Improvement of n-Butene Yield in Dimethyl Ether-to-Olefin Reaction Using Ferrierite Zeolite Catalysts
Abstract
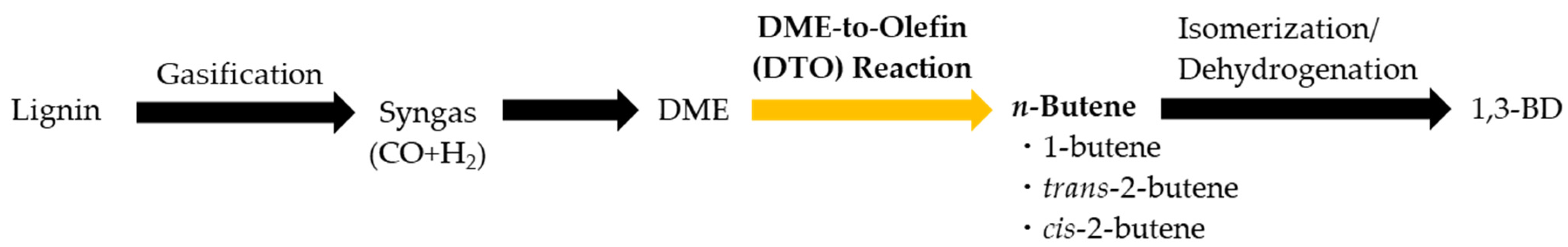
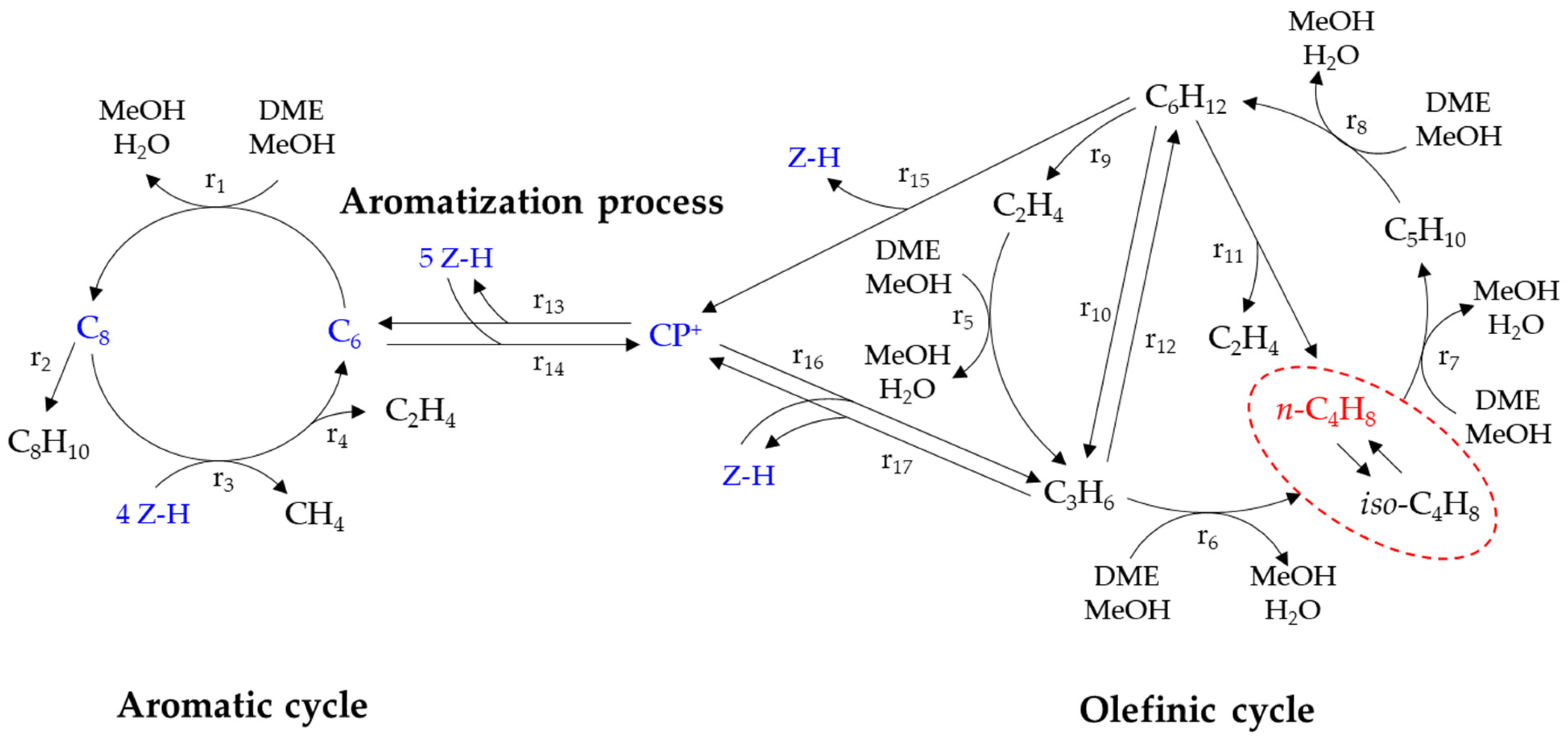
1. Introduction
2. Results and Discussion
2.1. Characterization
2.2. DTO Reactions
2.3. Correlation between n-Butene Yield and Physicochemical Properties of Zeolite
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterization
3.3. DTO Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cespi, D.; Passarini, F.; Vassura, I.; Cavani, F. Butadiene from biomass, life cycle perspective to address sustainability in the chemical industry. Green Chem. 2016, 18, 1625–1638. [Google Scholar] [CrossRef]
- Al-Douri, A.; Sengupta, D.; El-Halwagi, M.M. Shale gas monetization—A review of downstream processing to chemicals and fuels. J. Nat. Gas Sci. Eng. 2017, 45, 436–455. [Google Scholar] [CrossRef]
- Abdelrahman, O.A.; Park, D.S.; Vinter, K.P.; Spanjers, C.S.; Ren, L.; Cho, H.J.; Vlachos, G.V.; Fan, W.; Tsapatsis, M.; Dauenhauer, P.J. Biomass-derived butadiene by dehydra-decyclization of tetrahydrofuran. ACS Sustain. Chem. Eng. 2017, 5, 3732–3736. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, Z.; Liu, S.; Cui, L.; Dai, Q.; He, J.; Dong, W.; Bai, C. Synthesis of 1,3-butadiene and its 2-substituted monomers for synthetic rubbers. Catalysts 2019, 9, 97. [Google Scholar] [CrossRef]
- Reschetilowski, W.; Hauser, M.; Alscher, F.; Klauck, M.; Kalies, G. Studies on the binary MgO/SiO2 mixed oxide catalysts for the conversion of ethanol to 1,3-butadiene. Catalysts 2020, 10, 854. [Google Scholar] [CrossRef]
- Sun, D.; Li, Y.; Yang, C.; Su, Y.; Yamada, Y.; Sato, S. Production of 1,3-butadiene from biomass-derived C4 alcohols. Fuel Proc. Technol. 2020, 197, 106193. [Google Scholar] [CrossRef]
- Farzad, S.; Mandegari, M.A.; Görgens, J.F. Integrated techno-economic and environmental analysis of butadiene production from biomass. Bioresour. Technol. 2017, 239, 37–48. [Google Scholar] [CrossRef]
- Kuznetsov, A.; Kumar, G.; Ardagh, A.; Tsapatisis, M.; Zhang, Q.; Dauenhauer, P.J. On the economics and process design of renewable butadiene from biomass-derived furfural. ACS Sustain. Chem. Eng. 2020, 8, 3273–3282. [Google Scholar] [CrossRef]
- Liu, C.; Lu, X.; Yu, Z.; Xiong, J.; Bai, H.; Zhang, R. Production of levulinic acid from cellulose and cellulosic biomass in different catalytic systems. Catalysts 2020, 10, 1006. [Google Scholar] [CrossRef]
- Sobuś, N.; Czekaj, I. Catalytic transformation of biomass-derived hemicellulose sugars by the one-pot method into oxalic, lactic, and levulinic acids using a homogeneous H2SO4 catalyst. Catalysts 2023, 13, 349. [Google Scholar] [CrossRef]
- Hanaoka, T.; Fujimoto, S.; Yoshida, M. Efficiency estimation and improvement of the 1,3-butadiene production process from lignin via syngas through process simulation. Energy Fuel 2017, 31, 12965–12976. [Google Scholar] [CrossRef]
- Hanaoka, T.; Fujimoto, S.; Kihara, H. Improvement of the 1,3-butadiene production process from lignin—A comparison with the gasification power generation process. Renew. Energy 2019, 135, 1303–1313. [Google Scholar] [CrossRef]
- Hanaoka, T.; Fujimoto, S.; Kihara, H. Evaluation of n-butene synthesis from dimethyl ether in the production of 1,3-butadiene from lignin: A techno-economic analysis. Renew. Energy 2021, 163, 964–973. [Google Scholar] [CrossRef]
- Hanaoka, T.; Aoyagi, M.; Edashige, Y. n-Butene synthesis in the dimethyl ether-to-olefin reaction over zeolites. Catalysts 2021, 11, 743. [Google Scholar] [CrossRef]
- Khadzhiev, S.N.; Magomedova, M.V.; Peresypkina, E.G. Kinetic models of methanol and dimethyl ether conversion to olefins over zeolite catalysts (Review). Petrol. Chem. 2015, 55, 503–521. [Google Scholar] [CrossRef]
- Al-Dughaither, A.S.; de Lasa, H. Neat dimethyl ether conversion to olefins (DTO) over HZSM-5: Effect of SiO2/Al2O3 on porosity, surface chemistry, and reactivity. Fuel 2014, 138, 52–64. [Google Scholar] [CrossRef]
- Crodero-Lanzac, T.; Bodríguez-Cano, M.A.; Palomoto, J.; Valero-Romero, M.J.; Aguayo, A.T.; Bilbao, J.; Rodríguez-Mirasol, J.; Cordero, T. Binderless ZrO2/HZSM-5 fibrillar composites by electrospinning as catalysts for the dimethyl ether-to-olefins process. Microporous Mesoporous Mater. 2022, 342, 112102. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Zhang, Y.; Chevella, D.; Li, G.; Chen, Y.; Guo, X.; Liu, J.; Yu, J. A green route for the synthesis of nano-sized hierarchical ZSM-5 zeolite with excellent DTO catalytic performance. Chem. Eng. J. 2020, 388, 124322. [Google Scholar] [CrossRef]
- Gao, Y.; Cao, Y.; Chen, S.-L.; Wang, Y.; Zhu, R.; Sun, W.; Zhang, Q.; Fan, Y. Differences in product distribution measured with flame ionization detector gas chromatography and thermal conductivity detector gas chromatography during the di-methyl ether-to-olefins and methanol-to-olefins processes. Energy Fuel. 2017, 31, 13266–13272. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, W.; Yang, L.; Xie, T.; Li, W.; Yu, D.; Fang, Y. Effect of morphology and acidity control of Ni-SAPO-34 zeolite on catalytic performance of dimethyl ether to olefins. J. Solid State Chem. 2021, 303, 122503. [Google Scholar] [CrossRef]
- Kubota, Y.; Inagaki, S. High-performance catalysts with MSE-type zeolite framework. Top. Catal. 2015, 58, 480–493. [Google Scholar] [CrossRef]
- Park, S.; Inagaki, S.; Kubota, Y. Selective formation of light olefins from dimethyl ether over MCM-68 modified with phosphate species. Catal. Today 2016, 265, 218–224. [Google Scholar] [CrossRef]
- Han, Q.; Park, S.; Inagaki, S.; Kubota, Y. Selective production of light olefins over MSE-type zeolite catalyst. J. Jpn. Pet. Inst. 2017, 60, 288–300. [Google Scholar] [CrossRef]
- Han, Q.; Enoeda, K.; Inagaki, S.; Kubota, Y. Catalytic performance of Ce-modified MCM-68 zeolite in the dimethyl ether-to-olefin reaction: Impact of high calcination temperature. Chem. Lett. 2017, 46, 1434–1437. [Google Scholar] [CrossRef]
- Ahmed, M.H.M.; Muraza, O.; Al-Amer, A.M.; Sugiura, Y.; Nishiyama, N. Development of desilicated EU-1 zeolite and its application in conversion of dimethyl ether to olefins. Microporous Mesoporous Mater. 2015, 207, 9–16. [Google Scholar] [CrossRef]
- Ahmed, M.H.M.; Muraza, O.; Al-Amer, A.M.; Miyake, K.; Nishiyama, N. Development of hierarchical EU-1 zeolite by sequential alkaline and acid treatments for selective dimethyl ether to propylene (DTP). Appl. Catal. A Gen. 2015, 497, 127–134. [Google Scholar] [CrossRef]
- Liu, Q.; Yoshida, Y.; Nakazawa, N.; Inagaki, S.; Kubota, Y. The synthesis of YNU-5 zeolite and its application to the catalysis in the dimethyl ether-to-olefin reaction. Materials 2020, 13, 2030. [Google Scholar] [CrossRef] [PubMed]
- Nasser, G.; Kurniawan, T.; Miyake, K.; Galadima, A.; Hirota, Y.; Nishiyama, N.; Muraza, O. Dimethyl ether to olefins over dealuminated mordenite (MOR) zeolites derived from natural minerals. J. Nat. Gas Sci. Eng. 2016, 28, 566–571. [Google Scholar] [CrossRef]
- Kurniawan, T.; Muraza, O.; Miyake, K.; Hakeem, A.S.; Hirota, Y.; Al-Amer, A.M.; Nishiyama, N. Conversion of dimethyl ether to olefins over nanosized mordenite fabricated by a combined high-energy ball milling with recrystallization. Ind. Eng. Chem. Res. 2017, 56, 4258–4266. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, Y.; Li, Z.; Wang, Y.; Yu, J. Synthesis of SAPO-18/34 intergrowth zeolites and their enhanced stability for dimethyl ether to olefins. RSC Adv. 2017, 7, 939–946. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, Y.; Li, Z.; Wang, Y.; Yu, J. Synthesis of AEI/CHA intergrowth zeolites by dual templates and their catalytic performance for dimethyl ether to olefins. Chem. Eng. J. 2017, 323, 295–303. [Google Scholar] [CrossRef]
- Lee, Y.; Park, M.B.; Kim, P.S.; Vicente, A.; Fernandez, C.; Nam, I.-S.; Hong, S.B. Synthesis and catalytic behavior of ferrierite zeolite nanoneedles. ACS Catal. 2013, 3, 617–621. [Google Scholar] [CrossRef]
- Zhang, S.; Wen, Z.; Yang, L.; Duan, C.; Lu, X.; Song, Y.; Ge, Q.; Fang, Y. Controllable synthesis of hierarchical porous petal-shaped SAPO-34 zeolite with excellent DTO performance. Microporous Mesoporous Mater. 2019, 274, 220–226. [Google Scholar] [CrossRef]
- Klemm, E.; Seitz, M.; Scheidat, H.; Emig, G. Controlling acidity and selectivity of HY-type zeolites by silanation. J. Catal. 1998, 173, 177–186. [Google Scholar] [CrossRef]
- Iida, A.; Nakamura, R.; Komura, K.; Sugi, Y. Remarkable improvement of catalytic performance in dimethyl ether to olefin reaction over CeO2-modified calcium-containing MFI type zeolite. Chem. Lett. 2008, 37, 494–495. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, H.; Wang, L.; Zhang, H.; Zhang, Y.; Xu, H.; Shen, W.; Tang, Y. Highly stable boron-modified hierarchical nanocrystalline ZSM-5 zeolite for the methanol to propylene reaction. Catal. Sci. Technol. 2014, 4, 2891–2895. [Google Scholar] [CrossRef]
- De Klerk, A. Isomerization of 1-butene to isobutene at low temperature. Ind. Eng. Chem. Res. 2004, 43, 6325–6330. [Google Scholar] [CrossRef]
- Dong, H.; Yu, H.; Wang, L.; Wang, Y.; Zhong, J.; Lian, Y. Catalytic performance of phosphorus modified HZSM-5 zeolite catalysts in the co-cracking reaction of n-hexane and methanol. Catal. Lett. 2022, 152, 1233–1243. [Google Scholar] [CrossRef]
- Niu, X.; Wang, K.; Bai, Y.; Du, Y.; Chen, Y.; Dong, M.; Fan, W. Selective formation para-xylene by methanol aromatization over phosphorus modified ZSM-5 zeolites. Catalysts 2020, 10, 484. [Google Scholar] [CrossRef]
- Omata, K.; Yamaguchi, Y.; Watanabe, Y.; Kodama, K.; Yamada, M. Artificial neural network (ANN)-aided optimization of ZSM-5 catalyst for the dimethyl ether to olefin (DTO) reaction from neat dimethyl ether (DME). Ind. Eng. Chem. Res. 2009, 48, 6256–6261. [Google Scholar] [CrossRef]
- Wei, P.; Zhu, X.; Wang, Y.; Chu, W.; Xie, S.; Yang, Z.; Liu, Z.; Li, Z.; Xu, L. Rapid synthesis of ferrierite zeolite through microwave assisted organic template free route. Microporous Mesoporous Mater. 2019, 279, 220–227. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquél, R.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Wang, X.; Lv, Y.; Zhu, S.; Wang, X.; Deng, C. Phosphoric acid modification of Hβ zeolite for guaiacol hydrodeoxygenation. Catalysts 2021, 11, 962. [Google Scholar] [CrossRef]
- Van der Bij, H.E.; Aramburo, L.R.; Arstad, B.; Dynes, J.J.; Wang, J.; Weckhuysen, B.M. Phosphatation of zeolite H-ZSM-5: A combined microscopy and spectroscopy study. ChemPhysChem 2014, 15, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Al-Dughaither, A.S.; de Lasa, H. HZSM-5 zeolites with different SiO2/Al2O3 ratios. Characterization and NH3 desorption kinetics. Ind. Eng. Chem. Res. 2014, 53, 15303–15316. [Google Scholar] [CrossRef]
- Vu, D.V.; Hirota, Y.; Nishiyama, N.; Egashira, Y.; Ueyama, K. High propylene selectivity in methanol-to-olefin reaction over H-ZSM-5 catalyst treated with phosphoric acid. J. Jpn. Pet. Inst. 2010, 53, 232–238. [Google Scholar] [CrossRef]
- Soh, J.C.; Chong, S.L.; Hossain, S.S.; Cheng, C.K. Catalytic ethylene production from ethanol dehydration over non-modified and phosphoric acid modified Zeolite H-Y (80) catalysts. Fuel Proc. Technol. 2017, 158, 85–95. [Google Scholar] [CrossRef]
- Olsbye, U.; Svelle, S.; Bjørgen, M.; Beato, P.; Janssens, T.V.W.; Joensen, F.; Bordiga, S.; Lillerud, K.P. Conversion of methanol to hydrocarbons: How zeolite cavity and pore size controls product selectivity. Angew. Chem. Int. Edit. 2012, 51, 5810–5831. [Google Scholar] [CrossRef]
- Magomedova, M.; Starozhiskaya, A.; Davidov, I.; Mximov, A.; Kravstov, M. Dual-cycle mechanism based kinetic model for DME-to-olefin synthesis on HZSM-5-type catalysts. Catalysts 2021, 11, 1459. [Google Scholar] [CrossRef]
- Hu, M.; Wang, C.; Gao, X.; Chu, Y.; Qi, G.; Wang, Q.; Xu, G.; Xu, J.; Deng, F. Establishing a link between the dual cycles in methanol-to-olefins conversion on H-ZSM-5: Aromatization of cycloalkenes. ACS Catal. 2020, 10, 4299–4305. [Google Scholar] [CrossRef]
- Sun, T.; Chen, W.; Xu, S.; Zheng, A.; Wu, X.; Zeng, S.; Wang, N.; Meng, X.; Wei, Y.; Liu, Z. The first carbon-carbon bond formation mechanism in methanol-to-hydrocarbons process over chabazite zeolite. Chem 2021, 7, 2415–2428. [Google Scholar] [CrossRef]
- Ortega, C.; Hessel, V.; Kolb, G. Dimethyl ether to hydrocarbons over ZSM-5: Kinetic study in an external recycle reactor. Chem. Eng. J. 2018, 354, 21–34. [Google Scholar] [CrossRef]
- Menges, M.; Kraushaar-Czarnetzki, B. Kinetics of methanol to olefins over AlPO4-bound ZSM-5 extrudates in a two-stage unit with dimethyl ether pre-reactor. Microporous Mesoporous Mater. 2012, 164, 172–181. [Google Scholar] [CrossRef]
- Hill, I.M.; Ng, Y.S.; Bhan, A. Kinetics of butene isomer methylation with dimethyl ether over zeolite catalysts. ACS Catal. 2012, 2, 1742–1748. [Google Scholar] [CrossRef]
- Khitev, Y.P.; Ivanova, I.I.; Kolyagin, Y.G.; Ponomareva, O.A. Skeletal isomerization of 1-butene over micro/mesoporous materials based on FER zeolite. Appl. Catal. A Gen. 2012, 441–442, 123–135. [Google Scholar] [CrossRef]
- Nawaz, Z.; Ziaoping, T.; Jie, Z.; Fei, W.; Naveed, S. Catalytic cracking of 1-hexene to propylene using SAPO-34 catalysts with different bulk topologies. Chin. J. Catal. 2009, 30, 1049–1057. [Google Scholar] [CrossRef]
- Sun, H.; Cao, L.; Zhang, Y.; Zhao, L.; Gao, J.; Xu, C. Effect of catalyst acidity and reaction temperature on hexene cracking reaction to produce propylene. Energy Fuel 2021, 35, 3296–3306. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, L.-L.; Li, G.-D.; Shang, Y.-S.; Zhao, X.-M.; Ma, T.; Zhang, L.-M.; Zhai, Y.-L.; Gong, Y.-J.; Xu, J.; et al. ZSM-5 extrudates modified with phosphorus as a super effective MTP catalyst: Impact of the acidity on binder. Fuel Proc. Technol. 2017, 168, 105–115. [Google Scholar] [CrossRef]



| Catalyst | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | |||
|---|---|---|---|---|---|
| Total 1 | External 2 | Micro 2 | Total 1 | Micro 2 | |
| P(0)_450 | 297 | 28 | 352 | 0.37 | 0.11 |
| P(1.4)_450 | 280 | 22 | 339 | 0.27 | 0.11 |
| P(3.3)_450 | 148 | 21 | 168 | 0.20 | 0.06 |
| P(6.9)_450 | 27 | 12 | 13 | 0.11 | 0.01 |
| P(12)_450 | 10 | 5 | 5 | 0.04 | 0.00 |
| P(0)_500 | 366 | 27 | 460 | 0.37 | 0.14 |
| P(1.1)_500 | 288 | 21 | 361 | 0.29 | 0.11 |
| P(3.7)_500 | 54 | 20 | 34 | 0.23 | 0.02 |
| P(6.9)_500 | 21 | 14 | 6 | 0.14 | 0.00 |
| P(12)_500 | 11 | 6 | 4 | 0.05 | 0.00 |
| P(0)_550 | 349 | 27 | 436 | 0.33 | 0.13 |
| P(1.1)_550 | 323 | 25 | 392 | 0.37 | 0.12 |
| P(3.3)_550 | 166 | 24 | 189 | 0.32 | 0.06 |
| P(6.9)_550 | 21 | 11 | 8 | 0.15 | 0.01 |
| P(12)_550 | 12 | 3 | 9 | 0.04 | 0.00 |
| P(0)_600 | 380 | 29 | 474 | 0.38 | 0.14 |
| P(1.2)_600 | 306 | 19 | 383 | 0.35 | 0.11 |
| P(3.3)_600 | 190 | 16 | 229 | 0.28 | 0.07 |
| P(6.7)_600 | 16 | 9 | 6 | 0.13 | 0.03 |
| P(12)_600 | 10 | 4 | 5 | 0.05 | 0.03 |
| Catalyst | Weak (mmol g−1) | Strong (mmol g−1) | Total (mmol g−1) |
|---|---|---|---|
| P(0)_450 | 1.45 | 1.43 | 2.87 |
| P(1.4)_450 | 1.39 | 1.30 | 2.69 |
| P(3.3)_450 | 1.08 | 1.02 | 2.10 |
| P(6.9)_450 | 0.74 | 0.83 | 1.57 |
| P(12)_450 | 0.54 | 0.60 | 1.14 |
| P(0)_500 | 1.40 | 1.31 | 2.70 |
| P(1.1)_500 | 1.34 | 1.15 | 2.49 |
| P(3.7)_500 | 1.12 | 0.83 | 1.95 |
| P(6.9)_500 | 0.89 | 0.66 | 1.55 |
| P(12)_500 | 0.50 | 0.38 | 0.88 |
| P(0)_550 | 1.45 | 1.37 | 2.82 |
| P(1.1)_550 | 1.24 | 1.06 | 2.29 |
| P(3.3)_550 | 1.10 | 0.86 | 1.96 |
| P(6.9)_550 | 0.63 | 0.54 | 1.17 |
| P(12)_550 | 0.26 | 0.12 | 0.37 |
| P(0)_600 | 1.32 | 1.03 | 2.35 |
| P(1.2)_600 | 1.19 | 0.89 | 2.08 |
| P(3.3)_600 | 1.01 | 0.73 | 1.74 |
| P(6.7)_600 | 0.70 | 0.59 | 1.29 |
| P(12)_600 | 0.33 | 0.20 | 0.53 |
| Catalyst | DME Conv. (%) | Product Yield (C-mol%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C1–C3 | C2= | C3= | MeOH | C4 1 | i-C4= | 1,3-BD | n-C4= 2 | C5 | C6≤ | ||
| P(0)_450 | 97.8 | 3.9 | 4.0 | 9.1 | 2.6 | 0.1 | 4.7 | 0.4 | 31.2 | 33.3 | 8.6 |
| P(1.4)_450 | 12.2 | 1.2 | 0.2 | 0.6 | 2.5 | 0.0 | 0.0 | 0.1 | 4.6 | 2.5 | 0.6 |
| P(3.3)_450 | 3.6 | 0.9 | 0.5 | 0.1 | 1.8 | 0.0 | 0.0 | 0.0 | 0.2 | 0.1 | 0.0 |
| P(6.9)_450 | 3.0 | 0.4 | 0.3 | 0.0 | 2.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| P(12)_450 | 1.9 | 0.2 | 0.0 | 0.1 | 1.2 | 0.0 | 0.0 | 0.0 | 0.2 | 0.1 | 0.0 |
| P(0)_500 | 89.5 | 4.9 | 4.8 | 7.2 | 4.1 | 0.3 | 5.8 | 0.3 | 27.8 | 28.8 | 5.7 |
| P(1.1)_500 | 12.6 | 1.2 | 0.1 | 0.5 | 3.0 | 0.0 | 0.1 | 0.1 | 4.5 | 2.5 | 0.6 |
| P(3.7)_500 | 3.2 | 0.9 | 0.3 | 0.1 | 1.5 | 0.0 | 0.0 | 0.0 | 0.3 | 0.1 | 0.0 |
| P(6.9)_500 | 2.9 | 0.7 | 0.3 | 0.0 | 1.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| P(12)_500 | 1.8 | 0.3 | 0.1 | 0.0 | 1.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| P(0)_550 | 93.8 | 6.0 | 5.3 | 7.3 | 5.0 | 0.3 | 5.7 | 0.4 | 29.8 | 28.6 | 5.3 |
| P(1.1)_550 | 7.7 | 1.1 | 0.1 | 0.3 | 2.2 | 0.0 | 0.0 | 0.0 | 2.5 | 1.1 | 0.3 |
| P(3.3)_550 | 3.5 | 0.9 | 0.3 | 0.1 | 2.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.1 | 0.0 |
| P(6.9)_550 | 2.3 | 0.5 | 0.2 | 0.0 | 1.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| P(12)_550 | 1.3 | 0.3 | 0.0 | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| P(0)_600 | 67.9 | 5.2 | 3.1 | 3.3 | 11.3 | 0.3 | 3.5 | 0.4 | 19.8 | 17.7 | 3.3 |
| P(1.2)_600 | 13.3 | 1.8 | 0.1 | 0.4 | 3.9 | 0.0 | 0.1 | 0.1 | 3.9 | 2.5 | 0.5 |
| P(3.3)_600 | 3.6 | 1.1 | 0.4 | 0.1 | 1.6 | 0.0 | 0.0 | 0.0 | 0.3 | 0.1 | 0.0 |
| P(6.7)_600 | 2.8 | 0.6 | 0.3 | 0.0 | 1.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| P(12)_600 | 3.2 | 0.9 | 0.3 | 0.1 | 1.5 | 0.0 | 0.0 | 0.0 | 0.3 | 0.1 | 0.0 |
| Selected Explanatory Variables | Tolerance |
|---|---|
| x1-x2 | 0.261 |
| x1-x3 | 0.163 |
| x1-x4 | 0.024 |
| x1-x5 | 0.245 |
| x1-x6 | 0.332 |
| x2-x3 | 0.104 |
| x2-x4 | 0.282 |
| x2-x5 | 0.078 |
| x2-x6 | 0.179 |
| x3-x4 | 0.173 |
| x3-x5 | 0.148 |
| x3-x6 | 0.314 |
| x4-x5 | 0.267 |
| x4-x6 | 0.352 |
| x5-x6 | 0.086 |
| Trial | Explanatory Variable | RSS | F-Ratio | Regression Equation | Contribution Rate (%) |
|---|---|---|---|---|---|
| 1 | None | 3086 | |||
| 2 | x1 | 1150 | 30.30 | y = −1.76 + 0.04 x1 | 49.9 |
| 3 | x1, x2 | 1119 | |||
| x1, x3 | 1141 | ||||
| x1, x5 | 1145 | ||||
| x1, x6 | 1062 | 1.41 | y = −7.01 + 0.03 x1 + 9.97 x6 | 53.7 | |
| 4 | x1, x6, x2 | 1060 | 0.03 | y = −6.81 + 0.03 x1 + 11.32 x6 − 0.10 x2 | 53.8 |
| x1, x6, x3 | 1018 | 0.68 | y = −5.08 + 0.04 x1 + 12.54 x6 − 31.74 x3 | 55.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanaoka, T.; Aoyagi, M.; Edashige, Y. Improvement of n-Butene Yield in Dimethyl Ether-to-Olefin Reaction Using Ferrierite Zeolite Catalysts. Catalysts 2023, 13, 1040. https://doi.org/10.3390/catal13071040
Hanaoka T, Aoyagi M, Edashige Y. Improvement of n-Butene Yield in Dimethyl Ether-to-Olefin Reaction Using Ferrierite Zeolite Catalysts. Catalysts. 2023; 13(7):1040. https://doi.org/10.3390/catal13071040
Chicago/Turabian StyleHanaoka, Toshiaki, Masaru Aoyagi, and Yusuke Edashige. 2023. "Improvement of n-Butene Yield in Dimethyl Ether-to-Olefin Reaction Using Ferrierite Zeolite Catalysts" Catalysts 13, no. 7: 1040. https://doi.org/10.3390/catal13071040
APA StyleHanaoka, T., Aoyagi, M., & Edashige, Y. (2023). Improvement of n-Butene Yield in Dimethyl Ether-to-Olefin Reaction Using Ferrierite Zeolite Catalysts. Catalysts, 13(7), 1040. https://doi.org/10.3390/catal13071040







