A Comparative Study of the Antiviral Properties of Thermally Sprayed Coatings against Human Coronavirus HCoV-229E
Abstract
1. Introduction
2. Results and Discussion
2.1. Phase Composition
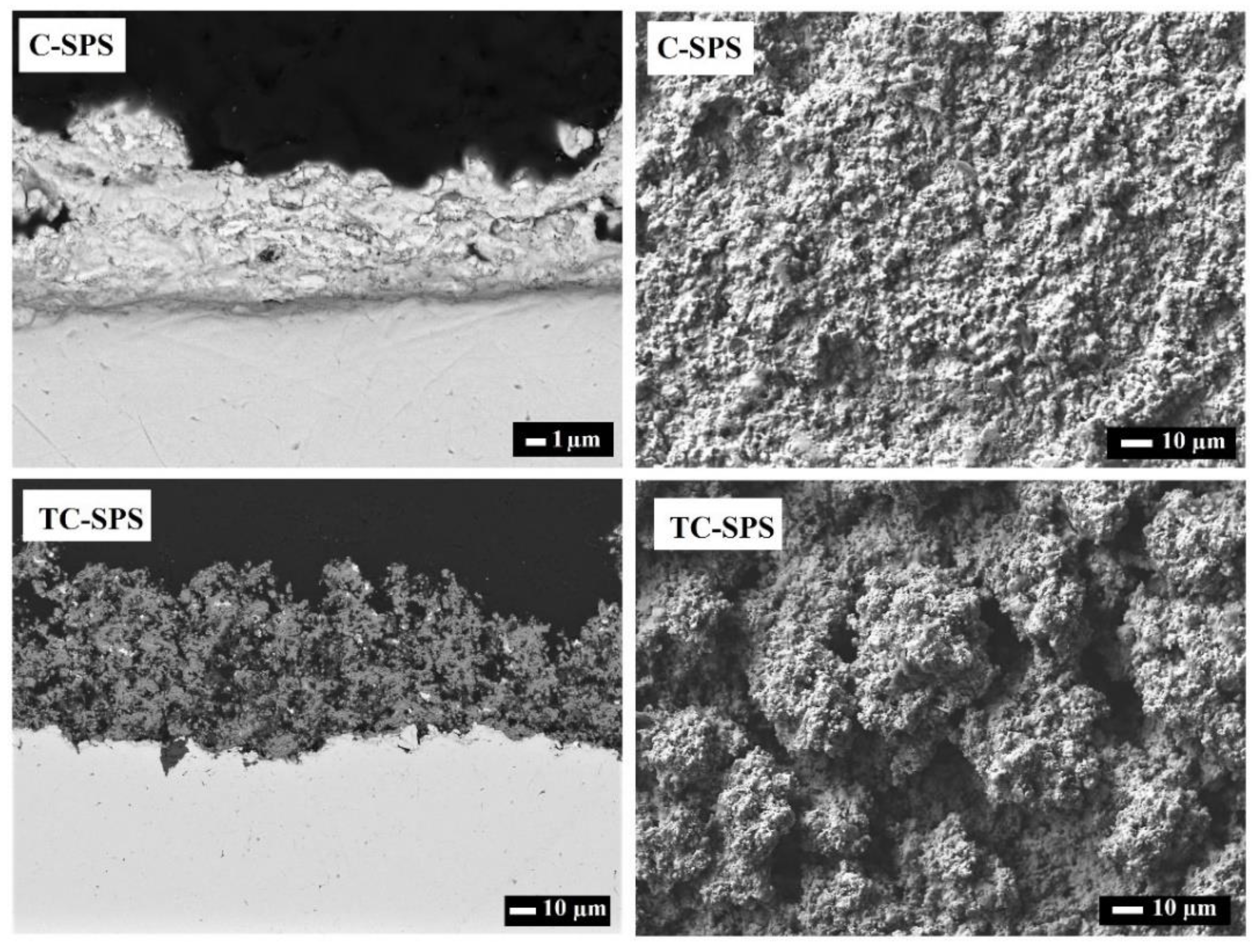
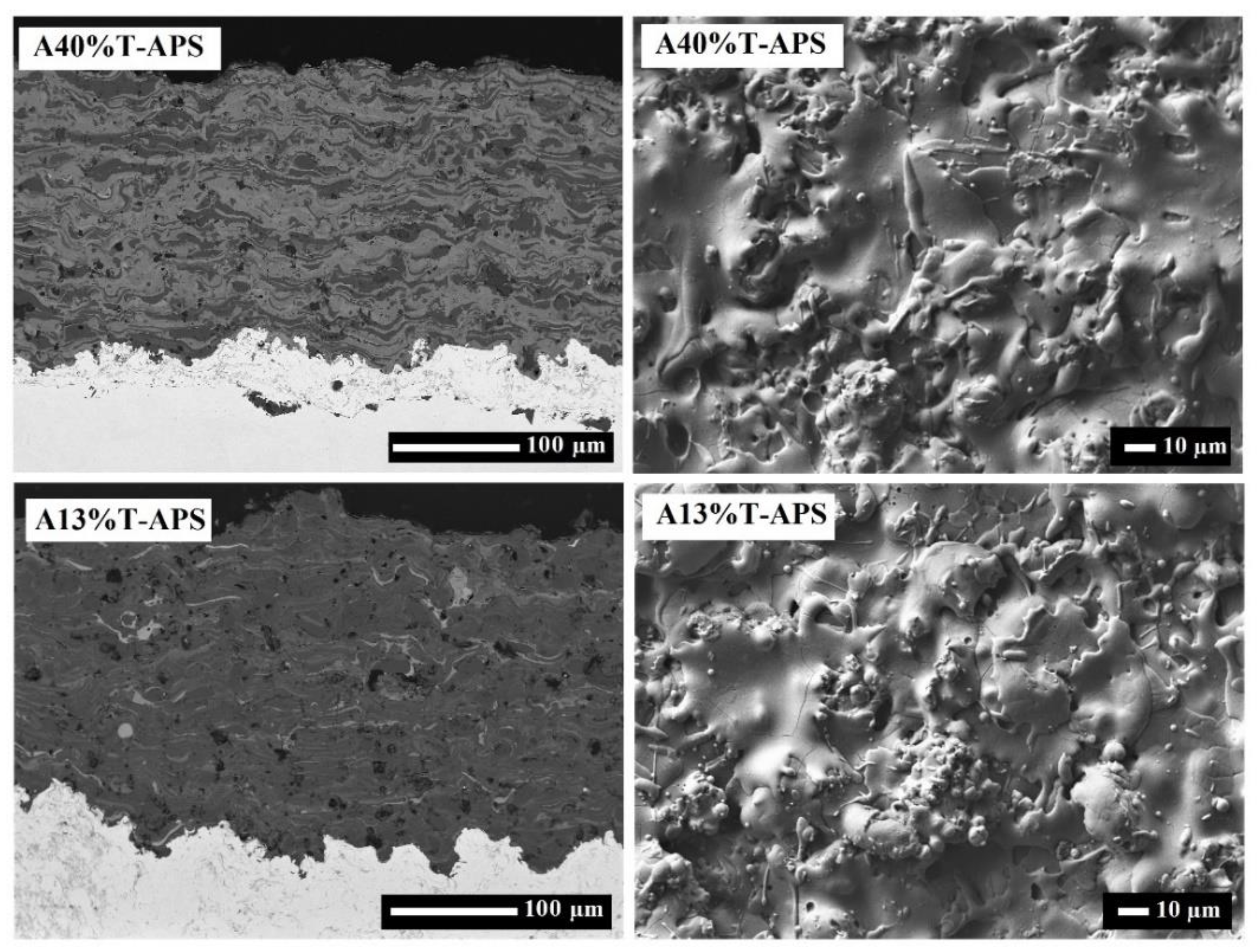
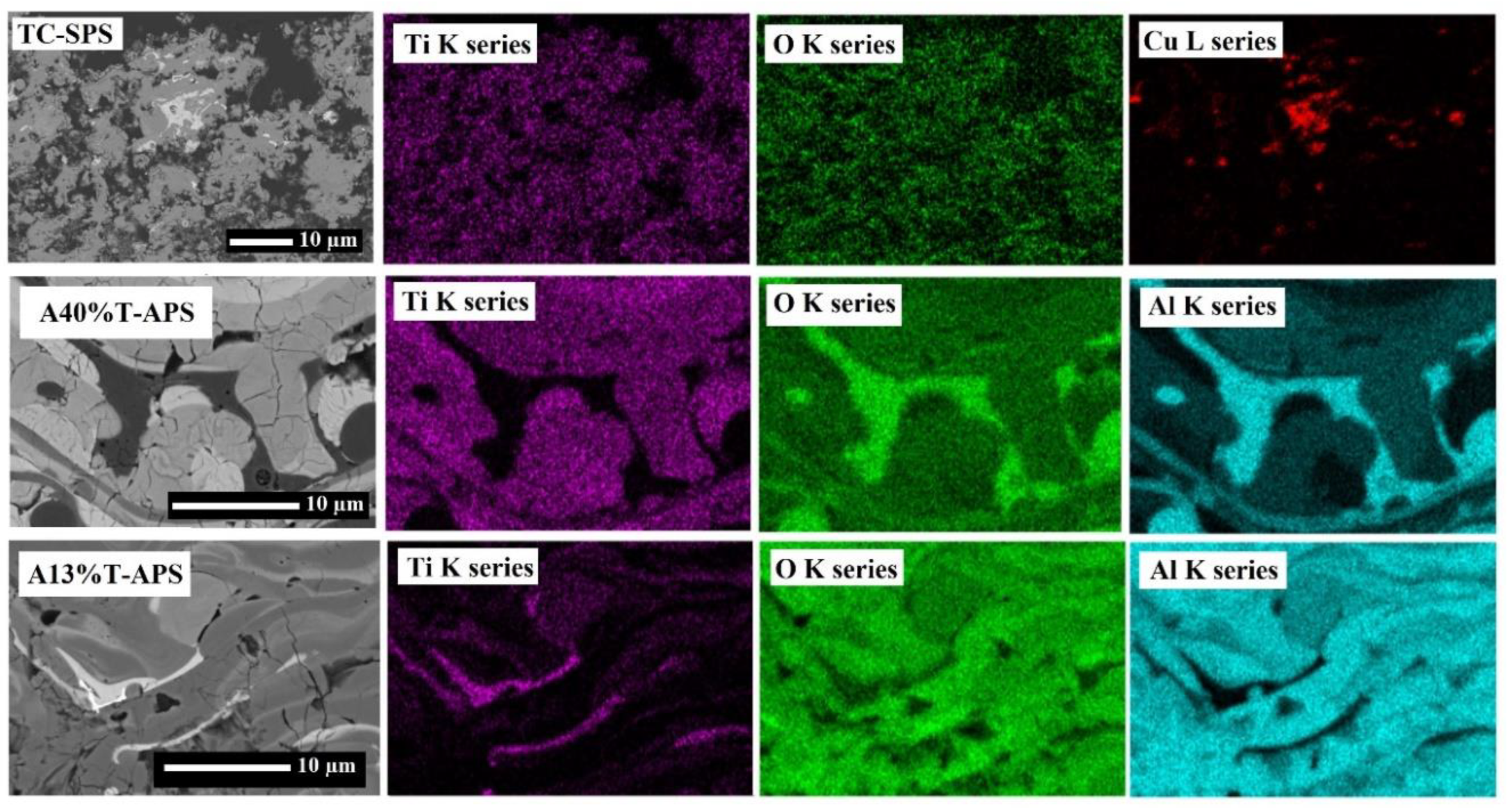
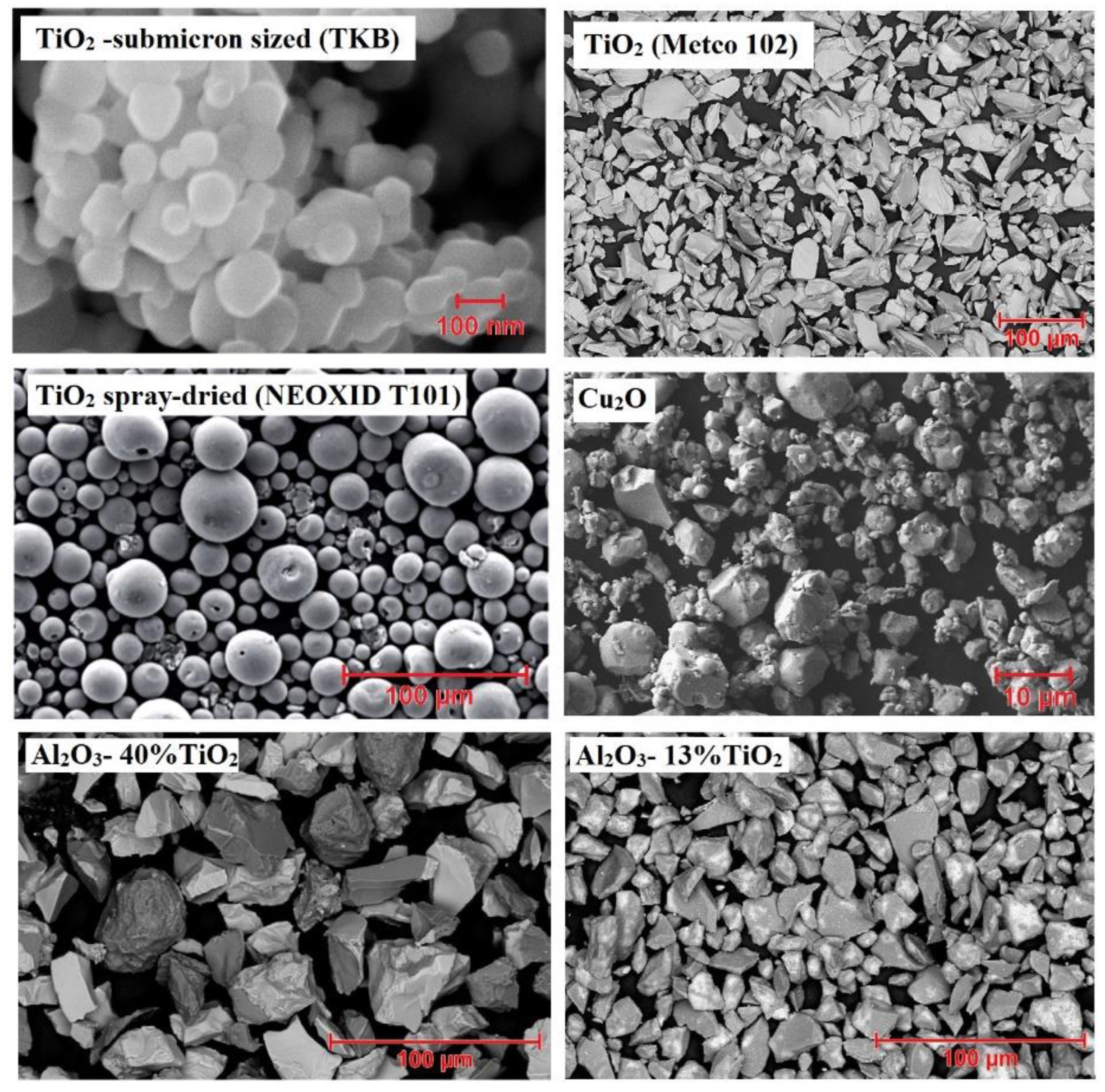
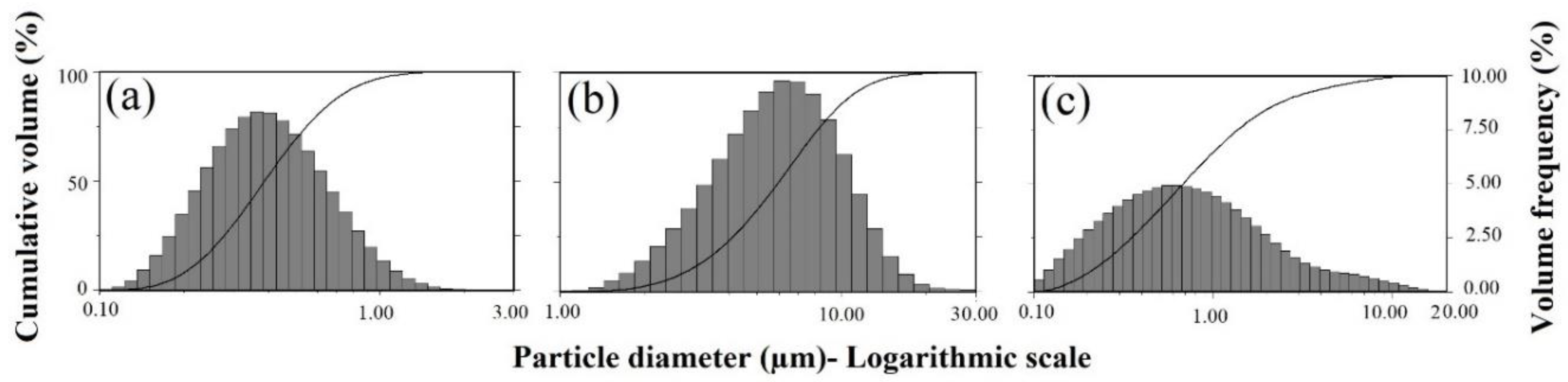
2.2. Microstructure and Morphology
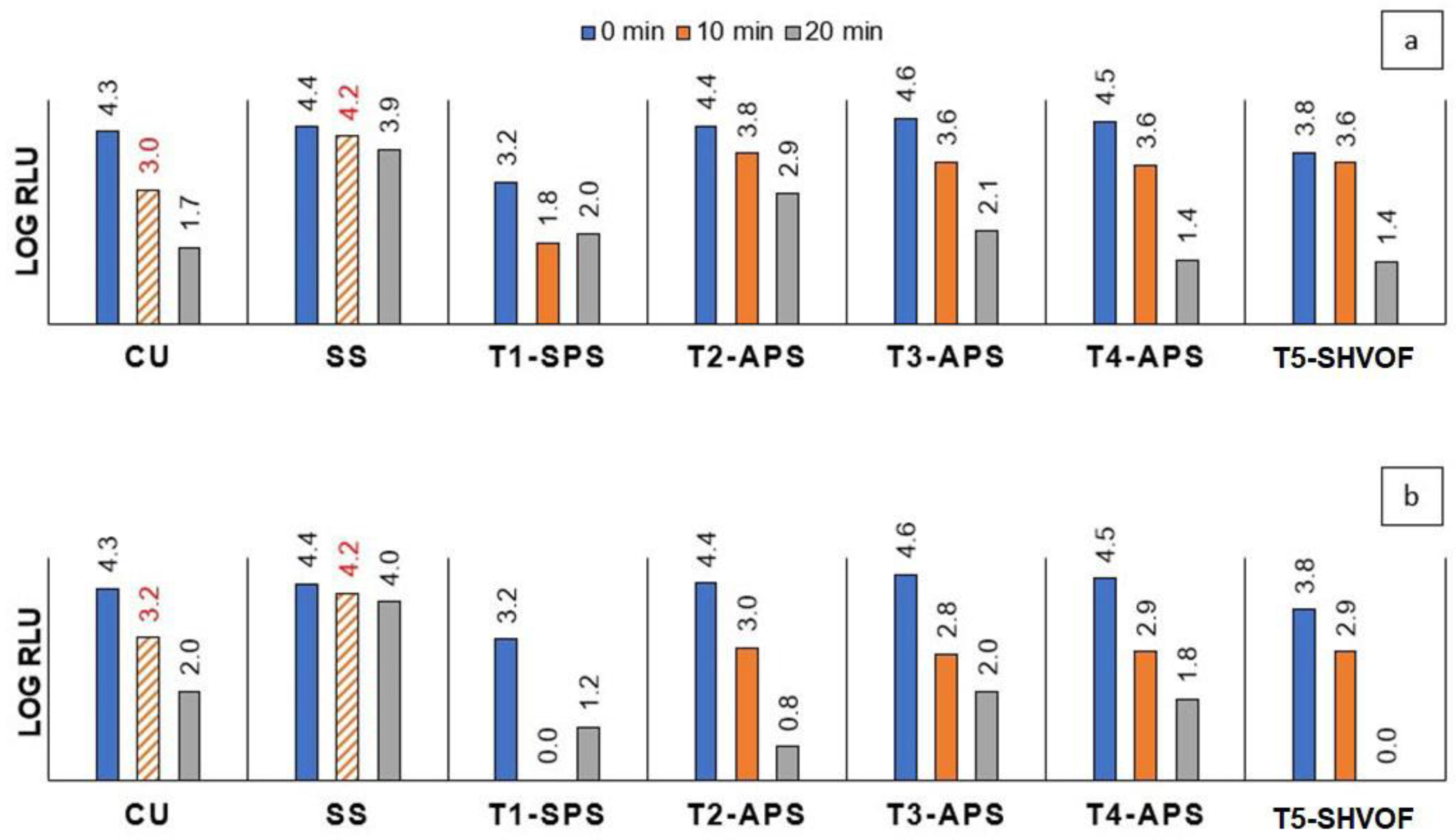
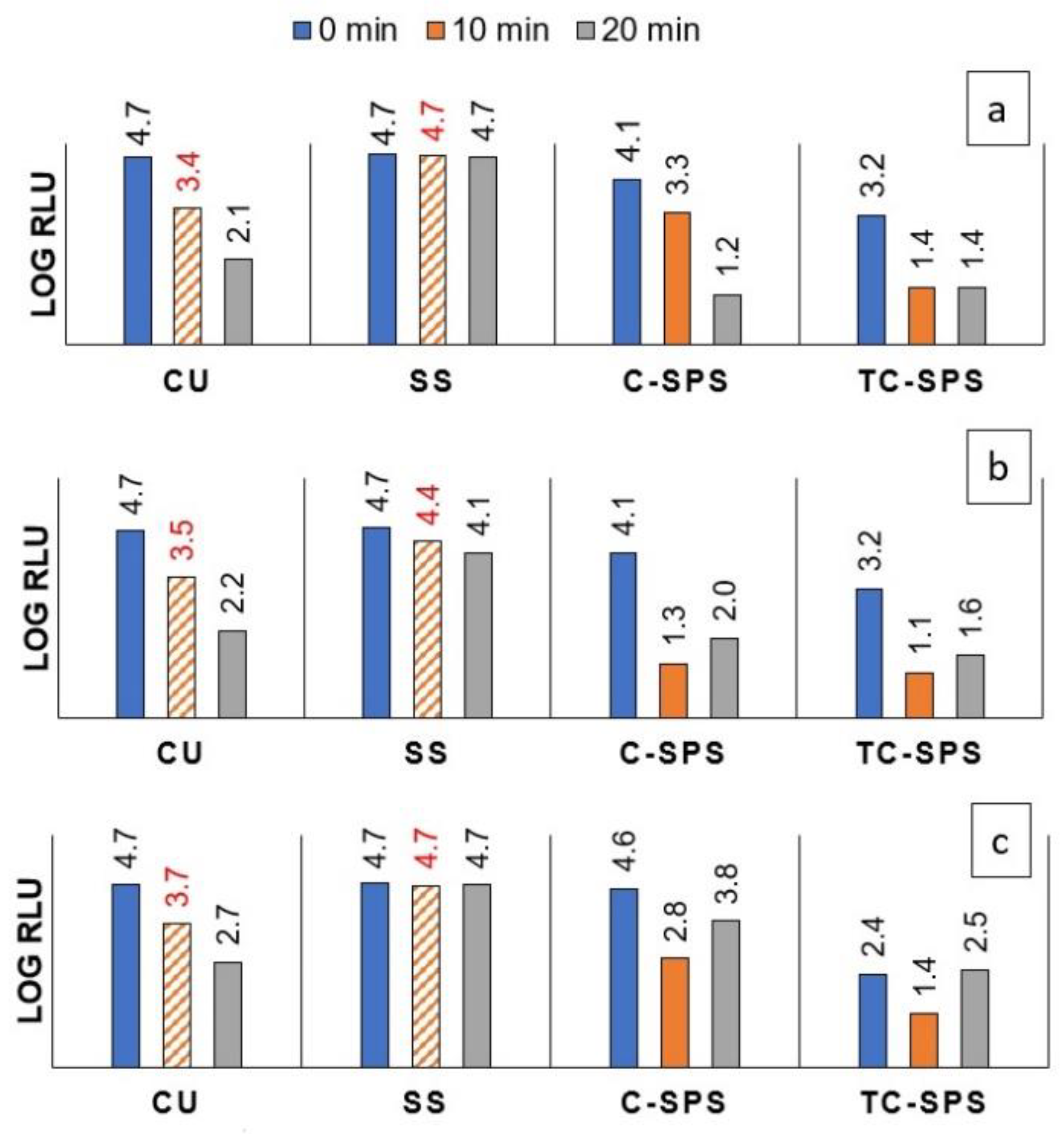
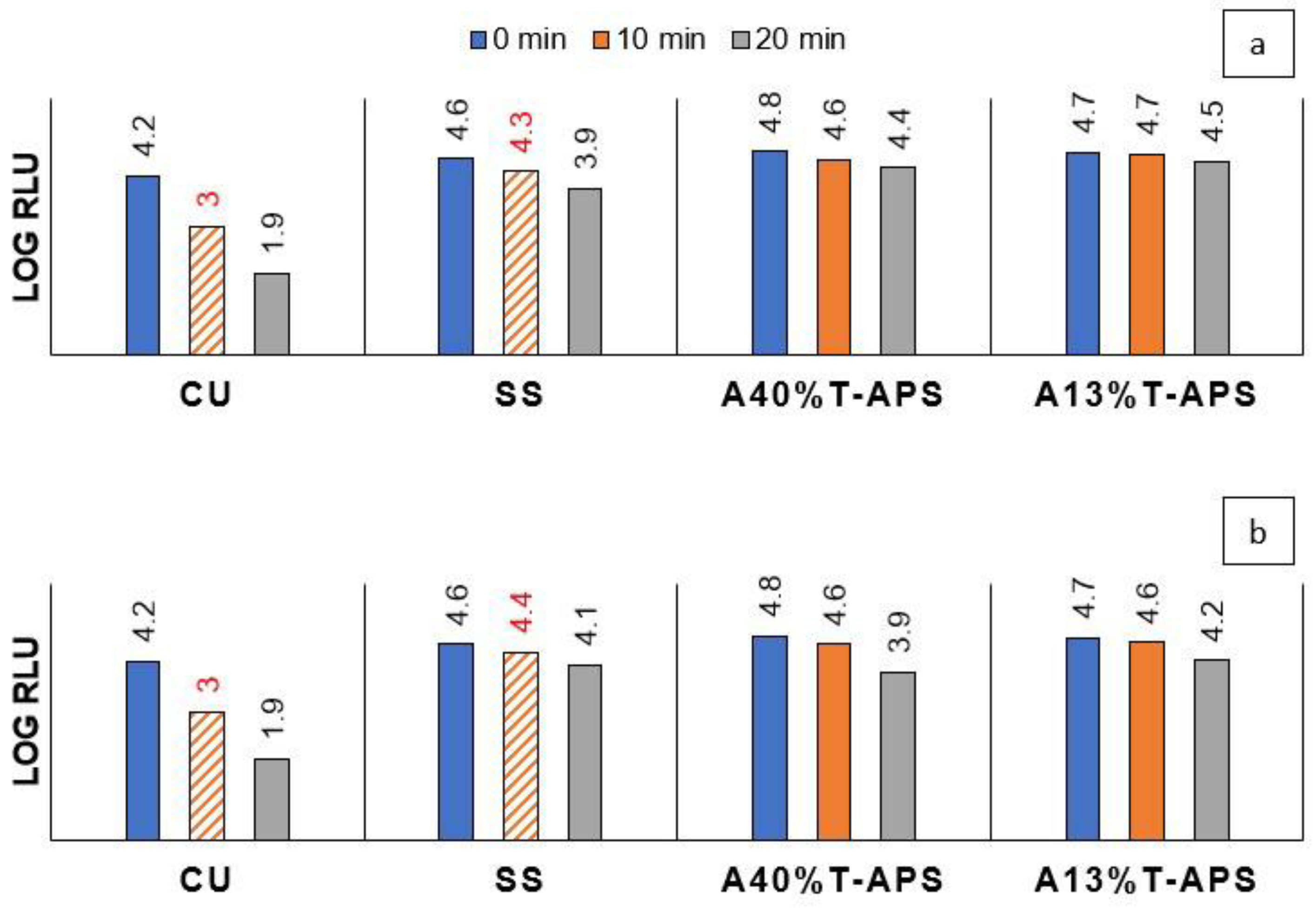
2.3. Antiviral Activity
2.3.1. TiO2 Coatings
2.3.2. TiO2-Cu2O and Cu2O Coatings
2.3.3. Al2O3-TiO2 Coatings
3. Materials and Methods
3.1. Preparation of Antiviral Coatings
3.2. Characterization of the Coatings
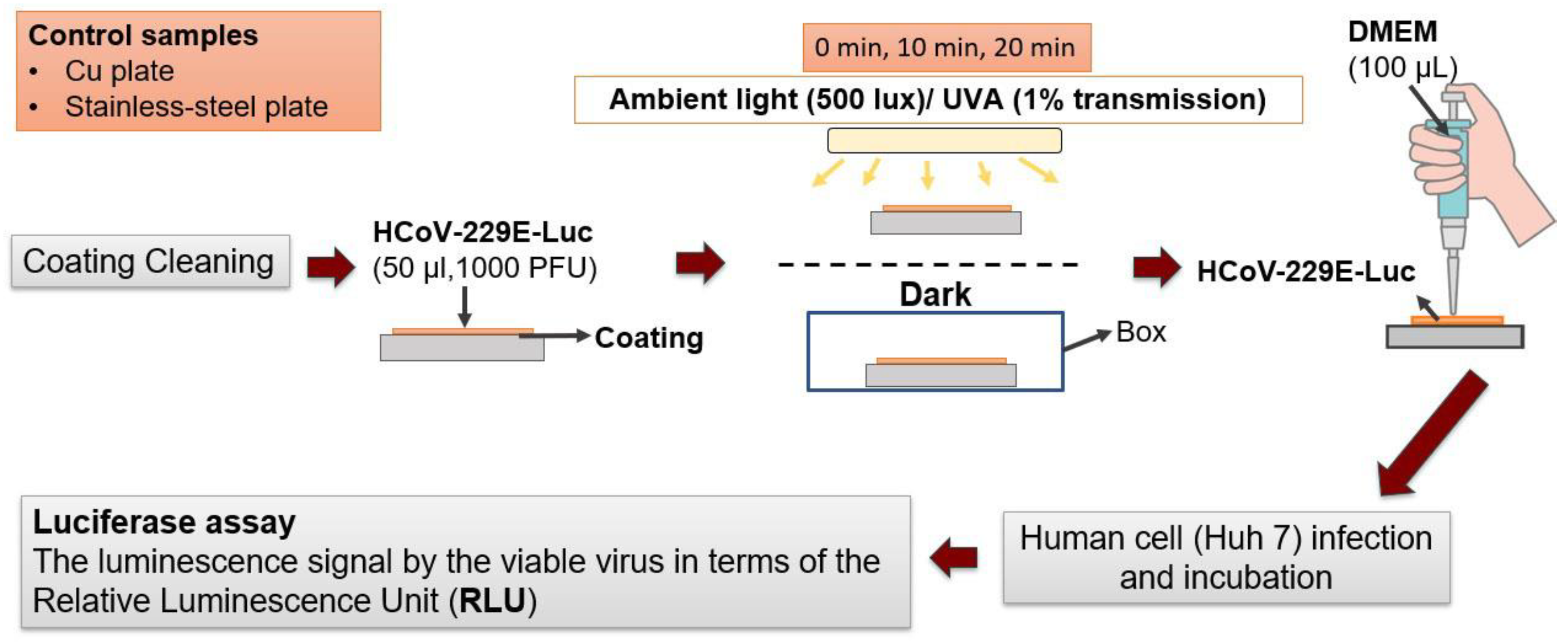
3.3. Antiviral Activity Assessment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19 Weekly Epidemiological Update; World Health Organization: Singapore, 2022; pp. 1–33. [Google Scholar]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Sunada, K.; Minoshima, M.; Hashimoto, K. Highly efficient antiviral and antibacterial activities of solid-state cuprous compounds. J. Hazard. Mater. 2012, 235–236, 265–270. [Google Scholar] [CrossRef]
- Morawska, L.; Cao, J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ. Int. 2020, 139, 105730. [Google Scholar] [CrossRef]
- Scully, J.R. The COVID-19 Pandemic, Part 1: Can Antimicrobial Copper-Based Alloys Help Suppress Infectious Transmission of Viruses Originating from Human Contact with High-Touch Surfaces? Corrosion 2020, 76, 523–527. [Google Scholar] [CrossRef]
- Warnes, S.L.; Little, Z.R.; Keevil, C.W. Human Coronavirus 229E Remains Infectious on Common Touch Surface Materials. mBio 2015, 6, e01697-15. [Google Scholar] [CrossRef]
- George, N.; Mahon, M.; McDonald, A. Bactericidal Performance of Flame-Sprayed Nanostructured Titania-Copper Composite Coatings. J. Therm. Spray Technol. 2010, 19, 1042–1053. [Google Scholar] [CrossRef]
- Versoza, M.; Jung, W.; Barabad, M.L.; Ko, S.; Kim, M.; Park, D. Reduction of Escherichia Coli Using Metal Plates with the Influenced of Applied Low Current and Physical Barrier of Filter Layers. Int. J. Environ. Res. Public Health 2019, 16, 3887. [Google Scholar] [CrossRef]
- Rakowska, P.D.; Tiddia, M.; Faruqui, N.; Bankier, C.; Pei, Y.; Pollard, A.J.; Zhang, J.; Gilmore, I.S. Antiviral surfaces and coatings and their mechanisms of action. Commun. Mater. 2021, 2, 53. [Google Scholar] [CrossRef]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Shuai, D.; Shen, Y.; Wang, D. Progress and challenges in photocatalytic disinfection of waterborne Viruses: A review to fill current knowledge gaps. Chem. Eng. J. 2019, 355, 399–415. [Google Scholar] [CrossRef]
- Cui, H.; Jiang, J.; Gu, W.; Sun, C.; Wu, D.; Yang, T.; Yang, G. Photocatalytic Inactivation Efficiency of Anatase Nano-TiO2 Sol on the H9N2 Avian Influenza Virus. Photochem. Photobiol. 2010, 86, 1135–1139. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Sunada, K.; Iyoda, T.; Hashimoto, K.; Fujishima, A. Photocatalytic bactericidal effect of TiO2 thin films: Dynamic view of the active oxygen species responsible for the effect. J. Photochem. Photobiol. A Chem. 1997, 106, 51–56. [Google Scholar] [CrossRef]
- Prakash, J.; Cho, J.; Mishra, Y.K. Photocatalytic TiO2 nanomaterials as potential antimicrobial and antiviral agents: Scope against blocking the SARS-COV-2 spread. Micro Nano Eng. 2022, 14, 100100. [Google Scholar] [CrossRef]
- Hajkova, P.; Spatenka, P.; Horsky, J.; Horska, I.; Kolouch, A. Photocatalytic Effect of TiO2 Films on Viruses and Bacteria. Plasma Process. Polym. 2007, 4, S397–S401. [Google Scholar] [CrossRef]
- Khatibnezhad, H.; Ambriz-Vargas, F.; Ben Ettouil, F.; Moreau, C. Role of phase content on the photocatalytic performance of TiO2 coatings deposited by suspension plasma spray. J. Eur. Ceram. Soc. 2022, 42, 2905–2920. [Google Scholar] [CrossRef]
- Wagstaffe, M.; Noei, H.; Stierle, A. Elucidating the Defect-Induced Changes in the Photocatalytic Activity of TiO2. J. Phys. Chem. C 2020, 124, 12539–12547. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef]
- Rauf, M.; Meetani, M.; Hisaindee, S. An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination 2011, 276, 13–27. [Google Scholar] [CrossRef]
- Shayegan, Z.; Haghighat, F.; Lee, C.-S.; Bahloul, A.; Huard, M. Effect of surface fluorination of P25-TiO2 on adsorption of indoor environment volatile organic compounds. Chem. Eng. J. 2018, 346, 578–589. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2010, 46, 855–874. [Google Scholar] [CrossRef]
- Stengl, V.; Ageorges, H.; Ctibor, P.; Murafa, N. Atmospheric plasma sprayed (APS) coatings of Al2O3–TiO2 system for photocatalytic application. Photochem. Photobiol. Sci. 2009, 8, 733–738. [Google Scholar] [CrossRef]
- Choi, H.; Stathatos, E.; Dionysiou, D.D. Sol–gel preparation of mesoporous photocatalytic TiO2 films and TiO2/Al2O3 composite membranes for environmental applications. Appl. Catal. B Environ. 2006, 63, 60–67. [Google Scholar] [CrossRef]
- Toma, F.-L.; Berger, L.-M.; Stahr, C.C.; Naumann, T.; Langner, S. Microstructures and Functional Properties of Suspension-Sprayed Al2O3 and TiO2 Coatings: An Overview. J. Therm. Spray Technol. 2010, 19, 262–274. [Google Scholar] [CrossRef]
- Borkow, G.; Zhou, S.S.; Page, T.; Gabbay, J. A Novel Anti-Influenza Copper Oxide Containing Respiratory Face Mask. PLoS ONE 2010, 5, e11295. [Google Scholar] [CrossRef]
- Borkow, G. Safety of Using Copper Oxide in Medical Devices and Consumer Products. Curr. Chem. Biol. 2012, 6, 86–92. [Google Scholar] [CrossRef]
- Qiu, X.; Miyauchi, M.; Sunada, K.; Minoshima, M.; Liu, M.; Lu, Y.; Li, D.; Shimodaira, Y.; Hosogi, Y.; Kuroda, Y.; et al. Hybrid CuxO/TiO2 Nanocomposites as Risk-Reduction Materials in Indoor Environments. ACS Nano 2012, 6, 1609–1618. [Google Scholar] [CrossRef]
- Liu, M.; Sunada, K.; Hashimoto, K.; Miyauchi, M. Visible-light sensitive Cu(ii)–TiO2with sustained anti-viral activity for efficient indoor environmental remediation. J. Mater. Chem. A 2015, 3, 17312–17319. [Google Scholar] [CrossRef]
- Jeffery, B.; Peppler, M.; Lima, R.S.; McDonald, A. Bactericidal Effects of HVOF-Sprayed Nanostructured TiO2 on Pseudomonas aeruginosa. J. Therm. Spray Technol. 2010, 19, 344–349. [Google Scholar] [CrossRef]
- Vardelle, A.M.; Moreau, C.; Akedo, J.; Ashrafizadeh, H.; Berndt, C.; Berghaus, J.O.; Boulos, M.; Brogan, J.; Bourtsalas, A.C.; Dolatabadi, A.; et al. The 2016 Thermal Spray Roadmap. J. Therm. Spray Technol. 2016, 25, 1376–1440. [Google Scholar] [CrossRef]
- Du, L.; Coyle, T.W.; Chien, K.; Pershin, L.; Li, T.; Golozar, M. Titanium Dioxide Coating Prepared by Use of a Suspension-Solution Plasma-Spray Process. J. Therm. Spray Technol. 2015, 24, 915–924. [Google Scholar] [CrossRef]
- Khatibnezhad, H.; Ambriz-Vargas, F.; Ben Ettouil, F.; Moreau, C. An investigation on the photocatalytic activity of sub-stoichiometric TiO2−x coatings produced by suspension plasma spray. J. Eur. Ceram. Soc. 2020, 41, 544–556. [Google Scholar] [CrossRef]
- Selvamani, V.; Zareei, A.; Elkashif, A.; Maruthamuthu, M.K.; Chittiboyina, S.; DeLisi, D.; Li, Z.; Cai, L.; Pol, V.G.; Seleem, M.; et al. Hierarchical Micro/Mesoporous Copper Structure with Enhanced Antimicrobial Property via Laser Surface Texturing. Adv. Mater. Interfaces 2020, 7, 1901890. [Google Scholar] [CrossRef]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef]
- Alebrahim, E.; Tarasi, F.; Rahaman, S.; Dolatabadi, A.; Moreau, C. Fabrication of titanium dioxide filtration membrane using suspension plasma spray process. Surf. Coat. Technol. 2019, 378, 124927. [Google Scholar] [CrossRef]
- Alebrahim, E.; Rahaman, S.; Moreau, C. TiO2 Photocatalytic Ultrafiltration Membrane Developed with Suspension Plasma Spray Process. Coatings 2022, 12, 1764. [Google Scholar] [CrossRef]
- Toma, F.-L.; Bertrand, G.; Begin, S.; Meunier, C.; Barres, O.; Klein, D.; Coddet, C. Microstructure and environmental functionalities of TiO2-supported photocatalysts obtained by suspension plasma spraying. Appl. Catal. B Environ. 2006, 68, 74–84. [Google Scholar] [CrossRef]
- Li, G.-R.; Lv, B.-W.; Yang, G.-J.; Zhang, W.-X.; Li, C.-X.; Li, C.-J. Relationship between Lamellar Structure and Elastic Modulus of Thermally Sprayed Thermal Barrier Coatings with Intra-splat Cracks. J. Therm. Spray Technol. 2015, 24, 1355–1367. [Google Scholar] [CrossRef]
- Ramakrishnan, G.; Dwivedi, G.; Sampath, S.; Orlov, A. Development and optimization of thermal sprayed ceramic microfiltration membranes. J. Membr. Sci. 2015, 489, 106–111. [Google Scholar] [CrossRef]
- Bono, N.; Ponti, F.; Punta, C.; Candiani, G. Effect of UV Irradiation and TiO2-Photocatalysis on Airborne Bacteria and Viruses: An Overview. Materials 2021, 14, 1075. [Google Scholar] [CrossRef]
- Gong, M.; Xiao, S.; Yu, X.; Dong, C.; Ji, J.; Zhang, D.; Xing, M. Research progress of photocatalytic sterilization over semiconductors. RSC Adv. 2019, 9, 19278–19284. [Google Scholar] [CrossRef]
- Han, R.; Coey, J.D.; O’Rourke, C.; Bamford, C.G.; Mills, A. Flexible, disposable photocatalytic plastic films for the destruction of viruses. J. Photochem. Photobiol. B Biol. 2022, 235, 112551. [Google Scholar] [CrossRef]
- Kozerski, S.; Toma, F.-L.; Pawlowski, L.; Leupolt, B.; Latka, L.; Berger, L.-M. Suspension plasma sprayed TiO2 coatings using different injectors and their photocatalytic properties. Surf. Coat. Technol. 2010, 205, 980–986. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, Y.; Li, H. Onion-like carbon-modified TiO2 coating by suspension plasma spray with enhanced photocatalytic performances. J. Nanopart. Res. 2019, 21, 182. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef]
- Fiorillo, L.; Cervino, G.; Matarese, M.; D’Amico, C.; Surace, G.; Paduano, V.; Fiorillo, M.T.; Moschella, A.; La Bruna, A.; Romano, G.L.; et al. COVID-19 Surface Persistence: A Recent Data Summary and Its Importance for Medical and Dental Settings. Int. J. Environ. Res. Public Health 2020, 17, 3132. [Google Scholar] [CrossRef]
- Justicia, I.; Ordejón, P.; Canto, G.; Mozos, J.L.; Fraxedas, J.; Battiston, G.A.; Gerbasi, R.; Figueras, A. Designed self-doped titanium oxide thin films for efficient visible-light photocatalysis. Adv. Mater. 2002, 14, 1399–1402. [Google Scholar] [CrossRef]
- Ager, F.; Justicia, I.; Gerbasi, R.; Battiston, G.; McSporran, N.; Figueras, A. RBS analysis of substoichiometric TiO2-anatase thin films for visible-light photocatalysis. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2006, 249, 490–492. [Google Scholar] [CrossRef]
- Vu, P.; Otto, N.; Vogel, A.; Kern, F.; Killinger, A.; Gadow, R. Efficiently quantifying the anatase content and investigating its effect on the photocatalytic activity of titania coatings by suspension plasma spraying. Surf. Coat. Technol. 2018, 371, 117–123. [Google Scholar] [CrossRef]
- Colmenares-Angulo, J.R.; Cannillo, V.; Lusvarghi, L.; Sola, A.; Sampath, S. Role of process type and process conditions on phase content and physical properties of thermal sprayed TiO2 coatings. J. Mater. Sci. 2009, 44, 2276–2287. [Google Scholar] [CrossRef]
- Toma, F.-L.; Sokolov, D.; Bertrand, G.; Klein, D.; Coddet, C.; Meunier, C. Comparison of the Photocatalytic Behavior of TiO2 Coatings Elaborated by Different Thermal Spraying Processes. J. Therm. Spray Technol. 2006, 15, 576–581. [Google Scholar] [CrossRef]
- Xiong, L.-B.; Li, J.-L.; Yang, B.; Yu, Y. Ti3+ in the Surface of Titanium Dioxide: Generation, Properties and Photocatalytic Application. J. Nanomater. 2012, 2012, 831524. [Google Scholar] [CrossRef]
- Amano, F.; Nakata, M.; Yamamoto, A.; Tanaka, T. Rutile titanium dioxide prepared by hydrogen reduction of Degussa P25 for highly efficient photocatalytic hydrogen evolution. Catal. Sci. Technol. 2016, 6, 5693–5699. [Google Scholar] [CrossRef]
- Yamasaki, R.S. Intensity variations of ultraviolet, visible, and near infrared bands of terrestrial solar radiation. J. Paint Technol. 1971, 43, 75–83. [Google Scholar]
- Miyauchi, M.; Sunada, K.; Hashimoto, K. Antiviral Effect of Visible Light-Sensitive CuxO/TiO2 Photocatalyst. Catalysts 2020, 10, 1093. [Google Scholar] [CrossRef]
- Vaßen, R.; Kaßner, H.; Mauer, G.; Stöver, D. Suspension Plasma Spraying: Process Characteristics and Applications. J. Therm. Spray Technol. 2009, 19, 219–225. [Google Scholar] [CrossRef]
- Pala, Z.; Shaw, E.; Murray, J.; Senin, N.; Hussain, T. Suspension high velocity oxy-fuel spraying of TiO2: A quantitative approach to phase composition. J. Eur. Ceram. Soc. 2017, 37, 801–810. [Google Scholar] [CrossRef]
- Meunier, T.; Desmarets, L.; Bordage, S.; Bamba, M.; Hervouet, K.; Rouillé, Y.; François, N.; Decossas, M.; Sencio, V.; Trottein, F.; et al. A Photoactivable Natural Product with Broad Antiviral Activity against Enveloped Viruses, including Highly Pathogenic Coronaviruses. Antimicrob. Agents Chemother. 2022, 66, e01581-21. [Google Scholar] [CrossRef]
- Smale, S.T. Luciferase Assay. Cold Spring Harb. Protoc. 2010, 2010, 54. [Google Scholar] [CrossRef]
- Noyce, J.O.; Michels, H.; Keevil, C.W. Inactivation of Influenza A Virus on Copper versus Stainless Steel Surfaces. Appl. Environ. Microbiol. 2007, 73, 2748–2750. [Google Scholar] [CrossRef]
- Mehtar, S.; Wiid, I.; Todorov, S. The antimicrobial activity of copper and copper alloys against nosocomial pathogens and Mycobacterium tuberculosis isolated from healthcare facilities in the Western Cape: An in-vitro study. J. Hosp. Infect. 2008, 68, 45–51. [Google Scholar] [CrossRef] [PubMed]
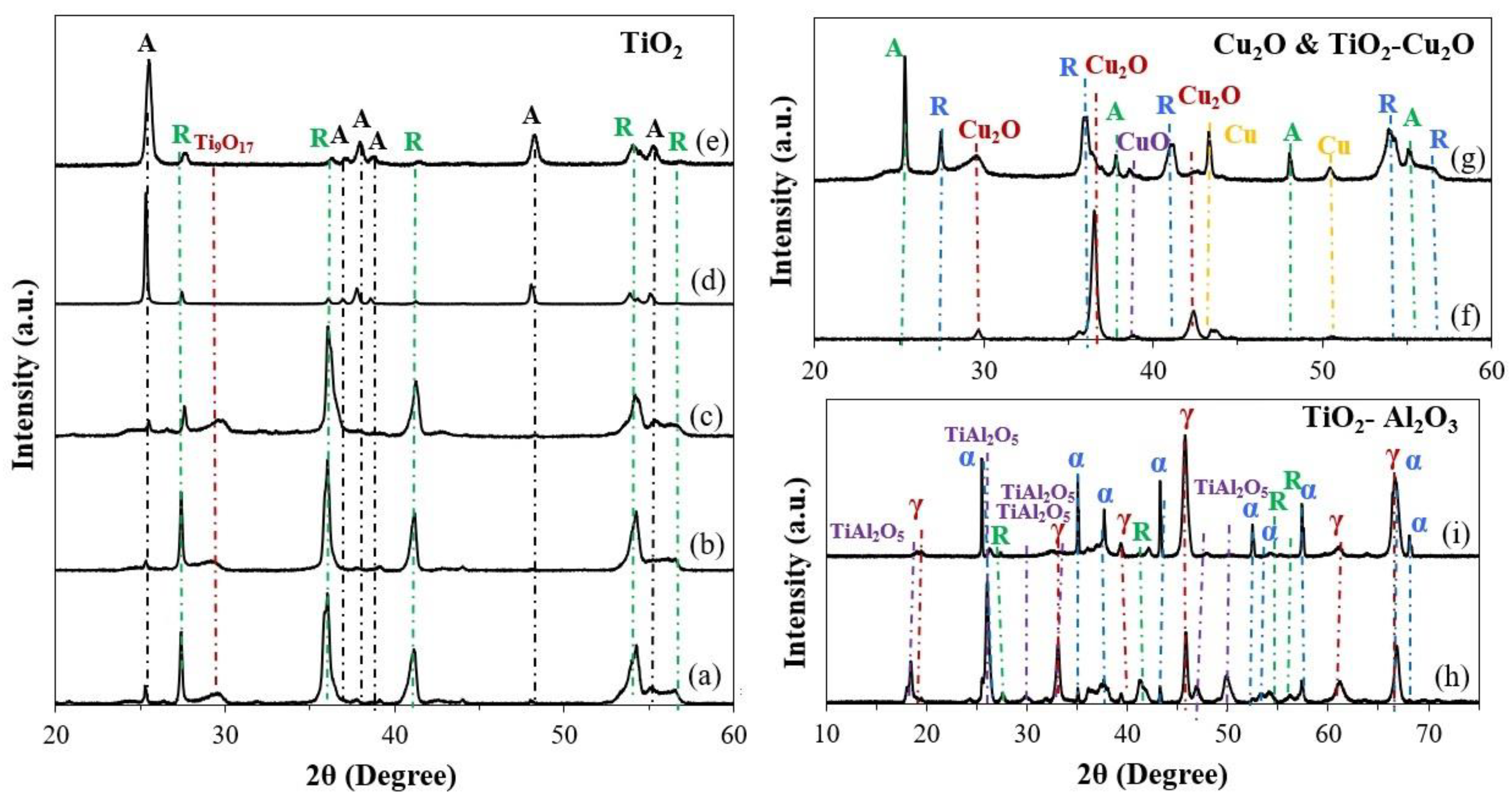
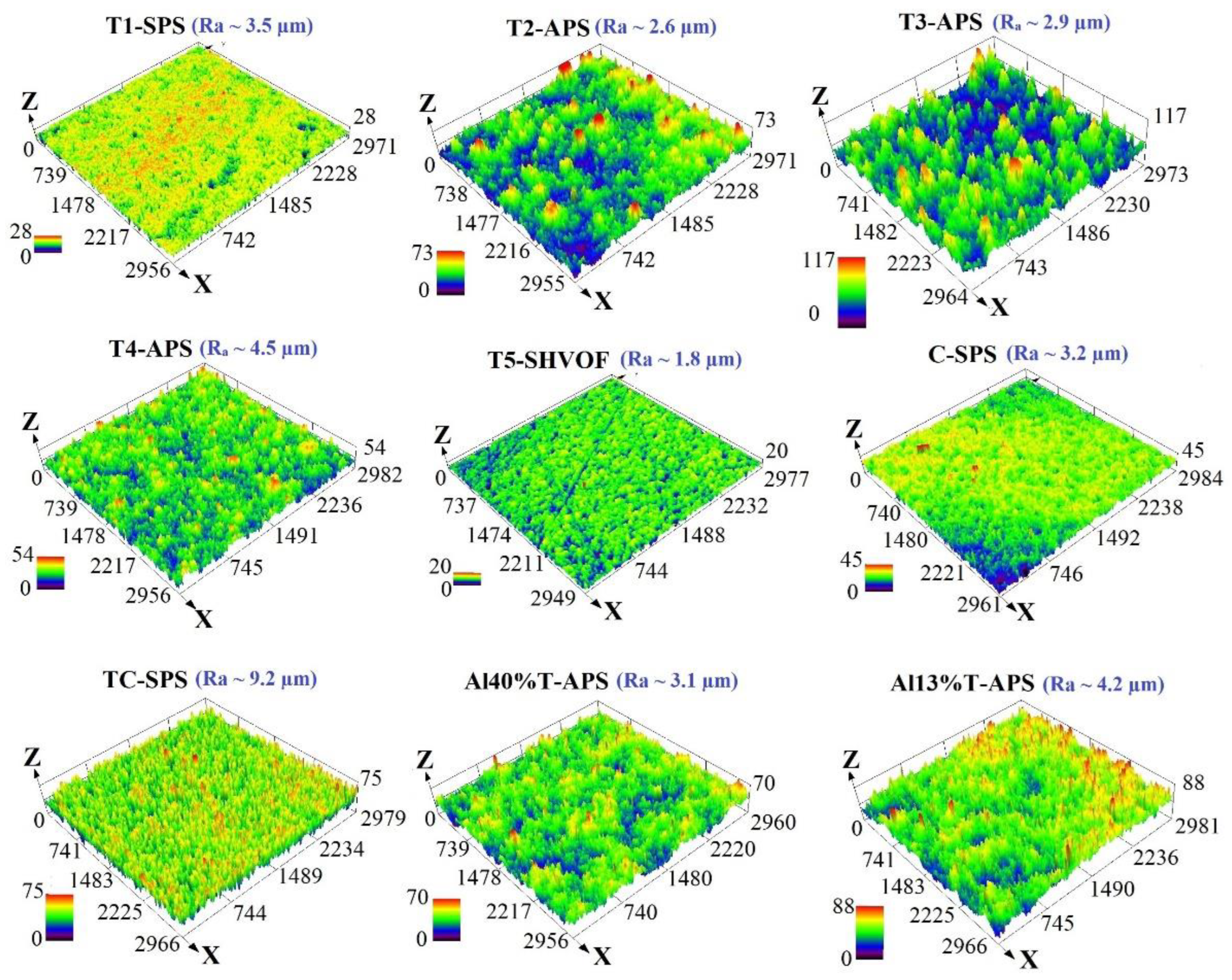
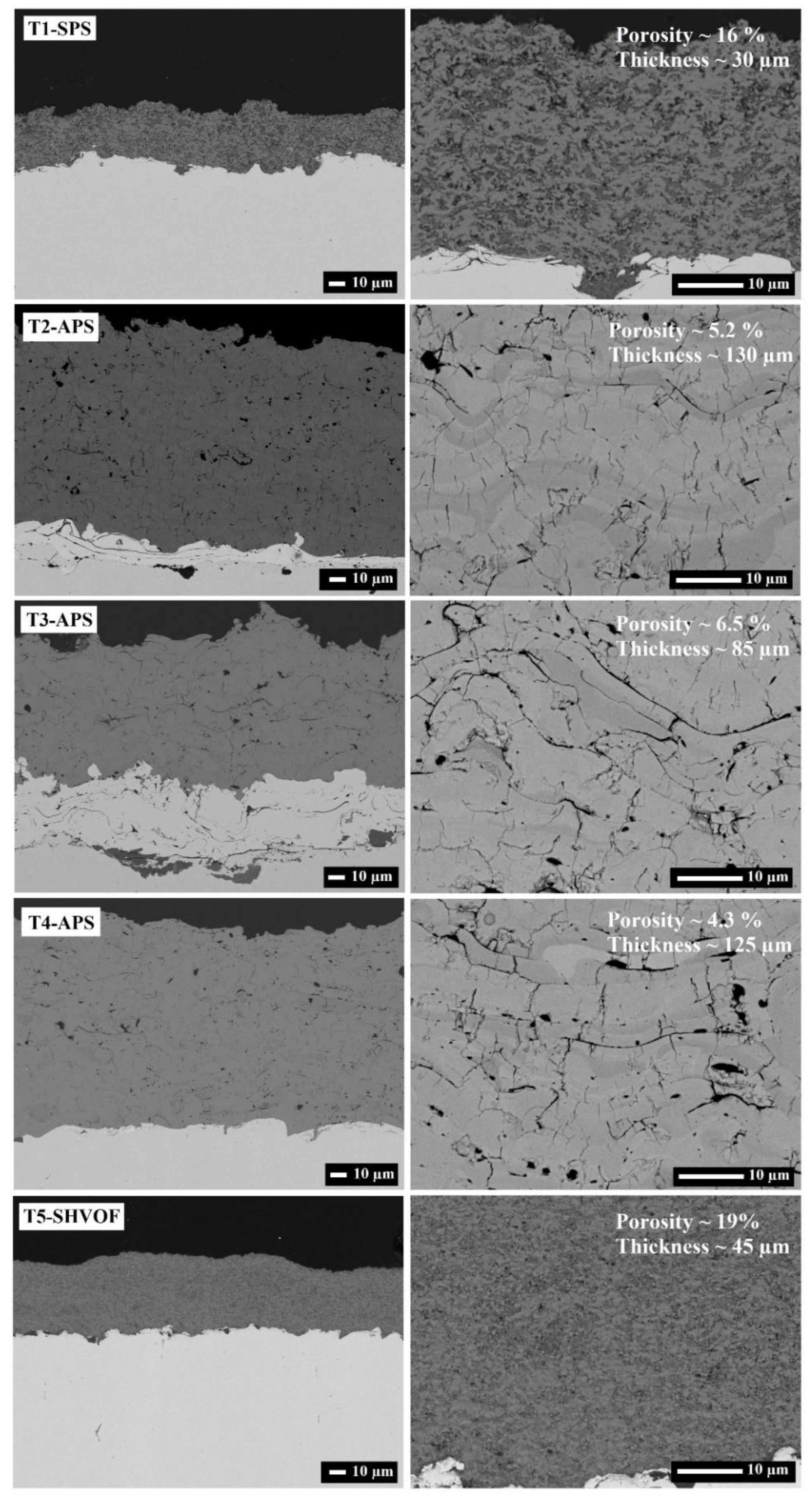
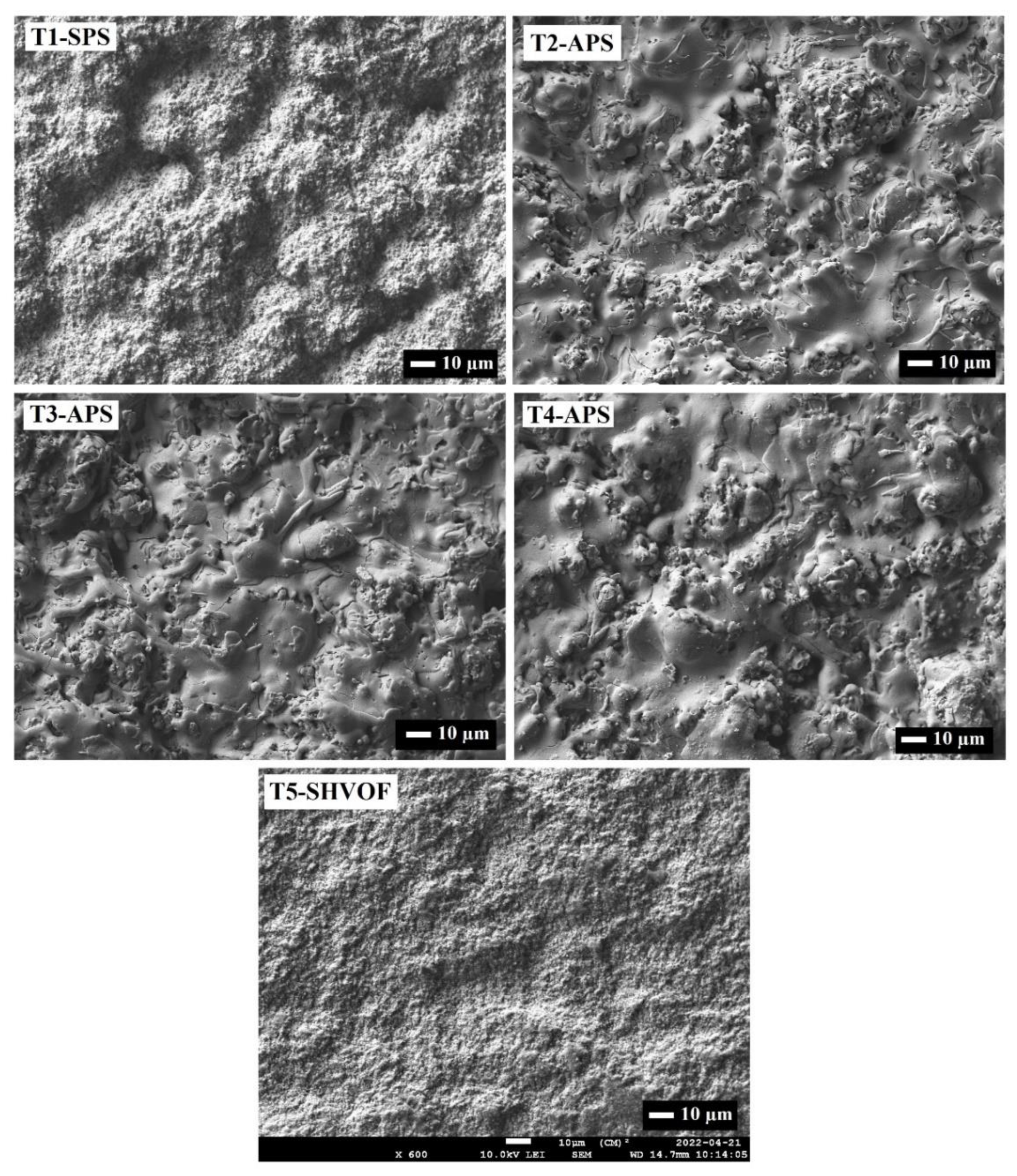


| Sample | %Anatase | %Rutile | %Ti9O17 | %Cu2O | %CuO | %Cu | %α-Al2O3 | %γ-Al2O3 | %TiAl2O5 |
|---|---|---|---|---|---|---|---|---|---|
| T1-SPS | 83 | 17 | - | - | - | - | - | - | - |
| T2-APS | 9.5 | 64.5 | 26 | - | - | - | - | - | - |
| T3-APS | 6 | 67.5 | 26.5 | - | - | - | - | - | - |
| T4-APS | 28 | 72 | - | - | - | - | - | - | - |
| T5-SHVOF | 82 | 18 | - | - | - | - | - | - | - |
| C-SPS | - | - | - | 65.1 | 18.2 | 16.7 | - | - | - |
| TC-SPS | 42 | 19 | - | 34 | - | 5 | - | - | - |
| A40%T-APS | - | 6 | - | - | - | - | 32.5 | 37 | 24.5 |
| A13%T-APS | - | 2 | - | - | - | - | 55 | 32 | 11 |
| Sample | Feedstock Material | Thermal Spray Process | Spray System | Powder Feed Rate (g/min) | Suspension Feed Rate (mL/min) | Current (A) | Spray Distance (mm) | Power (kW) |
|---|---|---|---|---|---|---|---|---|
| T1-SPS | TiO2-TKB | SPS | Axial IIITM | - | 30 | 220 | 75 | 77 |
| T2-APS | TiO2-Metco 102 | APS | SG-100 | 25 | - | 1000 | 100 | 35 |
| T3-APS | TiO2-Metco 102 | APS | 100 HE | 100 | - | 400 | 115 | 95 |
| T4-APS | TiO2-NEOXID T101 | APS | 3 MB | 20 | - | 500 | 75 | 29 |
| T5-SHVOF | TiO2-TKB | S-HVOF | ID-Nova | - | 37 | - | 30 | - |
| C-SPS | Cu2O-PI-KEM | SPS | 3MB | - | 35 | 500 | 50 | 17.5 |
| TC-SPS | TiO2- 25%Cu2O-TKB | SPS | 3 MB | - | 35 | 500 | 50 | 20 |
| A40%T-APS | Al2O3- 40%TiO2 (Amdry 6257) | APS | F4 | 30 | - | 480 | 120 | 30 |
| A13%T-APS | Al2O3- 13%TiO2 (Metco 130) | APS | 100 HE | 90 | - | 260 | 115 | 65 |
| Sample | Ar Flow Rate (L/min) | Secondary Gas Flow Rate (L/min) | ||
|---|---|---|---|---|
| H2 | He | N2 | ||
| T1-SPS | Gas flow (SLPM): 223, Gas mixture (%): Ar: 82/N2: 11/H2: 7 | |||
| T2-APS | 42.7 | - | 27.4 | - |
| T3-APS | 85 | 66 | - | 66 |
| T4-APS | 40 | 8 | - | - |
| C-SPS | 50 | - | 10 | - |
| TC-SPS | 50 | 1 | - | - |
| A40%T-APS | 40 | 8 | - | - |
| A13%T-APS | 86 | 56 | - | 56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alebrahim, E.; Khatibnezhad, H.; Bajgiran, M.M.; Solomon, M.; Liang, C.; Sagan, S.M.; Lima, R.S.; Oberste Berghaus, J.; Aghasibeig, M.; Moreau, C. A Comparative Study of the Antiviral Properties of Thermally Sprayed Coatings against Human Coronavirus HCoV-229E. Catalysts 2023, 13, 1141. https://doi.org/10.3390/catal13071141
Alebrahim E, Khatibnezhad H, Bajgiran MM, Solomon M, Liang C, Sagan SM, Lima RS, Oberste Berghaus J, Aghasibeig M, Moreau C. A Comparative Study of the Antiviral Properties of Thermally Sprayed Coatings against Human Coronavirus HCoV-229E. Catalysts. 2023; 13(7):1141. https://doi.org/10.3390/catal13071141
Chicago/Turabian StyleAlebrahim, Elnaz, Hediyeh Khatibnezhad, Morvarid Mohammadian Bajgiran, Magan Solomon, Chen Liang, Selena M. Sagan, Rogerio S. Lima, Jörg Oberste Berghaus, Maniya Aghasibeig, and Christian Moreau. 2023. "A Comparative Study of the Antiviral Properties of Thermally Sprayed Coatings against Human Coronavirus HCoV-229E" Catalysts 13, no. 7: 1141. https://doi.org/10.3390/catal13071141
APA StyleAlebrahim, E., Khatibnezhad, H., Bajgiran, M. M., Solomon, M., Liang, C., Sagan, S. M., Lima, R. S., Oberste Berghaus, J., Aghasibeig, M., & Moreau, C. (2023). A Comparative Study of the Antiviral Properties of Thermally Sprayed Coatings against Human Coronavirus HCoV-229E. Catalysts, 13(7), 1141. https://doi.org/10.3390/catal13071141








