Abstract
As energy demand increases, new energy conversion methods are also sought. In this study, two MnO2 and multiwalled carbon nanotube (MWCNT) composites were prepared and decorated with silver using magnetron sputtering, to evaluate their electrocatalytic activity towards the oxygen reduction reaction (ORR). Three nominal thicknesses of Ag layers were used, 5, 10 and 20 nm. The physicochemical characterisation was carried out using scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy, as well as X-ray photoelectron spectroscopy. The substrate materials (MnO2-MWCNT) were also investigated by X-ray diffraction analysis. The electrochemical studies of the ORR revealed that the activity and stability of the composite catalysts depend on the substrate material and the Ag layer thickness.
1. Introduction
Despite fossil fuels being cheaper and more abundant, alternative energy sources are in increasingly high demand. Fuel cell technology renders an alternative, showing considerable potential in the automotive industry. However, as noble metal catalysts such as Pt-based cathode materials used in the fuel cells are costly, cheaper alternatives which are stable and match Pt in electrocatalytic activity are actively sought [1]. For example, Ag shows competing activity in alkaline conditions and could be used in anion exchange membrane fuel cells (AEMFCs) as a cathode catalyst [2,3,4,5].
In general, the electrochemical oxygen reduction reaction (ORR) can follow two reaction pathways, proceeding either via direct four-electron transfer (Equation (1)) or via a peroxide intermediate (Equation (2)), which can be further reduced to OH− (Equation (3)):
O2 + 2H2O + 4e− = 4 OH−,
O2 + H2O + 2e− = HO2− + OH−,
HO2− + H2O + 2e− = 3 OH−.
The Ag-based catalyst’s oxygen reduction reaction activity and pathway depend on factors such as Ag size, shape and loading on chosen carbon substrates [6,7,8]. For example, smaller Ag nanoparticles (AgNPs) catalysed ORR via a 2+2e– pathway and larger ones via a four-electron pathway [7,9]. In the other works, it has been reported that O2 reduction on nanostructured Ag catalysts proceeds via a direct four-electron pathway rather than a 2e–+2e– one, avoiding the formation of hydroperoxide ions [10,11]. Another aspect of Ag structure dependence is the different ORR activity of Ag(hkl) single crystal planes. For example, Blizanac et al. ascribe the highest electrocatalytic activity to the crystal phase (110) and the lowest to the crystal phase (100), while on (111), they were reported to have intermediate activity [12,13]. Thus, Ag particle size and shape significantly influence Ag-catalysts’ electrocatalytic activity [14,15]. For instance, Wang et al. reported Ag decahedral nanoparticles of (111) crystal phase to be more active towards ORR than cubic AgNPs of (100) crystal phase [16]. Han et al. additionally showed that smaller AgNPs around 4.1 nm favoured a simultaneous two-electron and four-electron ORR pathway, while larger AgNPs around 174 nm favoured a four-electron pathway [7]. Treshchalov et al. further demonstrated high electrocatalytic activity and stability of AgNPs with a diameter of around 17 nm produced using the plasma jet method [17]. Besides, it has been shown that mass activity (MA) for oxygen reduction on Ag grows with the size reduction of AgNPs and Ag nanowires [18]. Lu et al. also found lower MA values for 3.3 nm AgNPs than for 0.7 nm Ag nanoclusters [19]. Ohyama et al. reported quantum size effects for AgNPs smaller than 3 nm, but not for AgNPs larger than 10 nm [15].
Moreover, the ORR activity and mechanism of Ag catalysts also depend on Ag loading. Bare carbon substrates tend to promote a two-electron ORR pathway yielding hydrogen peroxide [20,21]. Guo et al. reported that, in order to obtain an Ag catalyst that catalyses O2 reduction via a direct four-electron pathway, the Ag loading should be higher than 10 wt% and lower than 60 wt% [22]. If the Ag loading was lower than 10 wt%, the substrate started catalysing ORR via a two-electron pathway, producing more hydrogen peroxide [6]. Carbon support further plays a role in Ag catalyst activity. For example, an AEMFC obtained a maximum power density of 86 mW cm−2 with Ag catalysts supported on carbon nanocapsules [23]. Mesoporous carbon substrates have also been employed [3,24]. Maximum power densities of 310 mW cm−2 and 243 mW cm−2 at 65 °C were obtained with catalysts where AgNPs were deposited onto mesoporous carbon substrates [3]. Ag catalysts have also been prepared by depositing Ag onto the CNTs due to their high surface area, good electrical conductivity and chemical stability [25,26]. In addition, the synergistic effect between nitrogen species and AgNPs, on Ag catalyst deposited onto nitrogen-doped graphene, enhances the electrocatalytic activity [27,28,29].
Another approach for synergistic effects is to use transition metal oxides. One common type of metal oxide for that is MnxOy-s. There are multiple reasons to use manganese oxides; for example, they are low cost, abundant, show promising electrocatalytic performance, and the MnO2 d-band electronic structure is akin to platinum [30,31]. Ni et al. conducted electron paramagnetic resonance measurements and discovered that after Ag addition, the electrical resistance of α-MnO2 decreased twentyfold [30]. Furthermore, according to Ni et al., in composites of Ag and manganese oxide the ligand effect exists that affects the materials’ intrinsic activities, as the transition metal oxide changes the electronic structure of the metal and its d-band centre [30,32]. In addition, Yang et al. argue that the adsorption of oxygen to Ag is weak, and HO2− ion disproportionation is fast, while on manganese oxide, oxygen adsorption is strong and HO2− disproportionation is slow, thus combining them yields catalysts that follow indirect four-electron pathway [32,33]. In support of this, after Ag addition, Wang et al. reported increased current densities and decreased charge transfer resistance [31]. Sun et al. argue that MnO2 essentially catalyses the ORR via a two-electron pathway, and when doped with a high enough loading of silver, it starts to catalyse the ORR via a four-electron pathway [32]. Moreover, Ag catalyst activity is likely to increase by growing the number of electrochemically active sites resulting from Ag2O film formation on Ag particles caused by the proximity of Mn3O4 [34]. Ag2O also plays an important role in Ag-catalysts activity in ORR [22]. Liu et al. argue that the stability of this kind of composite might arise from negligible hydrogen peroxide formation. They say that Mn3O4 presence on the carbon substrate enhances O2 side-on adsorption, and small distances between Ag and Mn3O4 create an ensemble effect, where the hydrogen peroxide ion moves from the Mn3O4 surface to the Ag surface and disproportionates more easily [32,34,35]. Akbarian et al. claim that the Mn3O4 presence next to the Ag surface affects the Ag surface state so that oxygen vacancies start to form. These oxygen vacancies facilitate ORR by attracting electrons to the reaction centres or or by becoming additional active sites [36]. Different MnO2 structures with various crystal forms (α, β, and γ) have been shown to possess different catalytic abilities [37,38,39]. For example, a comparison of the ORR activity of MnO2 α-nanowires, α-nanorods, β-nanowires and β-nanorods revealed that structures with α crystal form showed higher activity than the structures with β form [30,31,32,33,38]. The electrocatalytic activity could also be increased by manganese oxide creating positive charge accumulation on the carbon support [32,40].
Therefore, this work aims to prepare active and stable Ag and MnO2_MWCNT composite catalysts for ORR. Two different MnO2_MWCNT support materials are prepared (MnO2_MWCNT1 and MnO2_MWCNT2), onto which Ag was deposited by a magnetron sputtering method, where the nominal thickness of Ag layer is 5 nm, 10 nm and 20 nm (named as Ag5/MnO2_MWCNT, Ag10/MnO2_MWCNT and Ag20/MnO2_MWCNT). The obtained catalysts show high mass activities, and Ag/MnO2_MWCNT1 catalysts show superior stability during the 1000 potential cycles of an accelerated stability test (AST), where catalysts with Ag nominal thickness of 10 nm and 5 nm only show a 4 mV and 2 mV decrease in their half-wave potentials.
2. Results and Discussion
2.1. Physicochemical Characterisation of Catalysts
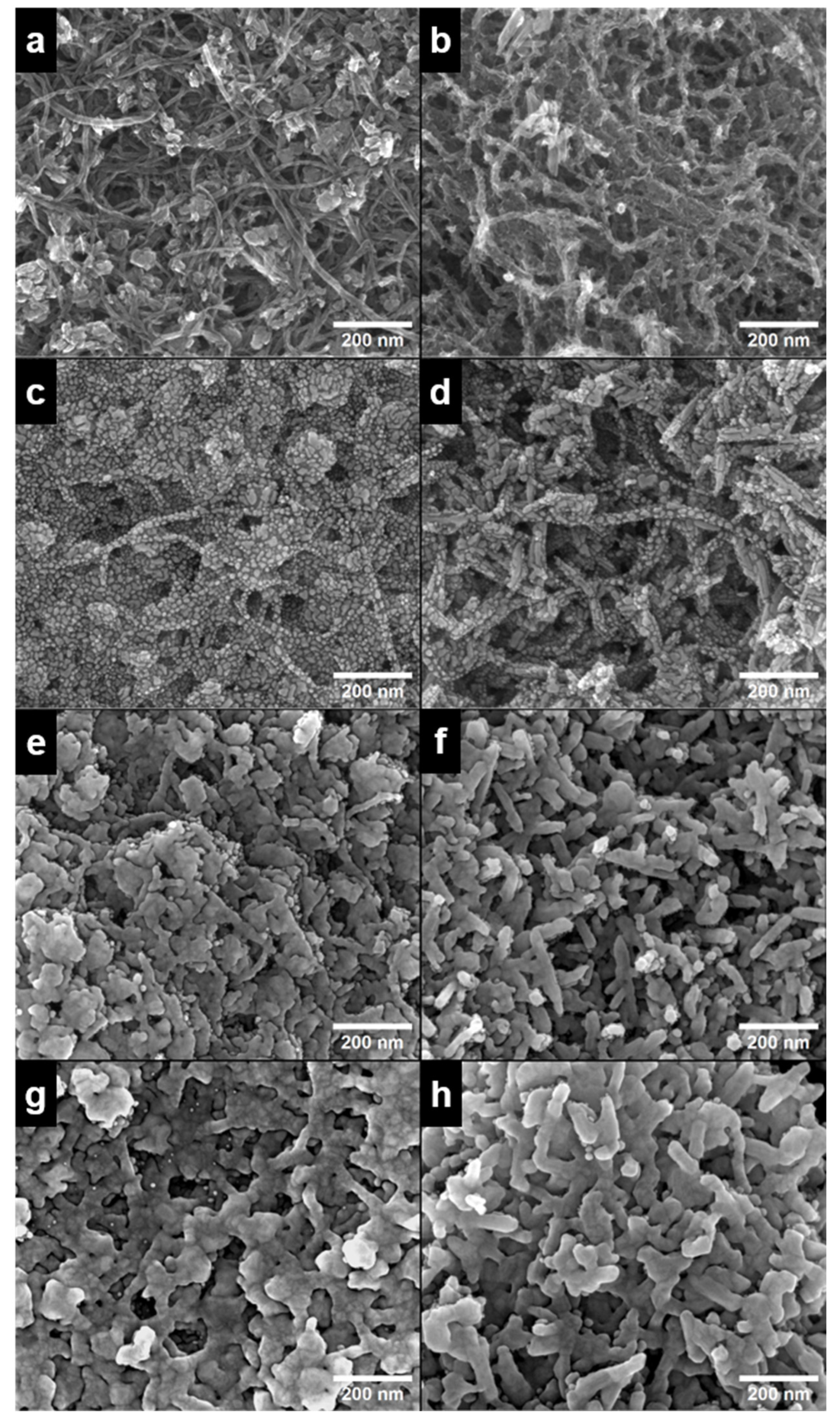
The surface morphology of MnO2-coated MWCNTs and Ag/MnO2_MWCNT catalysts with different Ag loading is shown in scanning electron microscopy (SEM) images in Figure 1. In addition, Figure S1 shows high-angle annular dark-field transmission electron microscopy (HAADF-TEM) images and EDS mappings of Ag-free MnO2_MWCNT1 and MnO2_MWCNT2 support materials. Evidently, in the case of MnO2_MWCNT2 (Figure 1b and Figure S1c,d), the MWCNTs seem to be covered with a more evenly distributed MnO2 layer, while MnO2 on MnO2_MWCNT1 (Figure 1a and Figure S1a,b) seems to be more in the form of particles. Thus, Ag/MnO2_MWCNT2 catalysts might have fewer defects where AgNPs have direct contact with MWCNTs, whereas Ag/MnO2_MWCNT1 possibly has more defects and, therefore, better contact of Ag with MWCNTs. Figure 1c–h show MnO2_MWCNT materials covered with Ag layers of different nominal thicknesses. At the thinnest layer of 5 nm of Ag, the substrate is covered with small AgNPs around 10 nm in size. At thicker layers of 10 nm and 20 nm, Ag particles merge and form an even layer covering the whole surface of both MnO2_MWCNT substrates. The average Ag particle size for Ag5/MnO2_MWCNT1 was 10 nm, and 13 nm for Ag5/MnO2_MWCNT2, which showed the slight effect of the substrate. At a nominal thickness of 10 nm and 20 nm, some sub-10 nm particles were still visible, but most of the Ag uniformly covered the electrode surface. Similar structures of metal layers have been observed previously when magnetron sputtering has been used to prepare Ag, Pt, Pd and Au coatings on MWCNT substrates [25,41,42,43,44,45,46,47].
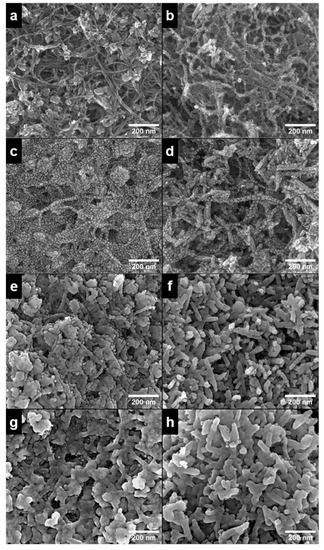
Figure 1.
SEM images of (a) MnO2_MWCNT1 and (b) MnO2_MWCNT2 substrates; (c,e,g) show Ag/MnO2_MWCNT1 images of nominal Ag layer thickness of 5, 10, and 20 nm, respectively; (d,f,h) show Ag/MnO2_MWCNT2 images of nominal Ag layer thickness of 5, 10 and 20 nm, respectively.
The Ag, Mn, and C contents in Ag/MnO2_MWCNT catalysts were determined by energy-dispersive X-ray spectroscopy (EDS) (Table 1). As expected, the Ag loadings on MnO2_MWCNT1 and MnO2_MWCNT2 match. It is also evident that the Mn/C ratio is higher for Ag/MnO2_MWCNT2 catalysts.

Table 1.
Ag, Mn, and C content in Ag/MnO2_MWCNT catalysts derived from EDS analysis. The loading of MnO2_MWCNT substrate materials is 0.25 mg cm−2.
The Ag loading was determined using X-ray fluorescence (XRF) on one additional sample (Si-plate) for each nominal Ag layer thickness. The following silver loadings were determined: 5.4 µg cm−2 for 5 nm, 10.7 µg cm−2 for 10 nm, and 22.9 µg cm−2 for 20 nm Ag layer. These values are in good agreement with the Ag loadings obtained from the EDS analysis, considering that the total catalyst loading was 250 µg cm−2 in all cases.
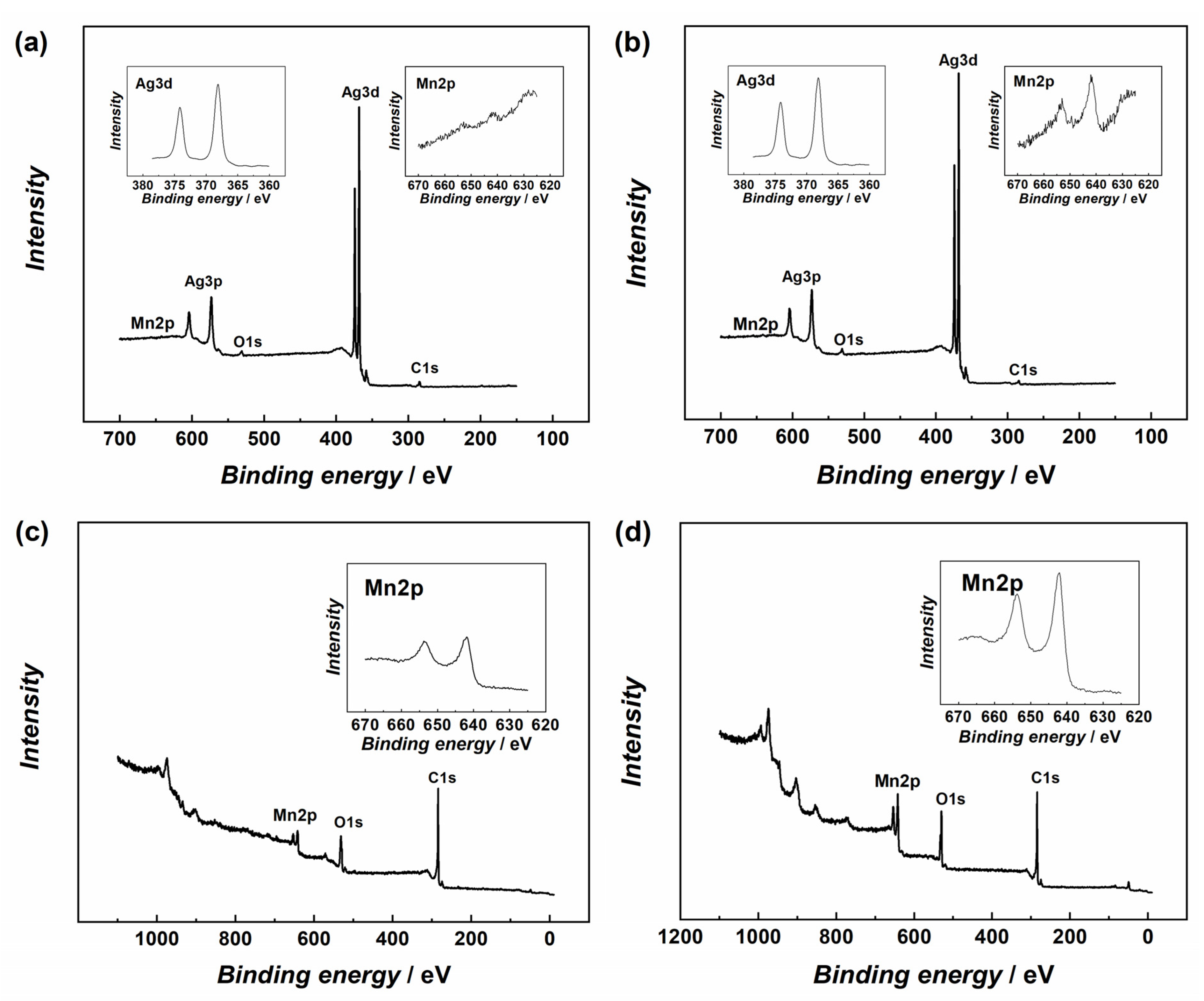
Figure 2 shows the X-ray photoelectron spectroscopy (XPS) results for the Ag10/MnO2_MWCNT catalysts and MnO2_MWCNT substrates. XPS peaks for Ag, C, Mn, and O were observed. The Ag3d doublet is at 368 and 374 eV, the C1s peak at 284 eV, the Mn2p doublet is at 641 and 653 eV, and the O1s peak at 531 eV. The separation energy for the Mn2p doublets’ two peaks, 11.8 eV, matches the reported data [48,49,50]. Low Mn and carbon signals in Figure 2a,b indicate that the substrate is almost completely covered with an Ag nanolayer.
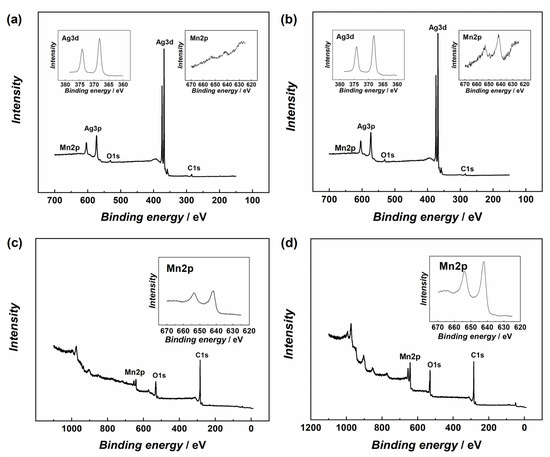
Figure 2.
XPS survey spectra of (a) Ag10/MnO2_MWCNT1 and (b) Ag10/MnO2_MWCNT2 samples, insets show detailed XPS spectra in the Ag3d and Mn2p regions. XPS survey spectra of Ag-free (c) MnO2_MWCNT1 and (d) MnO2_MWCNT2 substrate materials, insets show detailed XPS spectra in the Mn2p regions.
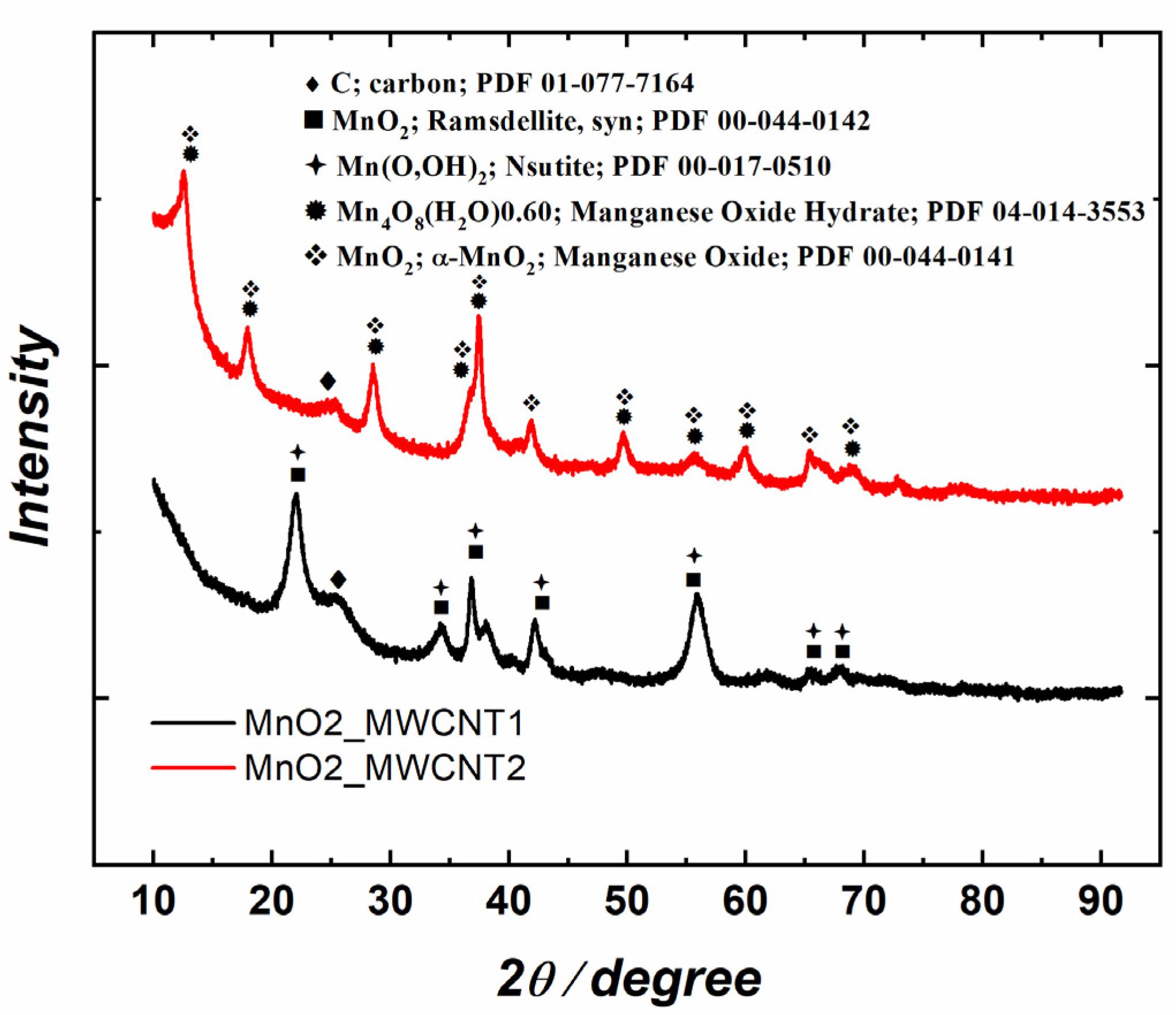
Figure 3 presents the X-ray diffraction (XRD) patterns of the substrates prior to the Ag deposition. MnO2 crystallite sizes ranged between 5 and 15 nm. Both materials consist mainly of MnO2 and its partially hydrated compounds, but the crystal structures differ. MnO2_MWCNT1 contains a mixture of orthorhombic (ramsdellite, PDF 00-044-0142) and hexagonal MnO2 as well as nsutites (Mn(O,OH)2). In MnO2_MWCNT2, MnO2 shows a tetragonal structure that matches well with α-MnO2 (PDF 00-044-0141) and may be hydrated to some extent. From Figure 3, it is also evident that since the C peak (25°2θ) of MnO2_MWCNT1 is higher than that of MnO2_MWCNT2, which is in accordance with the EDS results, where a lower Mn/C ratio was observed for Ag/MnO2_MWCNT1 catalysts.
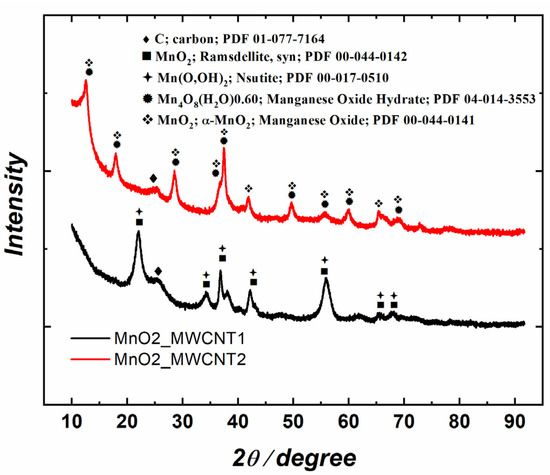
Figure 3.
XRD patterns of MnO2_MWCNT1 and MnO2_MWCNT2 samples.
2.2. Oxygen Reduction Reaction Studies
All prepared electrodes were subjected to electrochemical testing in an O2-saturated 0.1 M KOH solution for which rotating disk electrode (RDE) polarisation curves were measured at different electrode rotation rates. The obtained RDE polarisation data were analysed using the Koutecky–Levich (K-L) equation (Equation (4)):
where j is the ORR current density at a specific potential, jk and jd are the kinetic and diffusion-limited current densities, respectively, n is the number of electrons transferred per O2 molecule, F is the Faraday constant (96,485 C mol−1), is the concentration of O2 in bulk (1.2 × 10−6 mol cm−3) [51], k is the heterogeneous rate constant for ORR at a specific potential (cm s−1), is the diffusion coefficient of O2 (1.9 × 10−5 cm2 s−1) [51], ν is the kinematic viscosity of the solution (0.01 cm2 s−1) [52], and ω is the electrode rotation rate (rad s−1).
The ORR mass activities (MA) for the Ag-based catalysts were calculated at −0.2 V vs. SCE using Equation (5):
where Ik stands for the kinetic current and mAg is the mass of silver on the electrode determined from the XRF data.
MA = Ik/mAg,
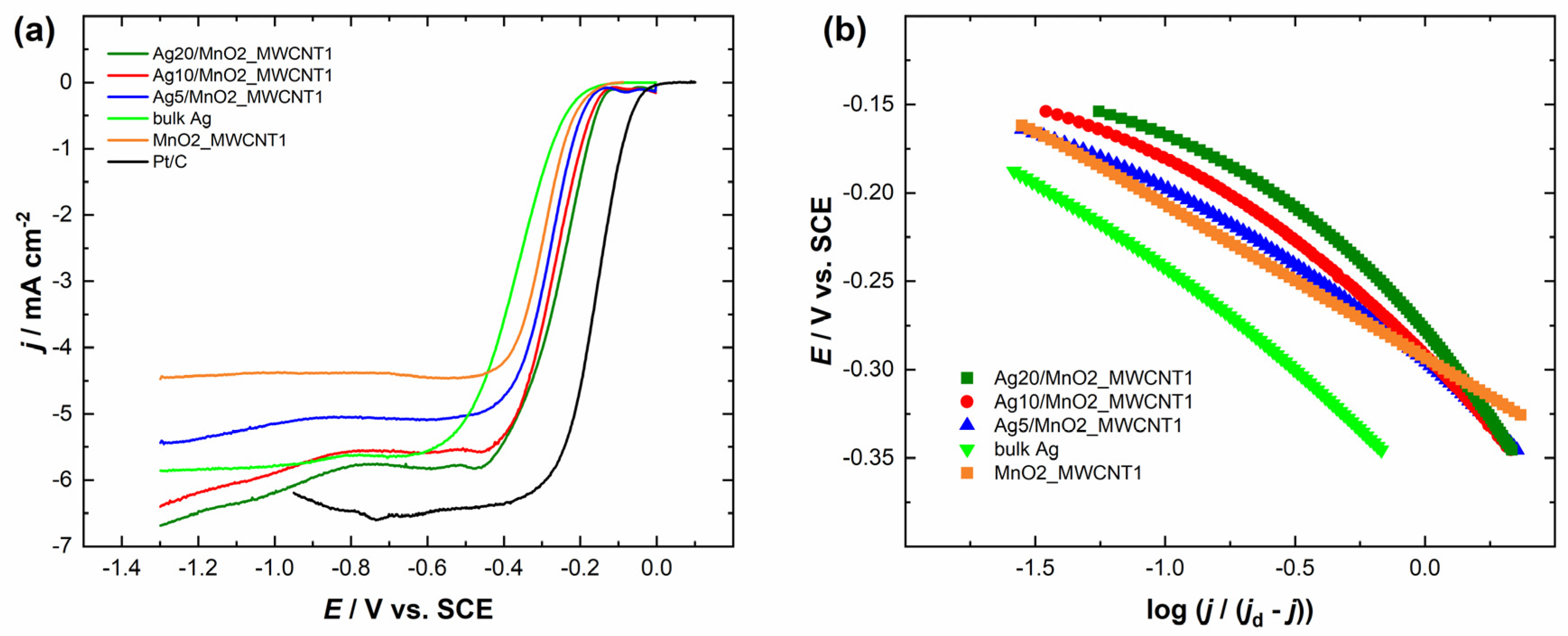
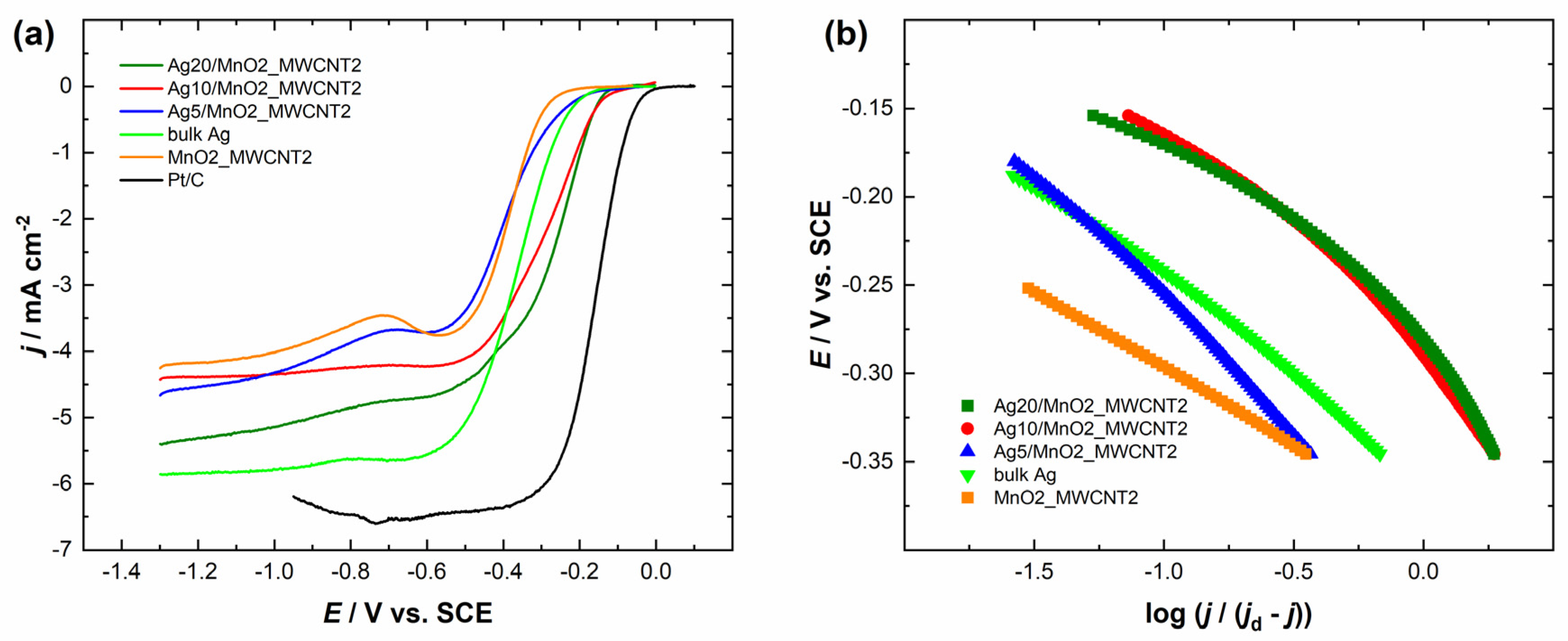
The comparison of the RDE polarisation curves at a single electrode rotation rate is presented in Figure 4a and Figure 5a. It can be observed that Ag/MnO2_MWCNT catalysts with an Ag layer thickness of 20 nm had the most positive half-wave potential (E1/2) of −0.29 V, although Ag20/MnO2_MWCNT1 catalyst showed a bigger MA value of 84 A g−1 compared to that of Ag20/MnO2_MWCNT2 around 61 A g−1 (see Table 1). Ag5/MnO2_MWCNT1 catalyst revealed the biggest MA of 126 A g−1, while Ag10/MnO2_MWCNT2 catalyst with Ag thickness of 10 nm had the biggest MA of 110 A g−1 compared to other Ag/MnO2_MWCNT2 catalysts. Ag/MnO2_MWCNT catalysts in terms of MA surpassed the results obtained with Ag nanowires [53], the Ag catalysts where Ag was chemically deposited onto nitrogen-doped graphene oxide [27], and Ag catalysts where Ag was deposited onto carbide-derived carbon [54]. A similar MA value of 94 A g−1 was obtained for Ag catalysts electrodeposited onto nitrogen-doped graphene oxide [55]. Both Ag5/MnO2_MWCNT catalysts had the most negative E1/2, and Ag5/MnO2_MWCNT2 had the smallest MA. All prepared Ag-based catalysts showed more positive half-wave potentials than the polycrystalline bulk Ag electrode. The half-wave potential of ORR on Ag10/MnO2_MWCNT1 and Ag5/MnO2_MWCNT1 catalysts obtained in the present study is in a similar range to those obtained earlier on Ag sputter deposited onto MWCNT with a nominal Ag layer thickness of 15 nm [25]. The E1/2 of bare Ag nanoparticles prepared by plasma-jet treatment was even lower than that for bulk Ag [17]. The high activity of Ag and MnO2 composite catalysts might come from an increased number of active sites on Ag2O layers formed on Ag particles in the presence of Mn oxides and particle-to-particle ligand effects due to the proximity of Ag particles [34]. Similar yet slightly higher E1/2 values than for bulk Ag were obtained for Ag thin films and electrodeposited Ag nanoparticles [55,56,57,58,59]. Compared to the commercial 20 wt% Pt/C catalyst, all the Ag/MnO2_MWCNT catalysts are less active for ORR.
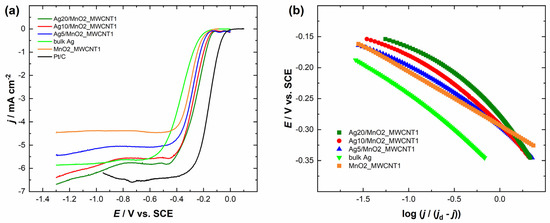
Figure 4.
(a) Comparison of the ORR polarization curves of the Ag/MnO2_MWCNT1 catalysts and Pt/C in O2-saturated 0.1 M KOH at 1900 rpm. ν = 10 mV s−1. (b) Mass-transfer corrected Tafel plots for ORR (data derived from (a)).
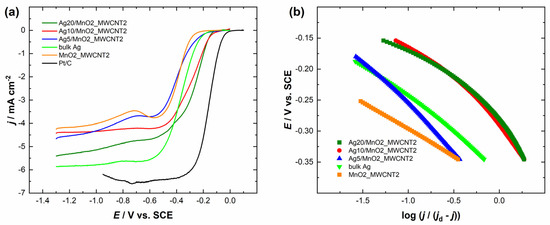
Figure 5.
(a) Comparison of the ORR polarization curves of the Ag/MnO2_MWCNT2 and Pt/C catalysts in O2-saturated 0.1 M KOH at 1900 rpm. ν = 10 mV s−1. (b) Mass-transfer corrected Tafel plots for ORR (data derived from (a)).
Electron transfer number values for ORR on the catalyst materials were obtained using the K–L equation (Equation (4)). For example, a set of ORR polarisation curves on Ag10/MnO2_MWCNT1 and the corresponding K–L plots are shown in Figure S2. These n values show overall tendencies for ORR since the K–L analysis does not differentiate between direct four-electron and 2+2-electron pathways. The n value for MnO2_MWCNT1 and MnO2_MWCNT2 materials was around three, suggesting that the hydrogen peroxide formation occurs on both MnO2_MWCNT supports and partial 2+2-electron pathway during the ORR is likely. The value of n was around four for all Ag/MnO2_MWCNT catalysts, indicating that in the presence of Ag, either less hydrogen peroxide formed or it was more easily reduced or disproportionated quicker. 5 wt% of Ag-MnO2 composite on single-walled carbon nanotubes and acetylene black are reported to catalyse the ORR by direct four-electron pathway, while the carbon nanotube-based composite is found to be more active overall [60]. Ag catalysts showed lower n values when Ag was electrodeposited on the glassy carbon (GC) electrodes and when Ag–MnxOy was deposited onto the reduced graphene oxide and nitrogen-doped graphene oxide substrates [59,61,62]. Ag catalysts deposited onto electrospun lignin-derived carbon fibre mats showed n values below four due to AgNPs at higher loadings growing in size and the diffusion effects of reacting species [63]. Guo et al. varied Ag loadings deposited onto conventional Vulcan carbon substrate and observed four-electron ORR for Ag catalysts where the loadings were 20 wt% and higher (up to 60 wt%) and 2+2-electron pathway for 10 wt% [22]. Fazil et al. prepared Ag catalysts on carbon nanotubes with 20 wt% and 40 wt % and, similarly to Guo et al., obtained n values close to four [26]. In a recent work where AgNPs were deposited onto two different mesoporous engineered catalyst supports with 40 wt% nominal Ag loading, n values close to four were also obtained [3].
Figure 4b and Figure 5b present mass-transfer corrected Tafel plots for ORR in 0.1 M KOH derived from the RDE data in Figure 4a and Figure 5a, respectively. The Tafel slope values for Ag/MnO2_MWCNT catalysts (in potential range from −0.15 V to −0.25 V vs. SCE) were similar and stayed between −81 and −94 mV regardless of the nominal thickness of the sputter-deposited Ag layers (Table 2). Tafel slope values of −91 mV and −163 mV were obtained for Ag-based catalysts where Ag was deposited onto MnO2 nanorods (Ag/MnO2) and clean MnO2 nanorods, respectively [64]. The Tafel slope value for polycrystalline bulk Ag electrode remained around −80 mV. Tafel slope value for bulk silver has been reported in the literature to be −90 mV and for 100 nm AgNPs, −120 mV [65,66]. Continuously changing Tafel slopes from −70 to −80 mV and from −120 to −130 mV were reported for Ag(hkl) single crystal facets, and their occurrence was explained by changes in the concentration of adsorbed oxygenated species and resulting changes in reaction steps and ORR mechanism [9,12,62]. Tafel slope values of Ag catalysts depend on the difference in catalyst structure [67]. AgNPs showed Tafel slope values of −95 and −134 mV, and Ag nanosheet arrays values of −60 and −120 mV. Substrates might play a role in different Tafel slope values [25]. For instance, −59 and −60 mV were obtained for Ag catalysts where Ag was deposited onto the nitrogen-doped graphene substrate [27,55]. It was reasoned that since the Tafel slope values for pure and Ag-covered substrates were very similar, the ORR might partially proceed on the substrate [27,68]. As the Tafel slope values of MnO2_MWCNT2 differ from that of Ag on this support, we can assume that the ORR proceeds mainly on the Ag surface. However, the same assumption cannot be made for MnO2_MWCNT1. The ORR on Ag catalysts might also be influenced by the Ag deposition method [58,59]. The Tafel slope values between −71 and −80 mV were obtained with Ag catalysts where AgNPs were synthesized using pulse charge deposition and vacuum dried onto GC electrodes [17]. As the Tafel slope values herein remained similar to that of bulk Ag, we expect the reaction mechanism to be the same on Ag/MnO2_MWCNT catalysts as on bulk Ag.

Table 2.
Kinetic parameters for ORR on Ag-based catalysts in 0.1 M KOH solution. ω = 1900 rpm.
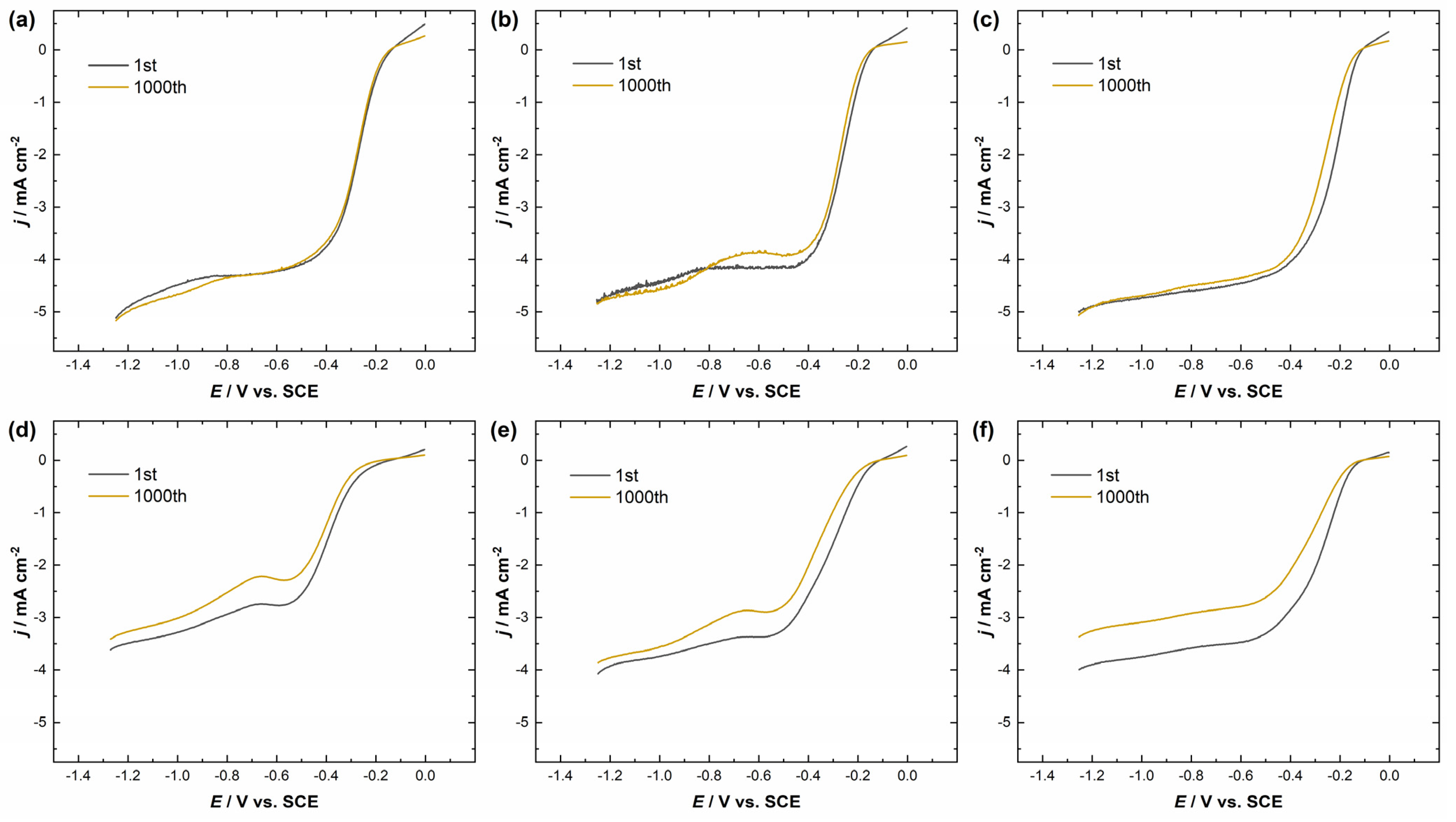
Accelerated stability tests (1000 potential cycles in the range from −1.3 V to 0 V vs. SCE at a scan rate of 100 mV s−1) of Ag/MnO2_MWCNT1 and Ag/MnO2_MWCNT2 catalysts were also conducted. Figure 6a–f shows the ORR polarization curves for catalysts with different nominal Ag layer thicknesses before and after the 1000 potential cycles at ω = 960 rpm. Regardless of Ag nominal thickness, Ag/MnO2_MWCNT1 catalysts showed higher stability than Ag/MnO2_MWCNT2 catalysts (see Table 2). A decrease in the half-wave potential values could be caused by silver oxidation, dissolution, and Ag particle re-organisation via redeposition. Ag/MnO2_MWCNT1 catalysts showed the highest stability when Ag layer nominal thicknesses were 5 nm and 10 nm, for which the E1/2 change was only 2 mV and 4 mV, respectively. There could be several additional reasons for that. For example, Liu et al. argue that the enhanced stability might come from a synergistic effect between Ag and Mn3O4 nanoparticles [34]. The synergistic effect is argued to include an ensemble effect between Ag and Mn3O4 nanoparticles, where on Mn3O4, hydroperoxide ions form and then diffuse or re-adsorb on AgNPs, where they can disproportionate or be further reduced to OH− ions [34,35,64]. Part of the synergistic effect might arise from the electron transfer from carbon substrate to Mn3O4, which creates a positive charge on adjacent carbon surfaces allowing for side-on O2 adsorption, where O-O bonding is weakened, and it facilitates the four-electron pathway [34,56]. Hu et al. argued that in the case of Ag/MnO2 catalysts, oxygen vacancies could play a role in the ORR invigorating synergistic effect [64]. In the case of Ag/MnO2_MWCNT2 catalysts, there may be fewer available carbon sites along MnO2, and only the ensemble effect between Ag and MnO2 might affect the electroreduction of the formed peroxide intermediates, which in turn could induce a greater decrease in half-wave potential after AST for Ag/MnO2_MWCNT2 catalysts.
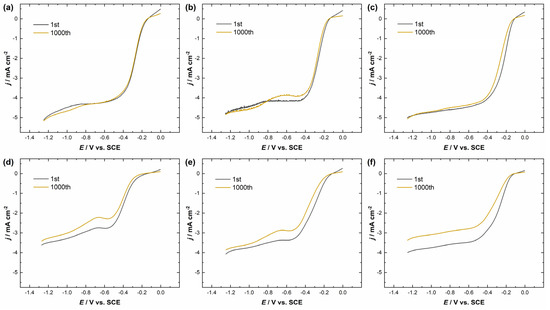
Figure 6.
RDE polarization curves for oxygen reduction on (a) Ag5/MnO2_MWCNT1, (b) Ag10/MnO2_MWCNT1, (c) Ag20/MnO2_MWCNT1, (d) Ag5/MnO2_MWCNT2, (e) Ag10/MnO2_MWCNT2, (f) Ag20/MnO2_MWCNT2 catalysts in O2-saturated 0.1 M KOH before and after the AST. ω = 960 rpm, ν = 10 mV s−1. Background current not subtracted.
3. Materials and Methods
3.1. Synthesis of MnO2_MWCNT1 and MnO2_MWCNT2
Acid treatment of commercial multiwalled carbon nanotubes (MWCNTs, Nanocyl, Sambreville, Belgium) was carried out according to a previously reported protocol [69]. MnO2_MWCNT1 substrate was synthesised using a previously published method [70] by adding 25 mg of acid-treated MWCNT to 20 mL of deionised (Milli-Q, Millipore, Inc., Burlington, MA, United States) water and sonicated for 30 min. 228.4 mg of MnSO4•H2O (≥98%, Sigma-Aldrich, St. Louis, MO, USA) was added to the suspension and sonicated further for 30 min. A fresh KMnO4 (Thermo Scientific, ACS reagent, Waltham, MA, USA) solution was prepared by dissolving 704.8 mg of KMnO4 in 30 mL of deionised water. Under vigorous stirring, MWCNT suspension and KMnO4 solution were heated up to 80 °C and KMnO4 solution was quickly added to the suspension. The synthesis suspension was continuously stirred for an hour until the purple colour disappeared. It was then washed, filtered with ethanol and deionised water and dried overnight at 60 °C.
MnO2_MWCNT2 was synthesised by a one-pot method where 10 mg of untreated MWCNTs were added to 10 mL of deionised water, then 100 mg of KMnO4 was added and dissolved, as reported previously [71]. The synthesis mixture was stirred at room temperature for 6 h. Next, 50 µL of concentrated H2SO4 (97% puriss, p.a., Honeywell, Charlotte, NC, USA) was added, and the synthesis mixture was stirred at room temperature for another hour. The solution was then heated to 80 °C and stirred for an additional 60 min. The synthesis mixture was then removed from the heat source, poured into a 1 L flask containing 0.5 L of deionised water and filled up to the 1 L mark. The suspension was filtered, washed with ethanol and a copious amount of deionised water and dried overnight at 60 °C.
3.2. Silver Deposition Using Magnetron Sputtering
Before the experiments, glassy carbon (GC, GC-20SS Tokai Carbon, Tokyo, Japan) electrodes (d = 5 mm) were polished on alumina slurries (1 and 0.3 μm, Buehler, Uzwil, Switzerland) and washed with 2-propanol and deionised water using sonication for 3 min in each solvent. 1 mg cm−3 MnO2_MWCNT inks were prepared in a 2-propanol-water mixture (80% deionised water and 20% 2-propanol) by adding 2 mL of a 2-propanol-water mixture and 2 µL of Nafion solution (5% in lower aliphatic alcohols, Sigma-Aldrich) to 2 mg of MnO2_MWCNT powder. 50 µL of ink was then drop-casted onto a freshly polished GC electrode to have a loading of 0.25 mg cm−2, and the electrode was dried under N2 gas flow [64,70]. Subsequently, Ag was sputter-deposited onto the substrates with nominal thicknesses of 5, 10, and 20 nm. Magnetron sputtering was carried out, as reported earlier [25,42,46], in this study using an Ag target in the Ar atmosphere. The pressure in the deposition chamber was 3 × 10−3 mbar, the DC power of 2 W, and the distance between the target and substrate was 6 cm. Si plates were used as additional substrates to determine the deposited Ag loading by X-ray fluorescence (XRF). A bulk polycrystalline silver disc (d = 5 mm, 99.95%, Alfa Aesar) electrode embedded into a Teflon holder was used for comparison.
3.3. Electrochemical Testing
The freshly prepared electrodes were subjected to electrochemical testing in freshly prepared 0.1 M KOH (Sigma-Aldrich, ≥85%) solution, which was either deaerated using Ar (99.999%, Linde Gas, Dublin, Ireland) or oxygenated using O2 (99.999% Linde Gas). The electrochemical measurements were conducted in a standard 3-electrode configuration where a saturated calomel electrode (SCE) was used as a reference electrode connected to the main cell via a Luggin capillary. Pt wire, separated via a glass frit, served as the counter electrode. All potentials herein were measured in reference to the SCE and are given with respect to the SCE unless otherwise stated.
The electrodes were first conditioned in an Ar-saturated 0.1 M KOH solution by recording 10 cyclic voltammograms in the potential range from −1.2 to 0 V vs. SCE. After that, background current was recorded in the potential range from −1.3 to 0 V vs. SCE. Then the solution was saturated with oxygen to carry out the ORR measurements using RDE at different electrode rotation rates (360–4600 rpm) from −1.3 to 0 V vs. SCE. ASTs were conducted at a constant (960 rpm) rotation rate in the potential range from −1.3 to 0 V vs. SCE for 1000 potential cycles at 100 mV s−1.
3.4. Catalyst Characterization
A scanning electron microscope (SEM, Helios Nanolab 600, FEI, Hillsboro, OR, USA) was used at an accelerating voltage of 10 kV to characterize the surface morphology of the prepared Ag-based catalysts. It was equipped with the energy-dispersive X-ray spectrometer INCA Energy 350 (Oxford Instruments, Abingdon, UK), which was used to determine the elemental composition of prepared catalysts. Before the SEM measurements, fresh MnO2_MWCNT inks were prepared in a 2-propanol-water mixture and drop-casted onto GC disks.
The prepared catalyst materials were also characterised by scanning transmission electron microscopy (STEM, Titan 200, FEI), equipped with energy dispersive X-ray spectrometer (EDS) Super-X™ system, operated at 200 kV.
The loadings of the Ag sputter-deposited onto Si plates were measured by XRF spectrometer Rigaku ZSX 400 (Tokyo, Japan) using ZSX Version 5.55 program with a thin film analysis option.
The powder samples were studied by XRD using a Bruker D8 Advance (Billerica, MA, USA) diffractometer with Ni-filtered CuKα radiation and a 1D detector. Scanning steps of 0.013°2θ from 3 to 92°2θ and a total counting time of 534 s per step were used. Bruker’s DIFFRAC.EVA software with ICDD database PDF-4+ (2023) was used for phase identification.
The X-ray photoelectron spectroscopy (XPS) analysis of catalysts was carried out by using samples prepared by drop-casting onto GC substrates, onto which Ag was then deposited. The measurements were conducted using SCIENTA SES-100 (VG Scienta, Uppsala, Sweden) instrument with Mg Kα X-ray source (incident energy of 1253.6 eV).
4. Conclusions
In this work, the synthesised catalyst materials possessed high electrocatalytic activity for ORR in an alkaline solution. The most active Ag-based catalyst was Ag5/MnO2_MWCNT1, with a mass activity of 126 A g−1 at −0.2 V vs. SCE. The Tafel slope values obtained in this work, from −81 mV to −94 mV, were close to the ones reported in the literature. The electron transfer number was close to four for all Ag catalysts. Two of the six prepared Ag-catalysts, Ag5/MnO2_MWCNT1 and Ag10/MnO2_MWCNT1, showed superior electrocatalytic stability during 1000 potential cycle AST due to possible synergistic ensemble effects. The half-wave potential changed for them by only 2 mV and 4 mV, respectively. The results obtained in this work show that these catalysts could be good candidates as cathode catalysts in anion exchange membrane fuel cells.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13060976/s1, Figure S1: HAADF-TEM image and EDS mapping of (a,b) MnO2_MWCNT1 and (c,d) MnO2_MWCNT2 sample; Figure S2: (a) a set of RDE polarization curves for oxygen reduction on an Ag10/MnO2_MWCNT1 catalyst, at various electrode rotation rates in O2-saturated 0.1 M KOH. ν = 10 mV s−1. (b) Koutecky-Levich plots derived from (a). Insets show the n-E dependence.
Author Contributions
Conceptualization, K.T. and J.M.L.; methodology, K.T., A.S., A.T. and V.K.; validation, J.M.L., A.S., H.E., A.K., J.A. and K.T.; formal analysis, J.M.L., J.K., P.R., J.A. and A.K.; investigation, J.M.L., J.K., P.R., A.K. and J.A.; resources, K.T., A.T., V.K. and J.A.; data curation, J.M.L.; writing—original draft preparation, J.M.L.; writing—review and editing, J.M.L., H.E., P.R., A.K., V.K., J.A., J.K., A.T., A.S. and K.T.; visualization, J.M.L.; supervision, H.E., A.S. and K.T.; funding acquisition, K.T., A.T., V.K. and J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Estonian Research Council, grants number PRG723 and PRG753 and by the EU through the European Regional Development Fund, grants number TK141 and TK134.
Data Availability Statement
Data is available from the authors on request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Hussain, S.; Erikson, H.; Kongi, N.; Sarapuu, A.; Solla-Gullón, J.; Maia, G.; Kannan, A.M.; Alonso-Vante, N.; Tammeveski, K. Oxygen reduction reaction on nanostructured Pt-based electrocatalysts: A review. Int. J. Hydrogen Energy 2020, 45, 31775–31797. [Google Scholar] [CrossRef]
- Hernandez-Rodriguez, M.A.; Goya, M.C.; Arevalo, M.C.; Rodriguez, J.L.; Pastor, E. Carbon supported Ag and Ag-Co catalysts tolerant to methanol and ethanol for the oxygen reduction reaction in alkaline media. Int. J. Hydrogen Energy 2016, 41, 19789–19798. [Google Scholar] [CrossRef]
- Linge, J.M.; Erikson, H.; Mooste, M.; Piirsoo, H.M.; Kaljuvee, T.; Kikas, A.; Aruväli, J.; Kisand, V.; Tamm, A.; Kannan, A.M.; et al. Ag nanoparticles on mesoporous carbon support as cathode catalyst for anion exchange membrane fuel cell. Int. J. Hydrogen Energy 2023, 48, 11058–11070. [Google Scholar] [CrossRef]
- Sleightholme, A.E.S.; Varcoe, J.R.; Kucernak, A.R. Oxygen reduction at the silver/hydroxide-exchange membrane interface. Electrochem. Commun. 2008, 10, 151–155. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, D.; Li, J.; Jonsson, M.; Jannasch, P.; Soroka, I.L. Using an ionomer as a size regulator in γ-radiation induced synthesis of Ag nanocatalysts for oxygen reduction reaction in alkaline solution. J. Colloid Interface Sci. 2023, 646, 381–390. [Google Scholar] [CrossRef]
- Campbell, F.W.; Belding, S.R.; Baron, R.; Xiao, L.; Compton, R.G. Hydrogen Peroxide Electroreduction at a Silver-Nanoparticle Array: Investigating Nanoparticle Size and Coverage Effects. J. Phys. Chem. C 2009, 113, 9053–9062. [Google Scholar] [CrossRef]
- Han, J.J.; Li, N.; Zhang, T.Y. Ag/C nanoparticles as an cathode catalyst for a zinc-air battery with a flowing alkaline electrolyte. J. Power Sources 2009, 193, 885–889. [Google Scholar] [CrossRef]
- Erikson, H.; Sarapuu, A.; Tammeveski, K. Oxygen Reduction Reaction on Silver Catalysts in Alkaline Media: A Minireview. ChemElectroChem 2019, 6, 73–86. [Google Scholar] [CrossRef]
- Šepa, D.; Vojnovíc, M.; Damjanovic, A. Oxygen reduction at silver electrodes in alkaline solutions. Electrochim. Acta 1970, 15, 1355–1366. [Google Scholar] [CrossRef]
- Chatenet, M.; Genies-Bultel, L.; Aurousseau, M.; Durand, R.; Andolfatto, F. Oxygen reduction on silver catalysts in solutions containing various concentrations of sodium hydroxide–comparison with platinum. J. Appl. Electrochem. 2002, 32, 1131–1140. [Google Scholar] [CrossRef]
- Singh, P.; Buttry, D.A. Comparison of Oxygen Reduction Reaction at Silver Nanoparticles and Polycrystalline Silver Electrodes in Alkaline Solution. J. Phys. Chem. C 2012, 116, 10656–10663. [Google Scholar] [CrossRef]
- Blizanac, B.B.; Ross, P.N.; Markovic, N.M. Oxygen reduction on silver low-index single-crystal surfaces in alkaline solution: Rotating ring Disk(Ag(hkl)) studies. J. Phys. Chem. B 2006, 110, 4735–4741. [Google Scholar] [CrossRef] [PubMed]
- Blizanac, B.B.; Ross, P.N.; Markovic, N.M. Oxygen electroreduction on Ag(111): The pH effect. Electrochim. Acta 2007, 52, 2264–2271. [Google Scholar] [CrossRef]
- Lee, C.L.; Chiou, H.P.; Syu, C.M.; Wu, C.C. Silver triangular nanoplates as electrocatalyst for oxygen reduction reaction. Electrochem. Commun. 2010, 12, 1609–1613. [Google Scholar] [CrossRef]
- Ohyama, J.; Okata, Y.; Watabe, N.; Katagiri, M.; Nakamura, A.; Arikawa, H.; Shimizu, K.; Takeguchi, T.; Ueda, W.; Satsuma, A. Oxygen reduction reaction over silver particles with various morphologies and surface chemical states. J. Power Sources 2014, 245, 998–1004. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Cui, X.Q.; Guan, W.M.; Zhang, L.; Fan, X.F.; Shi, Z.; Zheng, W.T. Shape-dependent catalytic activity of oxygen reduction reaction (ORR) on silver nanodecahedra and nanocubes. J. Power Sources 2014, 269, 152–157. [Google Scholar] [CrossRef]
- Treshchalov, A.; Erikson, H.; Puust, L.; Tsarenko, S.; Saar, R.; Vanetsev, A.; Tammeveski, K.; Sildos, I. Stabilizer-free silver nanoparticles as efficient catalysts for electrochemical reduction of oxygen. J. Colloid Interface Sci. 2017, 491, 358–366. [Google Scholar] [CrossRef]
- Alia, S.M.; Duong, K.; Liu, T.; Jensen, K.; Yan, Y.S. Supportless Silver Nanowires as Oxygen Reduction Reaction Catalysts for Hydroxide-Exchange Membrane Fuel Cells. ChemSusChem 2012, 5, 1619–1624. [Google Scholar] [CrossRef]
- Lu, Y.Z.; Chen, W. Size effect of silver nanoclusters on their catalytic activity for oxygen electro-reduction. J. Power Sources 2012, 197, 107–110. [Google Scholar] [CrossRef]
- Tammeveski, K.; Kontturi, K.; Nichols, R.J.; Potter, R.J.; Schiffrin, D.J. Surface redox catalysis for O-2 reduction on quinone-modified glassy carbon electrodes. J. Electroanal. Chem. 2001, 515, 101–112. [Google Scholar] [CrossRef]
- Kruusenberg, I.; Leis, J.; Arulepp, M.; Tammeveski, K. Oxygen reduction on carbon nanomaterial-modified glassy carbon electrodes in alkaline solution. J. Solid State Electrochem. 2010, 14, 1269–1277. [Google Scholar] [CrossRef]
- Guo, J.S.; Hsu, A.; Chu, D.; Chen, R.R. Improving Oxygen Reduction Reaction Activities on Carbon-Supported Ag Nanoparticles in Alkaline Solutions. J. Phys. Chem. C 2010, 114, 4324–4330. [Google Scholar] [CrossRef]
- Wang, Z.C.; Xin, L.; Zhao, X.S.; Qiu, Y.; Zhang, Z.Y.; Baturina, O.A.; Li, W.Z. Carbon supported Ag nanoparticles with different particle size as cathode catalysts for anion exchange membrane direct glycerol fuel cells. Renew. Energy 2014, 62, 556–562. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Li, H.N.; Hu, J.; Liu, B.Z.; Zhang, Q.R.; Fernandez, C.; Peng, Q.M. High oxygen reduction reaction activity of C-N/Ag hybrid composites for Zn-air battery. J. Alloys Compd. 2017, 694, 419–428. [Google Scholar] [CrossRef]
- Tammeveski, L.; Erikson, H.; Sarapuu, A.; Kozlova, J.; Ritslaid, P.; Sammelselg, V.; Tammeveski, K. Electrocatalytic oxygen reduction on silver nanoparticle/multi-walled carbon nanotube modified glassy carbon electrodes in alkaline solution. Electrochem. Commun. 2012, 20, 15–18. [Google Scholar] [CrossRef]
- Fazil, A.; Chetty, R. Synthesis and Evaluation of Carbon Nanotubes Supported Silver Catalyst for Alkaline Fuel Cell. Electroanalysis 2014, 26, 2380–2387. [Google Scholar] [CrossRef]
- Linge, J.M.; Erikson, H.; Sarapuu, A.; Merisalu, M.; Rahn, M.; Matisen, L.; Sammelselg, V.; Tammeveski, K. Electroreduction of oxygen on nitrogen-doped graphene oxide supported silver nanoparticles. J. Electroanal. Chem. 2017, 794, 197–203. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Chantaramethakul, J.; Chokradjaroen, C.; Ishizaki, T. In situ solution plasma synthesis of silver nanoparticles supported on nitrogen-doped carbons with enhanced oxygen reduction activity. Mater. Lett. 2019, 251, 135–139. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Lin, L.; Luo, S.; Meng, A.; Song, G.; Li, S. Carbon nanotubes-supported Ag/MoO2 or Ag/MnO2 heterostructures for a highly efficient oxygen reduction reaction. Mater. Charact. 2021, 176, 111147. [Google Scholar] [CrossRef]
- Ni, S.; Zhang, H.; Zhao, Y.; Li, X.; Sun, Y.; Qian, J.; Xu, Q.; Gao, P.; Wu, D.; Kato, K.; et al. Single atomic Ag enhances the bifunctional activity and cycling stability of MnO2. Chem. Eng. J. 2019, 366, 631–638. [Google Scholar] [CrossRef]
- Wang, L.; Kong, D.; Chen, F.; Cui, L.; Cai, Y.; Wang, H.; Zhong, X.; Huang, Y.; Li, Q.; Ma, Z.; et al. Strongly Coupled MnO2 Nanosheets/Silver Nanoparticles Hierarchical Spheres for Efficient Oxygen Reduction Reaction Electrocatalysis. Energy Fuels 2021, 35, 16829–16836. [Google Scholar] [CrossRef]
- Sun, H.; Hu, Z.; Yao, C.; Yu, J.; Du, Z. Silver Doped Amorphous MnO2 as Electrocatalysts for Oxygen Reduction Reaction in Al-Air Battery. J. Electrochem. Soc. 2020, 167, 080539. [Google Scholar] [CrossRef]
- Zamora Zeledón, J.A.; Gunasooriya, G.T.K.K.; Kamat, G.A.; Kreider, M.E.; Ben-Naim, M.; Hubert, M.A.; Avilés Acosta, J.E.; Nørskov, J.K.; Stevens, M.B.; Jaramillo, T.F. Engineering metal–metal oxide surfaces for high-performance oxygen reduction on Ag–Mn electrocatalysts. Energy Environ. Sci. 2022, 15, 1611–1629. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Song, W.; Wang, F.; Song, Y. The role of electronic interaction in the use of Ag and Mn3O4 hybrid nanocrystals covalently coupled with carbon as advanced oxygen reduction electrocatalysts. J. Mater. Chem. A 2014, 2, 17477–17488. [Google Scholar] [CrossRef]
- Slanac, D.A.; Lie, A.; Paulson, J.A.; Stevenson, K.J.; Johnston, K.P. Bifunctional Catalysts for Alkaline Oxygen Reduction Reaction via Promotion of Ligand and Ensemble Effects at Ag/MnOx Nanodomains. J. Phys. Chem. C 2012, 116, 11032–11039. [Google Scholar] [CrossRef]
- Akbarian, P.; Kheirmand, M.; Faraji, M. Facile electrochemical fabrication of high-performance graphene quantum dots-supported Mn3O4/Ag hybrid catalyst for oxygen reduction reaction in alkaline media. Int. J. Energy Res. 2022, 46, 23004–23019. [Google Scholar] [CrossRef]
- Xu, H.; Qu, Z.; Zhao, S.; Mei, J.; Quan, F.; Yan, N. Different crystal-forms of one-dimensional MnO2 nanomaterials for the catalytic oxidation and adsorption of elemental mercury. J. Hazard. Mater. 2015, 299, 86–93. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Ma, S.; Ke, B.; Yu, L.; Zeng, W.; Li, Y.; Wang, J. Insight into the effect of crystalline structure on the oxygen reduction reaction activities of one-dimensional MnO2. Phys. E Low-Dimens. Syst. Nanostructures 2019, 109, 191–197. [Google Scholar] [CrossRef]
- Cao, Y.L.; Yang, H.X.; Ai, X.P.; Xiao, L.F. The mechanism of oxygen reduction on MnO2-catalyzed air cathode in alkaline solution. J. Electroanal. Chem. 2003, 557, 127–134. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, X.; Nie, H.; Yao, Z.; Huang, S. Facile Construction of Manganese Oxide Doped Carbon Nanotube Catalysts with High Activity for Oxygen Reduction Reaction and Investigations into the Origin of their Activity Enhancement. ACS Appl. Mater. Interfaces 2011, 3, 2601–2606. [Google Scholar] [CrossRef]
- Hussain, S.; Erikson, H.; Kongi, N.; Merisalu, M.; Ritslaid, P.; Sammelselg, V.; Tammeveski, K. Heat-treatment effects on the ORR activity of Pt nanoparticles deposited on multi-walled carbon nanotubes using magnetron sputtering technique. Int. J. Hydrogen Energy 2017, 42, 5958–5970. [Google Scholar] [CrossRef]
- Jukk, K.; Kozlova, J.; Ritslaid, P.; Sammelselg, V.; Alexeyeva, N.; Tammeveski, K. Sputter-deposited Pt nanoparticle/multi-walled carbon nanotube composite catalyst for oxygen reduction reaction. J. Electroanal. Chem. 2013, 708, 31–38. [Google Scholar] [CrossRef]
- Jukk, K.; Alexeyeva, N.; Ritslaid, P.; Kozlova, J.; Sammelselg, V.; Tammeveski, K. Electrochemical Reduction of Oxygen on Heat-Treated Pd Nanoparticle/Multi-Walled Carbon Nanotube Composites in Alkaline Solution. Electrocatalysis 2013, 4, 42–48. [Google Scholar] [CrossRef]
- Alexeyeva, N.; Kozlova, J.; Sammelselg, V.; Ritslaid, P.; Mandar, H.; Tammeveski, K. Electrochemical and surface characterisation of gold nanoparticle decorated multi-walled carbon nanotubes. Appl. Surf. Sci. 2010, 256, 3040–3046. [Google Scholar] [CrossRef]
- Hussain, S.; Erikson, H.; Kongi, N.; Tarre, A.; Ritslaid, P.; Kook, M.; Rahn, M.; Merisalu, M.; Sammelselg, V.; Tammeveski, K. Improved ORR Activity and Long-Term Durability of Pt Nanoparticles Deposited on TiO2-Decorated Multiwall Carbon Nanotubes. J. Electrochem. Soc. 2019, 166, F1284–F1291. [Google Scholar] [CrossRef]
- Hussain, S.; Erikson, H.; Kongi, N.; Tarre, A.; Ritslaid, P.; Rahn, M.; Matisen, L.; Merisalu, M.; Sammelselg, V.; Tammeveski, K. Pt nanoparticles sputter-deposited on TiO2/MWCNT composites prepared by atomic layer deposition: Improved electrocatalytic activity towards the oxygen reduction reaction and durability in acid media. Int. J. Hydrogen Energy 2018, 43, 4967–4977. [Google Scholar] [CrossRef]
- Jukk, K.; Kongi, N.; Tarre, A.; Rosental, A.; Treshchalov, A.B.; Kozlova, J.; Ritslaid, P.; Matisen, L.; Sammelselg, V.; Tammeveski, K. Electrochemical oxygen reduction behaviour of platinum nanoparticles supported on multi-walled carbon nanotube/titanium dioxide composites. J. Electroanal. Chem. 2014, 735, 68–76. [Google Scholar] [CrossRef]
- Wang, J.-G.; Yang, Y.; Huang, Z.-H.; Kang, F. Rational synthesis of MnO2/conducting polypyrrole@carbon nanofiber triaxial nano-cables for high-performance supercapacitors. J. Mater. Chem. 2012, 22, 16943–16949. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, J.; Chen, S.; Hammond, P.T.; Shao-Horn, Y. Carbon Nanotube/Manganese Oxide Ultrathin Film Electrodes for Electrochemical Capacitors. ACS Nano 2010, 4, 3889–3896. [Google Scholar] [CrossRef]
- Li, Q.; Liu, J.; Zou, J.; Chunder, A.; Chen, Y.; Zhai, L. Synthesis and electrochemical performance of multi-walled carbon nanotube/polyaniline/MnO2 ternary coaxial nanostructures for supercapacitors. J. Power Sources 2011, 196, 565–572. [Google Scholar] [CrossRef]
- Davis, R.E.; Horvath, G.L.; Tobias, C.W. The solubility and diffusion coefficient of oxygen in potassium hydroxide solutions. Electrochim. Acta 1967, 12, 287–297. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 82nd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Linge, J.M.; Kozhemyakin, D.; Erikson, H.; Vlassov, S.; Kongi, N.; Tammeveski, K. Silver Nanowire-Based Catalysts for Oxygen Reduction Reaction in Alkaline Solution. ChemCatChem 2021, 13, 4364–4371. [Google Scholar] [CrossRef]
- Linge, J.M.; Erikson, H.; Merisalu, M.; Matisen, L.; Kaarik, M.; Leis, J.; Sammelselg, V.; Aruvali, J.; Kaljuvee, T.; Tammeveski, K. Oxygen Reduction on Silver Nanoparticles Supported on Carbide-Derived Carbons. J. Electrochem. Soc. 2018, 165, F1199–F1205. [Google Scholar] [CrossRef]
- Linge, J.M.; Erikson, H.; Merisalu, M.; Sammelselg, V.; Tammeveski, K. Oxygen reduction on silver catalysts electrodeposited on various nanocarbon supports. SN Appl. Sci. 2021, 3, 263. [Google Scholar] [CrossRef]
- Salome, S.; Rego, R.; Oliveira, M.C. Development of silver-gas diffusion electrodes for the oxygen reduction reaction by electrodeposition. Mater. Chem. Phys. 2013, 143, 109–115. [Google Scholar] [CrossRef]
- Linge, J.M.; Erikson, H.; Kasikov, A.; Rahn, M.; Sammelselg, V.; Tammeveski, K. Oxygen reduction reaction on thin-film Ag electrodes in alkaline solution. Electrochim. Acta 2019, 325, 134922. [Google Scholar] [CrossRef]
- Linge, J.M.; Erikson, H.; Kozlova, J.; Sammelselg, V.; Tammeveski, K. Oxygen reduction reaction on electrochemically deposited silver nanoparticles from non-aqueous solution. J. Electroanal. Chem. 2018, 810, 129–134. [Google Scholar] [CrossRef]
- Linge, J.M.; Erikson, H.; Kozlova, J.; Aruvali, J.; Sammelselg, V.; Tammeveski, K. Oxygen reduction on electrodeposited silver catalysts in alkaline solution. J. Solid State Electrochem. 2018, 22, 81–89. [Google Scholar] [CrossRef]
- Hu, F.-P.; Zhang, X.-G.; Xiao, F.; Zhang, J.-L. Oxygen reduction on Ag–MnO2/SWNT and Ag–MnO2/AB electrodes. Carbon 2005, 43, 2931–2936. [Google Scholar] [CrossRef]
- Wolf, S.; Roschger, M.; Genorio, B.; Kolar, M.; Garstenauer, D.; Bitschnau, B.; Hacker, V. Ag-MnxOy on Graphene Oxide Derivatives as Oxygen Reduction Reaction Catalyst in Alkaline Direct Ethanol Fuel Cells. Catalysts 2022, 12, 780. [Google Scholar] [CrossRef]
- Innocenti, M.; Zafferoni, C.; Lavacchi, A.; Becucci, L.; Di Benedetto, F.; Carretti, E.; Vizza, F.; Foresti, M.L. Electroactivation of Microparticles of Silver on Glassy Carbon for Oxygen Reduction and Oxidation Reactions. J. Electrochem. Soc. 2014, 161, D3018–D3024. [Google Scholar] [CrossRef]
- Lai, C.; Kolla, P.; Zhao, Y.; Fong, H.; Smirnova, A.L. Lignin-derived electrospun carbon nanofiber mats with supercritically deposited Ag nanoparticles for oxygen reduction reaction in alkaline fuel cells. Electrochim. Acta 2014, 130, 431–438. [Google Scholar] [CrossRef]
- He, M.; Turup, Z.; Jin, X.; Chen, F. Ag nanoparticle-loaded to MnO2 with rich oxygen vacancies and Mn3+ for the synergistically enhanced oxygen reduction reaction. Int. J. Hydrogen Energy 2023, in press. [CrossRef]
- Wiberg, G.K.H.; Mayrhofer, K.J.J.; Arenz, M. Investigation of the Oxygen Reduction Activity on Silver—A Rotating Disc Electrode Study. Fuel Cells 2010, 10, 575–581. [Google Scholar] [CrossRef]
- Wiberg, G.; Mayrhofer, K.; Arenz, M. Investigation of the Oxygen Reduction Activity of non-Platinum Catalysts—A RDE Methodology. ECS Trans. 2009, 19, 37–46. [Google Scholar] [CrossRef]
- Chao, Y.J.; Lyu, Y.P.; Wu, Z.W.; Lee, C.L. Seed-mediated growth of Ag nanocubes and their size-dependent activities toward oxygen reduction reaction. Int. J. Hydrogen Energy 2016, 41, 3896–3903. [Google Scholar] [CrossRef]
- Zhou, R.F.; Qiao, S.Z. Silver/Nitrogen-Doped Graphene Interaction and Its Effect on Electrocatalytic Oxygen Reduction. Chem. Mater. 2014, 26, 5868–5873. [Google Scholar] [CrossRef]
- Alexeyeva, N.; Tammeveski, K. Electrochemical reduction of oxygen on multiwalled carbon nanotube modified glassy carbon electrodes in acid media. Electrochem. Solid State Lett. 2007, 10, F18–F21. [Google Scholar] [CrossRef]
- Roy, A.; Ray, A.; Saha, S.; Ghosh, M.; Das, T.; Nandi, M.; Lal, G.; Das, S. Influence of electrochemical active surface area on the oxygen evolution reaction and energy storage performance of MnO2-multiwalled carbon nanotube composite. Int. J. Energy Res. 2021, 45, 16908–16921. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Geng, D.; Li, R.; Hong, H.; Chen, J.; Sun, X. One-pot synthesis of MnO2/graphene/carbon nanotube hybrid by chemical method. Carbon 2011, 49, 4434–4442. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).