Construction of Supported MnOx/MgAl Hydrotalcite Catalysts and Their Highly Efficient Catalytic Performance for Low-Temperature Formaldehyde Removal
Abstract
:1. Introduction
2. Results and Discussion
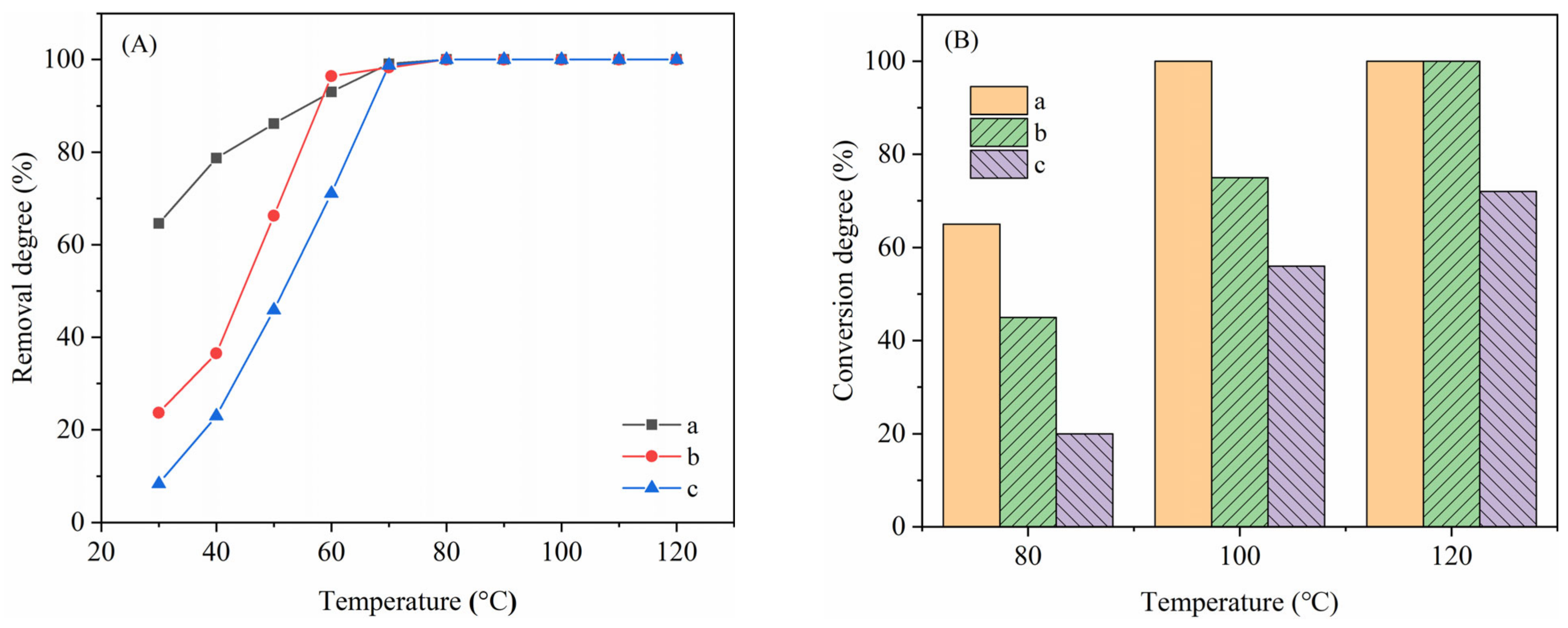
2.1. Catalytic Activity
2.2. Effect of MnOx Loading Content
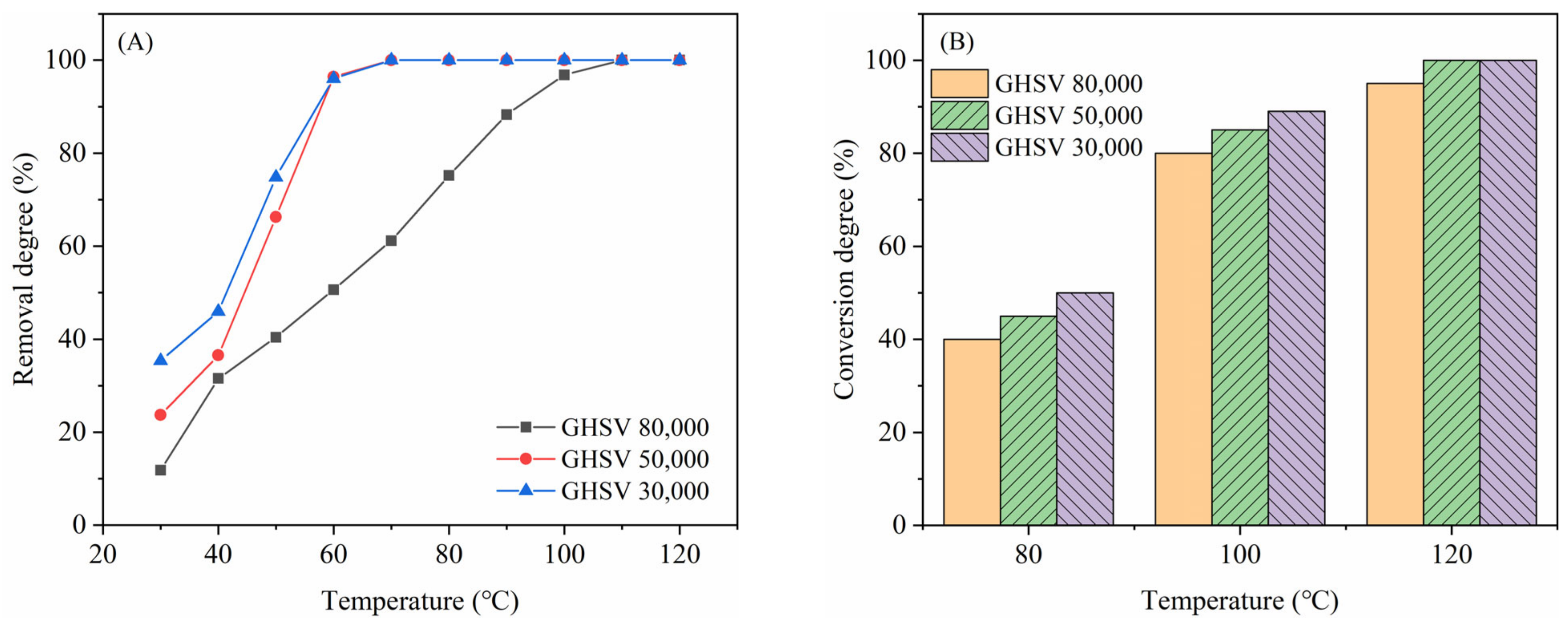
2.3. Adaptability to Different GHSV and Humidity
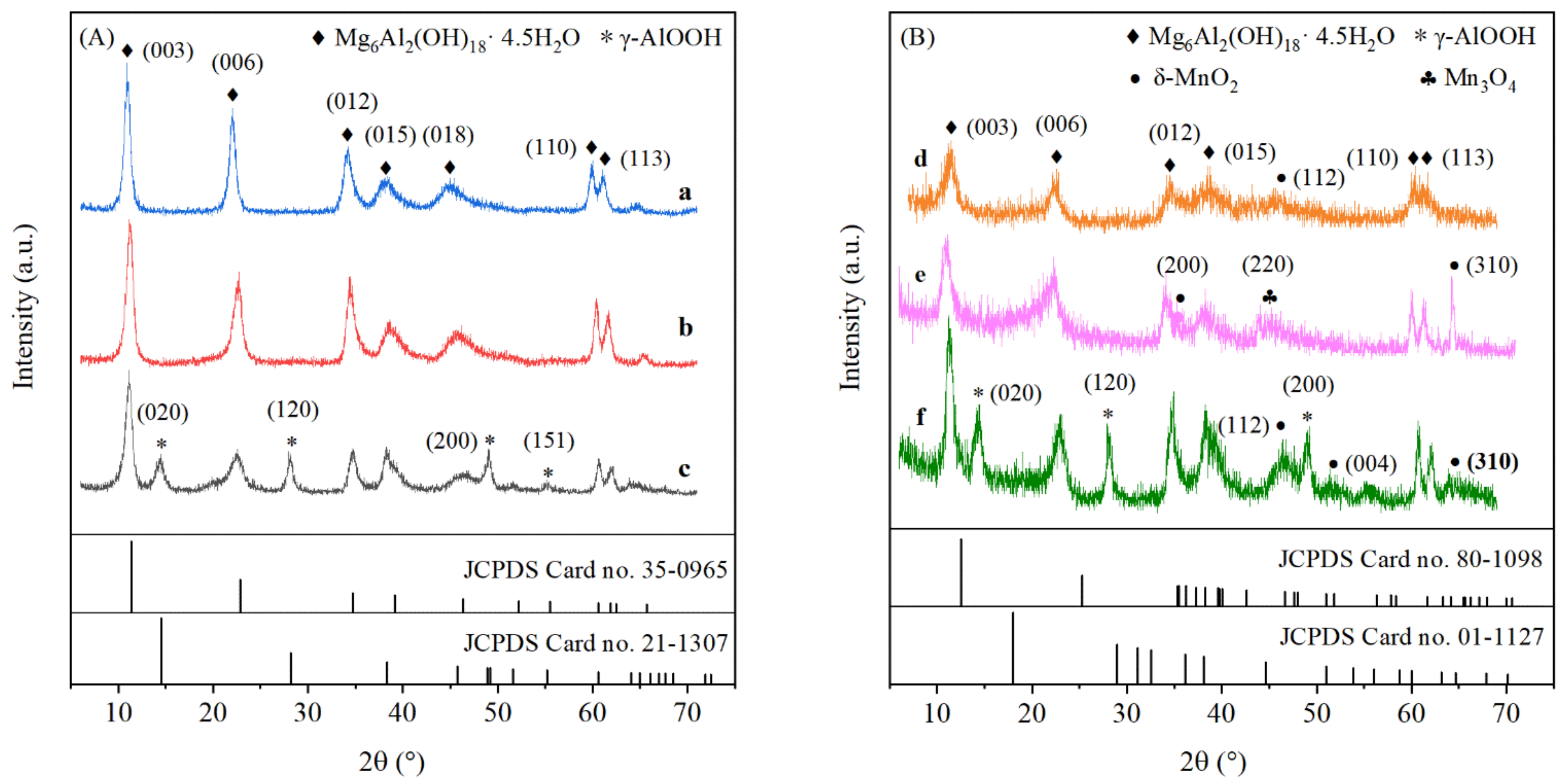
2.4. XRD Analyses
2.5. N2 Adsorption/Desorption Measurements
2.6. SEM Observations
2.7. Thermogravimetric Analysis
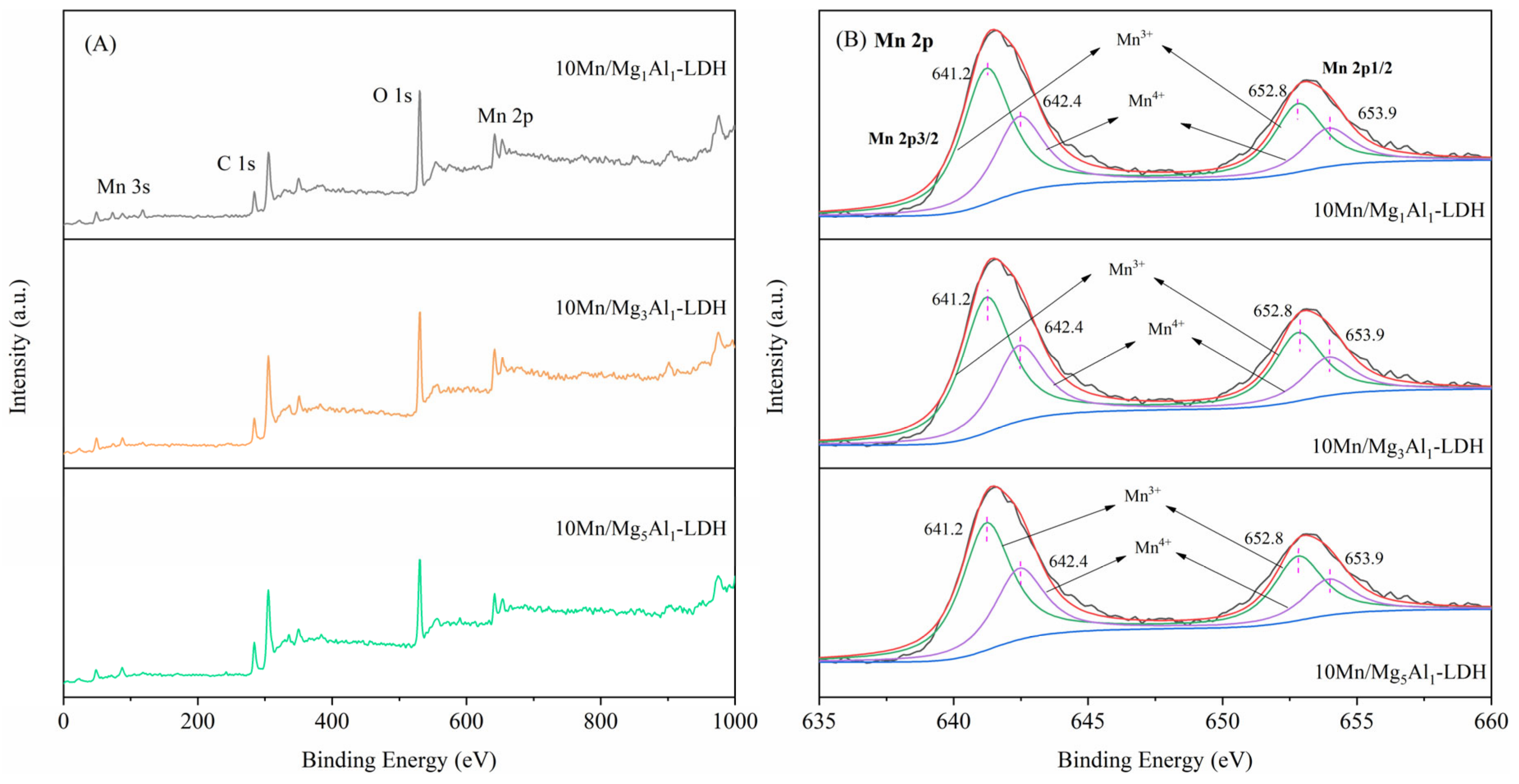
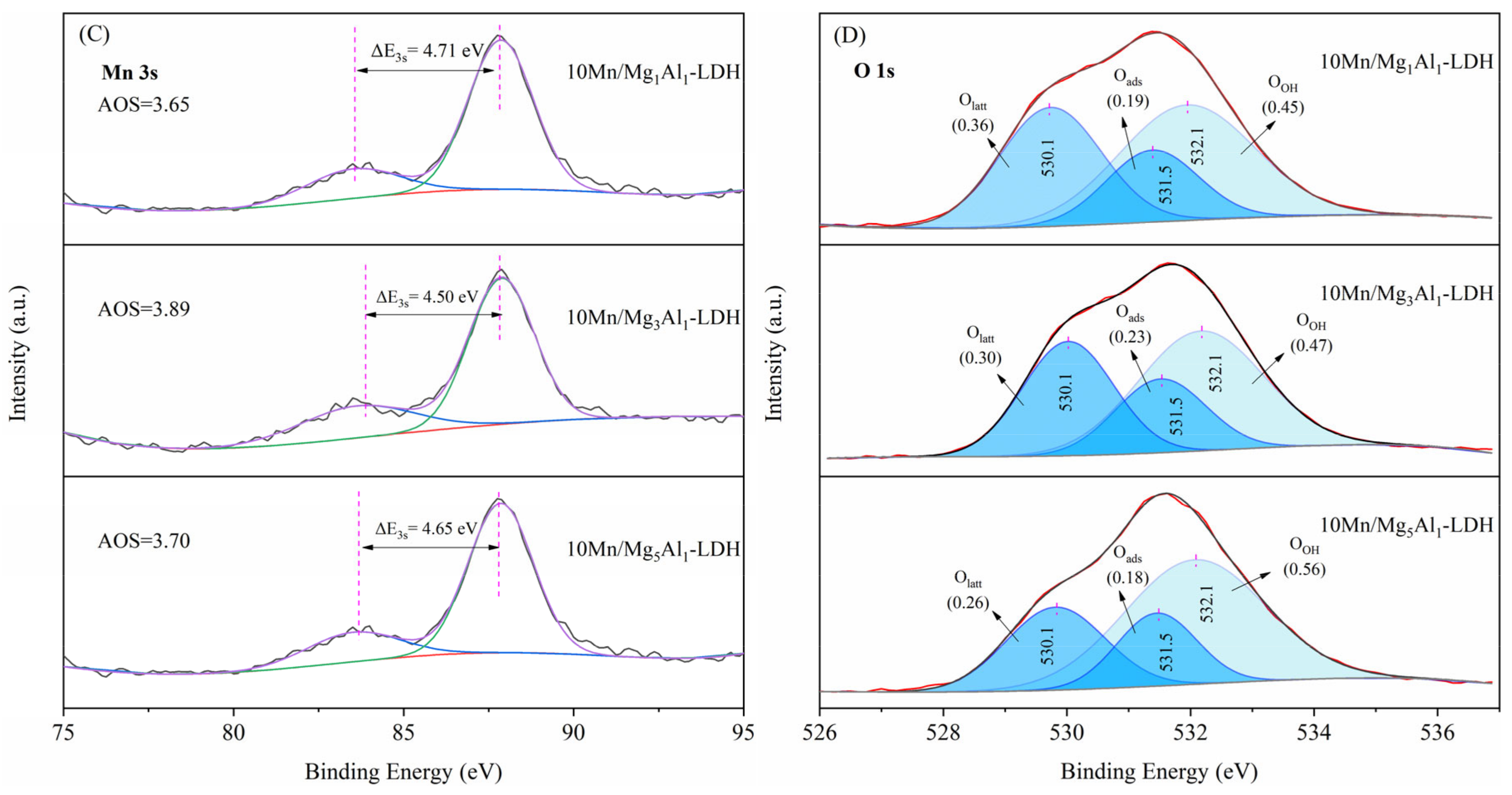
2.8. X-ray Photoelectron Spectroscopy
2.9. Fourier Transform Infrared Spectroscopy
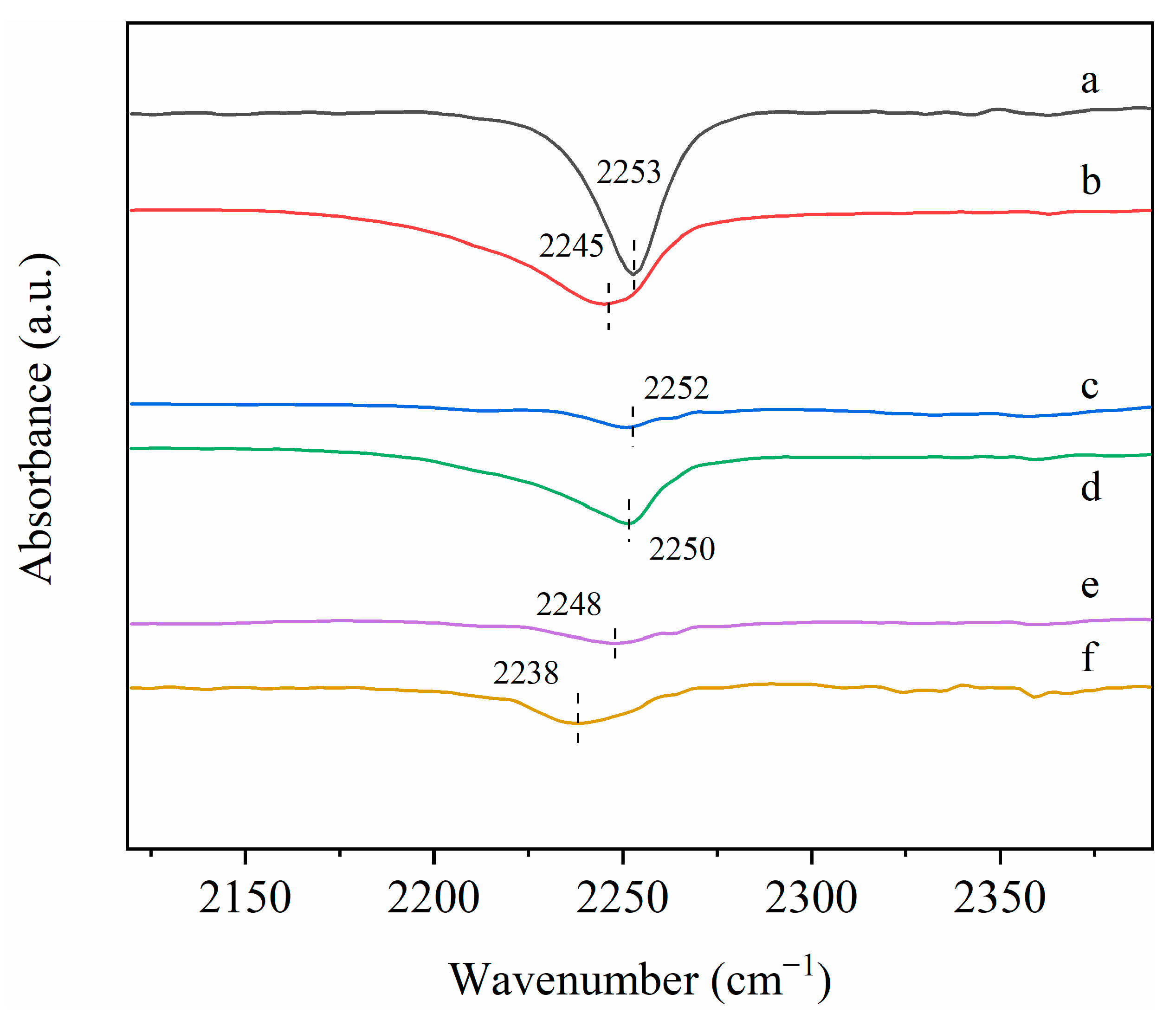
2.10. In Situ FITR Characterizations
2.10.1. Surface Basic Sites
2.10.2. Formaldehyde Oxidation
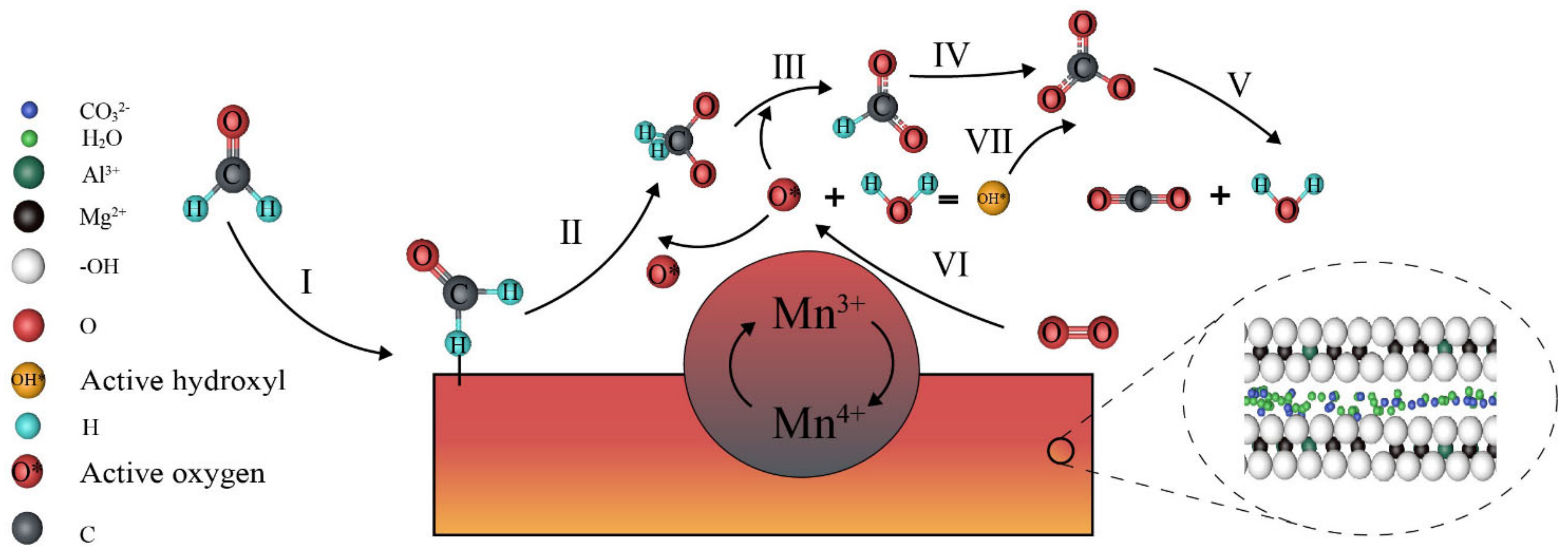
2.11. Brief Discussions about the Reaction Mechanism
3. Experimental Section
3.1. Catalysts Preparation
3.2. Catalyst Characterization
3.3. Catalytic Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Salthammer, T.; Mentese, S.; Marutzky, R. Formaldehyde in the indoor environment. Chem. Rev. 2010, 110, 2536–2572. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Li, D.; Zhang, P.; Li, J.; Wang, J.; Yu, J. Formaldehyde and volatile organic compound (VOC) emissions from particleboard: Identification of odorous compounds and effects of heat treatment. Build. Env. 2017, 117, 118–126. [Google Scholar] [CrossRef]
- Miao, L.; Wang, J.; Zhang, P. Review on manganese dioxide for catalytic oxidation of airborne formaldehyde. Appl. Surf. Sci. 2019, 466, 441. [Google Scholar] [CrossRef]
- Guo, J.; Lin, C.; Jiang, C.; Zhang, P. Review on noble metal-based catalysts for formaldehyde oxidation at room temperature. Appl. Surf. Sci. 2019, 475, 237–255. [Google Scholar] [CrossRef]
- Huang, H.; Leung, D.Y.C. Complete oxidation of formaldehyde at room temperature using TiO2 supported metallic Pd nanoparticles. ACS Catal. 2011, 1, 348–354. [Google Scholar] [CrossRef]
- Park, S.; Bae, I.; Nam, I.; Cho, B.K.; Jung, S.M.; Lee, J.-H. Oxidation of formaldehyde over Pd/Beta catalyst. Chem. Eng. J. 2012, 195–196, 392–402. [Google Scholar] [CrossRef]
- Chen, B.; Shi, C.; Crocker, M.; Wang, Y.; Zhu, A.-M. Catalytic removal of formaldehyde at room temperature over supported gold catalysts. Appl. Catal. B 2013, 132–133, 245–255. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Y.; Hu, H.; Wang, S.; Lin, Y.; Huang, Y. Achieving low temperature formaldehyde oxidation: A case study of NaBH 4 reduced cobalt oxide nanowires. Inorg. Chem. Commun. 2017, 82, 20–23. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, J.; Wang, R.; Wang, M. Highly efficient hydrogen production and formaldehyde degradation by Cu2O microcrystals. Appl. Catal. B 2015, 172–173, 1–6. [Google Scholar] [CrossRef]
- Liang, X.; Liu, P.; He, H.; Wei, G.; Chen, T.; Tan, W.; Tan, F.; Zhu, J.; Zhu, R. The variation of cationic microstructure in Mn-doped spinel ferrite during calcination and its effect on formaldehyde catalytic oxidation. J. Hazard. Mater. 2016, 306, 305–312. [Google Scholar] [CrossRef]
- Zhou, Y.; Wan, J.; Wang, X.; Deng, L.; Liu, Y.; Kan, J.; Yu, X.; Yang, X.; Wu, G. Promoting effect of Ce doping on catalytic performance and water resistance ability for toluene catalytic combustion over the cheap and efficient Mn8Ni2CeaOx catalysts. Mol. Catal. 2023, 540, 113019. [Google Scholar] [CrossRef]
- Sekine, Y. Oxidative decomposition of formaldehyde by metal oxides at room temperature. Atmos. Env. 2002, 36, 5543–5547. [Google Scholar] [CrossRef]
- Dai, Z.; Yu, X.; Huang, C.; Li, M.; Su, J.; Guo, Y.; Xu, H.; Ke, Q. Nanocrystalline MnO2 on an activated carbon fiber for catalytic formaldehyde removal. RSC Adv. 2016, 6, 97022–97029. [Google Scholar] [CrossRef]
- Fang, R.; Huang, H.; Ji, J.; He, M.; Feng, Q.; Zhan, Y.; Leung, D.Y. Efficient MnOx supported on coconut shell activated carbon for catalytic oxidation of indoor formaldehyde at room temperature. Chem. Eng. J. 2018, 334, 2050–2057. [Google Scholar] [CrossRef]
- Wang, C.; Chen, T.; Liu, H.; Xie, J.; Li, M.; Han, Z.; Zhao, Y.; He, H.; Zou, X.; Suib, S.L. Promotional catalytic oxidation of airborne formaldehyde over mineral-supported MnO2 at ambient temperature. Appl. Clay Sci. 2019, 182, 105289. [Google Scholar] [CrossRef]
- Han, Z.; Wang, C.; Zou, X.; Chen, T.; Dong, S.; Zhao, Y.; Xie, J.; Liu, H. Diatomite-supported birnessite-type MnO2 catalytic oxidation of formaldehyde: Preparation, performance and mechanism. Appl. Surf. Sci. 2020, 502, 144201. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Song, W.; Zhang, H.; Zhang, X.; Li, R.; Fan, C. Synthesis of MnO2 modified porous carbon spheres by preoxidation-assisted impregnation for catalytic oxidation of indoor formaldehyde. J. Porous Mater. 2020, 3, 801–815. [Google Scholar] [CrossRef]
- Gao, Z.; Liang, J.; Yao, J.; Meng, Q.; He, G.; Chen, H. Synthesis of Ce-doped NiAl LDH/RGO composite as an efficient photocatalyst for photocatalytic degradation of ciprofloxacin. J. Env. Chem. Eng. 2021, 9, 105405. [Google Scholar] [CrossRef]
- Zhitova, E.; Krivovichev, S.; Pekov, I.; Greenwell, H.C. Crystal chemistry of natural layered double hydroxides. 5. Single-crystal structure refinement of hydrotalcite, [Mg6Al2(OH)16](CO3)(H2O)4. Miner. Mag. 2019, 83, 269–280. [Google Scholar] [CrossRef]
- Zhang, H.; Kong, L.; Li, Z.; Liu, J.; Wang, W.; Wang, P.; Yang, B.; Shi, R.; Waterhouse, G.I.N.; Wen, X.-D.; et al. Preparation of highly dispersed Cu/ZnO/Al2O3 catalyst based on CuAl-LDH carrier and its catalytic performance. Chem. Ind. Eng. Prog. 2021, 40, 881–889. [Google Scholar]
- Naghel-Danaei, S.; Hosseini, S.A.; Niaei, A. Layered double hydroxides: Novel nanocatalysts for combustion of gaseous toluene from polluted air. Iran. J. Catal. 2020, 10, 227–233. [Google Scholar]
- Nie, L.; Yu, J.; Li, X.; Cheng, B.; Liu, G.; Jaroniec, M. Enhanced performance of NaOH-Modified Pt/TiO2 toward room temperature selective oxidation of formaldehyde. Environ. Sci. Technol. 2013, 47, 2777–2783. [Google Scholar] [CrossRef]
- Liu, F.; Rong, S.; Zhang, P.; Gao, L. One-step synthesis of nanocarbon-decorated MnO2 with superior activity for indoor formaldehyde removal at room temperature. Appl. Catal. B 2018, 235, 158–167. [Google Scholar] [CrossRef]
- Cui, W.; Yuan, X.; Wu, P.; Zheng, B.; Zhang, W.; Jia, M. Catalytic properties of γ-Al2O3 supported Pt-FeOx catalysts for complete oxidation of formaldehyde at ambient temperature. RSC Adv. 2015, 5, 104330–104336. [Google Scholar] [CrossRef]
- Wei, G.; Liu, P.; Chen, D.; Chen, T.; Liang, X.; Chen, H. Activity of manganese oxides supported on halloysite towards the thermal catalytic oxidation of formaldehyde: Constraint from the manganese precursor. Appl. Clay Sci. 2019, 182, 105280. [Google Scholar] [CrossRef]
- Averlant, R.; Royer, S.; Giraudon, J.; Bellat, J.; Bezverkhyy, I.; Weber, G.; Lamonier, J. Mesoporous silica-confined manganese oxide nanoparticles as highly efficient catalysts for the low-temperature elimination of formaldehyde. ChemCatChem 2014, 6, 152–161. [Google Scholar] [CrossRef]
- Huang, Y.; Ye, K.; Li, H.; Fan, W.; Zhao, F.; Zhang, Y.; Ji, H. A highly durable catalyst based on CoxMn3-xO4 nanosheets for low-temperature formaldehyde oxidation. Nano Res. 2016, 9, 3881–3892. [Google Scholar] [CrossRef]
- Yu, X.; He, J.; Wang, D.; Hu, Y.; Tian, H.; Dong, T.; He, Z. Preparation of Au0.5Pt0.5/MnO2/Cotton catalysts for decomposition of formaldehyde. J. Nanopart. Res. 2013, 15, 1–10. [Google Scholar] [CrossRef]
- Liu, P.; Wei, G.; Liang, X.; Chen, D.; He, H.; Chen, T.; Xi, Y.; Chen, H.; Han, D.; Zhu, J. Synergetic effect of Cu and Mn oxides supported on palygorskite for the catalytic oxidation of formaldehyde: Dispersion, microstructure, and catalytic performance. Appl. Clay Sci. 2018, 161, 265–273. [Google Scholar] [CrossRef]
- Liu, J.; Lv, G.; Gu, W.; Li, Z.; Tang, A.; Mei, L. A novel luminescence probe based on layered double hydroxides loaded with quantum dots for simultaneous detection of heavy metal ions in water. J. Mater. Chem. C 2017, 5, 5024–5030. [Google Scholar] [CrossRef]
- Yan, Z.; Xu, Z.; Yu, J.; Jaroniec, M. Effect of microstructure and surface hydroxyls on the catalytic activity of Au/AlOOH for formaldehyde removal at room temperature. J. Colloid. Interface Sci. 2017, 501, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zou, X.; Liu, H.; Chen, T.; Suib, S.L.; Chen, D.; Xie, J.; Li, M.; Sun, F. A highly efficient catalyst of palygorskite-supported manganese oxide for formaldehyde oxidation at ambient and low temperature: Performance, mechanism and reaction kinetics. Appl. Surf. Sci. 2019, 486, 420–430. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Wang, L.; Zhang, C.; He, H. Catalytic oxidation of formaldehyde over manganese oxides with different crystal structures. Catal. Sci. Technol. 2015, 5, 2305–2313. [Google Scholar] [CrossRef]
- Hosseini, S.; Ghasemi, E. Synthesis and characterization of hybrid MgAl-LDH@SiO2@CoAl2O4 pigment with high NIR reflectance for sustainable energy saving applications. Appl. Clay Sci. 2020, 193, 105674. [Google Scholar] [CrossRef]
- Ma, M.; Zhu, Q.; Jiang, Z.; Jian, Y.; Chen, C.; Liu, Q.; He, C. Achieving toluene efficient mineralization over K/a-MnO2 via oxygen vacancy modulation. J. Colloid. Interface Sci. 2021, 598, 238–249. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, B.; Liu, H.; He, B.; Ye, F.; Yu, L.; Sun, C.; Wen, H. The effect of acid/alkali treatment on the catalytic combustion activity of manganese oxide octahedral molecular sieves. RSC Adv. 2017, 7, 3958–3965. [Google Scholar] [CrossRef]
- Bai, B.; Qiao, Q.; Li, J.; Hao, J. Synthesis of three-dimensional ordered mesoporous MnO2 and its catalytic performance in formaldehyde oxidation. Chin. J. Catal. 2016, 1, 27–31. [Google Scholar] [CrossRef]
- Lu, L.; Tian, H.; He, J.; Yang, Q. Graphene-MnO2 hybrid nanostructure as a new catalyst for formaldehyde oxidation. J. Phys. Chem. C 2016, 120, 23660–23668. [Google Scholar] [CrossRef]
- Wan, J.; Tao, F.; Shi, Y.; Shi, Z.; Liu, Y.; Wu, G.; Kan, J.; Zhou, R. Designed preparation of nano rod shaped CeO2-MnOx catalysts with different Ce/Mn ratios and its highly efficient catalytic performance for chlorobenzene complete oxidation: New insights into structure–activity correlations. Chem. Eng. J. 2022, 433, 133788. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, G.; Zhang, P. Layered birnessite-type MnO2 with surface pits for enhanced catalytic formaldehyde oxidation activity. J. Mater. Chem. A 2017, 12, 5719. [Google Scholar] [CrossRef]
- Lv, T.; Peng, C.; Zhu, H.; Xiao, W. Heterostructured Fe2O3@SnO2 core-shell nanospindles for enhanced Room-temperature HCHO oxidation. Appl. Surf. Sci. 2018, 457, 83–92. [Google Scholar] [CrossRef]
- Kwon, D.W.; Seo, P.W.; Kim, G.J.; Hong, S.C. Characteristics of the HCHO oxidation reaction over Pt/TiO2 catalysts at room temperature: The effect of relative humidity on catalytic activity. Appl. Catal. B 2015, 163, 436–443. [Google Scholar] [CrossRef]
- Yan, Z.; Xu, Z.; Cheng, B.; Jiang, C. Co3O4 nanorod-supported Pt with enhanced performance for catalytic HCHO oxidation at room temperature. Appl. Surf. Sci. 2017, 404, 426–434. [Google Scholar] [CrossRef]
- Chen, B.; Zhu, X.; Crocker, M.; Wang, Y.; Shi, C. FeOx-supported gold catalysts for catalytic removal of formaldehyde at room temperature. Appl. Catal. B 2014, 154–155, 74–81. [Google Scholar] [CrossRef]
- Ye, J.; Cheng, B.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J. Flexible Mg-Al layered double hydroxide supported Pt on Al foil for use in room-temperature catalytic decomposition of formaldehyde. RSC Adv. 2016, 6, 3428–34287. [Google Scholar] [CrossRef]
- Timofeeva, M.; Timofeeva, M.; Kalashnikova, G.; Panchenko, V.N.; Nikolaev, A.I.; Gil, A. A layered titanosilicate AM-4 as a novel catalyst for the synthesis of 1-methoxy-2-propanole from propylene oxide and methano. Appl. Catal. A 2019, 587, 117240. [Google Scholar] [CrossRef]
- Zienkiewicz, J.; Kucharska, E.; Ptak, M. Mechanism of unusual isosymmetric order-disorder phase transition in [Dimethylhydrazinium] Mn (HCOO)3 hybrid perovskite probed by vibrational spectroscopy. Materials 2021, 14, 3984. [Google Scholar] [CrossRef]
- Ji, J.; Lu, X.; Chen, C.; He, M.; Huang, H. Potassium-modulated δ-MnO2 as robust catalysts for formaldehyde oxidation at room temperature. Appl. Catal. B 2020, 260, 118210. [Google Scholar] [CrossRef]




| Samples | SBET (m2/g) | Vpore (cm3/g) | Dpore (nm) |
|---|---|---|---|
| Mg1Al1-LDH | 15.44 | 0.260 | 32.72 |
| 10Mn/Mg1Al1-LDH | 16.78 | 0.204 | 23.92 |
| Mg3Al1-LDH | 21.80 | 0.151 | 13.16 |
| 10Mn/Mg3Al1-LDH | 25.65 | 0.182 | 14.15 |
| Mg5Al1-LDH | 10.24 | 0.077 | 59.59 |
| 10Mn/Mg5Al1-LDH | 14.63 | 0.078 | 10.81 |
| Samples | Surface Mn (at%) | Mn4+/Mn3+ | Olatt | Oads | OOH | AOS |
|---|---|---|---|---|---|---|
| 10Mn/Mg1Al1-LDH | 8.47% | 0.51 | 0.36 | 0.15 | 0.45 | 3.65 |
| 10Mn/Mg3Al1-LDH | 6.08% | 0.75 | 0.30 | 0.23 | 0.47 | 3.89 |
| 10Mn/Mg5Al1-LDH | 7.34% | 0.66 | 0.26 | 0.18 | 0.56 | 3.70 |
| Positions (cm−1) | Species | References |
|---|---|---|
| 3271~3525 | Symmetric stretching vibration of -OH in water molecules | [42,43] |
| 3090 | -CH stretching vibration in formaldehyde adsorbed on the catalyst surface | [44] |
| 2359 | -CO anti-symmetric stretching vibration in CO2 | [40] |
| 1577~1584 | -COC-antisymmetric stretching vibration in formate species | [45,47] |
| 1359~1389 | The antisymmetric vibration of carbonate | [42] |
| 1101~1116 | Intermediate dioxymethylene (DOM) species during formaldehyde conversion | [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Sun, Q.; Tian, J.; Wan, J.; Liu, Y.; Wang, X.; Kan, J.; Yang, X.; Wu, G. Construction of Supported MnOx/MgAl Hydrotalcite Catalysts and Their Highly Efficient Catalytic Performance for Low-Temperature Formaldehyde Removal. Catalysts 2023, 13, 1283. https://doi.org/10.3390/catal13091283
Yu X, Sun Q, Tian J, Wan J, Liu Y, Wang X, Kan J, Yang X, Wu G. Construction of Supported MnOx/MgAl Hydrotalcite Catalysts and Their Highly Efficient Catalytic Performance for Low-Temperature Formaldehyde Removal. Catalysts. 2023; 13(9):1283. https://doi.org/10.3390/catal13091283
Chicago/Turabian StyleYu, Xiankun, Qi Sun, Jingchen Tian, Jie Wan, Yanjun Liu, Xiaoli Wang, Jianfei Kan, Xiaojun Yang, and Gongde Wu. 2023. "Construction of Supported MnOx/MgAl Hydrotalcite Catalysts and Their Highly Efficient Catalytic Performance for Low-Temperature Formaldehyde Removal" Catalysts 13, no. 9: 1283. https://doi.org/10.3390/catal13091283
APA StyleYu, X., Sun, Q., Tian, J., Wan, J., Liu, Y., Wang, X., Kan, J., Yang, X., & Wu, G. (2023). Construction of Supported MnOx/MgAl Hydrotalcite Catalysts and Their Highly Efficient Catalytic Performance for Low-Temperature Formaldehyde Removal. Catalysts, 13(9), 1283. https://doi.org/10.3390/catal13091283







