Synthesis of Asymmetrical CsPbBr3/TiO2 Nanocrystals with Enhanced Stability and Photocatalytic Properties
Abstract
1. Introduction
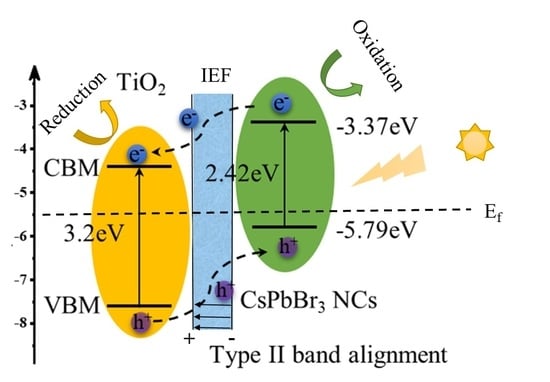
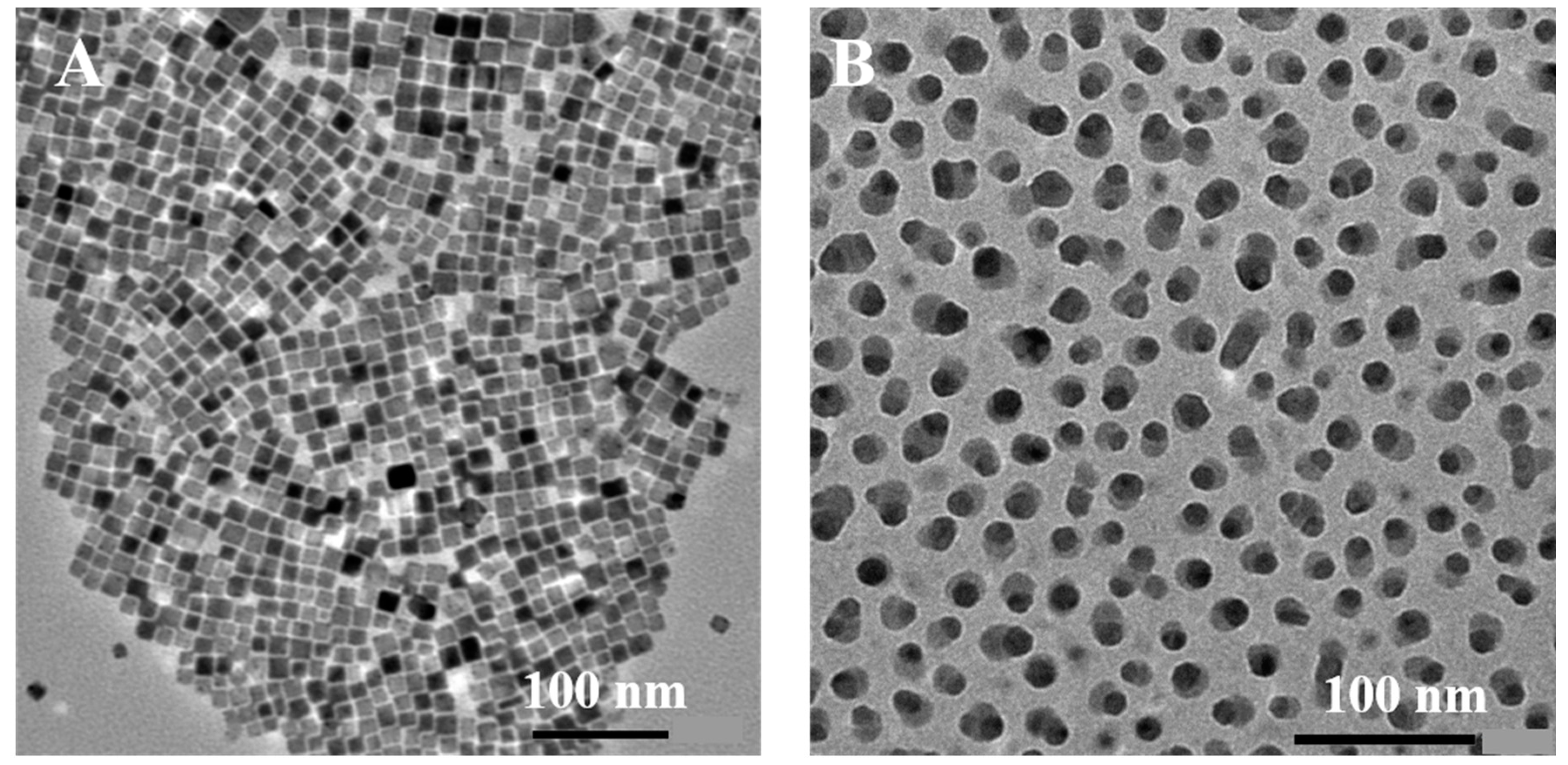
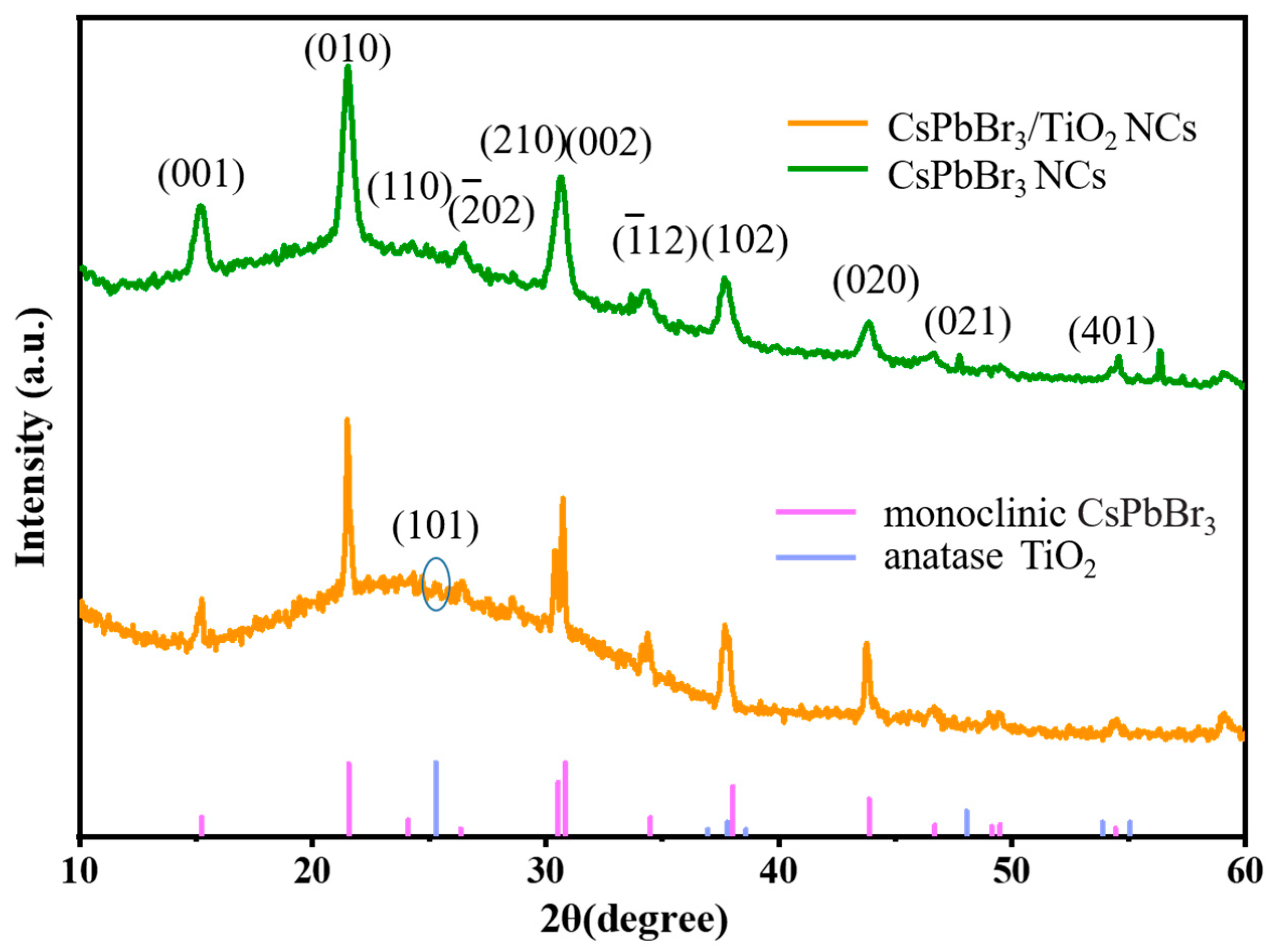
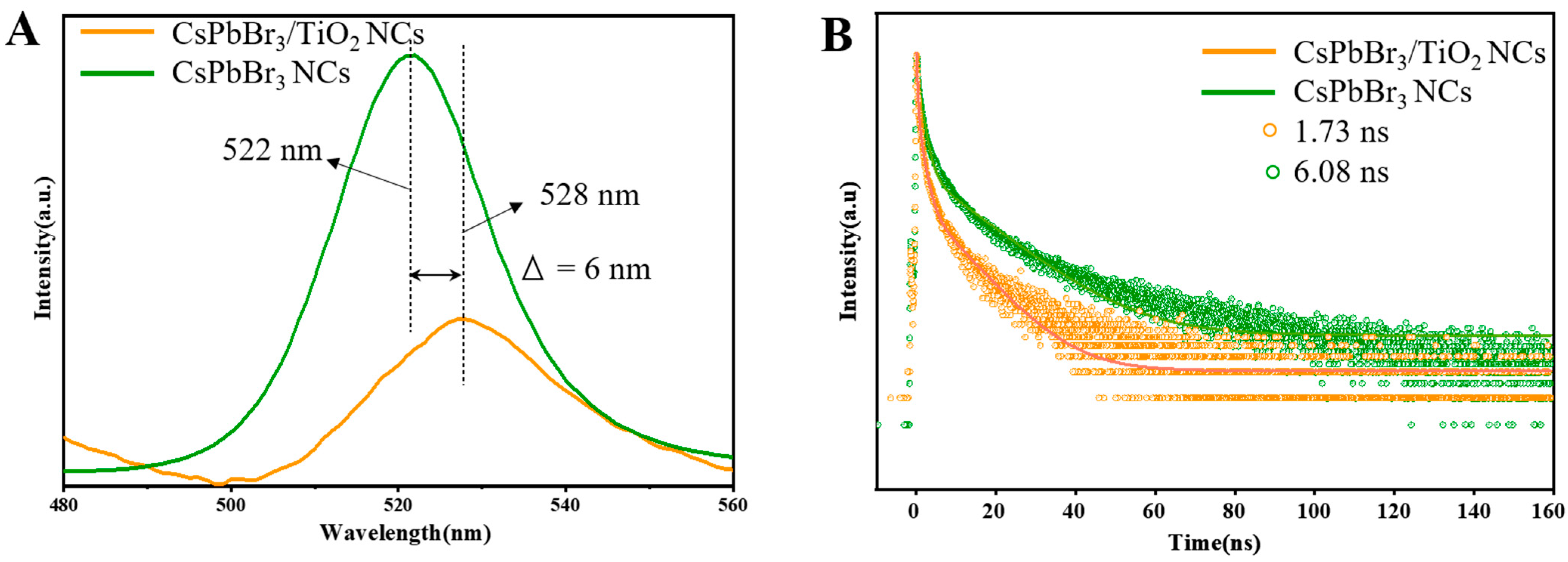
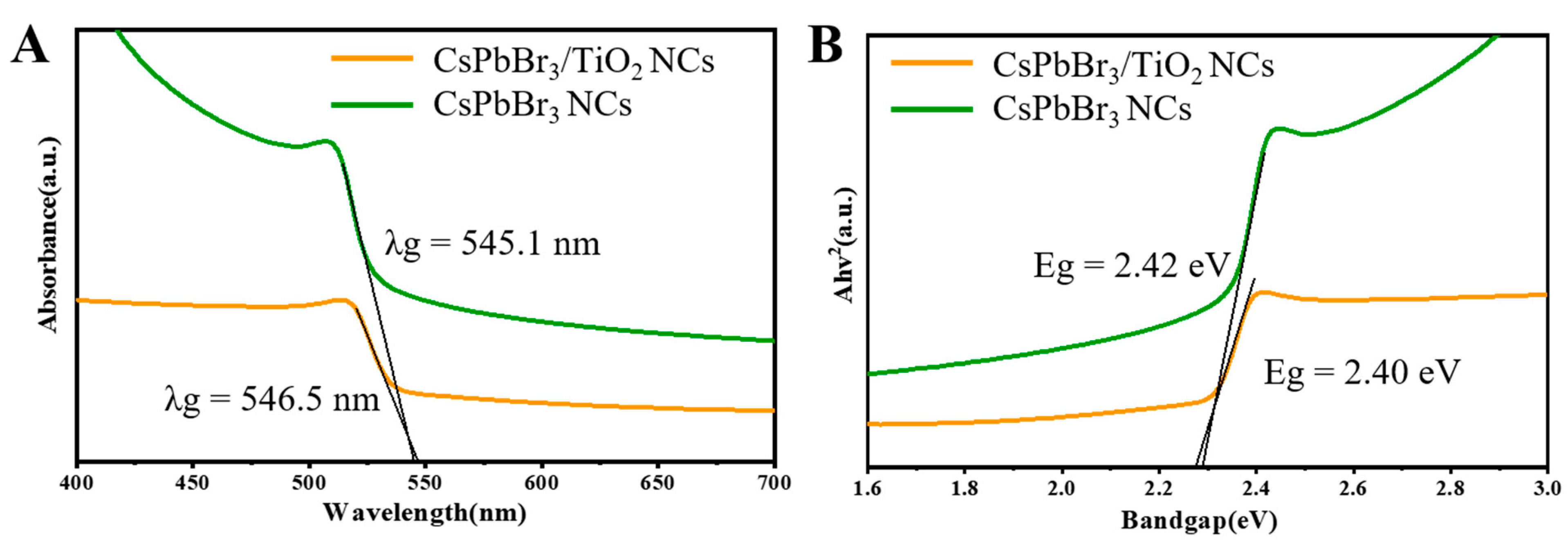
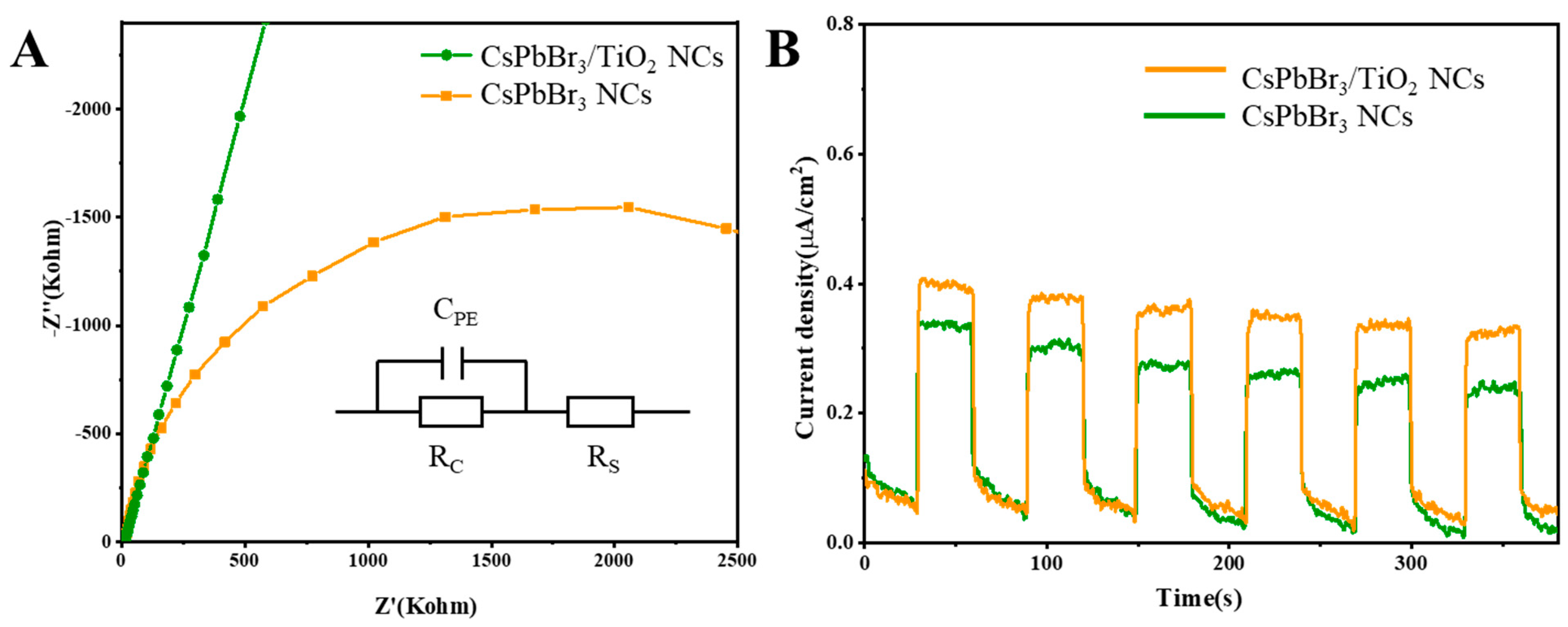
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. Characterization
3.3.1. Materials Characterization
3.3.2. Optical Characterization
3.3.3. (Photo)electrochemistry Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Swarnkar, A.; Marshall, A.R.; Sanehira, E.M.; Chernomordik, B.D.; Moore, D.T.; Christians, J.A.; Chakrabarti, T.; Luther, J. Quantum dot-induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science 2016, 354, 92–95. [Google Scholar] [CrossRef]
- Yang, W.S.; Park, B.W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H. Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum dot light-emitting diodes based on inorganic perovskite cesium lead halides (CsPbX3). Adv. Mater. 2016, 27, 7162–7167. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, L.; Wang, T.; Song, J.; Chen, J.; Xue, J.; Dong, Y.; Cai, B.; Shan, Q.; Han, B. 50-fold EQE improvement up to 6.27% of solution-processed all-inorganic perovskite CsPbBr3 QLEDs via surface ligand density control. Adv. Mater. 2017, 29, 1603885. [Google Scholar] [CrossRef]
- Zhi-Kuang, T.; Reza Saberi, M.; May Ling, L.; Pablo, D.; Ruben, H.; Felix, D.; Michael, P.; Aditya, S.; Pazos, L.M.; Dan, C. Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotechnol. 2018, 9, 687–692. [Google Scholar]
- Zhang, X.; Xu, B.; Zhang, J.; Gao, Y.; Zheng, Y.; Wang, K.; Sun, X. All-inorganic perovskite nanocrystals for high-efficiency light emitting diodes: Dual-phase CsPbBr3-CsPb2Br5 Composites. Adv. Funct. Mater. 2016, 26, 4595–4600. [Google Scholar] [CrossRef]
- Song, T.; Cheng, H.; Fu, C.; He, B.; Zou, B.J.N. Influence of the active layer nanomorphology on device performance for ternary PbSxSe1−X quantum dots based solution-processed infrared photodetector. Nanotechnology 2016, 27, 165202. [Google Scholar] [CrossRef]
- Chiba, T.; Hoshi, K.; Pu, Y.J.; Takeda, Y.; Kido, J. High efficiency perovskite quantum-dot light-emitting devices by effective washing process and interfacial energy level alignment. ACS Appl. Mater. Inter. 2017, 9, 18054. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Gu, Y.; Zou, Y.; Song, J.; Xu, L.; Li, J.; Xue, F.; Li, X.; Zeng, H. Improving all-Inorganic perovskite photodetectors by preferred orientation and plasmonic effect. Small 2016, 12, 5622–5632. [Google Scholar] [CrossRef] [PubMed]
- Veldhuis, S.A.; Boix, P.P.; Yantara, N.; Li, M.; Sum, T.C.; Mathews, N.; Mhaisalkar, S.G. Perovskite materials for light-emitting diodes and lasers. Adv. Mater. 2016, 28, 6804–6834. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhu, H.; Schrader, A.W.; Liang, D.; Jin, S. Nanowire lasers of formamidinium lead halide perovskites and their stabilized alloys with improved stability. Nano Lett. 2016, 16, 1000–1008. [Google Scholar] [CrossRef]
- Zhu, H.; Fu, Y.; Meng, F.; Wu, X.; Gong, Z.; Ding, Q.; Gustafsson, M.V.; Trinh, M.T.; Jin, S.; Zhu, X. Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat. Mater. 2015, 14, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Mathews, N.; Lim, S.S.; Yantara, N.; Liu, X.; Sabba, D. Low-temperature solution-processed wavelength-tunable perovskites for lasing. Nat.Mater. 2014, 13, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Cao, M.; Hu, H.; Di, Y.; Chen, M.; Li, P.; Wu, L.; Qiao, Z. One-pot synthesis of highly stable CsPbBr3@SiO2 core-shell nanoparticles. ACS Nano 2018, 12, 8579–8587. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano.Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Xu, Y.F.; Yang, M.Z.; Chen, H.Y.; Liao, J.F.; Kuang, D. Enhanced solar-driven gaseous CO2 conversion by CsPbBr3 nanocrystal/Pd nanosheet Schottky-junction photocatalyst. ACS Appl. Energy Mater. 2018, 1, 5083–5089. [Google Scholar] [CrossRef]
- Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Grotevent, M.J.; Kovalenko, M.V. Fast anion-exchange in highly luminescent nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef]
- Chen, S.; Yin, H.; Liu, P.; Wang, Y.; Zhao, H. Stabilization and performance enhancement strategies for halide perovskite photocatalysts. Adv. Mater. 2023, 35, 2203836. [Google Scholar] [CrossRef]
- Xiao, M.; Hao, M.; Lyu, M.; Moore, E.G.; Zhang, C.; Luo, B.; Hou, J.; Lipton-Duffin, J.; Wang, L. Surface ligands stabilized lead halide perovskite quantum dot photocatalyst for visible light-driven hydrogen generation. Adv. Funct. Mater. 2019, 29, 1905683. [Google Scholar] [CrossRef]
- Liu, C.; Zeng, Q.; Wei, H.; Yu, Y.; Zhao, Y.; Feng, T.; Yang, B. Metal halide perovskite nanocrystal solar cells: Progress and challenges. Small Methods 2020, 4, 2000419. [Google Scholar] [CrossRef]
- Imran, M.; Peng, L.; Pianetti, A.; Pinchetti, V.; Ramade, J.; Zito, J.; Di Stasio, F.; Buha, J.; Toso, S.; Song, J.; et al. Halide perovskite-lead chalcohalide nanocrystal heterostructures. J. Am. Chem. Soc. 2021, 143, 1435–1446. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Liao, Q. Embedding perovskite nanocrystals into a polymer matrix for tunable luminescence probes in cell imaging. Adv. Funct. Mater. 2017, 27, 1604382. [Google Scholar] [CrossRef]
- Wei, Y.; Deng, X.; Xie, Z.; Cai, X.; Liang, S.; Ma, P.A.; Hou, Z.Y.; Cheng, Z.Y.; Lin, J. Enhancing the stability of perovskite quantum dots by encapsulation in crosslinked polystyrene beads via a swelling–shrinking strategy toward superior water resistance. Adv. Funct. Mater. 2017, 27, 1703535. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Huang, J.; Cai, J.; Zhu, J.; Yang, X.; Shen, J.; Jiang, H.; Li, C. CsPbBr3 perovskite quantum dots-bsed monolithic electrospun fiber membrane as an ultrastable and utrasensitive fluorescent sensor in aqueous medium. J. Phys. Chem. Lett. 2016, 7, 4253–4258. [Google Scholar] [CrossRef]
- Raja, S.N.; Bekenstein, Y.; Koc, M.A.; Fischer, S.; Zhang, D.; Lin, L.; Ritchie, R.O.; Yang, P.; Alivisatos, A.P. Interfaces, Encapsulation of perovskite nanocrystals into macroscale polymer matrices: Enhanced stability and polarization. ACS Appl. Mater. Inter. 2016, 8, 35523–35533. [Google Scholar] [CrossRef]
- Hou, S.; Guo, Y.; Tang, Y.; Quan, Q. Interfaces, synthesis and stabilization of colloidal perovskite nanocrystals by multidentate polymer micelles. ACS Appl. Mater. Inter. 2017, 9, 18417. [Google Scholar] [CrossRef]
- Liao, H.; Guo, S.; Cao, S.; Wang, L.; Gao, F.; Yang, Z.; Zheng, J.; Yang, W. A general strategy for in situ growth of all-Inorganic CsPbX3 (X = Br, I, and Cl) perovskite nanocrystals in polymer fibers toward significantly enhanced water/thermal stabilities. Adv. Opt. Mater. 2018, 6, 1800346. [Google Scholar] [CrossRef]
- Yang, X.; Xu, T.; Zhu, Y.; Cai, J.; Gu, K.; Zhu, J.; Wang, Y.; Shen, J.; Li, C. Preparation of CsPbBr3@PS composite microspheres with high stability by electrospraying. J. Mater. Chem. C 2018, 6, 7971–7975. [Google Scholar] [CrossRef]
- Meyns, M.; Perálvarez, M.; Heuer-Jungemann, A.; Hertog, W.; Ibáñez, M.; Nafria, R.; Genç, A.; Arbiol, J.; Kovalenko, M.V. Carreras. Polymer-enhanced stability of inorganic perovskite nanocrystals and their application in color conversion LEDs. ACS Appl. Mat. Inte. 2016, 8, 19579–19586. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Long, K.; Huang, S.; Liang, L. Highly luminescent and ultrastable CsPbBr3 perovskite quantum dots incorporated into a silica/alumina monolith. Angew. Chem. 2017, 129, 8246–8250. [Google Scholar] [CrossRef]
- Wang, H.C.; Lin, S.Y.; Tang, A.C.; Singh, B.P.; Tong, H.C.; Chen, C.Y.; Lee, Y.C.; Tsai, T.L.; Liu, S. Mesoporous silica particles integrated with all-Inorganic CsPbBr3 perovskite quantum-dot nanocomposites (MP-PQDs) with high stability and wide color gamut used for backlight display. Angew. Chem. Int. 2016, 55, 7924–7929. [Google Scholar] [CrossRef]
- Dirin, D.N.; Protesescu, L.; Trummer, D.; Kochetygov, I.V.; Yakunin, S.; Krumeich, F.; Stadie, N.P.; Kovalenko, M.V. Harnessing defect-tolerance at the nanoscale: Highly luminescent lead halide perovskite nanocrystals in mesoporous silica matrixes. Nano Lett. 2016, 16, 5866–5874. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, Y.; Ruan, C.; Yin, C.; Wang, X.; Wang, Y.; Yu, W. Efficient and stable white LEDs with silica-coated inorganic perovskite quantum dots. Adv. Mater. 2016, 28, 10088–10094. [Google Scholar] [CrossRef] [PubMed]
- Loiudice, A.; Saris, S.; Oveisi, E.; Alexander, D.; Buonsanti, R. CsPbBr3 QD/AlOx inorganic nanocomposites with exceptional stability in water, light, and heat. Angew. Chem. 2017, 129, 11099. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Dvoranova, D.; Lajaunie, L.; Rozman, N.; Figueiredo, B.; Seabra, M.P.; Skapin, A.S.; Calvino, J.J.; Brezova, V.; Labrincha, J.A. Graphene-TiO2 hybrids for photocatalytic aided removal of VOCs and nitrogen oxides from outdoor environment. Chem. Eng. J. 2021, 405, 126651. [Google Scholar] [CrossRef]
- Jamjoum, H.A.A.; Umar, K.; Adnan, R.; Razali, M.R.; Mohamad Ibrahim, M.N. Synthesis, characterization, and photocatalytic activities of graphene oxide/metal oides nanocomposites: A review. Front. Chem. 2021, 9, 752276. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Khan, S.; Takagi, K.; Suzuki, N.; Teshima, K.; Terashima, C.; Fujishima, A. Photocatalytic degradation of bisphenol A using titanium dioxide@nanodiamond composites under UV light illumination. Colloid Interface Sci. 2021, 582, 1058–1066. [Google Scholar] [CrossRef]
- Li, Z.Z.; Hofman, E.; Li, J.; Davis, A. Photoelectrochemically active and environmentally stable CsPbBr3/TiO2 core/shell nanocrystals. Adv. Funct. Mater. 2017, 28, 1704288. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, M.; Yang, Z.; Qiu, H.; Bhatti, A. Trioctylphosphine-assisted pre-protection low-temperature solvothermal synthesis of highly stable CsPbBr3/TiO2 Nanocomposites. J. Phys. Chem. Lett. 2021, 12, 3786–3794. [Google Scholar] [CrossRef] [PubMed]
- Long, R.; Fang, W.H.; Prezhd, O.V. Strong interaction at the perovskite/TiO2 interface facilitates ultrafast photoinduced charge separation: A nonadiabatic molecular dynamics sudy. J. Phys. Chem. C 2017, 121, 3797–3806. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Chen, Y.; Gao, Y. Improved electroluminescence performance of quantum dot light-emitting diodes: A promising hole injection layer of Fe-doped NiO nanocrystals. Opt. Mater. 2020, 107, 110158. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Liu, J.; Wang, X.; He, J.; Li, Y.; Liu, Y. Synthesis of Asymmetrical CsPbBr3/TiO2 Nanocrystals with Enhanced Stability and Photocatalytic Properties. Catalysts 2023, 13, 1048. https://doi.org/10.3390/catal13071048
Liu W, Liu J, Wang X, He J, Li Y, Liu Y. Synthesis of Asymmetrical CsPbBr3/TiO2 Nanocrystals with Enhanced Stability and Photocatalytic Properties. Catalysts. 2023; 13(7):1048. https://doi.org/10.3390/catal13071048
Chicago/Turabian StyleLiu, Wanli, Jinfeng Liu, Xiaoqian Wang, Jiazhen He, Yuqing Li, and Yong Liu. 2023. "Synthesis of Asymmetrical CsPbBr3/TiO2 Nanocrystals with Enhanced Stability and Photocatalytic Properties" Catalysts 13, no. 7: 1048. https://doi.org/10.3390/catal13071048
APA StyleLiu, W., Liu, J., Wang, X., He, J., Li, Y., & Liu, Y. (2023). Synthesis of Asymmetrical CsPbBr3/TiO2 Nanocrystals with Enhanced Stability and Photocatalytic Properties. Catalysts, 13(7), 1048. https://doi.org/10.3390/catal13071048