Abstract
Pharmaceutical compounds (PCs) are one of the most notable water pollutants of the current age with severe impacts on the ecosystem. Hence, scientists and engineers are continuously working on developing different materials and technologies to eradicate PCs from aqueous media. Among various new-age materials, graphitic carbon nitride (g-C3N4) is one of the wonder substances with excellent catalytic property. The current review article describes the latest trend in the application of g-C3N4-based catalyst materials towards the degradation of various kinds of drugs and pharmaceutical products present in wastewater. The synthesis procedure of different g-C3N4-based catalysts is covered in brief, and this is followed by different PCs degraded as described by different workers. The applicability of these novel catalysts in the real field has been highlighted along with different optimization techniques in practice. Different techniques often explored to characterize the g-C3N4-based materials are also described. Finally, existing challenges in this field along with future perspectives are presented before concluding the article.
1. Introduction
Emerging contaminants are the most alarming water pollutants of the present era. Pharmaceutical compounds (PCs) have become one of the notable classes of emerging water pollutants [1]. These compounds have been detected in various concentration ranges (ng/L to µg/L) in natural water bodies. These compounds are reported to be present in natural water bodies, such as Taihu Lake in China and Mississippi River in the USA [2]. Moreover, an estimation has shown that till now at least 3000 species of PCs have been detected in water and wastewater [3]. Among various categories of drug compounds, nonsteroidal anti-inflammatory drugs and sulfonamides are often used for the treatment of various categories of diseases. However, the long-term existence of these compounds in natural water bodies leads to the chronic poisoning of the aquatic lives. Various drugs have been marked by several environmental agencies for their toxic effects. Naproxen (NPX), a drug often used to treat menstrual cramps, has been enlisted by US EPA as Class I in Current Contaminant Candidate List. Diclofenac (DC), a commonly used painkiller, is well known for its bioaccumulation and toxic characteristics. Evidence shows that it causes hemodynamic changes and thyroid tumor in humans [4].
Moreover, due to the COVID-19 pandemic, their concentration has increased drastically in the environmental matrices. Morales-Paredes et al. [5] in their recent review article mentioned the abnormal increase in the concentration range of these compounds in natural water and wastewater. Among several antiviral drugs used during the COVID-19 times, azithromycin and chloroquine are some of the most commonly used PCs. The concentration of azithromycin drug is increased by 217 times in comparison to the normal concentration (WWTP-river-estuary at Wuhan, China). Due to various toxic effects, its removal from water streams is of utmost importance to environmental scientists and engineers. Conventional biological water treatment processes, adsorption, and coagulation are not feasible for the ultimate destruction of these recalcitrant compounds in aqueous media.
Eradication of organic compounds is possible through photocatalytic reaction, which undoubtedly is one of the green waste management options. In this regard, it may be noted that g-C3N4 is a prominent photocatalyst of the new age. It provides an attractive option to the research community for synthesizing noble metal-free efficient semiconductor-based photocatalysts. It possesses several distinguishing features such as higher thermal and chemical stability, due to the presence of strong covalent bonds between the C and N atoms in the conjugated g-C3N4 framework in it. It is easily prepared and nontoxic in nature [6]. However, the high probability of charge recombination, small surface area, and low reusability are some of the major hindrances behind its large-scale application [7]. Often these hurdles are minimized by means of doping [8], co-doping, co-polymerization via hybridization, exfoliation [9,10], formation of heterojunction structures [11], etc. A significant amount of research work has been performed by scientists all over the globe in recent times regarding the modification of g-C3N4 and its application for the treatment of PC-bearing wastewater. The current review article aims to focus on the latest trends in the applications of g-C3N4-based composite photocatalysts towards PC wastewater remediation. Firstly, different types of g-C3N4-based catalysts and their novel synthesis procedures are discussed. After that, various types of g-C3N4-based catalysts applied for PC wastewater treatment in recent times have been elaborated. The next two sections deal with the optimization and applicability of different g-C3N4-based catalysts for real wastewater treatment. Several characterization procedures often followed to get better insight into the removal process are then mentioned. The last section mentions the current challenges and future perspectives. This is followed by the concluding remarks. Almost all the papers discussed here have been published within the last five years.
2. Synthesis of Different Types of g-C3N4-Based Catalysts for PC Degradation
Different types of g-C3N4-based catalysts have been reported in the literature regarding the degradation of PCs. In the current section of this article, three categories have been selected for distinguishing g-C3N4-based catalysts in respect of preparation technique. Firstly, they have been differentiated on the basis of the precursor material used for the production of g-C3N4. Different starting materials, such as melamine, urea, and dicyanamide, have been reported in the literature for the preparation of g-C3N4. Therefore, the first categorization describes the synthesis procedure of different novel catalysts from different precursor materials.
Moreover, it has been stated in the previous section that in comparison to the bare g-C3N4, composite formation with other materials and doping improved the catalytic efficiency. Hence, the next two subsections deal with the preparation procedure of several g-C3N4 composite and doped catalysts. As the classification is based on different criteria, a particular catalyst may satisfy more than one principle.
2.1. Various Precursors for Preparing g-C3N4
In g-C3N4, nitrogen is placed in a framework of graphite with a p-conjugated system and the distance between the two layers is 0.326 nm. g-C3N4 is often produced from a precursor material such as urea. Upon condensation of urea molecules, NH3 and CO2 gases are produced, which ultimately helps in the production of porous g-C3N4. However, in comparison to pure g-C3N4 catalysts, doped materials are often preferred by the research community for their improved photocatalytic features. Due to the doping with Na or K, the potentials of the valence band and conduction band for absorbing visible light are enhanced, which ultimately leads to increased photocatalytic activity. Guo et al. [12] reported the preparation of g-C3N4 nanosheets from urea. Briefly, urea was placed inside an alumina crucible and heated to 550 °C for 3 h at the rate of 5 °C/min. The obtained yellow powder was heated for the second time in a muffle furnace at 52 °C to complete the thermal polymerization reaction and g-C3N4 nanosheets were produced.
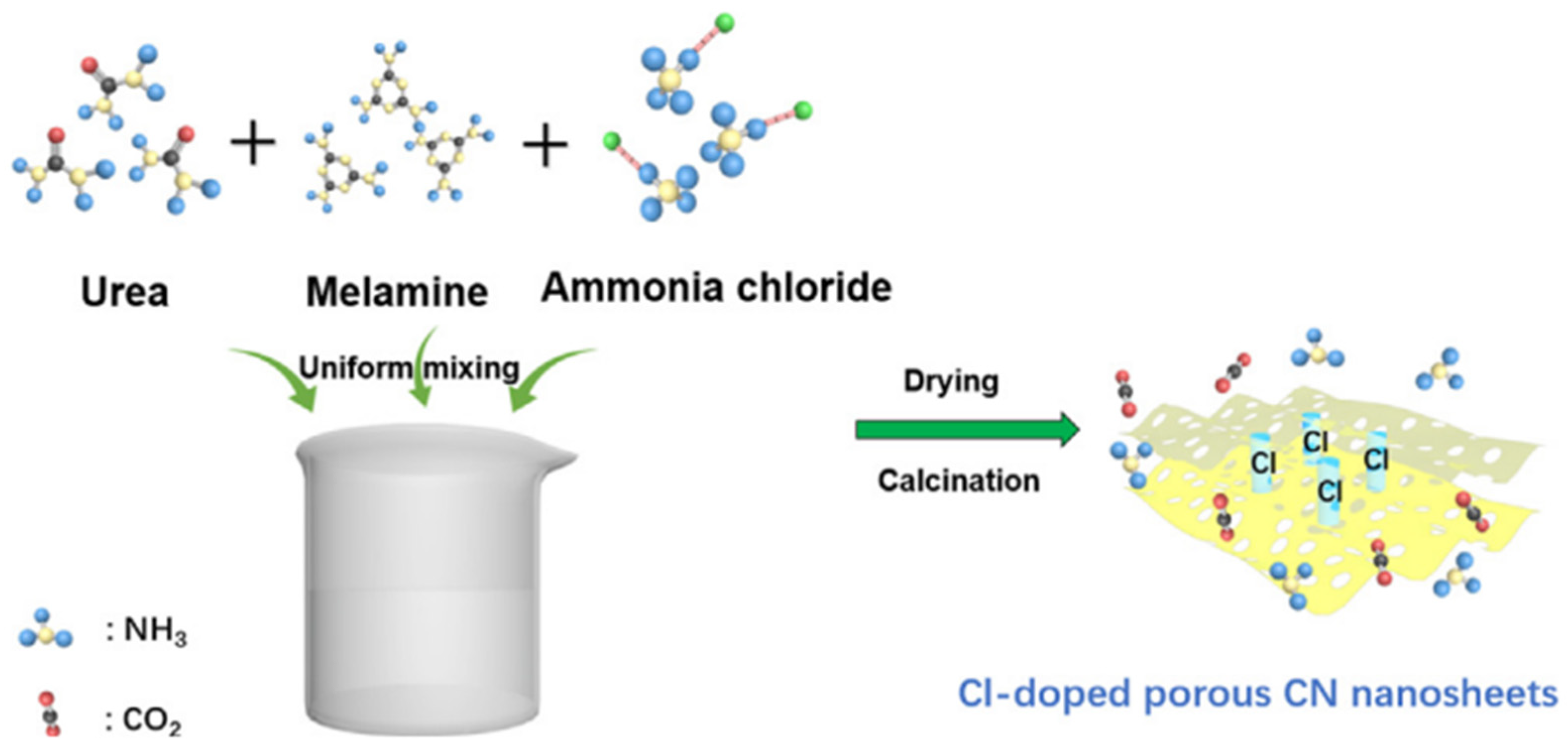
Many studies reported the synthesis of g-C3N4 from melamine as the precursor. Chi et al. [13] prepared a g-C3N4-based catalyst from melamine. Guo et al. [14] also prepared a g-C3N4-based novel photocatalyst from melamine and urea. Briefly, 7.704 g urea, 5.4 g melamine, and different amount of ammonium chloride (NH4Cl) were added to 90 mL deionized water. After stirring the solution for 30 min, it was dried at 80 °C to obtain the precursor powder. Then, the precursor powder was calcinated at 550 °C for 3 h at the heating rate of 0.5 °C/min. The whole schematic of the preparation of the Cl-doped g-C3N4 nanosheet catalyst is shown in Figure 1. Smykalova et al. [15] produced exfoliated g-C3N4 catalyst using melamine as the precursor.
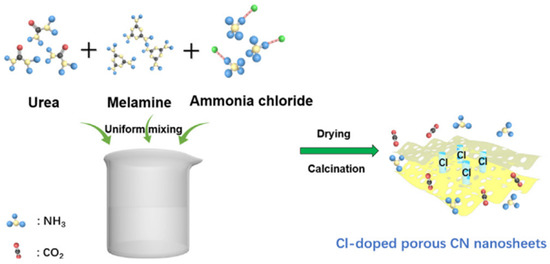
Figure 1.
Schematic illustration of the preparation for Cl-doped porous CN nanosheets [14].
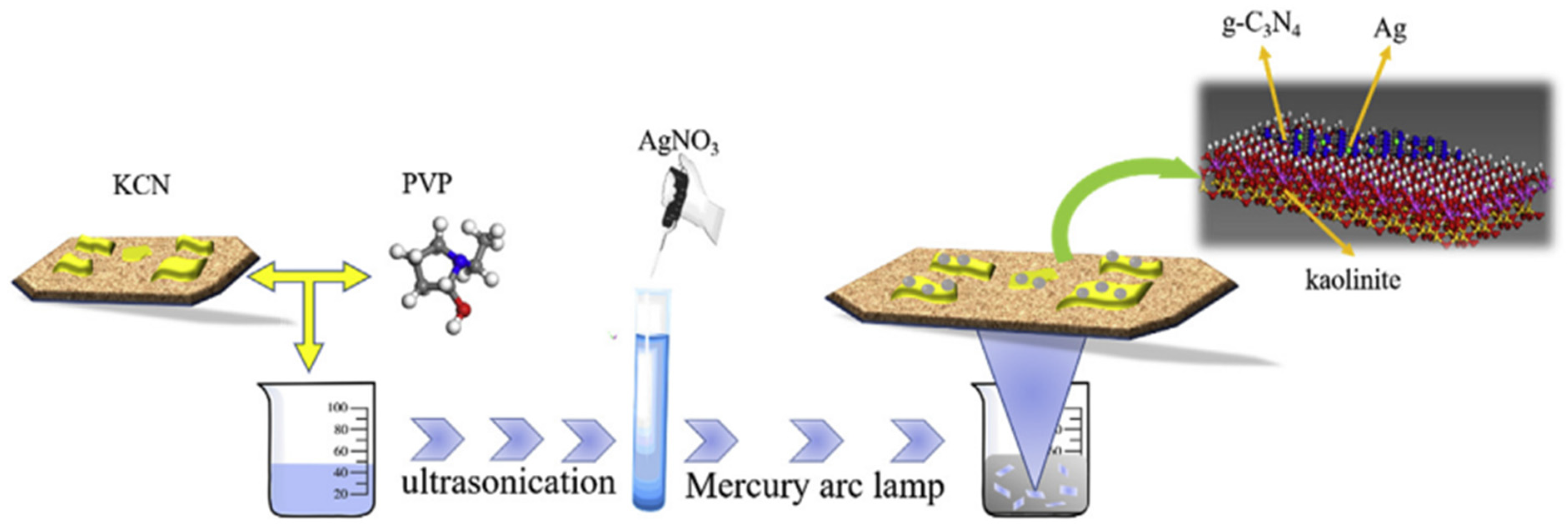
He et al. [16] prepared g-C3N4 powder from dicyanodiamine. Sixteen grams of dicyanodiamine was heated at the rate of 2.5 °C up to 600 °C and it was maintained for 4 h. After that, the heated powder was cooled and ground for further use. Wang et al. [17] also reported the preparation of g-C3N4 from dicyandiamide as the precursor. Sun et al. [18] produced bulk g-C3N4 from dicyandiamide as the precursor. To the prepared g-C3N4, kaolinite was loaded via the impregnation calcination process and the composite thus produced was named as g-C3N4/kaolinite (KCN). To it, Ag was loaded to form the ternary composite Ag/g-C3N4/kaolinite composite. The preparation procedure is illustrated in Figure 2.
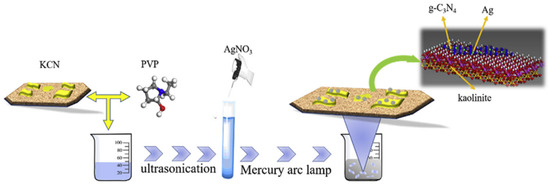
Figure 2.
Scheme of the synthetic process for the Ag/KCN-X photocatalysts [18].
2.2. Composite with Other Materials
Often g-C3N4 is utilized in making composite catalysts with other suitable materials. ZnO is an excellent semiconductor photocatalyst material and has been proven to be an efficient catalyst for degrading different categories of PCs. Its bandgap is 3.2 eV and there is a huge possibility of electron–hole recombination. Along with this, its optical corrosion is another major hindrance behind its usage for real field purposes. In this regard, composite with g-C3N4 offers a sustainable efficient photocatalyst with higher activity. Feyzi et al. [19] prepared ZnO/g-C3N4/zeolite P supported photocatalyst for photodegradation of the tetracycline (TC) molecule. The composite catalyst was synthesized in three steps. Firstly, ZnO was prepared by the sol–gel method. Then g-C3N4 was synthesized at a large scale by means of pyrolysis of urea. In the final step, the as-synthesized ZnO and g-C3N4 were mixed and added to zeolite for the production of the composite catalyst. Mirzaei et al. [20] synthesized a ZnO@g-C3N4 catalyst for the mineralization of a sulfamethoxazole (SMX) drug. Baladi et al. [21] reported the preparation and application of g-C3N4-CoFe2O4-ZnO photocatalyst for the degradation of the penicillin G antibiotic compound. Chen et al. [22] applied Ag-AgVO3/g-C3N4 photocatalyst for the degradation of TC antibiotic. g-C3N4/TiO2/CFs composite was synthesized by Guo et al. for TC elimination from wastewater [23]. Firstly, different quantity of oxamide and 10 g of urea were blended mechanically. After that, the mixture was subjected to calcination in a muffle furnace at 550 °C to produce g-C3N4. In order to synthesize the g-C3N4/TiO2 composite, the as-prepared g-C3N4 was dissolved in a previously prepared Ti(SO4)2 solution and the whole setup was subjected to ultra-sonification. The whole process was repeated in the presence of carbon fibers (CFs) to prepare the composite C3N4/TiO2/CFs. Kumar et al. [24] deployed BiOCl/g-C3N4/Cu2O/Fe3O4 ternary composite photocatalyst for oxidative degradation of SMX from wastewater.
2.3. Doping of g-C3N4-Based Catalysts
Doping and co-doping with various metals and metal oxides enhanced the catalytic activity of g-C3N4-based materials. Wang et al. [25] investigated the co-doping of bimetallic oxides and the effect of oxygen on the catalytic degradation performance of g-C3N4 on SMX removal. Liang et al. [26] prepared Ba-atom-embedded g-C3N4-based catalyst for the degradation of carbamazepine (CBZ) and DC drug molecules. The Ba atom got anchored onto the surface of the g-C3N4 by means of forming an ionic bond with the triazine ring. The catalyst was prepared by the thermal polymerization method. In this method, 2.52 g melamine was mixed with 60 mL of ethylene glycol. This solution was kept in the temperature range of 58–62 °C and was named as solution A. On the other hand, 28% 5 mL HNO3 was prepared and named as solution B, and 40 mL of Ba(NO3)2 (in the range of 10–25 mmol) solution as solution C. These three solutions (A, B, and C) were mixed to form a white hydrogel and allowed to stand for 1 h. Then it was filtered and washed thoroughly with ethanol. The solid residue was heated at 550 °C to produce the Ba-embedded g-C3N4 catalyst. Tian et al. [27] prepared a Se-doped g-C3N4 novel catalyst for SMX detoxification. Due to Se doping, nitrogen vacancy is created in the catalyst matrix to modulate the electron distribution of g-C3N4. Additionally, it also helped in exfoliation and creating a large specific surface area of the composite catalyst due to the large radius of the Se atom. Guo et al. [14] reported the preparation and application of a Cl-doped novel porous g-C3N4 nanosheet photocatalyst for TC degradation under the irradiation of visible light. For doping purposes, different amounts of Cl were added, and the composite was named accordingly as CN-Cl-0.1, CN-Cl-0.3, CN-Cl-0.5, etc.
3. Different Categories of g-C3N4-Based Catalysts Reported in the Literature for PC Degradation
It has been already mentioned in the previous section that although g-C3N4 is a promising photocatalytic material, several drawbacks hinder its application. Therefore, researchers look forward to constructing different types of composite materials for enhanced photocatalytic activity. The main purpose of developing a novel g-C3N4-based photocatalyst is to reduce the bandgap and prevent the electron–hole recombination. For this purpose, photocatalysts having Z-scheme and S-scheme heterostructures are synthesized. Most of the g-C3N4-based catalysts have been found to be efficient under visible light irradiation. However, in some cases, the inclusion of some external agents, such as H2O2, peroxymonosulfate (PMS), and ultrasound assistance, facilitates the degradation process.
3.1. g-C3N4-Based Z-Scheme Photocatalysts
Z-scheme photocatalysts can be categorized into traditional Z-scheme, direct Z-scheme, and all-solid-state Z-scheme photocatalysts. Traditional Z-scheme photocatalysts were first introduced by Bard in 1979 [28]. It resembles the photosynthetic activity performed by green plants. This type of photocatalysts consists of two semiconductors with suitable intermediate couples. The two semiconductors used in this system have staggered band structure configurations. However, the major disadvantage of this type of catalyst is that it is confined to the solution phase only. Moreover, there is also a possibility of the occurrence of many side reactions.
All-solid-state Z scheme is also known as the indirect Z scheme. The idea of the all-solid-state Z scheme started in the year of 2006. Tada et al. [29] synthesized CdS-Au-TiO2 ternary composite photocatalyst. In this composite, Au acts as the electron mediator. Noble metals, such as Ag, Au, and Cu nanoparticles, are often explored as the electron mediator. Other than these, carbon quantum dots, graphene, and carbon nanotubes are also used as the electron mediator. As the solid conductor is used in all-solid-state Z scheme, it can be easily utilized in liquid as well as in gas.
However, in all-solid-state Z-scheme-based catalysts, charge transfer is solely based on the conductor. To improve the situation, a direct Z scheme was proposed. In the direct Z-scheme mechanism, no intermediate redox couples exist. Many recent studies on the application of g-C3N4-based catalysts are developed based on a direct Z-scheme mechanism. The photodegradation of TC by applying 2D/2D MnIn2S2/g-C3N4 composite is based on the direct Z-scheme mechanism [30]. Photodegradation of DC via S, B-co-doped g-C3N4 nanotube@MnO2 catalyst also proceeded via a direct Z-scheme mechanism [3]. The main reactive species involved in the degradation mechanism were h+, O2·−, and SO4·−. Moreover, the catalyst showed excellent stability up to 10 cycles. Ghosh and Pal [8], in their recent work, reported the synthesis and application of composite formed by g-C3N4 nanosheet, tungsten oxide hydrate nanoplates, and carbon quantum dots towards TC degradation under visible light exposure. It worked on the principle of all-solid-state Z schemes, and the degradation proceeded with a rate constant of 0.044 min−1.
3.2. g-C3N4-Based S Scheme Photocatalysts
S scheme is the other name of the step scheme photocatalyst. An S scheme photocatalyst comprises an oxidation photocatalyst and a reduction photocatalyst with a staggered band structure. Its band structure is similar to that of the type II heterojunction but a completely different charge transfer route. Pham et al. [31] prepared S scheme α-Fe2O3/g-C3N4 nanocomposites as an efficient photocatalyst for the degradation of model antibiotic compounds, such as amoxicillin (AMX) and cefalexin (CFX). Ni et al. [32] prepared a novel g-C3N4/TiO2 catalyst for TCH degradation under UV light irradiation. The S-scheme heterostructure facilitated the formation of ·O2−, h+, and OH· reactive species which ultimately helped in degradation performance. Feyzi et al. [19] reported the application of S-scheme ZnO/g-C3N4/zeolite P supported catalyst for the degradation of TC molecule. The ternary composite catalyst was loaded on a plasma reactor for degradation purposes. Under optimized reaction conditions, 95.5% degradation efficiency was achieved. Guo et al. [23] synthesized an S-scheme-based novel g-C3N4/TiO2/CFs catalyst for the photocatalytic degradation of TCH. Under 350 W Xe light irradiation, 99.9% degradation was achieved within 90 min.
3.3. g-C3N4-Based Fenton-Type Catalysts
Fenton-type reactions are one of the powerful techniques for the degradation of organic pollutants. It deploys the generation of powerful hydroxyl radicals from H2O2 in the presence of Fe2+ as the catalyst. However, there exists a lot of technical problems with the homogeneous Fenton process and therefore, the scientists are continuously developing novel heterogeneous Fenton catalysts for degradation purposes. There are several reported studies on the application of heterogeneous Fenton catalysts for PC wastewater degradation. It is very important to note that g-C3N4 undoubtedly added a new dimension for synthesizing heterogeneous Fenton-type catalysts. In the traditional Fenton process, iron was used as the catalyst, and H2O2 was the oxidant. However, apart from iron, different other transition metals have also been explored as Fenton-type catalysts. Moreover, PMS has replaced H2O2 in many studies. Moreover, visible light irradiation also often enhances degradation and is known as photo-Fenton degradation. This section describes different Fenton, Fenton types, and PMS-mediated g-C3N4-based catalysts for PC degradation.
Zhang et al. [33] reported the application of MnO2/Mn-modified alkalinized g-C3N4 catalyst for TC degradation through the photo-Fenton process. Excellent degradation (96.7%) occurred due to the synergistic effect of the surface-grafted hydroxyl groups, charge transfer via the Z-scheme mechanism, and activation of H2O2 by the redox cycle of Mn(IV)/Mn(III)/Mn(II). He et al. [16] utilized g-C3N4/Fe3O4@MIL-100(Fe) composite for photo-Fenton detoxification of CIP from wastewater. In comparison to the bare g-C3N4 and Fe3O4@MIL-100(Fe), the composite catalyst showed promising performance, exhibiting 94.7% degradation of CIP and having an initial concentration of 200 mg/L in 120 min.
Mei et al. [34] explored a metal-free carboxyl-modified g-C3N4 catalyst for PMS activation in order to degrade model PC, CBZ. As the process did not involve any strong acids or solvents, it has been described as a green low-cost environmentally friendly system. Luo et al. [35] reported the successful application of a Co-MOF-based/g-C3N4 catalyst for degrading antidepressant PC venlafaxine in the presence of PMS. Wang and Wang [36] studied the degradation process of SMX in the presence of γ-Fe2O3/O-g-C3N4/biochar composite via PMS activation. SMX eradication followed a first-order kinetic model with a rate constant value of 0.153 min−1. Wang et al. [25] utilized a Fe-Co-O-co-doped g-C3N4 catalyst for PMS activation in order to degrade SMX molecules. Detailed experimental investigation revealed that both sulfate radical and singlet oxygen were present in the reaction mixture. However, the role of singlet oxygen in the SMX removal process was not clear. Superb degradation efficiency was attributed to the existence of the synergism between the metal oxide and O-g-C3N4.
Liu et al. [37] developed a novel Z-scheme Fe-g-C3N4/Bi2WO6 heterogeneous photo-Fenton catalyst for TC degradation purposes. Experimental investigation revealed that 1O2 and ·O2− were the predominant species participating in the degradation phenomenon. Li et al. [38] prepared a g-C3N4/MgO composite which acted as a Fenton-type catalyst for the oxidation of the SMX drug present in wastewater. The composite catalyst showed excellent performance towards the degradation. The H2O2 requirement was also less for oxidative degradation.
Li and Gan [39] applied Cu-doped g-C3N4 composite as the heterogeneous photo-Fenton-type catalyst for the degradation of different PCs at a very low concentration. Due to the doping, the bandgap got reduced from 2.79 eV to 2.17 eV, which proved beneficial for adsorbing visible sunlight for degradation purposes. Cao et al. [40] synthesized novel Fe/g-C3N4/kaolinite as the heterogeneous photo-Fenton catalyst for TCH degradation purposes. High degradation efficiency was achieved due to the large specific surface area of the kaolinite, which can result in the adsorption of the TCH molecule. Moreover, Fe(III) acted as the electron acceptor in the composite matrix, restricted the electron–hole recombination rate, and facilitated the photo-Fenton process. g-C3N4 nanosheets/schwertmannite nanocomposites were explored by Qiao et al. [41] for chlortetracycline eradication from wastewater through the photo-Fenton mechanism.
3.4. g-C3N4-Based Sonocatalysts for PCs Degradation
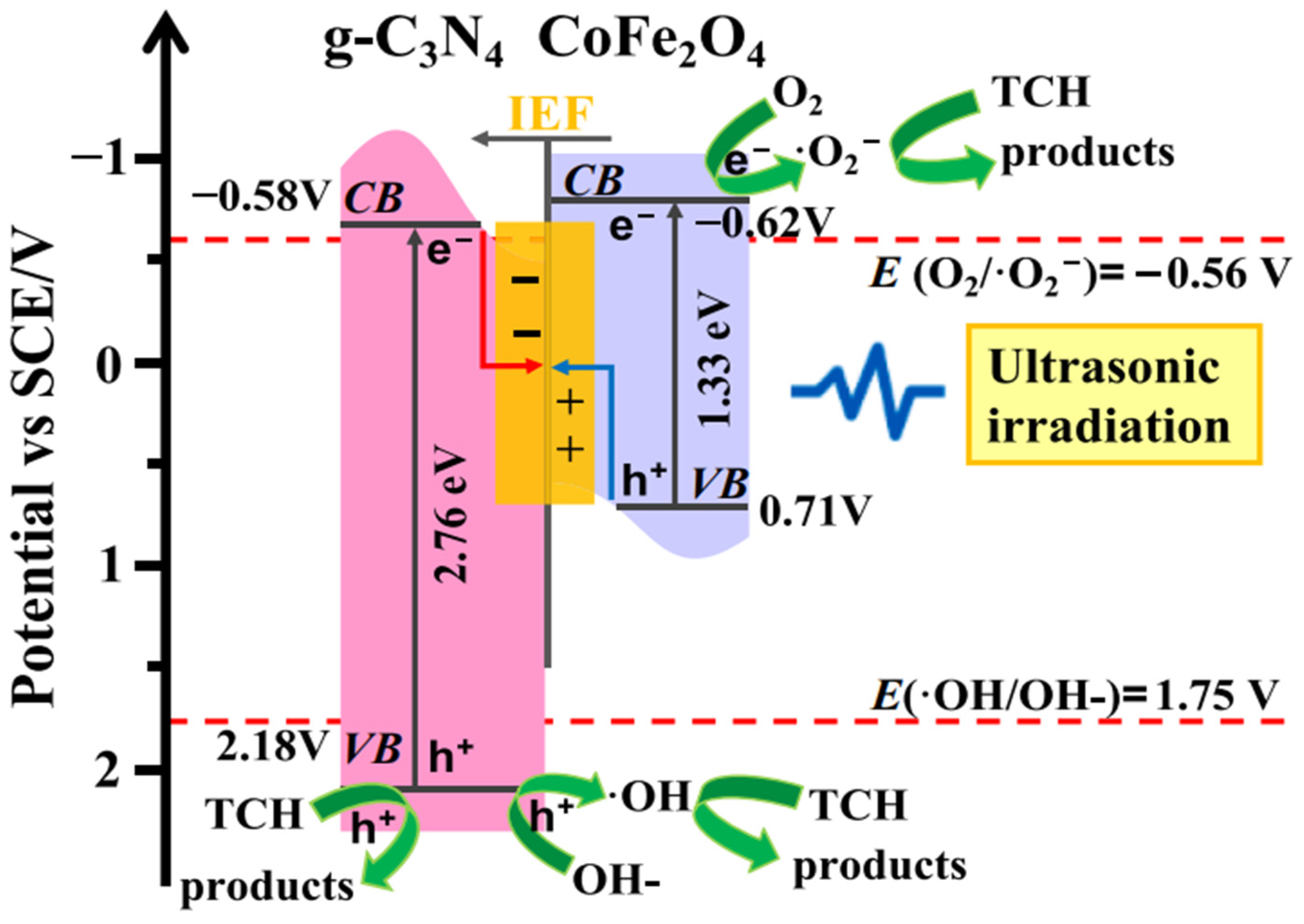
Ultrasound is often deployed for the destruction of stable organic molecules in wastewater streams. Experimentally it has been proved that sound waves having a frequency greater than 20 kHz have the capability of breaking organic pollutants of higher molecular weight into simpler products. Some g-C3N4-based sonocatalysts have also been reported in the literature related to the degradation of PCs. Zhang et al. [42] utilized CoFe2O4/g-C3N4 composite as the sonocatalyst for the degradation of the TCH molecule. Maximum sonocatalytic efficiency of 26.71% in 10 min was exhibited by the composite catalyst when the amount of CoFe2O4 in the catalyst matrix was 25%. Charge transfer and electron–hole separation mechanism proceeded via S scheme heterojunction. The mechanism is shown in Figure 3.
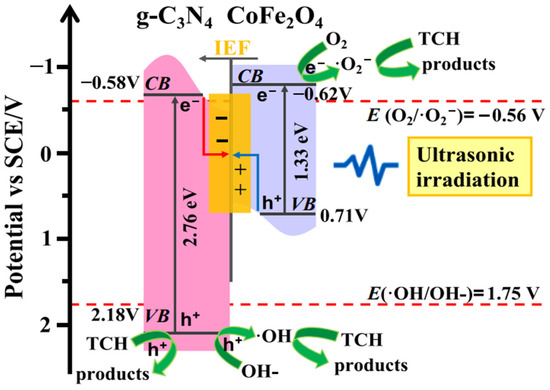
Figure 3.
Proposed mechanism for the sonocatalytic degradation of TCH with CFO/CN [42].
He et al. [43] prepared g-C3N4/MoS2 catalyst for levofloxacin (LFX) oxidation through a sonocatalytic mechanism. The as-prepared catalyst showed excellent degradation efficiency towards LFX (75.81%) with an initial concentration of 10 mg/L in 140 min with promising reusability. Experimental investigation revealed that both ·OH and ·O2− played a major role in the degradation process. The authors validated the sonocatalytic degradation via hotspot and sonoluminescence effect theory.
Vinesh et al. [44] described the application of r-GO supported g-C3N4 nanosheet catalyst for sonophotocatalytic degradation of TC. Influence of ultrasound generated more active sites in the catalyst, which ultimately improved PC degradation. For a TC sample having a 15 mg/L initial concentration, almost 90% degradation was achieved within 60 min of reaction under sonophotocatalysis. Gholami et al. [45] applied Zn-Cu-Mg mixed metal hydroxide/g-C3N4 composite as the effective sonophotocatalyst for the degradation of sulfadiazine (SDZ) drug from wastewater. When the content of mixed metal hydroxide remained at 15 wt%, maximum degradation efficiency of 93% was attained with an initial concentration of SDZ as 0.15 mM, solution pH 6.5, and ultrasonication power of 300 W.
4. Degradation of Different PCs
4.1. Tetracycline (TC)
TC is one of the most common drugs, often explored to test the catalytic efficiency of a newly developed catalyst. It is a broad-spectrum antibiotic. Its major functional groups include phenolic hydroxyl group, dimethylamino group, acylamino group, etc. Owing to the possession of both electron-rich and electron-deficit groups, it has three dissociation constants [1]. TC, OTC (oxytetracycline), and TCH (tetracycline hydrochloride) all belong to the TC group of drugs. Chen et al. [22] explored Ag-AgVO3/g-C3N4 photocatalyst for the degradation of TC in aqueous media. The photocatalytic activity of the composite catalyst was two times higher than that of the pristine g-C3N4. A highly promising MnIn2S4/g-C3N4 photocatalyst was developed by Chen et al. [30] and successively applied for TCH degradation. The composite catalyst exhibited higher degradation efficiency in comparison to the MnIn2S4 nanoflakes and mesoporous g-C3N4 nanosheets. The enhanced removal occurred due to the formation of the Z scheme which results in the transfer and effective separation of the photogenerated charge carriers. Chi et al. [13] reported the exploration of B/Na-co-doped g-C3N4 photocatalyst for the degradation of TC. The synergistic effect between the B/Na co-doping and the porous g-C3N4 nanosheet played a major role in the degradation process. Within a reaction time of 30 min, under visible light irradiation (λ = 430 nm), 78.39% degradation was achieved. Cl-doped g-C3N4-based catalysts were explored by Guo et al. [14] for TC degradation purposes. The optimized removal efficiency of 92% within 120 min of reaction time has been achieved. Due to the Cl doping, the electronic structure of g-C3N4 material was regulated. Moreover, because of the Cl doping, the specific surface area of the composite got increased and the recombination of hole–electrons on the catalyst surface was prevented. Thus ultimately, TC degradation was improved in comparison to the bulk g-C3N4 catalyst.
Jiang et al. [46] developed a nitrogen self-doped g-C3N4 nanosheet catalyst following a self-doping and thermal exfoliation procedure. Thus, the newly developed nitrogen-doped g-C3N4 photocatalyst showed enhanced visible light absorption, high specific surface area, and improved electron–hole separation in comparison to the bulk g-C3N4. Hence, it showed promising efficiency towards TC degradation. The authors proposed an interesting mechanism behind the photocatalytic degradation of TC. The whole degradation process progressed via three steps, such as light harvesting, photogenerated electron–hole pairs separation and transfer, and surface adsorption and redox reaction. Moreover, the catalyst showed excellent repeatability and only a negligible efficiency is lost during the fifth cycle of reuse.
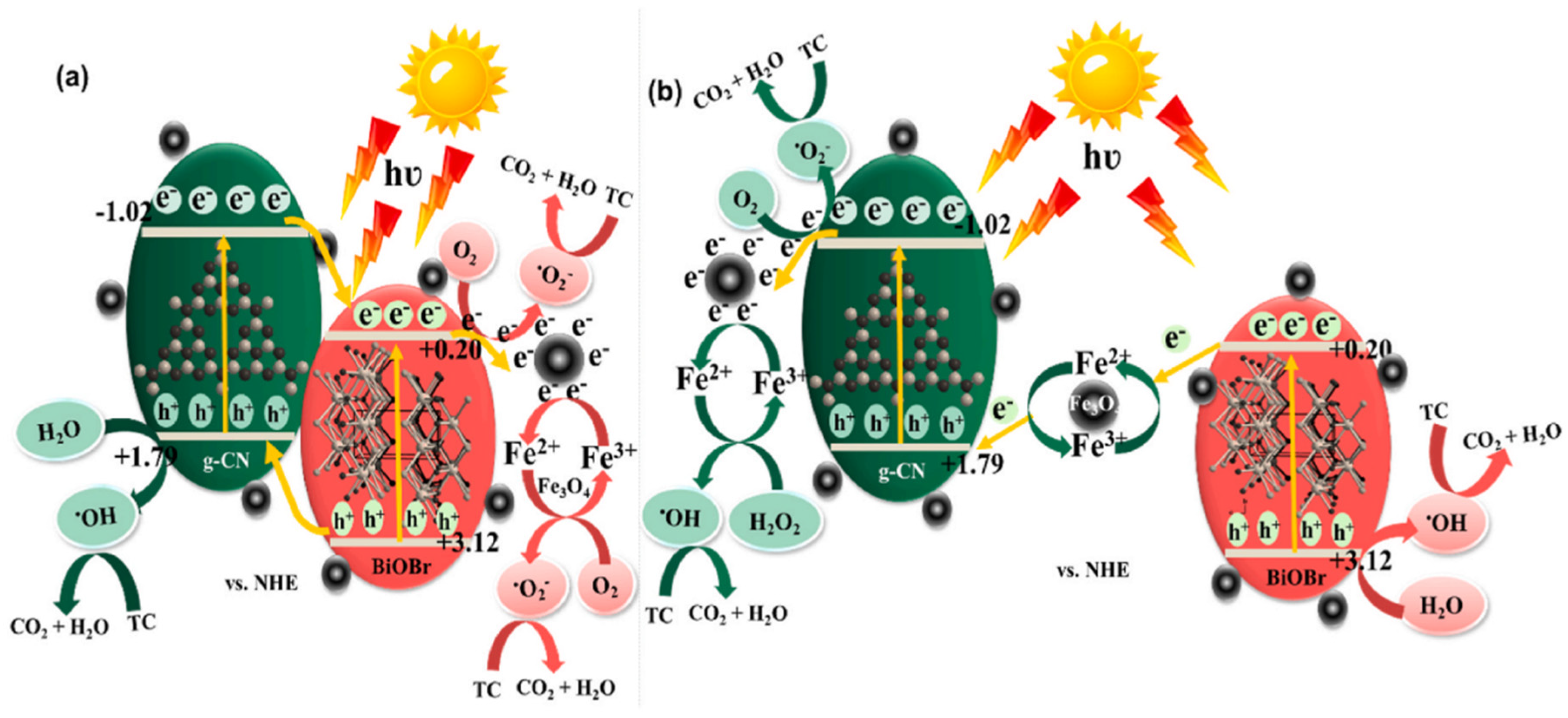
Palanivel et al. [47] reported the synthesis of a novel NiFe2O4-deposited S-doped g-C3N4 nanorod catalyst and deployed it for TC degradation through a photo-Fenton mechanism. Strong chemical interaction between the NiFe2O4 and sulfur-doped g-C3N4 helps in efficient visible light absorption, and electron–hole recombination is also prevented effectively. Preeyangha et al. [48] prepared g-C3N4/BiOBr/Fe3O4 nanocomposite photocatalyst for the degradation of TC under the irradiation of visible light. Complete degradation and 78% TOC removal were achieved within 60 min, where the reaction rate constant was found six times higher than that obtained with the bare g-C3N4 catalyst. Based on the radical scavenging investigations, it was observed that h+ has a major role in the degradation followed by ·O2− and OH·. Besides that, the ternary composite showed excellent recyclability also. The possible reaction mechanism is shown in Figure 4.
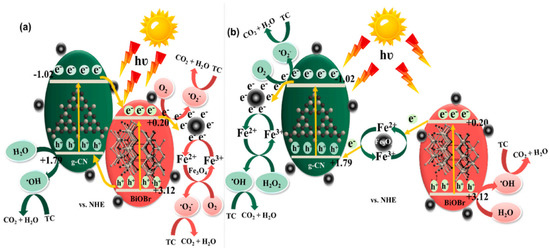
Figure 4.
The schematic representation of plausible charge transfer mechanism (a) type II and (b) Z-scheme heterojunction during the photocatalytic TC degradation over g-CN/BiOBr/Fe3O4 nanocomposites [48].
Various reported g-C3N4-based catalysts for TC removal are presented in Table 1.

Table 1.
A list of g-C3N4-based catalysts reported for TC degradation.
4.2. Diclofenac (DC)
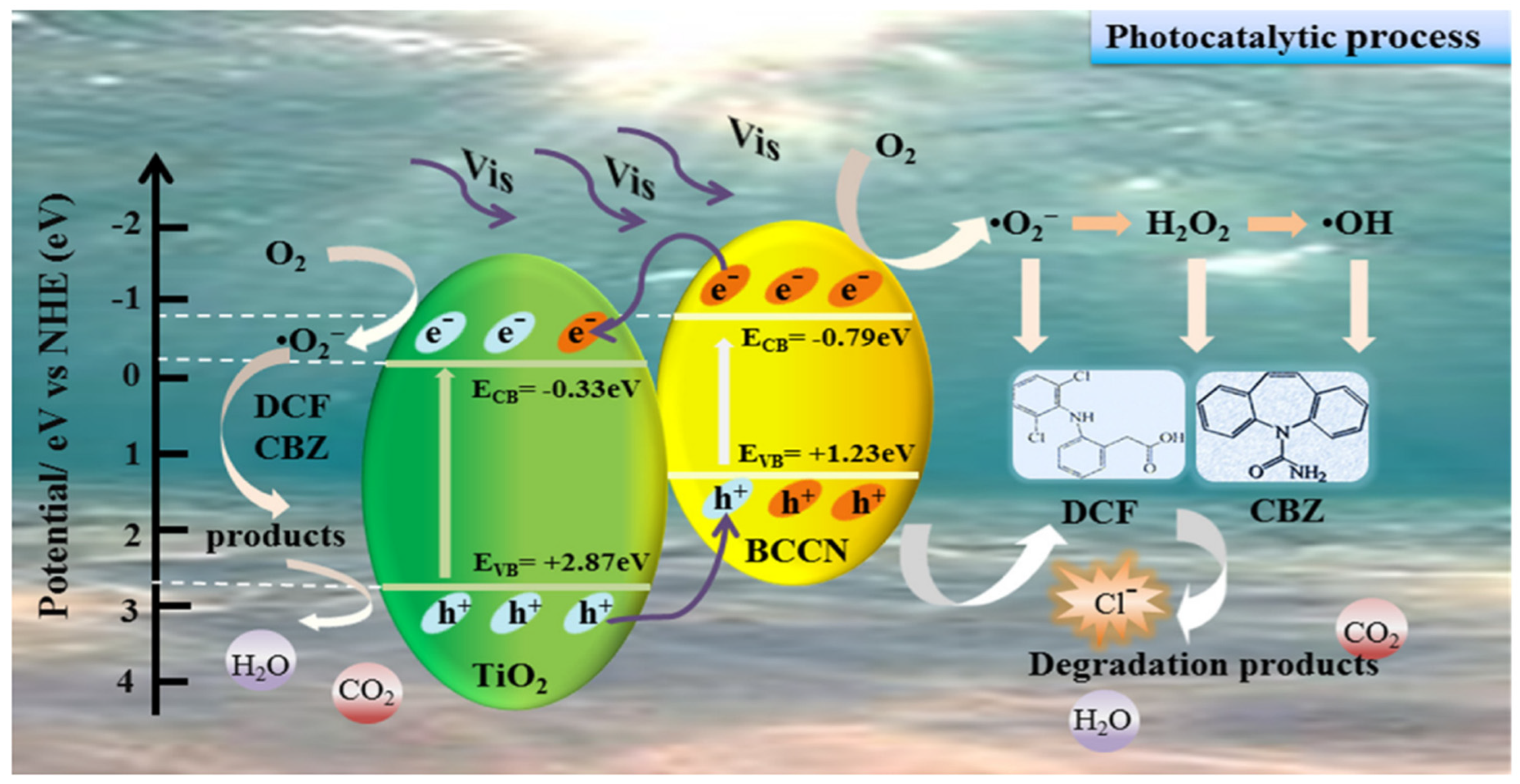
DC is also another drug like TC which is also investigated by researchers for degradation purposes. It is one of the notable members of the nonsteroidal anti-inflammatory drug group possessing high Kow and bioaccumulating potential power. Due to its wide unrestricted usage during the last four decades, it has become one of the significant emerging contaminants of the current era. Various reports are available in the literature describing the applicability of g-C3N4-based photocatalysts towards sustainable eradication of DC from wastewater. Li et al. [78] applied Fe oxide nanoclusters supported on g-C3N4 as a robust photocatalyst for DC degradation purposes. He et al. [4] prepared a heterostructure of Ti3C2/g-C3N4 photocatalyst for the degradation of DC. Polymeric g-C3N4 catalyst was explored by Papamichail et al. [79] for degradation of DC. Quantum carbon dots modified reduced ultrathin g-C3N4 photocatalyst was designed and applied by Jin et al. [80] for the oxidative degradation of DC from wastewater. Complete degradation of DC took place within 6 min. Hu et al. [81] deployed g-C3N4/TiO2 photocatalyst for the degradation of DC and CBM. The mechanism is shown in Figure 5.
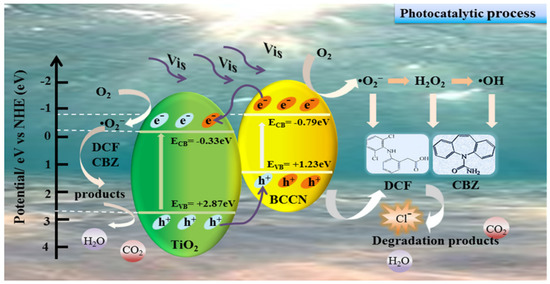
Figure 5.
Possible mechanism for the photodegradation of DCF and CBZ under LED lamp irradiation over 30% BCCNT composites [81].
A comprehensive list regarding the application of different g-C3N4-based photocatalysts for the degradation of DC is provided in Table 2.

Table 2.
A list of g-C3N4-based catalysts reported for DC degradation.
4.3. Sulfamethoxazole (SMX)
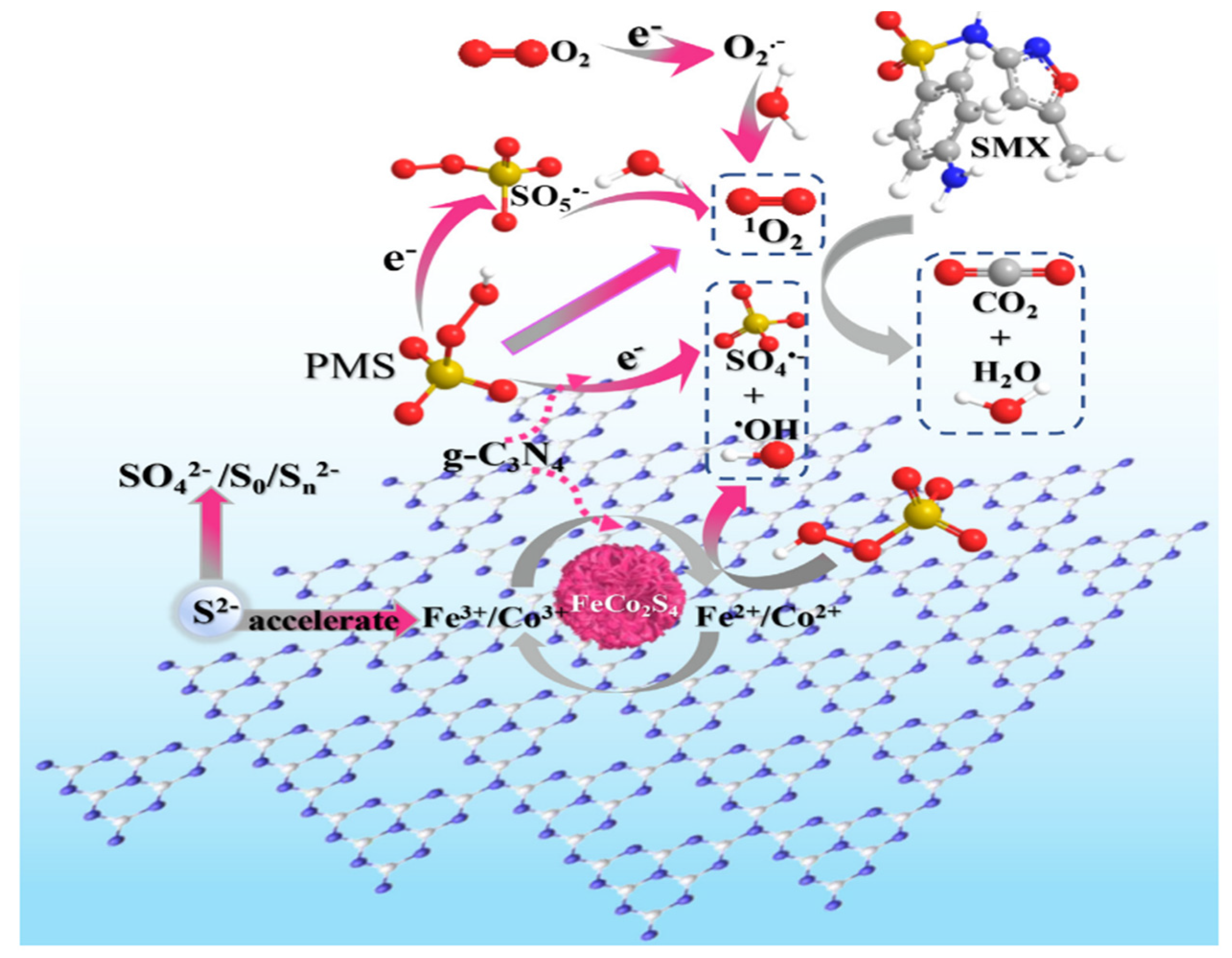
SMX is another widely used sulfonamide antibiotic. Due to its overuse, it often comes across in different quantities in different water bodies. In recent years, several studies report the catalytic elimination of sulfamethoxazole from water bodies by deploying g-C3N4-based composite catalysts. ZnIn2S4/g-C3N4 photocatalyst was utilized by Reddy et al. [87] to catalytically degrade SMX in a water medium. Tian et al. [27] fabricated a Se-doped g-C3N4 catalyst for the degradation of SMX through PMS activation. Experimental findings showed that 93% SMX can be degraded by the synthesized catalyst within 180 min with a reaction rate constant of 0.0149 min−1. The rate constant of the doped catalyst was four times higher in comparison to the bulk g-C3N4 catalyst. The inclusion of Se in the composite matrix created nitrogen vacancy to modulate the electron distribution of the g-C3N4 catalyst. Peng et al. [88] utilized a “trap-zap” catalyst for the degradative elimination of SMX through PMS activation. β-Cyclodextrin polymer composite with Fe-doped g-C3N4 catalyst was used for the degradation. The degradation rate constant by the β-CDPs/Fe-g-C3N4 catalyst was found as 0.132 min−1 which was 14.7 times and 2.2 times higher than that of the g-C3N4 and Fe-g-C3N4 catalyst. The inclusion of β-CDPs in the catalyst composition accelerated the electron transfer between the catalyst and PMS. Li et al. [89] applied a FeCo2S4-modified g-C3N4 catalyst for the degradation of the SMX drug. Optimized degradation efficiency has been obtained at the unadjusted pH of 6.5. On the other hand, acidic pH inhibited the degradation. Temperature played a major role in the degradation process. When the reaction temperature was increased from 10 °C to 40 °C, the degradation efficiency got enhanced from 61.2% to 99.9% with a rate constant value of 0.294 min−1. The schematic for the mechanism is shown in Figure 6.
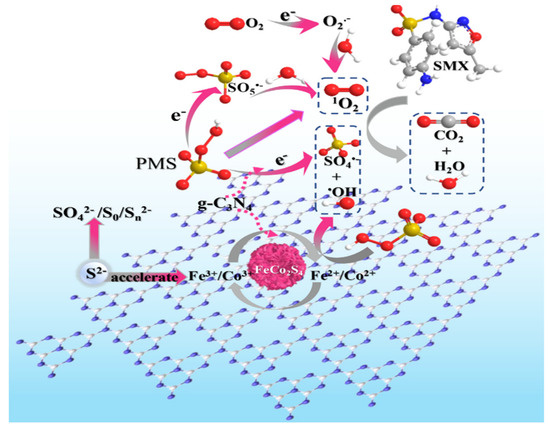
Figure 6.
Schematic illustration of the mechanism of PMS activation on FeCo2S4-CN [89].
A comprehensive list regarding the application of different g-C3N4-based photocatalysts for the degradation of SMX is provided in Table 3.

Table 3.
A list of g-C3N4-based catalysts reported for SMX degradation.
4.4. Ibuprofen (Ibu)
Ibu is also a nonsteroidal anti-inflammatory drug commonly used for pain relief, fever, and inflammation purposes. In recent years, some studies have been performed on using g-C3N4-based catalysts for degrading Ibu from aqueous media. Liu and Tang [97] reported the application of g-C3N4/Bi2WO6/rGO heterostructured nanocomposite for the degradation of Ibu. Ag/g-C3N4/kaolinite composite was applied by Sun et al. [18] for the eradication of Ibu. Mao et al. [98] reported the preparation and application of 1D/2D nanorod FeV3O8/g-C3N4 composite catalyst for the degradation of Ibu. In comparison to the g-C3N4 nanosheets, the catalytic activity of this nanocomposite was nearly four times higher. The authors proposed a Z-scheme mechanism for the composite. Meng et al. [99] utilized layered g-C3N4 and BiOBr photocatalysts for the degradation of Ibu in wastewater. A list is provided in Table 4 regarding the application of various g-C3N4-based catalysts towards catalytic eradication of Ibu from water.

Table 4.
A list of g-C3N4-based catalysts reported for Ibu degradation.
4.5. Other Drugs
Studies have also been conducted on the application of g-C3N4-based catalysts to degrade ciprofloxacin (CIP) from water bodies. Deng et al. [110] reported the preparation and application of Ag-modified phosphorus-doped ultrathin g-C3N4/BiVO4 photocatalyst for the degradation of CIP drug., In the study, more than 92% degradation efficiency was achieved under visible light and near-infrared light irradiation (λ > 420 nm, λ > 760 nm), with an initial concentration of CIP 10 mg/L. Zhang et al. [111] applied Fe3O4/CdS/g-C3N4 composite for the photocatalytic degradation of CIP under visible light irradiation. CdS itself is a photocatalyst. However, the addition of g-C3N4 in the composite improved its optical response and the incorporation of Fe3O4 nanoparticles helped in the easy recovery of the catalyst. Triclosan (TCS) is a famous nonionic broad-spectrum antimicrobial pharmaceutical compound. However, US Food and Drug Administration banned its usage in 2016 due to the health risk associated with it [112]. In recent years, some research groups also explored g-C3N4-based photocatalysts towards triclosan degradation in water. Wang et al. [113] reported the application of g-C3N4/MnFe2O4 catalyst for the degradation of TCS through PMS activation. The as-prepared catalyst showed promising behavior in terms of stability and metal leaching. Dechlorination, hydroxylation, and cyclization along with other bond-breaking mechanisms were attributed to the triclosan degradation purpose. In one of the recent articles, Yu et al. [114] described the highly efficient degradation performance of a novel catalyst g-C3N4/Bi2MoO6 towards TCS drug. TCS was converted to 2-phenoxyphenol under visible light irradiation. In 180 min, 95.5% degradation was achieved, which was 3.6 times higher in comparison to that obtained with pure g-C3N4 catalyst.
Mafa et al. [115] developed a multi-elemental doped g-C3N4 catalyst and tested it towards NPX degradation. Pure g-C3N4 catalyst showed poor performance in terms of degradation efficiency (21.5%). With rare earth metals loading (1%), the composite catalyst showed excellent behavior (92.9% efficiency) towards drug removal. A heterojunction was formed between the rare earth metal and the g-C3N4 surface which provided the defect for facilitating electron–hole separation. The degradation followed the Z-scheme mechanism with visible light absorption, with the participation of superoxide radicals.
Truong et al. [116] utilized ZnFe2O4/BiVO4/g-C3N4 photocatalyst for the efficient removal of lomefloxacin antibiotic degradation. Keeping the amount of ZnFe2O4, BiVO4, and g-C3N4 in the ratio 1:8:10, the optimized removal efficiency of 96.1% was obtained after keeping the set-up illuminated for 105 min.
Like the above-mentioned drugs, AMX is another commonly used drug which often occurs in the ecosystem. Mirzaei et al. [117] prepared a magnetic fluorinated mesoporous g-C3N4 catalyst for the AMX elimination purpose. Fluorination of the catalyst material provides a facilitating condition for the catalysis by even distribution in the aqueous medium. Due to the inclusion of the iron nanoparticles, the removal efficiency further got enhanced due to the formation of the heterostructure. However, on increasing the iron content, the catalytic efficiency got diminished due to the fact, that the nanoparticles covered the active sites. A list of g-C3N4-based catalysts for degrading other PCs is provided in Table 5.

Table 5.
A list of g-C3N4-based catalysts reported for other PC degradation.
4.6. Application on Multiple Compounds
In some studies, prepared g-C3N4-based catalysts have been applied on more than one PC rather than one compound. Dai et al. [123] applied surface hydroxylated g-C3N4 nanofibers for the catalytic degradation of TCH, DC, and metaprolol (MT). Within 60 min of reaction time, 97.3%, 88.9%, and 63.2% degradation of TC, DC, and MT, respectively, was achieved. Barium-embedded g-C3N4 was tested against CBM and DC degradation [26]. Thang et al. [124] prepared Ag/g-C3N4/ZnO nanorods photocatalyst and assigned it for the degradation of commercial drugs, such as paracetamol (PR), cefalexin (CF), and AMX. By applying only a catalyst dose of 0.08 g/L, the degradation of a target compound having a concentration of 40 mg/L was possible. Di et al. [2] applied g-C3N4/ZnFeMMO composites for the photocatalytic degradation of Ibu and sulfadiazine (SDZ) drugs. The Z-scheme mechanism was found to be appropriate for the overall process. The degradation of Ibu proceeded via h+ generation in the process while OH· was responsible for the elimination of SDZ. A list is presented in Table 6, regarding the application of g-C3N4-based catalysts for multiple PC degradation.

Table 6.
A list of g-C3N4-based catalysts reported for multiple PC degradation.
5. Optimization Techniques
Optimization of the photocatalyst is one of the pertinent areas for proper resource utilization. In the present era, multiparameter optimization has gained more predominance in comparison to single-parameter optimization. Response surface methodology (RSM) and artificial neural network (ANN) are commonly used for this purpose. Mirzaei et al. [20] used the RSM technique for the photodegradation of SMX by taking catalyst dose, solution pH, and airflow rate as the variables. The authors used central composite design (CCD) for RSM analysis and the catalyst dose, pH, and airflow rate were varied in the range of 0.4–0.8 g/L, 3–11, and 0.5–2.5 L/min, respectively. Twenty experiments were run for the purpose and the experimental values showed a good correlation with the predicted values (R2 = 0.9802). The optimum condition was found as 0.65 g/L of photocatalyst dose, pH of 5.6, and airflow rate of 1.89 L/min and under this condition, the removal efficiency achieved was 94%. Shanavas et al. [126] conducted an optimization study regarding the application of rGO-gC3N4-based catalysts for TC and CIP degradation. The reaction time, reaction temperature, and molar concentration were chosen as the variables for the purpose.
John et al. [82] explored RSM for the optimization of DC degradation. Four variables, namely, irradiation time, initial solution pH, initial DC concentration, and g-C3N4 loading, were chosen for the study. From the analysis, it was found that the optimum reaction conditions were obtained as irradiation time = 90 min, initial solution pH = 5, initial DC concentration = 5 ppm, and g-C3N4 loading = 0.3 g/g TiO2, and the maximum removal efficiency achieved was 93.49%.
Quarajehdaghi et al. [127] optimized photocatalytic degradation of CIP by application of CdS/g-C3N4/rGO/CMC catalyst using RSM and ANN approach. Four parameters were chosen for the RSM study, such as initial concentration of CIP, dose of catalyst, pH, and time of irradiation. Using the CCD model, 30 experiments were run. Predicted removal efficiency shows a good correlation with the experimental values. The quadratic model best fitted the trend with a high R2 value of 0.9827. From the RSM analysis, the condition for the optimized removal efficiency was determined as initial concentration of CIP = 7.89 mg/L, the dose of catalyst = 0.6 g/L, pH = 6.15, and irradiation time = 33.94 min. The predicted optimized removal was 84.25%, while experimentally, the removal was achieved at 81.93%. From the ANN study, the relative importance of different parameters on the degradation efficiency was determined. It was seen that the influence of the dose of catalyst was the highest (34%), followed by pH (30%), irradiation time (26%), and, lastly, initial concentration of CIP (10%). Furthermore, the values obtained from the ANN model were also compared with those obtained from the RSM model as well as with the experimental values. The values found from RSM, ANN, and experimental investigation were quite close to each other.
AttariKhasraghi et al. [128] also used RSM and ANN models for cefoperazone degradation using a zeolite-supported CdS/g-C3N4 catalyst. CCD model was used for the purpose using four variables, such as dose of catalyst, initial concentration of cefoperazone, pH, and time. The degradation percentages predicted by both the models (RSM and ANN) showed a good correlation with the experimentally obtained values. Using RSM analysis, the optimized condition was found as the dose of catalyst = 0.4 g/L, initial concentration of cefaperazone = 17 mg/L, pH = 9, and time = 80 min. The predicted maximum removal efficiency was 95.66%, while actually 93.23% was achieved. It proves the accuracy of the model.
6. Real Field Application
It is pertinent to check the performance of the catalyst towards real wastewater. For utilization of the novel g-C3N4-based catalyst in real wastewater treatment, firstly scaling up the process is mandatory. Plasma reactor is one of the innovative reactors which has the feasibility of being applied in the real field scenario. Feyzi et al. [19] used ZnO/g-C3N4/zeolite-P-supported catalyst in a dielectric barrier discharge plasma reactor for photodegradation of TC.
Kumar et al. [129] reported the application of novel g-C3N4/TiO2/Fe3O4@SiO2 photocatalyst for the degradation of Ibu in sewage. Ibu concentration was kept at 2 mg/L, while the dose of the photocatalyst was maintained at 1 g/L and 2 g/L. With a 1 g/L dose of catalyst, only 13% degradation efficiency was achieved, while with a 2 g/L dose, 92% efficiency was obtained. Nivetha et al. [122] tested the potency of the SnO2/g-C3N4 nanocomposite photocatalyst towards the degradation of real pharmaceutical effluent after being successful towards degrading AMX. Untreated pharmaceutical effluent shows a strong absorbance at 280 nm. However, after the application of the novel photocatalyst, the peak started decreasing with the fading of the solution visible to the naked eye.
Rapti et al. [130] investigated the photocatalytic efficiency of g-C3N4 and 1% MoS2/g-C3N4 catalysts towards 10 psychiatric drugs in hospital wastewater effluent. Experiments were conducted in a stainless-steel lamp reactor of volume 46 L provided with 10 UVA lamps and quartz filters connected to a propylene recirculation tank of volume 55–100 L. The composite catalyst (1% MoS2/g-C3N4) showed higher catalytic performance compared to that obtained with pure g-C3N4 catalyst. The degradation efficiency for every pharmaceutical compound varies in the range of 91–100%. Moreover, the reaction was also conducted in a solar simulator under the irradiation of 500 Wm−2, where the degradation efficiency was maintained in the range of 54–100%. Further, the study was escalated to the pilot wastewater treatment plant (parabolic reactor) established at University Hospital, Ioannina City. The degradation efficiency of the targeted pharmaceutical compounds was evaluated using natural sunlight as the source of irradiation. All the samples were analyzed using solid phase extraction followed by chromatographic measurements. Antonopoulou et al. [131] used a g-C3N4 catalyst under the irradiation of UVA light for the catalytic degradation of amisulpride, a psychiatric drug in distilled water as well as in municipal wastewater. High degradation percentage was maintained in both the distilled water and municipal wastewater matrix. However, a slower reaction rate was observed in the case of the latter due to the complex nature of the real wastewater.
Kumar et al. [132] developed a novel g-C3N4 nanorod catalyst by hydrothermal method. Thus, the prepared activated g-C3N4 catalyst exhibited higher degradation efficiency in comparison to that of the bare g-C3N4 material. The catalytic efficiency was tested towards 17α-ethinylestradiol in real hospital wastewater effluent. Within 45 min of reaction time, high removal percentage was attained.
Jin et al. [80] applied the as-prepared quantum carbon dot modified g-C3N4 photocatalyst for the oxidative degradation of DC in distilled water as well as DC spiked in tap water, wastewater treatment plant effluent, Pearl River water, lake water, and South China Sea water. In comparison to the distilled water, degradation efficiency got reduced in the range of 5–13% for different real water matrices. The reduction in removal efficiency might have caused due to the presence of several that act as light filters and electron trappers. Further investigation on the interference study revealed that certain anions such as chloride, sulfate, and nitrate had minimal adverse effects on the degradation efficiency. However, the presence of HCO3− caused a moderate hindrance to the reaction as it can quench OH· to form less reactive CO32−. In the presence of Cu2+ and Fe3+, removal efficiency got reduced significantly, as it reacted with O2· prior to the degradation of DC.
7. Characterization Techniques
7.1. Fourier Transformed Infrared Spectroscopy (FTIR)
FTIR analysis is a common tool for knowing the functional groups involved in the degradation process. In the work of Kumar et al. [132], strong peaks were noticed around 1515 cm−1 corresponding to the C-N= stretching vibration. The appearances of a double peak at 1276 and 1350 cm−1 are designated to the aromatic C-N stretching. In the photocatalytic degradation study of DC by reduced ultrathin g-C3N4 decorated with quantum carbon dots, FTIR spectra of ultrathin carbon nitride (UCN), reduced ultrathin carbon nitride (RUCN), and carbon quantum dots decorated ultrathin g-C3N4 were recorded [80]. In all the spectra, peaks appeared at 810 cm−1, 1200–1700 cm−1, and 3000–3700 cm−1 corresponding to the bending mode of triazine units, stretching vibration due to C-N heterocycles, and N-H vibrations, respectively.
7.2. Electron Microscopic Analysis
Morphological features of the g-C3N4 catalyst are obtained from electron microscopic analysis, such as scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Mafa et al. [115] conducted both SEM and TEM analyses of the different elements (Ce, Er, Gd, and Sm) doped g-C3N4 catalysts. The pure g-C3N4 catalyst exhibits a tubular structure with small openings. Due to doping, the tubular structure got transformed to flake-like structure. Cerium doping reduces the size of the flake. The SEM image is shown in Figure 7.

Figure 7.
FESEM micrograms of (a) CN, (b) CeCN, (c) ErCN, (d) GdCN, (e) SmCN, (f) 1RECN, (g) 3RECN, and (h) 5RECN photocatalysts [115].
In another study, Palanivel et al. found from the SEM image that the structure of S-doped g-C3N4 resembled to that of the nanorods [47]. On the other hand, NiFe2O4 nanoparticles possessed agglomerating structure. The nanorod structure helped in improving charge carrier mobility and facilitated electron channelization which ultimately made the composite a suitable photocatalyst. In the work of Peng et al. [88], the 3D porous structure of βCDPs/Fe-g-C3N4 catalyst was confirmed by both SEM and TEM analyses.
7.3. BET Surface Area Analysis
BET surface area analysis is important for providing insight into the porosity and surface area of the catalyst material. Mirzaei et al. [117] found the specific surface area of the prepared fluorinated g-C3N4 catalyst as ~243 m2/g. On the other hand, the specific surface area of bare g-C3N4 was found to be around 37.85 m2/g. Enhancement in the specific surface area of the g-C3N4 nanosheets occurred due to the acid-assisted hydrothermal treatment. The pore volume of the bare g-C3N4 and fluorinated catalyst was 0.082 cm3/g and 0.427 cm3/g, respectively. The nitrogen adsorption–desorption isotherm showed a similar trend with the type IV isotherm.
In the study of TC degradation by Ba-doped g-C3N4 catalyst, BET surface area analysis for g-C3N4 and the composite material was performed [49]. Pure g-C3N4 had a specific surface area of 9.98 m2/g. On the other hand, due to the composite formation, it got increased to 11.41 m2/g. Similar results were also found in the case of pore volume measurements (0.068 m3/g for g-C3N4 and 0.073 m3/g for the composite).
7.4. XPS Analysis
The chemical composition of the constructed photocatalyst and the oxidation states of the elements involved are obtained from the XPS analysis. Guo et al. [12] performed the XPS analysis of a 5% Cu3P-ZSO-CN catalyst. The survey spectra revealed the presence of the Zn 2p, Sn 3d, O 1s, P 2p, Cu 3d, C 1s, and N 1s, and no impurities were found in the catalyst. Furthermore, the high-resolution spectra of Zn 2p displayed strong peaks appearing at 1021.5 eV and 1044.8 eV corresponding to Zn 2p3/2 and Zn 2p1/2. In Sn 3d spectrum, peaks appeared at 486.4 eV and 494.7 eV denoting the presence of Sn 3d5/2 and Sn 3d3/2. Chen et al. [102] performed the XPS analysis of TiO2, g-C3N4, and TiO2/g-C3N4 (5% weight) in order to get an idea regarding the surface chemical properties.
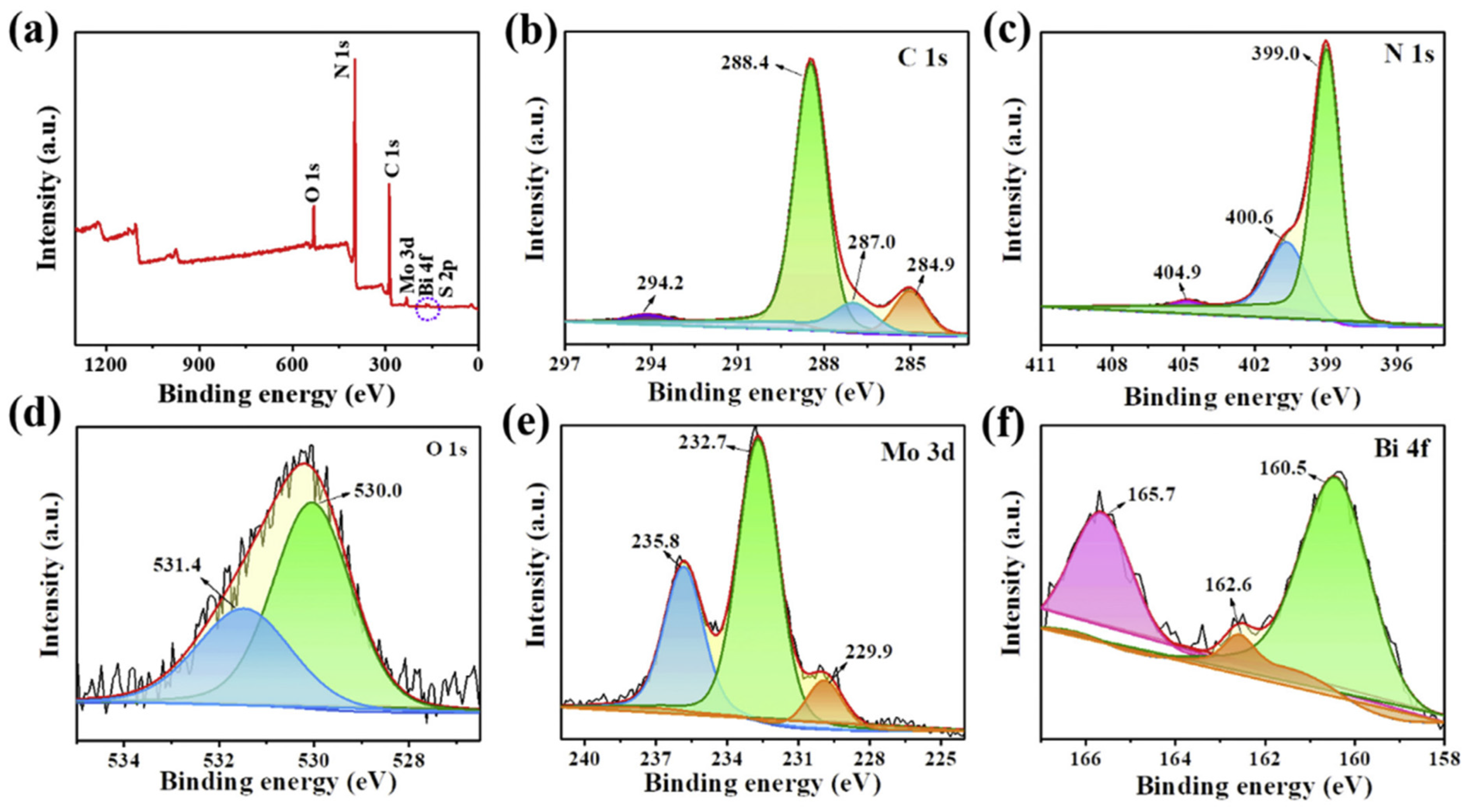
Liang et al. [96] conducted the XPS analysis in order to get a clear idea regarding the elemental composition as well as the surface chemical properties of the composite catalyst. From the survey spectrum, prominent peaks of the elements C, N, O, Mo, and Bi were visible. No signal corresponding to S and W was noticeable, which might be due to their presence in very low concentrations. In the O1s spectrum, peaks corresponding to W-O and Bi-O at 232.7 eV and 229.9 eV were visible. On the other hand, Mo 3d peaks at 232.7 eV and 229.9 eV, corresponding to Mo 3d3/2 and Mo 3d5/2, implying that Mo is in a +4 state, were observed. The XPS spectra are shown in Figure 8.
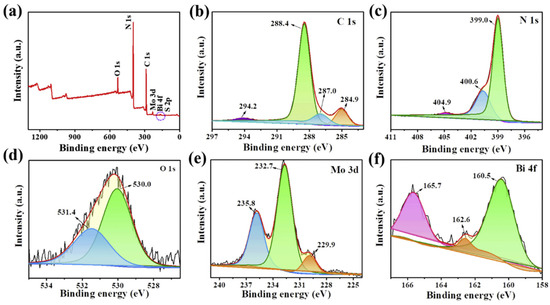
Figure 8.
XPS of (a) survey spectra, (b) C 1s, (c) N 1s, (d) O 1s, (e) Mo 3d, and (f) Bi 4f of CN-BM2 [96].
Kumar et al. [129] conducted an XPS analysis of the g-C3N4/TiO2/Fe3O4@SiO2 (gCTFS) catalyst. The wide-scan spectra indicated that the nano photocatalyst was composed of Fe, Si, Ti, C, N, and O. Among all these elements, the signal corresponding to N was very weak indicating that only a few nitrogen atoms were retained after calcination.
7.5. Diffuse Reflectance Spectra (DRS) Analysis
Optical features of the as-synthesized photocatalysts are often explored by conducting diffuse reflectance spectral (DRS) analysis. Baladi et al. [21] performed the DRS analysis of the pure g-C3N4, ZnO, and the composite prepared from both. The absorption edges for g-C3N4 and ZnO appeared at 445 nm and 405 nm corresponding to the bandgap of 2.78 eV and 3.1 eV. However, after forming the composite, a redshift in comparison to the ZnO appeared indicating a better electron–hole separation efficiency, and an enhanced visible light absorption by the composite photocatalyst. Moreover, the absorption intensity for the composite got increased with respect to the pure g-C3N4 indicating the presence of ZnO in the matrix.
Jin et al. [80] carried out the UV-vis DRS analysis of the as-prepared ultrathin g-C3N4 photocatalysts. Ultrathin g-C3N4 (UCN) showed a strong absorption edge at 460 nm while in the case of reduced UCN, the absorption edge showed a red shift due to the nitrogen defects. Moreover, due to the carbon quantum dot modification, it was shifted to 480 nm which indicates that the incorporation of CQD can enhance the spectral response.
Palanivel et al. [47] found that the absorption of bare g-C3N4 occurred at 467 nm with a bandgap of 2.65 eV. On the other hand, carbon nitride nanorod (CNNR) material shows strong absorption at 452 nm. It happened due to the quantum confinement effect. During the formation of the nanocomposite, its electronic state became discrete and hence a blue shift is observed in comparison to the bulk parent material. Moreover, due to sulfur doping, SCNNR showed a strong absorption at 479 nm with a bandgap of 2.58 eV. Due to the incorporation of the S atom, the band gap is shortened.
7.6. X-ray Diffraction (XRD) Analysis
XRD analysis is often explored by researchers for finding out the size of the photocatalyst material. It is based on the Debye–Scherer equation as follows:
Di et al. [2] performed the XRD analysis of g-C3N4/ZnMMO composite catalyst. In the XRD spectra of pure g-C3N4, two prominent peaks were observed at 13.2° and 27.4°. It refers to the (100) and (002) planes, respectively, which indicates interplanar repeated packing of tri-s-triazine rings and interlayer stacking of graphitic-like structure. In the XRD spectrum of ZnFeMMO, diffraction peaks corresponding to hexagonal wurtzite ZnO and ZnFe2O4 were clearly observed. On the other hand, after the formation of the composite between the two, the peaks got diminished in magnitude indicating a strong interaction between the two.
Kumar et al. [129] carried out the XRD analysis of Fe3O4, Fe3O4@SiO2, g-C3N4 nanosheets, and the composite catalyst with TiO2. In the spectrum of Fe3O4, five peaks at 30.1°, 35.5°, 43.1°, 57°, and 62.6° were observed corresponding to (220), (311), (400), (511), and (440) planes. However, in Fe3O4@SiO2, after forming a composite with SiO2, no characteristic peak due to SiO2 was observed. Finally, in the spectrum of the composite catalyst (g-C3N4/TiO2/Fe3O4@SiO2), peaks were found at 25.4°, 38°, 48°, 53.9°, 55.19°, 62.72°, 69°, 70.25°, and 75.3° corresponding to the TiO2.
7.7. Photoluminescence (PL) Spectroscopy
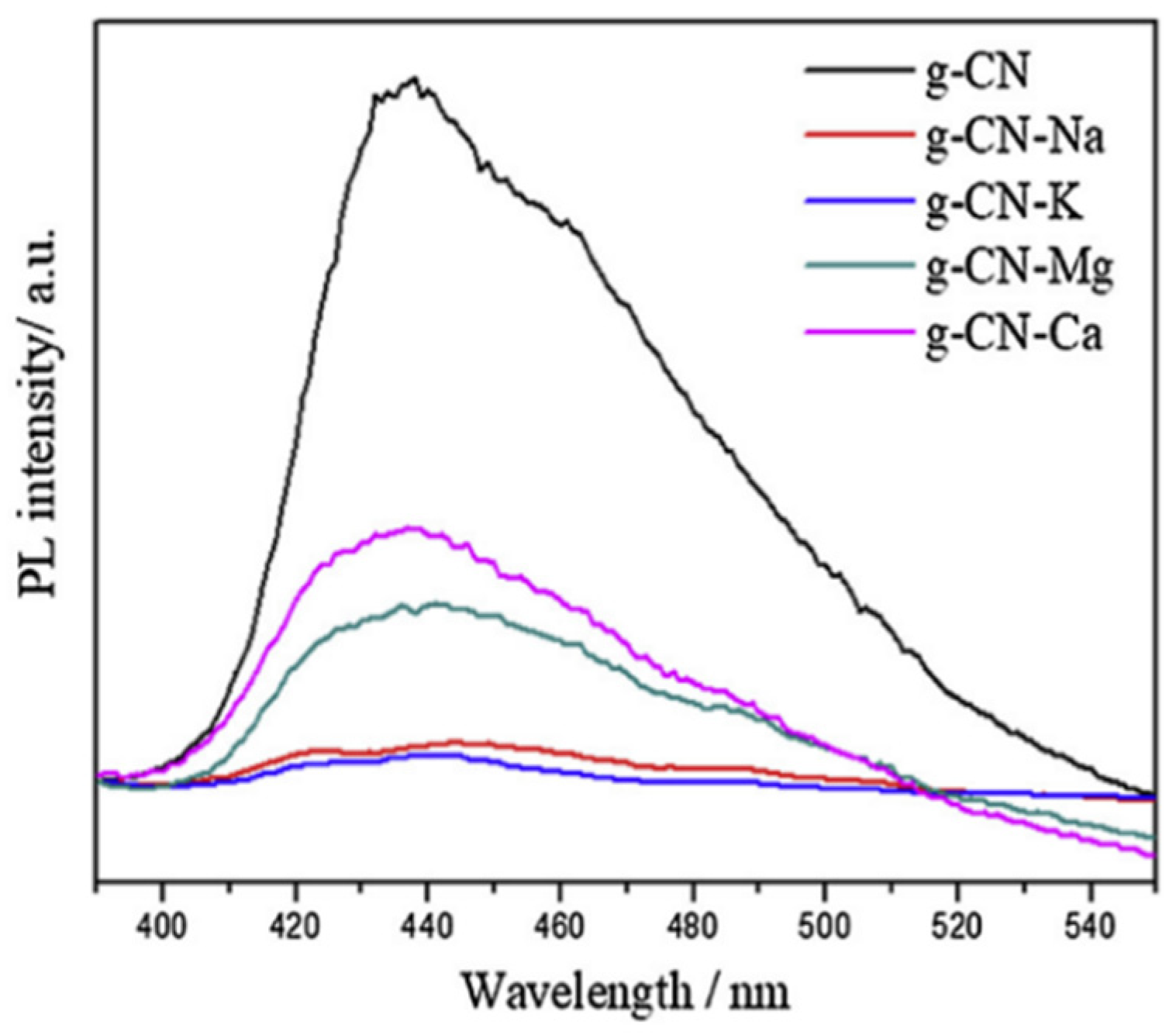
PL spectra are often explored by research groups to have more insight into the photocatalytic property of as-synthesized composite materials. Yan et al. [133] recorded the PL spectra of the pristine g-C3N4 material as well as the metal (Na, K, Ca, Mg) doped g-C3N4 matrix. A strong absorption peak around 440 nm is observed for the pure g-C3N4 while similar spectra with reduced intensity are being observed for the doped materials (as shown in Figure 9). Reduction in the PL intensity indicates that the electron–hole recombination is reduced as well as the lifetime of the charge carrier is increased. Mirzaei et al. [117] reported that due to the fluorination of the g-C3N4 catalyst, the intensity of the photoluminescence spectra got reduced.
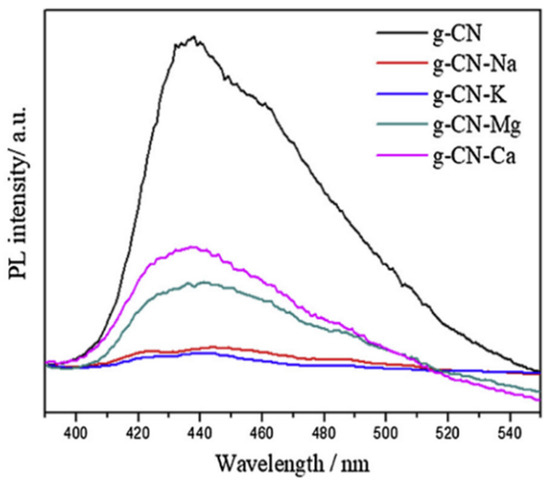
Figure 9.
PL spectra of pristine and doped g-C3N4 samples [133].
7.8. Identification of the Intermediate Products
In pharmaceutical degradation, the identification of the intermediate products constitutes an important study. In the photocatalytic degradation of Ibu by g-C3N4/TiO2/Fe3O4@SiO2 photocatalyst, the intermediate compounds were identified [129]. Peaks were obtained at m/z 237, 253, 241, 257, 165, and 163 as a result of the hydroxylation, decarboxylation, and demethylation. Moreover, according to the mass balance equation, 6.5 µmol of CO2 was obtained after the complete mineralization of 10 µmol of Ibu.
Huang et al. [134] identified the intermediate products by LC-MS analysis while degrading CBZ by a g-C3N4 heterogeneous catalyst through the Fenton process. A prominent peak was identified corresponding to an m/z ratio of 253 due to hydroxyl substitution reaction. During the reaction process, a loss of CONH2 occurred which was reflected by the strong signal at the m/z value of 193.
7.9. Photoelectrochemical Tests
Photoelectrochemical tests constitute an important characterization of the photocatalyst material as it gives insight into the charge transfer mechanism of the synthesized material. Guo et al. [14] showed that in comparison to the bare g-C3N4 catalyst, the Cl-doped catalyst revealed higher photocurrent density which implied that the electron–hole recombination could be prevented more effectively due to Cl doping. He et al. [4] carried out the photoelectrochemical test of the Ti3C2/g-C3N4 photocatalyst using Na2SO4 as the electrolyte. It was seen that the photocurrent response of the Ti3C2/g-C3N4 catalyst was higher in comparison to the bare g-C3N4 material. Due to the high conductivity of Ti3C2, photo-generated electrons from the surface of g-C3N4 got transported to the Ti3C2 surface resulting in high charge separation and greater photocurrent response. Furthermore, the Nyquist plot revealed that the impedance value of Ti3C2/g-C3N4 is lower than that of g-C3N4, which indicates the efficient generation of photoelectrons under visible light irradiation. Moreover, it also confirmed the 2D/2D nanostructure of the newly synthesized catalyst.
7.10. Electron Spin Resonance (ESR) Tests
The ESR technique is often explored by researchers to investigate the reactive oxygen species (ROS) involved in the degradation process. Chen et al. [30] used 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as a spin-trap chemical in the aqueous dispersion of methanol. Results showed peaks of both DMPO-·O2− and DMPO-·OH adducts appeared in the ESR spectrum. Hence, it can be concluded that under visible light irradiation, the MnISCN-20 photocatalyst was able to generate both superoxide and hydroxyl radicals to facilitate TC degradation. Cao et al. [51] utilized DMPO for trapping and detecting radicals such as ·OH, ·O2−, and SO4·− generated during the catalytic degradation of TCH, while 2,2,6,6-tetramethylpiperidine-1-oxyl for the detection of 1O2 radical. The results showed that the 4%P/CNK catalyst was efficient to generate ·OH, ·O2−, SO4·−, and 1O2 radicals for TCH degradation purposes under the combined action of visible light irradiation and PMS.
8. Future Perspective and Current Challenges
g-C3N4-based photocatalysts are undoubtedly promising materials for pharmaceutical wastewater treatment. Scientists are rigorously working in this field for further development of newer catalysts and their applicability for real field applications. In spite of various achievements, there still exist several challenges and hurdles which need to be overcome. Production of a highly stable g-C3N4-based photocatalyst with a narrow band gap is still a challenging task. More control over the surface defects and other properties has to be attained through rigorous research. Another aspect is the synthesis cost of g-C3N4-based catalysts causing the major hurdle behind their large-scale applications.
Balakrishnan et al. [7] in their recent review article highlighted serious challenges associated with the usage of g-C3N4 catalysts for environmental remediation purposes. One of the major problems associated with the g-C3N4-based catalysts is the high electron–hole recombination and significant loss of weight while reusing the catalyst as already mentioned in the introduction part. To improve light absorption, scientists are continuously developing novel techniques for reducing bandgap. Several reports on g-C3N4 showed a significant reduction in band gap that occurred due to the modification or immobilization. However, a detailed explanation behind this bandgap reduction is often not dealt with. Hayat et al. [135] also commented that the charge transfer mechanism of the g-C3N4-based catalysts is not always fully understood. Hence, a more rigorous effort is recommended.
Many g-C3N4-based catalysts have been proven to be toxic to the aquatic ecosystem. Hence, toxicity analysis should also be dealt with while applying these materials for pharmaceutical wastewater treatment.
Often magnetic catalysts are designed for various types of pollutant elimination. However, using a simple bar magnet may lead to severe weight loss during recyclability. Hence, proper design in this respect should be adopted.
g-C3N4-based photocatalytic membranes are often explored by researchers for pollutant degradation purposes. Fouling and low mass transfer issues often hinder their use in large-scale industrial purposes. In this respect, uncommon ceramic materials can be explored for the optimization of the cost.
Doping is often tried out to enhance the photocatalytic activity of the g-C3N4-based catalysts. Patnaik et al. [136], in their recent review article, mentioned that in many cases doped g-C3N4-based catalysts showed poor stability under extreme thermal conditions. Moreover, dopant atoms often become the new area for recombination. Hence, co-doping has been suggested by the authors rather than single-element doping. Although many studies are reported in the literature, still more research is required for controlled doping in order to produce the optimized catalyst.
Yuda and Kumar [137] highlighted some of the important limitations of using g-C3N4-based catalysts for real-field wastewater systems. Firstly, their stability under different environmental conditions is not often explored. Hence, stability against acidic conditions, temperature variation, etc., should be checked. Moreover, electron–hole separation can be easily understood in two-component heterostructure systems. However, it becomes complicated in the case of multicomponent systems.
Author Contributions
S.B.: Writing, original draft preparation, A.P.: Writing, reviewing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data is created.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AMX | amoxicillin |
| ANN | artificial neural network |
| CBZ | carbamazepine |
| CFX | cefalexin |
| CIP | ciprofloxacin |
| DC | diclofenac |
| LFX | levofloxacin |
| NPX | naproxen |
| OTC | oxytetracycline |
| PC | pharmaceutical compound |
| PMS | peroxymonosulfate |
| PR RSM | paracetamol response surface methodology |
| SDZ | sulfadiazine |
| SMX | sulfamethoxazole |
| TC | tetracycline |
| TCH | tetracycline hydrochloride |
| TCS | triclosan |
References
- Mahamallik, P.; Saha, S.; Pal, A. Tetracycline degradation in aquatic environment by highly porous MnO2 nanosheet assembly. Chem. Eng. J. 2015, 276, 155–165. [Google Scholar] [CrossRef]
- Di, G.; Zhu, Z.; Huang, Q.; Zhang, H.; Zhu, J.; Qiu, Y.; Yin, D.; Zhao, J. Targeted modulation of g-C3N4 photocatalytic performance for pharmaceutical pollutants in water using ZnFe-LDH derived mixed metal oxides: Structure-activity and mechanism. Sci. Total Environ. 2019, 650, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.D.; Nguyen, T.B.; Tran, L.H.; Nguyen, T.G.; Fatimah, I.; Kuncoro, E.P.; Doong, R. Z-scheme S, B co-doped g-C3N4 nanotube@MnO2 heterojunction with visible-light-responsive for enhanced photodegradation of diclofenac by peroxymonosulfate activation. Chem. Eng. J. 2023, 452, 139249. [Google Scholar] [CrossRef]
- He, J.; Yang, J.; Jiang, F.; Liu, P.; Zhu, M. Photo-assisted peroxymonosulfate activation via 2D/2D heterostructure of Ti3C2/g-C3N4 for degradation of diclofenac. Chemosphere 2020, 258, 127339. [Google Scholar] [CrossRef]
- Morales-Paredes, C.A.; Rodriguez-Diaz, J.M.; Boluda-Botella, N. Pharmaceutical compounds used in the COVID-19 pandemic: A review of their presence in water and treatment techniques for their elimination. Sci. Total Environ. 2022, 814, 152691. [Google Scholar] [CrossRef]
- Pattanayak, D.S.; Pal, D.; Mishra, J.; Thakur, C. Noble metal-free doped graphitic carbon nitride (g-C3N4) for efficient photodegradation of antibiotics: Progress, limitations, and future directions. Environ. Sci. Pollut. Res. 2023, 30, 25546–25558. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Chinthala, M.; Polagani, R.K.; Vo, D.N. Removal of tetracycline from wastewater using g-C3N4 based photocatalysts: A review. Environ. Res. 2023, 216, 114660. [Google Scholar] [CrossRef]
- Ghosh, U.; Pal, A. Insight into the multiple roles of nitrogen doped carbon quantum dots in an ultrathin 2D-0D-2D all-solid-state Z scheme heterostructure and its performance in tetracycline degradation under LED illumination. Chem. Eng. J. 2022, 431, 133914. [Google Scholar] [CrossRef]
- Ghosh, U.; Majumdar, A.; Pal, A. 3D macroporous architecture of self-assembled defect engineered ultrathin g-C3N4 nanosheets for tetracycline degradation under LED light irradiation. Mater. Res. Bull. 2021, 133, 111074. [Google Scholar] [CrossRef]
- Ghosh, U.; Pal, A. Drastically enhanced tetracycline degradation performance of a porous 2D g-C3N4 nanosheet photocatalyst in real water matrix: Influencing factors and mechanism insight. J. Water Process Eng. 2022, 50, 103315. [Google Scholar] [CrossRef]
- Majumdar, A.; Ghosh, U.; Pal, A. Novel 2D/2D g-C3N4/Bi4NbO8Cl nano-composite for enhanced photocatalytic degradation of oxytetracycline under visible LED light irradiation. J. Colloid Interface Sci. 2021, 584, 320–331. [Google Scholar] [CrossRef]
- Guo, F.; Huang, X.; Chen, Z.; Cao, L.; Cheng, X.; Chen, L.; Shi, W. Construction of Cu3P-ZnSnO3-g-C3N4 p-n-n heterojunction with multiple built-in electric fields for effectively boosting visible-light photocatalytic degradation of broad-spectrum antibiotics. Sep. Purif. Technol. 2021, 265, 118477. [Google Scholar] [CrossRef]
- Chi, X.; Liu, F.; Gao, Y.; Song, J.; Guan, R.; Yuan, H. An efficient B/Na co-doped porous g-C3N4 nanosheets photocatalyst with enhanced photocatalytic hydrogen evolution and degradation of tetracycline under visible light. Appl. Surf. Sci. 2022, 576, 151837. [Google Scholar] [CrossRef]
- Guo, F.; Li, M.; Ren, H.; Huang, X.; Shu, K.; Shi, W.; Lu, C. Facile bottom-up preparation of Cl-doped porous g-C3N4 nanosheets for enhanced photocatalytic degradation of tetracycline under visible light. Sep. Purif. Technol. 2019, 228, 115770. [Google Scholar] [CrossRef]
- Smykalova, A.; Sokolova, B.; Foniok, K.; Matejka, V.; Praus, P. Photocatalytic degradation of selected pharmaceuticals using g-C3N4 and TiO2 nanomaterials. Nanomaterials 2019, 9, 1194. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Jia, H.; Li, Z.; Miao, C.; Lu, R.; Zhang, S.; Zhang, Z. Magnetic recyclable g-C3N4/Fe3O4@MIL-100(Fe) ternary catalyst for photo-Fenton degradation of ciprofloxacin. J. Environ. Chem. Eng. 2022, 10, 108698. [Google Scholar] [CrossRef]
- Wang, F.; Feng, Y.; Chen, P.; Wang, Y.; Su, Y.; Zhang, Q.; Zeng, Y.; Xie, Z.; Liu, H.; Liu, Y.; et al. Photocatalytic degradation of fluoroquinolone antibiotics using ordered mesoporous g-C3N4 under simulated sunlight irradiation: Kinetics, mechanism, and antibacterial activity elimination. Appl. Catal. B Environ. 2018, 227, 114–122. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, X.; Dong, X.; Liu, X.; Tan, Y.; Yuan, F.; Zheng, S.; Li, C. Hierarchical assembly of highly efficient visible-light-driven Ag/g-C3N4/kaolinite composite photocatalyst for the degradation of ibuprofen. J. Mater. 2020, 6, 582–592. [Google Scholar] [CrossRef]
- Feyzi, L.; Rahemi, N.; Allahyari, S. Efficient degradation of tetracycline in aqueous solution using a coupled S-scheme ZnO/g-C3N4/zeolite P supported catalyst with water falling film plasma reactor. Process Saf. Environ. Prot. 2022, 161, 827–847. [Google Scholar] [CrossRef]
- Mirzaei, A.; Yerushalmi, L.; Chen, Z.; Haghighat, F. Photocatalytic degradation of sulfamethoxazole by hierarchical magnetic ZnO@g-C3N4: RSM optimization, kinetic study, reaction pathway and toxicity evaluation. J. Hazard. Mater. 2018, 359, 516–526. [Google Scholar] [CrossRef]
- Baladi, E.; Davar, F.; Hojjati-Najafabadi, A. Synthesis and characterization of g-C3N4-CoFe2O4-ZnO magnetic nanocomposites for enhancing photocatalytic activity with visible light for degradation of penicillin G antibiotic. Environ. Res. 2022, 215, 114270. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, B.; Pu, Q.; Chen, X.; Wen, G.; Li, Z. Preparation of Ag-AgVO3/g-C3N4 composite photo-catalyst and degradation characteristics of antibiotics. J. Hazard. Mater. 2019, 373, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, M.; Huang, X.; Wu, Y.; Li, L. S-scheme g-C3N4/TiO2/CFs heterojunction composites with multi-dimensional through-holes and enhanced visible-light photocatalytic activity. Ceram. Int. 2022, 48, 8196–8208. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Sharma, G.; Al-Muhtaseb, A.H.; Naushad, M.; Ghfar, A.A.; Stadler, F.J. Quaternary magnetic BiOCl/g-C3N4/Cu2O/Fe3O4 nano-junction for visible light and solar powered degradation of sulfamethoxazole from aqueous environment. Chem. Eng. J. 2018, 334, 462–478. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Wang, J. Peroxymonosulfate activation by Fe−Co−O-Codoped graphite carbon nitride for degradation of sulfamethoxazole. Environ. Sci. Technol. 2020, 54, 10361–10369. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, W.; Zhao, Z.; Liu, W.; Ye, J.; Tong, M.; Li, Y. Different degradation mechanisms of carbamazepine and diclofenac by single-atom Barium embedded g-C3N4: The role of photosensitation-like mechanism. J. Hazard. Mater. 2021, 416, 125936. [Google Scholar] [CrossRef]
- Tian, Y.; Tian, X.; Zeng, W.; Nie, Y.; Yang, C.; Dai, C.; Li, Y.; Lu, L. Enhanced peroxymonosulfate decomposition into ·OH and 1O2 for sulfamethoxazole degradation over Se doped g-C3N4 due to induced exfoliation and N vacancies formation. Sep. Purif. Technol. 2021, 267, 118664. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-scheme heterojunction photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Tada, H.; Mitsui, T.; Kiyonaga, T.; Akita, T.; Tanaka, K. All solid-state Z-scheme in Cds-Au-TiO2 three component nanojunction system. Nat. Mater. 2006, 5, 782–786. [Google Scholar] [CrossRef]
- Chen, W.; He, Z.; Huang, G.; Wu, C.; Chen, W.; Liu, X. Direct Z scheme 2D/2D MnIn2S4/g-C3N4 architectures with highly efficient photocatalytic activities towards treatment of pharmaceutical wastewater and hydrogen evolution. Chem. Eng. J. 2019, 359, 244–253. [Google Scholar] [CrossRef]
- Pham, V.V.; Truong, T.K.; Hai, L.V.; La, H.P.P.; Nguyen, H.T.; Lam, V.Q.; Tong, H.D.; Nguyen, T.Q.; Sabbah, A.; Chen, K.; et al. S-Scheme α-Fe2O3/g-C3N4 nanocomposites as heterojunction photocatalysts for antibiotic degradation. Appl. Nanomater. 2022, 5, 4506–4514. [Google Scholar] [CrossRef]
- Ni, S.; Fu, Z.; Li, L.; Ma, M.; Liu, Y. Step-scheme heterojunction g-C3N4/TiO2 for efficient photocatalytic degradation of tetracycline hydrochloride under UV light. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 129475. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, Y.; Deng, F.; Wang, M.; Chen, D. Porous Z-scheme MnO2/Mn-modified alkalinized g-C3N4 heterojunction with excellent Fenton-like photocatalytic activity for efficient degradation of pharmaceutical pollutants. Sep. Purif. Technol. 2020, 246, 116890. [Google Scholar] [CrossRef]
- Mei, X.; Chen, S.; Wang, G.; Chen, W.; Lu, W.; Zhang, B.; Fang, Y.; Qi, C. Metal-free carboxyl modified g-C3N4 for enhancing photocatalytic degradation activity of organic pollutants through peroxymonosulfate activation in wastewater under solar irradiation. J. Solid State Chem. 2022, 310, 123053. [Google Scholar] [CrossRef]
- Luo, J.; Dai, Y.; Xu, X.; Liu, Y.; Yang, S.; He, H.; Sun, C.; Xia, Q. Green and efficient synthesis of Co-MOF-based/g-C3N4 composite catalysts to activate peroxymonosulfate for degradation of the antidepressant venlafaxine. J. Colloid Interf. Sci. 2022, 610, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J. Magnetic 2D/2D oxygen doped g-C3N4/biochar composite to activate peroxymonosulfate for degradation of emerging organic pollutants. J. Hazard. Mater. 2022, 423, 127207. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Dai, H.; Tan, C.; Pan, Q.; Hu, F.; Peng, X. Photo-Fenton degradation of tetracycline over Z-scheme Fe-g-C3N4/Bi2WO6 heterojunctions: Mechanism insight, degradation pathways and DFT calculation. Appl. Catal. B Environ. 2022, 310, 121326. [Google Scholar] [CrossRef]
- Li, T.; Ge, L.; Peng, X.; Wang, W.; Zhang, W. Enhanced degradation of sulfamethoxazole by a novel Fenton-like system with significantly reduced consumption of H2O2 activated by g-C3N4/MgO composite. Water Res. 2021, 190, 116777. [Google Scholar] [CrossRef]
- Li, X.; Gan, X. Photo-Fenton degradation of multiple pharmaceuticals at low concentrations via Cu-doped-graphitic carbon nitride (g-C3N4) under simulated solar irradiation at a wide pH range. J. Environ. Chem. Eng. 2022, 10, 108290. [Google Scholar] [CrossRef]
- Cao, Z.; Jia, Y.; Wang, Q.; Cheng, H. High-efficiency photo-Fenton Fe/g-C3N4/kaolinite catalyst for tetracycline hydrochloride degradation. Appl. Clay Sci. 2021, 212, 106213. [Google Scholar] [CrossRef]
- Qiao, X.; Liu, X.; Zhang, W.; Cai, Y.; Zhong, Z.; Li, Y.; Lu, J. Superior photo-Fenton activity towards chlortetracycline degradation over novel g-C3N4 nanosheets/schwertmannite nanocomposites with accelerated Fe(III)/Fe(II) cycling. Sep. Purif. Technol. 2021, 279, 119760. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Zhang, K.; Zada, A.; Qi, K. Sonocatalytic degradation of tetracycline hydrochloride with CoFe2O4/g-C3N4 composite. Ultrason. Sonochem. 2023, 94, 106325. [Google Scholar] [CrossRef]
- He, Y.; Ma, Z.; Junior, L.B. Distinctive binary g-C3N4/MoS2 heterojunctions with highly efficient ultrasonic catalytic degradation for levofloxacin and methylene blue. Ceram. Int. 2020, 46, 12364–12372. [Google Scholar] [CrossRef]
- Vinesh, V.; Ashokkumar, M.; Neppolian, B. rGO supported self-assembly of 2D nano sheet of (g-C3N4) into rod-like nano structure and its application in sonophotocatalytic degradation of an antibiotic. Ultrason. Sonochem. 2020, 68, 105218. [Google Scholar] [CrossRef]
- Gholami, P.; Khataee, A.; Vahid, B.; Karimi, A.; Golizadeh, M.; Ritala, M. Sonophotocatalytic degradation of sulfadiazine by integration of microfibrillated carboxymethyl cellulose with Zn-Cu-Mg mixed metal hydroxide/g-C3N4 composite. Sep. Purif. Technol. 2020, 245, 116866. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Zeng, G.; Liang, J.; Wu, Z.; Yu, H.; Mo, D.; Wang, H.; Xiao, Z.; Zhou, C. Nitrogen self-doped g-C3N4 nanosheets with tunable band structures for enhanced photocatalytic tetracycline degradation. J. Colloid Interf. Sci. 2019, 536, 17–29. [Google Scholar] [CrossRef]
- Palanivel, B.; Shkir, M.; Alshahrani, T.; Mani, A. Novel NiFe2O4 deposited S-doped g-C3N4 nanorod: Visible-light-driven heterojunction for photo-Fenton like tetracycline degradation. Diam. Relat. Mater. 2021, 112, 108148. [Google Scholar] [CrossRef]
- Preeyanghaa, M.; Dhileepan, M.D.; Madhavan, J.; Neppolian, B. Revealing the charge transfer mechanism in magnetically recyclable ternary g-C3N4/BiOBr/Fe3O4 nanocomposite for efficient photocatalytic degradation of tetracycline antibiotics. Chemosphere 2022, 303, 135070. [Google Scholar] [CrossRef]
- Bui, T.S.; Bansal, P.; Lee, B.; Mahvelati-Shamsabadi, T. Facile fabrication of novel Ba-doped g-C3N4 photocatalyst with remarkably enhanced photocatalytic activity towards tetracycline elimination under visible-light irradiation. Appl. Surf. Sci. 2020, 506, 144184. [Google Scholar] [CrossRef]
- Cao, Y.; Alsharif, S.; El-Shafay, A.S. Preparation, suppressed the charge carriers recombination, and improved photocatalytic performance of g-C3N4/MoS2 p-n heterojunction photocatalyst for tetracycline and dyes degradation upon visible light. Mater. Sci. Semicond. Process. 2022, 144, 106569. [Google Scholar] [CrossRef]
- Cao, Z.; Zhao, Y.; Li, J.; Wang, Q.; Mei, Q.; Cheng, H. Rapid electron transfer-promoted tetracycline hydrochloride degradation: Enhanced activity in visible light-coupled peroxymonosulfate with PdO/g-C3N4/kaolinite catalyst. Chem. Eng. J. 2023, 457, 141191. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Jiang, L.; Yuan, X.; Liang, J.; Zhang, J.; Yu, H.; Chu, W.; Wu, Z.; Li, H.; et al. Strategic combination of nitrogen-doped carbon quantum dots and g-C3N4: Efficient photocatalytic peroxydisulfate for the degradation of tetracycline hydrochloride and mechanism insight. Sep. Purif. Technol. 2021, 272, 118947. [Google Scholar] [CrossRef]
- Guo, F.; Shi, W.; Wang, H.; Han, M.; Li, H.; Huang, H.; Liu, Y.; Kang, Z. Facile fabrication of a CoO/g-C3N4 p-n heterojunction with enhanced photocatalytic activity and stability for tetracycline degradation under visible light. Catal. Sci. Technol. 2017, 7, 3325–3331. [Google Scholar] [CrossRef]
- Guo, F.; Shi, W.; Li, M.; Shi, Y.; Wen, H. 2D/2D Z-scheme heterojunction of CuInS2/g-C3N4 for enhanced visible-light-driven photocatalytic activity towards the degradation of tetracycline. Sep. Purif. Technol. 2019, 210, 608–615. [Google Scholar] [CrossRef]
- Guo, F.; Huang, X.; Chen, Z.; Sun, H.; Chen, L. Prominent co-catalytic effect of CoP nanoparticles anchored on high-crystalline g-C3N4 nanosheets for enhanced visible-light photocatalytic degradation of tetracycline in wastewater. Chem. Eng. J. 2020, 395, 125118. [Google Scholar] [CrossRef]
- Guo, B.; Liu, B.; Wang, C.; Lu, J.; Wang, Y.; Yin, S.; Javed, M.S.; Han, W. Boosting photocharge separation in Z-schemed g-C3N4/RGO/ln2S3 photocatalyst for H2 evolution and antibiotic degradation. J. Ind. Eng. Chem. 2022, 110, 217–224. [Google Scholar] [CrossRef]
- He, Y.; Ma, B.; Yang, Q.; Tong, Y.; Ma, Z.; Junior, L.B.; Yao, B. Surface construction of a novel metal-free g-C3N4-based heterojunction photocatalyst for the efficient removal of bio-toxic antibiotic residues. Appl. Surf. Sci. 2022, 571, 151299. [Google Scholar] [CrossRef]
- Huang, H.; Liu, C.; Ou, H.; Ma, T.; Zhang, Y. Self-sacrifice transformation for fabrication of type-I and type-II heterojunctions in hierarchical BixOyIz/g-C3N4 for efficient visible-light photocatalysis. Appl. Surf. Sci. 2019, 470, 1101–1110. [Google Scholar] [CrossRef]
- Obregon, S.; Ruiz-Gomez, M.A.; Rodriguez-Gonzalez, V.; Vaquez, A.; Hernandez-Uresti, D.B. A novel type-II Bi2W2O9/g-C3N4 heterojunction with enhanced photocatalytic performance under simulated solar irradiation. Mater. Sci. Semicond. Process. 2020, 113, 105056. [Google Scholar] [CrossRef]
- Panneri, S.; Ganguly, P.; Mohan, M.; Nair, B.N.; Mohamed, A.A.P.; Warrier, K.G.; Hareesh, U.S. Photoregenerable, bifunctional granules of carbon-doped g-C3N4 as adsorptive photocatalyst for the efficient removal of tetracycline antibiotic. ACS Sustain. Chem. Eng. 2017, 5, 1610–1618. [Google Scholar] [CrossRef]
- Peng, X.; Wu, J.; Zhao, Z.; Wang, X.; Dai, H.; Xu, L.; Xu, G.; Jian, Y.; Hu, F. Activation of peroxymonosulfate by single-atom Fe-g-C3N4 catalysts for high efficiency degradation of tetracycline via nonradical pathways: Role of high-valent iron-oxo species and Fe–Nx sites. Chem. Eng. J. 2022, 427, 130803. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, F.; Wen, K.; Lu, J. Enhanced photocatalytic activity for tetracyclines degradation with Ag modified g-C3N4 composite under visible light. J. Photochem. Photobiol. A Chem. 2020, 389, 112217. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, X.; Guo, R.; Li, X.; Peng, Y.; Zhao, X.; Pu, X. Hollow mesoporous g-C3N4/Ag2CrO4 photocatalysis with direct Z-scheme: Excellent degradation performance for antibiotics and dyes. Sep. Purif. Technol. 2021, 270, 118797. [Google Scholar] [CrossRef]
- Shi, Y.; Li, J.; Sun, Y.; Wan, D.; Wan, H.; Wang, Y. FeOOH coupling and nitrogen vacancies functionalized g-C3N4 heterojunction for efficient degradation of antibiotics: Performance evaluation, active species evolution and mechanism insight. J. Alloy. Compd. 2022, 903, 163898. [Google Scholar] [CrossRef]
- Song, H.; Liu, L.; Wang, H.; Feng, B.; Xiao, M.; Tang, Y.; Qu, X.; Gai, H.; Huang, T. Adjustment of the band gap of co-doped KCl/NH4Cl/g-C3N4 for enhanced photocatalytic performance under visible light. Mater. Sci. Semicond. Process. 2021, 128, 105757. [Google Scholar] [CrossRef]
- Tian, Y.; Gao, Y.; Wang, Q.; Wang, Z.; Guan, R.; Shi, W. Potassium gluconate-cooperative pore generation based on g-C3N4 nanosheets for highly efficient photocatalytic hydrogen production and antibiotic degradation. J. Environ. Chem. Eng. 2022, 10, 107986. [Google Scholar] [CrossRef]
- Wang, W.; Zeng, Z.; Zeng, G.; Zhang, C.; Xiao, R.; Zhou, C.; Xiong, W.; Yang, Y.; Lei, L.; Liu, Y.; et al. Sulfur doped carbon quantum dots loaded hollow tubular g-C3N4 as novel photocatalyst for destruction of Escherichia coli and tetracycline degradation under visible light. Chem. Eng. J. 2019, 378, 122132. [Google Scholar] [CrossRef]
- Wang, W.; Fang, J.; Chen, H. Nano-confined g-C3N4 in mesoporous SiO2 with improved quantum size effect and tunable structure for photocatalytic tetracycline antibiotic degradation. J. Alloy. Compd. 2020, 819, 153064. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Guo, Y.; Shen, M.; Wang, M.; Li, B.; Shi, J. Multifunctional 2D porous g-C3N4 nanosheets hybridized with 3D hierarchical TiO2 microflowers for selective dye adsorption, antibiotic degradation and CO2 reduction. Chem. Eng. J. 2020, 396, 125347. [Google Scholar] [CrossRef]
- Wang, M.; Jin, C.; Kang, J.; Liu, J.; Tang, Y.; Li, Z.; Li, S. CuO/g-C3N4 2D/2D heterojunction photocatalysts as efficient peroxymonosulfate activators under visible light for oxytetracycline degradation: Characterization, efficiency and mechanism. Chem. Eng. J. 2021, 416, 128118. [Google Scholar] [CrossRef]
- Wu, K.; Chen, D.; Fang, J.; Wu, S.; Yang, F.; Zhu, X.; Fang, Z. One-step synthesis of sulfur and tungstate co-doped porous g-C3N4 microrods with remarkably enhanced visible-light photocatalytic performances. Appl. Surf. Sci. 2018, 462, 991–1001. [Google Scholar] [CrossRef]
- Wu, K.; Chen, D.; Lu, S.; Fang, J.; Zhu, X.; Yang, F.; Pan, T.; Fang, Z. Supramolecular self-assembly synthesis of noble-metal-free (C, Ce) co-doped g-C3N4 with porous structure for highly efficient photocatalytic degradation of organic pollutants. J. Hazard. Mater. 2020, 382, 121027. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zuo, H.; Du, H.; Zhang, S.; Wang, L.; Yan, Q. Construction of layered embedding dual Z-Scheme Bi2O2CO3/g-C3N4/Bi2O3: Tetracycline degradation pathway, toxicity analysis and mechanism insight. Sep. Purif. Technol. 2022, 282, 120096. [Google Scholar] [CrossRef]
- Xu, Y.; Ge, F.; Chen, Z.; Huang, S.; Wei, W.; Xie, M.; Xu, H.; Li, H. One-step synthesis of Fe-doped surface-alkalinized g-C3N4 and their improved visible-light photocatalytic performance. Appl. Surf. Sci. 2019, 469, 739–746. [Google Scholar] [CrossRef]
- Zhang, C.; Ouyang, Z.; Yang, Y.; Long, X.; Qin, L.; Wang, W.; Zhou, Y.; Qin, D.; Qin, F.; Lai, C. Molecular engineering of donor-acceptor structured g-C3N4 for superior photocatalytic oxytetracycline degradation. Chem. Eng. J. 2022, 448, 137370. [Google Scholar] [CrossRef]
- Zhao, C.; Ran, F.; Dai, L.; Li, C.; Zheng, C.; Si, C. Cellulose-assisted construction of high surface area Z-scheme C-doped g-C3N4/WO3 for improved tetracycline degradation. Carbohydr. Polym. 2021, 255, 117343. [Google Scholar] [CrossRef]
- Lu, C.; Wang, J.; Cao, D.; Guo, F.; Hao, X.; Li, D.; Shi, W. Synthesis of magnetically recyclable g-C3N4/NiFe2O4 S-scheme heterojunction photocatalyst with promoted visible-light-response photo-Fenton degradation of tetracycline. Mater. Res. Bull. 2023, 158, 112064. [Google Scholar] [CrossRef]
- Li, F.; Huang, T.; Sun, F.; Chen, L.; Li, P.; Shao, F.; Yang, X.; Liu, W. Ferric oxide nanoclusters with low-spin FeIII anchored g-C3N4 rod for boosting photocatalytic activity and degradation of diclofenac in water under solar light. Appl. Catal. B Environ. 2022, 317, 121725. [Google Scholar] [CrossRef]
- Papamichail, P.; Nannou, C.; Giannakoudakis, D.A.; Bikiaris, N.D.; Papoulia, C.; Pavlidou, E.; Lambropoulou, D.; Samanidou, V.; Deliyanni, E. Maximization of the photocatalytic degradation of diclofenac using polymeric g-C3N4 by tuning the precursor and the synthetic protocol. Catal. Today 2023, 418, 114075. [Google Scholar] [CrossRef]
- Jin, X.; Wu, Y.; Wang, Y.; Lin, Z.; Liang, D.; Zheng, X.; Wei, D.; Liu, H.; Lv, W.; Liu, G. Carbon quantum dots-modified reduced ultrathin g-C3N4 with strong photoredox capacity for broad spectrum-driven PPCPs remediation in natural water matrices. Chem. Eng. J. 2021, 420, 129935. [Google Scholar] [CrossRef]
- Hu, Z.; Cai, X.; Wang, Z.; Li, S.; Wang, Z.; Xie, X. Construction of carbon-doped supramolecule-based g-C3N4/TiO2 composites for removal of diclofenac and carbamazepine: A comparative study of operating parameters, mechanisms, degradation pathways. J. Hazard. Mater. 2019, 380, 120812. [Google Scholar] [CrossRef] [PubMed]
- John, P.; Johari, K.; Gnanasundaram, N.; Appusamy, A.; Thanabalan, M. Enhanced photocatalytic performance of visible light driven TiO2/g-C3N4 for degradation of diclofenac in aqueous solution. Environ. Technol. Innov. 2021, 22, 101412. [Google Scholar] [CrossRef]
- Han, Y.; Gan, L.; Gong, H.; Han, J.; Qiao, W.; Xu, L. Photoactivation of peroxymonosulfate by wood pulp cellulose biochar/g-C3N4 composite for diclofenac degradation: The radical and nonradical pathways. Biochar 2022, 4, 35. [Google Scholar] [CrossRef]
- Jimenez-Salcedo, M.; Monge, M.; Tena, M.T. The photocatalytic degradation of sodium diclofenac in different water matrices using g-C3N4 nanosheets: A study of the intermediate by-products and mechanism. J. Environ. Chem. Eng. 2021, 9, 105827. [Google Scholar] [CrossRef]
- Oliveros, A.N.; Pimentel, J.A.I.; Luna, M.D.G.; Garcia-Segura, S.; Abarca, R.R.M.; Doong, R. Visible-light photocatalytic diclofenac removal by tunable vanadium pentoxide/boron-doped graphitic carbon nitride composite. Chem. Eng. J. 2021, 403, 126213. [Google Scholar] [CrossRef]
- Muelas-Ramos, V.; Sampaio, M.J.; Silva, C.G.; Bedia, J.; Rodriguez, J.J.; Faria, J.L. Degradation of diclofenac in water under LED irradiation using combined g-C3N4/NH2-MIL-125 photocatalysts. J. Hazard. Mater. 2021, 416, 126199. [Google Scholar] [CrossRef]
- Reddy, C.V.; Kakarla, R.R.; Cheolho, B.; Shim, J.; Aminabhavi, T.M. Heterostructured 2D/2D ZnIn2S4/g-C3N4 nanohybrids for photocatalytic degradation of antibiotic sulfamethoxazole and photoelectrochemical properties. Environ. Res. 2023, 225, 115585. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Liao, J.; Chen, L.; Wu, X.; Zhang, X.; Sun, W.; Ge, C. Constructing a 3D interconnected “trap-zap” β-CDPs/Fe-g-C3N4 catalyst for efficient sulfamethoxazole degradation via peroxymonosulfate activation: Performance, mechanism, intermediates and toxicity. Chemosphere 2022, 294, 133780. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Pan, Y.; Xiong, Z.; Yao, G.; Xie, R.; Lai, B. Peroxymonosulfate activation on FeCo2S4 modified g-C3N4 (FeCo2S4-CN): Mechanism of singlet oxygen evolution for nonradical efficient degradation of sulfamethoxazole. Chem. Eng. J. 2020, 384, 123361. [Google Scholar] [CrossRef]
- Zhao, G.; Li, W.; Zhang, H.; Wang, W.; Ren, Y. Single atom Fe-dispersed graphitic carbon nitride (g-C3N4) as a highly efficient peroxymonosulfate photocatalytic activator for sulfamethoxazole degradation. Chem. Eng. J. 2022, 430, 132937. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, W.; Chen, L.; Deng, H.; Wan, J. A novel ternary visible-light-driven photocatalyst AgCl/Ag3PO4/g-C3N4: Synthesis, characterization, photocatalytic activity for antibiotic degradation and mechanism analysis. Catal. Commun. 2017, 100, 191–195. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Z.; Lv, M.; Wang, H.; Chen, D.; Feng, Y. Photodegradation performance and transformation mechanisms of sulfamethoxazole by porous g-C3N4 modified with ammonia bicarbonate. Sep. Purif. Technol. 2020, 235, 116172. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, W.; Chen, L.; Deng, H. Z-scheme mechanism of photogenerated carriers for hybrid photocatalyst Ag3PO4/g-C3N4 in degradation of sulfamethoxazole. J. Colloid Interface Sci. 2017, 487, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qi, J.; Tian, J.; Gao, S.; Cui, F. Construction of Ag/g-C3N4 photocatalysts with visible-light photocatalytic activity for sulfamethoxazole degradation. Chem. Eng. J. 2018, 341, 547–555. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Yan, Y.; Wang, W.; Ren, Y.; Li, X. Synthesis of highly porous g-C3N4 nanotubes for efficient photocatalytic degradation of sulfamethoxazole. Mater. Today Commun. 2021, 27, 102288. [Google Scholar] [CrossRef]
- Liang, H.; Guo, J.; Yu, M.; Zhou, Y.; Zhan, R.; Liu, C.; Niu, J. Porous loofah-sponge-like ternary heterojunction g-C3N4/Bi2WO6/MoS2 for highly efficient photocatalytic degradation of sulfamethoxazole under visible light irradiation. Chemosphere 2021, 279, 130552. [Google Scholar] [CrossRef]
- Liu, S.; Tang, W. Photodecomposition of ibuprofen over g-C3N4/Bi2WO6/rGO heterostructured composites under visible/solar light. Sci. Total Environ. 2020, 731, 139172. [Google Scholar] [CrossRef]
- Mao, S.; Liu, C.; Xia, M.; Wang, F.; Ju, X. Construction of a Z-scheme 1D/2D FeV3O8/g-C3N4 composite for ibuprofen degradation: Mechanism insight, theoretical calculation and degradation pathway. Catal. Sci. Technol. 2021, 11, 3466–3480. [Google Scholar] [CrossRef]
- Meng, F.; Wang, J.; Tian, W.; Zhang, H.; Liu, S.; Tan, X.; Wang, S. Effects of inter/intralayer adsorption and direct/indirect reaction on photo-removal of pollutants by layered g-C3N4 and BiOBr. J. Clean. Prod. 2021, 322, 129025. [Google Scholar] [CrossRef]
- Akbarzadeh, R.; Fung, C.S.L.; Rather, R.A.; Lo, I.M.C. One-pot hydrothermal synthesis of g-C3N4/Ag/AgCl/BiVO4 micro-flower composite for the visible light degradation of ibuprofen. Chem. Eng. J. 2018, 341, 248–261. [Google Scholar] [CrossRef]
- Cao, W.; Yuan, Y.; Yang, C.; Wu, S.; Cheng, J. In-situ fabrication of g-C3N4/MIL-68(In)-NH2 heterojunction composites with enhanced visible-light photocatalytic activity for degradation of ibuprofen. Chem. Eng. J. 2020, 391, 123608. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Yang, J.; Sun, Q.; Yang, Y.; Wu, X. Multiphase TiO2 surface coating g-C3N4 formed a sea urchin like structure with interface effects and improved visible-light photocatalytic performance for the degradation of ibuprofen. Int. J. Hydrog. Energy 2018, 43, 13284–13293. [Google Scholar] [CrossRef]
- Jimenez-Salcedo, M.; Monge, M.; Tena, M.T. Photocatalytic degradation of ibuprofen in water using TiO2/UV and g-C3N4/visible light: Study of intermediate degradation products by liquid chromatography coupled to high-resolution mass spectrometry. Chemosphere 2019, 215, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Salcedo, M.; Monge, M.; Tena, M.T. Combination of Au-Ag plasmonic nanoparticles of varied compositions with carbon nitride for enhanced photocatalytic degradation of Ibuprofen under visible light. Materials 2021, 14, 3912. [Google Scholar] [CrossRef]
- Liang, M.; Zhang, Z.; Long, R.; Wang, Y.; Yu, Y.; Pei, Y. Design of a Z-scheme g-C3N4/CQDs/CdIn2S4 composite for efficient visible-light-driven photocatalytic degradation of ibuprofen. Environ. Pollut. 2020, 259, 113770. [Google Scholar] [CrossRef]
- Liu, R.; Sun, L.; Qiao, Y.; Bie, Y.; Wang, P.; Zhang, X.; Zhang, Q. Efficient photocatalytic degradation of pharmaceutical pollutants using plasma-treated g-C3N4/TiO. Energy Technol. 2020, 8, 2000095. [Google Scholar] [CrossRef]
- Liu, S.; Tang, W.; Chou, P. Microwave-assisted synthesis of triple 2D g-C3N4/Bi2WO6/rGO composites for ibuprofen photodegradation: Kinetics, mechanism and toxicity evaluation of degradation products. Chem. Eng. J. 2020, 387, 124098. [Google Scholar] [CrossRef]
- Wang, J.; Tang, L.; Zeng, G.; Deng, Y.; Liu, Y.; Wang, L.; Zhou, Y.; Guo, Z.; Wang, J.; Zhang, C. Atomic scale g-C3N4/Bi2WO6 2D/2D heterojunction with enhanced photocatalytic degradation of ibuprofen under visible light irradiation. Appl. Catal. B Environ. 2017, 209, 285–294. [Google Scholar] [CrossRef]
- Wei, Q.; Xiong, S.; Li, W.; Jin, C.; Chen, Y.; Hou, L.; Wu, Z.; Pan, Z.; He, Q.; Wang, Y.; et al. Double Z-scheme system of α-SnWO4/UiO-66(NH2)/g-C3N4 ternary heterojunction with enhanced photocatalytic performance for ibuprofen degradation and H2 evolution. J. Alloy. Compd. 2021, 885, 160984. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, L.; Feng, C.; Zeng, G.; Wang, J.; Zhou, Y.; Liu, Y.; Peng, B.; Feng, H. Construction of plasmonic Ag modified phosphorous-doped ultrathin g-C3N4 nanosheets/BiVO4 photocatalyst with enhanced visible-near-infrared response ability for ciprofloxacin degradation. J. Hazard. Mater. 2018, 344, 758–769. [Google Scholar] [CrossRef]
- Zhang, N.; Li, X.; Wang, Y.; Zhu, B.; Yang, J. Fabrication of magnetically recoverable Fe3O4/CdS/g-C3N4 photocatalysts for effective degradation of ciprofloxacin under visible light. Ceram. Int. 2020, 46, 20974–20984. [Google Scholar] [CrossRef]
- Savunthari, K.V.; Arunagiri, D.; Shanmugam, S.; Ganesan, S.; Arasu, M.V.; Al-Dhabi, N.A.; Chi, N.T.L.; Ponnusamy, V.K. Green synthesis of lignin nanorods/g-C3N4 nanocomposite materials for efficient photocatalytic degradation of triclosan in environmental water. Chemosphere 2021, 272, 129801. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yue, M.; Han, Y.; Xu, X.; Yue, Q.; Xu, S. Highly-efficient degradation of triclosan attributed to peroxymonosulfate activation by heterogeneous catalyst g-C3N4/MnFe2O. Chem. Eng. J. 2020, 391, 123554. [Google Scholar] [CrossRef]
- Yu, B.; Yan, W.; Meng, Y.; Zhang, Y.; Li, X.; Zhong, Y.; Ding, J.; Zhang, H. Selected dechlorination of triclosan by high-performance g-C3N4/Bi2MoO6 composites: Mechanisms and pathways. Chemosphere 2023, 312, 137247. [Google Scholar] [CrossRef]
- Mafa, P.J.; Malfane, M.E.; Idris, A.O.; Liu, D.; Gui, J.; Mamba, B.B.; Kuvarega, A.T. Multi-elemental doped g-C3N4 with enhanced visible light photocatalytic Activity: Insight into naproxen Degradation, Kinetics, effect of Electrolytes, and mechanism. Sep. Purif. Technol. 2022, 282, 120089. [Google Scholar] [CrossRef]
- Truong, H.B.; Huy, B.T.; Ray, S.K.; Gyawali, G.; Lee, Y.; Cho, J.; Hur, J. Magnetic visible-light activated photocatalyst ZnFe2O4/BiVO4/g-C3N4 for decomposition of antibiotic lomefloxacin: Photocatalytic mechanism, degradation pathway, and toxicity assessment. Chemosphere 2022, 299, 134320. [Google Scholar] [CrossRef]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Magnetic fluorinated mesoporous g-C3N4 for photocatalytic degradation of amoxicillin: Transformation mechanism and toxicity assessment. Appl. Catal. B Environ. 2019, 242, 337–348. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Park, C.M. Rational design of a novel LaFeO3/g-C3N4/BiFeO3 double Z-scheme structure: Photocatalytic performance for antibiotic degradation and mechanistic insight. Chem. Eng. J. 2021, 423, 130076. [Google Scholar] [CrossRef]
- Xie, X.; Chen, C.; Wang, X.; Li, J.; Naraginti, S. Efficient detoxification of triclosan by a S–Ag/TiO2@g-C3N4 hybrid photocatalyst: Process optimization and bio-toxicity assessment. RSC Adv. 2019, 9, 20439–20449. [Google Scholar] [CrossRef]
- Su, Q.; Li, J.; Yuan, H.; Wang, B.; Wang, Y.; Li, Y.; Xing, Y. Visible-light-driven photocatalytic degradation of ofloxacin by g-C3N4/ NH2-MIL-88B(Fe) heterostructure: Mechanisms, DFT calculation, degradation pathway and toxicity evolution. Chem. Eng. J. 2022, 427, 131594. [Google Scholar] [CrossRef]
- Huang, D.; Sun, X.; Liu, Y.; Ji, H.; Liu, W.; Wang, C.; Ma, W.; Cai, Z. A carbon-rich g-C3N4 with promoted charge separation for highly efficient photocatalytic degradation of amoxicillin. Chin. Chem. Lett. 2021, 32, 2787–2791. [Google Scholar] [CrossRef]
- Nivetha, M.S.; Kumar, J.V.; Ajarem, J.S.; Allam, A.A.; Manikandan, V.; Arulmozhi, R.; Abirami, N. Construction of SnO2/g-C3N4 an effective nanocomposite for photocatalytic degradation of amoxicillin and pharmaceutical effluent. Environ. Res. 2022, 209, 112809. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Xiao, L.; Li, Q.; Hao, G.; Hu, Y.; Jiang, W. 0D/1D Co3O4 quantum dots/surface hydroxylated g-C3N4 nanofibers heterojunction with enhanced photocatalytic removal of pharmaceuticals and personal care products. Sep. Purif. Technol. 2022, 297, 121481. [Google Scholar] [CrossRef]
- Thang, N.Q.; Sabbah, A.; Chen, L.; Chen, K.; Thi, C.M.; Viet, P.V. High-efficient photocatalytic degradation of commercial drugs for pharmaceutical wastewater treatment prospects: A case study of Ag/g-C3N4/ZnO nanocomposite materials. Chemosphere 2021, 282, 130971. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, A.; Liu, Y.; Fu, Y.; Du, Y. Local surface plasmon resonance (LSPR)-coupled charge separation over g-C3N4-supported WO3/BiOCl heterojunction for photocatalytic degradation of antibiotics. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128818. [Google Scholar] [CrossRef]
- Shanavas, S.; Roopan, S.M.; Priyadharshan, A.; Devipriya, D.; Jayapandi, S.; Acevedo, R.; Anbarasan, P.M. Computationally guided synthesis of (2D/3D/2D) rGO/Fe2O3/g-C3N4 nanostructure with improved charge separation and transportation efficiency for degradation of pharmaceutical molecules. Appl. Catal. B Environ. 2019, 255, 117758. [Google Scholar] [CrossRef]
- Qarajehdaghi, M.; Mehrizad, A.; Gharbani, P.; Shahverdizadeh, G.H. Quaternary composite of CdS/g-C3N4/rGO/CMC as a susceptible visible-light photocatalyst for effective abatement of ciprofloxacin: Optimization and modeling of the process by RSM and ANN. Process Saf. Environ. Prot. 2023, 169, 352–362. [Google Scholar] [CrossRef]
- AttariKhasraghi, N.; Zare, K.; Mehrizad, A.; Modirshahla, N.; Behnajady, M.A. Zeolite 4A supported CdS/g-C3N4 type-II heterojunction: A novel visible-light-active ternary nanocomposite for potential photocatalytic degradation of cefoperazone. J. Mol. Liq. 2021, 342, 117479. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, M.; Zeng, X.; Lo, I.M.C. Development of g-C3N4/TiO2/Fe3O4@SiO2 heterojunction via sol-gel route: A magnetically recyclable direct contact Z-scheme nanophotocatalyst for enhanced photocatalytic removal of ibuprofen from real sewage effluent under visible light. Chem. Eng. J. 2018, 353, 645–656. [Google Scholar] [CrossRef]
- Rapti, I.; Boti, V.; Albanis, T.; Konstantinou, I. Photocatalytic degradation of psychiatric pharmaceuticals in hospital WWTP secondary effluents using g-C3N4 and g-C3N4/MoS2 catalysts in laboratory-scale pilot. Catalysts 2023, 13, 252. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Papadaki, M.; Rapti, I.; Konstantinou, I. Photocatalytic degradation of pharmaceutical amisulpride using g-C3N4 catalyst and UV-A irradiation. Catalysts 2023, 13, 226. [Google Scholar] [CrossRef]
- Kumar, V.V.; Avisar, D.; Prasanna, L.V.; Betzalel, Y.; Mamane, H. Rapid visible-light degradation of EE2 and its estrogenicity in hospital wastewater by crystalline promoted g-C3N. J. Hazard. Mater. 2020, 398, 122880. [Google Scholar]
- Yan, W.; Yan, L.; Jing, C. Impact of doped metals on urea-derived g-C3N4 for photocatalytic degradation of antibiotics: Structure, photoactivity and degradation mechanisms. Appl. Catal. B Environ. 2019, 244, 475–485. [Google Scholar] [CrossRef]
- Huang, Y.; Luo, X.; Du, Y.; Fu, Y.; Guo, X.; Zou, C.; Wu, Y. The role of iron-doped g-C3N4 heterogeneous catalysts in Fenton-like process investigated by experiment and theoretical simulation. Chem. Eng. J. 2022, 446, 137252. [Google Scholar] [CrossRef]
- Hayat, A.; Al-Sehemi, A.G.; El-Nasser, K.S.; Taha, T.A.; Al-Ghamdi, A.A.; Syed, J.A.S.; Amin, M.A.; Ali, T.; Bashir, T.; Palamanit, A.; et al. Graphitic carbon nitride (g-C3N4)-based semiconductor as a beneficial candidate in photocatalysis diversity. Int. J. Hydrog. Energy 2022, 47, 5142–5191. [Google Scholar] [CrossRef]
- Patnaik, S.; Sahoo, D.P.; Parida, K. Recent advances in anion doped g-C3N4 photocatalysts: A review. Carbon 2021, 172, 682–711. [Google Scholar] [CrossRef]
- Yuda, A.; Kumar, A. A review of g-C3N4 based catalysts for direct methanol fuel cells. Int. J. Hydrog. Energy 2022, 47, 3371–3395. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).