Polyol Synthesis of Ag-Doped Copper Oxide Nanoparticles as a Methylene Blue-Degrading Agent
Abstract
1. Introduction
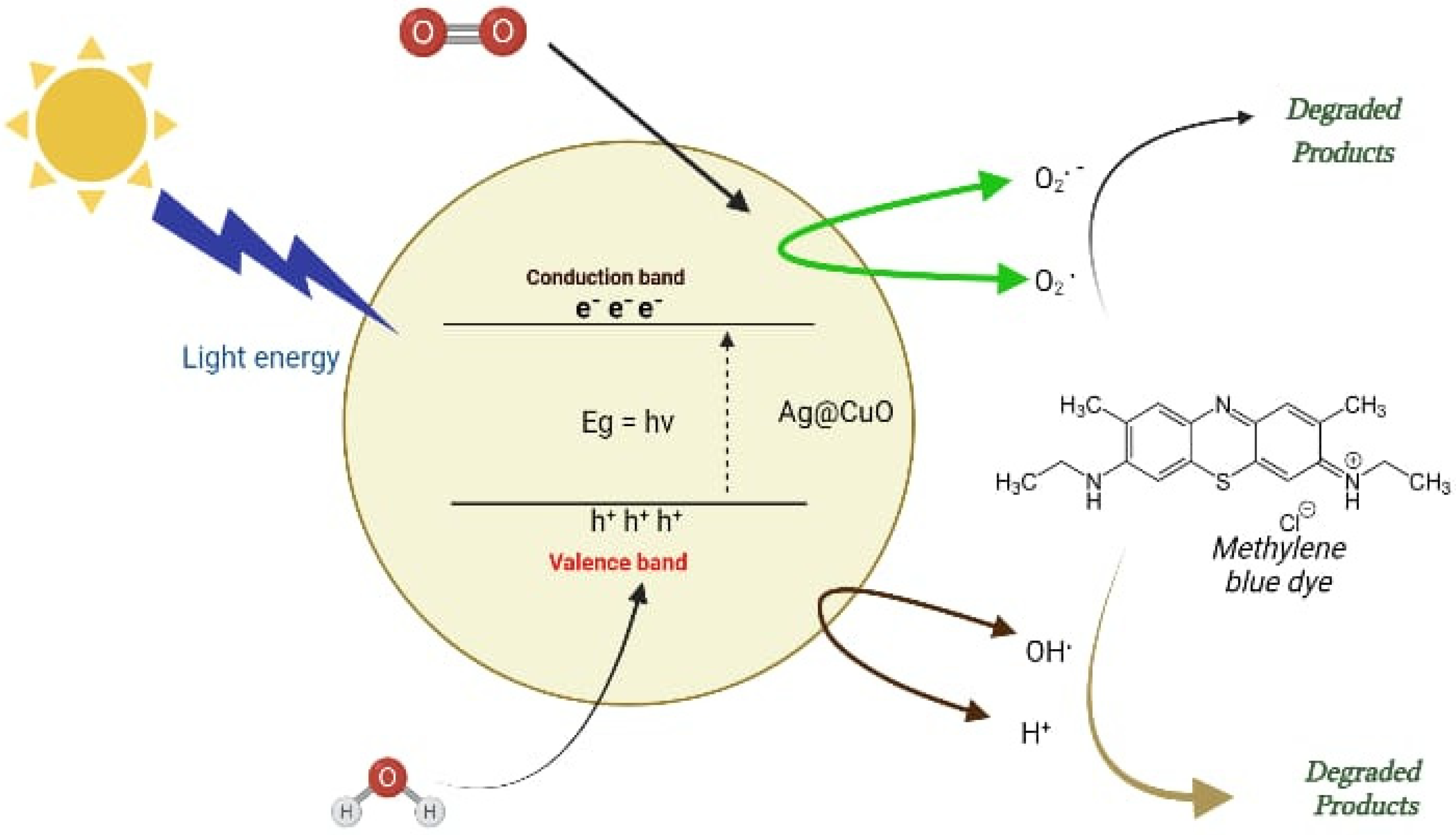
2. Results and Discussion
2.1. UV-Vis Absorption Spectroscopy Analysis
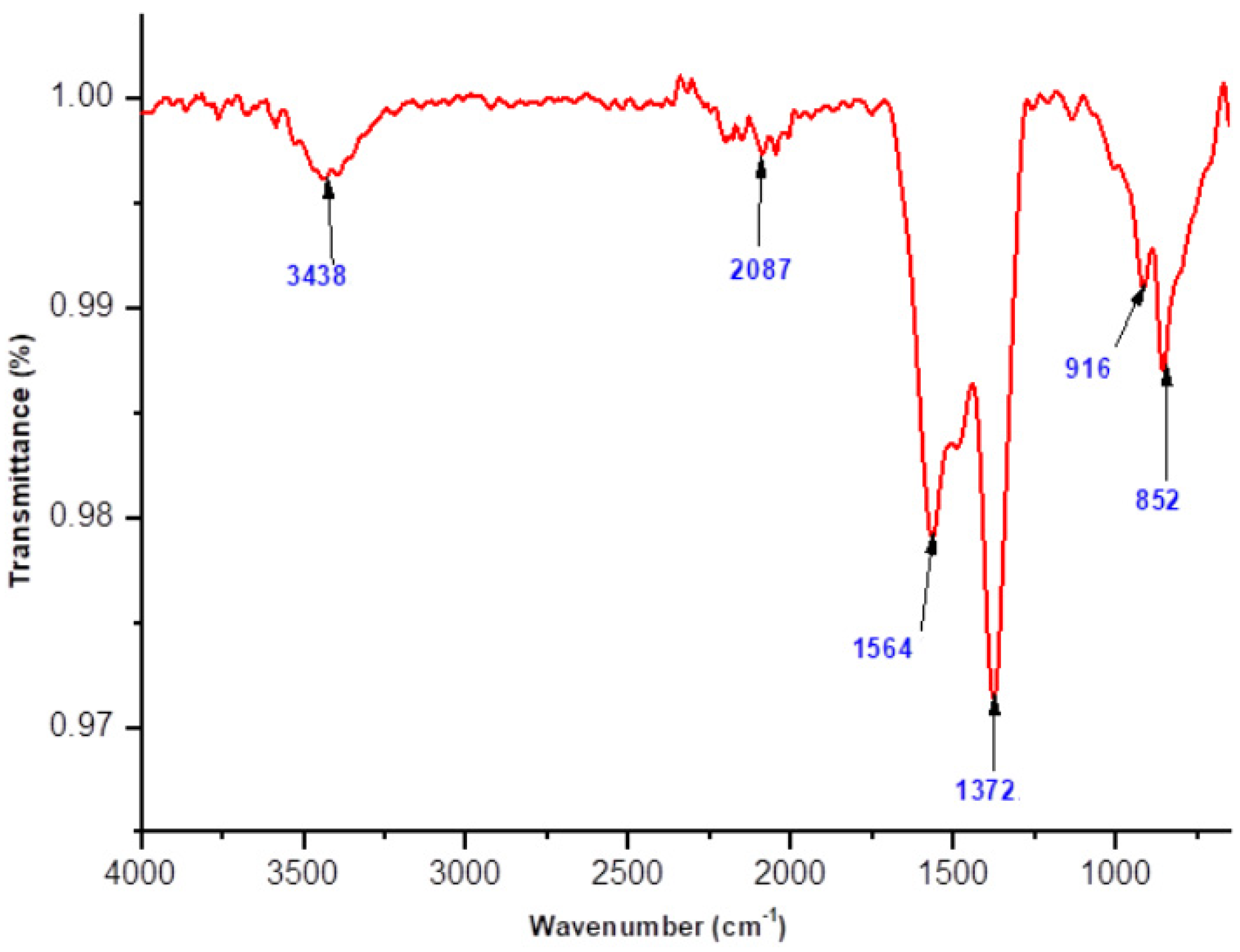
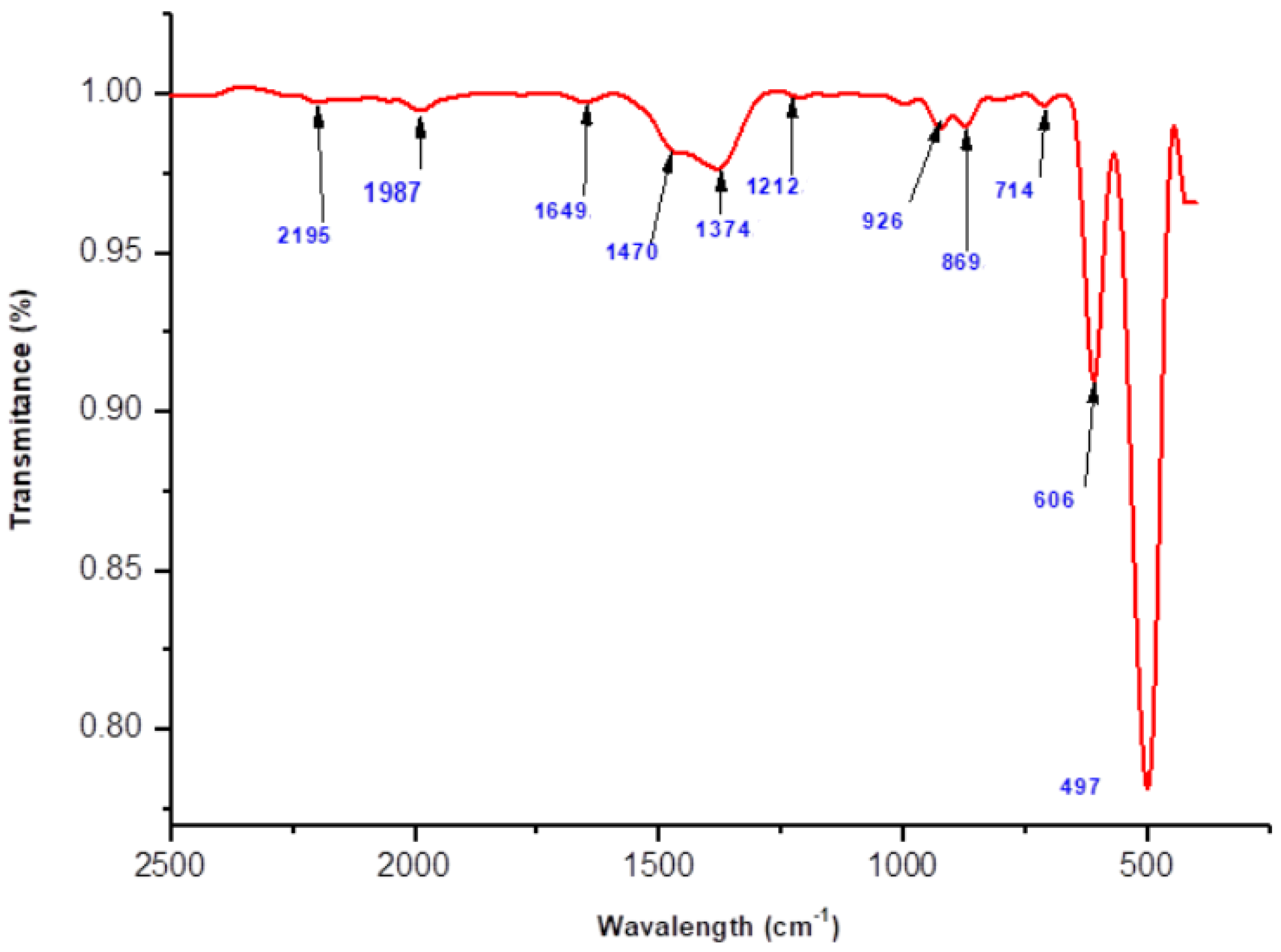
2.2. FT-IR Analysis
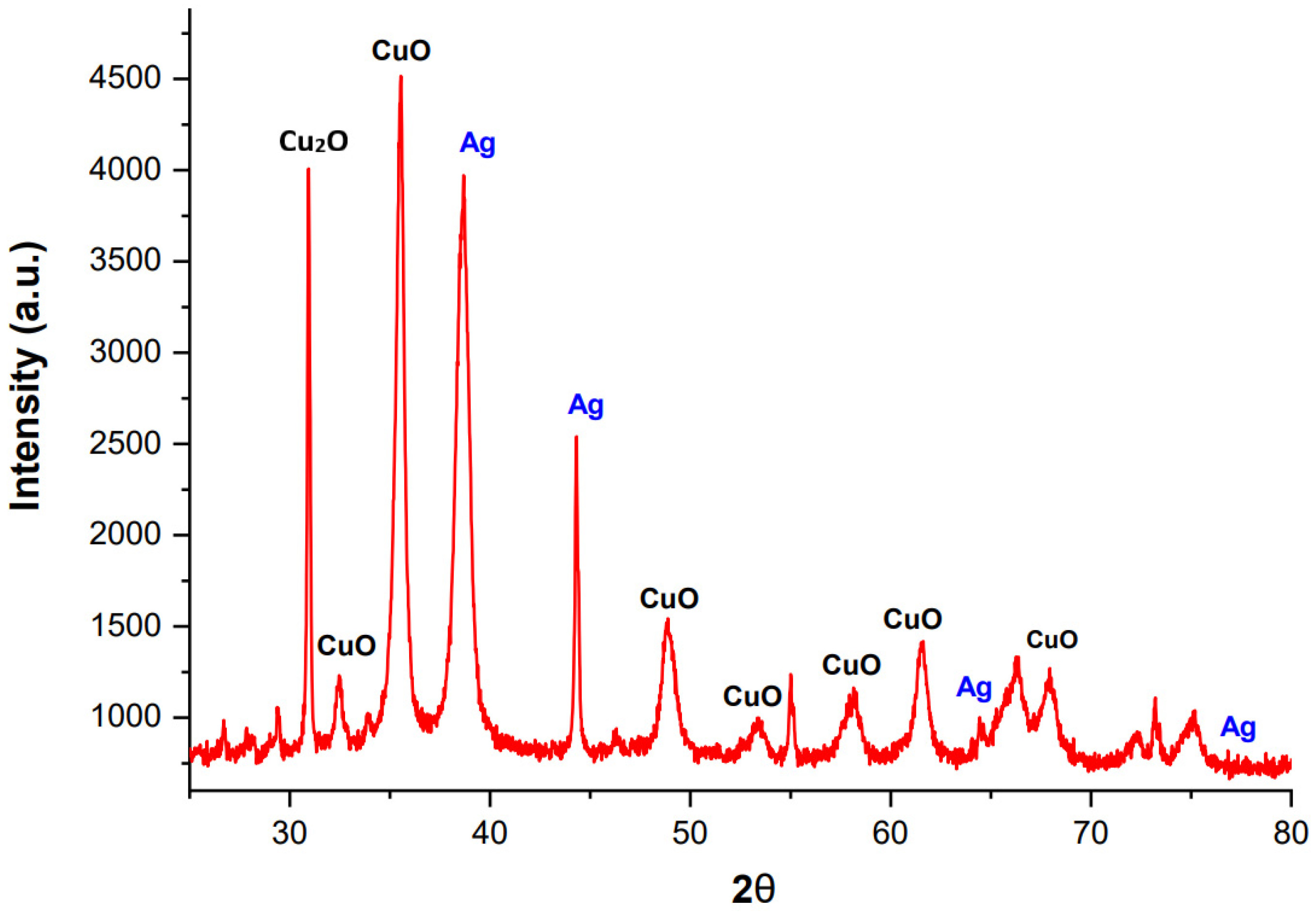
2.3. X-ray Diffraction Microscopy (XRD)
- λ = 1.54 A° (wavelength of X rays),
- n = order of diffraction (n = 1),
- d = distance between adjacent NPs layers,
- θ = diffraction angle
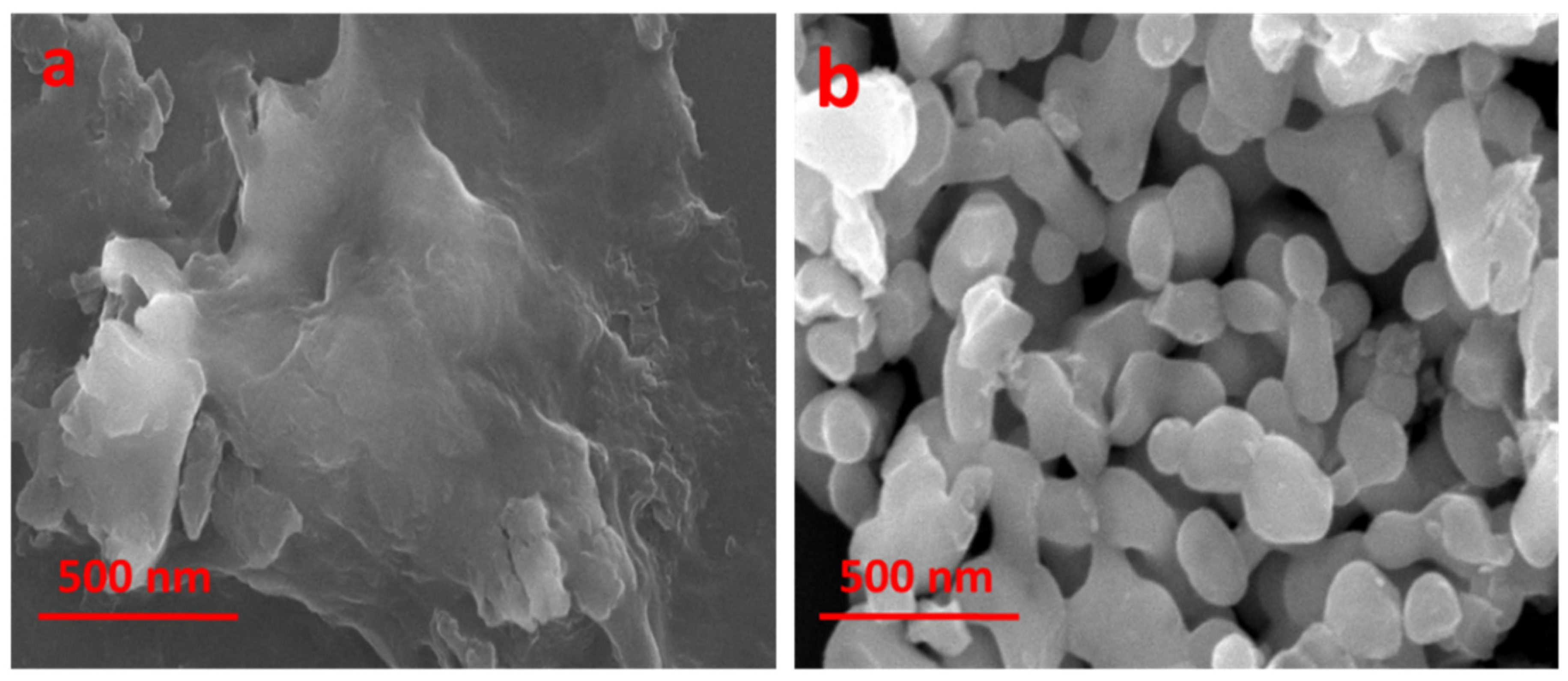
2.4. Field Emission Scanning Electron Microscopy (FE-SEM)
3. Materials and Methods
3.1. Materials
3.2. Preparation of Solutions
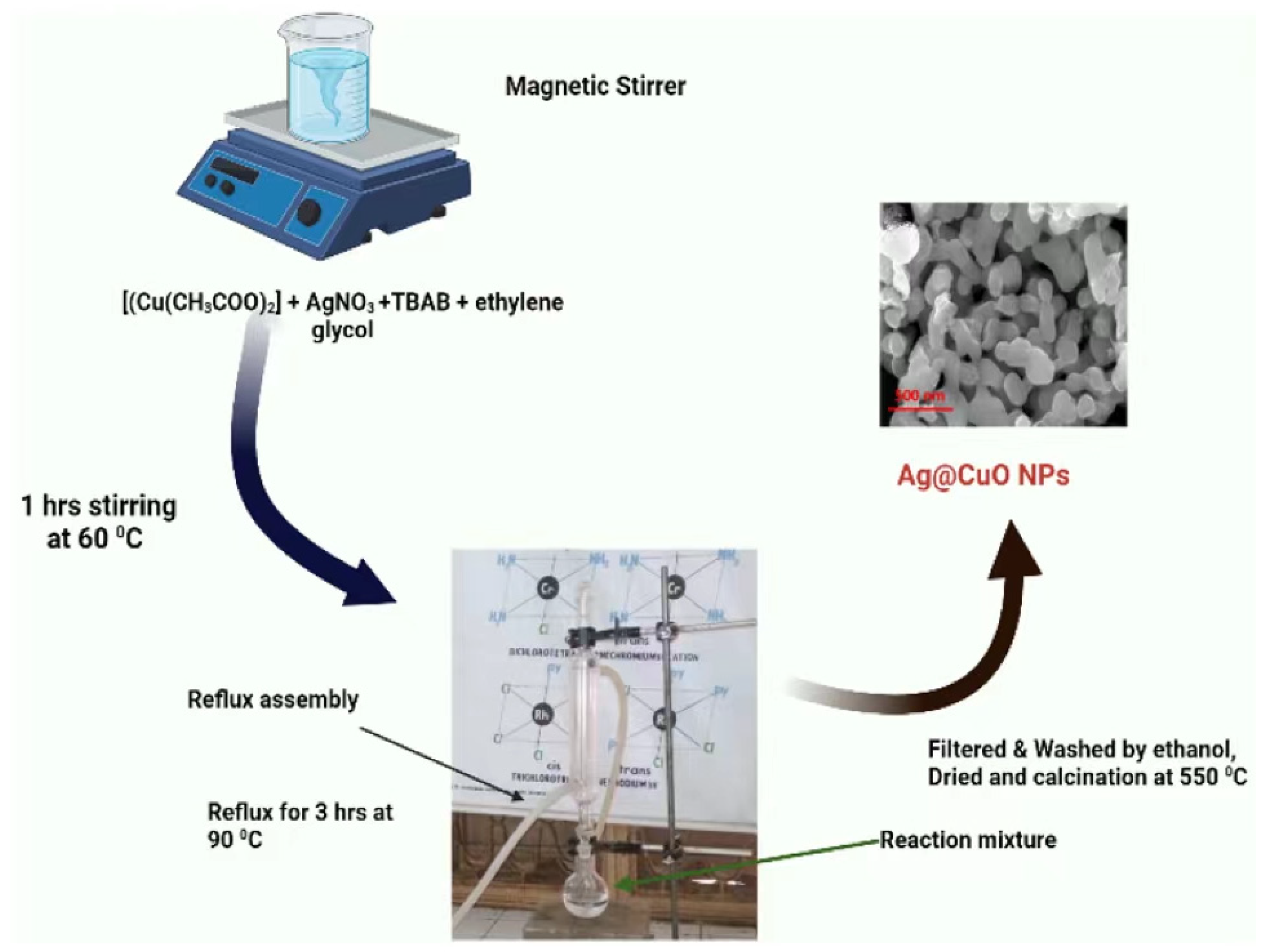
3.3. Synthesis of Polyol-Mediated Ag-CuO NPs Using the Refluxing Method
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Soni, S.A.; Jadhav, V.R.; Kere, T.A. Effect of Copper Substitution, Calcination Temperature, and Photo-sensitizers on Photocatalytic Activity of Cu0.05Zn0.95O. J. Chem. Environ. Sci. Appl. 2018, 5, 1–9. [Google Scholar] [CrossRef]
- Walunj, P.; Roy, A.; Jadhav, V.; Athare, P.; Dhaygude, A.; Aher, J.; Algethami, J.S.; Lokhande, D.; Alqahtani, M.S.; Bhagare, A.; et al. Polyol-mediated zinc oxide nanoparticles using the refluxing method as an efficient photocatalytic and antimicrobial agent. Front. Bioeng. Biotechnol. 2023, 11, 1177981. [Google Scholar] [CrossRef] [PubMed]
- Chakrabortty, S.; Kumar, R.; Nayak, J.; Jeon, B.H.; Dargar, S.K.; Tripathy, S.K.; Pal, P.; Ha, G.S.; Kim, K.H.; Jasiński, M. Green synthesis of MeOH derivatives through in situ catalytic transformations of captured CO2 in a membrane integrated photo-microreactor system: A state-of-art review for carbon capture and utilization. Renew. Sust. Energ. Rev. 2023, 182, 113417. [Google Scholar] [CrossRef]
- Kunwar, S.; Roy, A.; Bhusal, U.; Gacem, A.; Abdullah, M.M.; Sharma, P.; Yadav, K.K.; Rustagi, S.; Chatterjee, N.; Deshwal, V.K.; et al. Bio-Fabrication of Cu/Ag/Zn Nanoparticles and Their Antioxidant and Dye Degradation Activities. Catalysts 2023, 13, 891. [Google Scholar] [CrossRef]
- Ardila-Leal, L.D.; Poutou-Piñales, R.A.; Pedroza-Rodríguez, A.M.; Quevedo-Hidalgo, B.E. A brief history of colour, the environmental impact of synthetic dyes and removal by using laccases. Molecules 2021, 26, 3813. [Google Scholar] [CrossRef] [PubMed]
- Dikshith, T.S.S. Hazardous Chemicals: Safety Management and Global Regulations; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Elgarahy, A.M.; Elwakeel, K.Z.; Mohammad, S.H.; Elshoubaky, G.A. A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Clean. Eng. Technol. 2021, 4, 100209. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayak, A.; Agarwal, S. Chemical treatment technologies for wastewater recycling—An overview. Rsc. Adv. 2012, 2, 6380–6388. [Google Scholar] [CrossRef]
- Roy, A.; Elzaki, A.; Tirth, V.; Kajoak, S.; Osman, H.; Algahtani, A.; Islam, S.; Faizo, N.L.; Khandaker, M.U.; Islam, M.N.; et al. Biological synthesis of nanocatalysts and their applications. Catalysts 2021, 11, 1494. [Google Scholar] [CrossRef]
- Raizada, P.; Sudhaik, A.; Patial, S.; Hasija, V.; Khan, A.A.P.; Singh, P.; Gautam, S.; Kaur, M.; Nguyen, V.H. Engineering nanostructures of CuO-based photocatalysts for water treatment: Current progress and future challenges. Arab. J. Chem. 2020, 13, 8424–8457. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Haider, W. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: A review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef]
- Ameta, R.; Solanki, M.S.; Benjamin, S.; Ameta, S.C. Photocatalysis. In Advanced Oxidation Processes for Waste Water Treatment; Academic Press: Cambridge, MA, USA, 2018; pp. 135–175. [Google Scholar]
- Mani, V.M.; Kalaivani, S.; Sabarathinam, S.; Vasuki, M.; Soundari, A.J.P.G.; Das, M.A.; Elfasakhany, A.; Pugazhendhi, A. Copper oxide nanoparticles synthesized from an endophytic fungus Aspergillus terreus: Bioactivity and anti-cancer evaluations. Environ. Res. 2021, 201, 111502. [Google Scholar] [CrossRef] [PubMed]
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Ng, H.Y.; Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2021, 12, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Razali, M.; Kim, J.F.; Attfield, M.; Budd, P.M.; Drioli, E.; Lee, Y.M.; Szekely, G. Sustainable wastewater treatment and recycling in membrane manufacturing. Green Chem. 2015, 17, 5196–5205. [Google Scholar] [CrossRef]
- Parvathiraja, C.; Shailajha, S. Bioproduction of CuO and Ag/CuO heterogeneous photocatalysis-photocatalytic dye degradation and biological activities. Appl. Nanosci. 2021, 11, 1411–1425. [Google Scholar] [CrossRef]
- Chung, K.-H.; Kim, B.-J.; Park, Y.-K.; Kim, S.-C.; Jung, S.-C. Photocatalytic properties of amorphous N-doped TiO2 photocatalyst under visible light irradiation. Catalysts 2021, 11, 1010. [Google Scholar] [CrossRef]
- Cheriyamundath, S.; Vavilala, S.L. Nanotechnology-based wastewater treatment. Water Environ. J. 2021, 35, 123–132. [Google Scholar] [CrossRef]
- Mittal, S.; Roy, A. Fungus and plant-mediated synthesis of metallic nanoparticles and their application in degradation of dyes. In Photocatalytic Degradation of Dyes; Elsevier: Amsterdam, The Netherlands, 2021; pp. 287–308. [Google Scholar]
- Mondal, K.; Sharma, A. Recent advances in the synthesis and application of photocatalytic metal–metal oxide core–shell nanoparticles for environmental remediation and their recycling process. RSC Adv. 2016, 6, 83589–83612. [Google Scholar] [CrossRef]
- Roy, A.; Sharma, A.; Yadav, S.; Jule, L.T.; Krishnaraj, R. Nanomaterials for remediation of environmental pollutants. Bioinorg. Chem. Appl. 2021, 2021, 1764647. [Google Scholar] [CrossRef]
- Pawar, M.; Topcu Sendoğdular, S.; Gouma, P. A brief overview of TiO2 photocatalyst for organic dye remediation: Case study of reaction mechanisms involved in Ce-TiO2 photocatalysts system. J. Nanomater. 2018, 2018, 5953609. [Google Scholar] [CrossRef]
- Li, H.; Su, Z.; Hu, S.; Yan, Y. Free-standing and flexible Cu/Cu2O/CuO heterojunction net: A novel material as cost-effective and easily recycled visible-light photocatalyst. Appl. Catal. B Environ. 2017, 207, 134–142. [Google Scholar] [CrossRef]
- El Sayed, A.M.; Shaban, M. Structural, optical and photocatalytic properties of Fe and (Co, Fe) co-doped copper oxide spin coated films. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 638–646. [Google Scholar] [CrossRef]
- Maraj, M.; Raza, A.; Wang, X.; Chen, J.; Riaz, K.N.; Sun, W. Mo-doped CuO nanomaterial for photocatalytic degradation of water pollutants under visible light. Catalysts 2021, 11, 1198. [Google Scholar] [CrossRef]
- Devi, L.V.; Sellaiyan, S.; Selvalakshmi, T.; Zhang, H.J.; Uedono, A.; Sivaji, K.; Sankar, S. Synthesis, defect characterization and photocatalytic degradation efficiency of Tb doped CuO nanoparticles. Adv. Powder Technol. 2017, 28, 3026–3038. [Google Scholar] [CrossRef]
- Koffyberg, F.P.; Benko, F.A. A photoelectrochemical determination of the position of the conduction and valence band edges of p-type CuO. J. Appl. Phys. 1982, 53, 1173–1177. [Google Scholar] [CrossRef]
- Roy, A.; Murthy, H.A.; Ahmed, H.M.; Islam, M.N.; Prasad, R. Phytogenic Synthesized of Metal/Metal Oxide Nanoparticles for Degradation of Dyes. J. Renew. Mater. 2022, 10, 1–20. [Google Scholar] [CrossRef]
- Dasineh Khiavi, N.; Katal, R.; Kholghi Eshkalak, S.; Masudy-Panah, S.; Ramakrishna, S.; Jiangyong, H. Visible light driven heterojunction photocatalyst of CuO–Cu2O thin films for photocatalytic degradation of organic pollutants. Nanomaterials 2019, 9, 1011. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Hardy, J.S.; Scott Weil, K. Effects of CuO content on the wetting behavior and mechanical properties of a Ag–CuO braze for ceramic joining. J. Am. Ceram. Soc. 2005, 88, 2521–2527. [Google Scholar] [CrossRef]
- Pitrubhakta, J.R.; Kere, T.A.; Shinde, S.S.; Soni, S.A.; Jadhav, V.R. Synthesis, Characterization, and Gas Sensing performance of Nanometer TiO2 thick film by Hydrothermal method. Asian J. Res. Chem. 2020, 13, 360–364. [Google Scholar] [CrossRef]
- Jadhav, V.R. Mathematical treatment to understanding the concentration terms. Int. J. Res. Rev. 2019, 6, 1–4. [Google Scholar]
- Iqbal, S.; Javed, M.; Bahadur, A.; Qamar, M.A.; Ahmad, M.; Shoaib, M.; Raheel, M.; Ahmad, N.; Akbar, M.B.; Li, H. Controlled synthesis of Ag-doped CuO nanoparticles as a core with poly (acrylic acid) microgel shell for efficient removal of methylene blue under visible light. J. Mater. Sci. Mater. Electron. 2020, 31, 8423–8435. [Google Scholar] [CrossRef]
- Bishnoi, S.; Kumar, A.; Selvaraj, R. Facile synthesis of magnetic iron oxide nanoparticles using inedible Cynometra ramiflora fruit extract waste and their photocatalytic degradation of methylene blue dye. Mater. Res. Bull. 2018, 97, 121–127. [Google Scholar] [CrossRef]
- Ashraf, I.; Singh, N.B.; Agarwal, A. Green synthesis of iron oxide nanoparticles using Amla seed for methylene blue dye removal from water. Mater. Today Proc. 2023, 72, 311–316. [Google Scholar] [CrossRef]
- Dasgupta, N.; Nayak, M.A.; Gauthier, M. Starch-Stabilized Iron Oxide Nanoparticles for the Photocatalytic Degradation of Methylene Blue. Polysaccharides 2022, 3, 655–670. [Google Scholar] [CrossRef]
- Vanaja, M.; Paulkumar, K.; Baburaja, M.; Rajeshkumar, S.; Gnanajobitha, G.; Malarkodi, C.; Sivakavinesan, M.; Annadurai, G. Degradation of methylene blue using biologically synthesized silver nanoparticles. Bioinorg. Chem. Appl. 2014, 2014, 742346. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Yadav, V.K.; Gacem, A.; Ali, I.H.; Dave, D.; Khan, S.H.; Yadav, K.K.; Rather, S.-U.; Ahn, Y.; Son, C.T.; et al. Recent and emerging trends in remediation of methylene blue dye from wastewater by using zinc oxide nanoparticles. Water 2022, 14, 1749. [Google Scholar] [CrossRef]

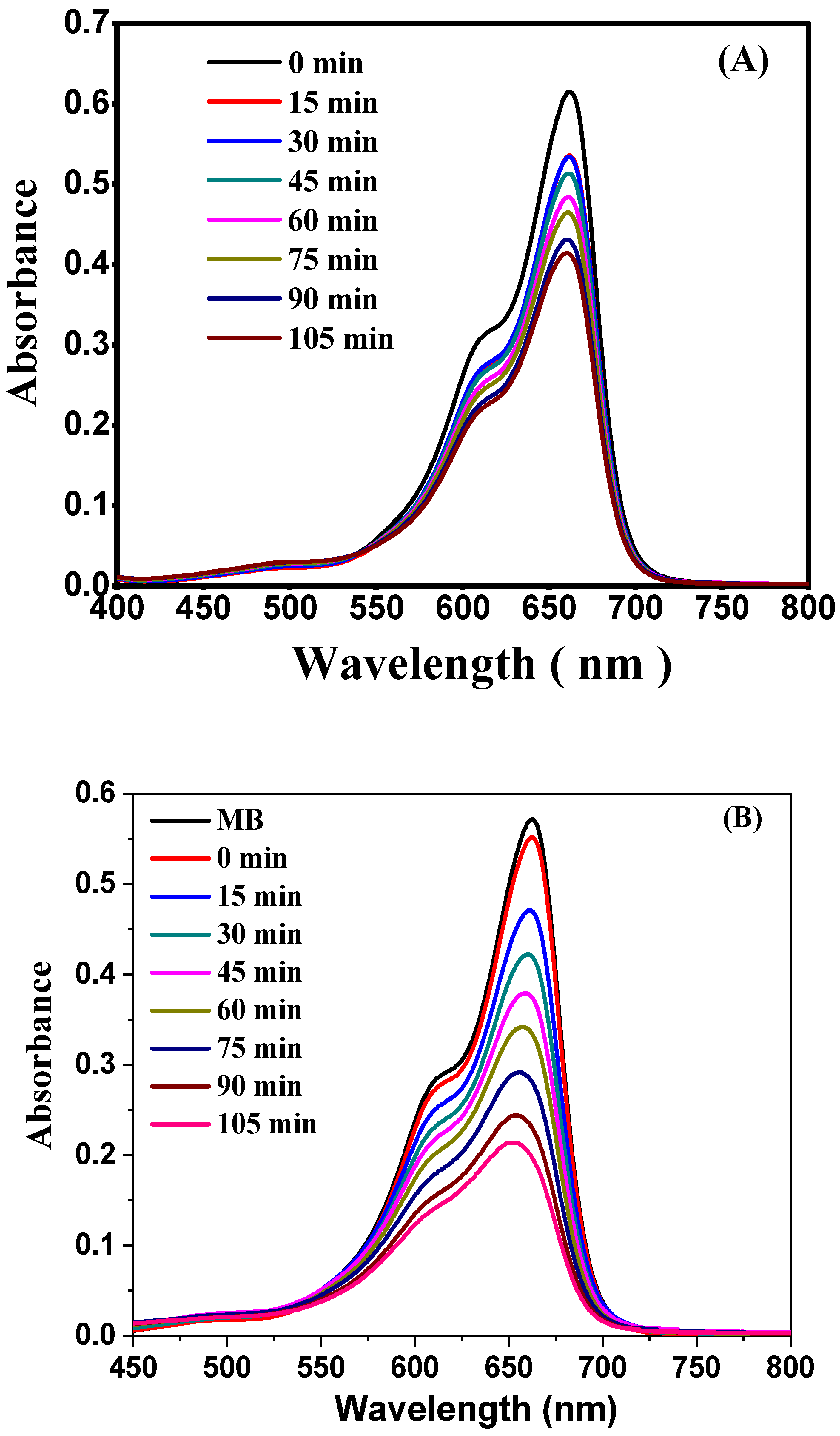

| Sr. No. | Time (min) | Absorbance (A) of MB | Rate Constant (K) min−1 | The Mean Value of Rate Constant (K) min−1 |
|---|---|---|---|---|
| 1 | 0 | 0.5560 | - | 8.641 × 10−3 |
| 2 | 15 | 0.4560 | 1.548 × 10−2 | |
| 3 | 30 | 0.4125 | 1.108 × 10−2 | |
| 4 | 45 | 0.3590 | 1.047 × 10−2 | |
| 5 | 60 | 0.3426 | 8.635 × 10−3 | |
| 6 | 75 | 0.2928 | 9.003 × 10−3 | |
| 7 | 90 | 0.2359 | 9.903 × 10−3 | |
| 8 | 105 | 0.2045 | 9.849 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baste, Y.; Jadhav, V.; Roy, A.; Alghamdi, S.; Abbas, M.; Algethami, J.S.; Almehmadi, M.; Allahyani, M.; Verma, D.; Yadav, K.K.; et al. Polyol Synthesis of Ag-Doped Copper Oxide Nanoparticles as a Methylene Blue-Degrading Agent. Catalysts 2023, 13, 1143. https://doi.org/10.3390/catal13071143
Baste Y, Jadhav V, Roy A, Alghamdi S, Abbas M, Algethami JS, Almehmadi M, Allahyani M, Verma D, Yadav KK, et al. Polyol Synthesis of Ag-Doped Copper Oxide Nanoparticles as a Methylene Blue-Degrading Agent. Catalysts. 2023; 13(7):1143. https://doi.org/10.3390/catal13071143
Chicago/Turabian StyleBaste, Yogeshwar, Vikram Jadhav, Arpita Roy, Saad Alghamdi, Mohamed Abbas, Jari S. Algethami, Mazen Almehmadi, Mamdouh Allahyani, Devvret Verma, Krishna Kumar Yadav, and et al. 2023. "Polyol Synthesis of Ag-Doped Copper Oxide Nanoparticles as a Methylene Blue-Degrading Agent" Catalysts 13, no. 7: 1143. https://doi.org/10.3390/catal13071143
APA StyleBaste, Y., Jadhav, V., Roy, A., Alghamdi, S., Abbas, M., Algethami, J. S., Almehmadi, M., Allahyani, M., Verma, D., Yadav, K. K., Jeon, B.-H., & Park, H.-K. (2023). Polyol Synthesis of Ag-Doped Copper Oxide Nanoparticles as a Methylene Blue-Degrading Agent. Catalysts, 13(7), 1143. https://doi.org/10.3390/catal13071143









