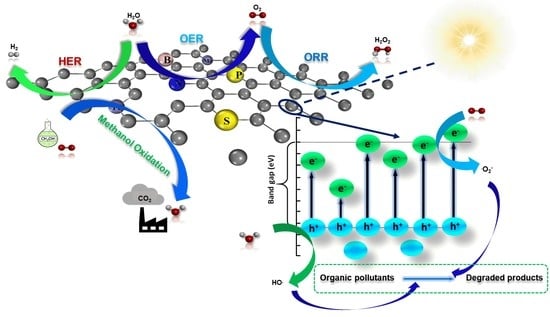
Catalytical Performance of Heteroatom Doped and Undoped Carbon-Based Materials
Abstract
1. Introduction
2. CBMs as Catalysts in Energy Conversion and Storage
2.1. Oxygen Evolution Reaction
2.1.1. Role of CBMs in the OER
2.1.2. Effect of Heteroatom Doping on OER
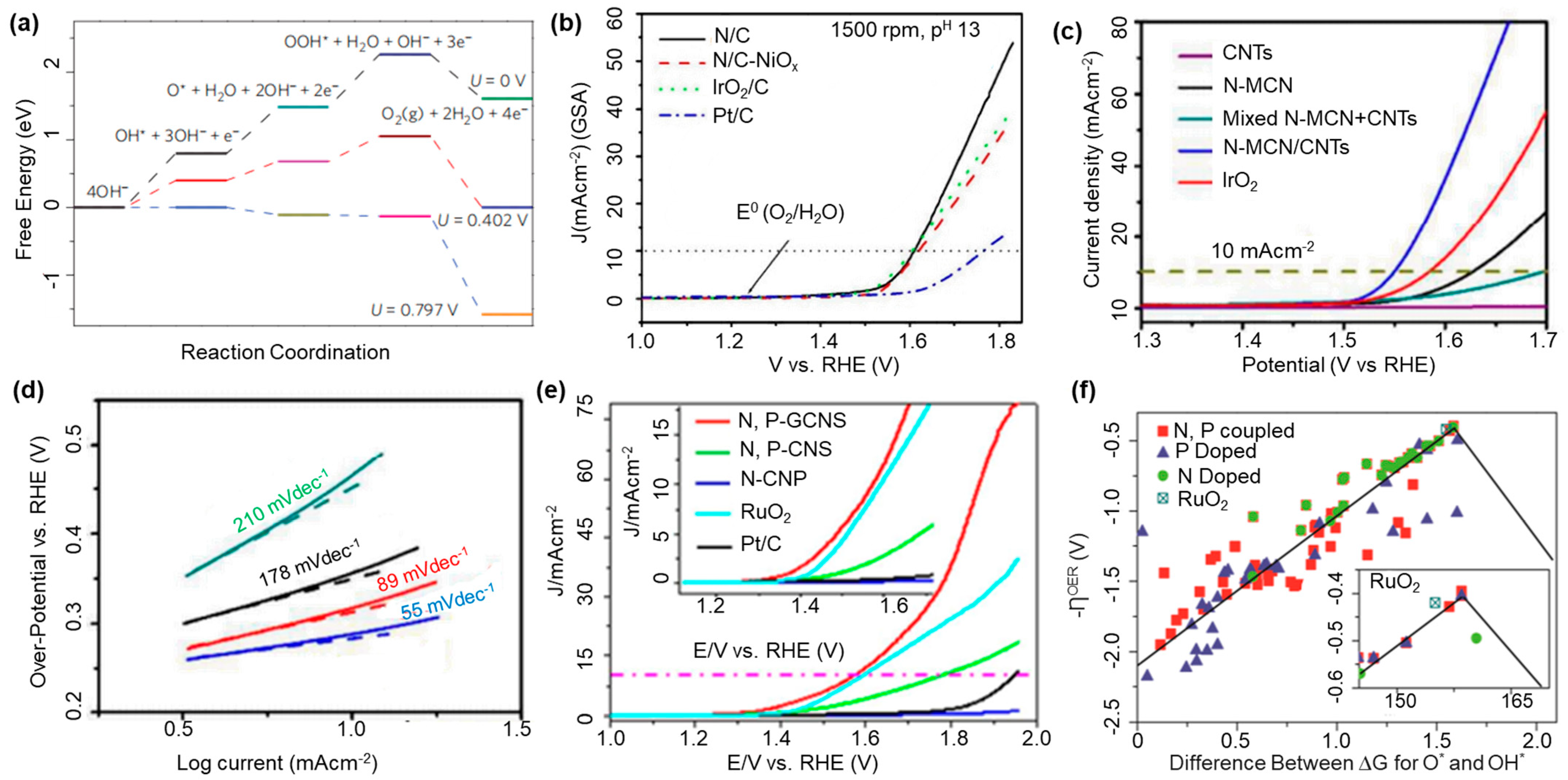
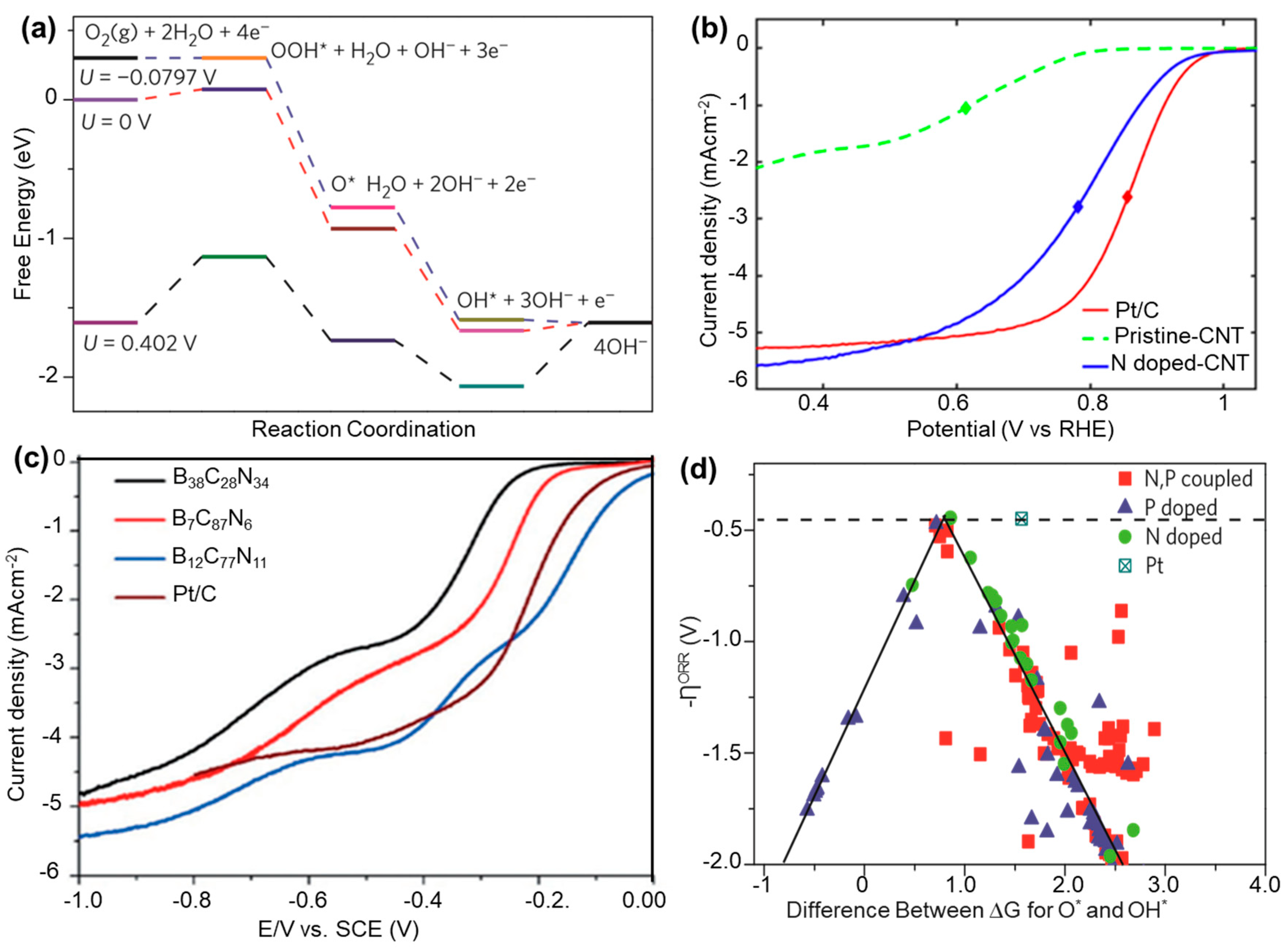
2.2. Oxygen Reduction Reaction (ORR)
2.2.1. Role of CBMs in an ORR
2.2.2. Effect of Heteroatom Doping on ORR
| Electrocatalyst for ORR | No. of Electrons Transferred | On-Set Potential (Eonset) (V) | Half-Wave Potential(E1/2) (V) | Limiting Current Density (mA/cm2) | Ref. | |
|---|---|---|---|---|---|---|
| Pt/C | 3.99 | 1.00 | 0.86 | −5.24 | [57] | |
| pristine CNT | - | 0.80 | 0.61 | −2.62 | ||
| N-doped CBMs | N-CNT | 3.92 | 0.96 | 0.78 | −5.82 | |
| N-mesoporous carbon | 4.02 | 0.949 | lesser as compared to Pt/C | ~−4.9 | [65] | |
| Pt/C | - | 0.95 | - | - | [59] | |
| Pristine Graphene | - | 0.82 | - | - | ||
| P-doped CBMs | P-graphene | 3.8 | 0.92 | - | −3.63 | |
| P-graphene/carbon black | 3.8 | 0.92 | - | −4.18 | ||
| N, P-co-doped CBMs | N, P doped graphene | 0.88 | 0.774 | - | [64] | |
| N, P-doped mesoporous carbon | ∼4.0 | 0.94 | 0.85 | comparable to that of Pt/C | [23] | |
| N, O-doped mesoporous carbon | 3.78–4 | 0.5–0.7 | - | ~−5.3 | [66] | |
| N, S, P co-doped graphene | >3.8 | 0.96 | 0.857 | - | [64] | |
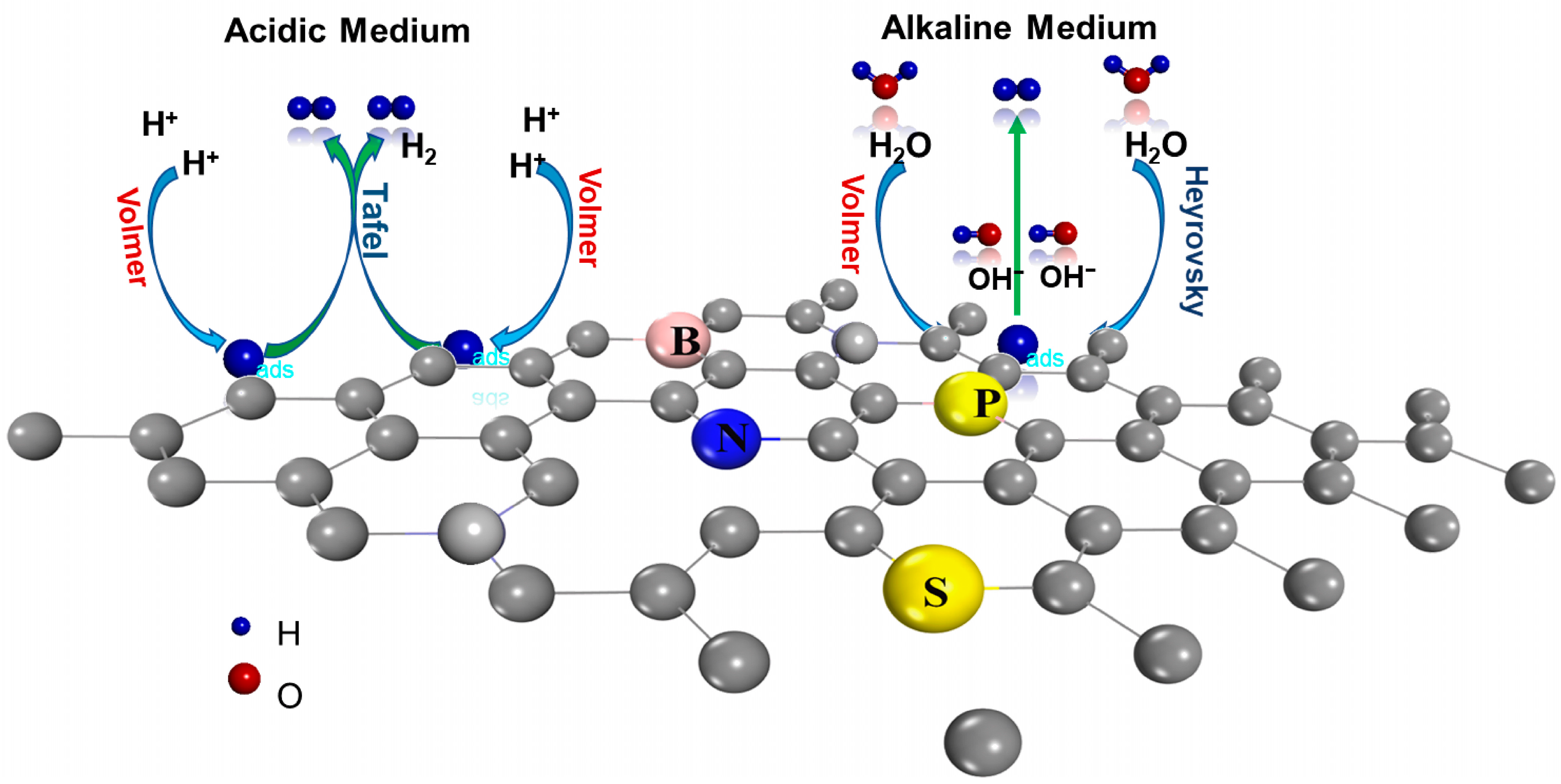
2.3. Hydrogen Evolution Reaction (HER)
2.3.1. Role of CBMs in a HER
2.3.2. Effect of Heteroatom Doping on HER
2.4. Methanol Oxidation
2.4.1. Role of CBMs in Methanol Oxidation
2.4.2. Effect of Heteroatom Doping on Methanol Oxidation
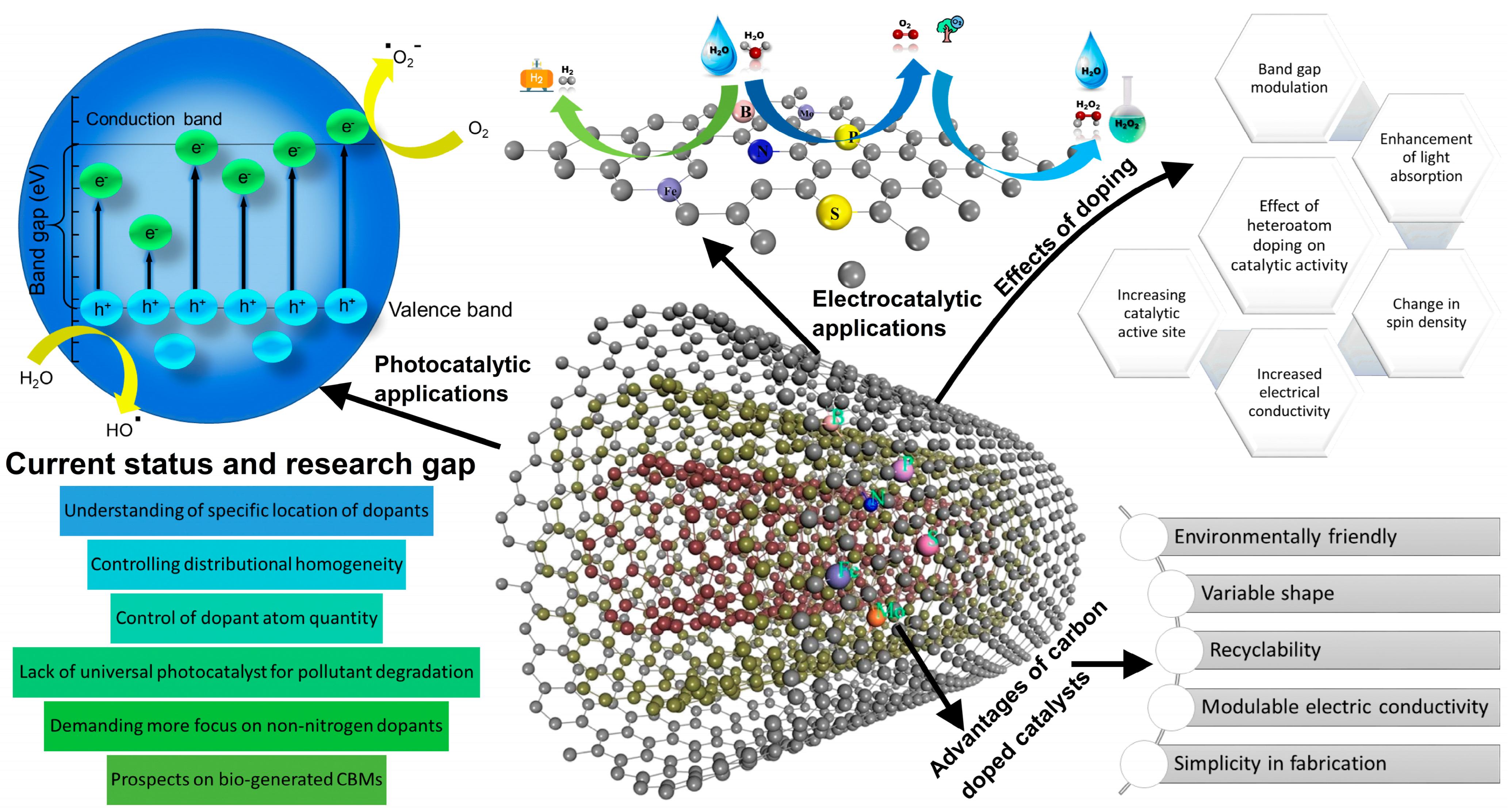
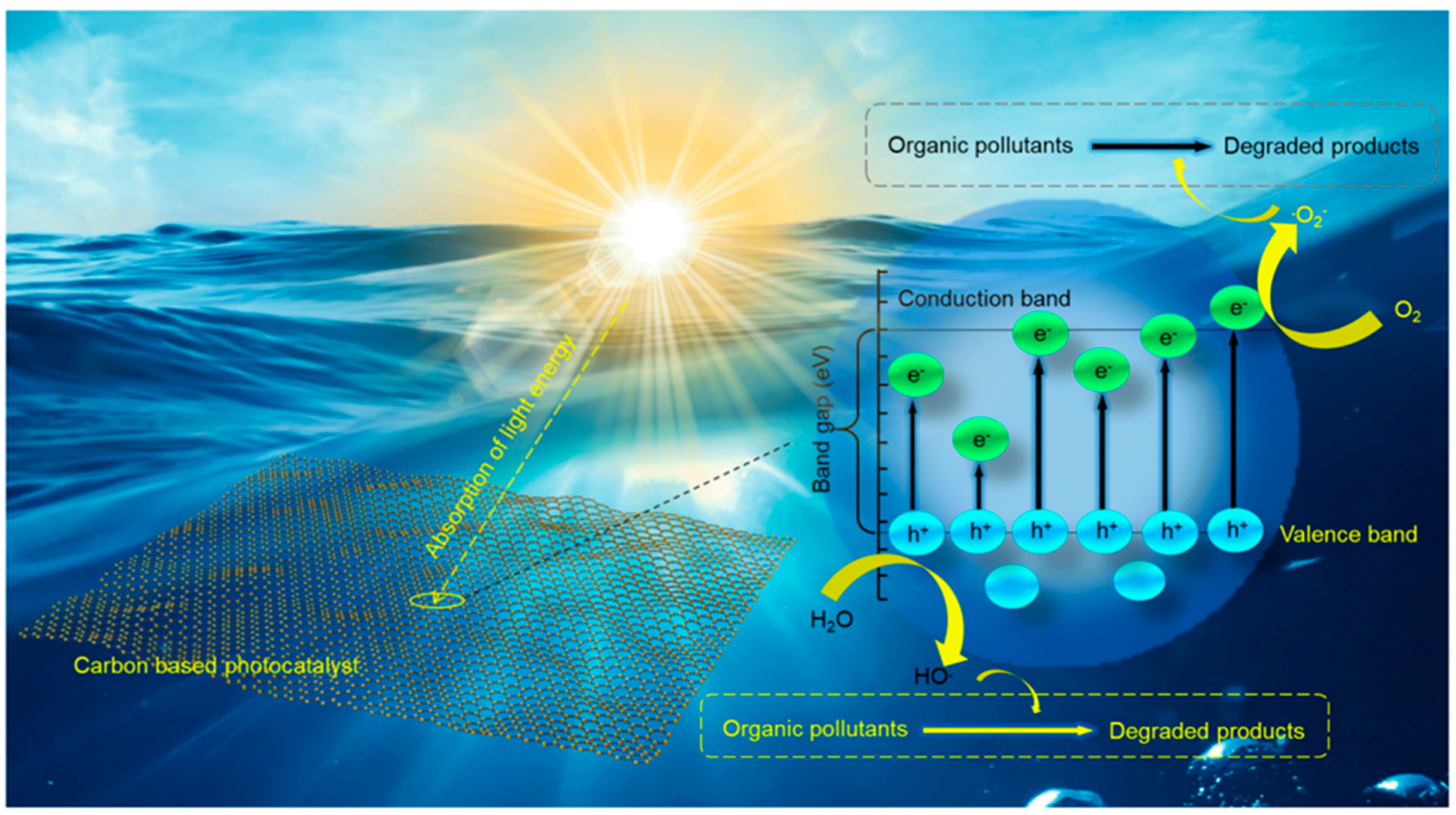
3. CBMs in Photocatalytic Decomposition of Organic Pollutants
3.1. Role of CBMs in the Photocatalysis Process
3.2. Research Progress of Heteroatom-Doped CBMs in Photocatalysis
4. Conclusions and Future Perspectives
- i.
- There is still a lack of understanding about the specific location of heteroatoms, the nature of their catalytic site in carbon materials and how they are doped. Combining experimental studies, state-of-the-art characterizations, and robust computational simulations is essential to a deeper understanding of the structure, mechanism, and thermodynamics of the catalytic core.
- ii.
- While N-doped CBMS has been extensively researched as catalysts in several organic transformations, it is evident from this work that there are other dopants with suitable physicochemical properties, such as B, P, and S, that may be included in the carbon core. Additionally, future research should investigate the usage of heteroatoms, including tellurium, selenium, and others.
- iii.
- Despite progress, controlling the number of heteroatoms, distributional homogeneity, bonding forms, and other aspects remains difficult. This is particularly true in situations where co-doping is involved. All of these factors influence the characteristics of doped carbons in catalytic processes, either directly or indirectly.
- iv.
- It is still difficult to accurately adjust the location and quantity of heteroatoms in the carbon basal plane as well as to establish a clear connection between both the doped structure and catalytic performance.
- v.
- Most of the research has focused on graphene and its derivatives (e.g., rGO, GCN, etc.) for evaluating the doping effects on their photocatalytic activity. The effect of heteroatom doping on other CBMs (such as CNT, activated carbon, and biochar) should also be studied.
- vi.
- More research is required to develop universal carbon-based photocatalysts that will be capable of degrading multiple types of pollutants (e.g., dyes, pesticides, surfactants, etc.) present in bodies of water.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, K.; Fu, B.; Luo, Z.; Xiong, R.; Men, Y.; Shen, H.; Li, B.; Shen, G.; Tao, S. Attributed radiative forcing of air pollutants from biomass and fossil burning emissions. Environ. Pollut. 2022, 306, 119378. [Google Scholar] [CrossRef]
- Olabi, A.; Abdelkareem, M.A. Renewable energy and climate change. Renew. Sustain. Energy Rev. 2022, 158, 112111. [Google Scholar] [CrossRef]
- Chen, T.W.; Anushya, G.; Chen, S.-M.; Kalimuthu, P.; Mariyappan, V.; Gajendran, P.; Ramachandran, R. Recent advances in nanoscale based electrocatalysts for metal-air battery, fuel cell and water-splitting applications: An overview. Materials 2022, 15, 458. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.-J.; Fan, Y.-J.; Wang, R.-X.; Xiang, S.; Tang, H.-G.; Sun, S.-G. A novel strategy for the synthesis of sulfur-doped carbon nanotubes as a highly efficient Pt catalyst support toward the methanol oxidation reaction. J. Mater. Chem. A 2017, 5, 19467–19475. [Google Scholar] [CrossRef]
- Boshir Ahmed, M.; Alom, J.; Hasan, M.S.; Asaduzzaman, M.; Rahman, M.S.; Hossen, R.; Abu Hasan Johir, M.; Taufiq Alam, M.; Zhou, J.L.; Zhu, Y.; et al. General Doping Chemistry of Carbon Materials. ChemNanoMat 2022, 9, e202200482. [Google Scholar] [CrossRef]
- Xiong, B.; Zhou, Y.; Zhao, Y.; Wang, J.; Chen, X.; O’Hayre, R.; Shao, Z. The use of nitrogen-doped graphene supporting Pt nanoparticles as a catalyst for methanol electrocatalytic oxidation. Carbon 2013, 52, 181–192. [Google Scholar] [CrossRef]
- Zhang, Q.; Guan, J. Recent progress in single-atom catalysts for photocatalytic water splitting. Sol. RRL 2020, 4, 2000283. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef]
- Chai, B.; Yan, J.; Wang, C.; Ren, Z.; Zhu, Y. Enhanced visible light photocatalytic degradation of Rhodamine B over phosphorus doped graphitic carbon nitride. Appl. Surf. Sci. 2017, 391, 376–383. [Google Scholar] [CrossRef]
- Yan, S.; Li, Z.; Zou, Z. Photodegradation of rhodamine B and methyl orange over boron-doped g-C3N4 under visible light irradiation. Langmuir 2010, 26, 3894–3901. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Zeng, G.; Wu, Z.; Liang, J.; Chen, X.; Leng, L.; Wang, H.; Wang, H. Metal-free efficient photocatalyst for stable visible-light photocatalytic degradation of refractory pollutant. Appl. Catal. B Environ. 2018, 221, 715–725. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, H.; Dai, Z.; Sun, H.; Liu, J.; Ao, Z.; Wang, S.; Han, C.; Liu, S. Nitrogen-doped carbon nanospheres-modified graphitic carbon nitride with outstanding photocatalytic activity. Nano-Micro Lett. 2020, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Torres, D.; Navlani-García, M.; Mori, K.; Kuwahara, Y.; Yamashita, H. Nitrogen-doped carbon materials as a promising platform toward the efficient catalysis for hydrogen generation. Appl. Catal. A Gen. 2019, 571, 25–41. [Google Scholar] [CrossRef]
- Zhang, Z.; Yi, G.; Li, P.; Zhang, X.; Fan, H.; Zhang, Y.; Wang, X.; Zhang, C. A minireview on doped carbon dots for photocatalytic and electrocatalytic applications. Nanoscale 2020, 12, 13899–13906. [Google Scholar] [CrossRef]
- Hu, C.; Dai, L. Doping of carbon materials for metal-free electrocatalysis. Adv. Mater. 2019, 31, 1804672. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Takeyasu, K.; Nakamura, J. Active sites and mechanism of oxygen reduction reaction electrocatalysis on nitrogen-doped carbon materials. Adv. Mater. 2019, 31, 1804297. [Google Scholar] [CrossRef]
- Zhang, W.; Xi, R.; Li, Y.; Zhang, Y.; Wang, P.; Hu, D. Recent development of transition metal doped carbon materials derived from biomass for hydrogen evolution reaction. Int. J. Hydrog. Energy 2022, 47, 32436–32454. [Google Scholar] [CrossRef]
- Shi, Z.; Yang, W.; Gu, Y.; Liao, T.; Sun, Z. Metal-nitrogen-doped carbon materials as highly efficient catalysts: Progress and rational design. Adv. Sci. 2020, 7, 2001069. [Google Scholar] [CrossRef]
- Wang, B.; Liu, B.; Dai, L. Non-N-Doped Carbons as Metal-Free Electrocatalysts. Adv. Sustain. Syst. 2021, 5, 2000134. [Google Scholar] [CrossRef]
- Suen, N.-T.; Hung, S.-F.; Quan, Q.; Zhang, N.; Xu, Y.-J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef]
- Tao, H.; Gao, Y.; Talreja, N.; Guo, F.; Texter, J.; Yan, C.; Sun, Z. Two-dimensional nanosheets for electrocatalysis in energy generation and conversion. J. Mater. Chem. A 2017, 5, 7257–7284. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Xu, Q.; Niu, J.; Xia, Z. N-doped graphene as catalysts for oxygen reduction and oxygen evolution reactions: Theoretical considerations. J. Catal. 2014, 314, 66–72. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Z.; Xia, Z.; Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 2015, 10, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Nakamura, R.; Kamiya, K.; Nakanishi, S.; Hashimoto, K. Nitrogen-doped carbon nanomaterials as non-metal electrocatalysts for water oxidation. Nat. Commun. 2013, 4, 2390. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jiao, Y.; Li, L.H.; Xing, T.; Chen, Y.; Jaroniec, M.; Qiao, S.Z. Toward design of synergistically active carbon-based catalysts for electrocatalytic hydrogen evolution. ACS Nano 2014, 8, 5290–5296. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Ma, R.; Lin, G.; Zhou, Y.; Liu, Q.; Zhang, T.; Shan, G.; Yang, M.; Wang, J. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. NPJ Comput. Mater. 2019, 5, 78. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, B.; Shen, X.; Liu, J.; Kong, X.; Chuang, S.S.C.; Yang, D.; Dong, A.; Peng, Z. A nitrogen-doped ordered mesoporous carbon/graphene framework as bifunctional electrocatalyst for oxygen reduction and evolution reactions. Nano Energy 2016, 30, 503–510. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, S.; Lin, X.; Liu, C.; Hu, S.; Huang, Q. N, P-doped multiphase transition metal sulfides are used for efficient electrocatalytic oxygen evolution reaction. Appl. Surf. Sci. 2022, 584, 152546. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Zhao, Q.; Zhang, X.; Liu, R.; Ge, X.; Wang, G.; Zhao, H.; Cai, W. Metal-organic framework derived nitrogen-doped porous carbon@graphene sandwich-like structured composites as bifunctional electrocatalysts for oxygen reduction and evolution reactions. Carbon 2016, 106, 74–83. [Google Scholar] [CrossRef]
- Li, X.; Fang, Y.; Zhao, S.; Wu, J.; Li, F.; Tian, M.; Long, X.; Jin, J.; Ma, J. Nitrogen-doped mesoporous carbon nanosheet/carbon nanotube hybrids as metal-free bi-functional electrocatalysts for water oxidation and oxygen reduction. J. Mater. Chem. A 2016, 4, 13133–13141. [Google Scholar] [CrossRef]
- Li, R.; Wei, Z.; Gou, X. Nitrogen and Phosphorus Dual-Doped Graphene/Carbon Nanosheets as Bifunctional Electrocatalysts for Oxygen Reduction and Evolution. ACS Catal. 2015, 5, 4133–4142. [Google Scholar] [CrossRef]
- Yuan, N.; Jiang, Q.; Li, J.; Tang, J. A review on non-noble metal based electrocatalysis for the oxygen evolution reaction. Arab. J. Chem. 2020, 13, 4294–4309. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Quan, X.; Chen, S.; Zhao, H.; Yu, H. Nitrogen and sulfur co-doped graphene/carbon nanotube as metal-free electrocatalyst for oxygen evolution reaction: The enhanced performance by sulfur doping. Electrochim. Acta 2016, 204, 169–175. [Google Scholar] [CrossRef]
- Wu, G.; Santandreu, A.; Kellogg, W.; Gupta, S.; Ogoke, O.; Zhang, H.; Wang, H.-L.; Dai, L. Carbon nanocomposite catalysts for oxygen reduction and evolution reactions: From nitrogen doping to transition-metal addition. Nano Energy 2016, 29, 83–110. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, T.; Xing, L.; Xu, K.; Tong, Y.; Xie, H.; Zhang, L.; Yan, W.; Chu, W.; Wu, C.; et al. Atomically Dispersed Iron-Nitrogen Species as Electrocatalysts for Bifunctional Oxygen Evolution and Reduction Reactions. Angew. Chem. (Int. Ed. Engl.) 2017, 56, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Yang Hong, B.; Miao, J.; Hung, S.-F.; Chen, J.; Tao Hua, B.; Wang, X.; Zhang, L.; Chen, R.; Gao, J.; Chen Hao, M.; et al. Identification of catalytic sites for oxygen reduction and oxygen evolution in N-doped graphene materials: Development of highly efficient metal-free bifunctional electrocatalyst. Sci. Adv. 2016, 2, e1501122. [Google Scholar] [CrossRef]
- Davodi, F.; Tavakkoli, M.; Lahtinen, J.; Kallio, T. Straightforward synthesis of nitrogen-doped carbon nanotubes as highly active bifunctional electrocatalysts for full water splitting. J. Catal. 2017, 353, 19–27. [Google Scholar] [CrossRef]
- Tian, G.-L.; Zhao, M.-Q.; Yu, D.; Kong, X.-Y.; Huang, J.-Q.; Zhang, Q.; Wei, F. Nitrogen-Doped Graphene/Carbon Nanotube Hybrids: In Situ Formation on Bifunctional Catalysts and Their Superior Electrocatalytic Activity for Oxygen Evolution/Reduction Reaction. Small 2014, 10, 2251–2259. [Google Scholar] [CrossRef]
- Jiang, H.; Gu, J.; Zheng, X.; Liu, M.; Qiu, X.; Wang, L.; Li, W.; Chen, Z.; Ji, X.; Li, J. Defect-rich and ultrathin N doped carbon nanosheets as advanced trifunctional metal-free electrocatalysts for ORR, OER and HER. Energy Environ. Sci. 2019, 12, 322–333. [Google Scholar] [CrossRef]
- Wahab, M.A.; Joseph, J.; Atanda, L.; Sultana, U.K.; Beltramini, J.N.; Ostrikov, K.; Will, G.; O’Mullane, A.P.; Abdala, A. Nanoconfined Synthesis of Nitrogen-Rich Metal-Free Mesoporous Carbon Nitride Electrocatalyst for the Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2020, 3, 1439–1447. [Google Scholar] [CrossRef]
- Ma, T.Y.; Ran, J.; Dai, S.; Jaroniec, M.; Qiao, S.Z. Phosphorus-Doped Graphitic Carbon Nitrides Grown In Situ on Carbon-Fiber Paper: Flexible and Reversible Oxygen Electrodes. Angew. Chem. Int. Ed. 2015, 54, 4646–4650. [Google Scholar] [CrossRef]
- Xiao, Z.; Huang, X.; Xu, L.; Yan, D.; Huo, J.; Wang, S. Edge-selectively phosphorus-doped few-layer graphene as an efficient metal-free electrocatalyst for the oxygen evolution reaction. Chem. Commun. (Camb. Engl.) 2016, 52, 13008–13011. [Google Scholar] [CrossRef]
- El-Sawy, A.M.; Mosa, I.M.; Su, D.; Guild, C.J.; Khalid, S.; Joesten, R.; Rusling, J.F.; Suib, S.L. Controlling the Active Sites of Sulfur-Doped Carbon Nanotube–Graphene Nanolobes for Highly Efficient Oxygen Evolution and Reduction Catalysis. Adv. Energy Mater. 2016, 6, 1501966. [Google Scholar] [CrossRef]
- Shinde, S.S.; Lee, C.-H.; Sami, A.; Kim, D.-H.; Lee, S.-U.; Lee, J.-H. Scalable 3-D Carbon Nitride Sponge as an Efficient Metal-Free Bifunctional Oxygen Electrocatalyst for Rechargeable Zn–Air Batteries. ACS Nano 2017, 11, 347–357. [Google Scholar] [CrossRef]
- Qu, K.; Zheng, Y.; Jiao, Y.; Zhang, X.; Dai, S.; Qiao, S.-Z. Polydopamine-Inspired, Dual Heteroatom-Doped Carbon Nanotubes for Highly Efficient Overall Water Splitting. Adv. Energy Mater. 2017, 7, 1602068. [Google Scholar] [CrossRef]
- Hu, C.; Dai, L. Multifunctional Carbon-Based Metal-Free Electrocatalysts for Simultaneous Oxygen Reduction, Oxygen Evolution, and Hydrogen Evolution. Adv. Mater. 2017, 29, 1604942. [Google Scholar] [CrossRef]
- Zhu, P.; Gao, J.; Chen, X.; Liu, S. An efficient metal-free bifunctional oxygen electrocatalyst of carbon co-doped with fluorine and nitrogen atoms for rechargeable Zn-air battery. Int. J. Hydrog. Energy 2020, 45, 9512–9521. [Google Scholar] [CrossRef]
- Li, Y.; Dai, H. Recent advances in zinc–air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B Environ. 2005, 56, 9–35. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, Z.; Dai, L. Carbon-based electrocatalysts for advanced energy conversion and storage. Sci. Adv. 2015, 1, e1500564. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, S.; Zhao, Y.; Zhu, L.; Chen, S.; Wang, X.; Wu, Q.; Ma, J.; Ma, Y.; Hu, Z. Boron-Doped Carbon Nanotubes as Metal-Free Electrocatalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2011, 50, 7132–7135. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Origin of the Electrocatalytic Oxygen Reduction Activity of Graphene-Based Catalysts: A Roadmap to Achieve the Best Performance. J. Am. Chem. Soc. 2014, 136, 4394–4403. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, Z.; Li, B.; Wang, G.; Leu, P.W. Identification of Efficient Active Sites in Nitrogen-Doped Carbon Nanotubes for Oxygen Reduction Reaction. J. Phys. Chem. C 2020, 124, 8689–8696. [Google Scholar] [CrossRef]
- Poh, H.L.; Šimek, P.; Sofer, Z.; Pumera, M. Sulfur-Doped Graphene via Thermal Exfoliation of Graphite Oxide in H2S, SO2, or CS2 Gas. ACS Nano 2013, 7, 5262–5272. [Google Scholar] [CrossRef]
- Zhang, C.; Mahmood, N.; Yin, H.; Liu, F.; Hou, Y. Synthesis of Phosphorus-Doped Graphene and its Multifunctional Applications for Oxygen Reduction Reaction and Lithium Ion Batteries. Adv. Mater. 2013, 25, 4932–4937. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.; Song, P.; Pan, J.; Zhuang, L.; Xu, W.; Xing, W. Fluorine-Doped Carbon Blacks: Highly Efficient Metal-Free Electrocatalysts for Oxygen Reduction Reaction. ACS Catal. 2013, 3, 1726–1729. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Xia, Z.; Roy, A.; Chang, D.W.; Baek, J.-B.; Dai, L. BCN Graphene as Efficient Metal-Free Electrocatalyst for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2012, 51, 4209–4212. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, Y.; Zhuang, X.; Li, S.; Wu, D.; Zhang, F.; Feng, X. Low-temperature synthesis of nitrogen/sulfur co-doped three-dimensional graphene frameworks as efficient metal-free electrocatalyst for oxygen reduction reaction. Carbon 2013, 62, 296–301. [Google Scholar] [CrossRef]
- Li, J.-S.; Li, S.-L.; Tang, Y.-J.; Li, K.; Zhou, L.; Kong, N.; Lan, Y.-Q.; Bao, J.-C.; Dai, Z.-H. Heteroatoms ternary-doped porous carbons derived from MOFs as metal-free electrocatalysts for oxygen reduction reaction. Sci. Rep. 2014, 4, 5130. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, J.; Cao, X.; Abbott, J.; Jin, C.; Wang, H.; Strasser, P.; Yang, R.; Chen, X.; Wu, G. N-, P-, and S-doped graphene-like carbon catalysts derived from onium salts with enhanced oxygen chemisorption for Zn-air battery cathodes. Appl. Catal. B Environ. 2019, 241, 442–451. [Google Scholar] [CrossRef]
- Gokhale, R.; Unni, S.M.; Puthusseri, D.; Kurungot, S.; Ogale, S. Synthesis of an efficient heteroatom-doped carbon electro-catalyst for oxygen reduction reaction by pyrolysis of protein-rich pulse flour cooked with SiO2 nanoparticles. Phys. Chem. Chem. Phys. 2014, 16, 4251–4259. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Voiry, D.; Chhowalla, M.; Asefa, T. Efficient Metal-Free Electrocatalysts for Oxygen Reduction: Polyaniline-Derived N- and O-Doped Mesoporous Carbons. J. Am. Chem. Soc. 2013, 135, 7823–7826. [Google Scholar] [CrossRef]
- Ahn, S.H.; Yoo, S.J.; Kim, H.-J.; Henkensmeier, D.; Nam, S.W.; Kim, S.-K.; Jang, J.H. Anion exchange membrane water electrolyzer with an ultra-low loading of Pt-decorated Ni electrocatalyst. Appl. Catal. B Environ. 2016, 180, 674–679. [Google Scholar] [CrossRef]
- Tuomi, S.; Guil-Lopez, R.; Kallio, T. Molybdenum carbide nanoparticles as a catalyst for the hydrogen evolution reaction and the effect of pH. J. Catal. 2016, 334, 102–109. [Google Scholar] [CrossRef]
- Gong, Q.; Cheng, L.; Liu, C.; Zhang, M.; Feng, Q.; Ye, H.; Zeng, M.; Xie, L.; Liu, Z.; Li, Y. Ultrathin MoS2(1–x)Se2x Alloy Nanoflakes For Electrocatalytic Hydrogen Evolution Reaction. ACS Catal. 2015, 5, 2213–2219. [Google Scholar] [CrossRef]
- Saadi, F.H.; Carim, A.I.; Verlage, E.; Hemminger, J.C.; Lewis, N.S.; Soriaga, M.P. CoP as an Acid-Stable Active Electrocatalyst for the Hydrogen-Evolution Reaction: Electrochemical Synthesis, Interfacial Characterization and Performance Evaluation. J. Phys. Chem. C 2014, 118, 29294–29300. [Google Scholar] [CrossRef]
- Vrubel, H.; Hu, X. Molybdenum Boride and Carbide Catalyze Hydrogen Evolution in both Acidic and Basic Solutions. Angew. Chem. Int. Ed. 2012, 51, 12703–12706. [Google Scholar] [CrossRef]
- Ma, L.; Fan, H.; Wang, J.; Zhao, Y.; Tian, H.; Dong, G. Water-assisted ions in situ intercalation for porous polymeric graphitic carbon nitride nanosheets with superior photocatalytic hydrogen evolution performance. Appl. Catal. B Environ. 2016, 190, 93–102. [Google Scholar] [CrossRef]
- Zeng, M.; Li, Y. Recent advances in heterogeneous electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 14942–14962. [Google Scholar] [CrossRef]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.-J.; Baek, J.-B. Metal-Free Catalysts for Oxygen Reduction Reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Liu, Q.; Cheng, N.; Asiri, A.M.; Sun, X. Activated carbon nanotubes: A highly-active metal-free electrocatalyst for hydrogen evolution reaction. Chem. Commun. 2014, 50, 9340–9342. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Li, L.H.; Han, Y.; Chen, Y.; Du, A.; Jaroniec, M.; Qiao, S.Z. Hydrogen evolution by a metal-free electrocatalyst. Nat. Commun. 2014, 5, 3783. [Google Scholar] [CrossRef]
- Tian, Y.; Wei, Z.; Wang, X.; Peng, S.; Zhang, X.; Liu, W.-m. Plasma-etched, S-doped graphene for effective hydrogen evolution reaction. Int. J. Hydrog. Energy 2017, 42, 4184–4192. [Google Scholar] [CrossRef]
- Ito, Y.; Cong, W.; Fujita, T.; Tang, Z.; Chen, M. High catalytic activity of nitrogen and sulfur co-doped nanoporous graphene in the hydrogen evolution reaction. Angew. Chem. 2015, 127, 2159–2164. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, Y.; Ao, Z.; Wang, G. Micelle-Template Synthesis of Nitrogen-Doped Mesoporous Graphene as an Efficient Metal-Free Electrocatalyst for Hydrogen Production. Sci. Rep. 2014, 4, 7557. [Google Scholar] [CrossRef]
- Hu, C.; Dai, L. Carbon-Based Metal-Free Catalysts for Electrocatalysis beyond the ORR. Angew. Chem. Int. Ed. 2016, 55, 11736–11758. [Google Scholar] [CrossRef]
- Ma, Y.-Y.; Wu, C.-X.; Feng, X.-J.; Tan, H.-Q.; Yan, L.-K.; Liu, Y.; Kang, Z.-H.; Wang, E.-B.; Li, Y.-G. Highly efficient hydrogen evolution from seawater by a low-cost and stable CoMoP@ C electrocatalyst superior to Pt/C. Energy Environ. Sci. 2017, 10, 788–798. [Google Scholar] [CrossRef]
- Liu, D.; Xu, G.; Yang, H.; Wang, H.; Xia, B.Y. Rational Design of Transition Metal Phosphide-Based Electrocatalysts for Hydrogen Evolution. Adv. Funct. Mater. 2023, 33, 2208358. [Google Scholar] [CrossRef]
- Sathe, B.R.; Zou, X.; Asefa, T. Metal-free B-doped graphene with efficient electrocatalytic activity for hydrogen evolution reaction. Catal. Sci. Technol. 2014, 4, 2023–2030. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, W.; Yang, L.; Li, L.; Zhang, Z.; Ke, Y.; Chen, S. Nitrogen and sulfur co-doped porous carbon derived from human hair as highly efficient metal-free electrocatalysts for hydrogen evolution reactions. J. Mater. Chem. A 2015, 3, 8840–8846. [Google Scholar] [CrossRef]
- Zhou, Y.; Leng, Y.; Zhou, W.; Huang, J.; Zhao, M.; Zhan, J.; Feng, C.; Tang, Z.; Chen, S.; Liu, H. Sulfur and nitrogen self-doped carbon nanosheets derived from peanut root nodules as high-efficiency non-metal electrocatalyst for hydrogen evolution reaction. Nano Energy 2015, 16, 357–366. [Google Scholar] [CrossRef]
- Wei, L.; Karahan, H.E.; Goh, K.; Jiang, W.; Yu, D.; Birer, Ö.; Jiang, R.; Chen, Y. A high-performance metal-free hydrogen-evolution reaction electrocatalyst from bacterium derived carbon. J. Mater. Chem. A 2015, 3, 7210–7214. [Google Scholar] [CrossRef]
- Jiang, H.; Zhu, Y.; Su, Y.; Yao, Y.; Liu, Y.; Yang, X.; Li, C. Highly dual-doped multilayer nanoporous graphene: Efficient metal-free electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 12642–12645. [Google Scholar] [CrossRef]
- Crabtree, R.H. Homogeneous transition metal catalysis of acceptorless dehydrogenative alcohol oxidation: Applications in hydrogen storage and to heterocycle synthesis. Chem. Rev. 2017, 117, 9228–9246. [Google Scholar] [CrossRef]
- Kakati, N.; Maiti, J.; Lee, S.H.; Jee, S.H.; Viswanathan, B.; Yoon, Y.S. Anode catalysts for direct methanol fuel cells in acidic media: Do we have any alternative for Pt or Pt–Ru? Chem. Rev. 2014, 114, 12397–12429. [Google Scholar] [CrossRef]
- Kamarudin, S.K.; Achmad, F.; Daud, W.R.W. Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices. Int. J. Hydrogen Energ. 2009, 34, 6902–6916. [Google Scholar] [CrossRef]
- Yuda, A.; Ashok, A.; Kumar, A. A comprehensive and critical review on recent progress in anode catalyst for methanol oxidation reaction. Catal. Rev. 2022, 64, 126–228. [Google Scholar] [CrossRef]
- Halder, A.; Sharma, S.; Hegde, M.S.; Ravishankar, N. Controlled attachment of ultrafine platinum nanoparticles on functionalized carbon nanotubes with high electrocatalytic activity for methanol oxidation. J. Phy. Chem. C 2009, 113, 1466–1473. [Google Scholar] [CrossRef]
- Su, F.; Tian, Z.; Poh, C.K.; Wang, Z.; Lim, S.H.; Liu, Z.; Lin, J. Pt nanoparticles supported on nitrogen-doped porous carbon nanospheres as an electrocatalyst for fuel cells. Chem. Mater. 2010, 22, 832–839. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Kuo, P.-L. The use of carbon nanotubes coated with a porous nitrogen-doped carbon layer with embedded Pt for the methanol oxidation reaction. J. Power Sources 2012, 198, 83–89. [Google Scholar] [CrossRef]
- Yang, G.; Li, Y.; Rana, R.K.; Zhu, J.-J. Pt–Au/nitrogen-doped graphene nanocomposites for enhanced electrochemical activities. J. Mater. Chem. A 2013, 1, 1754–1762. [Google Scholar] [CrossRef]
- Li, Y.-H.; Hung, T.-H.; Chen, C.-W. A first-principles study of nitrogen-and boron-assisted platinum adsorption on carbon nanotubes. Carbon 2009, 47, 850–855. [Google Scholar] [CrossRef]
- Ahmadi, R.; Amini, M.K. Synthesis and characterization of Pt nanoparticles on sulfur-modified carbon nanotubes for methanol oxidation. Int. J. Hydrog. Energy 2011, 36, 7275–7283. [Google Scholar] [CrossRef]
- Lv, R.; Cui, T.; Jun, M.S.; Zhang, Q.; Cao, A.; Su, D.S.; Zhang, Z.; Yoon, S.H.; Miyawaki, J.; Mochida, I. Open-ended, N-doped carbon nanotube–graphene hybrid nanostructures as high-performance catalyst support. Adv. Funct. Mater. 2011, 21, 999–1006. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, Z.; Lin, F.; Guo, F.; Alamry, K.A.; Taib, L.A.; Asiri, A.M.; Wang, X. Sulfur-doped carbon nitride polymers for photocatalytic degradation of organic pollutant and reduction of Cr (VI). Molecules 2017, 22, 572. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.; Zhang, B.; Yuan, M.; Ma, Y.; Kong, F. A facile strategy for photocatalytic degradation of seven neonicotinoids over sulfur and oxygen co-doped carbon nitride. Chemosphere 2020, 253, 126672. [Google Scholar] [CrossRef]
- Ortega-Liebana, M.C.; Hueso, J.L.; Ferdousi, S.; Arenal, R.; Irusta, S.; Yeung, K.L.; Santamaria, J. Extraordinary sensitizing effect of co-doped carbon nanodots derived from mate herb: Application to enhanced photocatalytic degradation of chlorinated wastewater compounds under visible light. Appl. Catal. B Environ. 2017, 218, 68–79. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Ma, Y.; Fang, S.; Kong, F.; Pang, X. Photocatalytic degradation of dinotefuran by layered phosphorus-doped carbon nitride and its mechanism. J. Photochem. Photobiol. A Chem. 2021, 414, 113287. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Saravanan, A.; Manohar, P.; Luong, J.H.; Gedanken, A. Photocatalytic degradation of organic dyes and antimicrobial activities by polyaniline–nitrogen-doped carbon dot nanocomposite. Nanomaterials 2021, 11, 1128. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Luo, W.; Hu, Y.; Dai, H.; Peng, X. Insight into the kinetics and mechanism of visible-light photocatalytic degradation of dyes onto the P doped mesoporous graphitic carbon nitride. J. Alloys Compd. 2019, 794, 594–605. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Al-Saeedi, S.I.; Almotairi, S.; Alshehri, S.M. Fabrication of MoS2/ZnS embedded in N/S doped carbon for the photocatalytic degradation of pesticide. Mater. Lett. 2020, 263, 127271. [Google Scholar] [CrossRef]
- Veerakumar, P.; Thanasekaran, P.; Subburaj, T.; Lin, K.-C. A Metal-free carbon-based catalyst: An overview and directions for future research. C 2018, 4, 54. [Google Scholar] [CrossRef]
- Jourshabani, M.; Shariatinia, Z.; Badiei, A. Controllable synthesis of mesoporous sulfur-doped carbon nitride materials for enhanced visible light photocatalytic degradation. Langmuir 2017, 33, 7062–7078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, X.; Guan, J.; Jiang, Y.; Hou, T.; Mu, X. Facile synthesis of phosphorus doped graphitic carbon nitride polymers with enhanced visible-light photocatalytic activity. Mater. Res. Bull. 2013, 48, 3485–3491. [Google Scholar] [CrossRef]
- Liu, J.; Song, Y.; Xu, H.; Zhu, X.; Lian, J.; Xu, Y.; Zhao, Y.; Huang, L.; Ji, H.; Li, H. Non-metal photocatalyst nitrogen-doped carbon nanotubes modified mpg-C3N4: Facile synthesis and the enhanced visible-light photocatalytic activity. J. Colloid Interface Sci. 2017, 494, 38–46. [Google Scholar] [CrossRef]
- Zhou, Y.; Zahran, E.M.; Quiroga, B.A.; Perez, J.; Mintz, K.J.; Peng, Z.; Liyanage, P.Y.; Pandey, R.R.; Chusuei, C.C.; Leblanc, R.M. Size-dependent photocatalytic activity of carbon dots with surface-state determined photoluminescence. Appl. Catal. B Environ. 2019, 248, 157–166. [Google Scholar] [CrossRef]
- Peng, Z.; Zhou, Y.; Ji, C.; Pardo, J.; Mintz, K.J.; Pandey, R.R.; Chusuei, C.C.; Graham, R.M.; Yan, G.; Leblanc, R.M. Facile synthesis of “boron-doped” carbon dots and their application in visible-light-driven photocatalytic degradation of organic dyes. Nanomaterials 2020, 10, 1560. [Google Scholar] [CrossRef]
- Yu, X.; Liu, J.; Yu, Y.; Zuo, S.; Li, B. Preparation and visible light photocatalytic activity of carbon quantum dots/TiO2 nanosheet composites. Carbon 2014, 68, 718–724. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Ma, D.-K.; Zhang, Y.-G.; Chen, W.; Huang, S.-M. N-doped carbon quantum dots for TiO2-based photocatalysts and dye-sensitized solar cells. Nano Energy 2013, 2, 545–552. [Google Scholar] [CrossRef]
- Joseph, S.; Abraham, S.; Abraham, T.; Priyanka, R.N.; Mathew, B. S-rGO modified sulphur doped carbon nitride with mixed-dimensional hierarchical nanostructures of silver vanadate for the enhanced photocatalytic degradation of pollutants in divergent fields. Appl. Surf. Sci. 2019, 495, 143478. [Google Scholar] [CrossRef]
- Sahu, R.S.; Shih, Y.-h.; Chen, W.-L. New insights of metal free 2D graphitic carbon nitride for photocatalytic degradation of bisphenol A. J. Hazard. Mater. 2021, 402, 123509. [Google Scholar] [CrossRef] [PubMed]
- Gar Alalm, M.; Tawfik, A.; Ookawara, S. Solar photocatalytic degradation of phenol by TiO2/AC prepared by temperature impregnation method. Desalination Water Treat. 2016, 57, 835–844. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, F.; Li, Y. Solid phase synthesis of nitrogen and phosphor co-doped carbon quantum dots for sensing Fe3+ and the enhanced photocatalytic degradation of dyes. Sens. Actuators B Chem. 2018, 255, 1105–1111. [Google Scholar] [CrossRef]
- Rahbar, M.; Mehrzad, M.; Behpour, M.; Mohammadi-Aghdam, S.; Ashrafi, M. S, N co-doped carbon quantum dots/TiO2 nanocomposite as highly efficient visible light photocatalyst. Nanotechnology 2019, 30, 505702. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Rao, L.; Wang, P.; Guo, Y.; Guo, X.; Zhang, L. Porous oxygen-doped carbon nitride: Supramolecular preassembly technology and photocatalytic degradation of organic pollutants under low-intensity light irradiation. Environ. Sci. Pollut. Res. 2019, 26, 15710–15723. [Google Scholar] [CrossRef] [PubMed]
- Abd Rani, U.; Ng, L.Y.; Ng, C.Y.; Mahmoudi, E.; Hairom, N.H.H. Photocatalytic degradation of crystal violet dye using sulphur-doped carbon quantum dots. Mater. Today Proc. 2021, 46, 1934–1939. [Google Scholar] [CrossRef]
- Qu, Y.; Xu, X.; Huang, R.; Qi, W.; Su, R.; He, Z. Enhanced photocatalytic degradation of antibiotics in water over functionalized N, S-doped carbon quantum dots embedded ZnO nanoflowers under sunlight irradiation. Chem. Eng. J. 2020, 382, 123016. [Google Scholar] [CrossRef]
- Guo, Z.; Ni, S.; Wu, H.; Wen, J.; Li, X.; Tang, T.; Li, M.; Liu, M. Designing nitrogen and phosphorus co-doped graphene quantum dots/g-C3N4 heterojunction composites to enhance visible and ultraviolet photocatalytic activity. Appl. Surf. Sci. 2021, 548, 149211. [Google Scholar] [CrossRef]
- Ishak, N.; Jeyalakshmi, V.; Setka, M.; Grandcolas, M.; Devadas, B.; Šoóš, M. Upgrading of g-C3N4 semiconductor by a Nitrogen-doped carbon material: A photocatalytic degradation application. J. Environ. Chem. Eng. 2023, 11, 109381. [Google Scholar] [CrossRef]
- Jiang, H.; Zhong, Y.; Tian, K.; Pang, H.; Hao, Y. Enhanced photocatalytic degradation of bisphenol A over N, S-doped carbon quantum dot-modified MIL-101 (Fe) heterostructure composites under visible light irradiation by persulfate. Appl. Surf. Sci. 2022, 577, 151902. [Google Scholar] [CrossRef]





| CBMs | Overpotential at 10 mAcm−2 in 0.1 M KOH (mV) | Tafel Slope mVdec−1 | Ref. | |
|---|---|---|---|---|
| N doped CBMs | N-doped mesoporous carbon nanosheet/carbon nanotube hybrid | 320 | 55 | [22,23,24,29,38,39,40] |
| N-doped mesoporous carbon nanosheet | 400 | 178 | ||
| N-doped Carbon Nanomaterials | 380 | - | ||
| Mixed N-doped mesoporous carbon nanosheet + carbon nanotube | 470 | 210 | ||
| N-doped mesoporous graphene | 324 | 67 | ||
| N-MWCNTs | 320 (1 M NaOH) | 68 | ||
| N-Graphene | 410 | - | ||
| N-CNTs | 390 | - | ||
| N-Graphene Nanoribbon | 405 | - | ||
| N-graphene/CNT hybrid | <110 | 83 | ||
| N-Graphene Nanoribbon | 360 (1 M KOH) | 47 | [38] | |
| N-Carbon nanosheet | 410 | 142 | [41] | |
| N- Graphitic mesoporous C3N4 | 376 | 52.4 | [42] | |
| P-doped CBMs | P-doped graphitic carbon nitride grown on carbon-fiber paper | 391 | 61.6 | [23,43,44] |
| P-graphene | 490 | - | ||
| P-Graphene | 330 (1 M KOH) | 62 | ||
| S-doped CBMs | S-CNTs | 350 (1 M KOH) | 95 | [45] |
| P-S co-doped CBMs | P, S-doped carbon nitride sponge | 330 | 64 | [46] |
| S-N co-doped CBMs | N/S-CNTs | 351(1 M KOH) | 56 | [35,47] |
| NS-graphene/CNT | 510 | 103 | ||
| N-S doped graphitic sheet | 230 | 71 | [48] | |
| -F co-doped CBMs | N-F co-doped carbon black | - | 69 | [49] |
| B-N co-doped CBMs | B/N-C | 270 (1 M KOH) | 100 | [34] |
| CBMs | Onset Potential (V) | Potential at 10 mAcm−2 (V) | Tafel Slope (mVdec−1) | Ref. | |
|---|---|---|---|---|---|
| Single heteroatom doped CBMs | N-doped mesoporous graphene | −0.15 | −0.24 | 109 | [79] |
| B-doped graphene | −0.22 | −0.47 | 99 | [83] | |
| O-doped CNTs | −0.05 | −0.22 | 71.3 | [75] | |
| Co-heteroatom doped CBMs | N, S co-doped porous carbons | −0.012 | −0.097 | 57.4 | [84] |
| N, S co-doped CNTs | −0.05 | −0.12 | 67.8 | [85] | |
| N, S co-doped nanoporous Graphene | −0.14 | −0.39 | 80.5 | [78] | |
| N, P co-doped nanoporous carbon | −0.076 | −0.204 | 58.4 | [86] | |
| N, P co-doped graphene | −0.2 | −0.42 | 91 | [26] | |
| N, P co-doped nanoporous Graphene | −0.12 | −0.213 | 79 | [87] | |
| Catalysts | Experimental Conditions (Catalyst Dosage, Pollutant conc. and Irradiation Source) | Degradation Efficiency | Ref. |
|---|---|---|---|
| Carbon dots (undoped) | 15 g/L catalyst 10 mg/L MB 10 mg/L RhB 310 W Hg-Xe lamp with a UV cutoff filter (λ < 420 nm) | 90% MB in 60 min | [110] |
| 50% RhB in 60 min | |||
| B-doped carbon dots | 2.5 mg/L MB 0.5 mg/L RhB 310 W Hg-Xe lamp (UV-Visible light irradiation) | 99.9% MB in 170 min 99.9% RhB in 170 min | [111] |
| Carbon dots (undoped) | 0.001 g/L catalyst 10 mg/L RhB 500 W Xe lamp with a cutoff filter (λ > 420 nm) | 0% in 60 min | [112] |
| Carbon dots/TNS | 95% in 60 min | ||
| Carbon dots (undoped) | 0.001 g/L catalyst 1 × 10−5 mol/L RhB 500 W Xe lamp with a cutoff filter (λ > 400 nm) | 0% in 30 min | [113] |
| Carbon dots/TiO2 | 95% in 30 min | ||
| Pristine g-C3N4 (undoped) | 0.01 g/L catalyst 10 mg/L methyl orange 300 W halogen lamp (λ > 400 nm) | 8.8% in 210 min | [107] |
| S doped g-C3N4 | 82.7% in 210 min | ||
| MoS2/ZnS embedded N, S co-doped graphitic carbon | Dicofol pesticide Visible light irradiation lamp | 84.5% in 90 min | [105] |
| S, O co-doped g-C3N4 | 150 g/L catalyst 2 mg/L nitenpyram 300 W Xe lamp (λ > 400 nm) | 91.4% in 30 min | [100] |
| P-doped carbon nitride (HPCN0.5) | 2 mg/L dinotefuran 300 W halogen lamp (λ > 400 nm) | 40.59% in 5 h | [102] |
| S doped rGO/S- g-C3N4/Ag3VO3 | 5 mg/L catalyst 10 mg/L methylene oxide 20 mg/L 2,4-dichlorophenoxy acetic acid (2,4-D) UV-Visible irradiation source (λ = 464–664 nm) | 90.1% 2,4-D in 80 min 90.3% MO in 12 min | [114] |
| N,P co-doped carbon nanodots (CNDs)@ TiO2 | 0.2 g/L catalyst 0.025 mmol/L 2,4-dichlorophenol Hg lamp with a cutoff filter (λ < 400 nm) | 40% in 2 h | [101] |
| Exfoliated graphitic carbon nitride | 0.5 g/L catalyst 2 mg/L Bisphenol A 300 W Xe lamp (λ > 420 nm) | 99.9% in 90 min | [115] |
| TiO2@activated carbon | 1.2 g/L catalyst 100 mg/L phenol Natural sunlight | 100% in 120 min | [116] |
| N, P co-doped carbon quantum dots @ TiO2 | 1 g/L catalyst 20 mg/L MB 300 W Xe lamp (λ > 420 nm) | 100% in 15 min | [117] |
| S, N co-doped carbon quantum dots @ TiO2 | 1 g/L catalyst 50 mg/L Acid red 88 Osram lamp (Visible light irradiation) | 77.29% in 120 min | [118] |
| Polyaniline @N-doped carbon nanodots (N/CNDs) | 0.5 g/L catalyst 0.1 g/L congo red White LED lamp (combination of λ = 450 nm and 550 nm) | 100% in 20 min | [103] |
| P-doped mesoporous graphitic C3N4 | 0.4 g/L catalyst 25 mg/L Brilliant ponceau-5R 500 W Xe lamp with a cutoff filter (λ > 420 nm) | 94.5% in 30 min | [104] |
| O-doped carbon nitride | 0.5 g/L catalyst 10 mg/L RhB 5 mg/L Tetracycline hydrochloride (TC-HCl) 300 W Xe lamp (λ > 420 nm) | 95% RhB in 6 h 70% TC-HCl in 6 h | [119] |
| S-doped carbon nitride polymeric micro rods | 0.25 g/L catalyst 1 × 10−5 mol/L RhB 300 W Xe lamp with a cutoff filter (λ > 420 nm) | 97% in 15 min | [99] |
| N-doped CNT/mpg-C3N4 | 0.5 g/L catalyst 10 mg/L RhB 300 W Xe lamp with a cutoff filter (λ > 400 nm) | 95% in 30 min | [109] |
| S-doped carbon quantum dots | 10 mg/L crystal violet 100 W UV-lamp (λ = 395 nm) | 99.7% in 200 min | [120] |
| N, S co-doped carbon quantum dots @ ZnO | 0.4 g/L catalyst 2 × 10−5 mol/L ciprofloxacin Natural sunlight | 85.8% in 50 min | [121] |
| N, P co-doped graphene quantum dots @ g-C3N4 | 1 g/L catalyst 10 mg/L MO 300 W Xe lamp (λ > 420 nm) | 97.0% in 8 min | [122] |
| N-doped g-C3N4 | 1 g/L catalyst Methylene blue Sunlight | 90.0% in 3 h | [123] |
| N, S-doped carbon quantum dot-modified MIL-101(Fe) heterostructure | 0.4 g/L catalyst Bisphenol A Visible light irradiation | 100% in 60 min | [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alom, J.; Hasan, M.S.; Asaduzaman, M.; Alam, M.T.; Belhaj, D.; Selvaraj, R.; Hossain, M.A.; Zargar, M.; Ahmed, M.B. Catalytical Performance of Heteroatom Doped and Undoped Carbon-Based Materials. Catalysts 2023, 13, 823. https://doi.org/10.3390/catal13050823
Alom J, Hasan MS, Asaduzaman M, Alam MT, Belhaj D, Selvaraj R, Hossain MA, Zargar M, Ahmed MB. Catalytical Performance of Heteroatom Doped and Undoped Carbon-Based Materials. Catalysts. 2023; 13(5):823. https://doi.org/10.3390/catal13050823
Chicago/Turabian StyleAlom, Jahangir, Md. Saif Hasan, Md. Asaduzaman, Mohammad Taufiq Alam, Dalel Belhaj, Raja Selvaraj, Md. Ashraf Hossain, Masoumeh Zargar, and Mohammad Boshir Ahmed. 2023. "Catalytical Performance of Heteroatom Doped and Undoped Carbon-Based Materials" Catalysts 13, no. 5: 823. https://doi.org/10.3390/catal13050823
APA StyleAlom, J., Hasan, M. S., Asaduzaman, M., Alam, M. T., Belhaj, D., Selvaraj, R., Hossain, M. A., Zargar, M., & Ahmed, M. B. (2023). Catalytical Performance of Heteroatom Doped and Undoped Carbon-Based Materials. Catalysts, 13(5), 823. https://doi.org/10.3390/catal13050823