Methane Combustion over Zeolite-Supported Palladium-Based Catalysts
Abstract
1. Introduction
2. Zeolite Types Used in Catalytic Methane Combustion
2.1. Small-Pore Zeolites with 8 Member Rings
2.2. Medium-Pore Zeolites with 10 Member Rings
2.3. Large-Pore Zeolites with 12 Member Rings
2.4. Mesoporous Zeolite and Mesoporous Silicas Catalysts
2.5. Other Noble Metal-Based Zeolite Catalysts
| Zeolite Framework | Catalyst | Catalyst Preparation Method | Pd Loading (wt%) | Feed Gas | GHSV a (mL/(gh)) | T50%/T90%b (°C) | TOF c (×10−2 s−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| MFI | Pd/ZSM-5(14) | Ion exchange | 3.39 | 1 vol% CH4 + 21 vol% O2 + N2 (balance) | 60,000 | 260/275 | 1.3 | [50] |
| MFI | Pd/Na-ZSM-5 | Ion exchange | 4.84 | 1 vol% CH4 + 18 vol% O2 + N2 (balance) | 60,000 | 285/290 | 1.2 | [74] |
| MFI | Pd/ZSM-5 | Ion exchange | 0.1 | 0.2 vol% CH4 + 18 vol% O2 + N2 (balance) | 120,000 | 370/>650 | - | [75] |
| MFI | Pd/ZSM-5 | Impregnation method | 0.55 | 0.2 vol% CH4 + 10 vol% O2 + N2 (balance) | 72,000 | 332/373 | - | [76] |
| MFI | Pd/Silicalite-1 | Ion exchange | 0.96 | 1 vol% CH4 + 10 vol% O2 + Ar (balance) | 3000 h−1 | 286/347 | - | [77] |
| MFI | Pd/S-1-in-6EN | One pot | 0.93 | 1 vol% CH4 + 20 vol% O2 + N2 (balance) | 60,000 | -/364 | 4.1 | [78] |
| FAU | PdMo/Y-zeolite | Ion exchange | 2 | 1 vol% CH4 + 4 vol% O2 + He (balance) | 120,000 | 410/- | - | [58] |
| FAU | Pd/Y-zeolite | Ion exchange | 2.5 | 1 vol% CH4 + 4 vol% O2 + He (balance) | 100,000 | 415/- | - | [79] |
| CHA | Pd/SZZ-13 | Ion exchange | 1.1 | 0.15 vol% CH4 + 5 vol% O2 + He (balance) | 200,000 | 317/- | 1.7 | [80] |
| LTA | Pd/LTA | Ion exchange | 1.0 | 0.15 vol% CH4 + 5 vol% O2 + He (balance) | 200,000 | 447/- | 1.5 | [80] |
| LTA | Pd/LTA | Impregnation method | 2.12 | 0.05 vol% CH4 + 8 vol% O2 + Ar (balance) + 5 vol% H2O | 27,200 | 388/- | - | [48] |
| CHA | Pd/SSZ-13 | Impregnation method | 1.06 | 0.05 vol% CH4 + 5 vol% O2 + Ar (balance) | 13,600 h−1 | 311/- | - | [81] |
| BEA | Pd/Beta-40 | Impregnation method | 1.0 | 0.05 vol% CH4 + 5 vol% O2 + Ar (balance) | 13,600 h−1 | 281/- | - | [81] |
| BEA | RD-Pd/D-Beta | Reduction deposition | 0.5 | 1 vol% CH4 + 21 vol% O2 + N2 (balance) | 30,000 | 333/< 375 | - | [55] |
| BEA | Pd(0.5)Co(1.0)/BEA | Impregnation method | 0.5 | 0.15 vol% CH4 + 5 vol% O2 + Ar (balance) | 99,900 | 352/420 | - | [82] |
| BEA | Pd/Beta-SH-HT | Mercaptosilane assisted | 0.5 | 1 vol% CH4 + 21 vol% O2 + N2 (balance) | 30,000 | 290/333 | - | [56] |
| CHA | Pd/SAPO-11 | Ion exchange | 1.1 | 0.4 vol% CH4 + 10 vol% O2 + N2 (balance) | 50,000 | 320/- | - | [71] |
| MOR | Pd/Na-MOR | Ion exchange | 1.0 | 1 vol% CH4 + 4 vol% O2 + N2 (balance) | 70,000 h−1 | 335/< 420 | - | [57] |
| Meso-pore | Pd/MCM-41 | Impregnation method | 1.36 | 2.5 vol% CH4 + 10 vol% O2 + Ar (balance) | 60,000 | 386/- | - | [67] |
| Mesopore | Pd/MCM-41 | Grafting | 1.0 | 1 vol% CH4 + 4 vol% O2 + He (balance) | 120,000 | 409/- | - | [64] |
| Meso-pore | Pd-5 wt% CeO2/SBA-15 | Impregnation method | 1.0 | 2 vol% CH4 + 21 vol% O2 + N2 (balance) | 72,000 | 342/406 | 1.64 | [66] |
| Meso-pore | Pd-5 wt% ZrO2/SBA-15 | Impregnation method | 0.5 | 2 vol% CH4 + 21 vol% O2 + N2 (balance) | 72,000 | 322/364 | 1.22 | [66] |
| Meso-pore | Pd-CeO2/SBA-15 | Impregnation method | 5% | 0.1 vol% CH4 + 10 vol% O2 + Ar (balance) | 180,000 | 290/<360 | - | [83] |
| Meso-pore | Pd@IMS-1 | Dry-gel conversion | 1.83 | 1 vol% CH4 + 20 vol% O2 + N2 (balance) | 36,000 | 261/318 | 1.5 | [68] |
| MFI | Pd/HZSM-5 | Impregnation method | 2.0 | 1 vol% CH4 + 4 vol% O2 + N2 (balance) | 30,000 | -/400 | - | [84] |
| MFI | Pd/TS-1 | Impregnation method | 1.4 | 0.7 vol% CH4 + 20 vol% O2 + N2 (balance) | 200,000 | -/430 | 0.7 | [85] |
| MFI | Pd/HZSM-5 | Impregnation method | 1 | 0.7 vol% CH4 + 20 vol% O2 + N2 (balance) + 3 vol% H2O | 200,000 | -/- | 4.1 | [86] |
| MFI | Pd/S-1-in | In situ method | 0.123 | 1 vol% CH4 + 20 vol% O2 + N2 (balance) | 16,000 | 329/382 | 3.22 | [87] |
| MFI | Pd@S-1 | Hydrothermal method | 0.6 | 1 vol% CH4 + 16 vol% O2 + N2 (balance) | 50,000 | 323/< 380 | 2.6 | [53] |
| MFI | Pd@H-ZSM-5 | Hydrothermal method | 0.7 | 1 vol% CH4 + 5 vol% O2 + He (balance) | 60,000 h−1 | 350/400 | - | [88] |
| MFI | PdCo@MFI | One-pot synthesis | 0.54 | 1 vol% CH4 + 20 vol% O2 + N2 (balance) | 30,000 | -/< 400 | - | [89] |
| MFI | Pd/NaLa-ZSM-5 | Impregnation method | 2.0 | 0.15 vol% CH4 + 5 vol% O2 + N2 (balance) | 60,000 | 264/296 | - | [90] |
| MFI | Pd/ZSM-5-Si | Colloidal immersion | 1.0 | 0.15 vol% CH4 + 5 vol% O2 + N2 (balance) | 60,000 | 288/331 | - | [91] |
3. Physicochemical Parameters
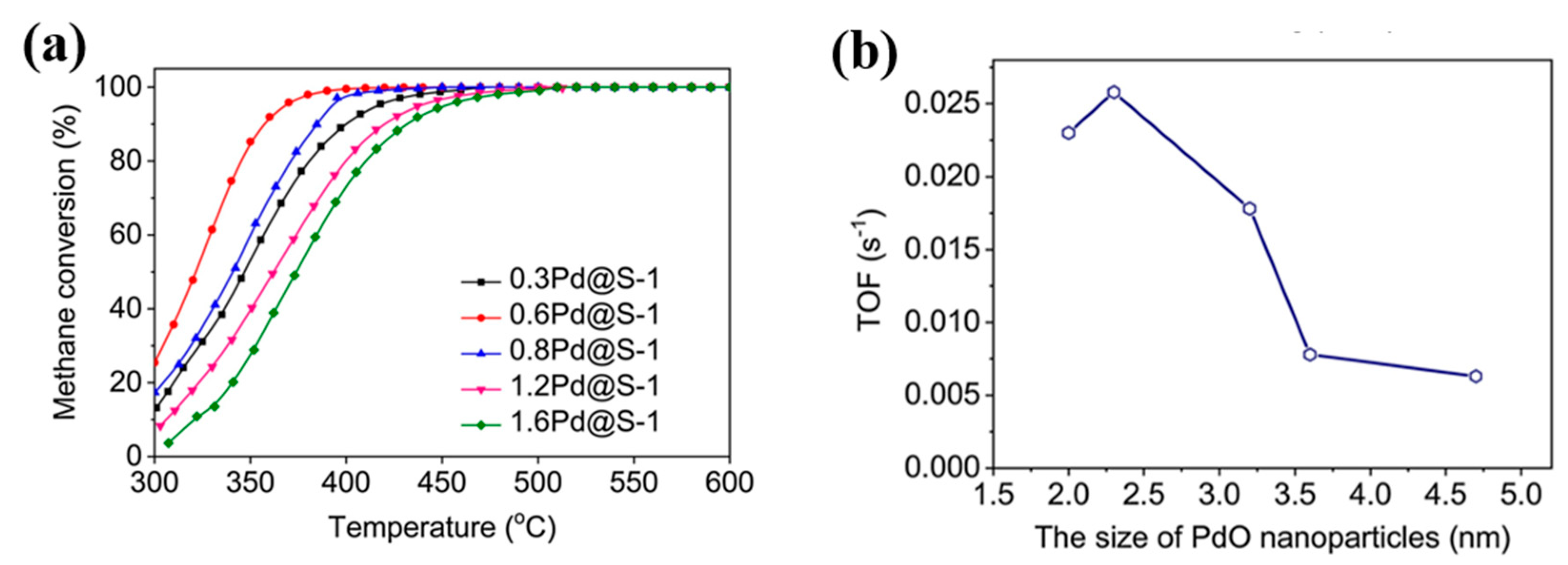
3.1. Active Site and Metal Particle Size
3.2. Si/Al Ratio and Acid Site
3.3. The Presence of Alkaline Metal Counter Ions
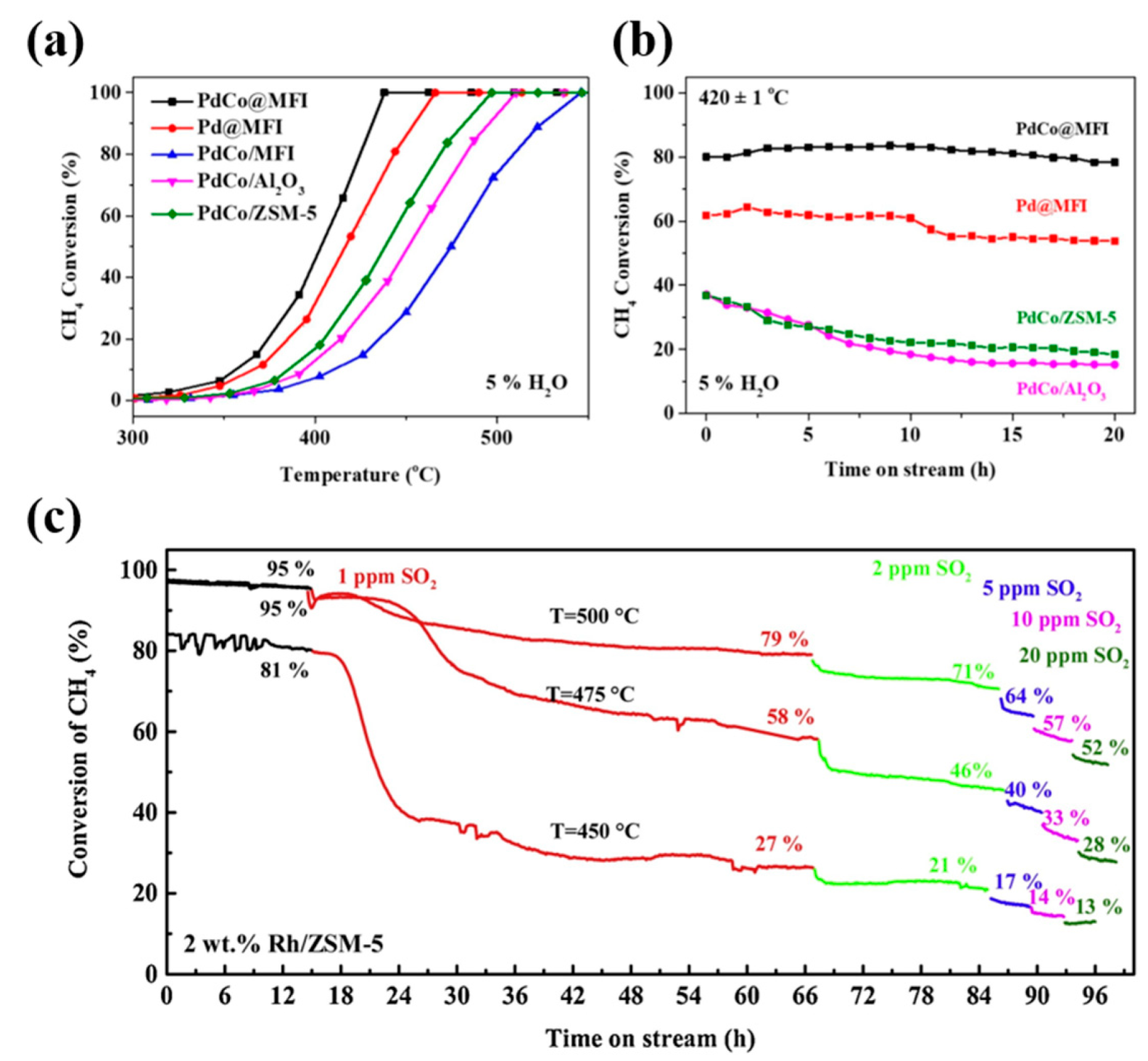
3.4. Incorporation of a Second Base Metal
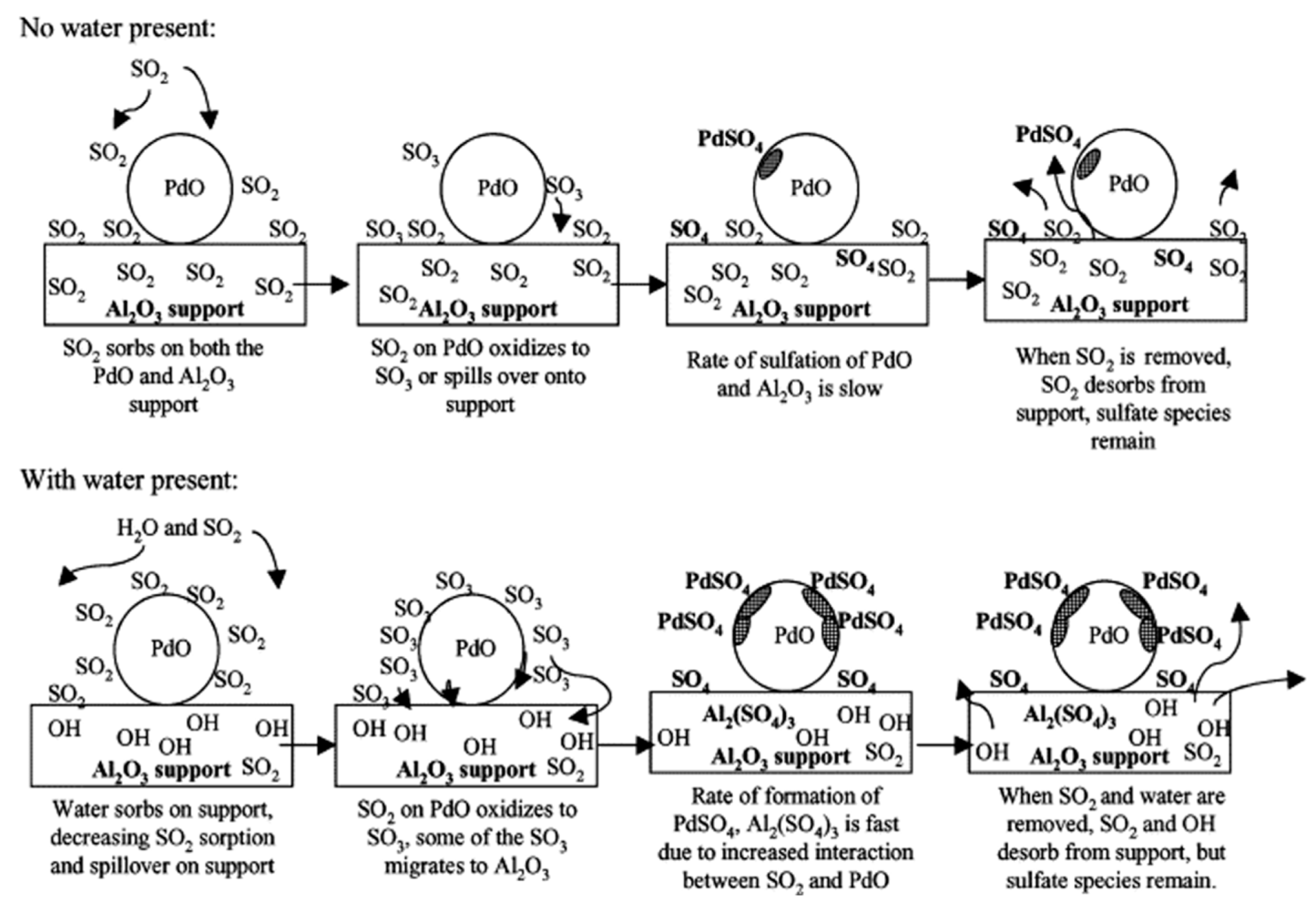
4. Effects of Water and Sulfur Dioxide on Catalytic Performance
5. Deactivation by Sintering
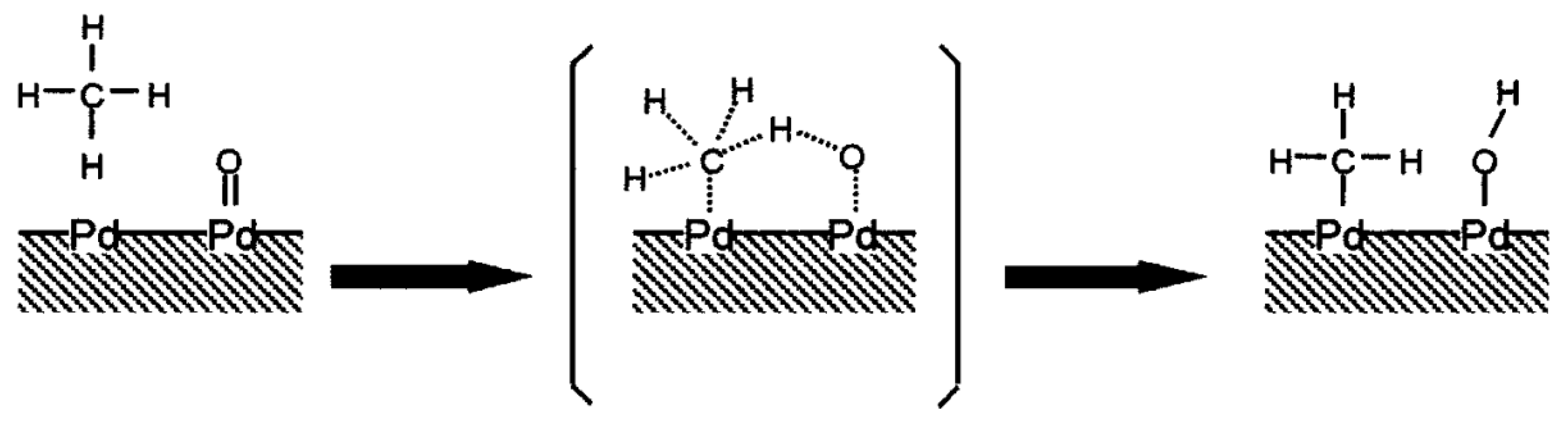
6. Catalytic Methane Combustion Mechanism
7. Summary and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ai, X.Y.; Hu, C.; Yang, Y.R.; Zhang, L.Y.; Liu, H.L.; Zhang, J.Q.; Chen, X.; Bai, G.Q.; Xiao, W. Quantification of Central and Eastern China’s atmospheric CH4 enhancement changes and its contributions based on machine learning approach. J. Environ. Sci. 2023, 138, 236–248. [Google Scholar] [CrossRef]
- Francoeur, C.B.; McDonald, B.C.; Gilman, J.B.; Zarzana, K.J.; Dix, B.; Brown, S.S.; de Gouw, J.A.; Frost, G.J.; Li, M.; McKeen, S.A.; et al. Quantifying methane and ozone precursor emissions from oil and gas production regions across the contiguous US. Environ. Sci. Technol. 2021, 55, 9129–9139. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.B.; Jiang, L.; Li, D.Y.; Tian, S.P.; Zhu, X.; Wang, H.; He, C.; Li, K.Z. Progress and key challenges in catalytic combustion of lean methane. J. Energy Chem. 2022, 75, 173–215. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Fang, S.X.; Chen, J.M.; Lin, Y.; Chen, Y.Y.; Liang, R.S.; Jiang, K.; Parker, R.J.; Boesch, H.; Steinbacher, M.; et al. Observed changes in China’s methane emissions linked to policy drivers. Proc. Natl. Acad. Sci. USA 2022, 119, e2202742119. [Google Scholar] [CrossRef]
- He, L.; Fan, Y.L.; Bellettre, J.; Yue, J.; Luo, L.G. A review on catalytic methane combustion at low temperatures: Catalysts, mechanisms, reaction conditions and reactor designs. Renew. Sustain. Energy Rev. 2020, 119, 109589. [Google Scholar] [CrossRef]
- Farrauto, R.J. Low-temperature oxidation of methane. Science 2012, 337, 659–660. [Google Scholar] [CrossRef]
- Chen, J.H.; Arandiyan, H.; Gao, X.; Li, J.H. Recent advances in catalysts for methane combustion. Catal. Surv. Asia 2015, 19, 140–171. [Google Scholar] [CrossRef]
- Chen, J.J.; Zhong, J.W.; Wu, Y.; Hu, W.; Qu, P.F.; Xiao, X.; Zhang, G.C.; Liu, X.; Jiao, Y.; Zhong, L.; et al. Particle size effects in stoichiometric methane combustion: Structure-activity relationship of Pd catalyst supported on gamma-alumina. ACS Catal. 2020, 10, 10339–10349. [Google Scholar] [CrossRef]
- Wang, Y.; Arandiyan, H.; Scott, J.; Akia, M.; Dai, H.X.; Deng, J.G.; Aguey-Zinsou, K.; Amal, R. High performance Au–Pd supported on 3D hybrid strontium-substituted lanthanum manganite perovskite catalyst for methane combustion. ACS Catal. 2016, 6, 6935–6947. [Google Scholar] [CrossRef]
- Xiong, H.F.; Kunwar, D.; Jiang, D.; García-Vargas, C.E.; Li, H.; Du, C.; Canning, G.; Pereira-Hernandez, X.I.; Wan, Q.; Lin, S.; et al. Engineering catalyst supports to stabilize PdOx two-dimensional rafts for water-tolerant methane oxidation. Nat. Catal. 2021, 4, 830–839. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, X.; Zhao, X.T.; Yang, J.; Hou, Z.Q.; Bai, L.; Chang, H.Q.; Liu, Y.X.; Deng, J.G.; Guo, G.S.; et al. Preparation, characterization, and catalytic performance of PdPt/3DOM LaMnAl11O19 for the combustion of methane. Appl. Catal. A 2018, 562, 284–293. [Google Scholar] [CrossRef]
- Yang, J.; Hu, S.Y.; Shi, L.M.; Hoang, S.; Yang, W.W.; Fang, Y.R.; Liang, Z.F.; Pan, C.Q.; Zhu, Y.H.; Li, L.; et al. Oxygen vacancies and Lewis acid sites synergistically promoted catalytic methane combustion over perovskite oxides. Environ. Sci. Technol. 2021, 55, 9243–9254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.T.; Zhang, R.; Liu, Y.X.; Deng, J.G.; Xu, P.; Yang, J.; Han, Z.; Hou, Z.Q.; Dai, H.X.; Au, C.T. In-situ reduction-derived Pd/3DOM La0.6Sr0.4MnO3: Good catalytic stability in methane combustion. Appl. Catal. A 2018, 568, 202–212. [Google Scholar] [CrossRef]
- Gélin, P.; Primet, M. Complete oxidation of methane at low temperature over noble metal based catalysts: A review. Appl. Catal. B 2002, 39, 1–37. [Google Scholar] [CrossRef]
- Park, J.; Ahn, J.; Sim, H.; Seo, G.; Han, H.S.; Shin, C. Low-temperature combustion of methane using PdO/Al2O3 catalyst: Influence of crystalline phase of Al2O3 support. Catal. Commun. 2014, 56, 157–163. [Google Scholar] [CrossRef]
- Beck, I.E.; Bukhtiyarov, V.I.; Pakharukov, I.Y.; Zaikovsky, V.I.; Kriventsov, V.V.; Parmon, V.N. Platinum nanoparticles on Al2O3: Correlation between the particle size and activity in total methane oxidation. J. Catal. 2009, 268, 60–67. [Google Scholar] [CrossRef]
- Shi, W.; Xu, G.Y.; Han, X.W.; Wang, Y.J.; Liu, Z.; Xue, S.; Sun, N.N.; Shi, X.Y.; Yu, Y.B.; He, H. Nano-sized alumina supported palladium catalysts for methane combustion with excellent thermal stability. J. Environ. Sci. 2023, 126, 333–347. [Google Scholar] [CrossRef]
- Hou, Z.Q.; Dai, L.Y.; Deng, J.G.; Zhao, G.F.; Jing, L.; Wang, Y.; Yu, X.H.; Gao, R.Y.; Tian, X.R.; Dai, H.X.; et al. Electronically engineering water resistance in methane combustion with an atomically dispersed tungsten on PdO catalyst. Angew. Chem. Int. Ed. 2022, 61, e202201655. [Google Scholar] [CrossRef]
- Hou, Z.Q.; Liu, Y.X.; Deng, J.G.; Lu, Y.; Xie, S.H.; Fang, X.; Dai, H.X. Highly active and stable Pd–GaOx/Al2O3 catalysts derived from intermetallic Pd5Ga3 nanocrystals for methane combustion. ChemCatChem 2018, 10, 5637–5648. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, G.Y.; Zeng, L.Y.; Shi, W.; Wang, Y.J.; Sun, Y.W.; Yu, Y.B.; He, H. Anchoring Pt-doped PdO nanoparticles on γ-Al2O3 with highly dispersed La sites to create a methane oxidation catalyst. Appl. Catal. B 2023, 324, 122259. [Google Scholar] [CrossRef]
- Ma, X.Y.; Xu, H.; Liu, Z.; Liu, Y.F.; Wang, C.; Shen, M.Q.; Du, C.; Shan, B. Durable PdNi/Al2O3 catalysts with PdO–NiO and PdO–NiAl2O4 dual interfaces for methane combustion. ACS ES&T Eng. 2023, 3, 349–359. [Google Scholar]
- Poznyak, A.A.; Knörnschild, G.H.; Pligovka, A.N.; Larin, T.D. Anodic alumina prepared in aqueous solutions of chelating complex zinc and cobalt compounds. Tech. Phys. 2022, 67, 411–422. [Google Scholar] [CrossRef]
- Poznyak, A.; Knörnschild, G.; Karoza, A.; Norek, M.; Pligovka, A. Peculiar porous aluminum oxide films produced via electrochemical anodizing in malonic acid solution with arsenazo-I additive. Materials 2021, 14, 5118. [Google Scholar] [CrossRef]
- Chen, S.Y.; Li, S.D.; You, R.Y.; Guo, Z.Y.; Wang, F.; Li, G.X.; Yuan, W.T.; Zhu, B.; Gao, Y.; Zhang, Z.; et al. Elucidation of active sites for CH4 catalytic oxidation over Pd/CeO2 via tailoring metal-support interactions. ACS Catal. 2021, 11, 5666–5677. [Google Scholar] [CrossRef]
- Colussi, S.; Gayen, A.; Farnesi Camellone, M.; Boaro, M.; Llorca, J.; Fabris, S.; Trovarelli, A. Nanofaceted Pd-O sites in Pd-Ce surface superstructures: Enhanced activity in catalytic combustion of methane. Angew. Chem. Int. Ed. 2009, 48, 8481–8484. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.H.; Liu, Y.X.; Deng, J.G.; Zhao, X.; Yang, J.; Zhang, K.F.; Han, Z.; Dai, H.X. Three-dimensionally ordered macroporous CeO2-supported Pd@Co nanoparticles: Highly active catalysts for methane oxidation. J. Catal. 2016, 342, 17–26. [Google Scholar] [CrossRef]
- Yang, W.W.; Polo-Garzon, F.; Zhou, H.; Huang, Z.N.; Chi, M.F.; Meyer, H., III; Yu, X.B.; Li, Y.Y.; Wu, Z.L. Boosting the activity of Pd single atoms by tuning their local environment on ceria for methane combustion. Angew. Chem. Int. Ed. 2023, 62, e202217323. [Google Scholar] [CrossRef]
- Ding, Y.Q.; Jia, Y.Y.; Jiang, M.X.; Guo, Y.; Guo, Y.; Wang, L.; Ke, Q.P.; Ngoc Ha, M.; Dai, S.; Zhan, W.C. Superior catalytic activity of Pd-based catalysts upon tuning the structure of the ceria-zirconia support for methane combustion. Chem. Eng. J. 2021, 416, 129150. [Google Scholar] [CrossRef]
- Ding, Y.Q.; Wu, Q.Q.; Lin, B.; Guo, Y.L.; Guo, Y.; Wang, Y.S.; Wang, L.; Zhan, W.C. Superior catalytic activity of a Pd catalyst in methane combustion by fine-tuning the phase of ceria-zirconia support. Appl. Catal. B 2020, 266, 118631. [Google Scholar] [CrossRef]
- 2Yang, W.W.; Kim, M.Y.; Polo-Garzon, F.; Gong, J.; Jiang, X.; Huang, Z.N.; Chi, M.F.; Yu, X.B.; Wang, X.; Guo, Y.B.; et al. CH4 combustion over a commercial Pd/CeO2–ZrO2 three-way catalyst: Impact of thermal aging and sulfur exposure. Chem. Eng. J. 2023, 451, 138930. [Google Scholar]
- Huang, J.L.; Lin, J.; Chen, X.H.; Zheng, Y.; Xiao, Y.H.; Zheng, Y. Optimizing the microstructure of SnO2–CeO2 binary oxide supported palladium catalysts for efficient and stable methane combustion. ACS Appl. Mater. Interfaces 2022, 14, 16233–16244. [Google Scholar] [CrossRef] [PubMed]
- Takeguchi, T.; Takeoh, O.; Aoyama, S.; Ueda, J.; Kikuchi, R.; Eguchi, K. Strong chemical interaction between PdO and SnO2 and the influence on catalytic combustion of methane. Appl. Catal. A 2003, 252, 205–214. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.W.; Liao, X.M.; Liu, Y.M.; Hou, J.D.; Pham-Huu, C. Enhancing oxygen activation on high surface area Pd–SnO2 solid solution with isolated metal site catalysts for catalytic CH4 combustion. Appl. Surf. Sci. 2021, 564, 150368. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Wang, B.W.; Ma, J.; Zhan, W.C.; Wang, L.; Guo, Y.L.; Guo, Y.; Lu, G.Z. Catalytic combustion of methane over Pd/SnO2 catalysts. Chin. J. Catal. 2017, 38, 1322–1329. [Google Scholar] [CrossRef]
- Willis, J.J.; Gallo, A.; Sokaras, D.; Aljama, H.; Nowak, S.H.; Goodman, E.D.; Wu, L.; Tassone, C.J.; Jaramillo, T.F.; Abild-Pedersen, F.; et al. Systematic structure-property relationship studies in palladium-catalyzed methane complete combustion. ACS Catal. 2017, 7, 7810–7821. [Google Scholar] [CrossRef]
- Garbowski, E.; Feumi-Jantou, C.; Mouaddib, N.; Primet, M. Catalytic combustion of methane over palladium supported on alumina catalysts: Evidence for reconstruction of particles. Appl. Catal. A 1994, 109, 277–291. [Google Scholar] [CrossRef]
- Wang, Y.L.; Hu, P.; Yang, J.; Zhu, Y.A.; Chen, D. C–H bond activation in light alkanes: A theoretical perspective. Chem. Soc. Rev. 2021, 50, 4299–4358. [Google Scholar] [CrossRef]
- Murata, K.; Mahara, Y.; Ohyama, J.; Yamamoto, Y.; Arai, S.; Satsuma, A. The metal–support -interaction concerning the particle size effect of Pd/Al2O3 on methane combustion. Angew. Chem. Int. Ed. 2017, 56, 15993–15997. [Google Scholar] [CrossRef]
- Hong, E.; Kim, C.; Lim, D.; Cho, H.; Shin, C. Catalytic methane combustion over Pd/ZrO2 catalysts: Effects of crystalline structure and textural properties. Appl. Catal. B 2018, 232, 544–552. [Google Scholar] [CrossRef]
- Schwartz, W.R.; Ciuparu, D.; Pfefferle, L.D. Combustion of methane over palladium-based catalysts: Catalytic deactivation and role of the support. J. Phys. Chem. C 2012, 116, 8587–8593. [Google Scholar] [CrossRef]
- Smit, B. Molecular simulations of zeolites: Adsorption, diffusion, and shape selectivity. Chem. Rev. 2008, 108, 4125–4184. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.Y.; Feng, J.L.; Huo, Q.S.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Kresge, A.C.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Kleitz, F.; Hei Choi, S.; Ryoo, R. Cubic Ia3d large mesoporous silica: Synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes. Chem. Commun. 2003, 2136–2137. [Google Scholar] [CrossRef]
- Cui, Y.R.; Chen, J.Z.; Peng, B.; Kovarik, L.; Devaraj, A.; Li, Z.; Ma, T.; Wang, Y.L.; Szanyi, J.; Miller, J.T.; et al. Onset of high methane combustion rates over supported palladium catalysts: From isolated Pd cations to PdO nanoparticles. JACS Au 2021, 1, 396–408. [Google Scholar] [CrossRef]
- Friberg, I.; Clark, A.H.; Ho, P.H.; Sadokhina, N.; Smales, G.J.; Woo, J.; Auvray, X.; Ferri, D.; Nachtegaal, M.; Kröcher, O.; et al. Structure and performance of zeolite supported Pd for complete methane oxidation. Catal. Today 2021, 382, 3–12. [Google Scholar] [CrossRef]
- Leistner, K.; Kumar, A.; Kamasamudram, K.; Olsson, L. Mechanistic study of hydrothermally aged Cu/SSZ-13 catalysts for ammonia-SCR. Catal. Today 2018, 307, 55–64. [Google Scholar] [CrossRef]
- Friberg, I.; Wang, A.; Olsson, L. Hydrothermal aging of Pd/LTA monolithic catalyst for complete CH4 oxidation. Catalysts 2020, 10, 517. [Google Scholar] [CrossRef]
- Argauer, R.J.; Landolt, G.R. Crystalline Zeolite ZSM-5 and Method of Preparing the Same. U.S. Patent 3702886DA, 14 November 1972. [Google Scholar]
- Li, Y.; Armor, J.N. Catalytic combustion of methane over palladium exchanged zeolites. Appl. Catal. B 1994, 3, 275–282. [Google Scholar] [CrossRef]
- Liu, C.; Yu, K.; Zhang, Y.; Zhu, X.; He, F.; Eliasson, B. Remarkable improvement in the activity and stability of Pd/HZSM-5 catalyst for methane combustion. Catal. Commun. 2003, 4, 303–307. [Google Scholar] [CrossRef]
- Hosseiniamoli, H.; Setiawan, A.; Adesina, A.A.; Kennedy, E.M.; Stockenhuber, M. The stability of Pd/TS-1 and Pd/silicalite-1 for catalytic oxidation of methane-understanding the role of titanium. Catal. Sci. Technol. 2020, 10, 1193–1204. [Google Scholar] [CrossRef]
- Wang, W.Y.; Zhou, W.; Li, W.; Xiong, X.W.; Wang, Y.H.; Cheng, K.J.; Kang, J.; Zhang, Q.H.; Wang, Y. In-situ confinement of ultrasmall palladium nanoparticles in silicalite-1 for methane combustion with excellent activity and hydrothermal stability. Appl. Catal. B 2020, 276, 119142. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Sun, L.W.; Hu, X.F.; Zhang, Y.B.; Tian, H.Y.; Yang, X.G. Anti-sintering Pd@silicalite-1 for methane combustion: Effects of the moisture and SO2. Appl. Surf. Sci. 2019, 494, 1044–1054. [Google Scholar] [CrossRef]
- Zhang, L.L.; Chen, J.F.; Guo, X.M.; Yin, S.M.; Zhang, M.; Rui, Z.B. Combination of reduction-deposition Pd loading and zeolite dealumination as an effective route for promoting methane combustion over Pd/Beta. Catal. Today 2021, 376, 119–125. [Google Scholar] [CrossRef]
- Zhang, L.L.; Chen, J.F.; Yang, H.; Wang, X.; Rui, Z.B. In situ mercaptosilane-assisted confinement of Pd nanoparticles in Beta for high-efficient methane oxidation. Catal. Today 2022, 400, 124–131. [Google Scholar] [CrossRef]
- Petrov, A.W.; Ferri, D.; Krumeich, F.; Nachtegaal, M.; van Bokhoven, J.A.; Kröcher, O. Stable complete methane oxidation over palladium based zeolite catalysts. Nat. Commun. 2018, 9, 2545. [Google Scholar] [CrossRef] [PubMed]
- Zina, M.S.; Ghorbel, A. Preparation and characterization of bimetallic PdMo/Y-zeolite: Catalytic properties in methane combustion. Solid State Sci. 2004, 6, 973–980. [Google Scholar] [CrossRef]
- Song, H.; Rioux, R.M.; Hoefelmeyer, J.D.; Komor, R.; Niesz, K.; Grass, M.; Yang, P.; Somorjai, G.A. Hydrothermal growth of mesoporous SBA-15 silica in the presence of PVP-stabilized Pt nanoparticles: Synthesis, characterization, and catalytic properties. J. Am. Chem. Soc. 2006, 128, 3027–3037. [Google Scholar] [CrossRef]
- Hussain, M.; Deorsola, F.A.; Russo, N.; Fino, D.; Pirone, R. Abatement of CH4 emitted by CNG vehicles using Pd-SBA-15 and Pd-KIT-6 catalysts. Fuel 2015, 149, 2–7. [Google Scholar] [CrossRef]
- Yuranov, I.; Moeckli, P.; Suvorova, E.; Buffat, P.; Kiwi-Minsker, L.; Renken, A. Pd/SiO2 catalysts: Synthesis of Pd nanoparticles with the controlled size in mesoporous silicas. J. Mol. Catal. A 2003, 192, 239–251. [Google Scholar] [CrossRef]
- Murthy, P.R.; Zhang, J.; Li, W. Exceptionally stable sol-immobilization derived Pd/SBA-15 catalysts for methane combustion. Catal. Sci. Technol. 2021, 11, 3609–3618. [Google Scholar] [CrossRef]
- Li, C.S.; Tang, B.Y.; Li, W.Z.; Lu, Q.; Yuan, L. Palladium nanoparticles encapsulated in surface-defected SBA-15 for lean methane oxidation. ACS Appl. Nano Mater. 2022, 5, 13055–13068. [Google Scholar] [CrossRef]
- Zribi, S.; Albela, B.; Bonneviot, L.; Zina, M.S. Surface engineering and palladium dispersion in MCM-41 for methane oxidation. Appl. Catal. A 2015, 502, 195–203. [Google Scholar] [CrossRef]
- Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Hernandez Garrido, J.C.; Venezia, A.M. Pd (1 wt%)/LaMn0.4Fe0.6O3 catalysts supported over silica SBA-15: Effect of perovskite loading and support morphology on methane oxidation activity and SO2 tolerance. Top. Catal. 2012, 55, 782–791. [Google Scholar] [CrossRef]
- Yin, F.X.; Ji, S.F.; Wu, P.Y.; Zhao, F.Z.; Li, C.Y. Deactivation behavior of Pd-based SBA-15 mesoporous silica catalysts for the catalytic combustion of methane. J. Catal. 2008, 257, 108–116. [Google Scholar] [CrossRef]
- Ruiz, J.A.; Fraga, M.A.; Pastore, H.O. Methane combustion over Pd supported on MCM-41. Appl. Catal. B 2007, 76, 115–122. [Google Scholar] [CrossRef]
- Peng, H.G.; Dong, T.; Yang, S.; Chen, H.; Yang, Z.Z.; Liu, W.M.; He, C.; Wu, P.; Tian, J.; Peng, Y.; et al. Intra-crystalline mesoporous zeolite encapsulation-derived thermally robust metal nanocatalyst in deep oxidation of light alkanes. Nat. Commun. 2022, 13, 295. [Google Scholar] [CrossRef]
- Zhang, Y.; Glarborg, P.; Andersson, M.P.; Johansen, K.; Torp, T.K.; Jensen, A.D.; Christensen, J.M. Sulfur poisoning and regeneration of Rh-ZSM-5 catalysts for total oxidation of methane. Appl. Catal. B 2020, 277, 119176. [Google Scholar] [CrossRef]
- Zhang, Y.; Glarborg, P.; Johansen, K.; Andersson, M.P.; Torp, T.K.; Jensen, A.D.; Christensen, J.M. A rhodium-based methane oxidation catalyst with high tolerance to H2O and SO2. ACS Catal. 2020, 10, 1821–1827. [Google Scholar] [CrossRef]
- Nomura, K.; Noro, K.; Nakamura, Y.; Yazawa, Y.; Yoshida, H.; Satsuma, A.; Hattori, T. Pd-Pt bimetallic catalyst supported on SAPO-5 for catalytic combustion of diluted methane in the presence of water vapor. Catal. Lett. 1998, 53, 167–169. [Google Scholar] [CrossRef]
- Osman, A.I.; Abu-Dahrieh, J.K.; Laffir, F.; Curtin, T.; Thompson, J.M.; Rooney, D.W. A bimetallic catalyst on a dual component support for low temperature total methane oxidation. Appl. Catal. B 2016, 187, 408–418. [Google Scholar] [CrossRef]
- Dai, Q.G.; Bai, S.; Lou, Y.; Wang, X.; Guo, Y.; Lu, G.Z. Sandwich-like PdO/CeO2 nanosheet@HZSM-5 membrane hybrid composite for methane combustion: Self-redispersion, sintering-resistance and oxygen, water-tolerance. Nanoscale 2016, 8, 9621–9628. [Google Scholar] [CrossRef] [PubMed]
- de Correa, C.M.; Ai Da Luz Villa, H. Combustion of methane over palladium ZSM-5 and mordenite catalysts. Appl. Catal. B 1996, 10, 313–323. [Google Scholar] [CrossRef]
- Okumura, K.; Shinohara, E.; Niwa, M. Pd loaded on high silica beta support active for the total oxidation of diluted methane in the presence of water vapor. Catal. Today 2006, 117, 577–583. [Google Scholar] [CrossRef]
- Ramadj, O.M.; Li, D.; Wang, X.; Zhang, B.; Lu, G. Role of acidity of catalysts on methane combustion over Pd/ZSM-5. Catal. Commun. 2007, 8, 880–884. [Google Scholar] [CrossRef]
- Xiao, C.; Yang, Y.; Meng, D.; Dong, L.; Luo, L.L.; Tan, Z.Y. Stable and active monolithic palladium catalyst for catalytic oxidation of methane using nanozeolite silicalite-1 coating on cordierite. Appl. Catal. A 2017, 531, 197–202. [Google Scholar] [CrossRef]
- Liu, M.Y.; Chen, X.M.; Wang, Z.K.; Zhang, Y.S.; Li, R.J.; Zheng, H.M.; Ye, D.Q.; Wu, J.L. Size-controlled palladium nanoparticles encapsulated in silicalite-1 for methane catalytic combustion. ACS Appl. Nano Mater. 2023, 6, 3637–3646. [Google Scholar] [CrossRef]
- Najar, H.; Saïd Zina, M.; Ghorbel, A. Catalytic activity of palladium supported on mesoporous modified Y-zeolite in methane combustion. Kinet. Catal. 2010, 51, 602–608. [Google Scholar] [CrossRef]
- Lim, J.B.; Jo, D.; Hong, S.B. Palladium-exchanged small-pore zeolites with different cage systems as methane combustion catalysts. Appl. Catal. B 2017, 219, 155–162. [Google Scholar] [CrossRef]
- Friberg, I.; Sadokhina, N.; Olsson, L. The effect of Si/Al ratio of zeolite supported Pd for complete CH4 oxidation in the presence of water vapor and SO2. Appl. Catal. B 2019, 250, 117–131. [Google Scholar] [CrossRef]
- Chen, J.; Giewont, K.; Walker, E.A.; Lee, J.; Niu, Y.; Kyriakidou, E.A. Cobalt-induced PdO formation in low-loading Pd/BEA catalysts for CH4 oxidation. ACS Catal. 2021, 11, 13066–13076. [Google Scholar] [CrossRef]
- Dai, Y.; Pavan Kumar, V.; Zhu, C.; MacLachlan, M.J.; Smith, K.J.; Wolf, M.O. Mesoporous silica-supported nanostructured PdO/CeO2 catalysts for low-temperature methane oxidation. ACS Appl. Mater. Interfaces 2018, 10, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Yu, K.L.; Zhang, Y.P.; Zhu, X.L.; He, F.; Eliasson, B. Characterization of plasma treated Pd/HZSM-5 catalyst for methane combustion. Appl. Catal. B 2004, 47, 95–100. [Google Scholar] [CrossRef]
- Setiawan, A.; Friggieri, J.; Hosseiniamoli, H.; Kennedy, E.M.; Dlugogorski, B.Z.; Adesina, A.A.; Stockenhuber, M. Towards understanding the improved stability of palladium supported on TS-1 for catalytic combustion. Phys. Chem. Chem. Phys. 2016, 18, 10528–10537. [Google Scholar] [CrossRef]
- Hosseiniamoli, H.; Bryant, G.; Kennedy, E.M.; Mathisen, K.; Nicholson, D.; Sankar, G.; Setiawan, A.; Stockenhuber, M. Understanding structure-function relationships in zeolite-supported Pd catalysts for oxidation of ventilation air methane. ACS Catal. 2018, 8, 5852–5863. [Google Scholar] [CrossRef]
- Niu, R.Y.; Liu, P.C.; Li, W.; Wang, S.; Li, J.P. High performance for oxidation of low-concentration methane using ultra-low Pd in silicalite-1 zeolite. Microporous Mesoporous Mater. 2019, 284, 235–240. [Google Scholar] [CrossRef]
- Gao, M.Y.; Gong, Z.M.; Weng, X.F.; Shang, W.X.; Chai, Y.C.; Dai, W.L.; Wu, G.J.; Guan, N.J.; Li, L.D. Methane combustion over palladium catalyst within the confined space of MFI zeolite. Chin. J. Catal. 2021, 42, 1689–1699. [Google Scholar] [CrossRef]
- Tang, X.; Lou, Y.; Zhao, R.L.; Tang, B.J.; Guo, W.Y.; Guo, Y.L.; Zhan, W.C.; Jia, Y.Y.; Wang, L.; Dai, S.; et al. Confinement of subnanometric PdCo bimetallic oxide clusters in zeolites for methane complete oxidation. Chem. Eng. J. 2021, 418, 129398. [Google Scholar] [CrossRef]
- Xie, Y.Q.; Zhang, L.; Jiang, Y.; Han, S.; Wang, L.; Meng, X.J.; Xiao, F.X. Enhanced catalytic performance of methane combustion over zeolite-supported Pd catalysts with the lanthanum. Catal. Today 2021, 364, 16–20. [Google Scholar] [CrossRef]
- Xie, Y.Q.; Meng, X.J.; Gao, S.; Wu, Z.; Xiao, F.S. Catalytic performances in methane combustion over Pd nanoparticles supported on pure silica zeolites with different structures. Microporous Mesoporous Mater. 2022, 346, 112298. [Google Scholar] [CrossRef]
- Fan, C.; Yang, L.; Luo, L.; Wu, Z.W.; Qin, Z.F.; Zhu, H.Q.; Fan, W.B.; Wang, J.G. A highly active Pd/H-ZSM-5 catalyst in lean methane combustion prepared via a sol-gel method and treated by reduction-oxidation. New J. Chem. 2020, 44, 3940–3949. [Google Scholar] [CrossRef]
- Lou, Y.; Ma, J.; Hu, W.; Dai, Q.G.; Wang, L.; Zhan, W.C.; Guo, Y.; Cao, X.; Guo, Y.; Hu, P. Low-temperature methane combustion over Pd/H-ZSM-5: Active Pd sites with specific electronic properties modulated by acidic sites of H-ZSM-5. ACS Catal. 2016, 6, 8127–8139. [Google Scholar] [CrossRef]
- Luo, L.; Wang, S.; Wu, Z.W.; Qin, Z.F.; Dong, M.; Wang, J.G.; Fan, W.B. Influence of the ZSM-5 support acidity on the catalytic performance of Pd/ZSM-5 in lean methane oxidation. Chem. Res. Chin. Univ. 2022, 38, 229–236. [Google Scholar] [CrossRef]
- Losch, P.; Huang, W.; Vozniuk, O.; Goodman, E.D.; Schmidt, W.; Cargnello, M. Modular Pd/Zeolite composites demonstrating the key role of support hydrophobic/hydrophilic character in methane catalytic combustion. ACS Catal. 2019, 9, 4742–4753. [Google Scholar] [CrossRef]
- Luo, L.; Wang, S.; Fan, C.; Yang, L.; Wu, Z.W.; Qin, Z.F.; Zhu, H.Q.; Fan, W.B.; Wang, J.G. Promoting effect of alkali metal cations on the catalytic performance of Pd/H-ZSM-5 in the combustion of lean methane. Appl. Catal. A 2020, 602, 117678. [Google Scholar] [CrossRef]
- Shi, C.K.; Yang, L.F.; Cai, J.X. Low-temperature complete combustion of methane over CeO2-promoted Pd/HZSM-5 catalyst with enhanced activity and stability. Chem. Lett. 2003, 32, 50–51. [Google Scholar] [CrossRef]
- Shi, C.K.; Yang, L.F.; Wang, Z.C.; He, X.; Cai, J.; Li, G.; Wang, X.S. Promotion effects of ZrO2 on the Pd/HZSM-5 catalyst for low-temperature catalytic combustion of methane. Appl. Catal. A 2003, 243, 379–388. [Google Scholar] [CrossRef]
- Shi, C.K.; Yang, L.F.; He, X.; Cai, J.X. Enhanced activity and stability of Zr-promoted Pd/HZSM-5 catalyst for low-temperature methane combustion. Chem. Commun. 2002, 2006–2007. [Google Scholar] [CrossRef][Green Version]
- Di Carlo, G.; Melaet, G.; Kruse, N.; Liotta, L.F.; Pantaleo, G.; Venezia, A.M. Combined sulfating and non-sulfating support to prevent water and sulfur poisoning of Pd catalysts for methane combustion. Chem. Commun. 2010, 46, 6317–6319. [Google Scholar] [CrossRef]
- Barrett, W.; Shen, J.; Hu, Y.; Hayes, R.E.; Scott, R.W.J.; Semagina, N. Understanding the role of SnO2 support in water-tolerant methane combustion: In situ observation of Pd(OH)2 and comparison with Pd/Al2O3. ChemCatChem 2020, 12, 944–952. [Google Scholar] [CrossRef]
- Persson, K.; Pfefferle, L.D.; Schwartz, W.; Ersson, A.; Järås, S.G. Stability of palladium-based catalysts during catalytic combustion of methane: The influence of water. Appl. Catal. B 2007, 74, 242–250. [Google Scholar] [CrossRef]
- Sharma, R.K.; Zhou, B.; Tong, S.; Chuang, K.T. Catalytic destruction of volatile organic compounds using supported platinum and palladium hydrophobic catalysts. Ind. Eng. Chem. Res. 1995, 34, 4310–4317. [Google Scholar] [CrossRef]
- Li, T.; Beck, A.; Krumeich, F.; Artiglia, L.; Ghosalya, M.K.; Roger, M.; Ferri, D.; Kröcher, O.; Sushkevich, V.; Safonova, O.V.; et al. Stable palladium oxide clusters encapsulated in silicalite-1 for complete methane oxidation. ACS Catal. 2021, 11, 7371–7382. [Google Scholar] [CrossRef]
- Petrov, A.W.; Ferri, D.; Tarik, M.; Kröcher, O.; Van Bokhoven, J.A. Deactivation aspects of methane oxidation catalysts based on palladium and ZSM-5. Top. Catal. 2017, 60, 123–130. [Google Scholar] [CrossRef]
- Wu, Z.X.; Deng, J.G.; Liu, Y.X.; Xie, S.H.; Jiang, Y.; Zhao, X.T.; Yang, J.; Arandiyan, H.; Guo, G.S.; Dai, H.X. Three-dimensionally ordered mesoporous Co3O4-supported Au-Pd alloy nanoparticles: High-performance catalysts for methane combustion. J. Catal. 2015, 332, 13–24. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Y.X.; Deng, J.X.; Jing, L.; Wu, L.; Dai, H.X. Catalytic performance and SO2 resistance of zirconia-supported platinum-palladium bimetallic nanoparticles for methane combustion. Catal. Today 2022, 402, 138–148. [Google Scholar] [CrossRef]
- Auvinen, P.; Hirvi, J.T.; Kinnunen, N.M.; Suvanto, M. PdSO4 surfaces in methane oxidation catalysts: DFT studies on stability, reactivity, and water inhibition. ACS Catal. 2020, 10, 12943–12953. [Google Scholar] [CrossRef]
- Mowery, D.L.; McCormick, R.L. Deactivation of alumina supported and unsupported PdO methane oxidation catalyst: The effect of water on sulfate poisoning. Appl. Catal. B 2001, 34, 287–297. [Google Scholar] [CrossRef]
- Chin, Y.; Buda, C.; Neurock, M.; Iglesia, E. Consequences of metal-oxide interconversion for C–H bond activation during CH4 reactions on Pd catalysts. J. Am. Chem. Soc. 2013, 135, 15425–15442. [Google Scholar] [CrossRef]
- Xu, P.; Wu, Z.X.; Deng, J.G.; Liu, Y.X.; Xie, S.H.; Guo, G.S.; Dai, H.X. Catalytic performance enhancement by alloying Pd with Pt on ordered mesoporous manganese oxide for methane combustion. Chin. J. Catal. 2017, 38, 92–105. [Google Scholar] [CrossRef]
- Carrillo, C.; Johns, T.R.; Xiong, H.; DeLaRiva, A.; Challa, S.R.; Goeke, R.S.; Artyushkova, K.; Li, W.; Kim, C.H.; Datye, A.K. Trapping of mobile Pt species by PdO nanoparticles under oxidizing conditions. J. Phys. Chem. Lett. 2014, 5, 2089–2093. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.M.; Wang, N.; Bing, Q.; Si, R.; Liu, J.; Bai, R.; Zhang, P.; Jia, M.; Yu, J.H. Subnanometric hybrid Pd-M(OH)2, M = Ni, Co, clusters in zeolites as highly efficient nanocatalysts for hydrogen generation. Chem 2017, 3, 477–493. [Google Scholar] [CrossRef]
- Wang, N.; Sun, Q.M.; Bai, R.; Li, X.; Guo, G.; Yu, J.H. In situ confinement of ultrasmall Pd clusters within nanosized silicalite-1 zeolite for highly efficient catalysis of hydrogen generation. J. Am. Chem. Soc. 2016, 138, 7484–7487. [Google Scholar] [CrossRef] [PubMed]
- Su, S.C.; Carstens, J.N.; Bell, A.T. A study of the dynamics of Pd oxidation and PdO reduction by H2 and CH4. J. Catal. 1998, 176, 125–135. [Google Scholar] [CrossRef]
- Müller, C.A.; Maciejewski, M.; Koeppel, R.A.; Tschan, R.; Baiker, A. Role of lattice oxygen in the combustion of methane over PdO/ZrO2: Combined pulse TG/DTA and MS study with 18O-labeled catalyst. J. Phys. Chem. 1996, 100, 20006–20014. [Google Scholar] [CrossRef]
- Ciuparu, D.; Altman, E.; Pfefferle, L. Contributions of lattice oxygen in methane combustion over PdO-based catalysts. J. Catal. 2001, 203, 64–74. [Google Scholar] [CrossRef]
- Au-Yeung, J.; Chen, K.D.; Bell, A.T.; Iglesia, E. Isotopic studies of methane oxidation pathways on PdO catalysts. J. Catal. 1999, 188, 132–139. [Google Scholar] [CrossRef]
- Müller, C.A.; Maciejewski, M.; Koeppel, R.A.; Baiker, A. Combustion of methane over palladium/zirconia derived from a glassy Pd-Zr alloy: Effect of Pd particle size on catalytic behavior. J. Catal. 1997, 166, 36–43. [Google Scholar] [CrossRef]
- Fujimoto, K.; Ribeiro, F.H.; Avalos-Borja, M.; Iglesia, E. Structure and reactivity of PdOx/ZrO2 catalysts for methane oxidation at low temperatures. J. Catal. 1998, 179, 431–442. [Google Scholar] [CrossRef]
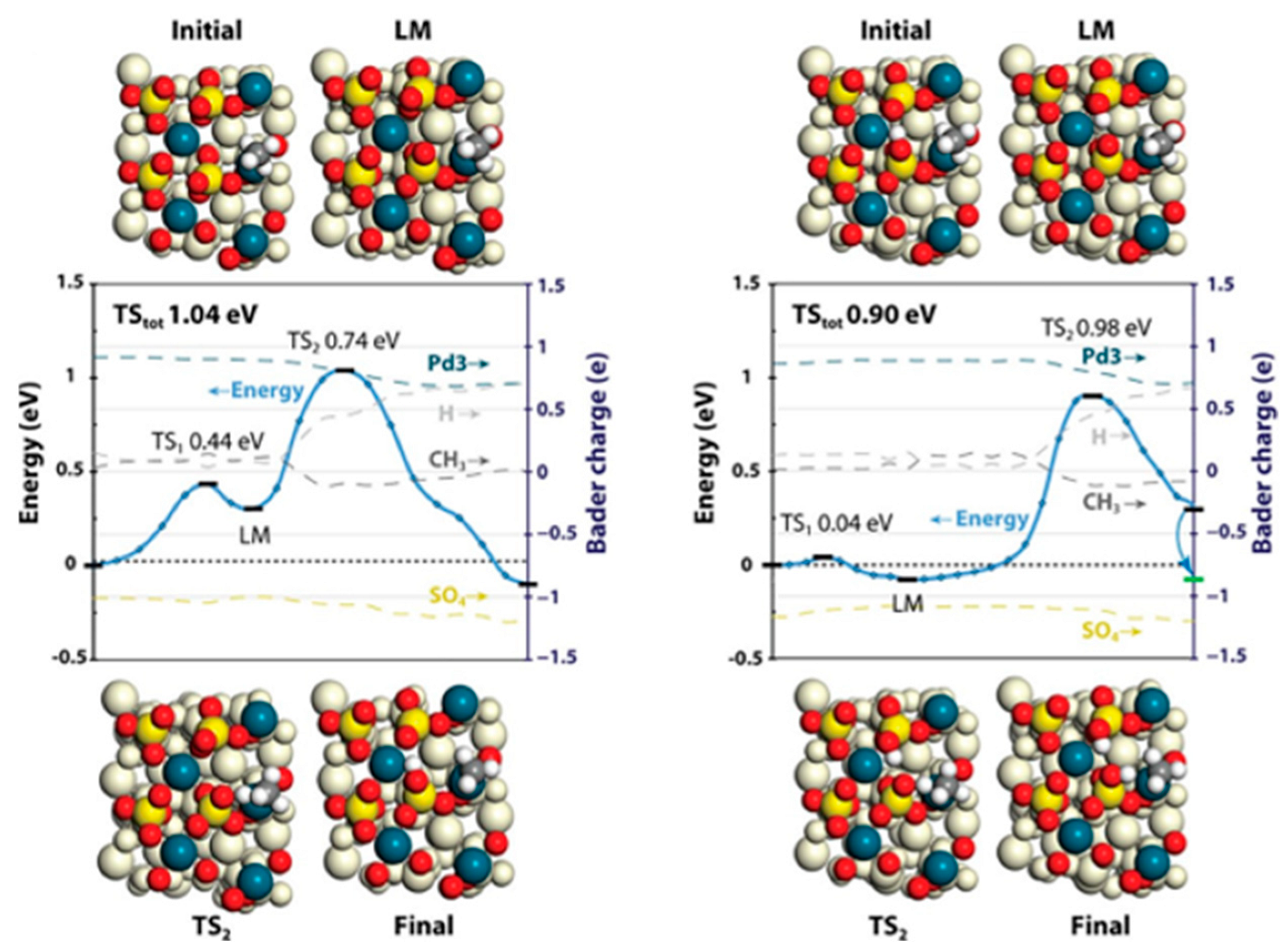
- Xue, W.J.; Mei, D.H. Mechanistic understanding of methane combustion over H-SSZ-13 zeolite encapsulated palladium nanocluster catalysts. Chem. Eng. J. 2022, 444, 136671. [Google Scholar] [CrossRef]
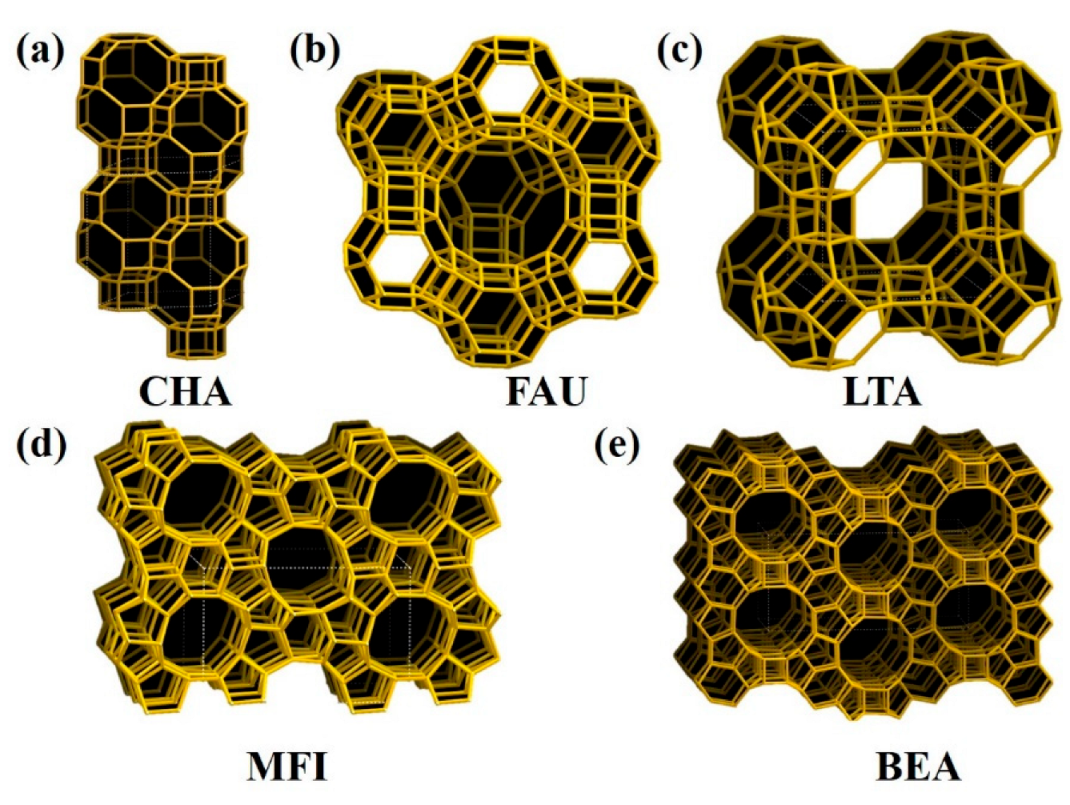



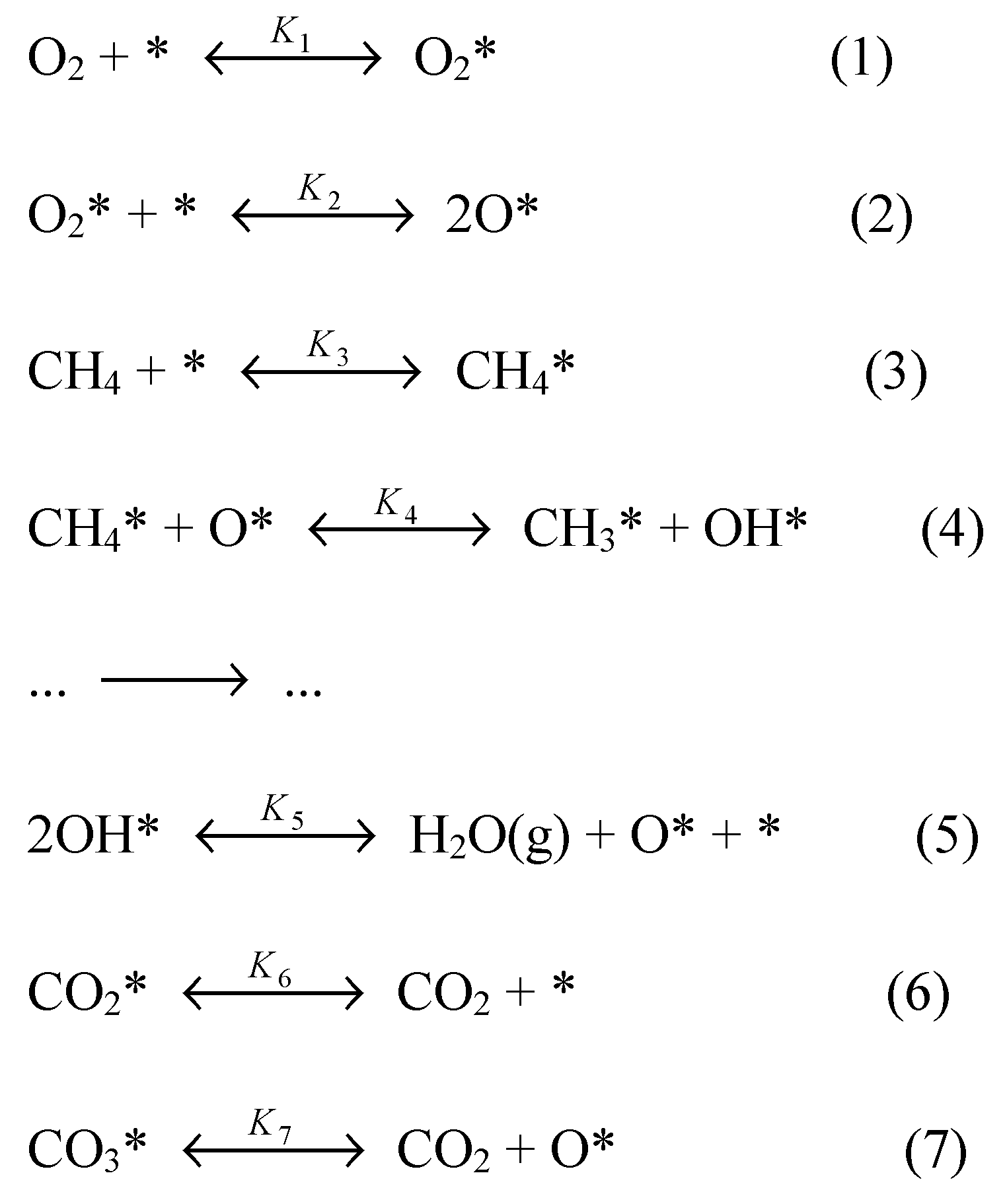
| Framework Type | Material Name | Dimensionality | Ring Type (Pore Type) | Limiting Pore Size (nm) |
|---|---|---|---|---|
| CHA | SSZ-13 | 3D | 8/6/4 (micropore) | 0.38 × 0.38 |
| LTA | LTA | 3D | 8/6/4 (micropore) | 0.41 × 0.41 |
| MFI | ZSM-5 | 3D | 10/6/5/4 (micropore) | 0.53 × 0.56 |
| BEA | Beta | 3D | 12/6/5/4 (micropore) | 0.66 × 0.67 |
| FAU | X, Y | 3D | 12/6/4 (micropore) | 0.33 × 0.33 |
| SBA-15 | - | 1D | -/-/- (mesopore) | 4.6–10.0 [42] |
| MCM-41 | - | 1D | -/-/- (mesopore) | 10.0 [43] |
| KIT-6 | - | 3D | -/-/- (mesopore) | 4.0–12.0 [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, J.; Liu, Y.; Deng, J.; Jing, L.; Hou, Z.; Wei, L.; Wang, Z.; Dai, H. Methane Combustion over Zeolite-Supported Palladium-Based Catalysts. Catalysts 2023, 13, 1251. https://doi.org/10.3390/catal13091251
Tao J, Liu Y, Deng J, Jing L, Hou Z, Wei L, Wang Z, Dai H. Methane Combustion over Zeolite-Supported Palladium-Based Catalysts. Catalysts. 2023; 13(9):1251. https://doi.org/10.3390/catal13091251
Chicago/Turabian StyleTao, Jinxiong, Yuxi Liu, Jiguang Deng, Lin Jing, Zhiquan Hou, Lu Wei, Zhiwei Wang, and Hongxing Dai. 2023. "Methane Combustion over Zeolite-Supported Palladium-Based Catalysts" Catalysts 13, no. 9: 1251. https://doi.org/10.3390/catal13091251
APA StyleTao, J., Liu, Y., Deng, J., Jing, L., Hou, Z., Wei, L., Wang, Z., & Dai, H. (2023). Methane Combustion over Zeolite-Supported Palladium-Based Catalysts. Catalysts, 13(9), 1251. https://doi.org/10.3390/catal13091251