154.03 | 511.78 |
| 50 | 0.0074 | 0.98 | 50.82 | 103.54 | −163.13 | 93.67 | 311.22 |
| Temperature (°C) | kd 1 (min−1) | R2 | ΔHd 2 (kJ/mol) | ΔGd 3 (kJ/mol) | ΔSd 4 (J/(K·mol)) | t1/2 5 (min) | D-Value 6 (min) |
|---|---|---|---|---|---|---|---|
| 30 | 0.0030 | 0.99 | 41.33 | 99.25 | −191.04 | 231.05 | 767.67 |
| 40 | 0.0054 | 0.96 | 41.25 | 101.08 | −191.05 | 128.36 | 426.48 |
| 50 | 0.0088 | 0.99 | 41.17 | 103.08 | −191.58 | 78.77 | 261.70 |
| Temperature (°C) | kL (min−1) | R2 | kd (min−1) | R2 |
|---|---|---|---|---|
| 20 | 0.079 | 0.96 | 0.0043 | 0.85 |
| 30 | 0.0808 | 0.99 | 0.0040 | 0.99 |
| 40 | 0.087 | 0.96 | 0.0035 | 0.93 |
| 50 | 0.0879 | 0.95 | 0.0024 | 0.95 |
| 60 | 0.070 | 0.91 | 0.0023 | 0.87 |
| 70 | 0.0491 | 0.97 | 0.0068 | 0.96 |
| Temperature (°C) | ΔG°L (kJ/mol) | ΔG°d (kJ/mol) | ΔG°RT (kJ/mol) | ΔH°L 1 (kJ/mol) | ΔH°d (kJ/mol) | ΔH°RT (kJ/mol) | ΔS°L (J/(K·mol)) | ΔS°d (J/(K·mol)) | ΔS°RT (J/(K·mol)) |
|---|---|---|---|---|---|---|---|---|---|
| 20 | −87.9 | −95.0 | 7.1 | 29.9 | 14.3 | 15.6 | 401.9 | 372.9 | 29.0 |
| 30 | −90.9 | −98.5 | 7.6 | 29.9 | 14.3 | 15.6 | 398.6 | 372.2 | 26.4 |
| 40 | −93.8 | −102.2 | 8.4 | 29.9 | 14.3 | 15.6 | 395.1 | 372.1 | 23.0 |
| 50 | −96.9 | −106.6 | 9.7 | 29.9 | 14.3 | 15.6 | 392.3 | 374.1 | 18.2 |
| 60 | −100.6 | −110.1 | 9.5 | 29.9 | 14.3 | 15.6 | 391.7 | 373.3 | 18.4 |
| 70 | −104.7 | −110.4 | 5.6 | 29.9 | 14.3 | 15.6 | 392.3 | 363.3 | 29.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Carvalho-Silva, J.; da Silva, M.F.; de Lima, J.S.; Porto, T.S.; de Carvalho, L.B., Jr.; Converti, A. Thermodynamic and Kinetic Investigation on Aspergillus ficuum Tannase Immobilized in Calcium Alginate Beads and Magnetic Nanoparticles. Catalysts 2023, 13, 1304. https://doi.org/10.3390/catal13091304
de Carvalho-Silva J, da Silva MF, de Lima JS, Porto TS, de Carvalho LB Jr., Converti A. Thermodynamic and Kinetic Investigation on Aspergillus ficuum Tannase Immobilized in Calcium Alginate Beads and Magnetic Nanoparticles. Catalysts. 2023; 13(9):1304. https://doi.org/10.3390/catal13091304
Chicago/Turabian Stylede Carvalho-Silva, Jônatas, Milena Fernandes da Silva, Juliana Silva de Lima, Tatiana Souza Porto, Luiz Bezerra de Carvalho, Jr., and Attilio Converti. 2023. "Thermodynamic and Kinetic Investigation on Aspergillus ficuum Tannase Immobilized in Calcium Alginate Beads and Magnetic Nanoparticles" Catalysts 13, no. 9: 1304. https://doi.org/10.3390/catal13091304
APA Stylede Carvalho-Silva, J., da Silva, M. F., de Lima, J. S., Porto, T. S., de Carvalho, L. B., Jr., & Converti, A. (2023). Thermodynamic and Kinetic Investigation on Aspergillus ficuum Tannase Immobilized in Calcium Alginate Beads and Magnetic Nanoparticles. Catalysts, 13(9), 1304. https://doi.org/10.3390/catal13091304









