Methane Production from Biomass by Thermochemical Conversion: A Review
Abstract
1. Introduction
2. The Route of Gasification for SNG Production and Its Methanation Reaction
3. Methanation Catalysts
4. Gas Conditioning
5. Environmental and Economic Assessment
6. Other Thermochemical Methods
7. Summary and Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kambolis, A.; Schildhauer, T.J.; Kröcher, O. CO Methanation for Synthetic Natural Gas Production. Chimia 2015, 69, 608. [Google Scholar] [CrossRef] [PubMed]
- Kopyscinski, J.; Schildhauer, T.J.; Biollaz, S.M.A. Production of synthetic natural gas (SNG) from coal and dry biomass—A technology review from 1950 to 2009. Fuel 2010, 89, 1763–1783. [Google Scholar] [CrossRef]
- Kolb, S.; Plankenbühler, T.; Hofmann, K.; Bergerson, J.; Karl, J. Life cycle greenhouse gas emissions of renewable gas technologies: A comparative review. Renew. Sustain. Energy Rev. 2021, 146, 111147. [Google Scholar] [CrossRef]
- Pucker, J.; Zwart, R.; Jungmeier, G. Greenhouse gas and energy analysis of substitute natural gas from biomass for space heat. Biomass Bioenergy 2012, 38, 95–101. [Google Scholar] [CrossRef]
- Walspurger, S.; Haije, W.G.; Louis, B. CO2 Reduction to Substitute Natural Gas: Toward a Global Low Carbon Energy System. Isr. J. Chem. 2014, 54, 1432–1442. [Google Scholar] [CrossRef]
- Vitasari, C.R.; Jurascik, M.; Ptasinski, K.J. Exergy analysis of biomass-to-synthetic natural gas (SNG) process via indirect gasification of various biomass feedstock. Energy 2011, 36, 3825–3837. [Google Scholar] [CrossRef]
- Feng, F.; Song, G.H.; Shen, L.H.; Xiao, J. Energy efficiency analysis of biomass-based synthetic natural gas production process using interconnected fluidized beds and fluidized bed methanation reactor. Clean Technol. Environ. Policy 2016, 18, 965–971. [Google Scholar] [CrossRef]
- Schildhauer, T.J.; Biollaz, S.M.A. Reactors for Catalytic Methanation in the Conversion of Biomass to Synthetic Natural Gas (SNG). Chimia 2015, 69, 603. [Google Scholar] [CrossRef]
- Billig, E.; Thraen, D. Renewable methane—A technology evaluation by multi-criteria decision making from a European perspective. Energy 2017, 139, 468–484. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Ducey, T.; Ro, K.S.; Hunt, P.G. Livestock waste-to-bioenergy generation opportunities. Bioresour. Technol. 2008, 99, 7941–7953. [Google Scholar] [CrossRef]
- Haiduc, A.G.; Brandenberger, M.; Suquet, S.; Vogel, F.; Bernier-Latmani, R.; Ludwig, C. SunCHem: An integrated process for the hydrothermal production of methane from microalgae and CO2 mitigation. J. Appl. Phycol. 2009, 21, 529–541. [Google Scholar] [CrossRef]
- Ren, J.; Liu, Y.; Zhao, X.; Cao, J. Methanation of syngas from biomass gasification: An overview. Int. J. Hydrog. Energy 2020, 45, 4223–4243. [Google Scholar] [CrossRef]
- Rehling, B.; Hofbauer, H.; Rauch, R.; Aichernig, C. BioSNG—Process simulation and comparison with first results from a 1-MW demonstration plant. Biomass Convers. Biorefinery 2011, 1, 111–119. [Google Scholar] [CrossRef]
- The Technical Choices for the 12 MW Bio-SNG Demonstration in the Netherlands. Available online: http://www.biosng.com/fileadmin/biosng/user/documents/presentations/The_technical_choices_for_the_12_MW_Bio-Methane_demonstration.pdf (accessed on 6 April 2023).
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Bassano, C.; Deiana, P.; Lietti, L.; Visconti, C.G. P2G movable modular plant operation on synthetic methane production from CO2 and hydrogen from renewables sources. Fuel 2019, 253, 1071–1079. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Q.; Gu, F.; Liu, B.; Zhong, Z.; Su, F. Recent advances in methanation catalysts for the production of synthetic natural gas. Rsc Adv. 2015, 5, 22759–22776. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Ping, Y.; Hu, D.; Xu, G.; Gu, F.; Su, F. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas. Rsc Adv. 2012, 2, 2358–2368. [Google Scholar] [CrossRef]
- Razzaq, R.; Zhu, H.; Jiang, L.; Muhammad, U.; Li, C.; Zhang, S. Catalytic Methanation of CO and CO2 in Coke Oven Gas over Ni–Co/ZrO2–CeO2. Ind. Eng. Chem. Res. 2013, 52, 2247–2256. [Google Scholar] [CrossRef]
- Habazaki, H.; Yamasaki, M.; Zhang, B.; Kawashima, A.; Kohno, S.; Takai, T.; Hashimoto, K. Co-methanation of carbon monoxide and carbon dioxide on supported nickel and cobalt catalysts prepared from amorphous alloys. Appl. Catal. A Gen. 1998, 172, 131–140. [Google Scholar] [CrossRef]
- Salbrechter, K.; Schubert, T. Combination of b-Fuels and e-Fuels—A Technological Feasibility Study. Energies 2021, 14, 5250. [Google Scholar] [CrossRef]
- Jaffar, M.M.; Nahil, M.A.; Williams, P.T. Methane Production from the Pyrolysis—Catalytic Hydrogenation of Waste Biomass: Influence of Process Conditions and Catalyst Type. Energy Fuels 2019, 33, 7443–7457. [Google Scholar] [CrossRef]
- Luterbacher, J.S.; Fröling, M.; Vogel, F.; Maréchal, F.; Tester, J.W. Hydrothermal Gasification of Waste Biomass: Process Design and Life Cycle Asessment. Environ. Sci. Technol. 2009, 43, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.M.; Wissing, L.; Skjøth-Rasmussen, M.S. High temperature methanation: Catalyst considerations. Catal. Today 2013, 215, 233–238. [Google Scholar] [CrossRef]
- Bligaard, T.; Nørskov, J.K.; Dahl, S.; Matthiesen, J.; Christensen, C.H.; Sehested, J. The Brønsted–Evans–Polanyi relation and the volcano curve in heterogeneous catalysis. J. Catal. 2004, 224, 206–217. [Google Scholar] [CrossRef]
- Czekaj, I.; Loviat, F.; Raimondi, F.; Wambach, J.; Biollaz, S.; Wokaun, A. Characterization of surface processes at the Ni-based catalyst during the methanation of biomass-derived synthesis gas: X-ray photoelectron spectroscopy (XPS). Appl. Catal. A Gen. 2007, 329, 68–78. [Google Scholar] [CrossRef]
- Sehested, J. Four challenges for nickel steam-reforming catalysts. Catal. Today 2006, 111, 103–110. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, G.; Xu, S. Simultaneous Tar Reforming and Syngas Methanation for Bio-Substitute Natural Gas. Ind. Eng. Chem. Res. 2018, 57, 10905–10914. [Google Scholar] [CrossRef]
- Yuan, C.; Yao, N.; Wang, X.; Wang, J.; Lv, D.; Li, X. The SiO2 supported bimetallic Ni–Ru particles: A good sulfur-tolerant catalyst for methanation reaction. Chem. Eng. J. 2015, 260, 1–10. [Google Scholar] [CrossRef]
- Xing, W.; Liu, Y.; Zhang, W.; Sun, Y.; Kai, X.; Yang, T. Study on Methanation Performance of Biomass Gasification Syngas Based on a Ni/Al2O3 Monolithic Catalyst. ACS Omega 2020, 5, 28597–28605. [Google Scholar] [CrossRef]
- Shinde, V.M.; Madras, G. CO methanation toward the production of synthetic natural gas over highly active Ni/TiO2 catalyst. AICHE J. 2014, 60, 1027–1035. [Google Scholar] [CrossRef]
- Kamata, H.; Tian, Z.Q.; Izumi, Y.; Choong, C.K.S.; Chang, J.; Schreyer, M.; Chen, L.; Borgna, A. Dispersed and high loading Ni catalyst stabilized in porous SiO2 matrix for substituted natural gas production. Catal. Today 2018, 299, 193–200. [Google Scholar] [CrossRef]
- Ding, M.; Tu, J.; Zhang, Q.; Wang, M.; Tsubaki, N.; Wang, T.; Ma, L. Enhancement of methanation of bio-syngas over CeO2–modified Ni/Al2O3 catalysts. Biomass Bioenergy 2016, 85, 12–17. [Google Scholar] [CrossRef]
- Ding, M.; Tu, J.; Wang, T.; Ma, L.; Wang, C.; Chen, L. Bio-syngas methanation towards synthetic natural gas (SNG) over highly active Al2O3–CeO2 supported Ni catalyst. Fuel Process. Technol. 2015, 134, 480–486. [Google Scholar] [CrossRef]
- Baidya, T.; Cattolica, R.; Seiser, R. Ni-Ru-MgO catalyst with high activity and stability for methanation of syngas and producer gas. Catal. Today 2022, 397–399, 69–80. [Google Scholar] [CrossRef]
- Rönsch, S.; Ortwein, A. Methanisierung von Synthesegasen—Grundlagen und Verfahrensentwicklungen. Chem-Ing-Tech 2011, 83, 1200–1208. [Google Scholar] [CrossRef]
- Lei, Y.; Cant, N.W.; Trimm, D.L. Activity patterns for the water gas shift reaction over supported precious metal catalysts. Catal. Lett. 2005, 103, 133–136. [Google Scholar] [CrossRef]
- Smith, R.J.B.; Loganathan, M.; Shantha, M.S. A Review of the Water Gas Shift Reaction Kinetics. Int. J. Chem. React. Eng. 2010, 8. [Google Scholar] [CrossRef]
- Bukur, D.B.; Todic, B.; Elbashir, N. Role of water-gas-shift reaction in Fischer–Tropsch synthesis on iron catalysts: A review. Catal. Today 2016, 275, 66–75. [Google Scholar] [CrossRef]
- Martínez, I.; Romano, M.C. Flexible sorption enhanced gasification (SEG) of biomass for the production of synthetic natural gas (SNG) and liquid biofuels: Process assessment of stand-alone and power-to-gas plant schemes for SNG production. Energy 2016, 113, 615–630. [Google Scholar] [CrossRef]
- Bartik, A.; Fuchs, J.; Pacholik, G.; Föttinger, K.; Hofbauer, H.; Müller, S.; Benedikt, F. Experimental investigation on the methanation of hydrogen-rich syngas in a bubbling fluidized bed reactor utilizing an optimized catalyst. Fuel Process. Technol. 2022, 237, 107402. [Google Scholar] [CrossRef]
- Ding, W.; Zhang, X.; Zhao, B.; Zhou, W.; Xu, A.; Chen, L.; Sun, L.; Yang, S.; Guan, H.; Xie, X.; et al. TG-FTIR and thermodynamic analysis of the herb residue pyrolysis with in-situ CO2 capture using CaO catalyst. J. Anal. Appl. Pyrol. 2018, 134, 389–394. [Google Scholar] [CrossRef]
- Molino, A.; Braccio, G. Synthetic natural gas SNG production from biomass gasification—Thermodynamics and processing aspects. Fuel 2015, 139, 425–429. [Google Scholar] [CrossRef]
- Haro, P.; Johnsson, F.; Thunman, H. Improved syngas processing for enhanced Bio-SNG production: A techno-economic assessment. Energy 2016, 101, 380–389. [Google Scholar] [CrossRef]
- Dong, X.; Song, M.; Jin, B.; Zhou, Z.; Yang, X. The Synergy Effect of Ni-M (M = Mo, Fe, Co, Mn or Cr) Bicomponent Catalysts on Partial Methanation Coupling with Water Gas Shift under Low H2/CO Conditions. Catalysts 2017, 7, 51. [Google Scholar] [CrossRef]
- Bareschino, P.; Mancusi, E.; Tregambi, C.; Pepe, F.; Urciuolo, M.; Brachi, P.; Ruoppolo, G. Integration of biomasses gasification and renewable-energies-driven water electrolysis for methane production. Energy 2021, 230, 120863. [Google Scholar] [CrossRef]
- Zhang, J.; Fatah, N.; Capela, S.; Kara, Y.; Guerrini, O.; Khodakov, A.Y. Kinetic investigation of carbon monoxide hydrogenation under realistic conditions of methanation of biomass derived syngas. Fuel 2013, 111, 845–854. [Google Scholar] [CrossRef]
- Bai, X.; Wang, S.; Sun, T.; Wang, S. The sintering of Ni/Al2O3 methanation catalyst for substitute natural gas production. React. Kinet. Mech. Catal. 2014, 112, 437–451. [Google Scholar] [CrossRef]
- Sarić, M.; Dijkstra, J.W.; Haije, W.G. Economic perspectives of Power-to-Gas technologies in bio-methane production. J. CO2 Util. 2017, 20, 81–90. [Google Scholar] [CrossRef]
- Gutiérrez-Martín, F.; Rodríguez-Antón, L.M. Power-to-SNG technologies by hydrogenation of CO2 and biomass resources: A comparative chemical engineering process analysis. Int. J. Hydrog. Energy 2019, 44, 12544–12553. [Google Scholar] [CrossRef]
- Giglio, E.; Vitale, G.; Lanzini, A.; Santarelli, M. Integration between biomass gasification and high-temperature electrolysis for synthetic methane production. Biomass Bioenergy 2021, 148, 106017. [Google Scholar] [CrossRef]
- Heath, G.A.; O’Donoughue, P.; Arent, D.J.; Bazilian, M. Harmonization of initial estimates of shale gas life cycle greenhouse gas emissions for electric power generation. Proc. Natl. Acad. Sci. USA 2014, 111, E3167–E3176. [Google Scholar] [CrossRef]
- Skorek-Osikowska, A. Thermodynamic and environmental study on synthetic natural gas production in power to gas approaches involving biomass gasification and anaerobic digestion. Int. J. Hydrog. Energy 2022, 47, 3284–3293. [Google Scholar] [CrossRef]
- Bargiacchi, E.; Candelaresi, D.; Valente, A.; Spazzafumo, G.; Frigo, S. Life Cycle Assessment of Substitute Natural Gas production from biomass and electrolytic hydrogen. Int. J. Hydrog. Energy 2021, 46, 35974–35984. [Google Scholar] [CrossRef]
- Song, G.; Xiao, J.; Yu, Y.; Shen, L. A techno-economic assessment of SNG production from agriculture residuals in China. Energ. Source Part B 2016, 11, 465–471. [Google Scholar] [CrossRef]
- Schubert, M.; Müller, J.B.; Vogel, F. Continuous Hydrothermal Gasification of Glycerol Mixtures: Autothermal Operation, Simultaneous Salt Recovery, and the Effect of K3PO4 on the Catalytic Gasification. Ind. Eng. Chem. Res. 2014, 53, 8404–8415. [Google Scholar] [CrossRef]
- Waldner, M.H.; Vogel, F. Renewable Production of Methane from Woody Biomass by Catalytic Hydrothermal Gasification. Ind. Eng. Chem. Res. 2005, 44, 4543–4551. [Google Scholar] [CrossRef]
- Kruse, A. Hydrothermal biomass gasification. J. Supercrit. Fluids 2009, 47, 391–399. [Google Scholar] [CrossRef]
- Lewandowski, W.M.; Ryms, M.; Kosakowski, W. Thermal Biomass Conversion: A Review. Processes 2020, 8, 516. [Google Scholar] [CrossRef]
- Kirubakaran, V.; Sivaramakrishnan, V.; Nalini, R.; Sekar, T.; Premalatha, M.; Subramanian, P. A review on gasification of biomass. Renew. Sustain. Energy Rev. 2009, 13, 179–186. [Google Scholar] [CrossRef]
- Gassner, M.; Vogel, F.; Heyen, G.; Marechal, F. Optimal process design for the polygeneration of SNG, power and heat by hydrothermal gasification of waste biomass: Thermo-economic process modelling and integration. Energ. Environ. Sci. 2011, 4, 1726–1741. [Google Scholar] [CrossRef]
- Dreher, M.; Johnson, B.; Peterson, A.A.; Nachtegaal, M.; Wambach, J.; Vogel, F. Catalysis in supercritical water: Pathway of the methanation reaction and sulfur poisoning over a Ru/C catalyst during the reforming of biomolecules. J. Catal. 2013, 301, 38–45. [Google Scholar] [CrossRef]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, J.M.J.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energ. Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Vogel, F.D.R.; Waldner, M.H.; Rouff, A.A.; Rabe, S. Synthetic natural gas from biomass by catalytic conversion in supercritical water. Green Chem. 2007, 9, 616–619. [Google Scholar] [CrossRef]
- Yeh, T.M.; Dickinson, J.G.; Franck, A.; Linic, S.; Thompson, L.T.; Savage, P.E. Hydrothermal catalytic production of fuels and chemicals from aquatic biomass. J. Chem. Technol. Biotechnol. 2013, 88, 13–24. [Google Scholar] [CrossRef]
- Üremek Cengiz, N.; Eren, S.; Sağlam, M.; Yüksel, M.; Ballice, L. Influence of temperature and pressure on hydrogen and methane production in the hydrothermal gasification of wood residues. J. Supercrit. Fluids 2016, 107, 243–249. [Google Scholar] [CrossRef]
- Gassner, M.; Vogel, F.; Heyen, G.; Marechal, F. Optimal process design for the polygeneration of SNG, power and heat by hydrothermal gasification of waste biomass: Process optimisation for selected substrates. Energ. Environ. Sci. 2011, 4, 1742–1758. [Google Scholar] [CrossRef]
- Ondze, F.; Ferrasse, J.; Boutin, O.; Ruiz, J.; Charton, F. Process simulation and energetic analysis of different supercritical water gasification systems for the valorisation of biomass. J. Supercrit. Fluids 2018, 133, 114–121. [Google Scholar] [CrossRef]
- Karayıldırım, T.; Sınag, A.; Kruse, A. Char and Coke Formation as Unwanted Side Reaction of the Hydrothermal Biomass Gasification. Chem. Eng. Technol. 2008, 31, 1561–1568. [Google Scholar] [CrossRef]
- Görling, M.; Larsson, M.; Alvfors, P. Bio-methane via fast pyrolysis of biomass. Appl. Energ. 2013, 112, 440–447. [Google Scholar] [CrossRef]
- Yun, H.A.H.; Ramírez-Solís, S.; Dupont, V. Bio-CH4 from palm empty fruit bunch via pyrolysis-direct methanation: Full plant model and experiments with bio-oil surrogate. J. Clean. Prod. 2020, 244, 118737. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, M.; Wang, F. Oxygen-vacancy-mediated catalytic methanation of lignocellulose at temperatures below 200 °C. Joule 2021, 5, 3031–3044. [Google Scholar] [CrossRef]
- Walspurger, S.; Elzinga, G.D.; Dijkstra, J.W.; Sarić, M.; Haije, W.G. Sorption enhanced methanation for substitute natural gas production: Experimental results and thermodynamic considerations. Chem. Eng. J. 2014, 242, 379–386. [Google Scholar] [CrossRef]
- Leimert, J.M.; Neubert, M.; Treiber, P.; Dillig, M.; Karl, J. Combining the Heatpipe Reformer technology with hydrogen-intensified methanation for production of synthetic natural gas. Appl. Energ. 2018, 217, 37–46. [Google Scholar] [CrossRef]
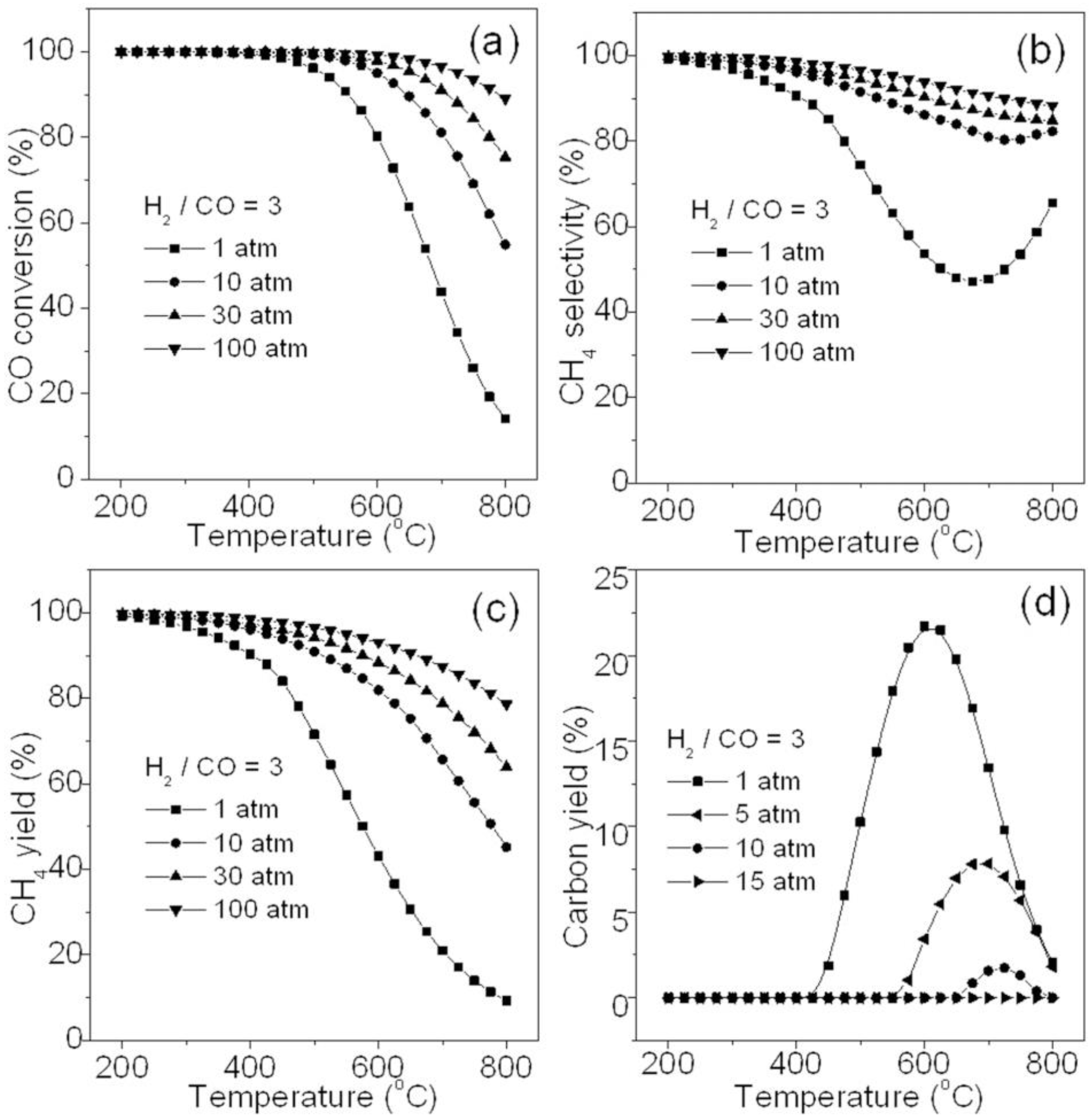



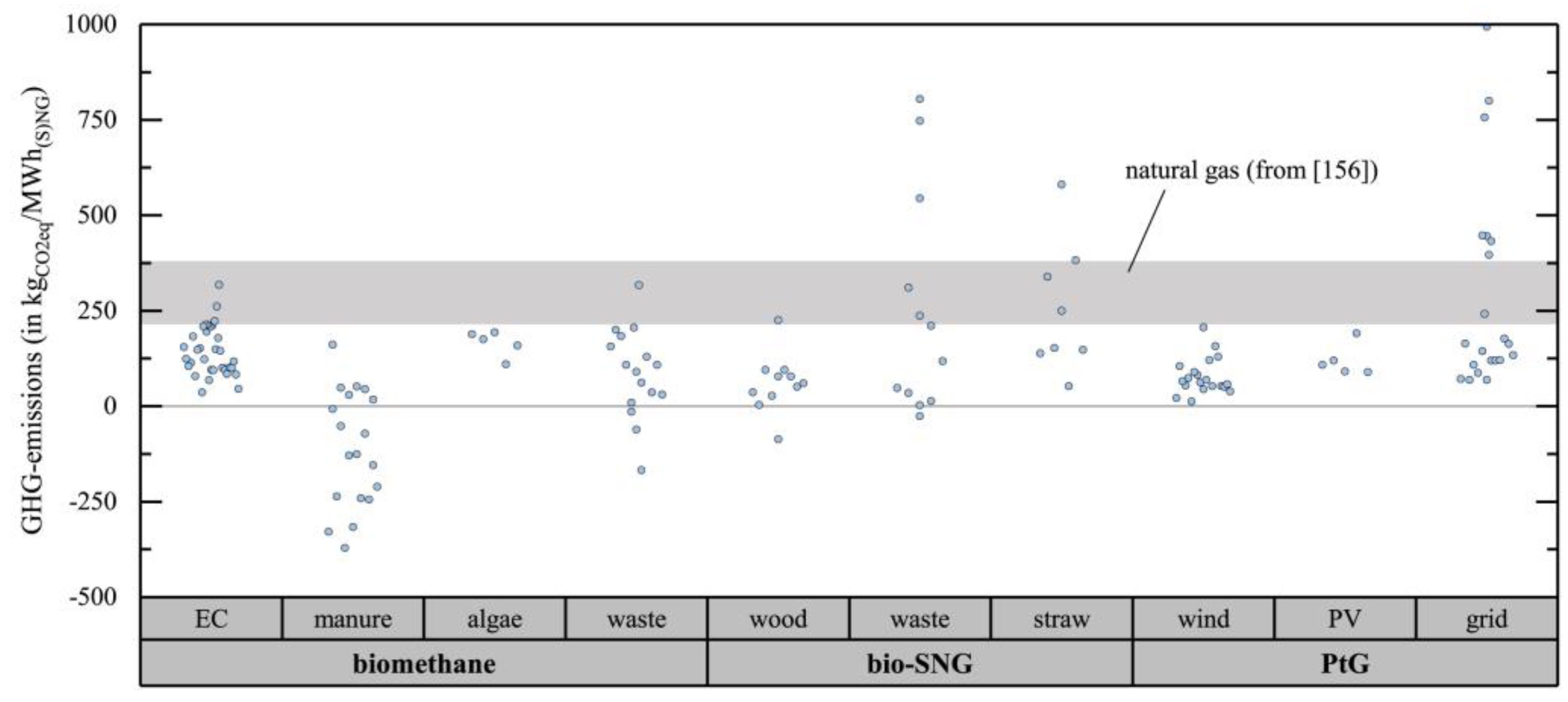

| Technology * | Advantage | Disadvantage | |
|---|---|---|---|
| Anaerobic digestion | Mature technology Low cost More types of feeds | Low energy efficiency Long retention time | |
| Gasification | High energy efficiency Short retention time | High gasification temperature Challenges for catalyst selection and stability | |
| Sorption enhanced reforming | High methane yield CO2 absorption | Need for suitable adsorbent | |
| Power-to-Gas | Very high methane yield Accommodating excess power and intermittent renewable energy | High investment Limited by electricity prices and sources Complex systems | |
| Hydrothermal gasification | High energy efficiency Low conversion temperature Less tar and coke Process wet biomass | High pressure High investment Corrosion of reactor Challenges for catalyst selection and stability | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Ye, X.; Wang, Y.; Wang, L. Methane Production from Biomass by Thermochemical Conversion: A Review. Catalysts 2023, 13, 771. https://doi.org/10.3390/catal13040771
Wu Y, Ye X, Wang Y, Wang L. Methane Production from Biomass by Thermochemical Conversion: A Review. Catalysts. 2023; 13(4):771. https://doi.org/10.3390/catal13040771
Chicago/Turabian StyleWu, Yuke, Xinchen Ye, Yutong Wang, and Lian Wang. 2023. "Methane Production from Biomass by Thermochemical Conversion: A Review" Catalysts 13, no. 4: 771. https://doi.org/10.3390/catal13040771
APA StyleWu, Y., Ye, X., Wang, Y., & Wang, L. (2023). Methane Production from Biomass by Thermochemical Conversion: A Review. Catalysts, 13(4), 771. https://doi.org/10.3390/catal13040771








