g-C3N4 for Photocatalytic Degradation of Parabens: Precursors Influence, the Radiation Source and Simultaneous Ozonation Evaluation
Abstract
1. Introduction
2. Results and Discussion
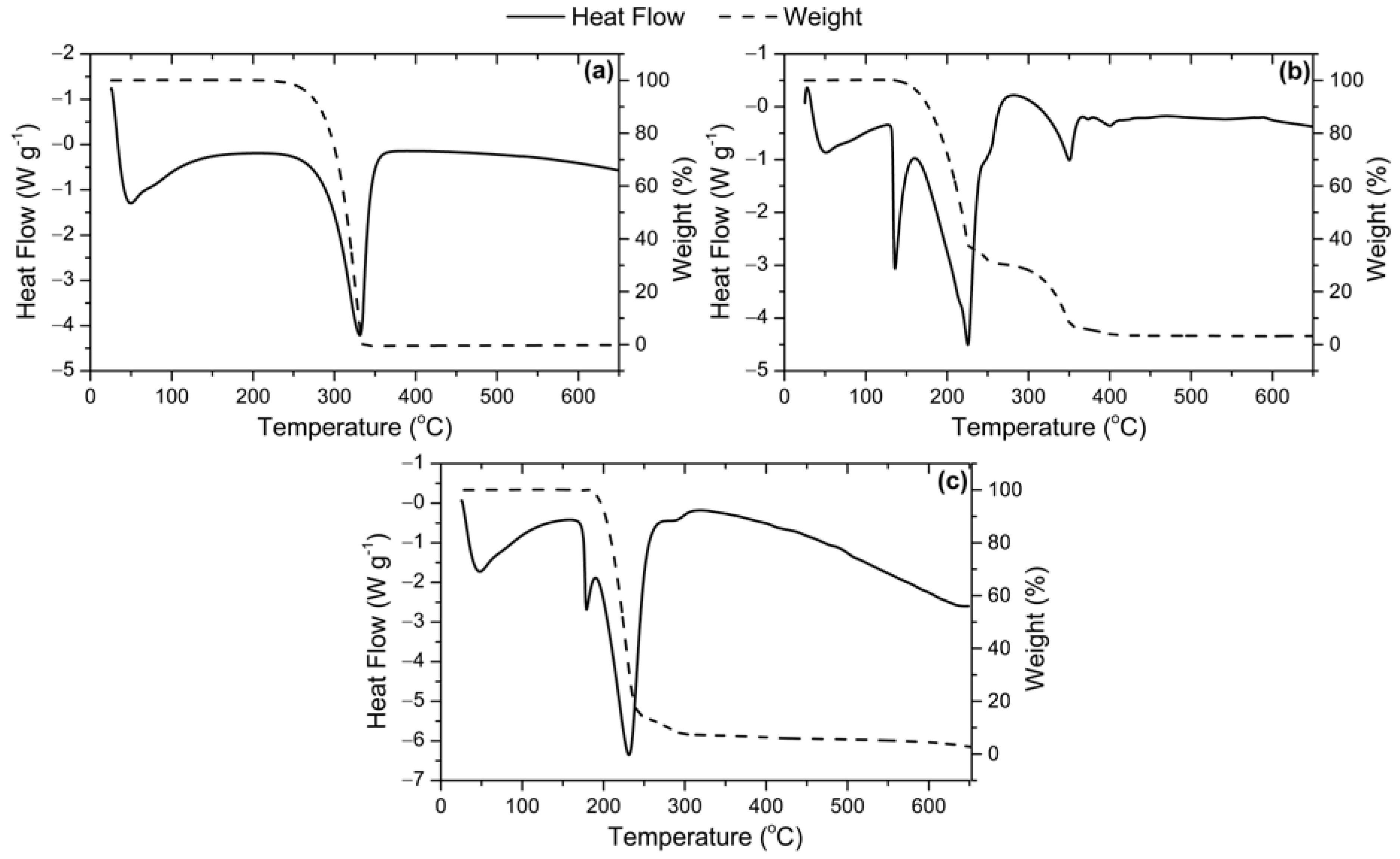
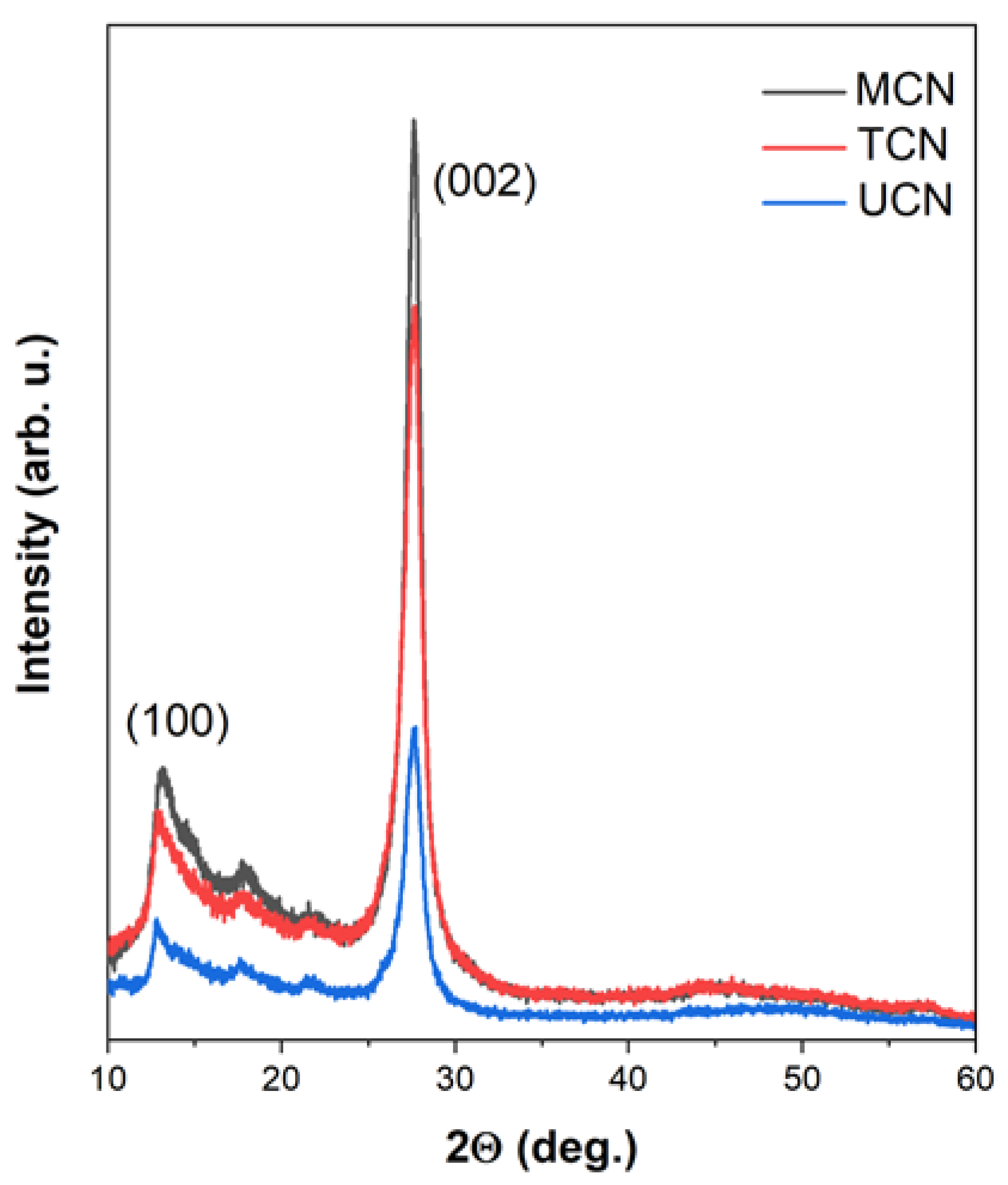
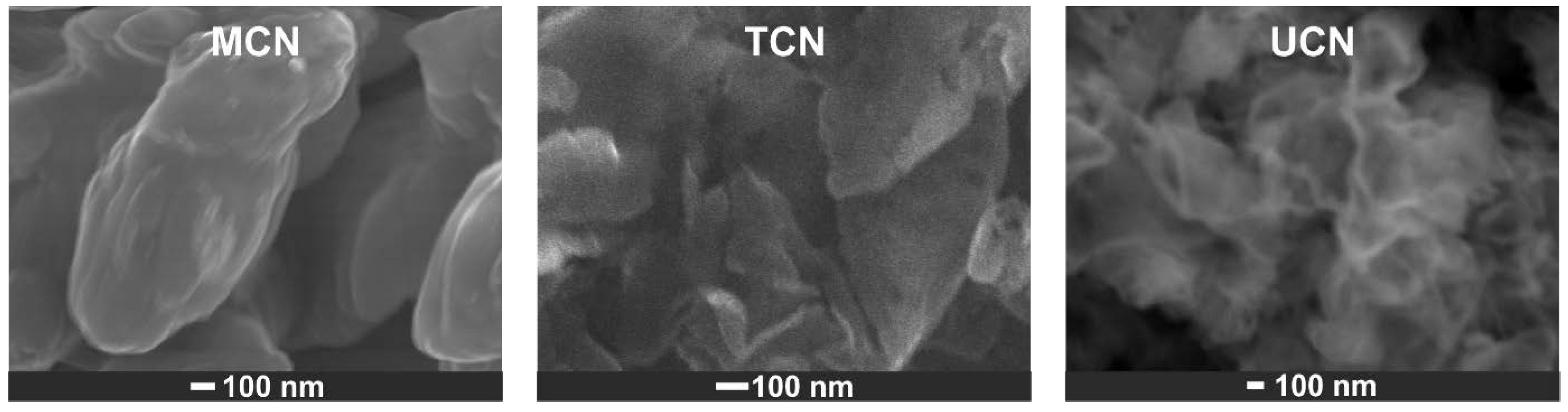
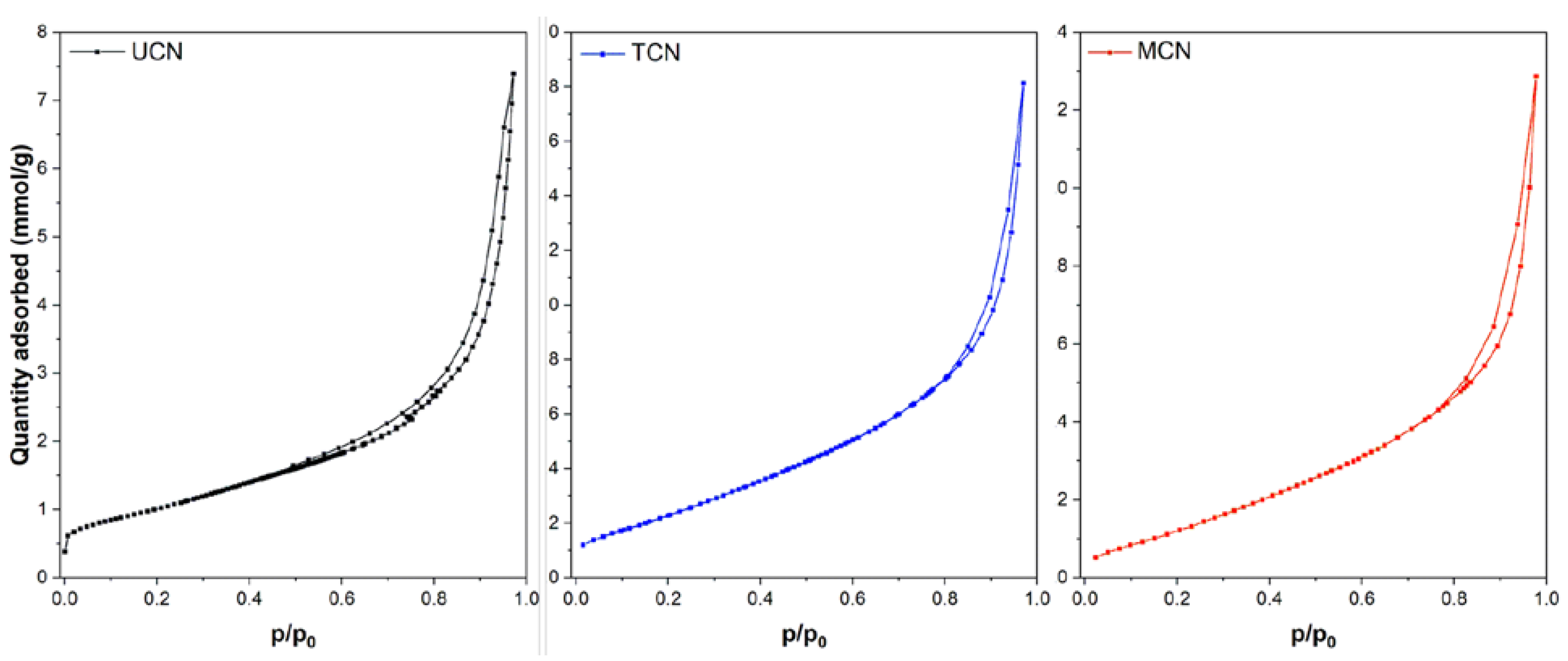
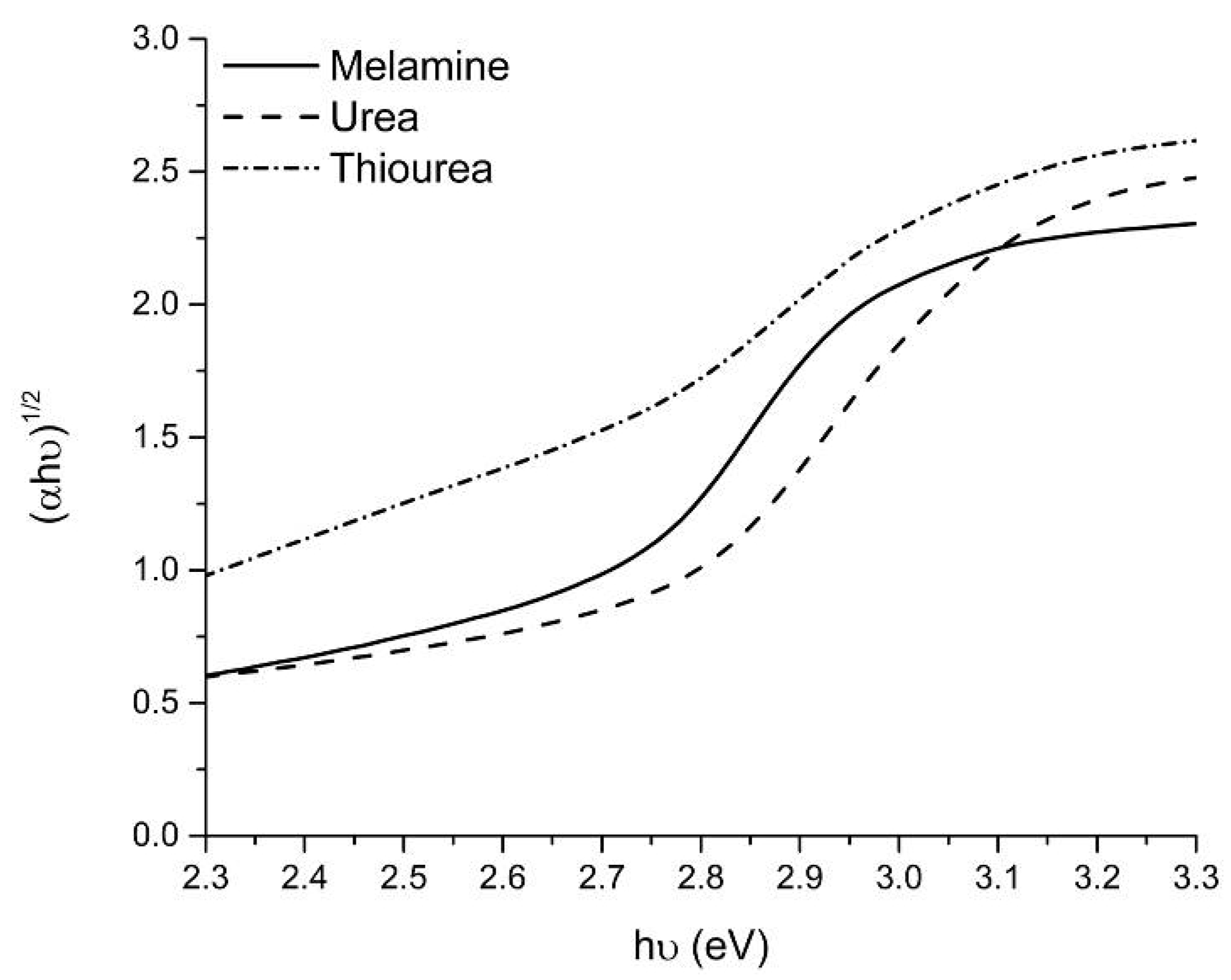
2.1. Catalysts Characterization
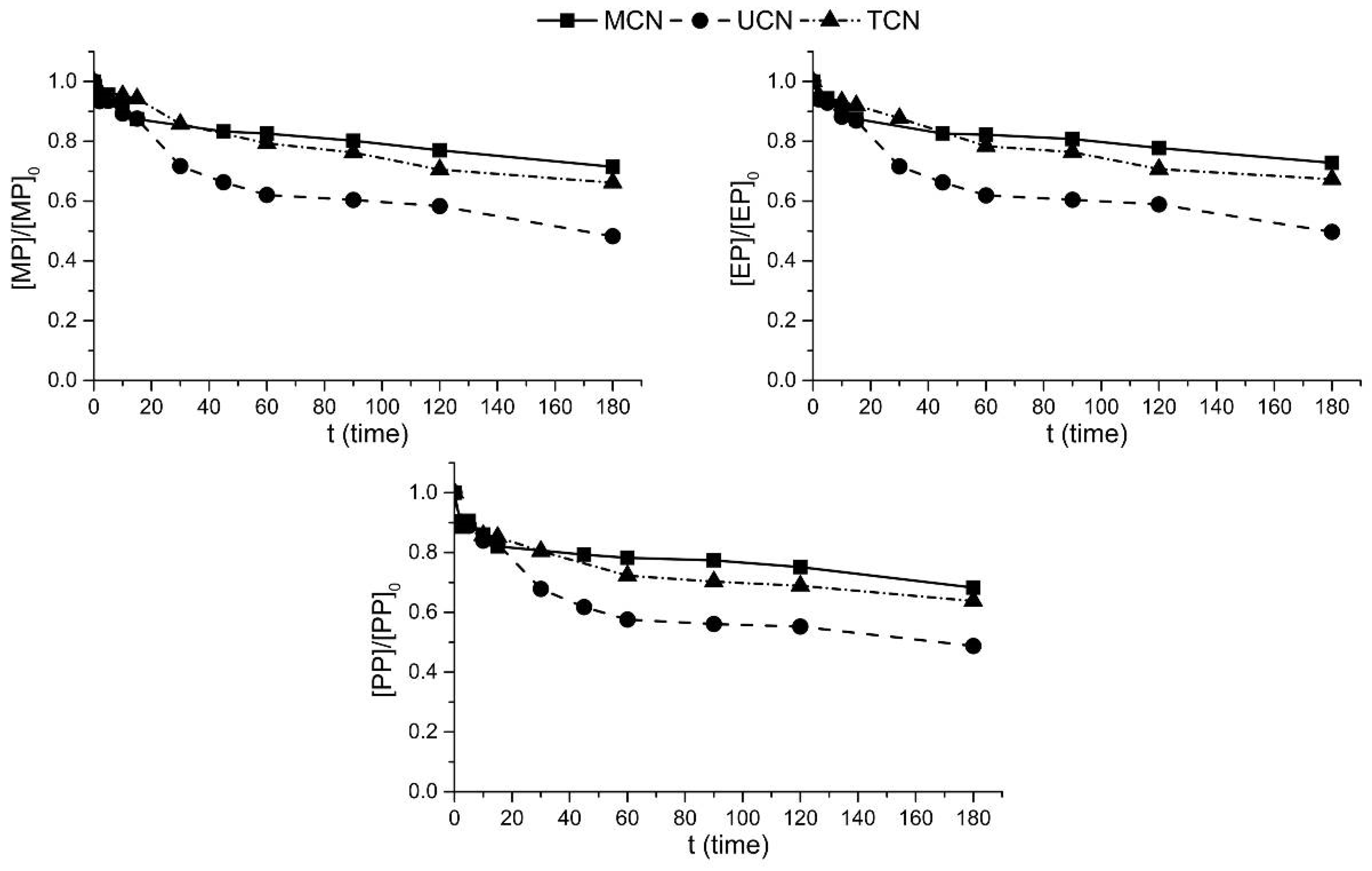
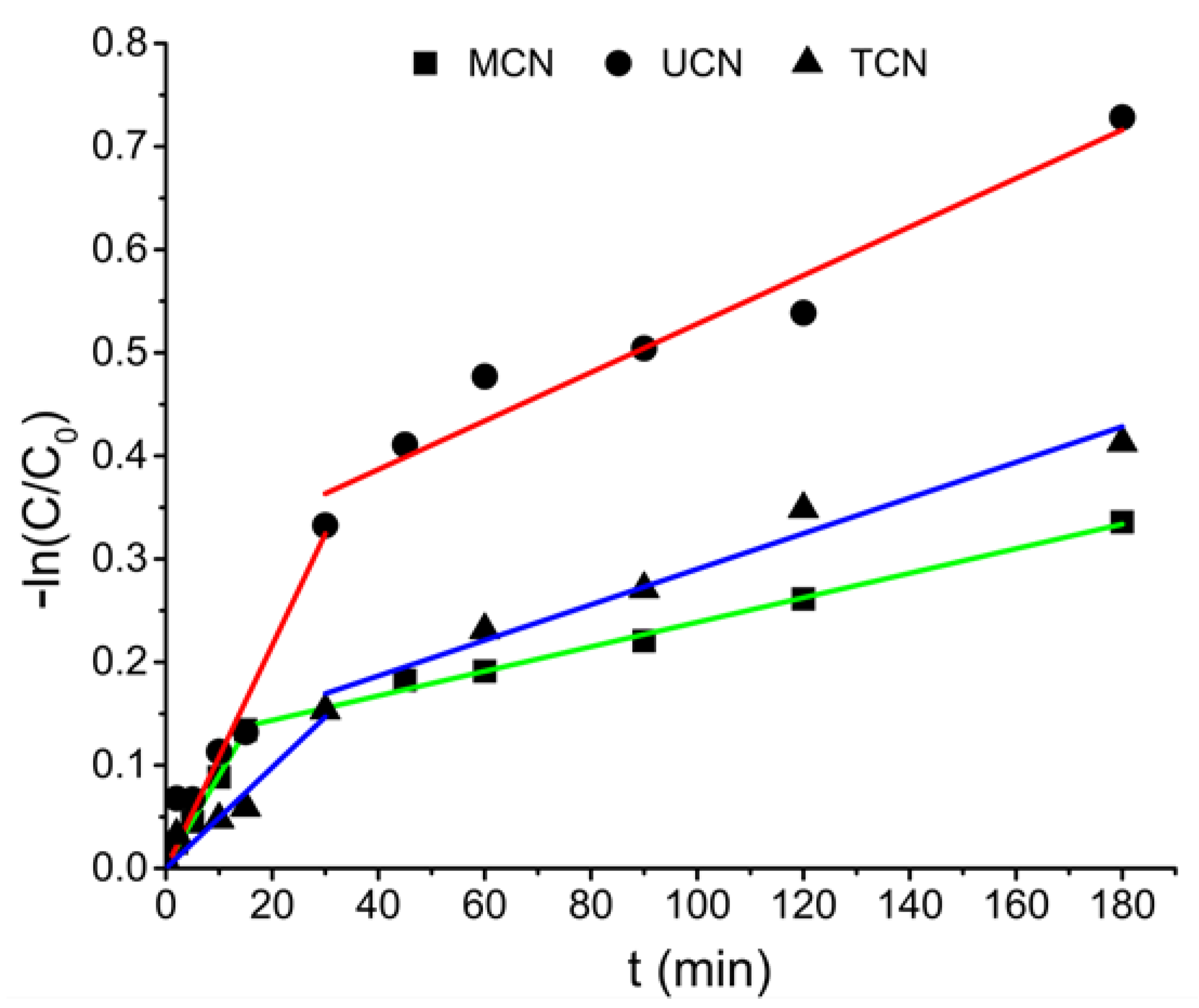
2.2. Photocatalytic Oxidation under Different Radiations
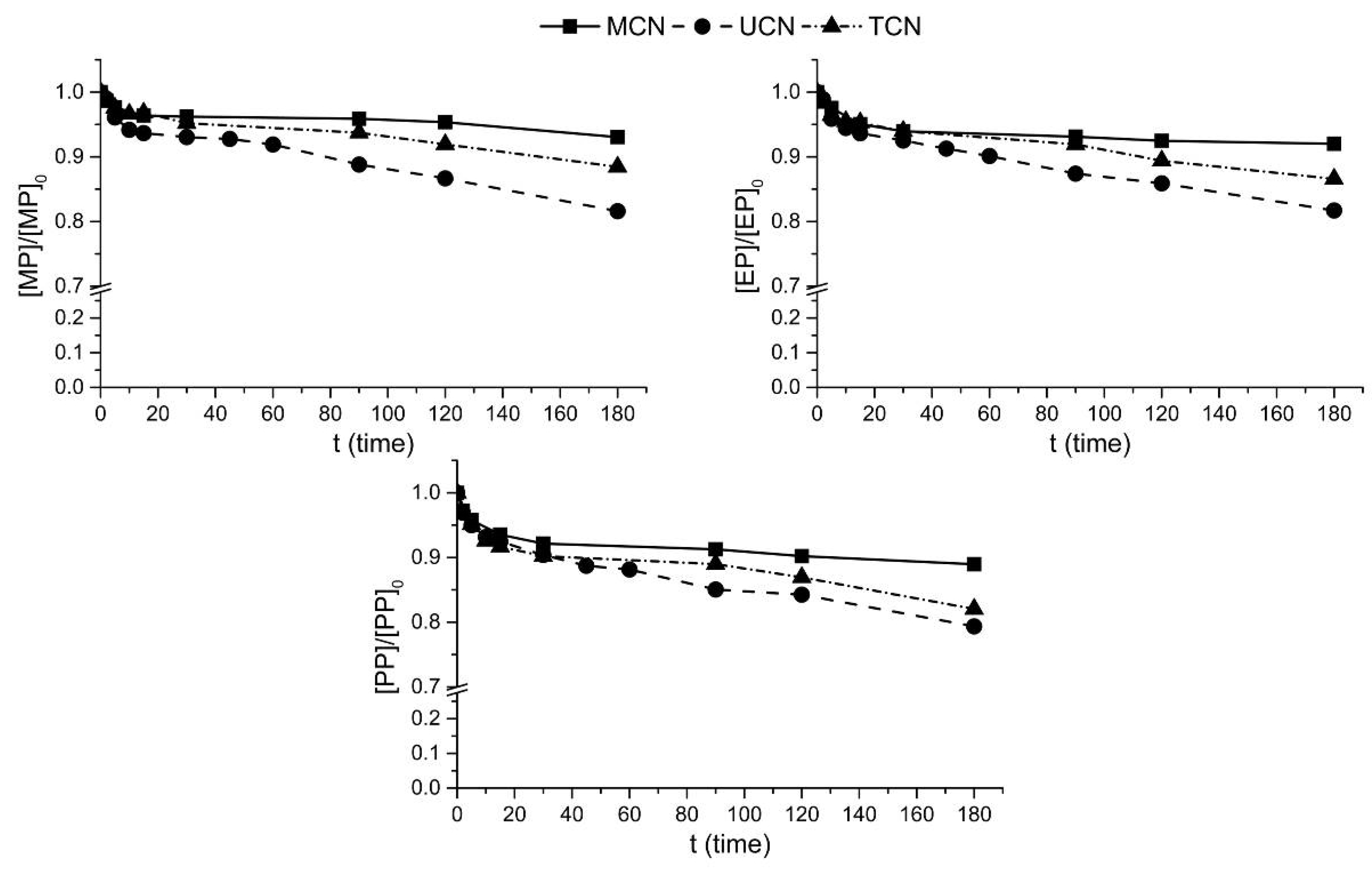
2.3. Photocatalytic Ozonation
3. Materials and Methods
3.1. Chemicals
3.2. Catalysts Synthesis
3.3. Photocatalytic and Ozonation Studies
3.4. Characterization Techniques
3.5. Analytical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- UN-Water. Summary Progress Update 2021: SDG 6—Water and Sanitation for All; UN-Water: Geneva, Switzerland, 2021. [Google Scholar]
- Stefano, P.H.P.; Roisenberg, A.; Santos, M.R.; Dias, M.A.; Montagner, C.C. Unraveling the Occurrence of Contaminants of Emerging Concern in Groundwater from Urban Setting: A Combined Multidisciplinary Approach and Self-Organizing Maps. Chemosphere 2022, 299, 134395. [Google Scholar] [CrossRef]
- Marson, E.O.; Paniagua, C.E.S.; Gomes Júnior, O.; Gonçalves, B.R.; Silva, V.M.; Ricardo, I.A.; Maria, M.C.; Amorim, C.C.; Trovó, A.G. A Review toward Contaminants of Emerging Concern in Brazil: Occurrence, Impact and Their Degradation by Advanced Oxidation Process in Aquatic Matrices. Sci. Total Environ. 2022, 836, 155605. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.M.; Tabatabaeian, M.; Chavoshani, A.; Amjadi, E.; Hashemi, M.; Ebrahimpour, K.; Klishadi, R.; Khazaei, S.; Mansourian, M. Paraben Content in Adjacent Normal-Malignant Breast Tissues from Women with Breast Cancer. Biomed. Environ. Sci. 2019, 32, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Jamal, A.; Rastkari, N.; Dehghaniathar, R.; Aghaei, M.; Nodehi, R.N.; Nasseri, S.; Kashani, H.; Yunesian, M. Prenatal Exposure to Parabens and Anthropometric Birth Outcomes: A Systematic Review. Environ. Res. 2019, 173, 419–431. [Google Scholar] [CrossRef]
- Cantarella, M.; Gorrasi, G.; Di Mauro, A.; Scuderi, M.; Nicotra, G.; Fiorenza, R.; Scirè, S.; Scalisi, M.E.; Brundo, M.V.; Privitera, V.; et al. Mechanical Milling: A Sustainable Route to Induce Structural Transformations in MoS2 for Applications in the Treatment of Contaminated Water. Sci. Rep. 2019, 9, 974. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Y.; Cong, S.; Xia, S.; Zou, D. Review of Mo-Based Materials in Heterogeneous Catalytic Oxidation for Wastewater Purification. Sep. Purif. Technol. 2023, 312, 123345. [Google Scholar] [CrossRef]
- Feijoo, S.; Yu, X.; Kamali, M.; Appels, L.; Dewil, R. Generation of Oxidative Radicals by Advanced Oxidation Processes (AOPs) in Wastewater Treatment: A Mechanistic, Environmental and Economic Review; Springer: Dordrecht, The Netherlands, 2023; Volume 22, ISBN 0123456789. [Google Scholar]
- Vaz, T.; Domingues, E.; Gomes, J.; Martins, R.C. Evaluation of the Activation Procedure on Oxone Efficiency for Synthetic Olive Mill Wastewater Treatment. Catalysts 2022, 12, 291. [Google Scholar] [CrossRef]
- Loeb, S.K.; Alvarez, P.J.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Li-Puma, G.; Quan, X.; et al. The Technology Horizon for Photocatalytic Water Treatment: Sunrise or Sunset? Environ. Sci. Technol. 2019, 53, 2937–2947. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhang, M.; Xu, L.; Wang, S.; Yang, T.; Wu, M.; Lu, W.; Li, Y.; Yu, D. Unraveling Timescale-Dependent Fe-MOFs Crystal Evolution for Catalytic Ozonation Reactivity Modulation. J. Hazard. Mater. 2022, 431, 128575. [Google Scholar] [CrossRef]
- Yang, T.; Yu, D.; Wang, D.; Yang, T.; Li, Z.; Wu, M.; Petru, M.; Crittenden, J. Accelerating Fe(Ⅲ)/Fe(Ⅱ) Cycle via Fe(Ⅱ) Substitution for Enhancing Fenton-like Performance of Fe-MOFs. Appl. Catal. B Environ. 2021, 286, 119859. [Google Scholar] [CrossRef]
- Ioannidi, A.; Frontistis, Z.; Mantzavinos, D. Destruction of Propyl Paraben by Persulfate Activated with UV-A Light Emitting Diodes. J. Environ. Chem. Eng. 2018, 6, 2992–2997. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, P.; Xie, P.; Shang, R.; Wang, Z.; Wang, S. Heat-Activated Persulfate Oxidation of Methyl- and Ethyl-Parabens: Effect, Kinetics, and Mechanism. Chemosphere 2017, 168, 1628–1636. [Google Scholar] [CrossRef]
- Zúñiga-Benítez, H.; Muñoz-Calderón, A.; Peñuela, G.A. Removal of a Mix of Benzophenones and Parabens Using Solar Photo-Fenton and a Cylinder Parabolic Collector in Aqueous Solutions. J. Environ. Chem. Eng. 2018, 6, 7347–7357. [Google Scholar] [CrossRef]
- Steter, J.R.; Brillas, E.; Sirés, I. Solar Photoelectro-Fenton Treatment of a Mixture of Parabens Spiked into Secondary Treated Wastewater Effluent at Low Input Current. Appl. Catal. B Environ. 2018, 224, 410–418. [Google Scholar] [CrossRef]
- Lee, W.; Marcotullio, S.; Yeom, H.; Son, H.; Kim, T.H.; Lee, Y. Reaction Kinetics and Degradation Efficiency of Halogenated Methylparabens during Ozonation and UV/H2O2 Treatment of Drinking Water and Wastewater Effluent. J. Hazard. Mater. 2022, 427, 127878. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.A.; Pham, T.T.; Nguyen-Phu, H.; Shin, E.W. The Effect of Graphitic Carbon Nitride Precursors on the Photocatalytic Dye Degradation of Water-Dispersible Graphitic Carbon Nitride Photocatalysts. Appl. Surf. Sci. 2021, 537, 148027. [Google Scholar] [CrossRef]
- Fernandes, E.; Gomes, J.; Martins, R.C. Semiconductors Application Forms and Doping Benefits to Wastewater Treatment: A Comparison of TiO2, WO3, and g-C3N4. Catalysts 2022, 12, 1218. [Google Scholar] [CrossRef]
- Cao, H.; Wang, J.; Kim, J.H.; Guo, Z.; Xiao, J.; Yang, J.; Chang, J.; Shi, Y.; Xie, Y. Different Roles of Fe Atoms and Nanoparticles on g-C3N4 in Regulating the Reductive Activation of Ozone under Visible Light. Appl. Catal. B Environ. 2021, 296, 120362. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, W.; Liao, G.; Li, X.; Wang, J.; Tang, Y.; Li, L. Strategy for Improving Photocatalytic Ozonation Activity of g-C3N4 by Halogen Doping for Water Purification. Appl. Catal. B Environ. 2022, 306, 121133. [Google Scholar] [CrossRef]
- Liao, G.; Zhu, D.; Li, L.; Lan, B. Enhanced Photocatalytic Ozonation of Organics by g-C3N4 under Visible Light Irradiation. J. Hazard. Mater. 2014, 280, 531–535. [Google Scholar] [CrossRef]
- Su, Q.; Sun, J.; Wang, J.; Yang, Z.; Cheng, W.; Zhang, S. Urea-Derived Graphitic Carbon Nitride as an Efficient Heterogeneous Catalyst for CO2 Conversion into Cyclic Carbonates. Catal. Sci. Technol. 2014, 4, 1556–1562. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, Y.; Fan, J.; Xue, Y.; Chang, H.; Masubuchi, Y.; Yin, S. Synthesis of Graphitic Carbon Nitride from Different Precursors by Fractional Thermal Polymerization Method and Their Visible Light Induced Photocatalytic Activities. J. Alloys Compd. 2018, 735, 1297–1305. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Ghandi, K. A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)–Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing. Nanomaterials 2022, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Gross, P.; Höppe, H.A. Biuret—A Crucial Reaction Intermediate for Understanding Urea Pyrolysis to Form Carbon Nitrides: Crystal-Structure Elucidation and In Situ Diffractometric, Vibrational and Thermal Characterisation. Chem. A Eur. J. 2020, 26, 14366–14376. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, Q.; Chai, G.; Liang, M.; Dong, G.; Zhang, Q.; Qiu, J. Synthesis and Luminescence Mechanism of Multicolor-Emitting g-C3N4 Nanopowders by Low Temperature Thermal Condensation of Melamine. Sci. Rep. 2013, 3, 1943. [Google Scholar] [CrossRef]
- Xin, G.; Meng, Y. Pyrolysis Synthesized g-C3N4 for Photocatalytic Degradation of Methylene Blue. J. Chem. 2013, 2013, 187912. [Google Scholar] [CrossRef]
- Jiang, X.; Li, J.; Fang, J.; Gao, L.; Cai, W.; Li, X.; Xu, A.; Ruan, X. The Photocatalytic Performance of g-C3N4 from Melamine Hydrochloride for Dyes Degradation with Peroxymonosulfate. J. Photochem. Photobiol. A Chem. 2017, 336, 54–62. [Google Scholar] [CrossRef]
- Petala, A.; Frontistis, Z.; Antonopoulou, M.; Konstantinou, I.; Kondarides, D.I.; Mantzavinos, D. Kinetics of Ethyl Paraben Degradation by Simulated Solar Radiation in the Presence of N-Doped TiO2 Catalysts. Water Res. 2015, 81, 157–166. [Google Scholar] [CrossRef]
- Álvarez, M.A.; Ruidíaz-Martínez, M.; Cruz-Quesada, G.; López-Ramón, M.V.; Rivera-Utrilla, J.; Sánchez-Polo, M.; Mota, A.J. Removal of Parabens from Water by UV-Driven Advanced Oxidation Processes. Chem. Eng. J. 2020, 379, 122334. [Google Scholar] [CrossRef]
- Fernandes, E.; Martins, R.C.; Gomes, J. Photocatalytic Ozonation of Parabens Mixture Using 10% N-TiO2 and the Effect of Water Matrix. Sci. Total Environ. 2020, 718, 137321. [Google Scholar] [CrossRef]
- Papailias, I.; Giannakopoulou, T.; Todorova, N.; Demotikali, D.; Vaimakis, T.; Trapalis, C. Effect of Processing Temperature on Structure and Photocatalytic Properties of g-C3N4. Appl. Surf. Sci. 2015, 358, 278–286. [Google Scholar] [CrossRef]
- Schachtner, J.; Bayer, P.; Von Wangelin, A.J. A Flow Reactor Setup for Photochemistry of Biphasic Gas/Liquid Reactions. Beilstein J. Org. Chem. 2016, 12, 1798–1811. [Google Scholar] [CrossRef]
- Hassani, A.; Eghbali, P.; Metin, Ö. Sonocatalytic Removal of Methylene Blue from Water Solution by Cobalt Ferrite/Mesoporous Graphitic Carbon Nitride (CoFe2O4/Mpg-C3N4) Nanocomposites: Response Surface Methodology Approach. Environ. Sci. Pollut. Res. 2018, 25, 32140–32155. [Google Scholar] [CrossRef] [PubMed]
- Rajeshwari, M.R.; Kokilavani, S.; Khan, S.S. Recent Developments in Architecturing the g-C3N4 Based Nanostructured Photocatalysts: Synthesis, Modifications and Applications in Water Treatment. Chemosphere 2021, 291, 132735. [Google Scholar] [CrossRef] [PubMed]
- Moschogiannaki, M.; Frontistis, Z.; Kiriakidis, G.; Mantzavinos, D.; Binas, V. Porous CoxNi1-XTiO3 Nanorods for Solar Photocatalytic Degradation of Ethyl Paraben. J. Mater. 2020, 6, 788–799. [Google Scholar] [CrossRef]
- Peñas-Garzón, M.; Sampaio, M.J.; Wang, Y.L.; Bedia, J.; Rodriguez, J.J.; Belver, C.; Silva, C.G.; Faria, J.L. Solar Photocatalytic Degradation of Parabens Using UiO-66-NH2. Sep. Purif. Technol. 2022, 286, 120467. [Google Scholar] [CrossRef]
- Bolton, J.R.; Bircher, K.G.; Tumas, W.; Tolman, C.A. Figures-of-Merit for the Technical Development and Application of Advanced Oxidation Technologies for Both Electric- and Solar-Driven Systems. Pure Appl. Chem. 2001, 73, 627–637. [Google Scholar] [CrossRef]
- Dhaka, S.; Kumar, R.; Lee, S.H.; Kurade, M.B.; Jeon, B.H. Degradation of Ethyl Paraben in Aqueous Medium Using Advanced Oxidation Processes: Efficiency Evaluation of UV-C Supported Oxidants. J. Clean. Prod. 2018, 180, 505–513. [Google Scholar] [CrossRef]
- Mergenbayeva, S.; Atabaev, T.S.; Vakros, J.; Mantzavinos, D.; Poulopoulos, S.G. Photocatalytic Degradation of 4-Tert-Butylphenol Using Solar Light Responsive Ag2CO3. Catalysts 2022, 12, 1523. [Google Scholar] [CrossRef]
- Fernandes, E.; Drosopoulou, S.; Mazierski, P.; Miodynska, M.; Gołaszewska, D.; Zaleska-Medynska, A.; Martins, R.C.; Gomes, J. Carbon Nitride Photoactivation Evaluation and Degradation of a Mixture of Parabens by Ozone Assistance. J. Water Process Eng. 2022, 49, 103018. [Google Scholar] [CrossRef]
- Acosta-Herazo, R.; Mueses, M.Á.; Puma, G.L.; Machuca-Martínez, F. Impact of Photocatalyst Optical Properties on the Efficiency of Solar Photocatalytic Reactors Rationalized by the Concepts of Initial Rate of Photon Absorption (IRPA) Dimensionless Boundary Layer of Photon Absorption and Apparent Optical Thickness. Chem. Eng. J. 2019, 356, 839–849. [Google Scholar] [CrossRef]
- Grčić, I.; Li Puma, G. Six-Flux Absorption-Scattering Models for Photocatalysis under Wide-Spectrum Irradiation Sources in Annular and Flat Reactors Using Catalysts with Different Optical Properties. Appl. Catal. B Environ. 2017, 211, 222–234. [Google Scholar] [CrossRef]
- Miodyńska, M.; Mikolajczyk, A.; Mazierski, P.; Klimczuk, T.; Lisowski, W.; Trykowski, G.; Zaleska-Medynska, A. Lead-Free Bismuth-Based Perovskites Coupled with g-C3N4: A Machine Learning Based Novel Approach for Visible Light Induced Degradation of Pollutants. Appl. Surf. Sci. 2022, 588, 152921. [Google Scholar] [CrossRef]
- Undergound, W. Solar Radiation Forecast. Available online: https://www.wunderground.com/dashboard/pws/ICOIMBRA41 (accessed on 3 June 2022).





| k1 × 10−3 (min) | k2 × 10−3 (min) | ||
|---|---|---|---|
| MCN | MP | 8.72 | 1.19 |
| EP | 9.62 | 0.79 | |
| PP | 14.58 | 1.19 | |
| UCN | MP | 10.82 | 2.35 |
| EP | 9.87 | 1.94 | |
| PP | 11.79 | 1.56 | |
| TCN | MP | 4.90 | 1.73 |
| EP | 4.86 | 1.33 | |
| PP | 8.72 | 1.04 |
| k1 × 10−3 (min) | k2 × 10−3 (min) | ||
|---|---|---|---|
| MCN | MP | 2.76 | 0.19 |
| EP | 3.60 | 0.14 | |
| PP | 5.02 | 0.24 | |
| UCN | MP | 5.09 | 0.91 |
| EP | 5.05 | 0.82 | |
| PP | 6.24 | 0.84 | |
| TCN | MP | 2.72 | 0.50 |
| EP | 3.91 | 0.56 | |
| PP | 6.66 | 0.64 |
| k × 10−3 (min) | ||
|---|---|---|
| MCN | MP | 2.22 |
| EP | 2.11 | |
| PP | 2.42 | |
| UCN | MP | 14.41 |
| EP | 14.32 | |
| PP | 14.36 | |
| TCN | MP | 5.63 |
| EP | 5.56 | |
| PP | 6.20 |
| Radiation Source | Contaminant | Removal (%) | EEO (kWh m−3 Order−1) | Energy Consumption (kWh m−3) | Cost (EUR m−3) |
|---|---|---|---|---|---|
| UVA | MP | 51.7 | 4.91 | 27 | 6.4 |
| EP | 50.3 | 4.89 | |||
| PP | 51.3 | 4.85 | |||
| Visible | MP | 18.4 | 5.12 | 39 | 9.3 |
| EP | 18.3 | 5.08 | |||
| PP | 20.6 | 5.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, E.; Mazierski, P.; Klimczuk, T.; Zaleska-Medynska, A.; Martins, R.C.; Gomes, J. g-C3N4 for Photocatalytic Degradation of Parabens: Precursors Influence, the Radiation Source and Simultaneous Ozonation Evaluation. Catalysts 2023, 13, 789. https://doi.org/10.3390/catal13050789
Fernandes E, Mazierski P, Klimczuk T, Zaleska-Medynska A, Martins RC, Gomes J. g-C3N4 for Photocatalytic Degradation of Parabens: Precursors Influence, the Radiation Source and Simultaneous Ozonation Evaluation. Catalysts. 2023; 13(5):789. https://doi.org/10.3390/catal13050789
Chicago/Turabian StyleFernandes, Eryk, Paweł Mazierski, Tomasz Klimczuk, Adriana Zaleska-Medynska, Rui C. Martins, and João Gomes. 2023. "g-C3N4 for Photocatalytic Degradation of Parabens: Precursors Influence, the Radiation Source and Simultaneous Ozonation Evaluation" Catalysts 13, no. 5: 789. https://doi.org/10.3390/catal13050789
APA StyleFernandes, E., Mazierski, P., Klimczuk, T., Zaleska-Medynska, A., Martins, R. C., & Gomes, J. (2023). g-C3N4 for Photocatalytic Degradation of Parabens: Precursors Influence, the Radiation Source and Simultaneous Ozonation Evaluation. Catalysts, 13(5), 789. https://doi.org/10.3390/catal13050789











