Cobalt-/pH-Modified V2O5-MoO3/TiO2 Catalyst with Enhanced Activity for the Low-Temperature Selective Catalytic Reduction Process
Abstract
1. Introduction
2. Results and Discussion
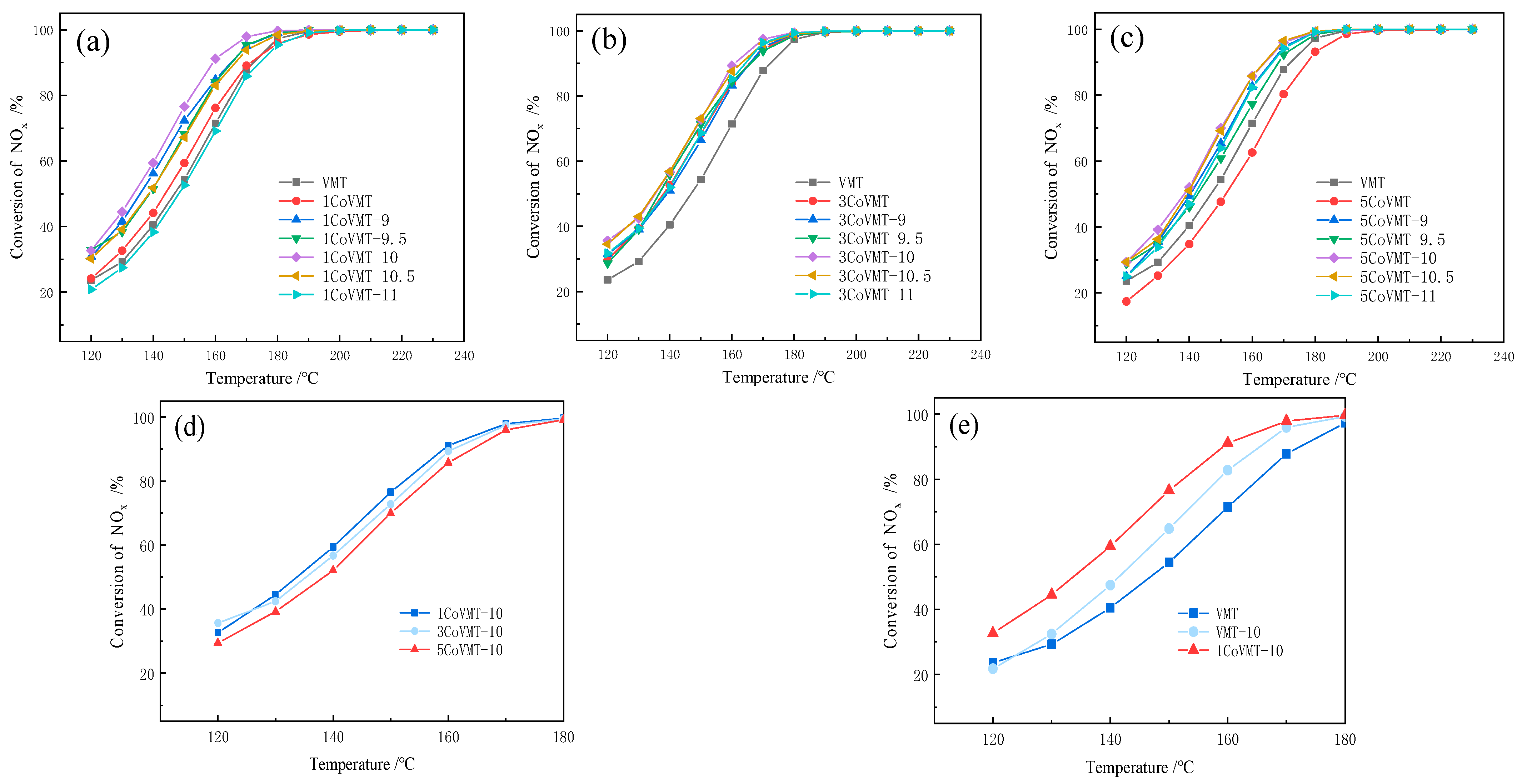
2.1. NH3-SCR Activity
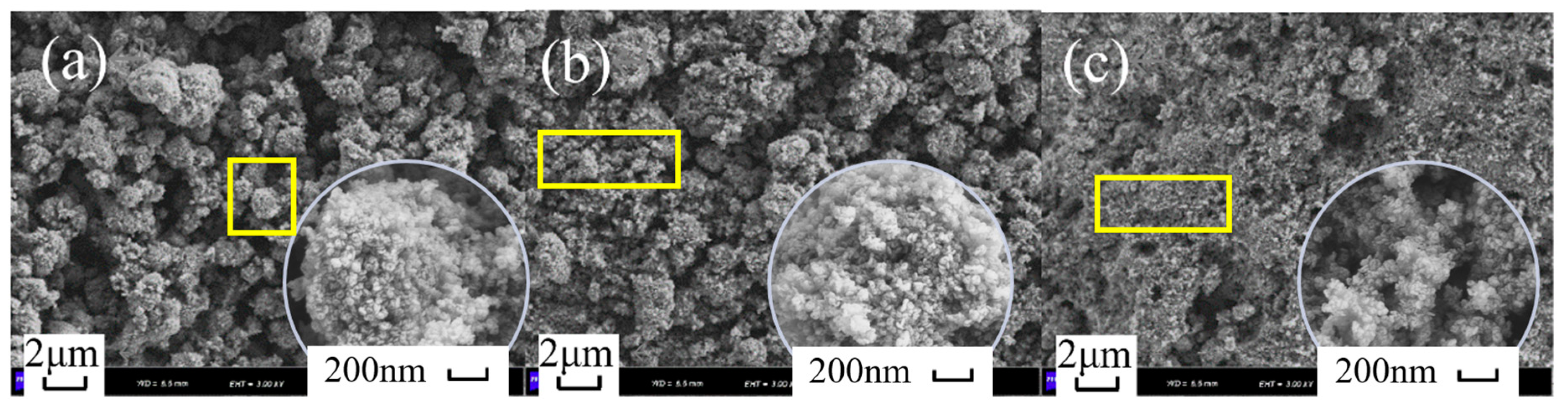
2.2. Textural Properties
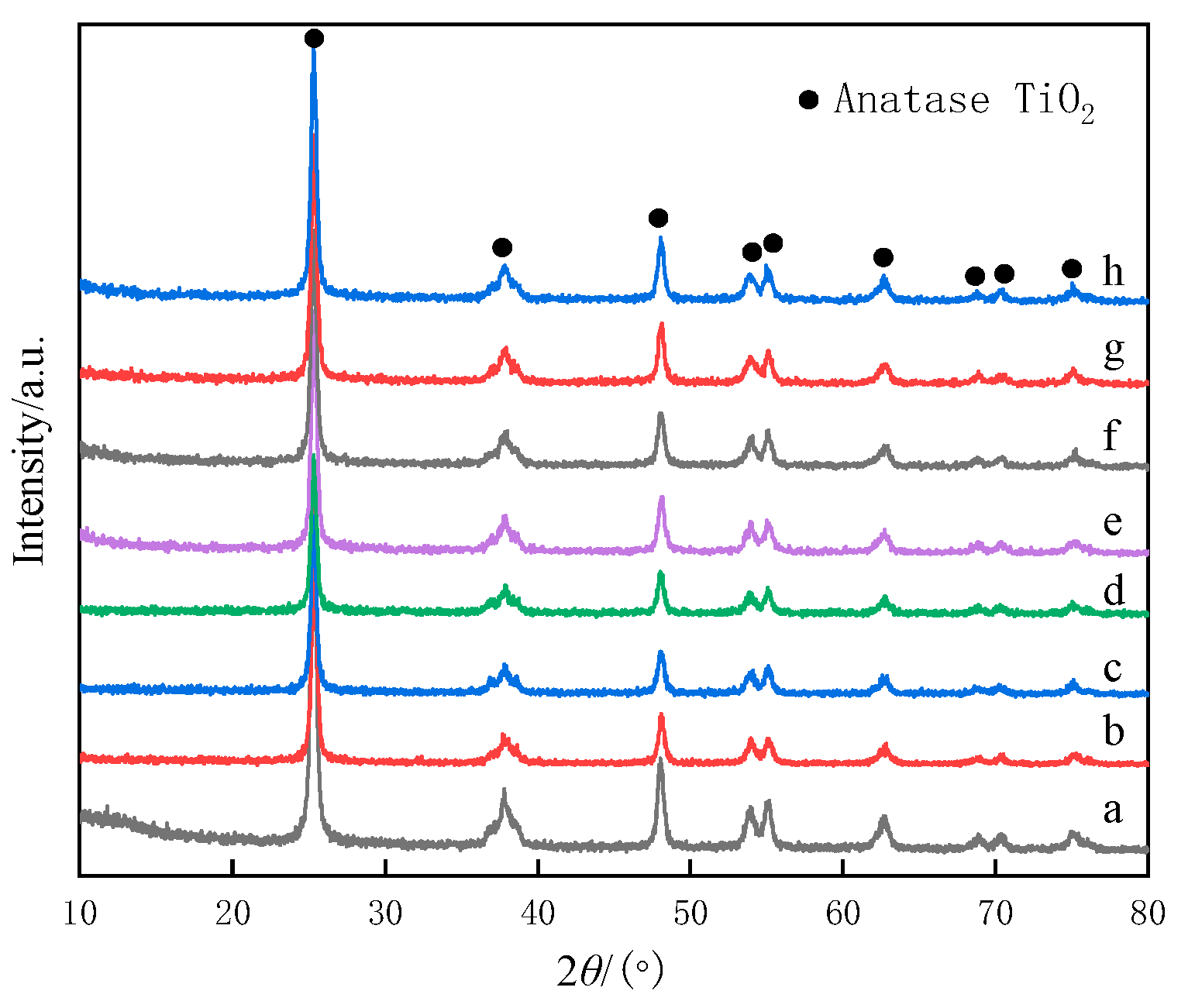
2.3. XRD Measurement
2.4. H2-TPR Experiments
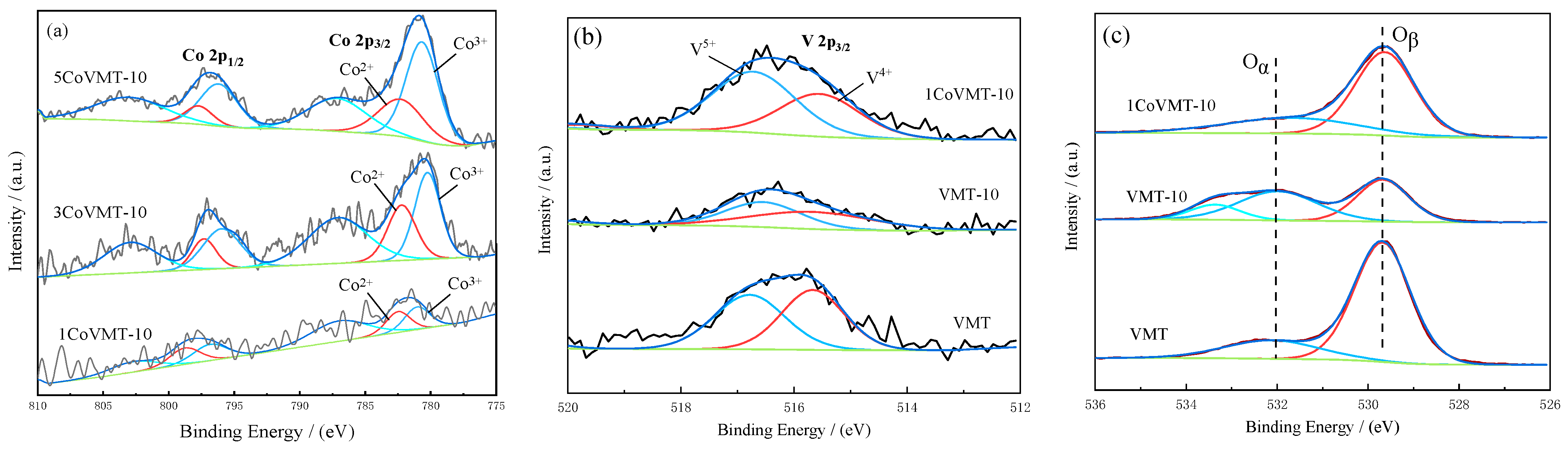
2.5. XPS Measurement
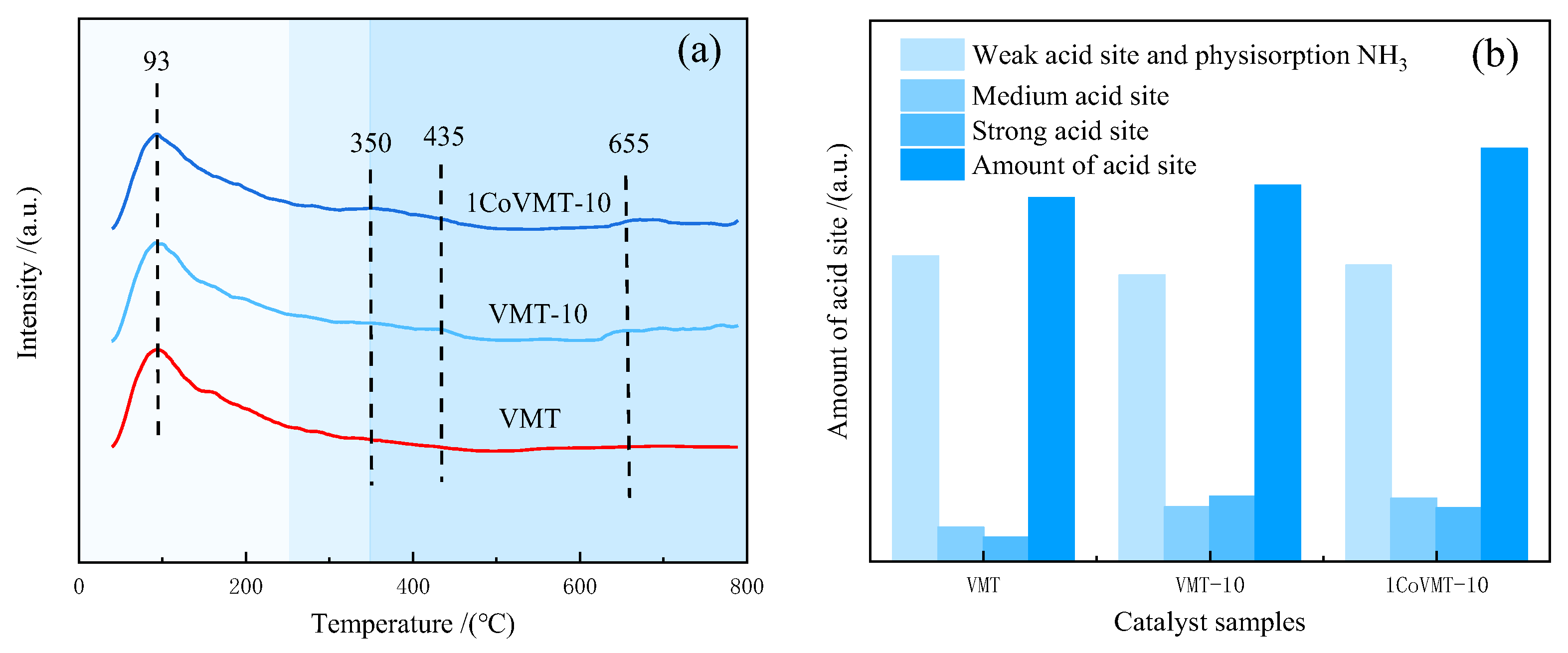
2.6. NH3-TPD Measurement
2.7. In Situ Drifts Measurement
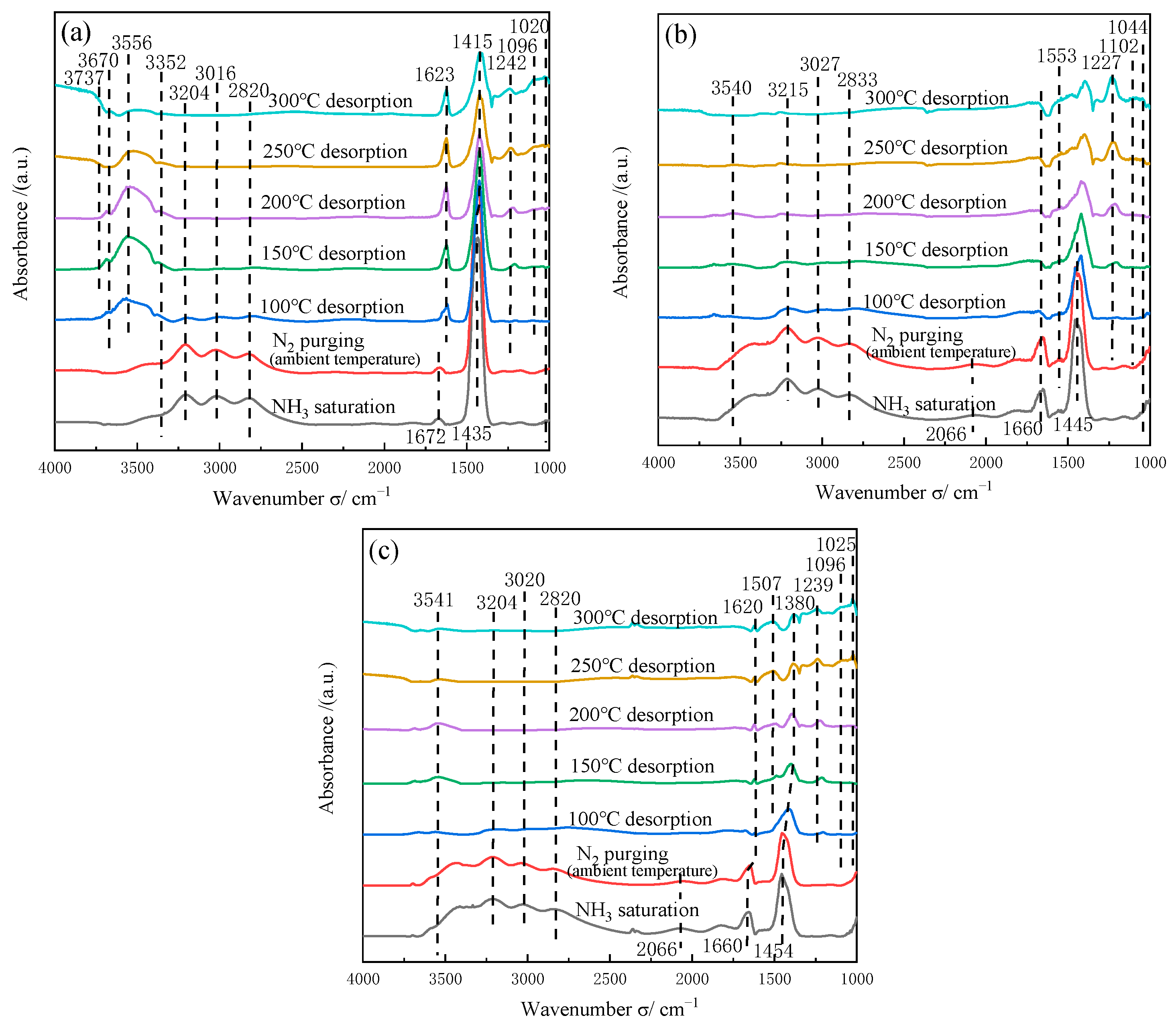
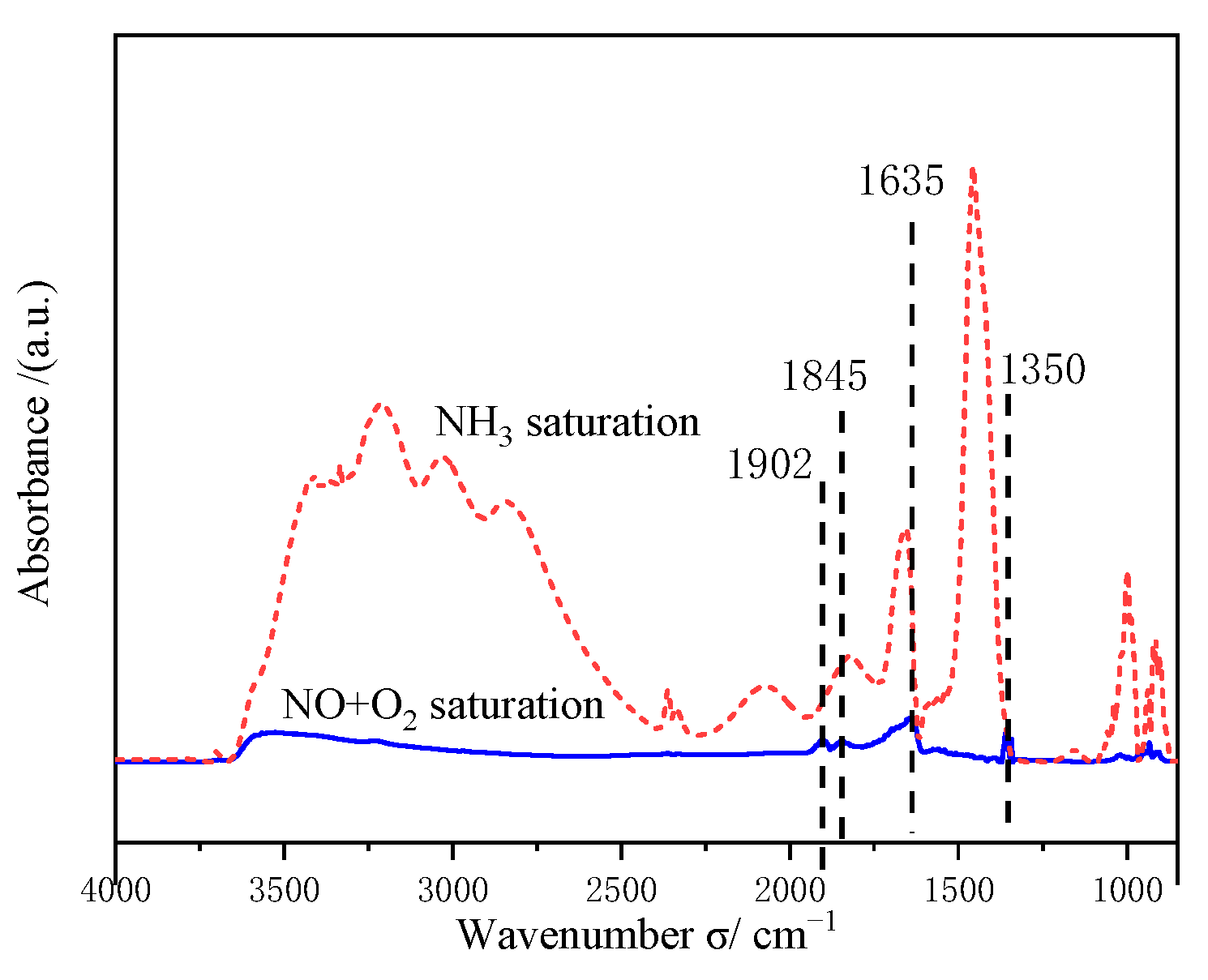
2.7.1. Adsorption of NH3
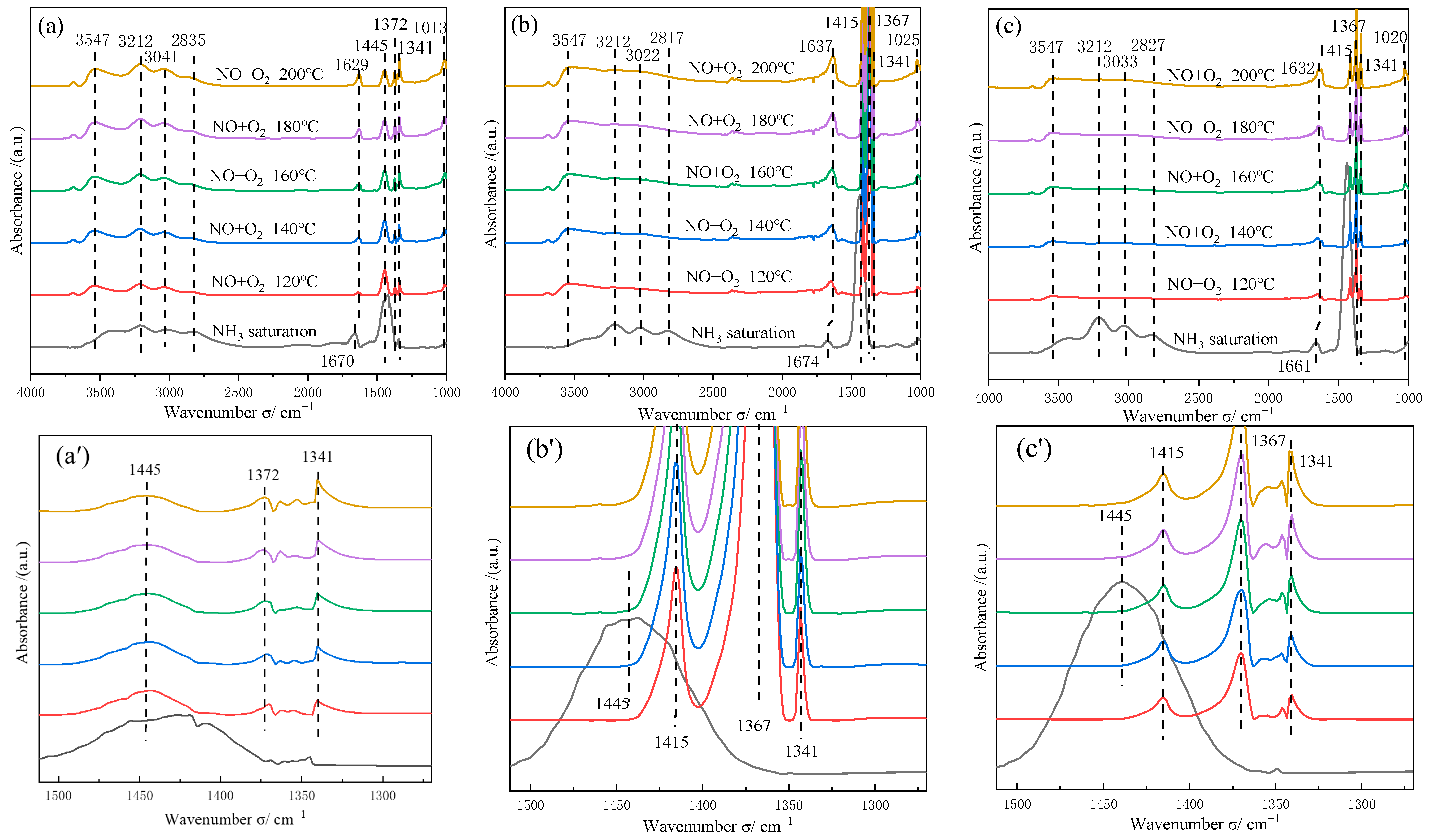
2.7.2. Reaction of NH3 with NO+O2
2.7.3. Reaction of NH3 and NO+O2 on 1CoVMT-10
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalytic Activity
3.3. Structure Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, Y.X.; Liang, H.L.; Chen, X.; Chen, C.; Wang, X.Z.; Dai, C.Y.; Hu, L.M.; Chen, Y.F. Effect of preparation methods on denitration performance of V-Mo/TiO2 catalyst. J. Fuel Chem. Technol. 2020, 48, 189–196. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Jensen, A.D.; Johnsson, J.E. Deactivation of V2O5-WO3-TiO2 SCR catalyst at a biomass-fired combined heat and power plant. Appl. Catal. B-Environ. 2005, 60, 253–264. [Google Scholar] [CrossRef]
- Nova, I.; Dall Acqua, L.; Lietti, L.; Giamello, E.; Forzatti, P. Study of thermal deactivation of a de-NOx commercial catalyst. Appl. Catal. B-Environ. 2001, 35, 31–42. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.Y.; Zhong, Q. Promotional effect of F-doped V2O5-WO3/TiO2 catalyst for NH3-SCR of NO at low-temperature. Appl. Catal. A.-Gen. 2012, 435–436, 156–162. [Google Scholar] [CrossRef]
- Zhao, W.; Zhong, Q.; Pan, Y.X.; Zhang, R. Systematic effects of S-doping on the activity of V2O5/TiO2 catalyst for low-temperature NH3-SCR. Chem. Eng. J. 2013, 228, 815–823. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, X.D.; Ran, R.; Si, Z.C.; Ma, Z.R.; Wang, B.D.O.; Weng, D. Effects of MoOx on dispersion of vanadia and low-temperature NH3-SCR activity of titania supported catalysts: Liquid acidity and steric hindrance. Appl. Surf. Sci. 2022, 585, 152710. [Google Scholar] [CrossRef]
- Jae, G.H.; Mahboob, U.; Chun, M.; Yong, S.C.; Seong, G.S.; Min, C.S.; Young, S.C.; Kim, D. Low-temperature shift DeNOx activity of Nanoflake V2O5 loaded WO3/TiO2 as NH3-SCR catalyst. Inorg. Chem. Commun. 2022, 137, 109191. [Google Scholar] [CrossRef]
- Liu, F.; He, H.; Lian, Z.; Shan, W.; Xie, L.; Asakura, K.; Yang, W.; Deng, H. Highly dispersed iron vanadate catalyst supported on TiO2 for the selective catalytic reduction of NOx with NH3. J. Catal. 2013, 307, 340–351. [Google Scholar] [CrossRef]
- Lónyi, F.; Solt, H.E.; Valyon, J.; Boix, A.; Gutierrez, L.B. The SCR of NO with methane over In,H- and Co,In,H-ZSM-5 catalysts: The promotional effect of cobalt. Appl. Catal. B-Environ. 2012, 117–118, 212–223. [Google Scholar] [CrossRef]
- Kubacka, A.; Janas, J.; Sulikowski, B. In/Co-ferrierite: A highly active catalyst for the CH4-SCR NO process under presence of steam. Appl. Catal. B-Environ. 2006, 69, 43–48. [Google Scholar] [CrossRef]
- Xue, H.Y.; Guo, X.M.; Meng, T.; Mao, D.S.; Ma, Z. NH3-SCR of NO over M/ZSM-5 (M = Mn, Co, Cu) catalysts: An in-situ DRIFTS study. Surf. Interfaces 2022, 29, 101722. [Google Scholar] [CrossRef]
- Kimihiro, A.; Chie, O.; Shinji, I.; Yasushi, S.; Masashi, I. Potassium-doped Co3O4 catalyst for direct decomposition of N2O. Appl. Catal. B-Environ. 2008, 78, 242–249. [Google Scholar] [CrossRef]
- Bo, Z.; Zhu, J.; Yang, S.; Yang, H.; Yan, J.; Cen, K. Enhanced plasma-catalytic decomposition of toluene over Co-Ce binary metal oxide catalysts with high energy efficiency. RSC Adv. 2019, 9, 7447–7456. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Han, W.; Tang, Z. Tailored design of high-stability CoMn1.5Ox@TiO2 double-wall nanocages derived from Prussian blue analogue for catalytic combustion of o-dichlorobenzene. Appl. Catal. B-Environ. 2020, 276, 119133. [Google Scholar] [CrossRef]
- Zhao, H.W.; Yang, G.P.; Hill, A.J.; Luo, B.E.; Jing, G.H. One-step ion-exchange from Na-SSZ-13 to Cu-SSZ-13 for NH3-SCR by adjusting the pH value of Cu-exchange solution: The effect of H+ ions on activity and hydrothermal stability. Microporous Mesoporous Mat. 2021, 324, 111271. [Google Scholar] [CrossRef]
- Fan, A.; Jing, Y.; Guo, J.; Shi, X.; Yuan, S.; Li, J. Investigation of Mn doped perovskite La-Mn oxides for NH3-SCR activity and SO2/H2O resistance. Fuel 2022, 310, 122237. [Google Scholar] [CrossRef]
- Besselmann, S.; Löffler, E.; Muhler, M. On the role of monomeric vanadyl species in toluene adsorption and oxidation on V2O5/TiO2 catalysts: A Raman and in situ DRIFTS study. Mol. Catal. 2000, 162, 401–411. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, B.; Du, J.; Tang, Q.; Liu, Z.H.; Liu, R.L.; Tao, C.Y. The monolithic cordierite supported V2O5-MoO3/TiO2 catalyst for NH3-SCR. Chem. Eng. J. 2016, 294, 264–272. [Google Scholar] [CrossRef]
- Pulido Melián, E.; González Díaz, O.; Ortega Méndez, A.; López, C.R.; Nereida Suárez, M.; Doña Rodríguez, J.M.; Navío, J.A.; Fernández Hevia, D.; Pérez Peña, J. Efficient and affordable hydrogen production by water photo-splitting using TiO2-based photocatalysts. Int. J. Hydrogen Energy 2013, 38, 2144–2155. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Kiwi-Minsker, L.; Rainone, F.; Renken, A. Characterization of surface vanadia forms on V/Ti-oxide catalyst via temperature-programmed reduction in hydrogen and spectroscopic methods. J. Catal. 2002, 205, 115–122. [Google Scholar] [CrossRef]
- Tang, F.S.; Zhuang, K.; Yang, F.; Yang, L.L.; Xu, B.L.; Qiu, J.H.; Fan, Y.N. Effect of dispersion state and surface properties of supported vanadia on the activity of V2O5/TiO2 catalysts for the selective catalytic reduction of NO by NH3. Chin. J. Catal. 2012, 33, 933–940. [Google Scholar] [CrossRef]
- Topsoe, N.Y.; Topsoe, H.; Dumesic, J.A. Vanadia/titania catalysts for selective catalytic reduction (SCR) of nitric-oxide by ammonia: I. combined temperature-programmed in-situ FTIR and on-line mass-spectroscopy studies. J. Catal. 1995, 151, 226–240. [Google Scholar] [CrossRef]
- Yu, C.; Huang, B.; Dong, L.; Chen, F.; Yang, Y.; Fan, Y.; Yang, Y.; Liu, X.; Wang, X. Effect of Pr/Ce addition on the catalytic performance and SO2 resistance of highly dispersed MnOx/SAPO-34 catalyst for NH3-SCR at low temperature. Chem. Eng. J. 2017, 316, 1059–1068. [Google Scholar] [CrossRef]
- Li, X.; Niu, Y.; Li, J.; Yang, M.; Chen, R.; Shao, D.; Zheng, X.; Zhang, C.; Qi, Y. Trace Co doping improves NH3-SCR performance and poisoning resistance of Ce-Mn-based catalysts. Chem. Eng. J. 2023, 454, 140180. [Google Scholar] [CrossRef]
- Chen, Q.; Guo, R.; Wang, Q.; Pan, W.; Yang, N.; Lu, C.; Wang, S. The promotion effect of Co doping on the K resistance of Mn/TiO2 catalyst for NH3-SCR of NO. J. Taiwan Inst. Chem. Eng. 2016, 64, 116–123. [Google Scholar] [CrossRef]
- Wu, R.; Li, L.; Zhang, N.; He, J.; Song, L.; Zhang, G.; Zhang, Z.; He, H. Enhancement of low-temperature NH3-SCR catalytic activity and H2O & SO2 resistance over commercial V2O5-MoO3/TiO2 catalyst by high shear-induced doping of expanded graphite. Catal. Today 2021, 376, 302–310. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Z.; Zhang, C.; Chen, L.; Tang, J.; Liao, Y.; Ma, X. Multiple pollutants control of NO, benzene and toluene from coal-fired plant by Mo/Ni impregnated TiO2-based NH3-SCR catalyst: A DFT supported experimental study. Appl. Surf. Sci. 2022, 599, 153986. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Li, J.; Zhao, S.; Wang, J.; Chu, C.; Li, C. Promotional mechanisms of activity and SO2 tolerance of Co-or Ni-doped MnOx-CeO2 catalysts for SCR of NOx with NH3 at low temperature. Chem. Eng. J. 2017, 317, 20–31. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, X. Effect of promoter thallium for a novel selectivity oxidation catalyst studied by X-ray photoelectron spectroscopy. J. Mol. Catal. 2003, 201, 161–166. [Google Scholar] [CrossRef]
- Xi, X.Y.; Zeng, F.; Zhang, H.; Wu, X.F.; Ren, J.; Bisswanger, T.; Stampfer, C.; Hofmann, J.P.; Palkovits, R.; Heeres, H.J. CO2 hydrogenation to higher alcohols over K-promoted bimetallic Fe-In catalysts on a Ce-ZrO2 support. ACS Sustain. Chem. Eng. 2021, 9, 6235–6249. [Google Scholar] [CrossRef]
- Qiu, L.; Pang, D.D.; Zhang, C.L.; Meng, J.J.; Zhu, R.S.; Ouyang, F. In situ IR studies of Co and Ce doped Mn/TiO2 catalyst for low-temperature selective catalytic reduction of NO with NH3. Appl. Surf. Sci. 2015, 357, 189–196. [Google Scholar] [CrossRef]
- Kang, S.H.; Ryu, J.H.; Kim, J.H.; Sai Prasad, P.S.; Bae, J.W.; Cheon, J.Y.; Jun, K.W. ZSM-5 supported cobalt catalyst for the direct production of gasoline range hydrocarbons by Fischer-Tropsch synthesis. Catal. Lett. 2011, 141, 1464–1471. [Google Scholar] [CrossRef]
- Lonyi, F.; Valyon, J. On the interpretation of the NH3-TPD patterns of H-ZSM-5 and H-mordenite. Microporous Mesoporous Mat. 2001, 47, 293–301. [Google Scholar] [CrossRef]
- Dong, G.; Zhang, Y.F.; Zhao, Y.; Bai, Y. Effect of the pH value of precursor solution on the catalytic performance of V2O5-WO3/TiO2 in the low temperature NH3-SCR of NOx. J. Fuel Chem. Technol. 2014, 42, 1455–1463. [Google Scholar] [CrossRef]
- Centeno, M.A.; Carrizosa, I.; Odriozola, J.A. In situ DRIFTS study of the SCR reaction of NO with ammonia over a high loading (15% weight) vanadia-titania catalyst. Phys. Chem. Chem. Phys. 1999, 1, 349–354. [Google Scholar] [CrossRef]
- Chen, Z.D.; Li, N.; Zhang, K.; Hou, L.M.; Wu, W.F. In-situ infrared spectroscopic study of the mechanism of the low temperature selective catalytic reduction of NO surface by Mn/bastnaesite concentrate. Int. J. Hydrogen Energy 2022, 47, 24777–24795. [Google Scholar] [CrossRef]
- Wang, J.P.; Yan, Z.; Liu, L.L.; Chen, Y.; Zhang, Z.T.; Wang, X.D. In situ DRIFTS investigation on the SCR of NO with NH3 over V2O5 catalyst supported by activated semi-coke. Appl. Surf. Sci. 2014, 313, 660–669. [Google Scholar] [CrossRef]
- Dall’Acqua, L.; Nova, I.; Lietti, L.; Ramis, G.; Busca, G.; Giamello, E. Spectroscopic characterisation of MoO3/TiO2 denox-scr catalysts: Redox and coordination properties. Phys. Chem. Chem. Phys. 2000, 2, 4991–4998. [Google Scholar] [CrossRef]
- Sun, D.; Liu, Q.; Liu, Z.; Gui, G.; Huang, Z. An In Situ DRIFTS Study on SCR of NO with NH3 Over V2O5/AC Surface. Catal. Lett. 2009, 132, 122–126. [Google Scholar] [CrossRef]
- Primet, M.; Pichat, P.; Mathieu, M.V. Infrared study of the surface of titanium dioxides. I. Hydroxyl groups. J. Phys. Chem. 1971, 75, 1216–1220. [Google Scholar] [CrossRef]
- Liu, K.; Liu, F.; Xie, L.; Shan, W.; He, H. DRIFTS study of a Ce-W mixed oxide catalyst for the selective catalytic reduction of NOx with NH3. Catal. Sci. Technol. 2015, 5, 2290–2299. [Google Scholar] [CrossRef]
- Karami, A.; Salehi, V. The influence of chromium substitution on an iron-titanium catalyst used in the selective catalytic reduction of NO. J. Catal. 2012, 292, 32–43. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Ge, M. DRIFT Study on Cerium-Tungsten/Titiania Catalyst for Selective Catalytic Reduction of NOx with NH3. Environ. Sci. Technol. 2010, 44, 9590–9596. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Voskoboinikov, T.; Sachtler, W.M.H. Reduction of NOx over Fe/ZSM-5 catalysts: Mechanistic causes of activity differences between alkanes. Catal. Today 1999, 54, 483–494. [Google Scholar] [CrossRef]
- Frost, R.L. An infrared and Raman spectroscopic study of the uranyl micas. Spectroc. Acta Part A-Molec. Biomolec. Spectr. 2004, 60, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Slager, T.L.; Armor, J.N. Selective reduction of NOx by methane on Co-Ferrierites: II. catalyst characterization. J. Catal. 1994, 150, 388–399. [Google Scholar] [CrossRef]
- Aylor, A.W.; Lobree, L.J.; Reimer, J.A.; Bell, A.T. An infrared study of NO reduction by CH4 over Co-ZSM-5. Stud. Surf. Sci. Catal. 1996, 101, 661–670. [Google Scholar] [CrossRef]
- Amblard, M.; Burch, R.; Southward, B.W.L. A study of the mechanism of selective conversion of ammonia to nitrogen on Ni/γ-Al2O3 under strongly oxidising conditions. Catal. Today 2000, 59, 365–371. [Google Scholar] [CrossRef]
- Zhu, Z.P.; Liu, Z.Y.; Niu, H.X.; Liu, S.J. Promoting effect of SO2 on activated carbon-supported vanadia catalyst for NO reduction by NH3 at low temperatures. J. Catal. 1999, 187, 245–248. [Google Scholar] [CrossRef]
- Abello, L.; Husson, E.; Repelin, Y.; Lucazeau, G. Vibrational spectra and valence force field of crystalline V2O5. Spectroc. Acta Part A-Molec. Biomolec. Spectr. 1983, 39, 641–651. [Google Scholar] [CrossRef]
- Topsøe, N.; Topsøe, H. Combined in-situ FTIR and on-line activity studies: Applications to vanadia-titania DeNOx catalyst. Catal. Today 1991, 9, 77–82. [Google Scholar] [CrossRef]
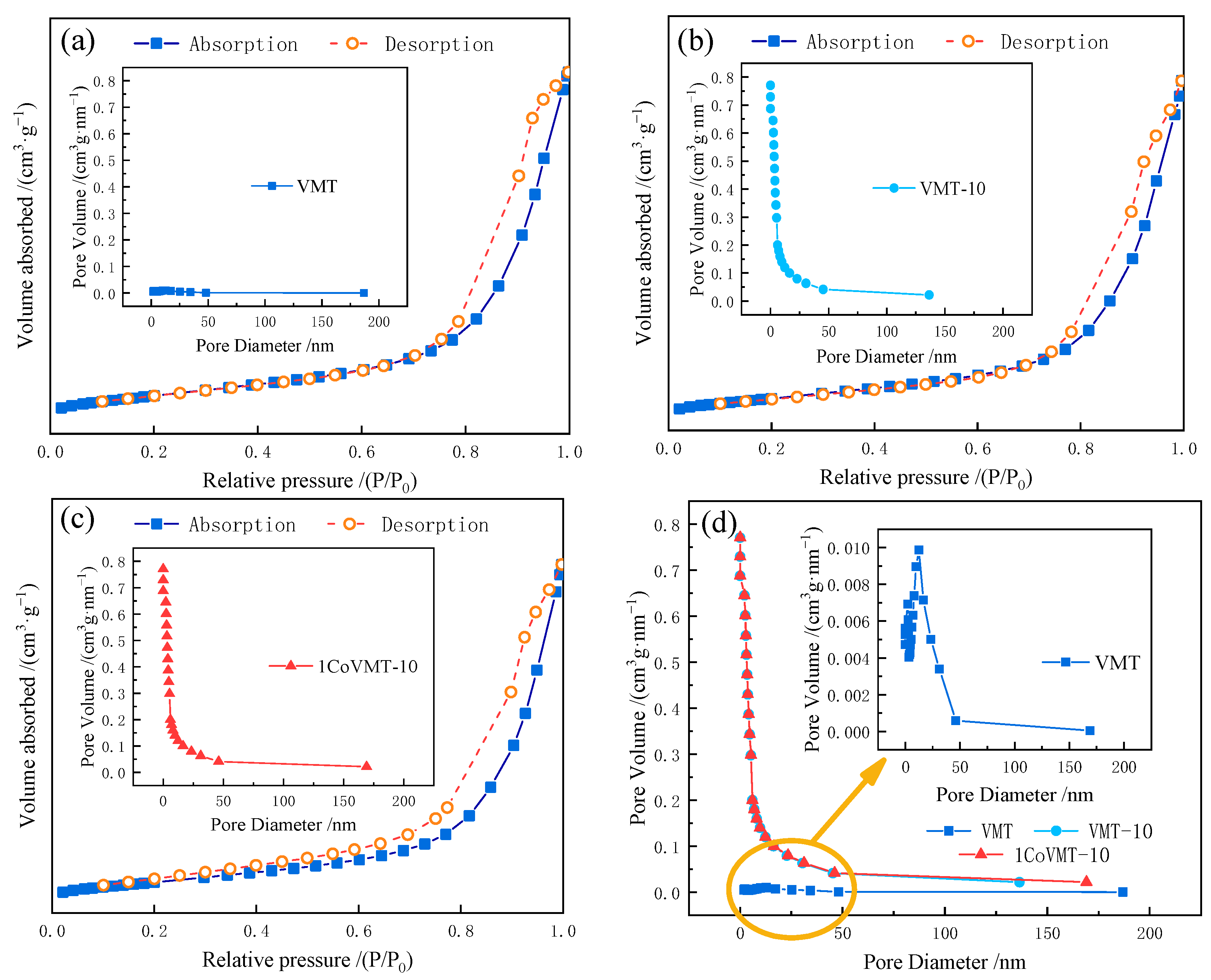

| Sample | pH Value | Specific Surface Area A/(m2·g−1) | Pore Volume v/(cm3·g–1) | Average Pore Diameter d/(nm) |
|---|---|---|---|---|
| VMT | 2.5 | 72 | 0.31 | 17.1 |
| VMT-10 | 10 | 78 | 0.37 | 18.8 |
| 1CoVMT-10 | 10 | 75 | 0.37 | 19.9 |
| Sample | Binding Energy (eV) | Ratio of Valence State | ||||
|---|---|---|---|---|---|---|
| Co 2p Co2+ Co3+ | Oα Oβ | V 2p V4+ V5+ | V5+/ V4+ | Oα/(Oβ + Oα) | Co2+/ Co3+ | |
| VMT | — | 532.5 529.6 | 515.7 516.8 | 1.02 | 14.11 | — |
| VMT-10 | — | 532.0 529.8 | 515.7 516.6 | 1.12 | 58.34 | — |
| 1CoVMT-10 | 782.2 779.9 | 532.3 529.5 | 515.6 516.7 | 1.49 | 16.30 | 5.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Zhang, Y.; Fan, X.; Li, J. Cobalt-/pH-Modified V2O5-MoO3/TiO2 Catalyst with Enhanced Activity for the Low-Temperature Selective Catalytic Reduction Process. Catalysts 2023, 13, 844. https://doi.org/10.3390/catal13050844
Wang R, Zhang Y, Fan X, Li J. Cobalt-/pH-Modified V2O5-MoO3/TiO2 Catalyst with Enhanced Activity for the Low-Temperature Selective Catalytic Reduction Process. Catalysts. 2023; 13(5):844. https://doi.org/10.3390/catal13050844
Chicago/Turabian StyleWang, Ruonan, Yanli Zhang, Xing Fan, and Jian Li. 2023. "Cobalt-/pH-Modified V2O5-MoO3/TiO2 Catalyst with Enhanced Activity for the Low-Temperature Selective Catalytic Reduction Process" Catalysts 13, no. 5: 844. https://doi.org/10.3390/catal13050844
APA StyleWang, R., Zhang, Y., Fan, X., & Li, J. (2023). Cobalt-/pH-Modified V2O5-MoO3/TiO2 Catalyst with Enhanced Activity for the Low-Temperature Selective Catalytic Reduction Process. Catalysts, 13(5), 844. https://doi.org/10.3390/catal13050844






