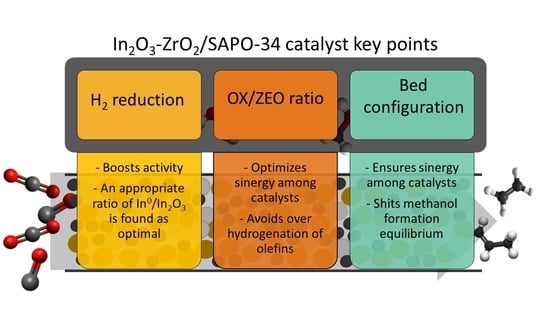
Setting up In2O3-ZrO2/SAPO-34 Catalyst for Improving Olefin Production via Hydrogenation of CO2/CO Mixtures
Abstract
1. Introduction
2. Results and Discussion
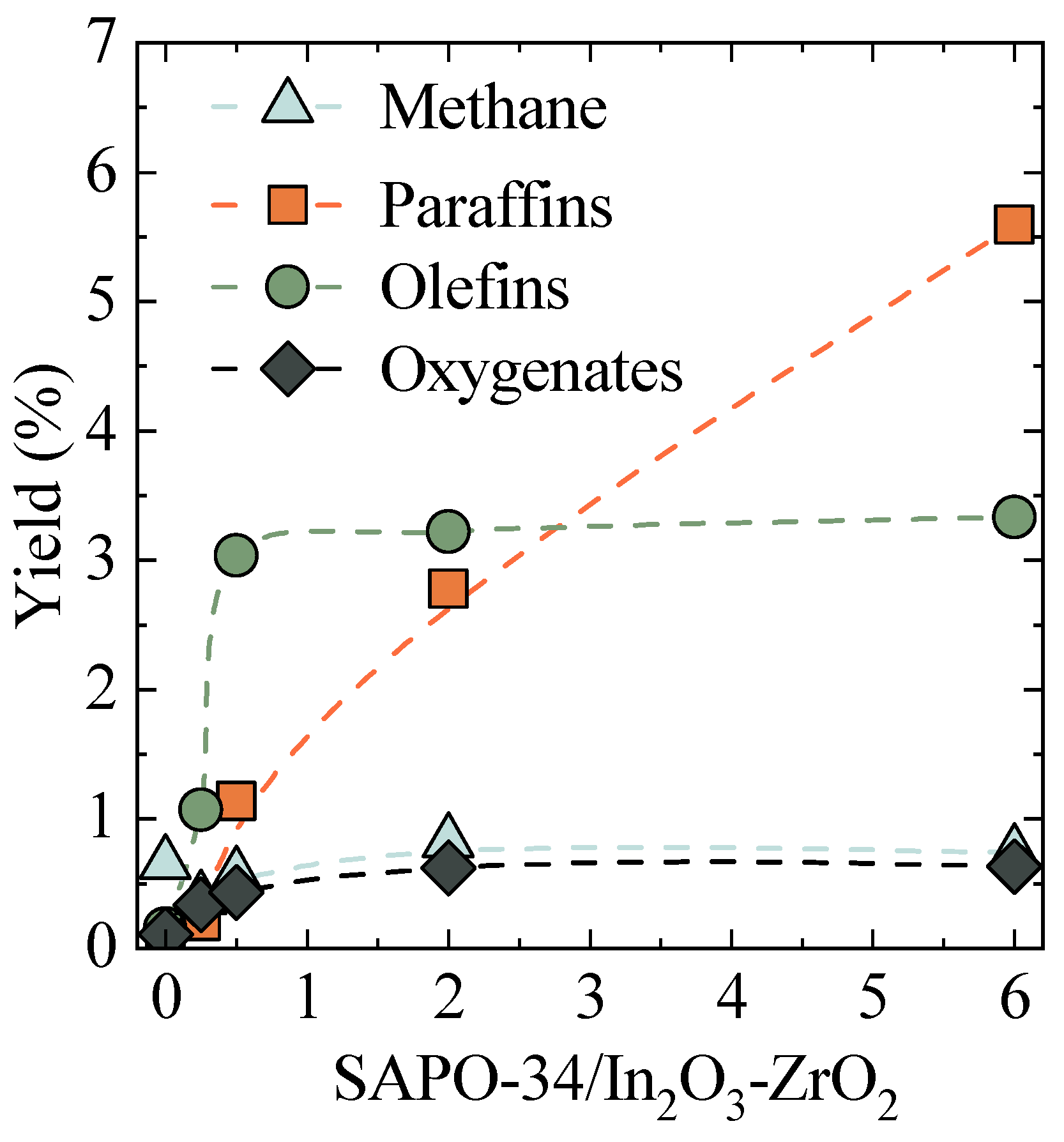
2.1. Optimal SAPO-34 to In2O3-ZrO2 Ratio
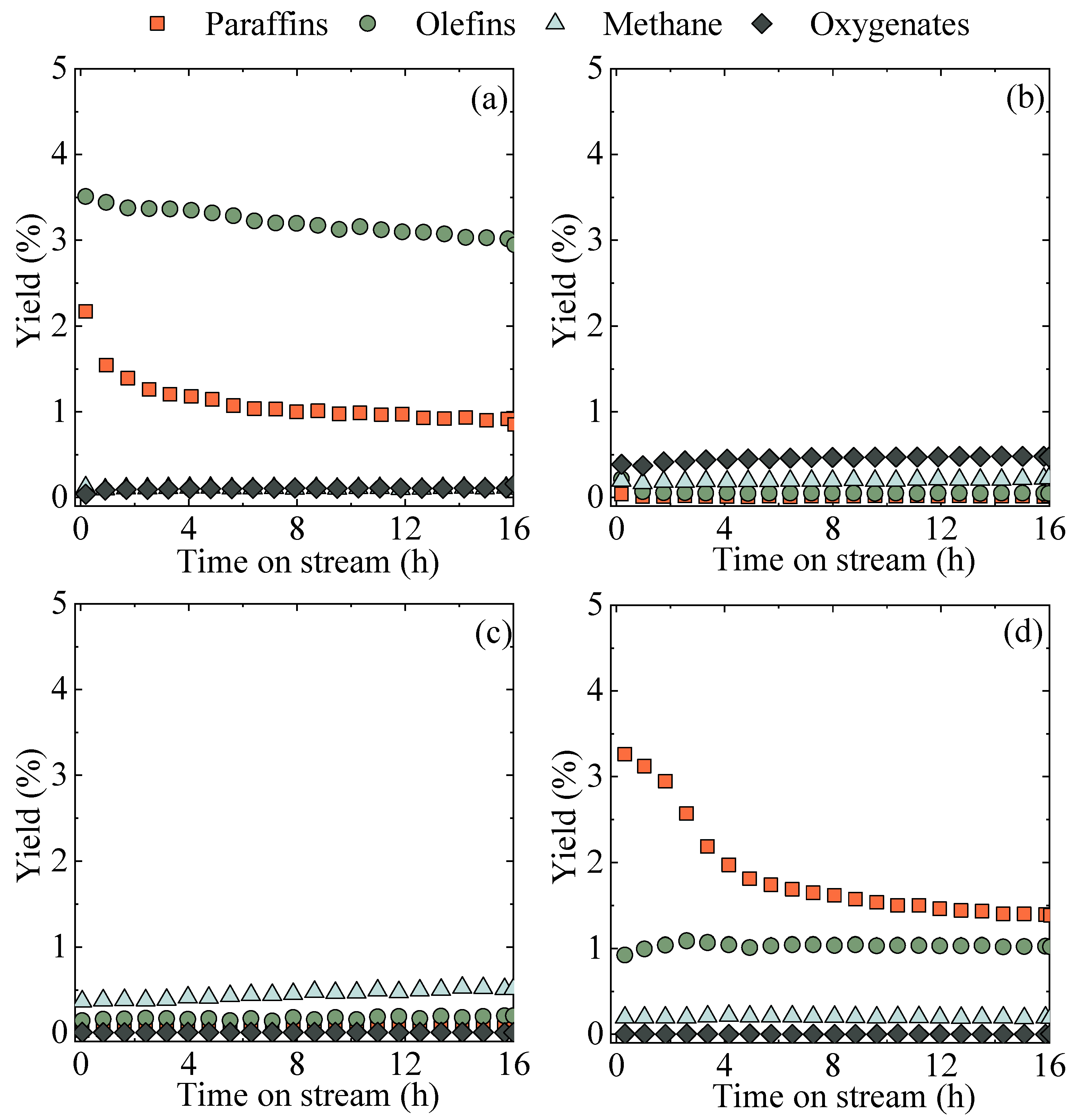
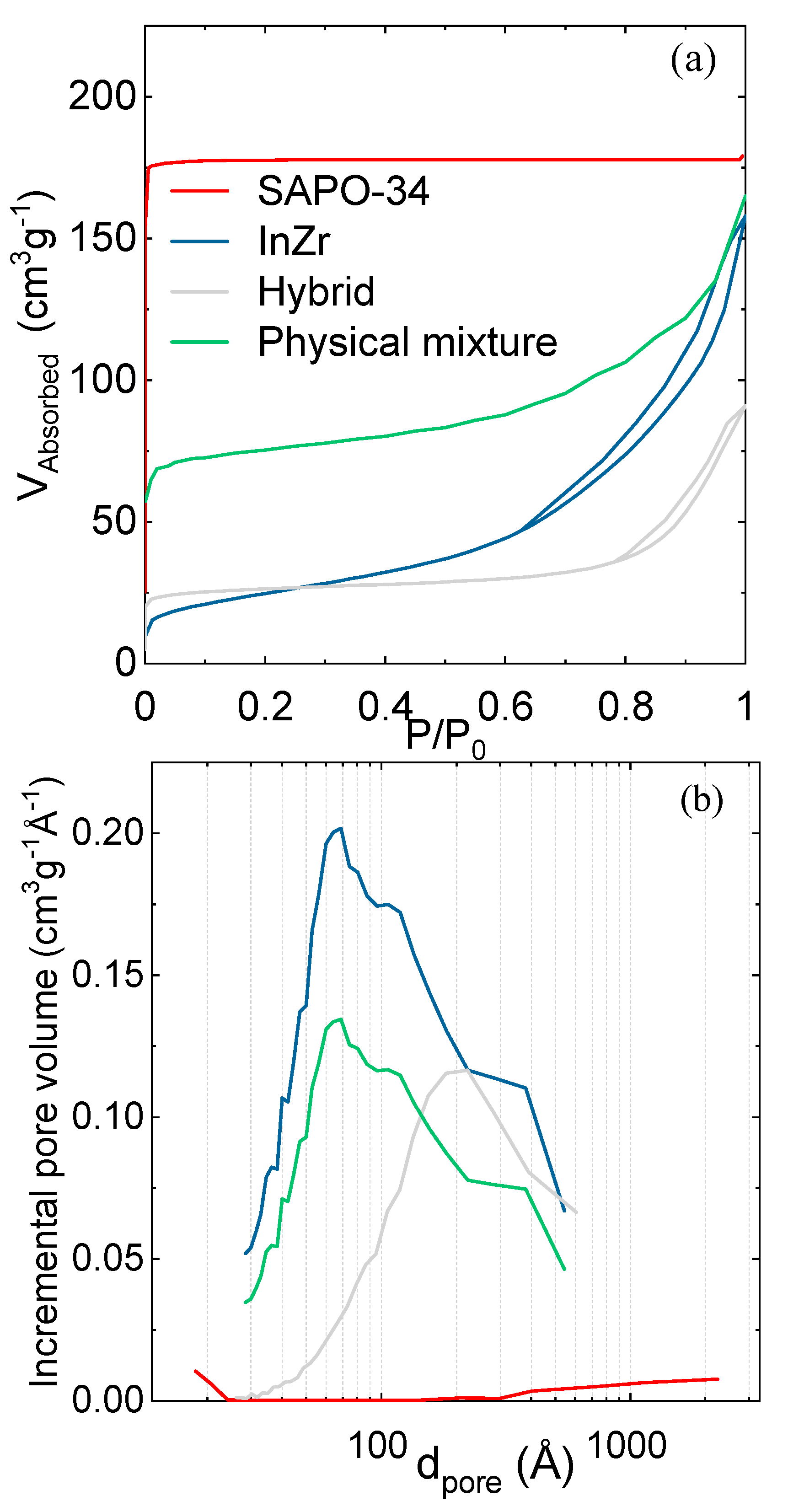
2.2. Configuration of the Catalyst and Catalytic Bed
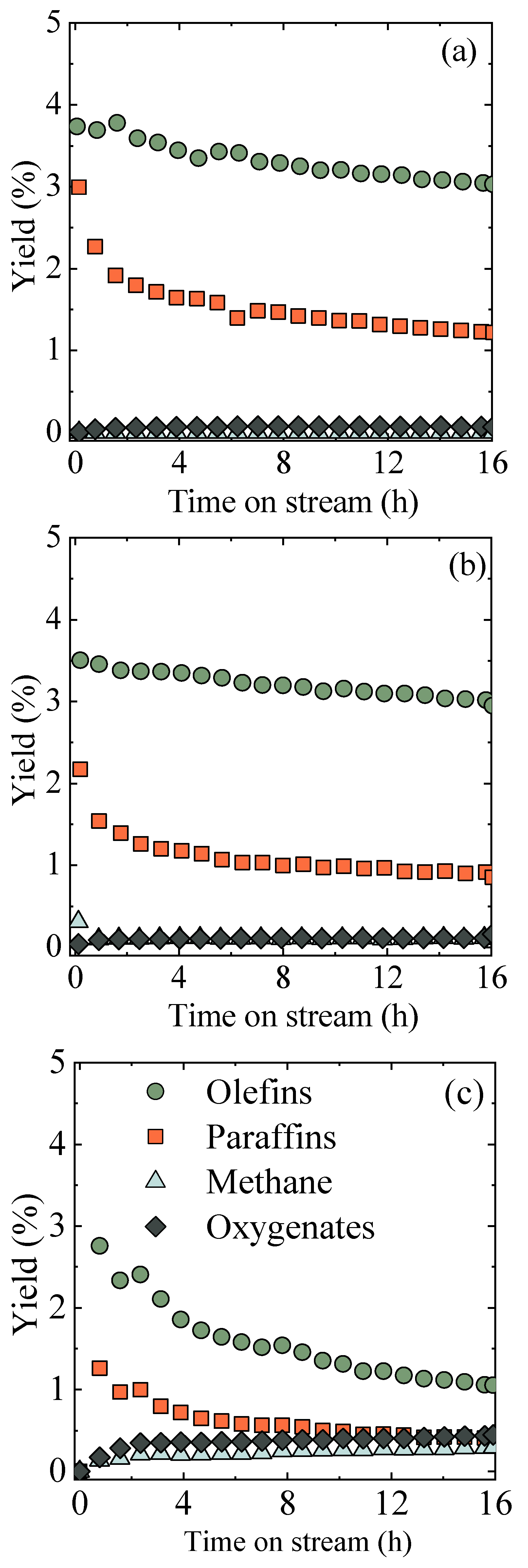
2.3. Catalyst Reduction Treatment
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Reaction and Analysis Equipment
3.4. Reaction Indices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Energy Agency. Net Zero by 2050—Analysis—IEA. Available online: https://www.iea.org/reports/world-energy-outlook-2021 (accessed on 2 July 2023).
- Cordero-Lanzac, T.; Ramirez, A.; Navajas, A.; Gevers, L.; Brunialti, S.; Gandía, L.M.; Aguayo, A.T.; Sarathy, S.M.; Gascon, J. A Techno-Economic and Life Cycle Assessment for the Production of Green Methanol from CO2: Catalyst and Process Bottlenecks. J. Energy Chem. 2022, 68, 255–266. [Google Scholar] [CrossRef]
- Alabdullah, M.A.; Gomez, A.R.; Vittenet, J.; Bendjeriou-Sedjerari, A.; Xu, W.; Abba, I.A.; Gascon, J. A Viewpoint on the Refinery of the Future: Catalyst and Process Challenges. ACS Catal. 2020, 10, 8131–8140. [Google Scholar] [CrossRef]
- Gholami, Z.; Gholami, F.; Tišler, Z.; Vakili, M. A Review on the Production of Light Olefins Using Steam Cracking of Hydrocarbons. Energies 2021, 14, 8190. [Google Scholar] [CrossRef]
- Gholami, Z.; Gholami, F.; Tišler, Z.; Tomas, M.; Vakili, M. A Review on Production of Light Olefins via Fluid Catalytic Cracking. Energies 2021, 14, 1089. [Google Scholar] [CrossRef]
- Tian, P.; Wei, Y.; Ye, M.; Liu, Z. Methanol to Olefins (MTO): From Fundamentals to Commercialization. ACS Catal. 2015, 5, 1922–1938. [Google Scholar] [CrossRef]
- Pawelec, B.; Guil-López, R.; Mota, N.; Fierro, J.L.G.; Yerga, R.M.N. Catalysts for the Conversion of CO2 to Low Molecular Weight Olefins—A Review. Materials 2021, 14, 6952. [Google Scholar] [CrossRef] [PubMed]
- Orege, J.I.; Liu, N.; Amoo, C.C.; Wei, J.; Ge, Q.; Sun, J. Boosting CO2 Hydrogenation to High-Value Olefins with Highly Stable Performance over Ba and Na Co-Modified Fe Catalyst. J. Energy Chem. 2023, 80, 614–624. [Google Scholar] [CrossRef]
- Guo, S.; Wang, H.; Qin, Z.; Li, Z.; Wang, G.; Dong, M.; Fan, W.; Wang, J. Feasibility, Limit, and Suitable Reaction Conditions for the Production of Alcohols and Hydrocarbons from CO and CO2 through Hydrogenation, a Thermodynamic Consideration. Ind. Eng. Chem. Res. 2022, 61, 17027–17038. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, W.; Yang, Y.; Cheng, K.; Kang, J.; Zhang, L.; Zhang, G.; Min, X.; Zhang, Q.; Wang, Y. Design of Efficient Bifunctional Catalysts for Direct Conversion of Syngas into Lower Olefins via Methanol/Dimethyl Ether Intermediates. Chem. Sci. 2018, 9, 4708–4718. [Google Scholar] [CrossRef]
- Al-Qadri, A.A.; Nasser, G.A.; Adamu, H.; Muraza, O.; Saleh, T.A. CO2 Utilization in Syngas Conversion to Dimethyl Ether and Aromatics: Roles and Challenges of Zeolites-Based Catalysts. J. Energy Chem. 2023, 79, 418–449. [Google Scholar] [CrossRef]
- Tsoukalou, A.; Abdala, P.M.; Stoian, D.; Huang, X.; Willinger, M.G.; Fedorov, A.; Müller, C.R. Structural Evolution and Dynamics of an In2O3 Catalyst for CO2 Hydrogenation to Methanol: An Operando XAS-XRD and in Situ TEM Study. J. Am. Chem. Soc. 2019, 141, 13497–13505. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, G.; Zhu, J.; Zhang, X.; Ding, F.; Zhang, A.; Guo, X.; Song, C. CO2 Hydrogenation to Methanol over In2O3-Based Catalysts: From Mechanism to Catalyst Development. ACS Catal. 2021, 11, 1406–1423. [Google Scholar] [CrossRef]
- Ye, J.; Liu, C.; Ge, Q. DFT Study of CO2 Adsorption and Hydrogenation on the In2O3 Surface. J. Phys. Chem. C 2012, 116, 7817–7825. [Google Scholar] [CrossRef]
- Ye, J.; Liu, C.; Mei, D.; Ge, Q. Active Oxygen Vacancy Site for Methanol Synthesis from CO2 Hydrogenation on In2O3 (110): A DFT Study. ACS Catal. 2013, 3, 1296–1306. [Google Scholar] [CrossRef]
- Sun, K.; Fan, Z.; Ye, J.; Yan, J.; Ge, Q.; Li, Y.; He, W.; Yang, W.; Liu, C.-j. Hydrogenation of CO2 to Methanol over In2O3 Catalyst. J. CO2 Util. 2015, 12, 1–6. [Google Scholar] [CrossRef]
- Martin, O.; Martín, A.J.; Mondelli, C.; Mitchell, S.; Segawa, T.F.; Hauert, R.; Drouilly, C.; Curulla-Ferré, D.; Pérez-Ramírez, J. Indium Oxide as a Superior Catalyst for Methanol Synthesis by CO2 Hydrogenation. Angew. Chemie 2016, 128, 6369–6373. [Google Scholar] [CrossRef]
- Dang, S.; Gao, P.; Liu, Z.; Chen, X.; Yang, C.; Wang, H.; Zhong, L.; Li, S.; Sun, Y. Role of Zirconium in Direct CO2 Hydrogenation to Lower Olefins on Oxide/Zeolite Bifunctional Catalysts. J. Catal. 2018, 364, 382–393. [Google Scholar] [CrossRef]
- Portillo, A.; Ateka, A.; Ereña, J.; Bilbao, J.; Aguayo, A.T. Role of Zr Loading into In2O3 Catalysts for the Direct Conversion of CO2/CO Mixtures into Light Olefins. J. Environ. Manag. 2022, 316, 115329. [Google Scholar] [CrossRef]
- Numpilai, T.; Wattanakit, C.; Chareonpanich, M.; Limtrakul, J.; Witoon, T. Optimization of Synthesis Condition for CO2 Hydrogenation to Light Olefins over In2O3 Admixed with SAPO-34. Energy Convers. Manag. 2019, 180, 511–523. [Google Scholar] [CrossRef]
- Araújo, T.P.; Shah, A.; Mondelli, C.; Stewart, J.A.; Ferré, D.C.; Pérez-Ramírez, J. Impact of Hybrid CO2-CO Feeds on Methanol Synthesis over In2O3-Based Catalysts. Appl. Catal. B Environ. 2021, 285, 119878. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, K.; Kang, J.; Zhou, C.; Subramanian, V.; Zhang, Q.; Wang, Y. New Horizon in C1 Chemistry: Breaking the Selectivity Limitation in Transformation of Syngas and Hydrogenation of CO2 into Hydrocarbon Chemicals and Fuels. Chem. Soc. Rev. 2019, 48, 3193–3228. [Google Scholar] [CrossRef]
- Cai, D.; Cai, Y.; Tan, K.B.; Zhan, G. Recent Advances of Indium Oxide-Based Catalysts for CO2 Hydrogenation to Methanol: Experimental and Theoretical. Materials 2023, 16, 2803. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.; Li, S.; Yang, C.; Chen, X.; Li, X.; Zhong, L.; Gao, P.; Sun, Y. Selective Transformation of CO2 and H2 into Lower Olefins over In2O3-ZnZrOx/SAPO-34 Bifunctional Catalysts. ChemSusChem 2019, 12, 3582–3591. [Google Scholar] [CrossRef] [PubMed]
- Martín, N.; Portillo, A.; Ateka, A.; Cirujano, F.G.; Oar-Arteta, L.; Aguayo, A.T.; Dusselier, M. MOF-Derived/Zeolite Hybrid Catalyst for the Production of Light Olefins from CO2. ChemCatChem 2020, 12, 5750–5758. [Google Scholar] [CrossRef]
- Portillo, A.; Ateka, A.; Ereña, J.; Bilbao, J.; Aguayo, A.T. Alternative Acid Catalysts for the Stable and Selective Direct Conversion of CO2/CO Mixtures into Light Olefins. Fuel Process. Technol. 2022, 238, 107513. [Google Scholar] [CrossRef]
- Hemelsoet, K.; Van Der Mynsbrugge, J.; De Wispelaere, K.; Waroquier, M.; Van Speybroeck, V. Unraveling the Reaction Mechanisms Governing Methanol-to-Olefins Catalysis by Theory and Experiment. ChemPhysChem 2013, 14, 1526–1545. [Google Scholar] [CrossRef]
- Bjørgen, M.; Svelle, S.; Joensen, F.; Nerlov, J.; Kolboe, S.; Bonino, F.; Palumbo, L.; Bordiga, S.; Olsbye, U. Conversion of Methanol to Hydrocarbons over Zeolite H-ZSM-5: On the Origin of the Olefinic Species. J. Catal. 2007, 249, 195–207. [Google Scholar] [CrossRef]
- Gao, J.; Jia, C.; Liu, B. Direct and Selective Hydrogenation of CO2 to Ethylene and Propene by Bifunctional Catalysts. Catal. Sci. Technol. 2017, 7, 5602–5607. [Google Scholar] [CrossRef]
- Gao, P.; Dang, S.; Li, S.; Bu, X.; Liu, Z.; Qiu, M.; Yang, C.; Wang, H.; Zhong, L.; Han, Y.; et al. Direct Production of Lower Olefins from CO2 Conversion via Bifunctional Catalysis. ACS Catal. 2018, 8, 571–578. [Google Scholar] [CrossRef]
- Portillo, A.; Parra, O.; Ereña, J.; Aguayo, A.T.; Bilbao, J.; Ateka, A. Effect of Water and Methanol Concentration in the Feed on the Deactivation of In2O3-ZrO2/SAPO-34 Catalyst in the Conversion of CO2/CO to Olefins by Hydrogenation. Fuel 2023, 346, 128298. [Google Scholar] [CrossRef]
- Gao, P.; Li, S.; Bu, X.; Dang, S.; Liu, Z.; Wang, H.; Zhong, L.; Qiu, M.; Yang, C.; Cai, J.; et al. Direct Conversion of CO2 into Liquid Fuels with High Selectivity over a Bifunctional Catalyst. Nat. Chem. 2017, 9, 1019–1024. [Google Scholar] [CrossRef]
- Han, Z.; Tang, C.; Wang, J.; Li, L.; Li, C. Atomically Dispersed Ptn+ Species as Highly Active Sites in Pt/In2O3 Catalysts for Methanol Synthesis from CO2 Hydrogenation. J. Catal. 2021, 394, 236–244. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, A.; Jiang, X.; Song, C.; Guo, X. Highly Selective Conversion of CO2 to Lower Hydrocarbons (C2-C4) over Bifunctional Catalysts Composed of In2O3-ZrO2 and Zeolite. J. CO2 Util. 2018, 27, 81–88. [Google Scholar] [CrossRef]
- Li, G.; Jiao, F.; Pan, X.; Li, N.; Miao, D.; Li, L.; Bao, X. Role of SAPO-18 Acidity in Direct Syngas Conversion to Light Olefins. ACS Catal. 2020, 10, 12370–12375. [Google Scholar] [CrossRef]
- Ateka, A.; Pérez-Uriarte, P.; Gamero, M.; Ereña, J.; Aguayo, A.T.; Bilbao, J. A Comparative Thermodynamic Study on the CO2 Conversion in the Synthesis of Methanol and of DME. Energy 2017, 120, 796–804. [Google Scholar] [CrossRef]
- Qi, L.; Li, J.; Wang, L.; Wang, C.; Xu, L.; Liu, Z. Comparative Investigation of the Deactivation Behaviors over HZSM-5 and HSAPO-34 Catalysts during Low-Temperature Methanol Conversion. Catal. Sci. Technol. 2017, 7, 2022–2031. [Google Scholar] [CrossRef]
- Aguayo, A.T.; del Campo, A.E.S.; Gayubo, A.G.; Tarrío, A.; Bilbao, J. Deactivation by Coke of a Catalyst Based on a SAPO-34 in the Transformation of Methanol into Olefins. J. Chem. Technol. Biotechnol. 1999, 74, 315–321. [Google Scholar] [CrossRef]
- Pérez-Uriarte, P.; Ateka, A.; Gamero, M.; Aguayo, A.T.; Bilbao, J. Effect of the Operating Conditions in the Transformation of DME to Olefins over a HZSM-5 Zeolite Catalyst. Ind. Eng. Chem. Res. 2016, 55, 6569–6578. [Google Scholar] [CrossRef]
- Sierra, I.; Ereña, J.; Aguayo, A.T.; Arandes, J.M.; Olazar, M.; Bilbao, J. Co-Feeding Water to Attenuate Deactivation of the Catalyst Metallic Function (CuO–ZnO–Al2O3) by Coke in the Direct Synthesis of Dimethyl Ether. Appl. Catal. B Environ. 2011, 106, 167–173. [Google Scholar] [CrossRef]
- Gayubo, A.G.; Aguayo, A.T.; Del Campo, A.E.S.; Tarrío, A.M.; Bilbao, J. Kinetic Modeling of Methanol Transformation into Olefins on a SAPO-34 Catalyst. Ind. Eng. Chem. Res. 2000, 39, 292–300. [Google Scholar] [CrossRef]
- Cordero-Lanzac, T.; Aguayo, A.T.; Gayubo, A.G.; Bilbao, J. Consideration of the Activity Distribution Using the Population Balance Theory for Designing a Dual Fluidized Bed Reactor-Regenerator System. Application to the MTO Process. Chem. Eng. J. 2021, 405, 126448. [Google Scholar] [CrossRef]
- Portillo, A.; Ateka, A.; Ereña, J.; Aguayo, A.T.; Bilbao, J. Conditions for the Joint Conversion of CO2 and Syngas in the Direct Synthesis of Light Olefins Using In2O3–ZrO2/SAPO-34 Catalyst. Ind. Eng. Chem. Res. 2021, 2022, 10365–10376. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Mohammadi, M.; Sedighi, M. Sustainable Production of Light Olefins from Greenhouse Gas CO2 over SAPO-34 Supported Modified Cerium Oxide. Microporous Mesoporous Mater. 2020, 297, 110029. [Google Scholar] [CrossRef]
- Sánchez-Contador, M.; Ateka, A.; Aguayo, A.T.; Bilbao, J. Direct Synthesis of Dimethyl Ether from CO and CO2 over a Core-Shell Structured CuO-ZnO-ZrO2@SAPO-11 Catalyst. Fuel Process. Technol. 2018, 179, 258–268. [Google Scholar] [CrossRef]
- Frei, M.S.; Capdevila-Cortada, M.; García-Muelas, R.; Mondelli, C.; López, N.; Stewart, J.A.; Ferré, D.C.; Pérez-Ramírez, J. Mechanism and Microkinetics of Methanol Synthesis via CO2 Hydrogenation on Indium Oxide. J. Catal. 2018, 361, 313–321. [Google Scholar] [CrossRef]
- Chen, T.Y.; Cao, C.; Chen, T.B.; Ding, X.; Huang, H.; Shen, L.; Cao, X.; Zhu, M.; Xu, J.; Gao, J.; et al. Unraveling Highly Tunable Selectivity in CO2 Hydrogenation over Bimetallic In-Zr Oxide Catalysts. ACS Catal. 2019, 9, 8785–8797. [Google Scholar] [CrossRef]


| Treatment | In0/In2O3 Ratio |
|---|---|
| Without treatment | 0 |
| Partial reduction | 0.04 |
| Complete reduction | Infinite |
| Catalyst | SBET (m2 g−1) | Vmicropore (cm3 g−1) | Vpore (cm3 g−1) | dp (Å) |
|---|---|---|---|---|
| In2O3-ZrO2 | 86 | - | 0.23 | 90 |
| SAPO-34 | 652 | 0.22 | 0.23 | 15 |
| Hybrid In2O3-ZrO2/SAPO-34 | 98 | 0.03 | 0.13 | 32 |
| In2O3-ZrO2 | 123 |
| SAPO-34 | 778 |
| Hybrid In2O3-ZrO2/SAPO-34 | 319 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portillo, A.; Parra, O.; Aguayo, A.T.; Ereña, J.; Bilbao, J.; Ateka, A. Setting up In2O3-ZrO2/SAPO-34 Catalyst for Improving Olefin Production via Hydrogenation of CO2/CO Mixtures. Catalysts 2023, 13, 1101. https://doi.org/10.3390/catal13071101
Portillo A, Parra O, Aguayo AT, Ereña J, Bilbao J, Ateka A. Setting up In2O3-ZrO2/SAPO-34 Catalyst for Improving Olefin Production via Hydrogenation of CO2/CO Mixtures. Catalysts. 2023; 13(7):1101. https://doi.org/10.3390/catal13071101
Chicago/Turabian StylePortillo, Ander, Onintze Parra, Andrés T. Aguayo, Javier Ereña, Javier Bilbao, and Ainara Ateka. 2023. "Setting up In2O3-ZrO2/SAPO-34 Catalyst for Improving Olefin Production via Hydrogenation of CO2/CO Mixtures" Catalysts 13, no. 7: 1101. https://doi.org/10.3390/catal13071101
APA StylePortillo, A., Parra, O., Aguayo, A. T., Ereña, J., Bilbao, J., & Ateka, A. (2023). Setting up In2O3-ZrO2/SAPO-34 Catalyst for Improving Olefin Production via Hydrogenation of CO2/CO Mixtures. Catalysts, 13(7), 1101. https://doi.org/10.3390/catal13071101