Assessing the Efficacy of A Mo2C/Peroxydisulfate System for Tertiary Wastewater Treatment: A Study of Losartan Degradation, E. coli Inactivation, and Synergistic Effects
Abstract
:1. Introduction
2. Results and Discussion
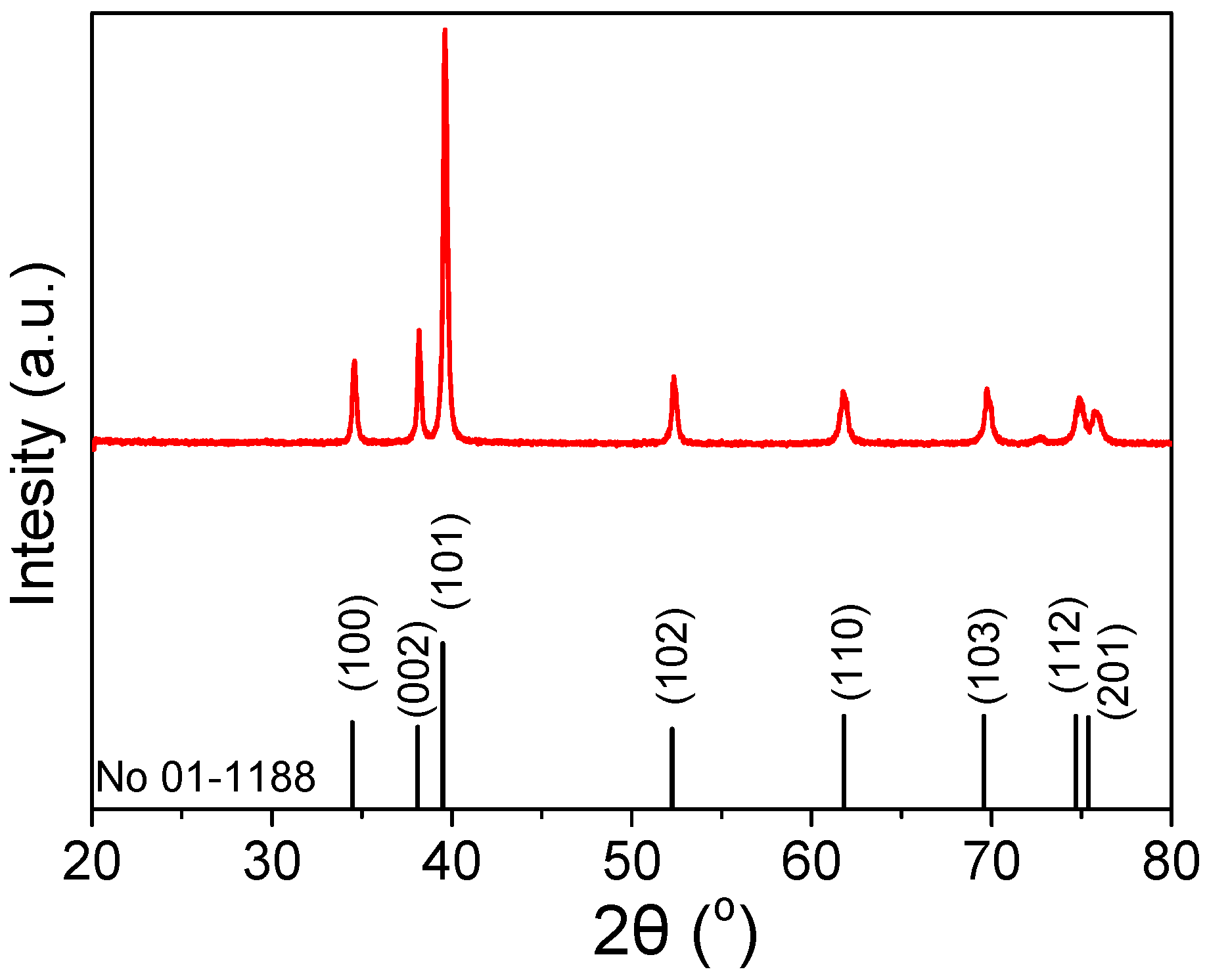
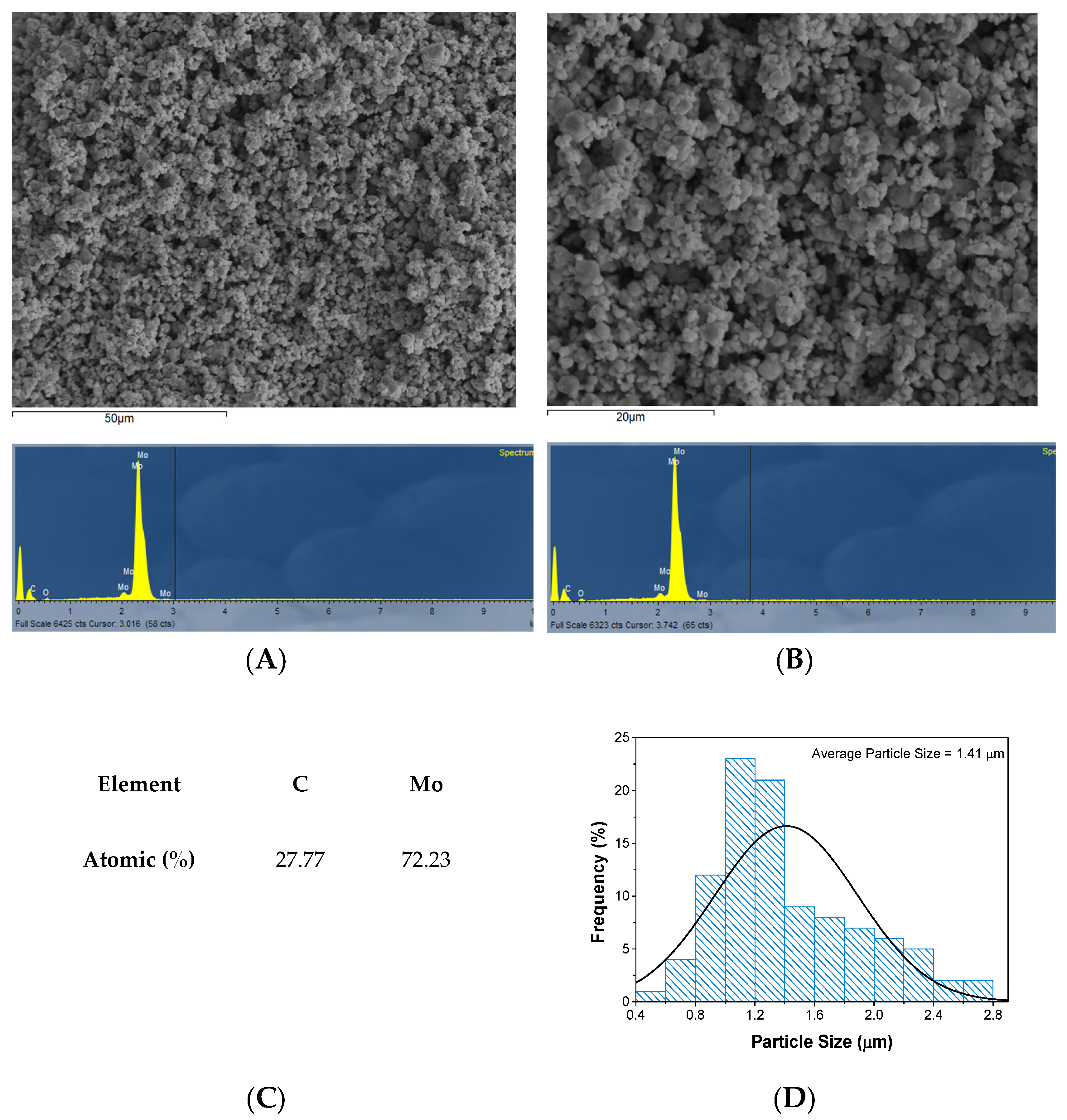
2.1. Mo2C Characterization
2.2. Catalytic Results
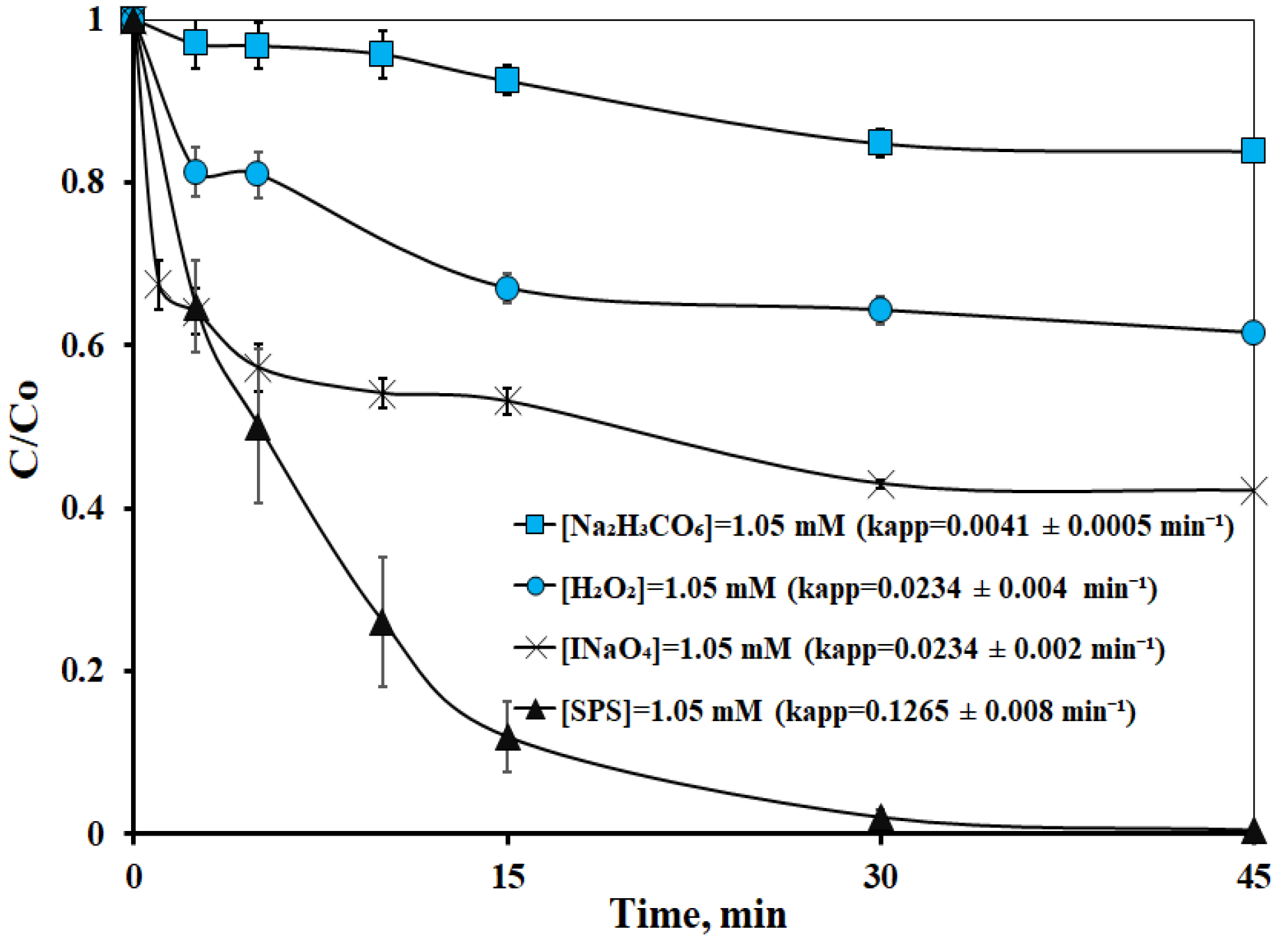
2.2.1. Activation of Various Types of Oxidants
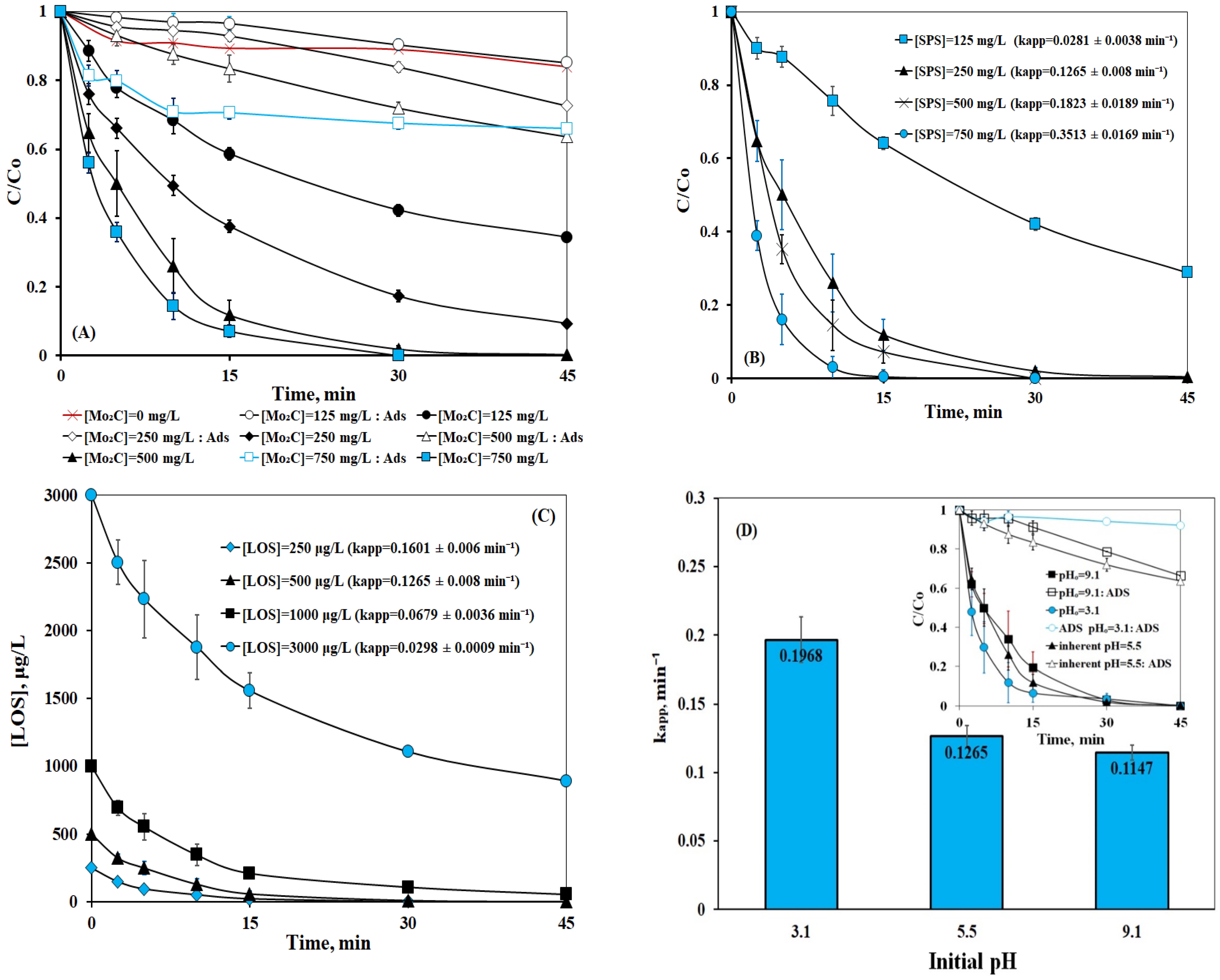
2.2.2. Effect of Operating Parameters (Initial Concentration of Catalyst, Persulfate, Losartan, and pH)
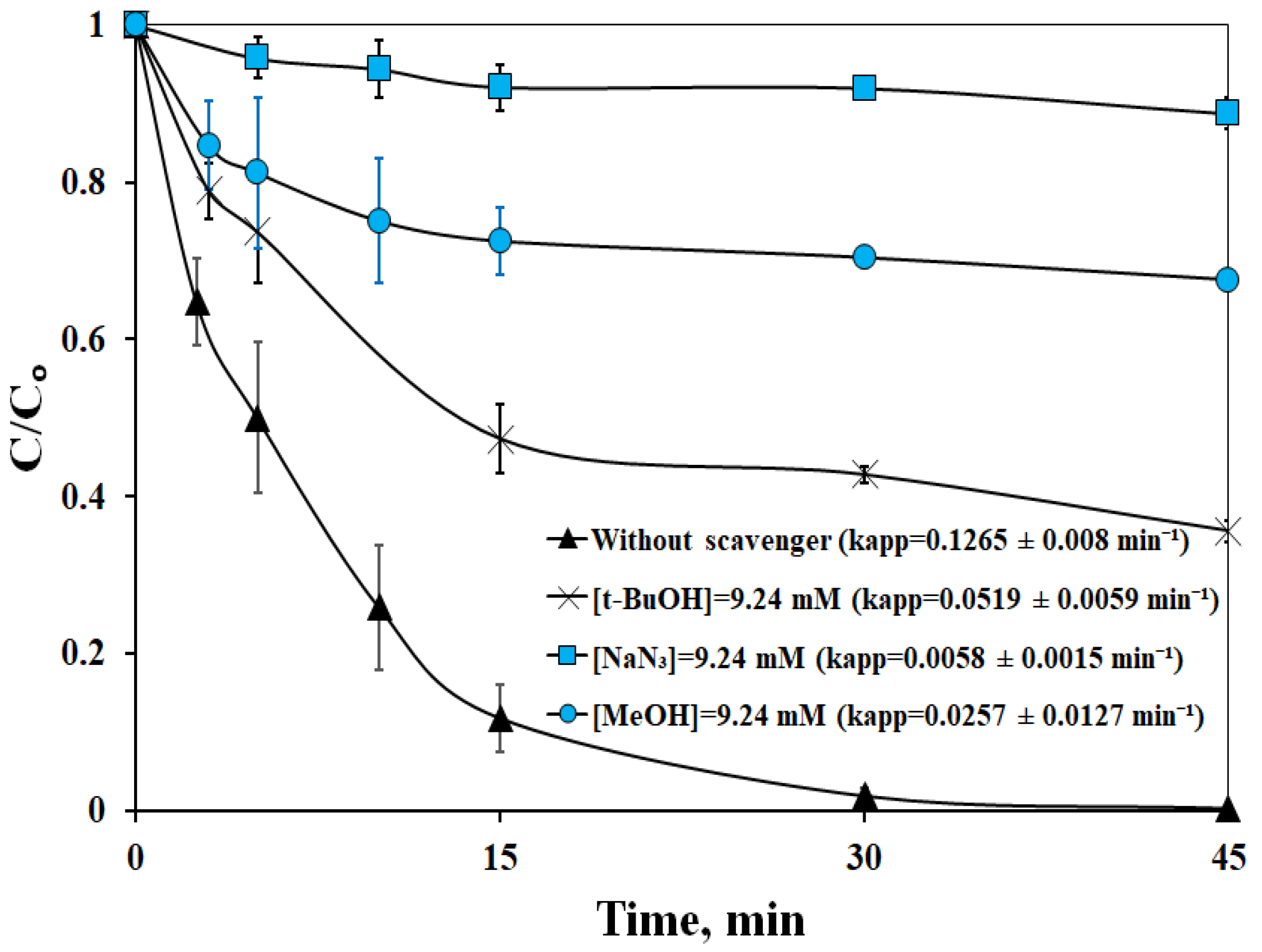
2.2.3. Effect of Scavengers
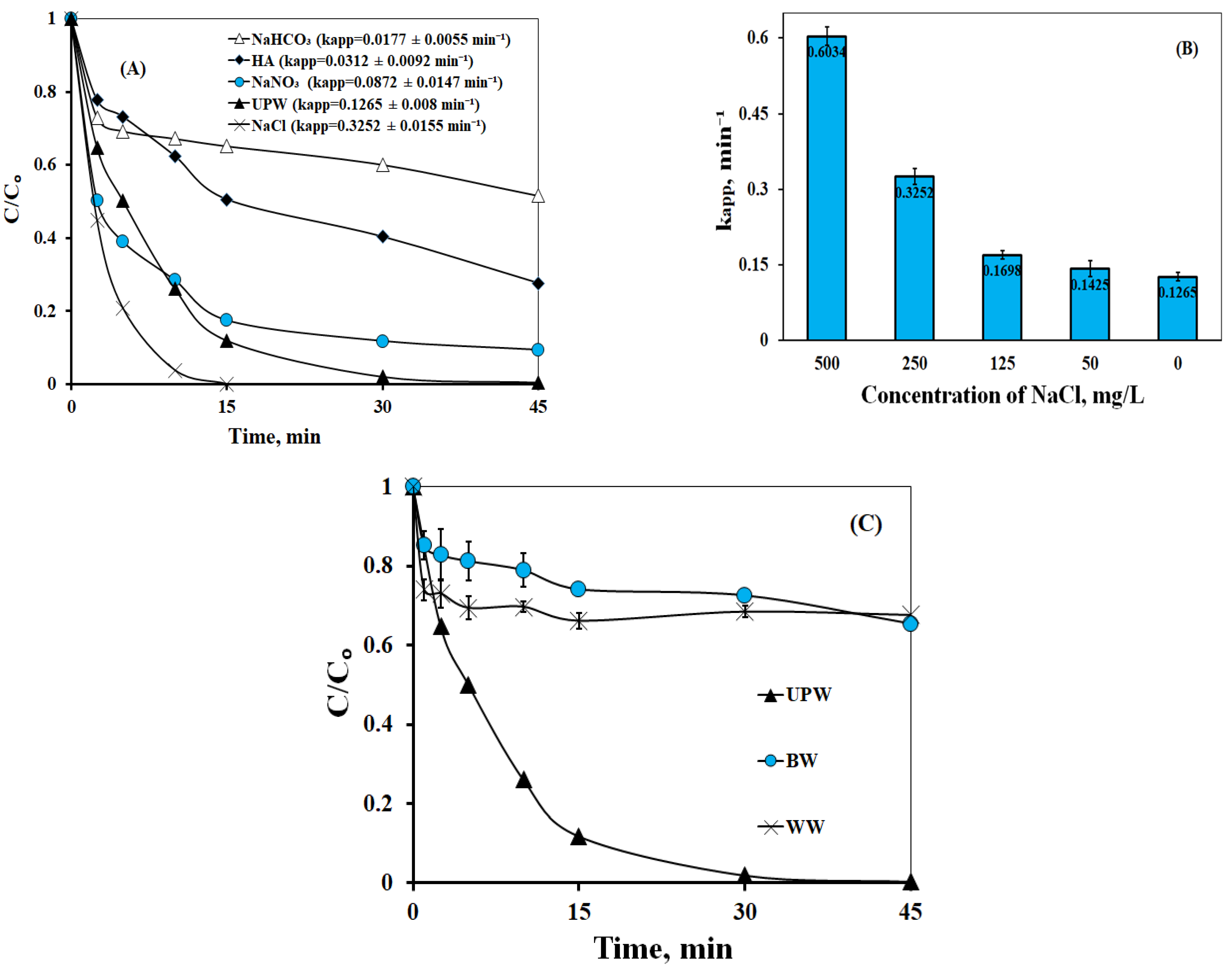
2.2.4. Effect of Water Matrix
2.2.5. Reuse
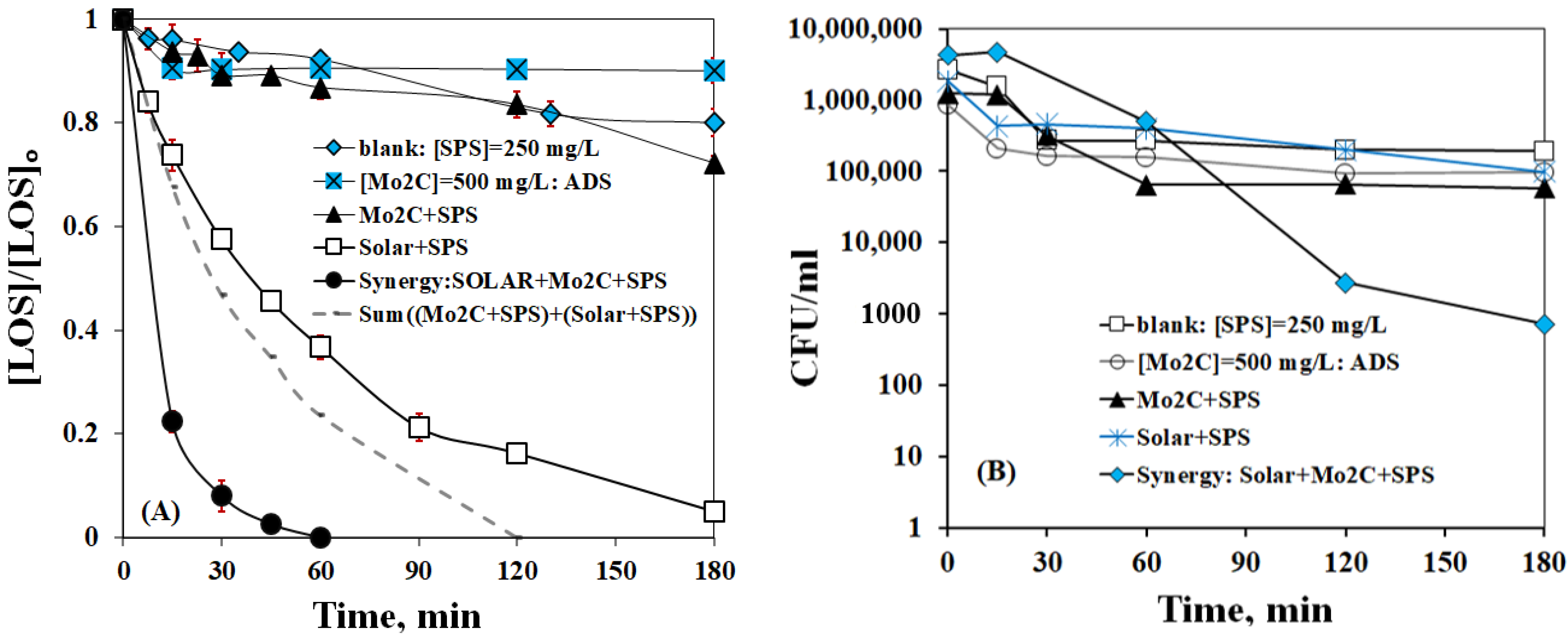
2.3. Disinfection and Synergistic Effects with Simulated Solar Irradiation
3. Materials and Methods
3.1. Materials
3.2. Procedure of Degradation Experiments
3.3. Experimental Procedure of Disinfection
3.4. Analytical Methods
3.5. Mo2C Characterization
3.6. Data Analysis
4. Conclusions
- -
- The investigated system was capable of eliminating 500 μg/L of LOS in less than 45 min. This concentration is well over the upper limit typically found in surface waters and/or secondary treated effluents, which implies that milder treatment conditions would suffice to deal with environmentally relevant concentrations.
- -
- Oxidation adhered to pseudo-first-order kinetics, and the apparent kinetic constant decreased with increasing LOS concentration and increased in acidic pH.
- -
- The presence of organic matter and carbonate impeded LOS degradation in the experiments conducted in secondary effluent and bottled water; moreover, the presence of E. coli slowed down LOS decomposition, while E. coli removal was only 1.23 log after 180 min. This clearly highlights the critical role of the environmentally relevant water matrix.
- -
- Sulfate radicals, hydroxyl radicals, and singlet oxygen participated in LOS destruction, but singlet oxygen emerged as the predominant species. In this respect, the possible interplay between catalyst, oxidants, and the target and non-target species may be quite complicated.
- -
- Experiments conducted in the presence of simulated solar irradiation demonstrated a significant synergy and efficiency improvement, leading to complete LOS elimination in 60 min and nearly a 4-log reduction of E. coli in 180 min. This implies that process coupling may be a step in the right direction in terms of enhancing treatment efficiency; nonetheless, this should be complemented by cost-efficient strategies that can be offered by the application of renewable energy sources.
- -
- Though the use of 2D materials in environmental remediation appears as a promising strategy, further research is needed to examine the operation of similar systems under continuous flow, and scaling up is required. Future work must also delve deeper into the mechanism and transformation products, as well as investigate toxicity and Mo leaching during the treatment.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ekramul Mahmud, H.N.M.; Huq, A.K.O.; Yahya, R.B. The removal of heavy metal ions from wastewater/aqueous solution using polypyrrole-based adsorbents: A review. RSC Adv. 2016, 6, 14778–14791. [Google Scholar] [CrossRef]
- Priya, A.K.; Gnanasekaran, L.; Rajendran, S.; Qin, J.; Vasseghian, Y. Occurrences and removal of pharmaceutical and personal care products from aquatic systems using advanced treatment- A review. Environ. Res. 2022, 204, 112298. [Google Scholar] [CrossRef]
- Asghar, M.A.; Zhu, Q.; Sun, S.; Peng, Y.; Shuai, Q. Suspect screening and target quantification of human pharmaceutical residues in the surface water of Wuhan, China, using UHPLC-Q-Orbitrap HRMS. Sci. Total Environ. 2018, 635, 828–837. [Google Scholar] [CrossRef]
- Busch, W.; Schmidt, S.; Kühne, R.; Schulze, T.; Krauss, M.; Altenburger, R. Micropollutants in European rivers: A mode of action survey to support the development of effect-based tools for water monitoring: Micropollutants in European rivers: A mode-of-action. Environ. Toxicol. Chem. 2016, 35, 1887–1899. [Google Scholar] [CrossRef] [PubMed]
- Kane, S.P. Valsartan, ClinCalc DrugStats Database, Version 2022.08. ClinCalc. Available online: https://clincalc.com/DrugStats/Drugs/Valsartan (accessed on 29 July 2023).
- Statista: Number of Losartan Potassium Prescriptions in the U.S. from 2004 to 2020 (in Million). Available online: https://www.statista.com/statistics/781681/losartan-potassium-prescriptions-number-in-the-us/ (accessed on 29 July 2023).
- Centers for Disease Control and Prevention. Hypertension Cascade: Hypertension Prevalence, Treatment and Control Estimates Among U.S. Adults Aged 18 Years and Older Applying the Criteria from the American College of Cardiology and American Heart Association’s 2017 Hypertension Guideline—NHANES 2017–2020. Atlanta, GA: 12 May 2023. Available online: https://www.cdc.gov/bloodpressure/facts.htm#:~:text=This%20includes%2037%20million%20U.S.%20adults.&text=About%2034%20million%20adults%20who,140%2F90%20mmHg%20or%20higher (accessed on 6 July 2023).
- Rouette, J.; McDonald, E.G.; Schuster, T.; Brophy, J.M.; Azoulay, L. Treatment and prescribing trends of antihypertensive drugs in 2.7 million UK primary care patients over 31 years: A population-based cohort study. BMJ Open 2022, 12, e057510. [Google Scholar] [CrossRef] [PubMed]
- Cortez, F.S.; Souza, L.S.; Guimarães, L.L.; Almeida, J.E.; Pusceddu, F.H.; Maranho, L.A.; Mota, L.G.; Nobre, C.R.; Moreno, B.B.; Abessa, D.M.S.; et al. Ecotoxicological effects of losartan on the brown mussel Perna perna and its occurrence in seawater from Santos Bay (Brazil). Sci. Total Environ. 2018, 637–638, 1363–1371. [Google Scholar] [CrossRef]
- Mandaric, L.; Diamantini, E.; Stella, E.; Cano-Paoli, K.; Valle-Sistac, J.; Molins- Delgado, D.; Bellin, A.; Chiogna, G.; Majone, B.; Diaz-Cruz, M.S.; et al. Contamination sources and distribution patterns of pharmaceuticals and personal care products in Alpine rivers strongly affected by tourism. Sci. Total Environ. 2017, 590–591, 484–494. [Google Scholar] [CrossRef]
- Nuel, M.; Laurent, J.; Bois, P.; Heintz, D.; Wanko, A. Seasonal and ageing effect on the behaviour of 86 drugs in a full-scale surface treatment wetland: Removal efficiencies and distribution in plants and sediments. Sci. Total Environ. 2018, 615, 1099–1109. [Google Scholar] [CrossRef]
- Ashfaq, M.; Li, Y.; Wang, Y.; Chen, W.; Wang, H.; Chen, X.; Wu, W.; Huang, Z.; Yu, C.-P.; Sun, Q. Occurrence, fate, and mass balance of different classes of pharmaceuticals and personal care products in an anaerobic-anoxic-oxic wastewater treatment plant in Xiamen, China. Water Res. 2017, 123, 655–667. [Google Scholar] [CrossRef]
- Botero-Coy, A.M.; Martínez-Pachon, D.; Boix, C.; Rincon, R.J.; Castillo, N.; Arias- Marín, L.P.; Manrique-Losada, L.; Torres-Palma, R.; Moncayo-Lasso, A.; Hernandez, F. An investigation into the occurrence and removal of pharmaceuticals in Colombian wastewater’. Sci. Total Environ. 2018, 642, 842–853. [Google Scholar] [CrossRef]
- Castro, G.; Rodríguez, I.; Ramil, M.; Cela, R. Selective determination of sartan drugs in environmental water samples by mixed-mode solid-phase extraction and liquid chromatography tandem mass spectrometry. Chemosphere 2019, 224, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Isaza-Pineda, L.; Moncayo-Lasso, A.; Hernández, F.; Ibáñez, M.; Torres-Palma, R.A. Comparative degradation of two highly consumed antihypertensives in water by sonochemical process. Determination of the reaction zone, primary degradation products and theoretical calculations on the oxidative process. Ultrason. Sonochem. 2019, 58, 104635. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Dulova, N. UV-assisted chemical oxidation of antihypertensive losartan in water. J. Environ. Manag. 2020, 261, 110170. [Google Scholar] [CrossRef]
- Matsoukas, J.; Apostolopoulos, V.; Zulli, A.; Moore, G.; Kelaidonis, K.; Moschovou, K.; Mavromoustakos, T. From Angiotensin II to Cyclic Peptides and Angiotensin Receptor Blockers (ARBs): Perspectives of ARBs in COVID-19 Therapy. Molecules 2021, 26, 618. [Google Scholar] [CrossRef]
- Gurke, R.; R¨oßler, M.; Marx, C.; Diamond, S.; Schubert, S.; Oertel, R.; Fauler, J. Occurrence and removal of frequently prescribed pharmaceuticals and corresponding metabolites in wastewater of a sewage treatment plant. Sci. Total Environ. 2015, 532, 762–770. [Google Scholar] [CrossRef]
- Ladhari, A.; La Mura, G.; Di Marino, C.; Di Fabio, G.; Zarrelli, A. Sartans: What they are for, how they degrade, where they are found and how they transform. Sustain. Chem. Pharm. 2021, 20, 100409. [Google Scholar] [CrossRef]
- Osorio, V.; Larrañaga, A.; Aceña, J.; P’erez, S.; Barcel’o, D. Concentration and risk of pharmaceuticals in freshwater systems are related to the population density and the livestock units in Iberian Rivers. Sci. Total Environ. 2016, 540, 267–277. [Google Scholar] [CrossRef]
- Adams, E.; Neves, B.B.; Prola, L.D.T.; De Liz, M.V.; Martins, L.R.R.; Ramsdorf, W.A.; De Freitas, A.M. Ecotoxicity and genotoxicity assessment of losartan after UV/H2O2 and UVC/photolysis treatments. Environ. Sci. Pollut. Res. 2021, 28, 23812–23821. [Google Scholar] [CrossRef]
- Czekalski, N.; Imminger, S.; Salhi, E.; Veljkovic, M.; Kleffel, K.; Drissner, D.; Hammes, F.; Bürgmann, H.; Von Gunten, U. Inactivation of Antibiotic Resistant Bacteria and Resistance Genes by Ozone: From Laboratory Experiments to Full-Scale Wastewater Treatment. Environ. Sci. Technol. 2016, 50, 11862–11871. [Google Scholar] [CrossRef]
- Iakovides, I.C.; Michael-Kordatou, I.; Moreira, N.F.F.; Ribeiro, A.R.; Fernandes, T.; Pereira, M.F.R.; Nunes, O.C.; Manaia, C.M.; Silva, A.M.T.; Fatta-Kassinos, D. Continuous ozonation of urban wastewater: Removal of antibiotics, antibiotic-resistant Escherichia coli and antibiotic resistance genes and phytotoxicity. Water Res. 2019, 159, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Nnadozie, C.F.; Odume, O.N. Freshwater environments as reservoirs of antibiotic resistant bacteria and their role in the dissemination of antibiotic resistance genes. Environ. Pollut. 2019, 254, 113067. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, P.; Venieri, D.; Mantzavinos, D. Advanced Oxidation Processes for Water and Wastewater Viral Disinfection. A Systematic Review. Food Environ. Virol. 2021, 13, 283–302. [Google Scholar] [CrossRef] [PubMed]
- García-Espinoza, J.D.; Robles, I.; Durán-Moreno, A.; Godínez, L.A. Photo-assisted electrochemical advanced oxidation processes for the disinfection of aqueous solutions: A review. Chemosphere 2021, 274, 129957. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, X.; Zhou, X.; Wang, R.; Wang, S.; Ren, N.; Ho, S.-H. Advanced oxidation processes for water disinfection: Features, mechanisms and prospects. J. Chem. Eng. 2021, 409, 128207. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef]
- Chen, X.; Gudda, F.O.; Hu, X.; Waigi, M.G.; Gao, Y. Degradation of bisphenol A in an oxidation system constructed from Mo2C MXene and peroxymonosulfate. npj Clean Water 2022, 5, 66. [Google Scholar] [CrossRef]
- Guo, S.; Wang, H.; Yang, W.; Fida, H.; You, L.; Zhou, K. Scalable synthesis of Ca-doped α-Fe2O3 with abundant oxygen vacancies for enhanced degradation of organic pollutants through peroxymonosulfate activation. Appl. Catal. B 2020, 262, 118250. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Forouzesh, M.; Ebadi, A.; Aghaeinejad-Meybodi, A. Degradation of metronidazole antibiotic in aqueous medium using activated carbon as a persulfate activator. Sep. Purif. Technol. 2019, 210, 145–151. [Google Scholar] [CrossRef]
- Bekris, L.; Frontistis, Z.; Trakakis, G.; Sygellou, L.; Galiotis, C.; Mantzavinos, D. Graphene: A new activator of sodium persulfate for the advanced oxidation of parabens in water. Water Res. 2017, 126, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lai, L.; Wan, Y.; He, Y.; Yao, G.; Lai, B. Molybdenum disulfide (MoS2): A versatile activator of both peroxymonosulfate and persulfate for the degradation of carbamazepine. Chem. Eng. J. 2020, 384, 123264. [Google Scholar] [CrossRef]
- Alaba, P.A.; Abbas, A.; Huang, J.; Daud, W.M.A.W. Molybdenum carbide nanoparticle: Understanding the surface properties and reaction mechanism for energy production towards a sustainable future. Renew. Sust. Energ. Rev. 2018, 91, 287–300. [Google Scholar] [CrossRef]
- Yang, L.; Chen, H.; Jia, F.; Peng, W.; Tian, X.; Xia, L.; Wu, X.; Song, S. Emerging Hexagonal Mo2C Nanosheet with (002) Facet Exposure and Cu Incorporation for Peroxymonosulfate Activation Toward Antibiotic Degradation. ACS Appl. Mater. Interfaces 2021, 13, 14342–14354. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Liang, Y.; Yang, B.; Jia, F.; Song, S. In Situ Reduction of Au(I) for Efficient Recovery of Gold from Thiosulfate Solution by the 3D MoS2/Chitosan Aerogel. ACS Sustain. Chem. Eng. 2020, 8, 3673–3680. [Google Scholar] [CrossRef]
- Zhu, L.; Ji, J.; Liu, J.; Mine, S.; Matsuoka, M.; Zhang, J.; Xing, M. Designing 3D-MoS2 Sponge as Excellent Cocatalysts in Advanced Oxidation Processes for Pollutant Control. Angew. Chem. Int. Ed. 2020, 59, 13968–13976. [Google Scholar] [CrossRef]
- Ji, J.; Aleisa, R.M.; Duan, H.; Zhang, J.; Yin, Y.; Xing, M. Metallic Active Sites on MoO2(110) Surface to Catalyze Advanced Oxidation Processes for Efficient Pollutant Removal. Iscience 2020, 23, 100861. [Google Scholar] [CrossRef]
- Bao, Y.; Chen, T.; Zhu, Z.; Zhang, H.; Qiu, Y.; Yin, D. Mo2C/C catalyst as efficient peroxymonosulfate activator for carbamazepine degradation. Chemosphere 2022, 287, 132047. [Google Scholar] [CrossRef]
- De Andrade, J.R.; Vieira, M.G.A.; Da Silva, M.G.C.; Wang, S. Oxidative degradation of pharmaceutical losartan potassium with N-doped hierarchical porous carbon and peroxymonosulfate. J. Chem. Eng. 2020, 382, 122971. [Google Scholar] [CrossRef]
- Salazar, C.; Contreras, N.; Mansilla, H.D.; Yáñez, J.; Salazar, R. Electrochemical degradation of the antihypertensive losartan in aqueous medium by electro-oxidation with boron-doped diamond electrode. J. Hazard. Mater. 2016, 319, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Guateque-Londoño, J.F.; Serna-Galvis, E.A.; Ávila-Torres, Y.; Torres-Palma, R.A. Degradation of losartan in fresh urine by sonochemical and photochemical advanced oxidation processes. Water 2020, 12, 3398. [Google Scholar] [CrossRef]
- Martínez-Pach’on, D.; Espinosa-Barrera, P.; Rinc’on-Ortíz, J.; Moncayo-Lasso, A. Advanced oxidation of antihypertensives losartan and valsartan by photo-electro- Fenton at near-neutral pH using natural organic acids and a dimensional stable anode-gas diffusion electrode (DSA-GDE) system under light emission diode (LED) lighting. Environ. Sci. Pollut. Res. 2019, 26, 4426–4437. [Google Scholar] [CrossRef]
- Ioannidi, A.; Arvaniti, O.S.; Nika, M.-C.; Aalizadeh, R.; Thomaidis, N.S.; Mantzavinos, D.; Frontistis, Z. Removal of drug losartan in environmental aquatic matrices by heat-activated persulfate: Kinetics, transformation products and synergistic effects. Chemosphere 2022, 287, 131952. [Google Scholar] [CrossRef] [PubMed]
- Ioannidi, A.A.; Vakros, J.; Frontistis, Z.; Mantzavinos, D. Tailoring the Biochar Physicochemical Properties Using a Friendly Eco-Method and Its Application on the Oxidation of the Drug Losartan through Persulfate Activation. Catalysts 2022, 12, 1245. [Google Scholar] [CrossRef]
- García, G.; Guillén-Villafuerte, O.; Rodríguez, J.L.; Arévalo, M.C.; Pastor, E. Electrocatalysis on metal carbide materials. Int. J. Hydrog. Energy. 2016, 41, 19664–19673. [Google Scholar] [CrossRef]
- Ma, L.; Ting, L.R.L.; Molinari, V.; Giordano, C.; Yeo, B.S. Efficient hydrogen evolution reaction catalyzed by molybdenum carbide and molybdenum nitride nanocatalysts synthesized via the urea glass route. J. Mater. Chem. A 2015, 3, 8361–8368. [Google Scholar] [CrossRef]
- García, G.; Roca-Ayats, M.; Guillén-Villafuerte, O.; Rodríguez, J.L.; Arévalo, M.C.; Pastor, E. Electrochemical performance of α-Mo2C as catalyst for the hydrogen evolution reaction. J. Electroanal. Chem. 2017, 793, 235–241. [Google Scholar] [CrossRef]
- Kim, J.; Lee, D.H.; Yang, Y.; Chen, K.; Liu, C.; Kang, J.; Li, O.L. Hybrid Molybdenum Carbide/Heteroatom-Doped Carbon Electrocatalyst for Advanced Oxygen Evolution Reaction in Hydrogen Production. Catalysts 2020, 10, 1290. [Google Scholar] [CrossRef]
- Han, M.; Wang, H.; Jin, W.; Chu, W.; Xu, Z. The performance and mechanism of iron-mediated chemical oxidation: Advances in hydrogen peroxide, persulfate and percarbonate oxidation. J. Environ. Sci. 2023, 128, 181–202. [Google Scholar] [CrossRef]
- Lee, C.; Yoon, J. Application of photoactivated periodate to the decolorization of reactive dye: Reaction parameters and mechanism. J. Photochem. Photobiol., A 2004, 165, 35–41. [Google Scholar] [CrossRef]
- Ioannidi, A.; Frontistis, Z.; Mantzavinos, D. Destruction of propyl paraben by persulfate activated with UV-A light emitting diodes. J. Environ. Chem. Eng. 2018, 6, 2992–2997. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Botero-Coy, A.M.; Rosero-Moreano, M.; Lee, J.; Hernández, F.; Torres-Palma, R.A. An Initial Approach to the Presence of Pharmaceuticals in Wastewater from Hospitals in Colombia and Their Environmental Risk. Water 2022, 14, 950. [Google Scholar] [CrossRef]
- Avramiotis, E.; Frontistis, Z.; Manariotis, I.D.; Vakros, J.; Mantzavinos, D. Oxidation of Sulfamethoxazole by Rice Husk Biochar-Activated Persulfate. Catalysts 2021, 11, 850. [Google Scholar] [CrossRef]
- Tang, H.; Zhao, Y.; Shan, S.; Yang, X.; Liu, D.; Cui, F.; Xing, B. Theoretical insight into the adsorption of aromatic compounds on graphene oxide. Environ. Sci. Nano 2018, 5, 2357–2367. [Google Scholar] [CrossRef]
- Mrozik, W.; Minofar, B.; Thongsamer, T.; Wiriyaphong, N.; Khawkomol, S.; Plaimart, J.; Vakros, J.; Karapanagioti, H.; Vinitnantharat, S.; Werner, D. Valorisation of agricultural waste derived biochars in aquaculture to remove organic micropollutants from water—Experimental study and molecular dynamics simulations. J. Environ. Manag. 2021, 300, 113717. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.; Lang, J.; Liao, Z.; Khan, A.; Ifthikar, J.; Lv, Z.; Long, S.; Chen, Z.; Chen, Z. Activation of persulfate by CuOx@Co-LDH: A novel heterogeneous system for contaminant degradation with broad pH window and controlled leaching. J. Chem. Eng. 2018, 335, 548–559. [Google Scholar] [CrossRef]
- Shahzad, A.; Ali, J.; Ifthikar, J.; Aregay, G.G.; Zhu, J.; Chen, Z.; Chen, Z. Non-radical PMS activation by the nanohybrid material with periodic confinement of reduced graphene oxide (rGO) and Cu hydroxides. J. Hazard. Mater. 2020, 392, 122316. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Li, G.; An, T.; Zhao, H.; Wong, P.K. Catalyst-free activation of persulfate by visible light for water disinfection: Efficiency and mechanisms. Water Res. 2019, 157, 106–118. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Li, G.; Wong, P.K.; An, T. Visible light activation of persulfate by magnetic hydrochar for bacterial inactivation: Efficiency, recyclability and mechanisms. Water Res. 2020, 176, 115746. [Google Scholar] [CrossRef]
- Dong, J.; Shi, Y.; Huang, C.; Wu, Q.; Zeng, T.; Yao, W. A New and stable Mo-Mo2C modified g-C3N4 photocatalyst for efficient visible light photocatalytic H2 production. Appl. Catal. B. 2019, 243, 27–35. [Google Scholar] [CrossRef]
- Cold Spring Harbor Protocols. Available online: http://cshprotocols.cshlp.org/content/2006/1/pdb.rec8141.full?text_only=true (accessed on 24 May 2023).
- Sanders, E.R. Aseptic Laboratory Techniques: Plating Methods. J. Vis. Exp. 2012, 63, e3064. [Google Scholar] [CrossRef]
- Venieri, D.; Karapa, A.; Panagiotopoulou, M.; Gounaki, I. Application of activated persulfate for the inactivation of fecal bacterial indicators in water. J. Environ. Manage. 2020, 261, 110223. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-Y.A.; Chen, Y.-C.; Lin, Y.-F. LaMO3 perovskites (M = Co, Cu, Fe and Ni) as heterogeneous catalysts for activating peroxymonosulfate in water. Chem. Eng. Sci 2017, 160, 96–105. [Google Scholar] [CrossRef]
- Gkika, C.; Petala, A.; Frontistis, Z.; Bampos, G.; Hela, D.; Konstantinou, I.; Mantzavinos, D. Heterogeneous activation of persulfate by lanthanum strontium cobaltite for sulfamethoxazole degradation. Catal. Today 2021, 361, 130–138. [Google Scholar] [CrossRef]
| Process | Conditions | LOS Removal | Reference |
|---|---|---|---|
| Electrochemical oxidation | [LOS]o = 377 μM, [Na2SO4] = 0.05 M, BDD anode, SS cathode, J = 80 mA/cm2, pH = 7 | 100% in 180 min (UPW) - | [44] |
| Ultrasound | [LOS]o = 40 μM, P = 88 W/L, pH = 6.5, 20 °C | 100% in 120 min (UPW) 100% in 120 min (hospital wastewater) | [16] |
| UVC/Fe2+/H2O2 | [LOS]o = 40 μM, [Fe2+] = 40 μM, [H2O2] = 400 μΜ, pH = 6.1, 23 °C | 76% TOC in 120 min (UPW) 30% TOC in 120 min (groundwater) | [17] |
| N-doped porous carbon and PMS | [LOS]o = 40 μM, [catalyst] = 26 mg/L, [PMS] = 6.5 mM, pH = 5.2, 25 °C | 100% (240 min) 96% in 240 min (20 mg/L humic acid) | [43] |
| UVC/H2O2 | [LOS]o = 43.4 μM, [H2O2] = 500 μM pH 6.1 | 65.7% in 20 min (distilled water) ≈27% in 20 min (fresh urine) | [45] |
| Photoelectron Fenton | [LOS]o = 45 μM, [Na2SO4] = 0.05 M, [Fe2+] = 36 μM, BDD anode, SS cathode, j = 5 mA/cm2, UVA = 1.4 W/m2, pH = 3 | ≈95% (30 min) - | [46] |
| Heat/US/SPS | [LOS]o = 1.18 μM, [SPS] = 200 μΜ, US = 36 W/L, 20 kHz, 50 °C, pH = 5.25 | 100% in 15 min (UPW) 60% in 45 min (WW) | [47] |
| Biochar from spent malt rootlet treated with acid and SPS | [LOS]o = 0.59 μM, [SPS] = 1.05 mΜ, [Acid-C] = 90 mg/L pH = 5.6, 25 °C | 91% in 90 min (UPW) 20% in 90 min (WW) | [48] |
| Mo2C and SPS | [LOS]o = 1.18 μM, [SPS] = 1.05 mM, [Mo2C] = 500 mg/L pH = 5.5, 25 °C | 100% in 45 min (UPW) 34% in 45 min (WW) | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioannidi, A.A.; Vlachodimitropoulou, M.; Frontistis, Z.; Petala, A.; Koutra, E.; Kornaros, M.; Mantzavinos, D. Assessing the Efficacy of A Mo2C/Peroxydisulfate System for Tertiary Wastewater Treatment: A Study of Losartan Degradation, E. coli Inactivation, and Synergistic Effects. Catalysts 2023, 13, 1285. https://doi.org/10.3390/catal13091285
Ioannidi AA, Vlachodimitropoulou M, Frontistis Z, Petala A, Koutra E, Kornaros M, Mantzavinos D. Assessing the Efficacy of A Mo2C/Peroxydisulfate System for Tertiary Wastewater Treatment: A Study of Losartan Degradation, E. coli Inactivation, and Synergistic Effects. Catalysts. 2023; 13(9):1285. https://doi.org/10.3390/catal13091285
Chicago/Turabian StyleIoannidi, Alexandra A., Maria Vlachodimitropoulou, Zacharias Frontistis, Athanasia Petala, Eleni Koutra, Michael Kornaros, and Dionissios Mantzavinos. 2023. "Assessing the Efficacy of A Mo2C/Peroxydisulfate System for Tertiary Wastewater Treatment: A Study of Losartan Degradation, E. coli Inactivation, and Synergistic Effects" Catalysts 13, no. 9: 1285. https://doi.org/10.3390/catal13091285
APA StyleIoannidi, A. A., Vlachodimitropoulou, M., Frontistis, Z., Petala, A., Koutra, E., Kornaros, M., & Mantzavinos, D. (2023). Assessing the Efficacy of A Mo2C/Peroxydisulfate System for Tertiary Wastewater Treatment: A Study of Losartan Degradation, E. coli Inactivation, and Synergistic Effects. Catalysts, 13(9), 1285. https://doi.org/10.3390/catal13091285