Synthesis of CaCO3/Cu2O/GO Nanocomposite Catalysts for Hydrogen Production from NaBH4 Methanolysis
Abstract
1. Introduction
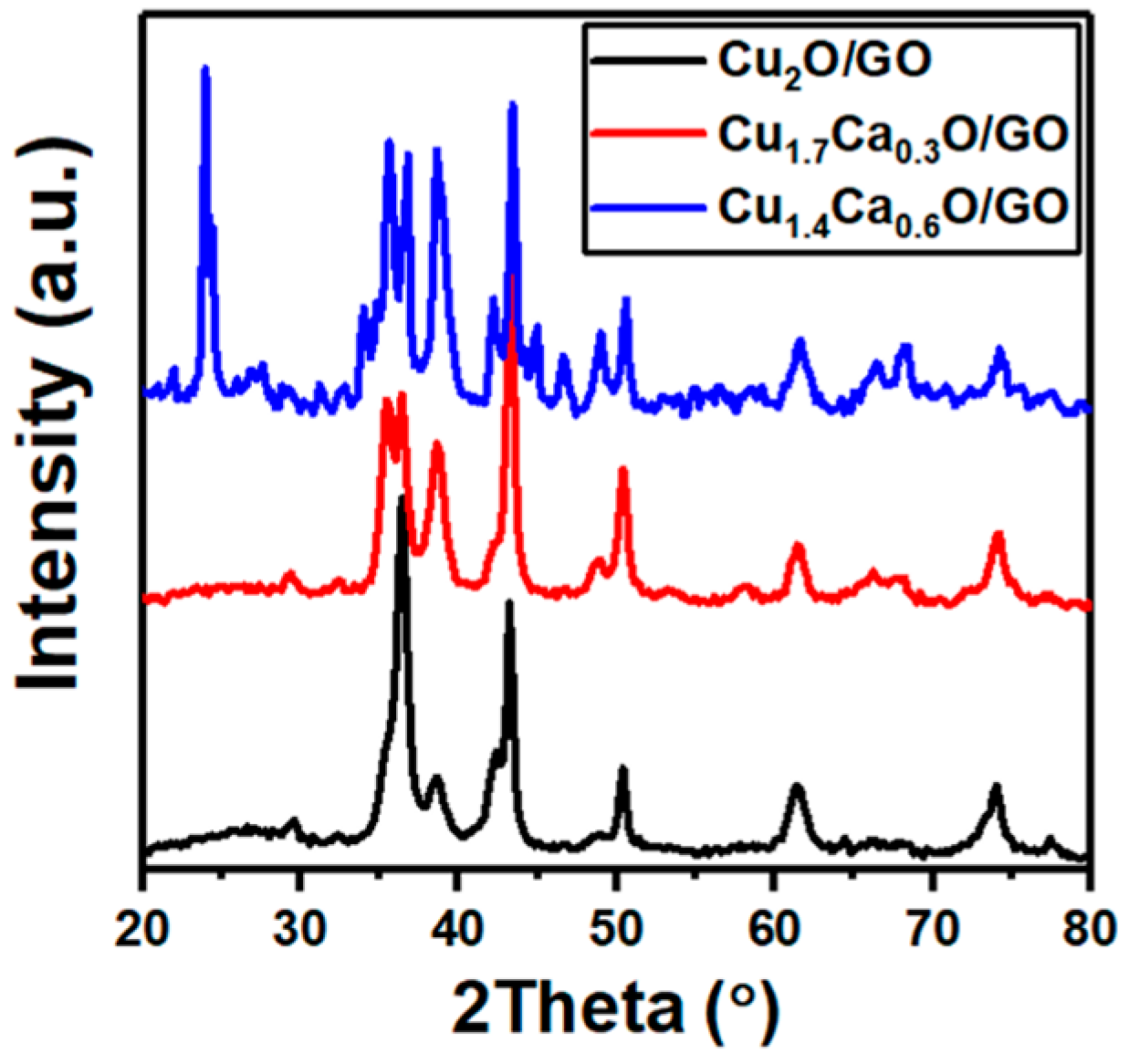
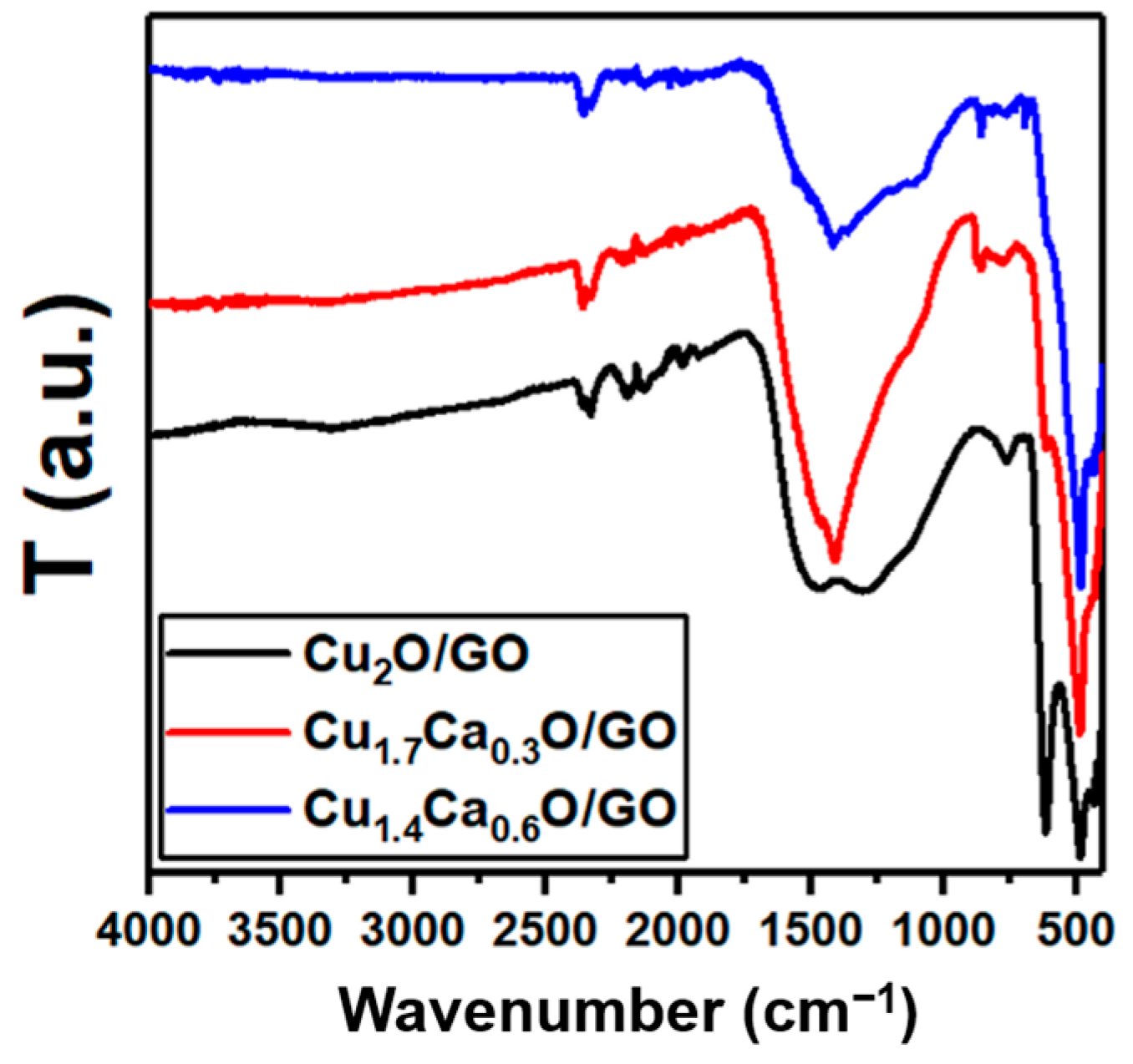
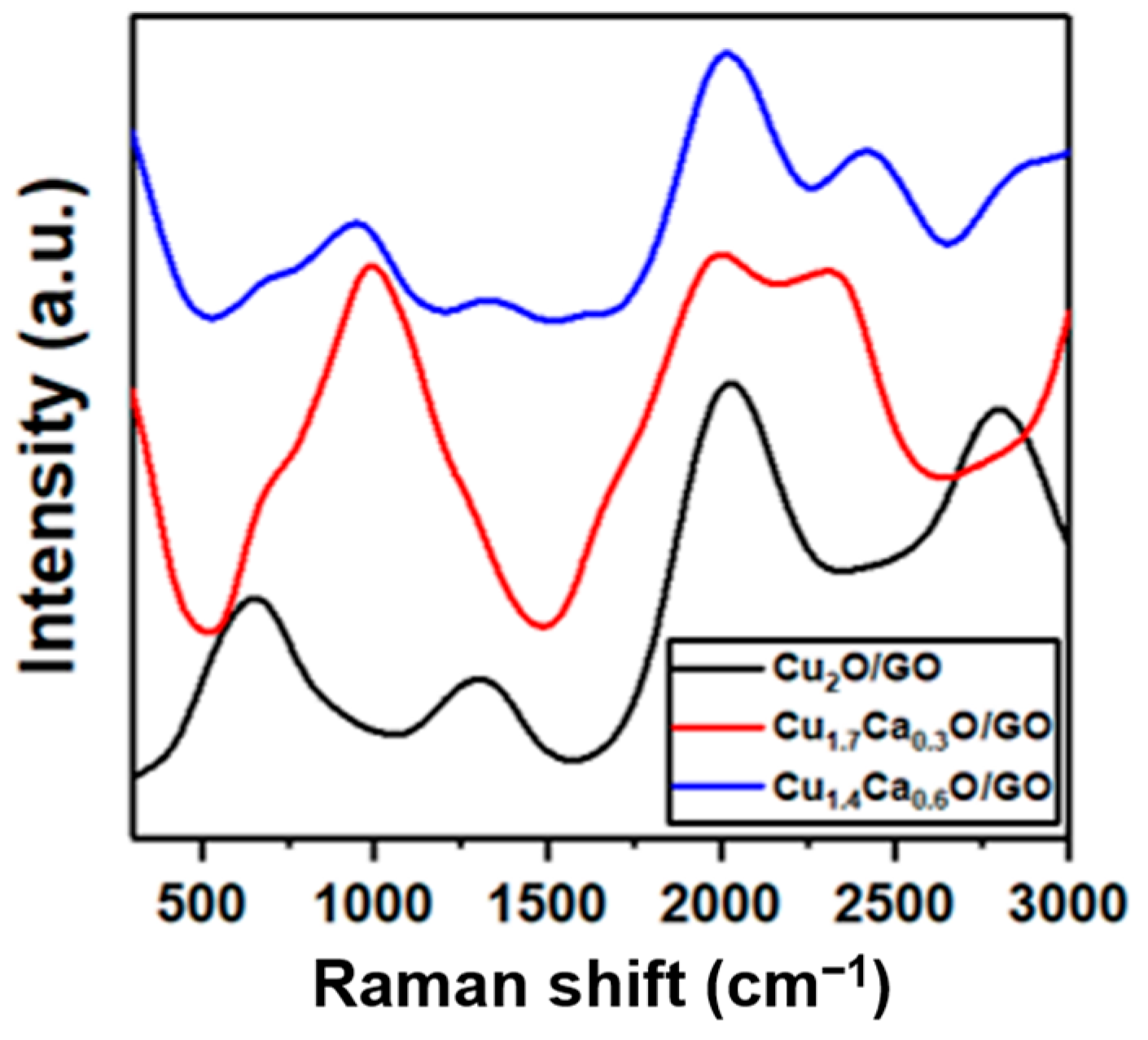
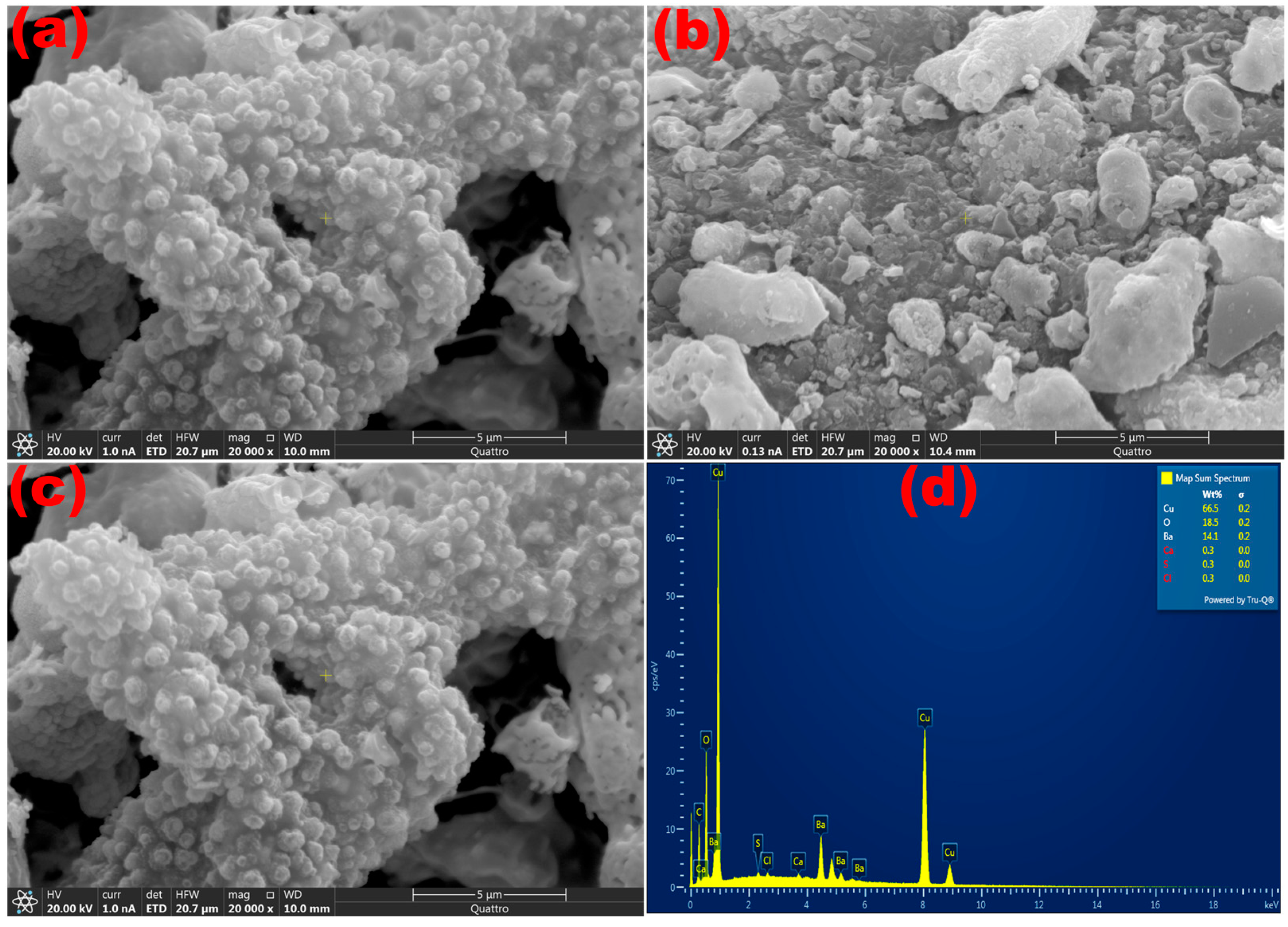
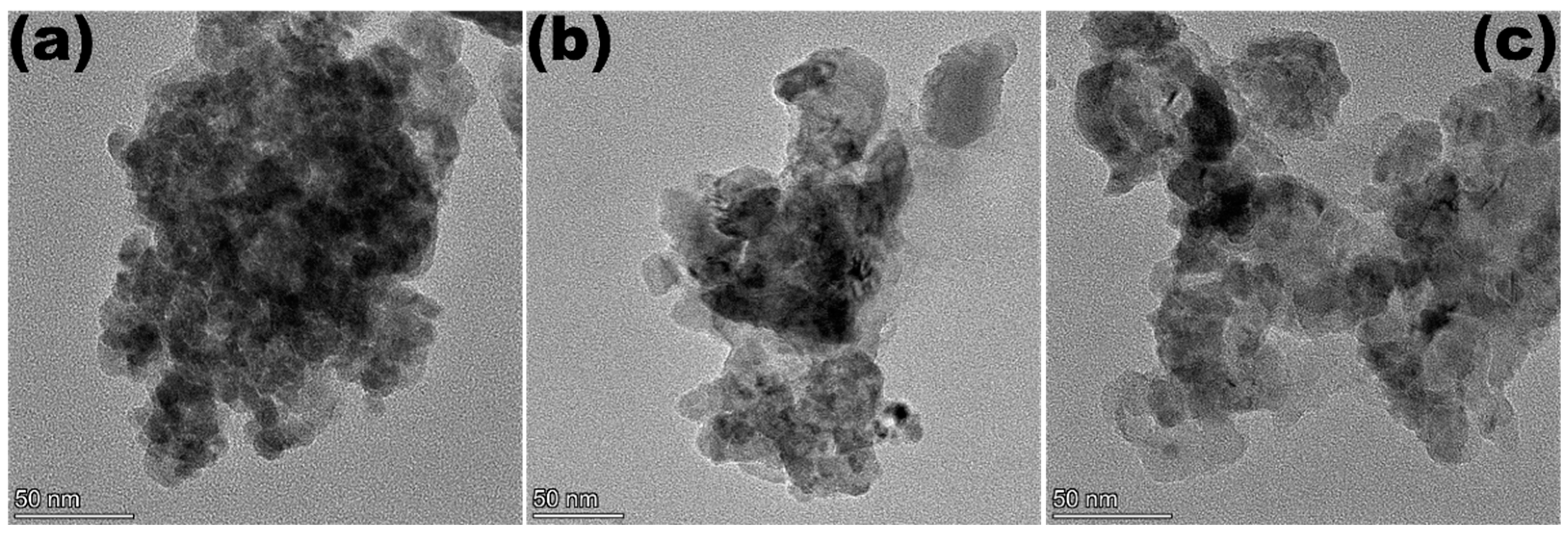
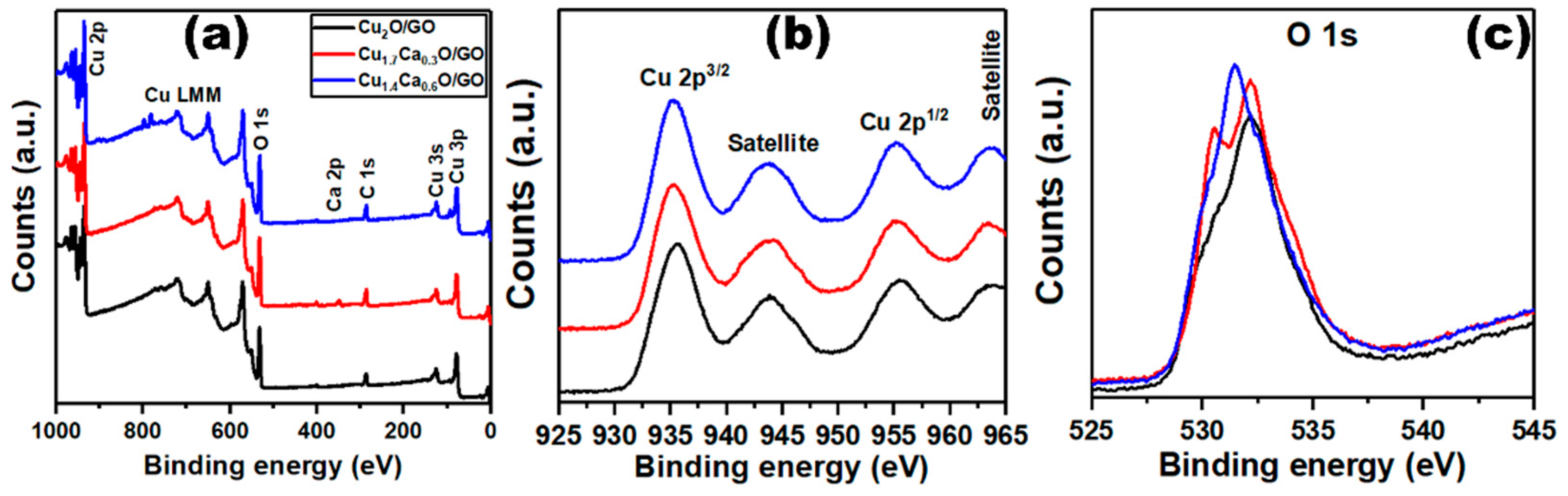
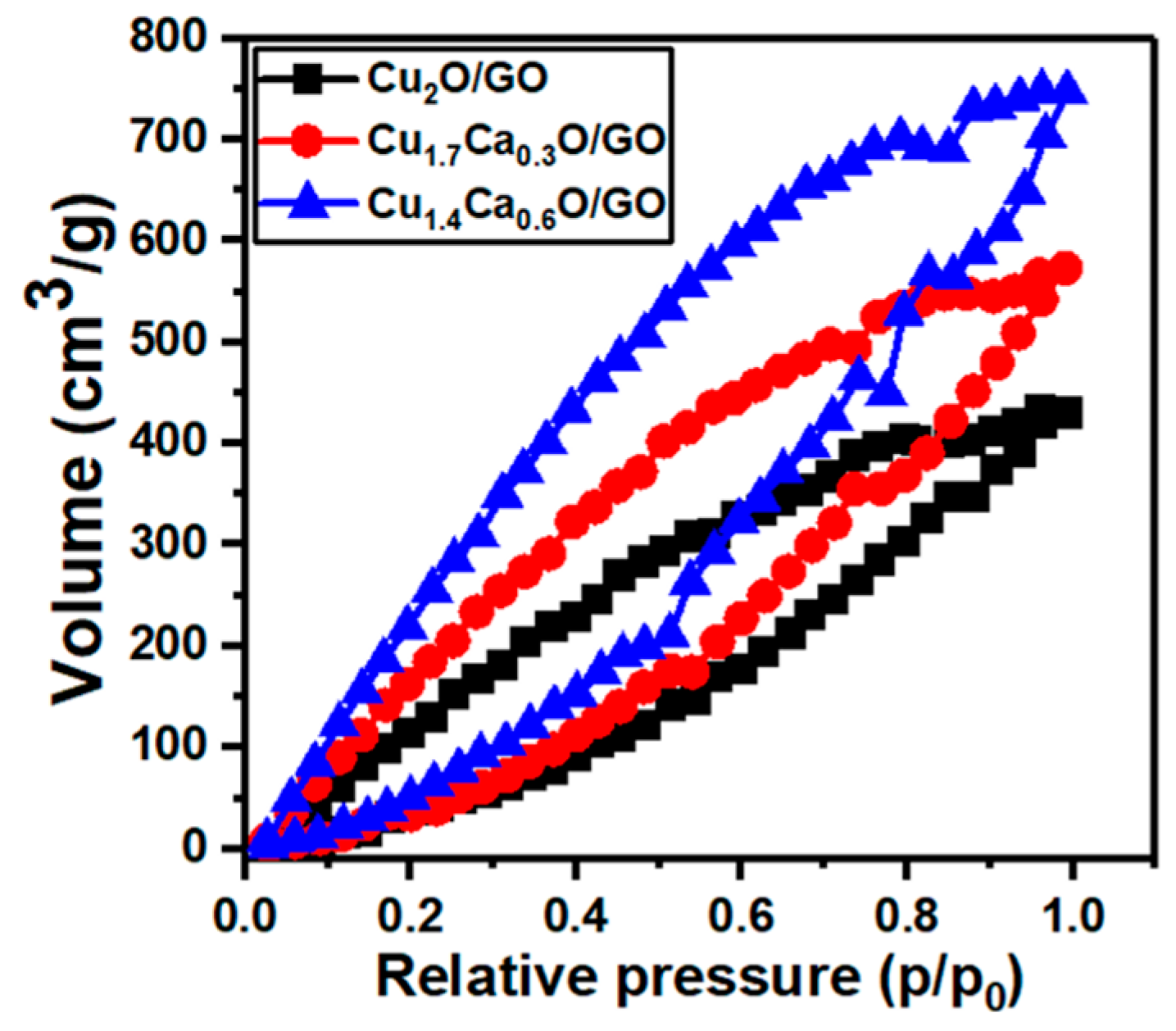
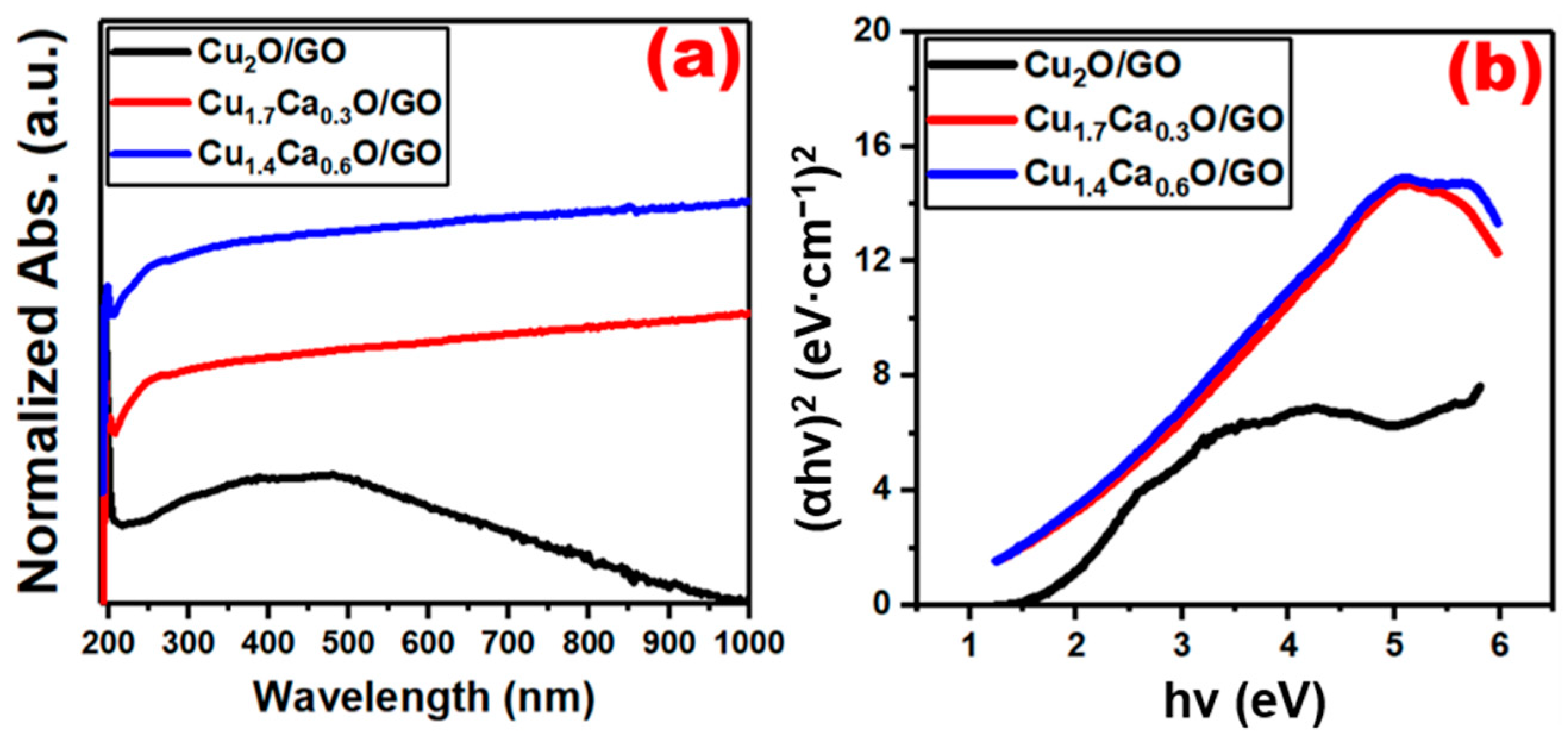
2. Results and Discussion
3. Experimental Procedure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Xia, C.; Joo, S.-W.; Hojjati-Najafabadi, A.; Xie, H.; Wu, Y.; Mashifana, T.; Vasseghian, Y. Latest advances in layered covalent organic frameworks for water and wastewater treatment. Chemosphere 2023, 329, 138580. [Google Scholar] [CrossRef] [PubMed]
- Barreto, L.; Makihira, A.; Riahi, K. The hydrogen economy in the 21st century: A sustainable development scenario. Int. J. Hydrog. Energy 2003, 28, 267–284. [Google Scholar] [CrossRef]
- Armaroli, N.; Balzani, V. The hydrogen issue. ChemSusChem 2011, 4, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.; Guo, L.; Zhao, L.; Zhang, X.; Liu, H.; Li, M.; Shen, S.; Liu, G.; Hu, X.; Zhang, X. Efficient solar hydrogen production by photocatalytic water splitting: From fundamental study to pilot demonstration. Int. J. Hydrog. Energy 2010, 35, 7087–7097. [Google Scholar] [CrossRef]
- Navya, P.N.; Daima, H.K. Rational engineering of physicochemical properties of nanomaterials for biomedical applications with nanotoxicological perspectives. Nano Converg. 2016, 3, 1. [Google Scholar] [CrossRef]
- Baladi, E.; Davar, F.; Hojjati-Najafabadi, A. Synthesis and characterization of g-C3N4-CoFe2O4-ZnO magnetic nanocomposites for enhancing photocatalytic activity with visible light for degradation of penicillin G antibiotic. Environ. Res. 2022, 215, 114270. [Google Scholar] [CrossRef]
- Mansoorianfar, M.; Nabipour, H.; Pahlevani, F.; Zhao, Y.; Hussain, Z.; Hojjati-Najafabadi, A.; Hoang, H.Y.; Pei, R. Recent progress on adsorption of cadmium ions from water systems using metal-organic frameworks (MOFs) as an efficient class of porous materials. Environ. Res. 2022, 214, 114113. [Google Scholar] [CrossRef]
- Asha, A.B.; Narain, R. Nanomaterials properties. In Polymer Science and Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 343–359. [Google Scholar]
- Haridas, D.; Gupta, V.; Sreenivas, K. Enhanced catalytic activity of nanoscale platinum islands loaded onto SnO2 thin film for sensitive LPG gas sensors. Bull. Mater. Sci. 2008, 31, 397–400. [Google Scholar] [CrossRef]
- Chavali, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef]
- Comini, E. Metal oxide nano-crystals for gas sensing. Anal. Chim. Acta 2006, 568, 28–40. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Zhou, G.; Yin, L.-C.; Ren, W.; Li, F.; Cheng, H.-M. Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 2011, 1, 107–131. [Google Scholar] [CrossRef]
- Mondal, K.; Sharma, A. Recent advances in the synthesis and application of photocatalytic metal–metal oxide core–shell nanoparticles for environmental remediation and their recycling process. RSC Adv. 2016, 6, 83589–83612. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, G.; Rawat, M. A brief review on synthesis and characterization of copper oxide nanoparticles and its applications. J. Bioelectron. Nanotechnol. 2016, 1, 9. [Google Scholar]
- Danish, M.S.S.; Estrella, L.L.; Alemaida, I.M.A.; Lisin, A.; Moiseev, N.; Ahmadi, M.; Nazari, M.; Wali, M.; Zaheb, H.; Senjyu, T. Photocatalytic Applications of Metal Oxides for Sustainable Environmental Remediation. Metals 2021, 11, 80. [Google Scholar] [CrossRef]
- Lu, C.; Qi, L.; Yang, J.; Wang, X.; Zhang, D.; Xie, J.; Ma, J. One-Pot Synthesis of Octahedral Cu2O Nanocages via a Catalytic Solution Route. Adv. Mater. 2005, 17, 2562–2567. [Google Scholar] [CrossRef]
- Kale, A.J.; Chaurasiya, R.; Dixit, A. Inorganic lead-Free Cs2AuBiCl6 perovskite absorber and Cu2O hole transport material based single-junction solar cells with 22.18% power conversion efficiency. Adv. Theory Simul. 2021, 4, 2000224. [Google Scholar] [CrossRef]
- Luo, Z.; Fu, L.; Zhu, J.; Yang, W.; Li, D.; Zhou, L. Cu2O as a promising cathode with high specific capacity for thermal battery. J. Power Sources 2019, 448, 227569. [Google Scholar] [CrossRef]
- Rai, B. Cu2O solar cells: A review. Sol. Cells 1988, 25, 265–272. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef] [PubMed]
- Hojjati-Najafabadi, A.; Aygun, A.; Tiri, R.N.E.; Gulbagca, F.; Lounissaa, M.I.; Feng, P.; Karimi, F.; Sen, F. Bacillus thuringiensis Based Ruthenium/Nickel Co-Doped Zinc as a Green Nanocatalyst: Enhanced Photocatalytic Activity, Mechanism, and Efficient H2 Production from Sodium Borohydride Methanolysis. Ind. Eng. Chem. Res. 2023, 62, 4655–4664. [Google Scholar] [CrossRef]
- Chen, W.; Ouyang, L.; Liu, J.; Yao, X.; Wang, H.; Liu, Z.; Zhu, M. Hydrolysis and regeneration of sodium borohydride (NaBH4)—A combination of hydrogen production and storage. J. Power Sources 2017, 359, 400–407. [Google Scholar] [CrossRef]
- Nabid, M.R.; Bide, Y.; Kamali, B. Hydrogen release from sodium borohydride by Fe2O3@nitrogen-doped carbon core-shell nanosheets as reasonable heterogeneous catalyst. Int. J. Hydrog. Energy 2019, 44, 25662–25670. [Google Scholar] [CrossRef]
- Tamboli, A.H.; Chaugule, A.A.; Sheikh, F.A.; Chung, W.-J.; Kim, H. Synthesis and application of CeO2-NiO loaded TiO2 nanofiber as novel catalyst for hydrogen production from sodium borohydride hydrolysis. Energy 2015, 89, 568–575. [Google Scholar] [CrossRef]
- Ugale, A.D.; Ghodke, N.P.; Kang, G.-S.; Nam, K.-B.; Bhoraskar, S.V.; Mathe, V.L.; Yoo, J.B. Cost-effective synthesis of carbon loaded Co3O4 for controlled hydrogen generation via NaBH4 hydrolysis. Int. J. Hydrog. Energy 2021, 47, 16–29. [Google Scholar] [CrossRef]
- Patil, K.N.; Prasad, D.; Bhagyashree; Manoorkar, V.K.; Nabgan, W.; Nagaraja, B.M.; Jadhav, A.H. Engineered nano-foam of tri-metallic (FeCuCo) oxide catalyst for enhanced hydrogen generation via NaBH4 hydrolysis. Chemosphere 2021, 281, 130988. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Kamanina, O.A.; Saverina, E.A.; Rybochkin, P.V.; Arlyapov, V.A.; Vereshchagin, A.N.; Ananikov, V.P. Preparation of Hybrid Sol-Gel Materials Based on Living Cells of Microorganisms and Their Application in Nanotechnology. Nanomaterials 2022, 12, 1086. [Google Scholar] [CrossRef] [PubMed]
- Vincze, T.; Micjan, M.; Pavuk, M.; Weis, M. Fabrication of cupric oxide-based transistors by a sol–gel technique using a binary solvent mixture. J. Mater. Sci. Mater. Electron. 2022, 33, 7701–7707. [Google Scholar] [CrossRef]
- Rahman, M.A.; Islam, M.T.; Singh, M.J.; Hossain, I.; Rmili, H.; Samsuzzaman, M. Magnetic, dielectric and structural properties of CoxZn(0.90−x)Al0.10Fe2O4 synthesized by sol–gel method with application as flexible microwave substrates for microstrip patch antenna. J. Mater. Res. Technol. 2022, 16, 934–943. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Davar, F. Synthesis of copper and copper(I) oxide nanoparticles by thermal decomposition of a new precursor. Mater. Lett. 2009, 63, 441–443. [Google Scholar] [CrossRef]
- Galván-Ruiz, M.; Hernández, J.; Baños, L.; Noriega-Montes, J.; Rodríguez-García, M.E. Characterization of Calcium Carbonate, Calcium Oxide, and Calcium Hydroxide as Starting Point to the Improvement of Lime for Their Use in Construction. J. Mater. Civ. Eng. 2009, 21, 694–698. [Google Scholar] [CrossRef]
- El-Sheikh, S.; El-Sherbiny, S.; Barhoum, A.; Deng, Y. Effects of cationic surfactant during the precipitation of calcium carbonate nano-particles on their size, morphology, and other characteristics. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 422, 44–49. [Google Scholar] [CrossRef]
- Alshammari, A.H.; Alshammari, M.; Alhassan, S.; Alshammari, K.; Alotaibi, T.; Taha, T.A.M. MoO3/S@g-C3N4 Nanocomposite Structures: Synthesis, Characterization, and Hydrogen Catalytic Performance. Nanomaterials 2023, 13, 820. [Google Scholar] [CrossRef]
- Abdullah, M.; Ansari, M.Z.; Ahamd, Z.; John, P.; Manzoor, S.; Shawky, A.M.; Hegazy, H.; Chughtai, A.H.; Ashiq, M.N.; Taha, T. Ag2Se/SnTe nanorod as potential candidate for energy conversion system developed via hydrothermal route. Ceram. Int. 2023, 49, 6780–6789. [Google Scholar] [CrossRef]
- Alshammari, A.H.; Alshammari, M.; Ibrahim, M.; Alshammari, K.; Taha, T.A.M. New Hybrid PVC/PVP Polymer Blend Modified with Er2O3 Nanoparticles for Optoelectronic Applications. Polymers 2023, 15, 684. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, K. FTIR spectroscopic study of CO2 adsorption/desorption on MgO/CaO catalysts. J. Phys. Chem. 1992, 9038, 9035–9038. [Google Scholar]
- Draou, K.; Bellakhal, N.; Chéron, B.; Brisset, J. Heat transfer to metals in low pressure oxygen plasma: Application to oxidation of the 90Cu–10Zn alloy. Mater. Chem. Phys. 1999, 58, 212–220. [Google Scholar] [CrossRef]
- Li, B.; Li, Y.; Zhao, Y.; Sun, L. Shape-controlled synthesis of Cu2O nano/microcrystals and their antibacterial activity. J. Phys. Chem. Solids 2013, 74, 1842–1847. [Google Scholar] [CrossRef]
- Sahoo, P.; Ishihara, S.; Yamada, K.; Deguchi, K.; Ohki, S.; Tansho, M.; Shimizu, T.; Eisaku, N.; Sasai, R.; Labuta, J.; et al. Rapid Exchange between Atmospheric CO2 and Carbonate Anion Intercalated within Magnesium Rich Layered Double Hydroxide. ACS Appl. Mater. Interfaces 2014, 6, 18352–18359. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Yang, L.; Wu, Y. Fabrication of a graphene–cuprous oxide composite. J. Solid State Chem. 2009, 182, 2486–2490. [Google Scholar] [CrossRef]
- Powell, D.; Compaan, A.; Macdonald, J.R.; Forman, R.A. Raman-scattering study of ion-implantation-produced damage in Cu2O. Phys. Rev. B 1975, 12, 20. [Google Scholar] [CrossRef]
- Claramunt, S.; Varea, A.; López-Díaz, D.; Velázquez, M.M.; Cornet, A.; Cirera, A. The Importance of Interbands on the Interpretation of the Raman Spectrum of Graphene Oxide. J. Phys. Chem. C 2015, 119, 10123–10129. [Google Scholar] [CrossRef]
- Muzyka, R.; Drewniak, S.; Pustelny, T.; Chrubasik, M.; Gryglewicz, G. Characterization of Graphite Oxide and Reduced Graphene Oxide Obtained from Different Graphite Precursors and Oxidized by Different Methods Using Raman Spectroscopy. Materials 2018, 11, 1050. [Google Scholar] [CrossRef] [PubMed]
- Rajasekar, P.; Rao, G.; Kumar, A.S.; Prakash, J.; Rathinasabapathi, P.; Venkatasubbu, G.D. Interaction of BSA with graphene oxide: Influence on the bioactivity of graphene oxide. Diam. Relat. Mater. 2023, 132, 109629. [Google Scholar] [CrossRef]
- López-Díaz, D.; Delgado-Notario, J.A.; Clericò, V.; Diez, E.; Merchán, M.D.; Velázquez, M.M. Towards Understanding the Raman Spectrum of Graphene Oxide: The Effect of the Chemical Composition. Coatings 2020, 10, 524. [Google Scholar] [CrossRef]
- Kasturi, S.; Torati, S.R.; Eom, Y.J.; Ahmad, S.; Lee, B.J.; Yu, J.S.; Kim, C. Real-time monitored photocatalytic activity and electrochemical performance of an rGO/Pt nanocomposite synthesized via a green approach. RSC Adv. 2020, 10, 13722–13731. [Google Scholar] [CrossRef]
- Platzman, I.; Brener, R.; Haick, H.; Tannenbaum, R. Oxidation of Polycrystalline Copper Thin Films at Ambient Conditions. J. Phys. Chem. C 2008, 112, 1101–1108. [Google Scholar] [CrossRef]
- Ni, M.; Ratner, B.D. Differentiating calcium carbonate polymorphs by surface analysis techniques—An XPS and TOF-SIMS study. Surf. Interface Anal. 2008, 40, 1356–1361. [Google Scholar] [CrossRef]
- Bayat, F.; Sheibani, S. Enhancement of photocatalytic activity of CuO-Cu2O heterostructures through the controlled content of Cu2O. Mater. Res. Bull. 2022, 145, 111561. [Google Scholar] [CrossRef]
- Aguirre, M.E.; Zhou, R.; Eugene, A.J.; Guzman, M.I.; Grela, M.A. Cu2O/TiO2 heterostructures for CO2 reduction through a direct Z-scheme: Protecting Cu2O from photocorrosion. Appl. Catal. B Environ. 2017, 217, 485–493. [Google Scholar] [CrossRef]
- Saeed, H.; Ikram, M.; Haider, A.; Naz, S.; Ul-Hamid, A.; Nabgan, W.; Haider, J.; Ibrahim, S.; Ullah, H.; Khan, S. Efficient dye degradation in the presence of reducing agent and bactericidal behavior with in silico molecular docking of z-scheme P3HT/g-C3N4 doped CuO heterojunction. Surf. Interfaces 2023, 38, 102804. [Google Scholar] [CrossRef]
- Ma, T.; Chang, P.R.; Zheng, P.; Ma, X. The composites based on plasticized starch and graphene oxide/reduced graphene oxide. Carbohydr. Polym. 2013, 94, 63–70. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, L.; Wang, J.; Yang, Z.; Long, M.; Hu, N.; Zhang, Y. Preparation of hollow porous Cu2O microspheres and photocatalytic activity under visible light irradiation. Nanoscale Res. Lett. 2012, 7, 347. [Google Scholar] [CrossRef] [PubMed]
- Dar, S.A.; Sharma, R.; Srivastava, V.; Sakalle, U.K. Investigation on the electronic structure, optical, elastic, mechanical, thermodynamic and thermoelectric properties of wide band gap semiconductor double perovskite Ba2InTaO6. RSC Adv. 2019, 9, 9522–9532. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, A.H.; Alshammari, K.; Alshammari, M.; Taha, T.A.M. Structural and Optical Characterization of g-C3N4 Nanosheet Integrated PVC/PVP Polymer Nanocomposites. Polymers 2023, 15, 871. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.M.; Taha, T.A.; Ezz-Eldin, F.M. Investigation of Sm2O3 effect on opto-electrical parameters and dielectric properties of some fluorophosphate glasses. J. Mater. Sci. Mater. Electron. 2021, 32, 28919–28934. [Google Scholar] [CrossRef]
- Ahmed, R.M.; Atta, M.M.; Taha, E.O. Optical spectroscopy, thermal analysis, and dynamic mechanical properties of graphene nano-platelets reinforced polyvinylchloride. J. Mater. Sci. Mater. Electron. 2021, 32, 22699–22717. [Google Scholar] [CrossRef]
- Wang, X.; Gong, J.; Dong, Y.; An, S.; Zhang, X.; Tian, J. Energy band engineering of hydroxyethyl group grafted on the edge of 3D g-C3N4 nanotubes for enhanced photocatalytic H2 production. Mater. Today Phys. 2022, 27, 100806. [Google Scholar] [CrossRef]
- Cafer, S.A.K.A. Highly active hydrogen generation from sodium borohydride methanolysis and ethylene glycolysis reactions using protonated chitosan-zeolite hybrid metal-free particles. Appl. Catal. B Environ. 2023, 325, 122335. [Google Scholar]
- Saka, C. Metal-free hybrid composite particles with phosphorus and oxygen-doped graphitic carbon nitride dispersed on kaolin for catalytic activity toward efficient hydrogen release. Int. J. Hydrog. Energy 2023, 48, 13864–13876. [Google Scholar] [CrossRef]
- Saka, C.; Kaya, M.; Bekiroğullari, M. Spirulina Platensis microalgae strain modified with phosphoric acid as a novel support material for Co-B catalysts: Its application to hydrogen production. Int. J. Hydrog. Energy 2019, 45, 2872–2883. [Google Scholar] [CrossRef]
- Fernandes, V.; Pinto, A.; Rangel, C. Hydrogen production from sodium borohydride in methanol–water mixtures. Int. J. Hydrog. Energy 2010, 35, 9862–9868. [Google Scholar] [CrossRef]
- Alshammari, A.H.; Alshammari, K.; Alotaibi, T.; Alshammari, M.; Alhassan, S.; Taha, T.A.M. In Situ Polycondensation Synthesis of NiS-g-C3N4 Nanocomposites for Catalytic Hydrogen Generation from NaBH4. Nanomaterials 2023, 13, 938. [Google Scholar] [CrossRef]
- Saka, C. g-C3N4 particles with boron and oxygen dopants/carbon vacancies for efficient dehydrogenation in sodium borohydride methanolysis. Int. J. Hydrog. Energy 2022, 47, 19016–19026. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Zhang, Y.; Luo, Y.; Zhu, H. Highly dispersed RuCo bimetallic nanoparticles supported on carbon black: Enhanced catalytic activity for hydrogen generation from NaBH4 methanolysis. J. Mater. Sci. 2018, 53, 6831–6841. [Google Scholar] [CrossRef]
- Wang, F.; Luo, Y.; Zhang, Y.; Wang, Y.; Zhu, H. Preparation of bush-like Ru/NiO-Ni foam catalyst and its performance in hydrogen production from sodium borohydride alcoholysis. Energy Fuels 2020, 34, 11365–11372. [Google Scholar] [CrossRef]
- Yan, K.; Li, Y.; Zhang, X.; Yang, X.; Zhang, N.; Zheng, J.; Chen, B.; Smith, K.J. Effect of preparation method on Ni2P/SiO2 catalytic activity for NaBH4 methanolysis and phenol hydrodeoxygenation. Int. J. Hydrog. Energy 2015, 40, 16137–16146. [Google Scholar] [CrossRef]
- Xie, L.; Wang, K.; Du, G.; Asiri, A.M.; Sun, X. 3D hierarchical CuO/Co3O4 core–shell nanowire array on copper foam for on-demand hydrogen generation from alkaline NaBH4 solution. RSC Adv. 2016, 6, 88846–88850. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Peng, X.; Jing, C.; Hu, W.; Tian, S.; Tian, J. In situ synthesis of cobalt-based tri-metallic nanosheets as highly efficient catalysts for sodium borohydride hydrolysis. Int. J. Hydrog. Energy 2016, 41, 219–226. [Google Scholar] [CrossRef]
- Guo, Y.; Dong, Z.; Cui, Z.; Zhang, X.; Ma, J. Promoting effect of W doped in electrodeposited Co-P catalysts for hydrogen generation from alkaline NaBH4 solution. Int. J. Hydrog. Energy 2012, 37, 1577–1583. [Google Scholar] [CrossRef]
- Patil, K.N.; Prasad, D.; Bhanushali, J.T.; Kim, H.; Atar, A.B.; Nagaraja, B.M.; Jadhav, A.H. Sustainable Hydrogen Generation by Catalytic Hydrolysis of NaBH4 Using Tailored Nanostructured Urchin-like CuCo2O4 Spinel Catalyst. Catal. Lett. 2019, 150, 586–604. [Google Scholar] [CrossRef]
- Kassem, A.A.; Abdelhamid, H.N.; Fouad, D.M.; Ibrahim, S.A. Metal-organic frameworks (MOFs) and MOFs-derived CuO@C for hydrogen generation from sodium borohydride. Int. J. Hydrog. Energy 2019, 44, 31230–31238. [Google Scholar] [CrossRef]
- Chinnappan, A.; Appiah-Ntiamoah, R.; Chung, W.-J.; Kim, H. Ionic liquid functionalized graphene oxide decorated with copper oxide nanostructures towards H2 generation from sodium borohydride. Int. J. Hydrog. Energy 2016, 41, 14491–14497. [Google Scholar] [CrossRef]
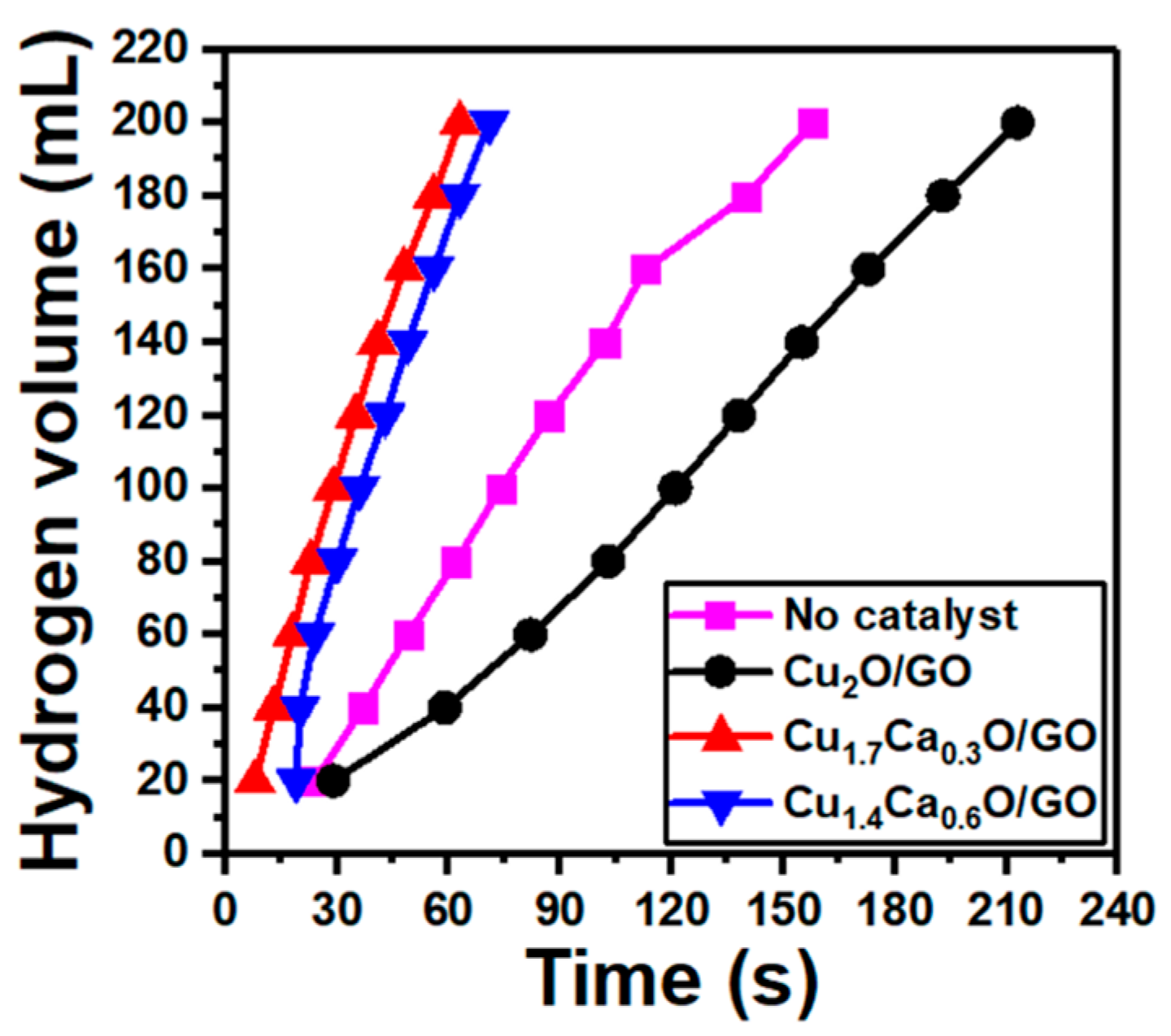
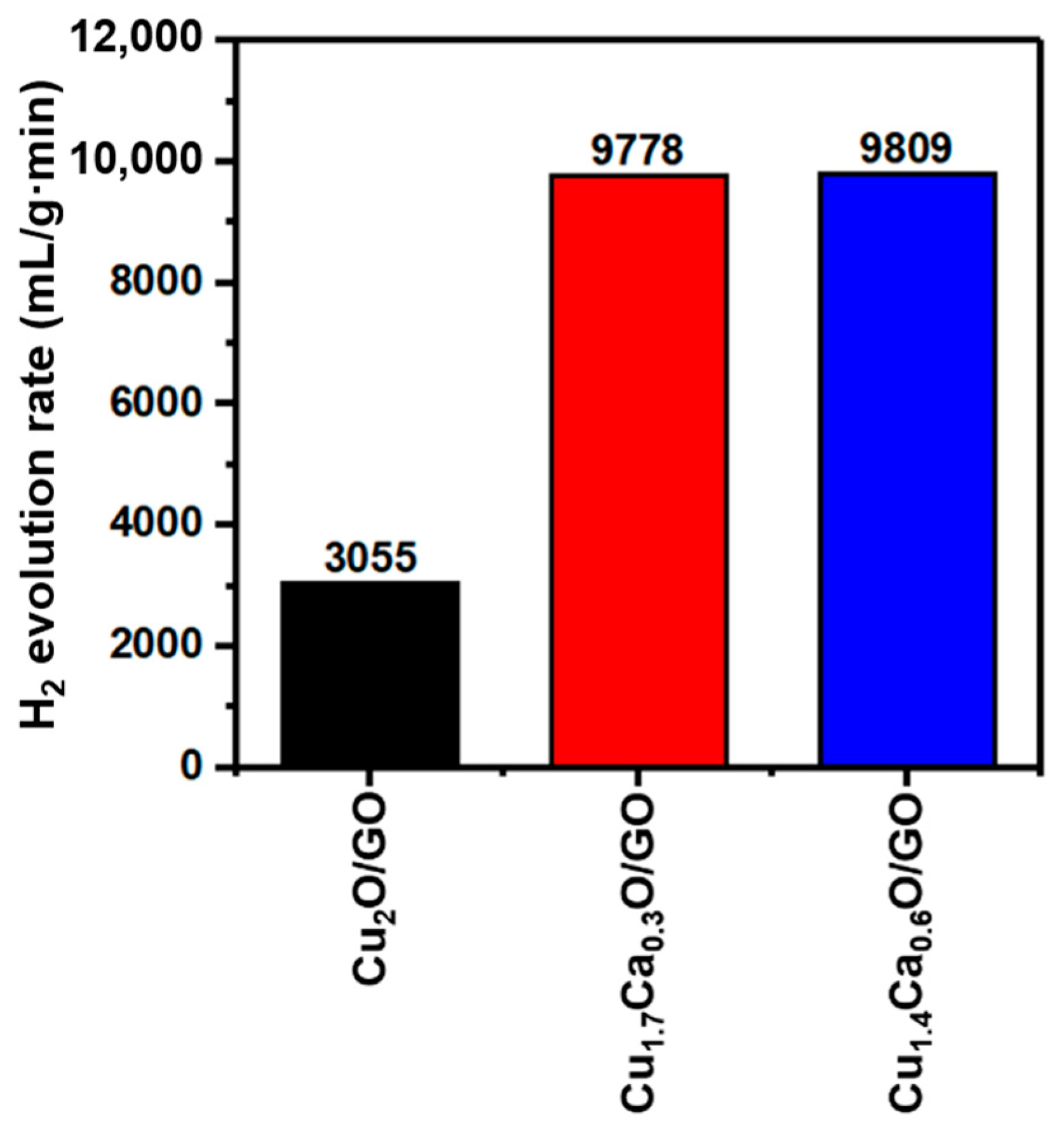
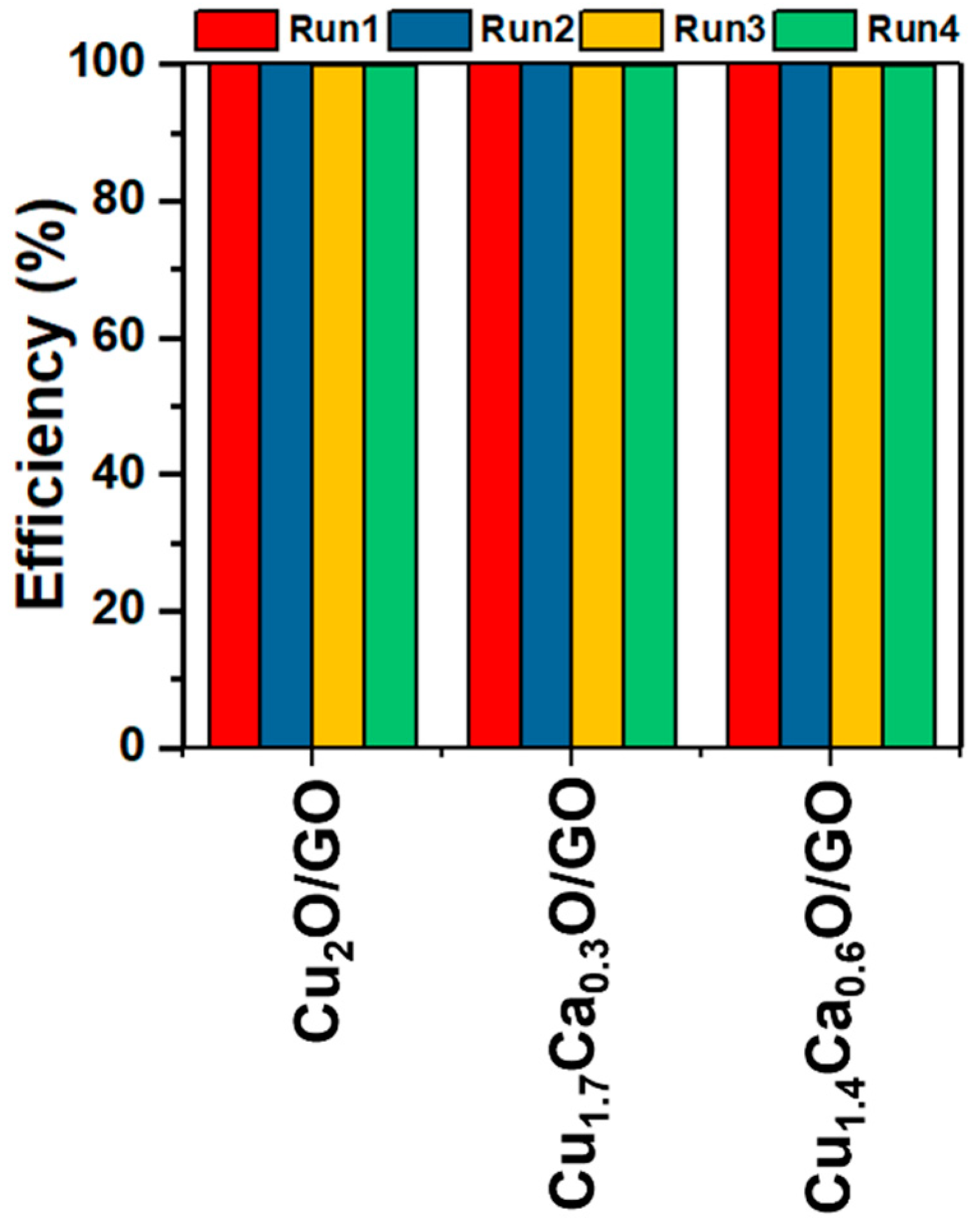
| Catalyst | Hydrogen Evolution Rate (mL/g·min) | Ref. |
|---|---|---|
| Ru5Co/C | 9360 | [67] |
| Ru/NiO-Ni | 6000 | [68] |
| Ni2P | 3700 | [69] |
| CuO/Co3O4/Cu | 6162 | [70] |
| Co-Cu-Ni | 5100 | [71] |
| Co-W-P-Cu | 5000 | [72] |
| CuCo2O | 1370 | [73] |
| CuO@C | 7240 | [74] |
| rGO-IL-CuO | 150 | [75] |
| Cu1.7Ca0.3O/GO | 9809 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshammari, M.; Alhassan, S.; Alshammari, K.; Alotaibi, T.; Taha, T.A.M.; Alshammari, A.H.; Ismael, A. Synthesis of CaCO3/Cu2O/GO Nanocomposite Catalysts for Hydrogen Production from NaBH4 Methanolysis. Catalysts 2023, 13, 1010. https://doi.org/10.3390/catal13061010
Alshammari M, Alhassan S, Alshammari K, Alotaibi T, Taha TAM, Alshammari AH, Ismael A. Synthesis of CaCO3/Cu2O/GO Nanocomposite Catalysts for Hydrogen Production from NaBH4 Methanolysis. Catalysts. 2023; 13(6):1010. https://doi.org/10.3390/catal13061010
Chicago/Turabian StyleAlshammari, Majed, Sultan Alhassan, Khulaif Alshammari, Turki Alotaibi, Taha Abdel Mohaymen Taha, Alhulw H. Alshammari, and Ali Ismael. 2023. "Synthesis of CaCO3/Cu2O/GO Nanocomposite Catalysts for Hydrogen Production from NaBH4 Methanolysis" Catalysts 13, no. 6: 1010. https://doi.org/10.3390/catal13061010
APA StyleAlshammari, M., Alhassan, S., Alshammari, K., Alotaibi, T., Taha, T. A. M., Alshammari, A. H., & Ismael, A. (2023). Synthesis of CaCO3/Cu2O/GO Nanocomposite Catalysts for Hydrogen Production from NaBH4 Methanolysis. Catalysts, 13(6), 1010. https://doi.org/10.3390/catal13061010







