Heterogeneous Copper Catalysts in the Aqueous Phase Hydrogenation of Maltose to Sorbitol
Abstract
1. Introduction
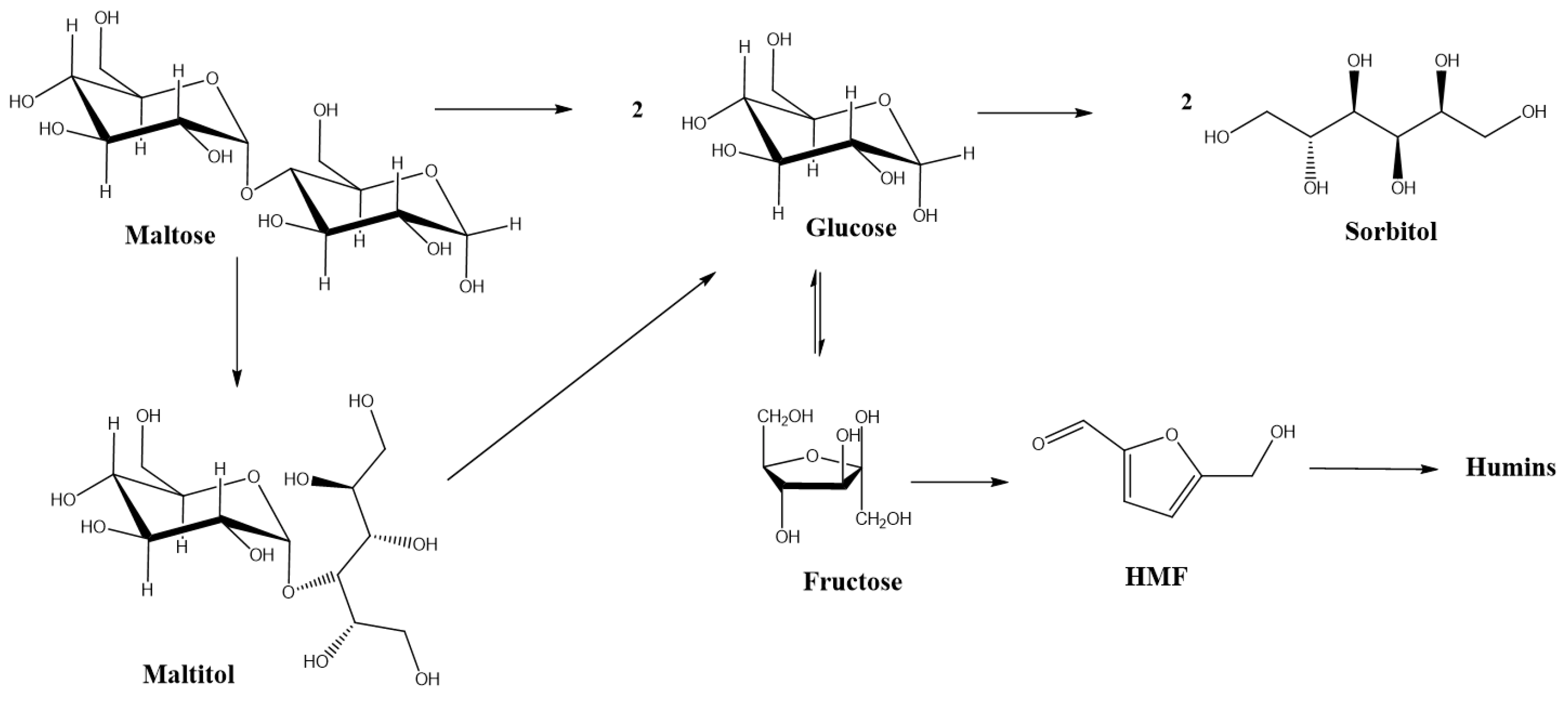
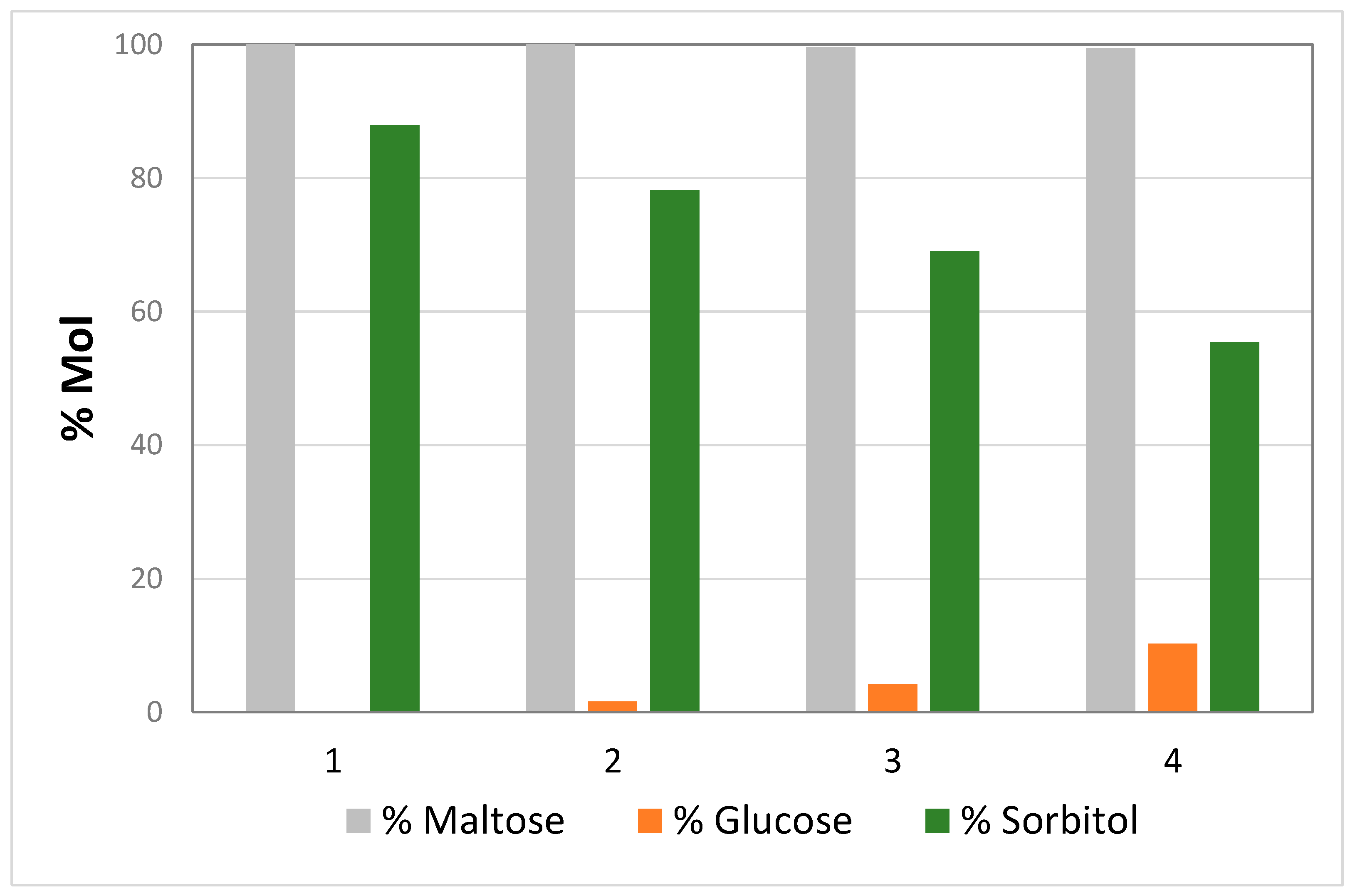
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. Catalyst Characterisation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass Volume I. In Results of Screening for Potential Candidates from Sugars and Synthesis Gas; U.S. Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 2004. [Google Scholar] [CrossRef]
- Saha, B.; Abu-Omar, M.M. Advances in 5-Hydroxymethylfurfural Production from Biomass in Biphasic Solvents. Green Chem. 2014, 16, 24–38. [Google Scholar] [CrossRef]
- Polidoro, D.; Perosa, A.; Barbaro, E.; Feltracco, M.; Argiriadis, E.; Selva, M. Multiphase Hydrogenation of D-Glucosamine Hydrochloride, N-Acetyl-d-Glucosamine, d-Glucose, and d-Maltose over Ru/C with Integrated Catalyst Recovery. ACS Sustain. Chem. Eng. 2022, 10, 2844–2858. [Google Scholar] [CrossRef]
- Bonnin, I.; Méreau, R.; Tassaing, T.; Jérôme, F.; De Oliveira Vigier, K. Hydrogenation of Sugars to Sugar Alcohols in the Presence of a Recyclable Ru/Al2O3 Catalyst Commercially Available. ACS Sustain. Chem. Eng. 2021, 9, 9240–9247. [Google Scholar] [CrossRef]
- Redina, E.; Tkachenko, O.; Salmi, T. Recent Advances in C5 and C6 Sugar Alcohol Synthesis by Hydrogenation of Monosaccharides and Cellulose Hydrolytic Hydrogenation over Non-Noble Metal Catalysts. Molecules 2022, 27, 1353. [Google Scholar] [CrossRef]
- Denkhaus, E.; Salnikow, K. Nickel Essentiality, Toxicity, and Carcinogenicity. Crit. Rev. Oncol. Hematol. 2002, 42, 35–56. [Google Scholar]
- Silvester, L.; Ramos, F.; Heyte, S.; Araque, M.; Paul, S.; Wojcieszak, R. Fully Integrated High-Throughput Methodology for the Study of Ni- and Cu- Supported Catalysts for Glucose Hydrogenation. Catal. Today 2019, 338, 72–80. [Google Scholar] [CrossRef]
- Sadier, A.; Shi, D.; Mamede, A.S.; Paul, S.; Marceau, E.; Wojcieszak, R. Selective Aqueous Phase Hydrogenation of Xylose to Xylitol over SiO2-Supported Ni and Ni-Fe Catalysts: Benefits of Promotion by Fe. Appl. Catal. B Environ. 2021, 298, 120564. [Google Scholar] [CrossRef]
- Fu, Y.; Ding, L.; Singleton, M.L.; Idrissi, H.; Hermans, S. Applied Catalysis B : Environmental Synergistic Effects Altering Reaction Pathways : The Case of Glucose Hydrogenation over Fe-Ni Catalysts. Appl. Catal. B Environ. 2021, 288, 119997. [Google Scholar] [CrossRef]
- Zelin, J.; Regenhardt, S.A.; Meyer, C.I.; Duarte, H.A.; Sebastian, V.; Marchi, A.J. Selective Aqueous-Phase Hydrogenation of D-Fructose into D-Mannitol Using a Highly Efficient and Reusable Cu-Ni/SiO2 Catalyst. Chem. Eng. Sci. 2019, 206, 315–326. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, Y.; Ding, M.; Zhao, J. Heterogeneous Cu Catalyst in Organic Transformations. Nano Res. 2022, 15, 2810–2833. [Google Scholar]
- Cavuoto, D.; Ardemani, L.; Ravasio, N.; Zaccheria, F.; Scotti, N. Some Insights into the Use of Heterogeneous Copper Catalysts in the Hydroprocessing of Levulinic Acid. Catalysts 2023, 13, 697. [Google Scholar]
- Ye, R.P.; Lin, L.; Li, Q.; Zhou, Z.; Wang, T.; Russell, C.K.; Adidharma, H.; Xu, Z.; Yao, Y.G.; Fan, M. Recent Progress in Improving the Stability of Copper-Based Catalysts for Hydrogenation of Carbon-Oxygen Bonds. Catal. Sci. Technol. 2018, 8, 3428–3449. [Google Scholar] [CrossRef]
- Esteban, J.; Yustos, P.; Ladero, M. Catalytic Processes from Biomass-Derived Hexoses and Pentoses: A Recent Literature Overview. Catalysts 2018, 8, 637. [Google Scholar]
- Upare, P.P.; Hwang, Y.K.; Kim, J.C.; Lee, J.H.; Kwak, S.K.; Hwang, D.W. A Robust and Highly Selective Catalytic System of Copper–Silica Nanocomposite and 1-Butanol in Fructose Hydrogenation to Mannitol. ChemSusChem 2020, 13, 5050–5057. [Google Scholar] [CrossRef]
- Saraiva, A.; Carrascosa, C.; Raheem, D.; Ramos, F.; Raposo, A. Maltitol: Analytical Determination Methods, Applications in the Food Industry, Metabolism and Health Impacts. Int. J. Environ. Res. Public Health 2020, 17, 5227. [Google Scholar]
- Zhang, J.; Li, J.; Wu, S.; Liu, Y. Efficient Conversion of Maltose into Sorbitol over Magnetic Catalyst in Extremely Low Acid. BioResources 2013, 8, 4676–4686. [Google Scholar] [CrossRef]
- Sulman, E.M.; Grigorev, M.E.; Doluda, V.Y.; Wärnå, J.; Matveeva, V.G.; Salmi, T.; Murzin, D.Y. Maltose Hydrogenation over Ruthenium Nanoparticles Impregnated in Hypercrosslinked Polystyrene. Chem. Eng. J. 2015, 282, 37–44. [Google Scholar] [CrossRef]
- Gromov, N.V.; Medvedeva, T.B.; Panchenko, V.N. One-Pot Hydrolysis-Hydrogenation of Potato Starch to Sorbitol Using Bifunctional Catalyst Ru/Cs3HSiW12O40. Catal. Ind. 2023, 15, 87–98. [Google Scholar] [CrossRef]
- Sadier, A.; Paul, S.; Marceau, E.; Wojcieszak, R. Ni-Fe Alloying Enhances the Efficiency of the Maltose Hydrogenation Process: The Role of Surface Species and Kinetic Study. Appl. Catal. B Environ. 2022, 313, 121446. [Google Scholar] [CrossRef]
- Araujo-Silva, R.; Mafra, A.C.O.; Rojas, M.J.; Kopp, W.; de Giordano, R.C.; Fernandez-Lafuente, R.; Tardioli, P.W. Maltose Production Using Starch from Cassava Bagasse Catalyzed by Cross-Linked β-Amylase Aggregates. Catalysts 2018, 8, 170. [Google Scholar] [CrossRef]
- Oh, Y.R.; Jang, Y.A.; Eom, G.T. Valorization of Waste Cooked Rice into Value-Added Maltobionic Acid Using Genetically Engineered Pseudomonas Taetrolens. ACS Sustain. Chem. Eng. 2022, 10, 810–815. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F.; et al. Food Waste as a Valuable Resource for the Production of Chemicals, Materials and Fuels. Current Situation and Global Perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar]
- Zaccheria, F.; Scotti, N.; Marelli, M.; Psaro, R.; Ravasio, N. Unravelling the Properties of Supported Copper Oxide: Can the Particle Size Induce Acidic Behaviour? Dalt. Trans. 2013, 42, 1319–1328. [Google Scholar] [CrossRef]
- Bellini, M.; Bianchi, S.; Zaccheria, F.; Ravasio, N. Tribology International Vegetable Oils as Triple Bottom Line Compliant Lubricants. Tribol. Int. 2021, 161, 107103. [Google Scholar] [CrossRef]
- Cavuoto, D.; Ravasio, N.; Scotti, N.; Gervasini, A.; Campisi, S.; Marelli, M.; Cappelletti, G.; Zaccheria, F. A Green Solvent Diverts the Hydrogenation of γ-Valerolactone to 1,4-Pentandiol over Cu/SiO2. Mol. Catal. 2021, 516, 111936. [Google Scholar] [CrossRef]
- Ravasio, N.; Zaccheria, F.; Allegrini, P.; Ercoli, M. Selective Hydrogenation of 4-(6-Methoxy-2-Naphtyl)-3-Buten-2-One to Nabumetone®. Catal. Today 2007, 121, 2–5. [Google Scholar] [CrossRef]
- Ravasio, N.; Antenori, M.; Gargano, M.; Mastrorilli, P. Cu/SiO2: An Improved Catalyst for the Chemoselective Hydrogenation of α,β-Unsaturated Ketones. Tetrahedron Lett. 1996, 37, 3529–3532. [Google Scholar] [CrossRef]
- Emeis, C.A. Determination of Integrated Molar Extinction Coefficients for Infrared Absorption Bands of Pyridine Adsorbed on Solid Acid Catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Mariani, M.; Zaccheria, F.; Psaro, R.; Ravasio, N. Some Insight into the Role of Different Copper Species as Acids in Cellulose Deconstruction. Catal. Commun. 2014, 44, 19–23. [Google Scholar] [CrossRef]
- Scotti, N.; Monticelli, D.; Zaccheria, F. Inorganica Chimica Acta Dispersed Copper Oxide : A Multifaceted Tool in Catalysis. Inorganica Chim. Acta 2012, 380, 194–200. [Google Scholar] [CrossRef]
- Shendurse, A.M.; Khedkar, C.D. Glucose: Properties and Analysis, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 3, ISBN 9780123849533. [Google Scholar]
- Scotti, N.; Finocchio, E.; Evangelisti, C.; Marelli, M.; Psaro, R.; Ravasio, N.; Zaccheria, F. Some Insight on the Structure/Activity Relationship of Metal Nanoparticles in Cu/SiO2 Catalysts. Chin. J. Catal. 2019, 40, 1788–1794. [Google Scholar] [CrossRef]
- Scotti, N.; Zaccheria, F.; Evangelisti, C.; Psaro, R.; Ravasio, N. Dehydrogenative Coupling Promoted by Copper Catalysts: A Way to Optimise and Upgrade Bio-Alcohols. Catal. Sci. Technol. 2017, 7, 1386–1393. [Google Scholar] [CrossRef]
- Gervasini, A.; Manzoli, M.; Martra, G.; Ponti, A.; Ravasio, N.; Sordelli, L.; Zaccheria, F. Dependence of Copper Species on the Nature of the Support for Dispersed CuO Catalysts. J. Phys. Chem. B 2006, 110, 7851–7861. [Google Scholar] [CrossRef]
- Cavuoto, D.; Zaccheria, F.; Marelli, M.; Evangelisti, C.; Piccolo, O.; Ravasio, N. The Role of Support Hydrophobicity in the Selective Hydrogenation of Enones and Unsaturated Sulfones over Cu/SiO2 Catalysts. Catalysts 2020, 10, 515. [Google Scholar] [CrossRef]
- Li, G.; Wang, B.; Resasco, D.E. Water-Mediated Heterogeneously Catalyzed Reactions. ACS Catal. 2020, 10, 1294–1309. [Google Scholar] [CrossRef]
- Lin, L.; Ge, Y.; Zhang, H.; Wang, M.; Xiao, D.; Ma, D. Heterogeneous Catalysis in Water. JACS Au 2021, 1, 1834–1848. [Google Scholar]
- Xu, C.; Yan, Z.; Yu, J.; Wang, X.; Ban, H.; Wang, Y.; Li, C. Applied Catalysis A, General Development of Stable Water-Resistant Cu-Based Catalyst for Methanol Synthesis. Appl. Catal. A Gen. 2021, 623, 118299. [Google Scholar] [CrossRef]


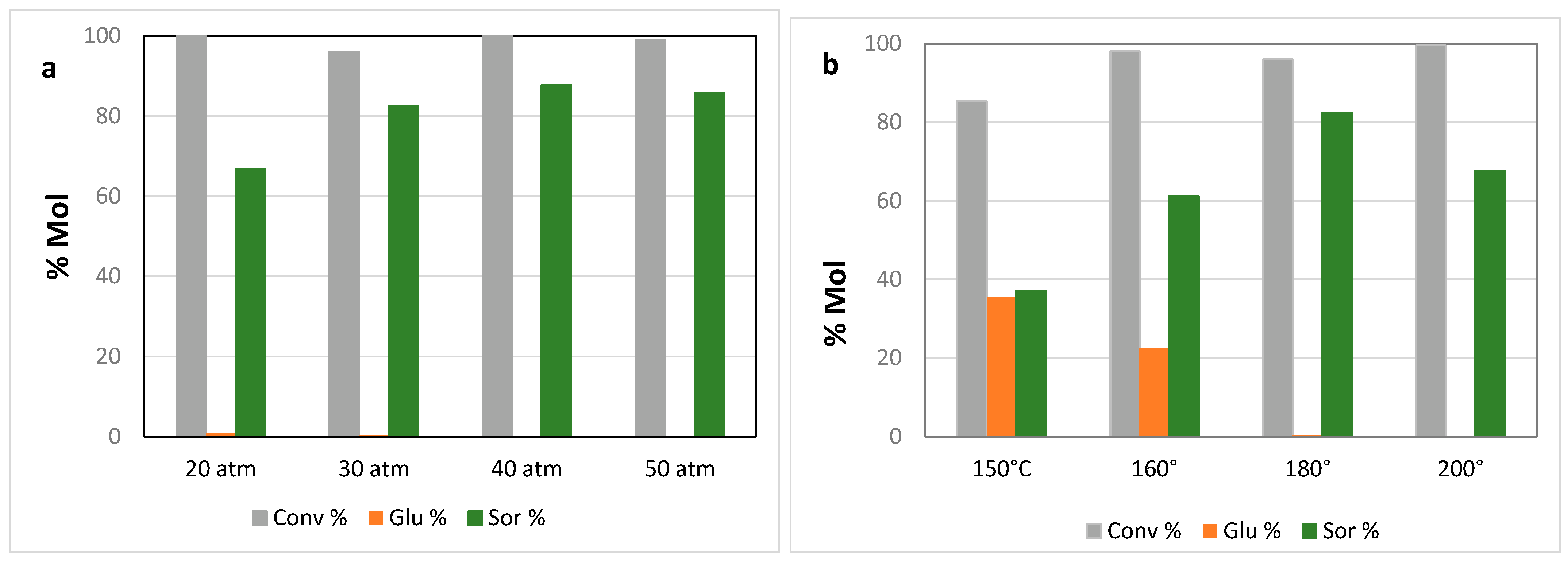
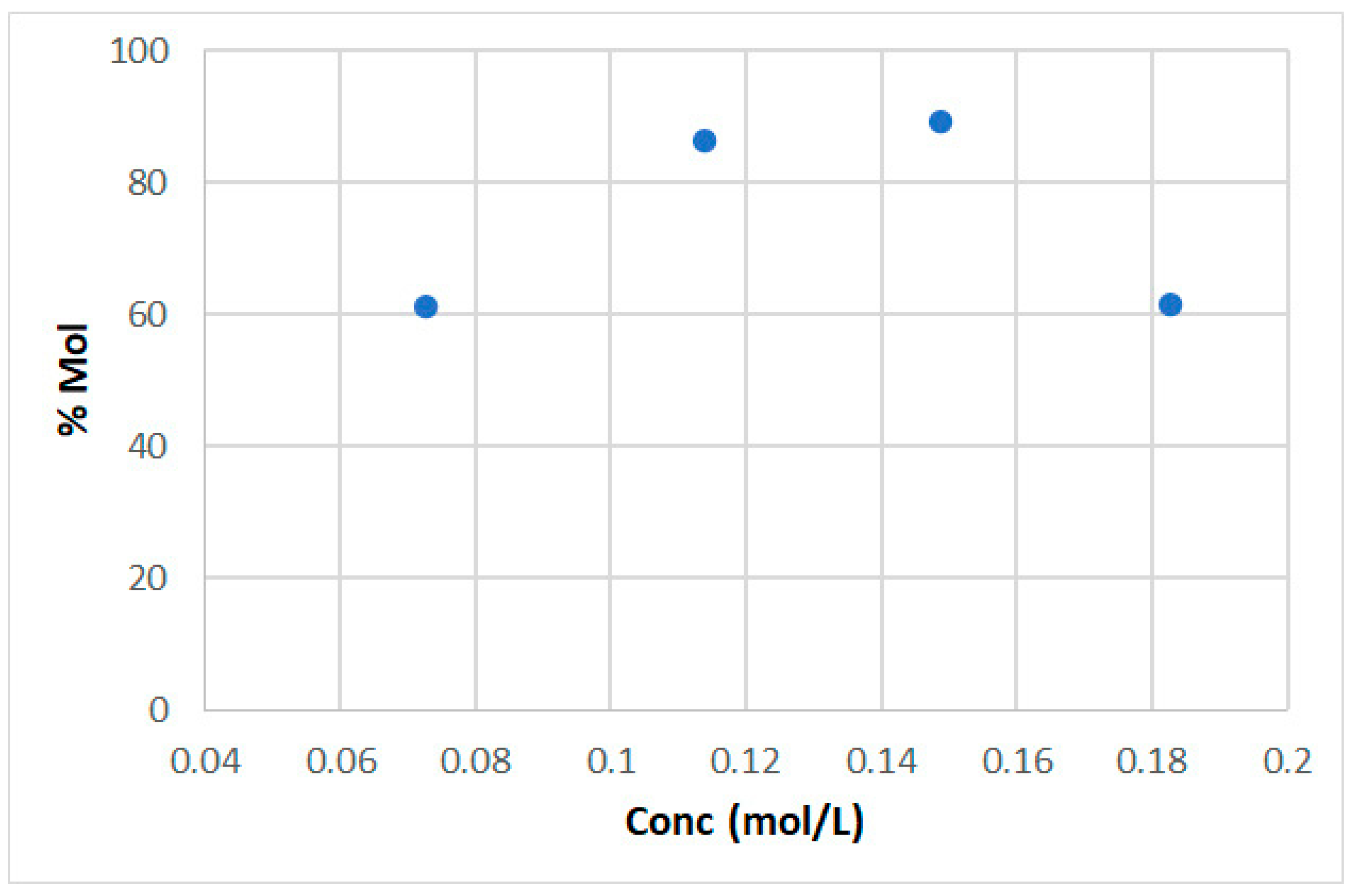
| Entry | Catalyst | Conversion | % Glucose | % Sorbitol | % HMF | Sorbitol Sel % |
|---|---|---|---|---|---|---|
| 1 | Cu/Al2O3 | 99.72 | 0.00 | 16.03 | - | 16.07 |
| 2 | Al2O3 | 90.94 | 7.45 | - | 8.83 | - |
| 3 | Cu/SiO2-Al2O3 | 99.64 | 22.22 | 3.05 | 2.7 | 3.06 |
| 4 | SiO2-Al2O3 | 95.42 | 1.01 | - | 20.77 | - |
| 5 | Cu/SiO2 A | 98.05 | 0 | 86.32 | - | 88.03 |
| 6 | SiO2 A | 99.43 | 39.47 | - | 21.37 | - |
| 7 | Cu/SiO2 B | 100 | 0.45 | 76.68 | - | 76.68 |
| 8 | SiO2 B | 99.46 | 45.41 | - | 19.91 | - |
| 9 | No cat | 98.70 | 45.99 | - | 20.66 | - |
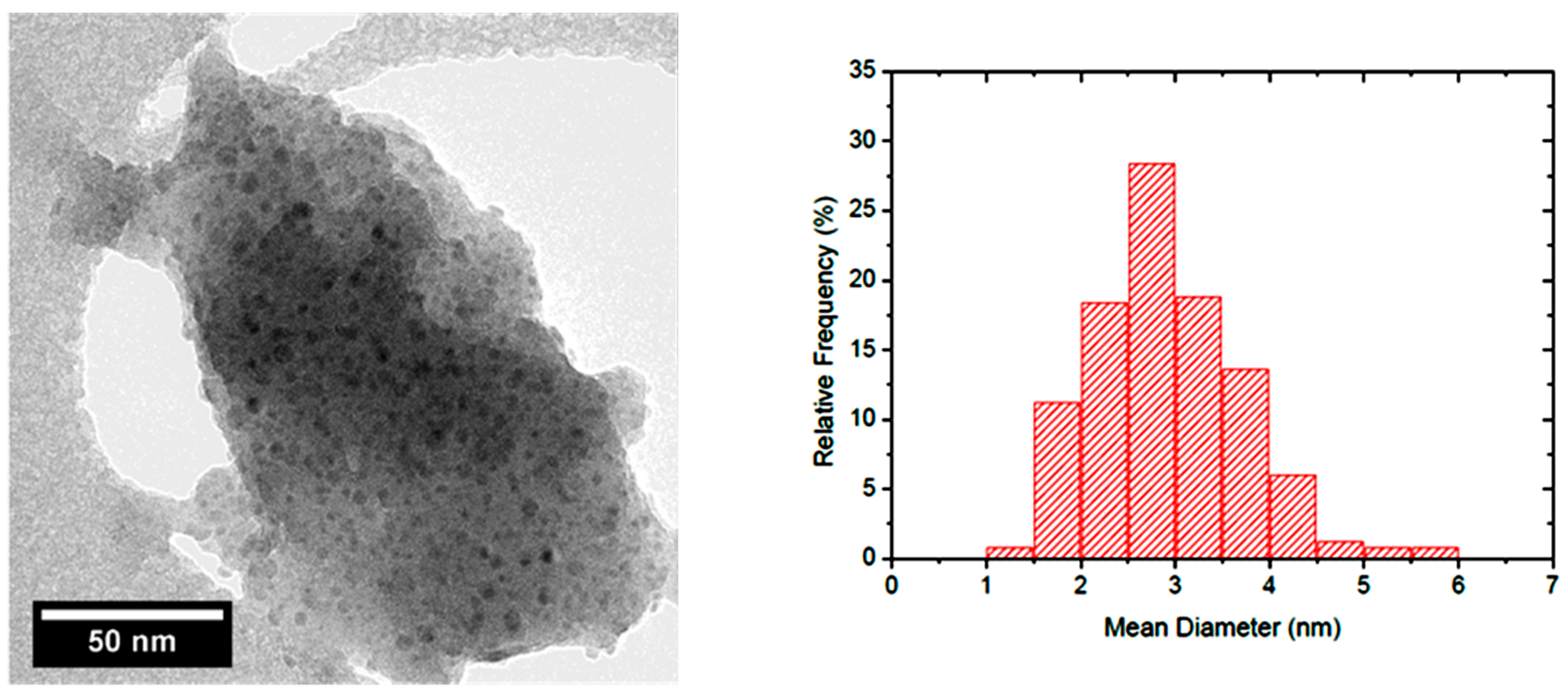
| Catalyst | Surface Area (m2/g) | Adsorbed Py (mmolPy/gcat) | Mean Particle ø (nm) | -OH Density (mmolOH/g) |
|---|---|---|---|---|
| Cu/Al2O3 | 110 | 0.069 | 3.7 | 2.02 |
| Cu/SiO2-Al2O3 | 247 | 0.119 | 3.2 ÷ 13 | 4.81 |
| Cu/SiO2 A | 318 | 0.061 | 2.9 | 3.80 |
| Cu/SiO2 B | 336 | 0.047 | 2.4 | 1.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pappalardo, V.; Zaccheria, F.; Scotti, N.; Ravasio, N. Heterogeneous Copper Catalysts in the Aqueous Phase Hydrogenation of Maltose to Sorbitol. Catalysts 2023, 13, 1183. https://doi.org/10.3390/catal13081183
Pappalardo V, Zaccheria F, Scotti N, Ravasio N. Heterogeneous Copper Catalysts in the Aqueous Phase Hydrogenation of Maltose to Sorbitol. Catalysts. 2023; 13(8):1183. https://doi.org/10.3390/catal13081183
Chicago/Turabian StylePappalardo, Valeria, Federica Zaccheria, Nicola Scotti, and Nicoletta Ravasio. 2023. "Heterogeneous Copper Catalysts in the Aqueous Phase Hydrogenation of Maltose to Sorbitol" Catalysts 13, no. 8: 1183. https://doi.org/10.3390/catal13081183
APA StylePappalardo, V., Zaccheria, F., Scotti, N., & Ravasio, N. (2023). Heterogeneous Copper Catalysts in the Aqueous Phase Hydrogenation of Maltose to Sorbitol. Catalysts, 13(8), 1183. https://doi.org/10.3390/catal13081183