Two-in-One Electrons Trapped Fe-BiOCl-Vo Nanosheets for Promoting Photocatalytic-Fenton Degradation Performances of Phenol
Abstract
:1. Introduction
2. Results and Discussion
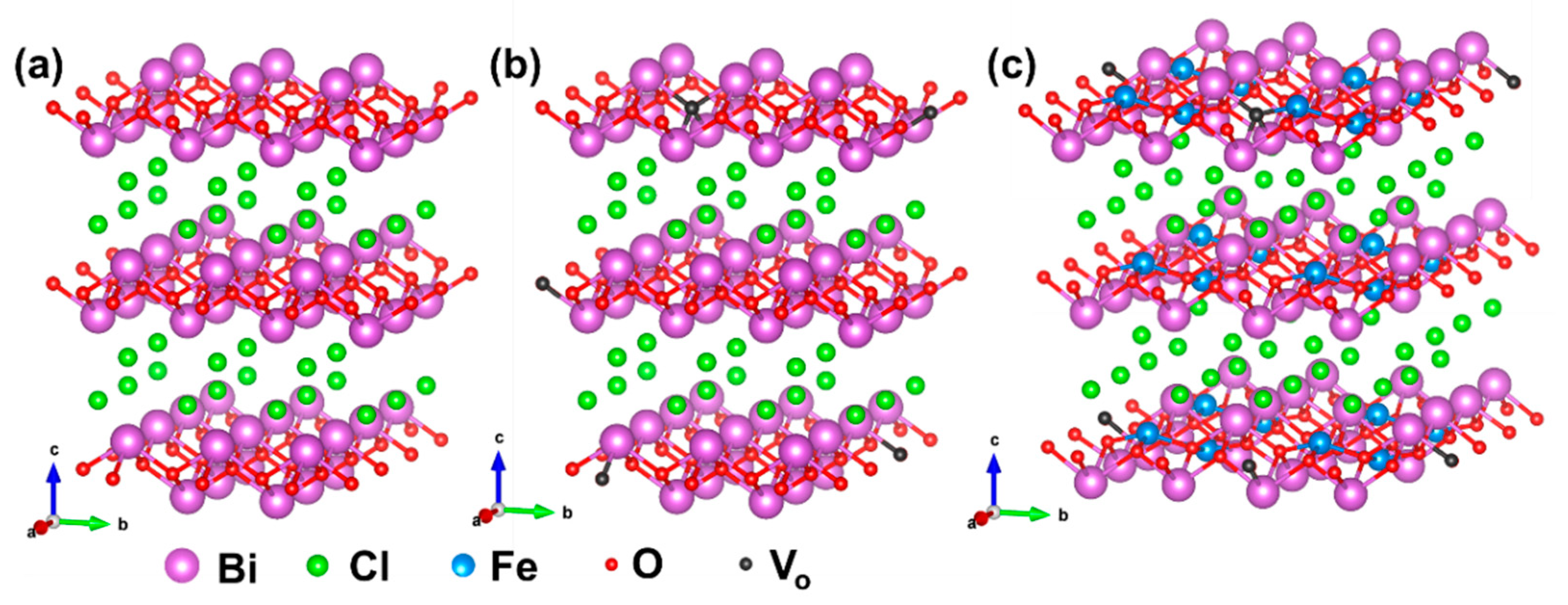
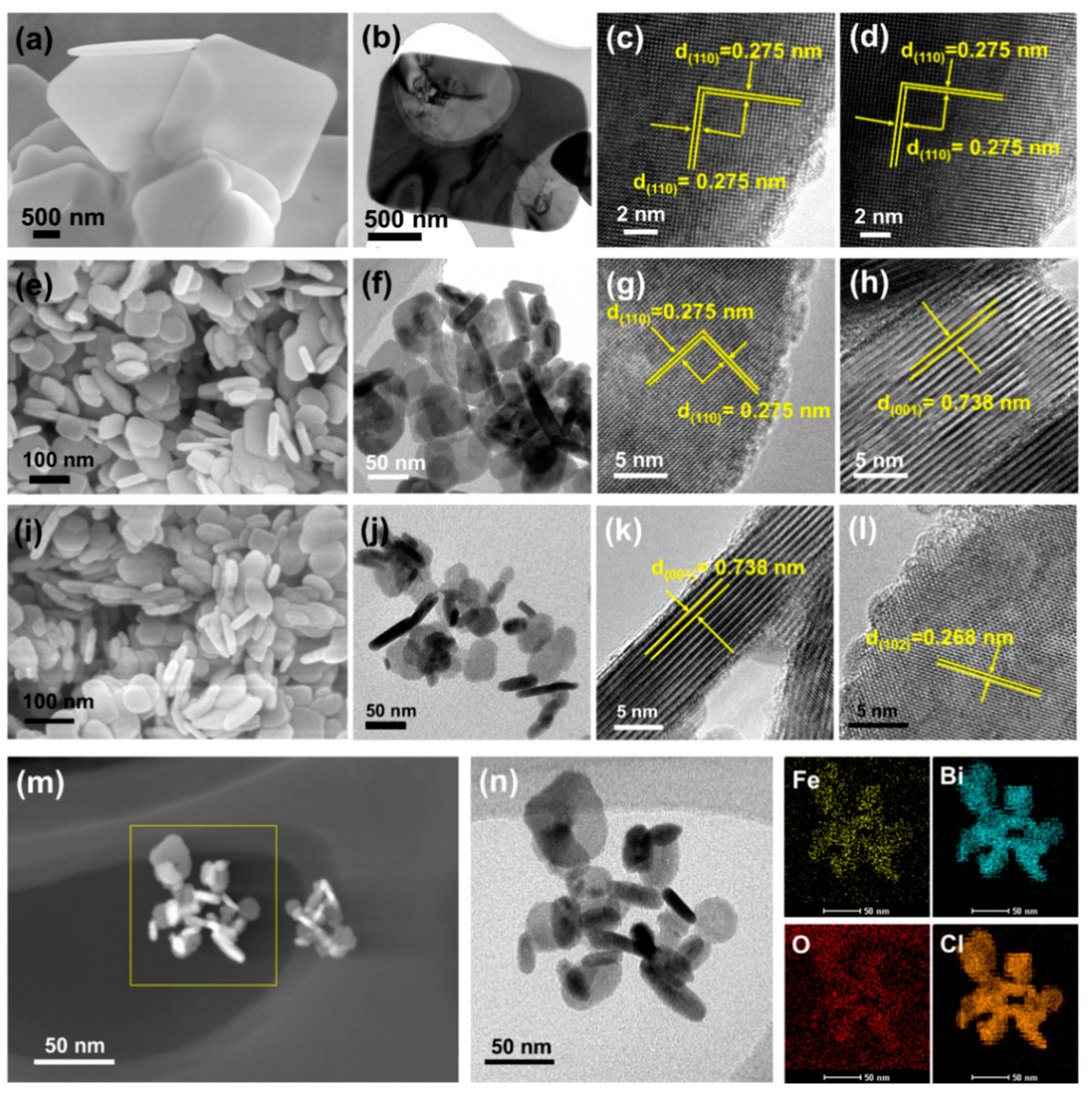
2.1. Structure Differential of Fe-BiOCl-Vo Nanosheets
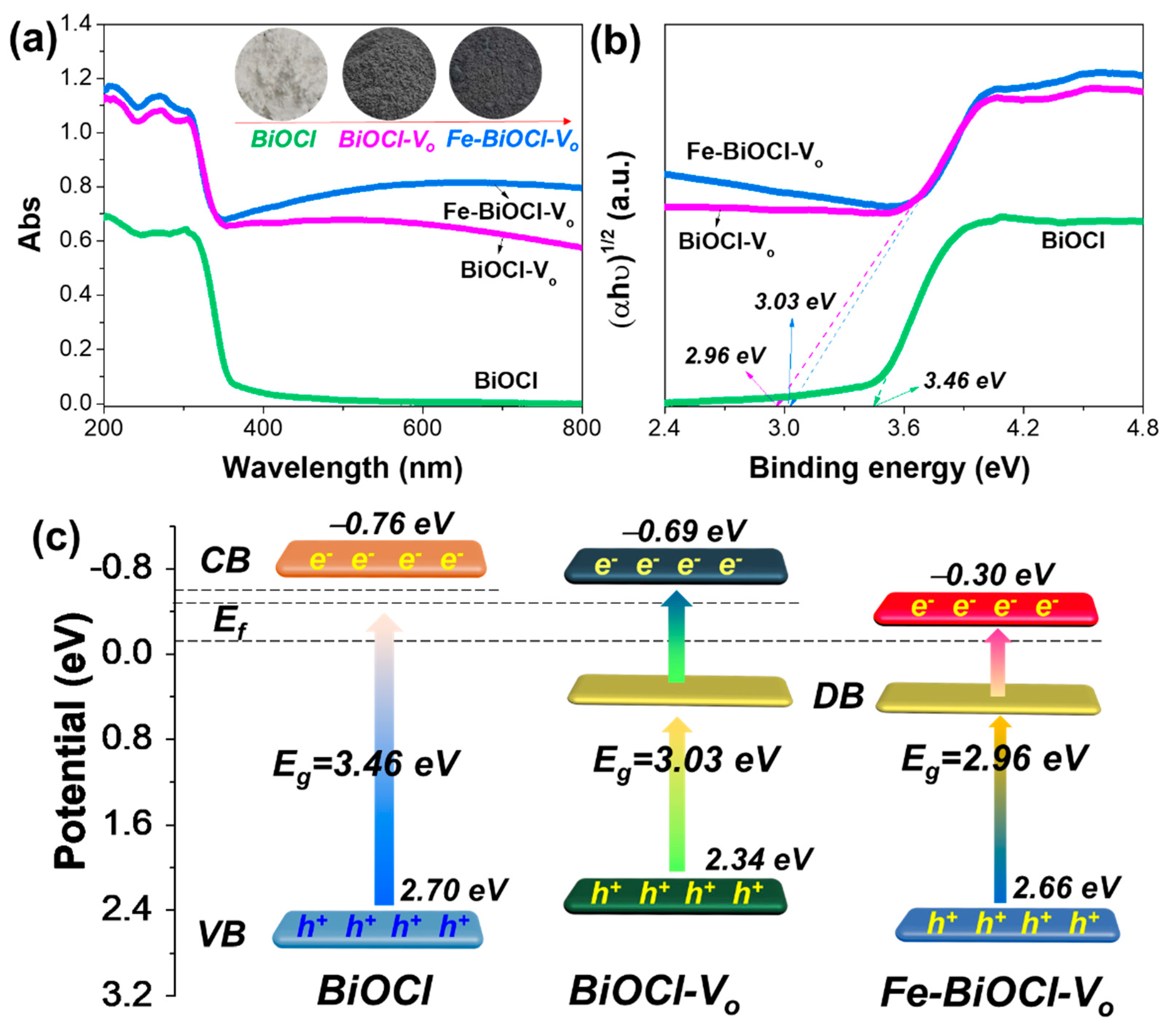
2.2. Band Structure Evolution Fe-BiOCl-Vo Nanosheets
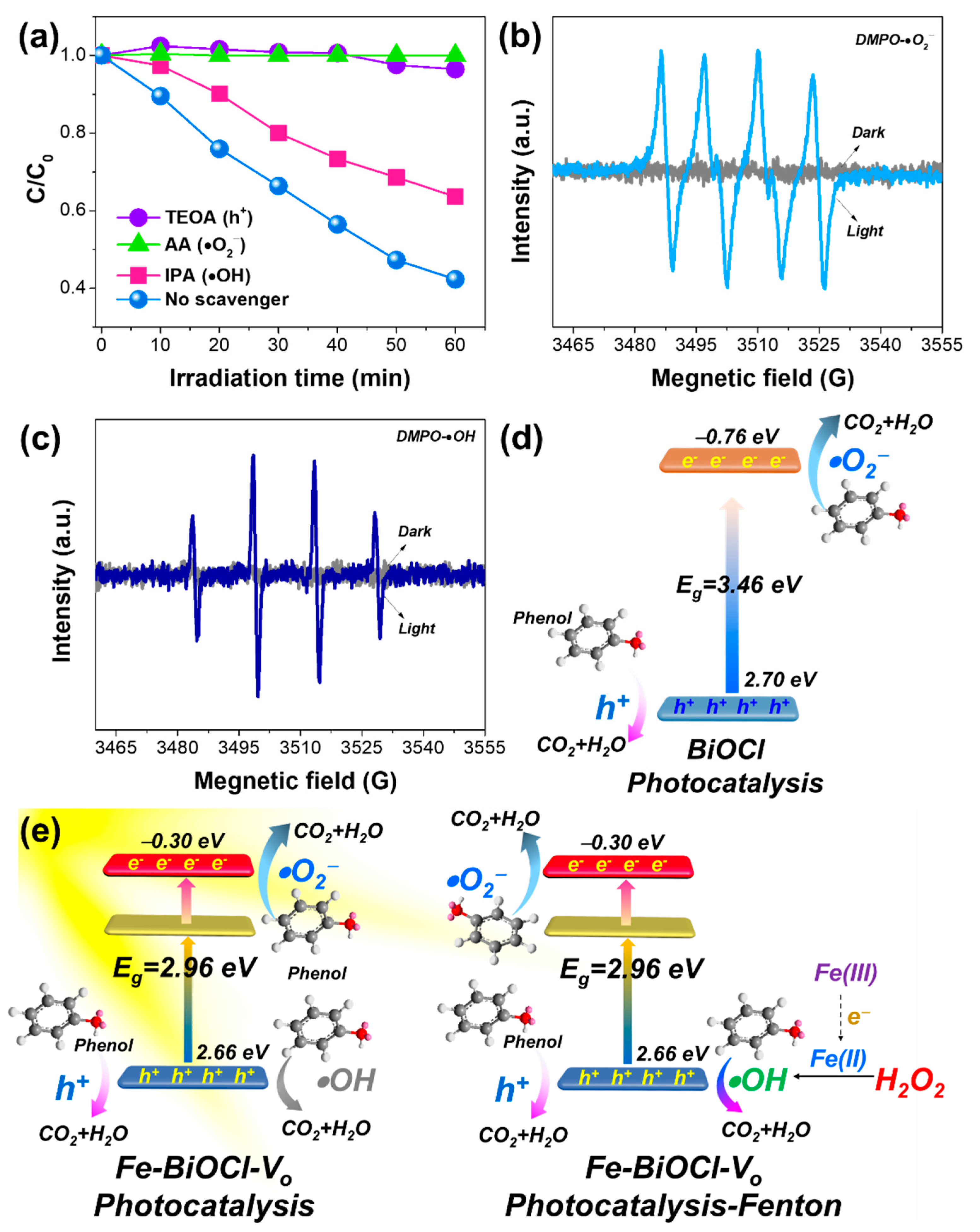
2.3. Enhanced Photocatalytic-Fenton Performances of Fe-BiOCl-Vo Nanosheets
3. Experimental
3.1. Preparation of Fe-BiOCl-Vo, BiOCl-Vo, and BiOCl Nanosheets
3.2. Characterization
3.3. Photocatalytic and Photocatalytic-Fenton Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sunidhi, B.; Subhankar, C. Fluoroquinolone antibiotics: Occurrence, mode of action, resistance, environmental detection, and remediation—A comprehensive review. Environ. Pollut. 2022, 315, 120440. [Google Scholar]
- Olivier, L.; René, M. Treatment of organic pollution in industrial saline wastewater: A literature review. Water Res. 2006, 40, 3671–3682. [Google Scholar]
- Imran, A.; Mohd, A.; Tabrez, A.K. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar]
- Zhang, K.; Liang, J.; Wang, S.; Liu, J.; Ren, K.; Zheng, X.; Luo, H.; Peng, Y.; Zou, X.; Bo, X.; et al. BiOCl Sub-microcrystals induced by citric acid and their high photocatalytic activities. Cryst. Growth Des. 2012, 12, 793–803. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.Q.; Fan, C.M.; Hao, X.G.; Ding, G.Y.; Wang, Y.F. Synthesis of BiOCl photocatalyst by a low-cost, simple hydrolytic technique and its excellent photocatalytic activity. Int. J. Min. Met. Mater. 2012, 19, 467–472. [Google Scholar] [CrossRef]
- Wei, Z.; Li, W.L.; Hu, J.S.; Ma, X.G.; Zhu, Y.F. Interfacial internal electric field and oxygen vacancies synergistically enhance photocatalytic performance of bismuth oxychloride. J. Hazard. Mater. 2021, 402, 123470. [Google Scholar] [CrossRef]
- Deng, F.; Zhang, Q.; Yang, L.; Luo, X.; Wang, A.; Luo, S.; Dionysiou, D.D. Visible-light-responsive graphene-functionalized Bi-bridge Z-scheme black BiOCl/Bi2O3 heterojunction with oxygen vacancy and multiple charge transfer channels for efficient photocatalytic degradation of 2-nitrophenol and industrial wastewater treatment. Appl. Catal. B Environ. 2018, 238, 61–69. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Ai, Z.H.; Jia, F.L.; Zhang, L.Z. Oxygen Vacancy-Mediated Photocatalysis of BiOCl: Reactivity, Selectivity, and Perspectives. Angew. Chem. Int. Ed. 2018, 57, 122–138. [Google Scholar] [CrossRef]
- Cai, Y.J.; Li, D.Y.; Sun, J.Y.; Chen, M.D.; Li, Y.R.; Zou, Z.W.; Zhang, H.; Xu, H.M.; Xia, D.S. Synthesis of BiOCl nanosheets with oxygen vacancies for the improved photocatalytic properties. Appl. Surf. Sci. 2018, 439, 697–704. [Google Scholar] [CrossRef]
- Wu, Z.H.; Li, Z.F.; Tian, Q.Y.; Liu, J.; Zhang, S.M.; Xu, K.Q.; Shen, J.; Zhang, S.Y.; Wu, W. Protonated branched polyethyleneimine induces the shape evolution of BiOCl and exposed {010} facet of BiOCl nanosheets. Cryst. Growth Des. 2018, 18, 5479–5491. [Google Scholar] [CrossRef]
- Zeng, X.X.; Gong, X.F.; Wan, Y.Q.; He, R.Y.; Xu, Z.D. Formation of oxygen vacancies on the {010} Facets of BiOCl and visible light activity for degradation of ciprofloxacin. Chem. Res. Chin. Univ. 2018, 34, 711–718. [Google Scholar] [CrossRef]
- Wang, L.; Lv, D.D.; Dong, F.; Wu, X.L.; Cheng, N.Y.; Scott, J.; Xu, X.; Hao, W.C.; Du, Y. Boosting visible-light-driven photo-oxidation of BiOCl by promoted charge separation via vacancy engineering. ACS Sustain. Chem. Eng. 2019, 7, 3010–3017. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, J.; Li, R.; Zhang, C.; Zhang, H.; Han, P.; Fan, C. DFT + U predictions: Structural stability, electronic and optical properties, oxidation activity of BiOCl photocatalysts with 3D transition metals doping. J. Mater. Sci. 2018, 53, 4494. [Google Scholar] [CrossRef]
- Tian, F.; Li, G.F.; Zhao, H.P.; Chen, F.X.; Li, M.; Liu, Y.L.; Chen, R. Residual Fe enhances the activity of BiOCl hierarchical nanostructure for hydrogen peroxide activation. J. Catal. 2019, 370, 265–273. [Google Scholar] [CrossRef]
- Wu, Z.; Shen, J.; Li, W.; Li, J.; Xia, D.; Xu, D.; Zhang, S.; Zhu, Y. Electron self-sufficient core-shell BiOCl@Fe-BiOCl nanosheets boosting Fe(III)/Fe(II) recycling and synergetic photocatalysis-Fenton for enhanced degradation of phenol. Appl. Catal. B Environ. 2023, 330, 122642. [Google Scholar] [CrossRef]
- Vorontsov, A.V. Advancing Fenton and photo-Fenton water treatment through the catalyst design. J. Hazard. Mater. 2019, 372, 103–112. [Google Scholar] [CrossRef]
- Liu, T.; Qu, H.; Tian, J.X.; He, S.H.; Su, Y.; Su, H.Q. Preparation of organic-free two-dimensional kaolinite nanosheets by in situ interlayer Fenton reaction. Chemistryselect 2019, 4, 11604–11608. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, Y.; Huang, D.L.; Lai, C.; Zeng, G.M.; Huang, J.H.; Liu, Z.F.; Zhang, C.; Zhou, C.Y.; Qin, L.; et al. Prussian blue analogue derived magnetic Cu-Fe oxide as a recyclable photo-Fenton catalyst for the efficient removal of sulfamethazine at near neutral pH values. Chem. Eng. J. 2019, 362, 865–876. [Google Scholar] [CrossRef]
- Xiong, Z.W.; Wang, Z.; Murugananthan, M.; Zhang, Y.R. Construction of an in-situ Fenton-like system based on a g-C3N4 composite photocatalyst. J. Hazard. Mater. 2019, 373, 565–571. [Google Scholar] [CrossRef]
- Li, W.T.; Geng, X.; Xiao, F.; An, G.Y.; Wang, D.S. Fe-II/Fe-III doped Bi/BiOBr hierarchical microspheres as a highly efficient catalyst for degradation of organic contaminants at neutral pH: The role of visible light and H2O2. Chemcatchem 2017, 9, 3762–3771. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, F.; Cao, Y.H.; Zhang, F.Y.; Zou, Y.Z.; Huang, Z.A.; Ye, L.Q.; Zhou, Y. Interfacial oxygen vacancy engineered two-dimensional g-C3N4/BiOCl heterostructures with boosted photocatalytic conversion of CO2. ACS Appl. Energ. Mater. 2020, 3, 4610–4618. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Cai, L.; Zhang, Y.F.; Wei, Y. Bi5+, Bi(3-x)+, and oxygen vacancy induced BiOClxI1-x solid solution toward promoting visible-light driven photocatalytic activity. Chem. Eur. J. 2018, 24, 7434–7444. [Google Scholar] [CrossRef]
- Li, H.; Shang, H.; Cao, X.M.; Yang, Z.P.; Ai, Z.H.; Zhang, L. Oxygen vacancies mediated complete visible light NO oxidation via side-on bridging superoxide radicals. Environ. Sci. Technol. 2018, 52, 8659–8665. [Google Scholar] [CrossRef]
- Xiao, X.; Tu, S.H.; Lu, M.L.; Zhong, H.; Zheng, C.X.; Zuo, X.X.; Nan, J.M. Discussion on the reaction mechanism of the photocatalytic degradation of organic contaminants from a viewpoint of semiconductor photo-induced electrocatalysis. Appl. Catal. B Environ. 2016, 198, 124–132. [Google Scholar] [CrossRef]
- Guo, Y.; Shi, W.X.; Zhu, Y.F.; Xu, Y.P.; Cui, F.Y. Enhanced photoactivity and oxidizing ability simultaneously via internal electric field and valence band position by crystal structure of bismuth oxyiodide. Appl. Catal. B Environ. 2020, 262, 118262. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhu, J.; Zhu, G.; He, H. Strategies for enhancing the heterogeneous Fenton catalytic reactivity: A review. Appl. Catal. B Environ. 2019, 255, 117739. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, J.; Zhang, S.; Xia, D.; Wan, Q.; Wan, Y.; Nong, M.; Wu, Z. Two-in-One Electrons Trapped Fe-BiOCl-Vo Nanosheets for Promoting Photocatalytic-Fenton Degradation Performances of Phenol. Catalysts 2023, 13, 947. https://doi.org/10.3390/catal13060947
Long J, Zhang S, Xia D, Wan Q, Wan Y, Nong M, Wu Z. Two-in-One Electrons Trapped Fe-BiOCl-Vo Nanosheets for Promoting Photocatalytic-Fenton Degradation Performances of Phenol. Catalysts. 2023; 13(6):947. https://doi.org/10.3390/catal13060947
Chicago/Turabian StyleLong, Jinlin, Suizhao Zhang, Donghao Xia, Qi Wan, Yu Wan, Meiqiu Nong, and Zhaohui Wu. 2023. "Two-in-One Electrons Trapped Fe-BiOCl-Vo Nanosheets for Promoting Photocatalytic-Fenton Degradation Performances of Phenol" Catalysts 13, no. 6: 947. https://doi.org/10.3390/catal13060947
APA StyleLong, J., Zhang, S., Xia, D., Wan, Q., Wan, Y., Nong, M., & Wu, Z. (2023). Two-in-One Electrons Trapped Fe-BiOCl-Vo Nanosheets for Promoting Photocatalytic-Fenton Degradation Performances of Phenol. Catalysts, 13(6), 947. https://doi.org/10.3390/catal13060947




