Heterobinuclear Metallocomplexes as Photocatalysts in Organic Synthesis
Abstract
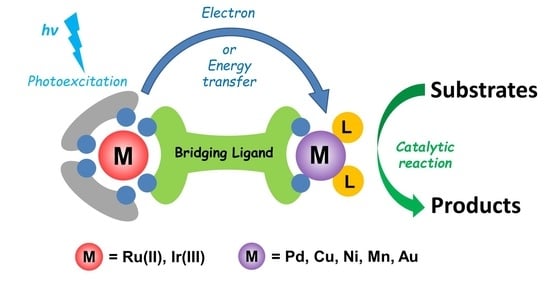
1. Introduction
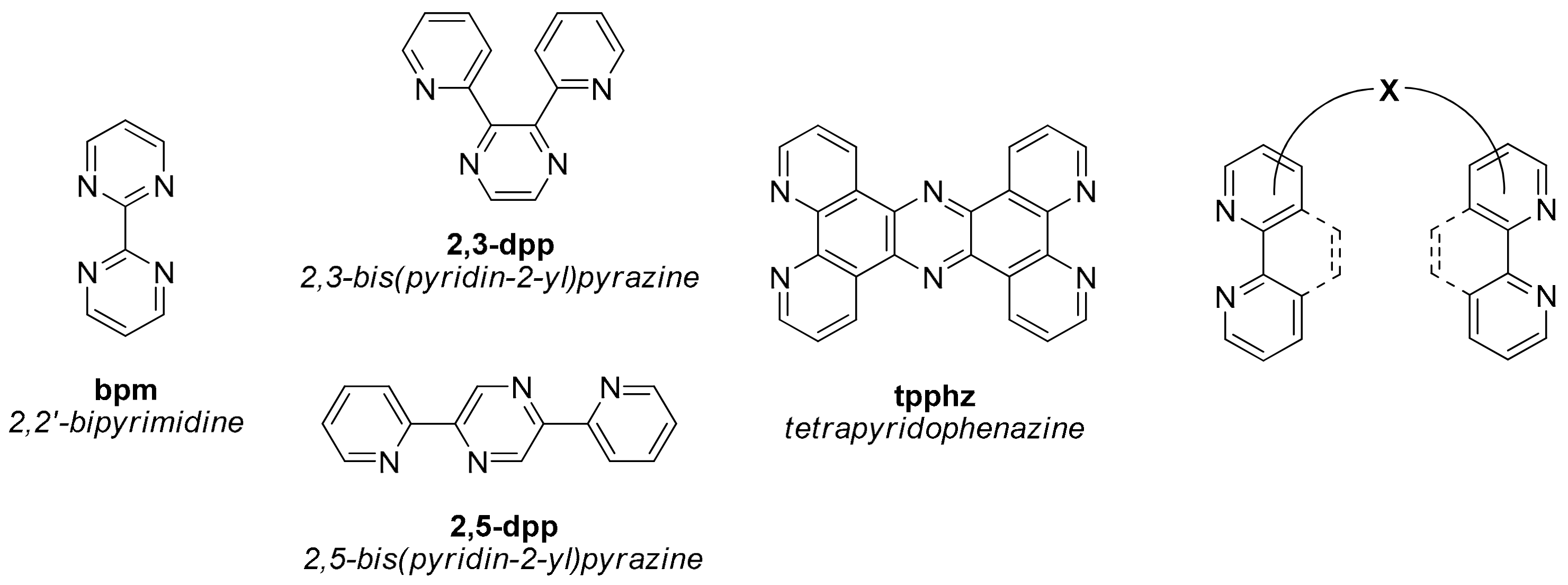
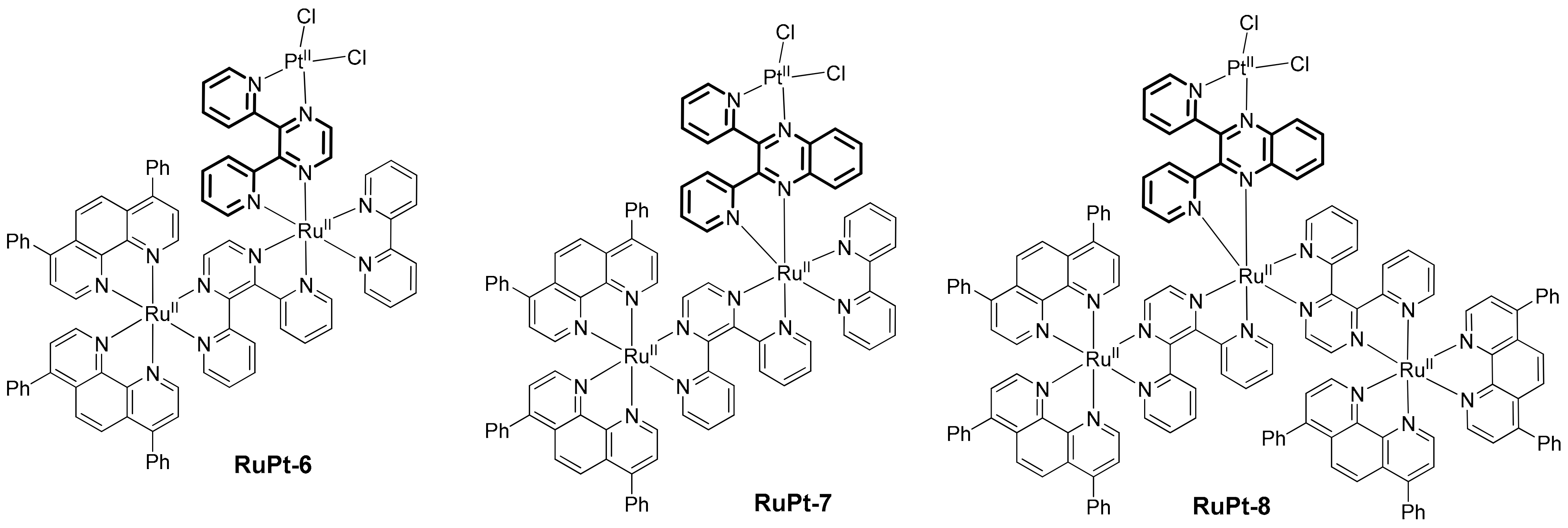
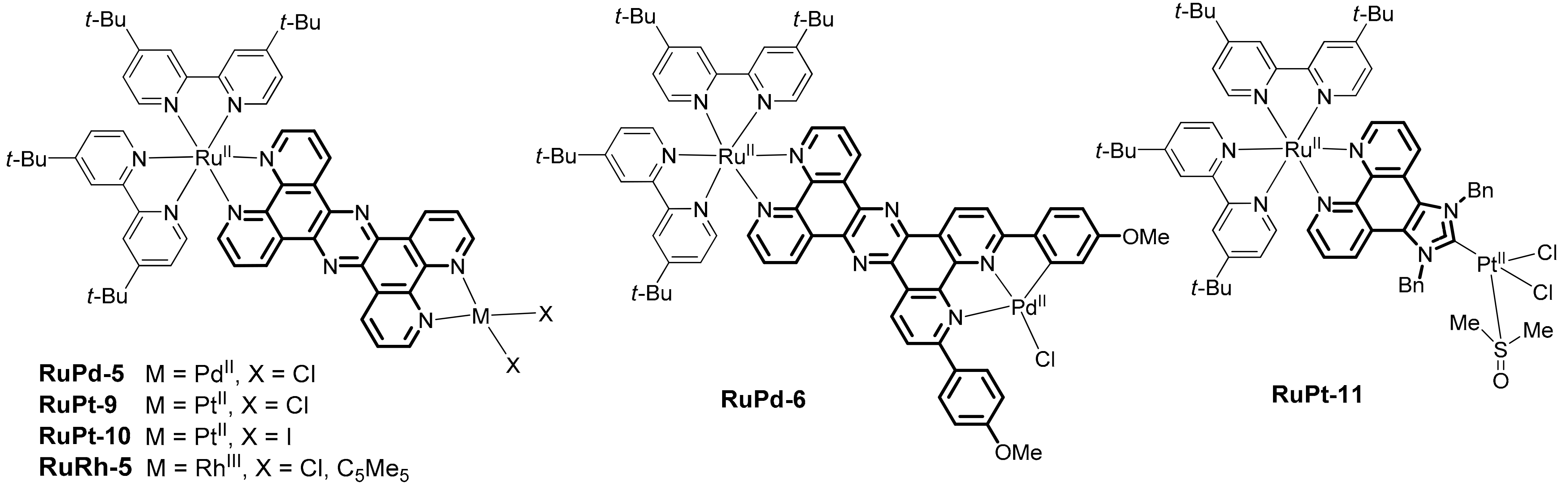
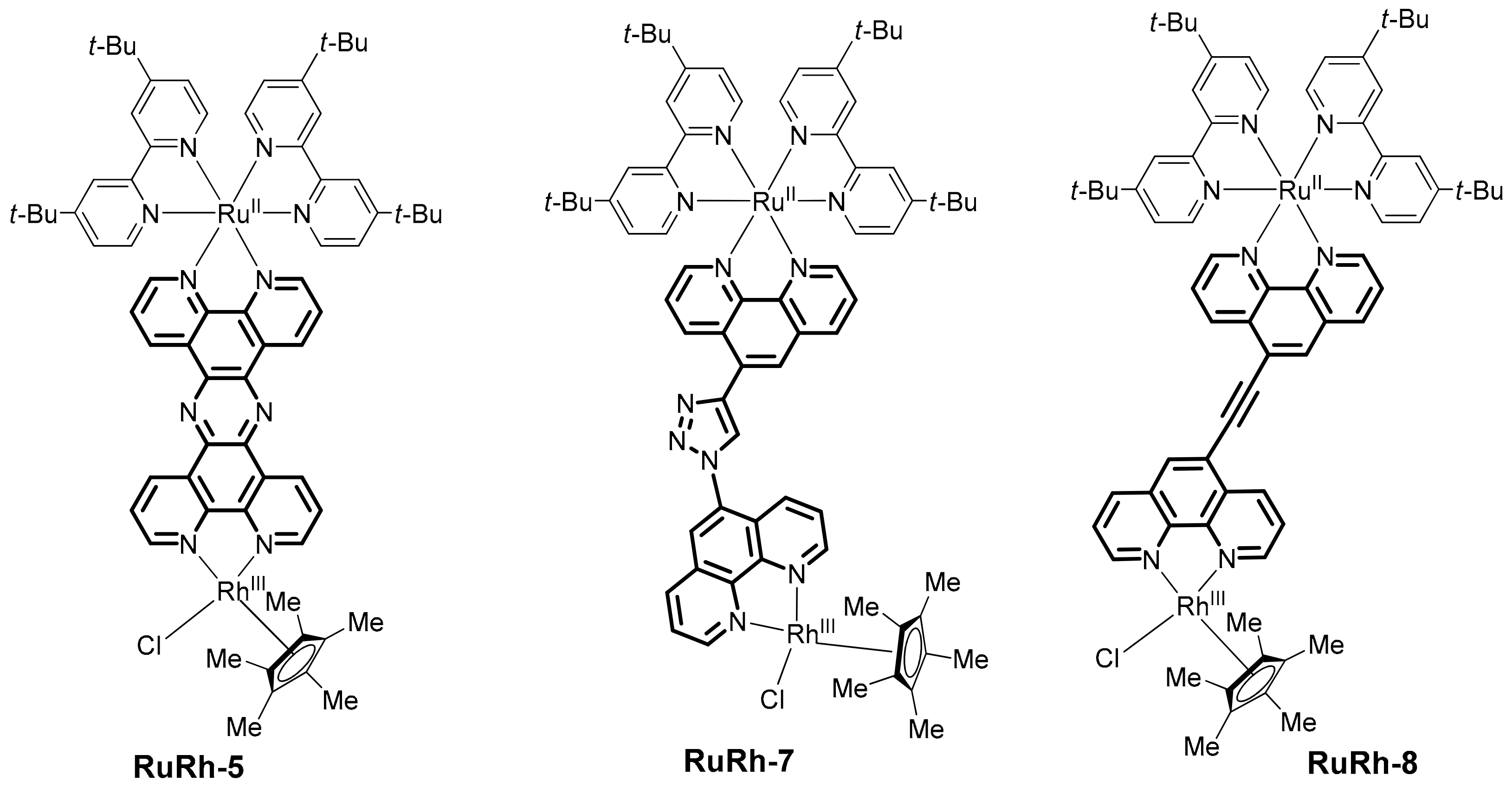
2. Heterobinuclear Photocatalysts
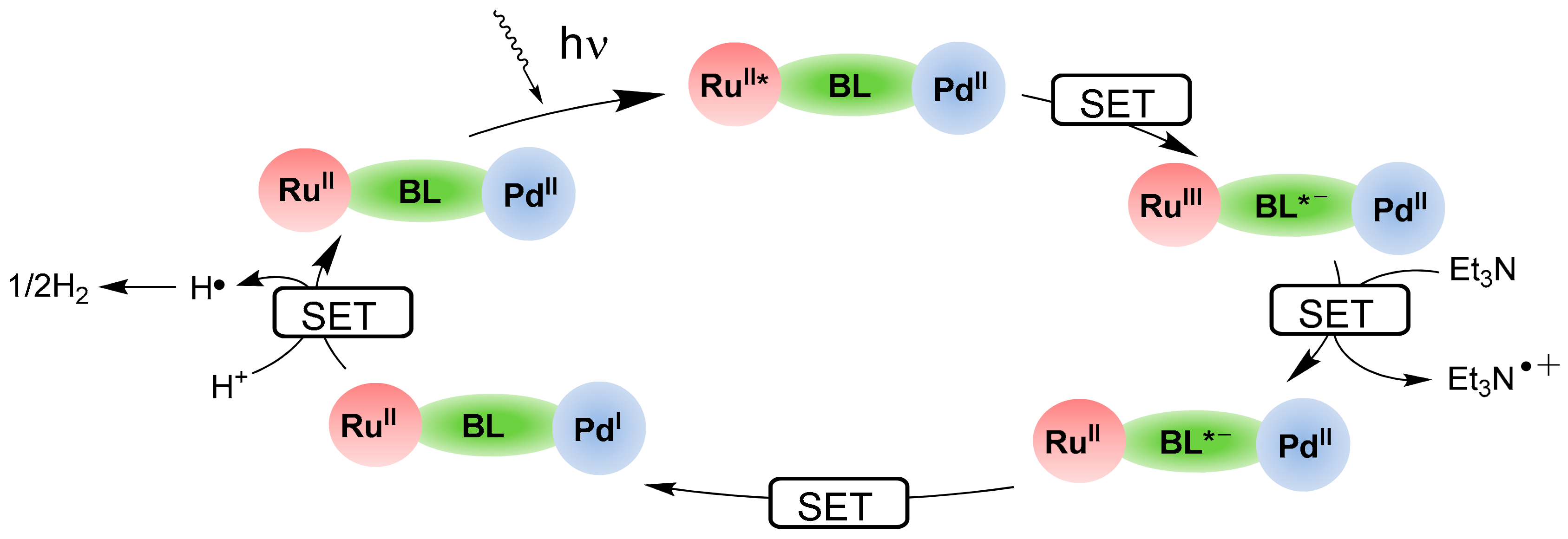
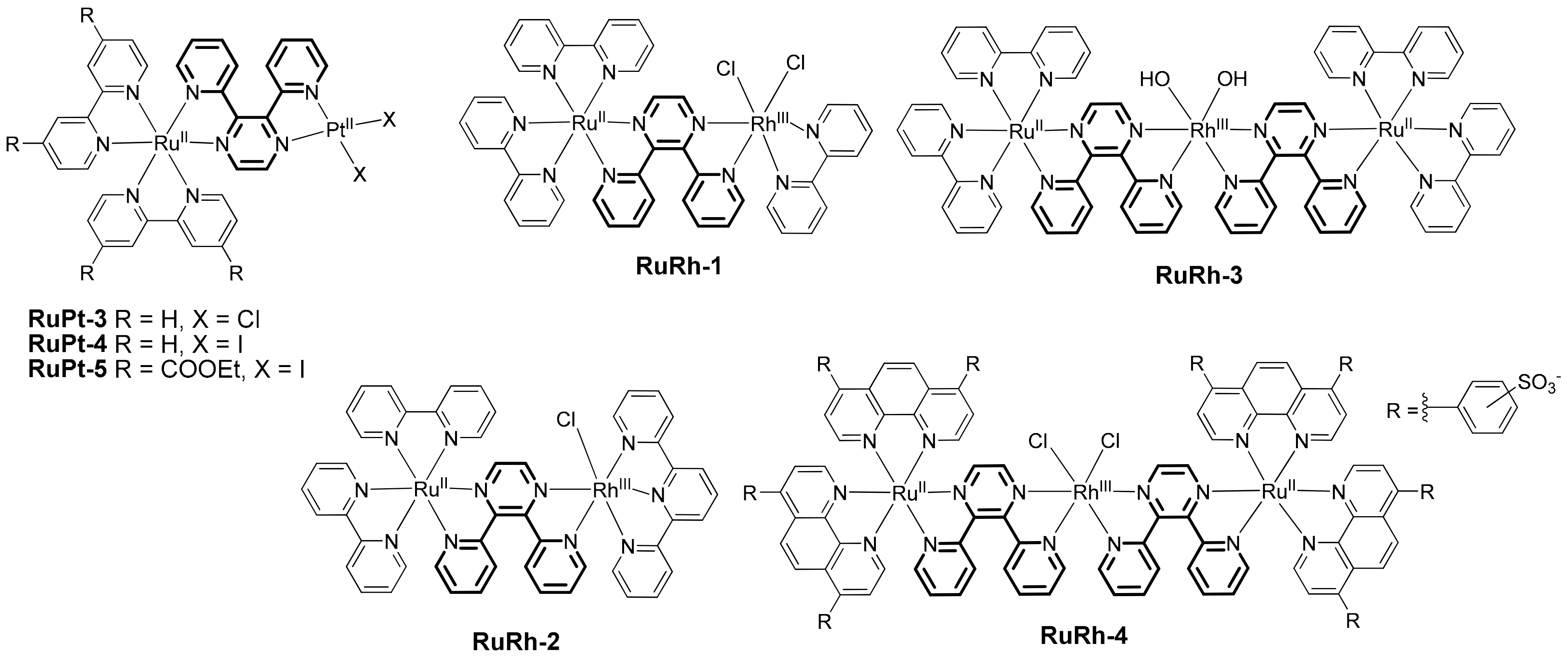
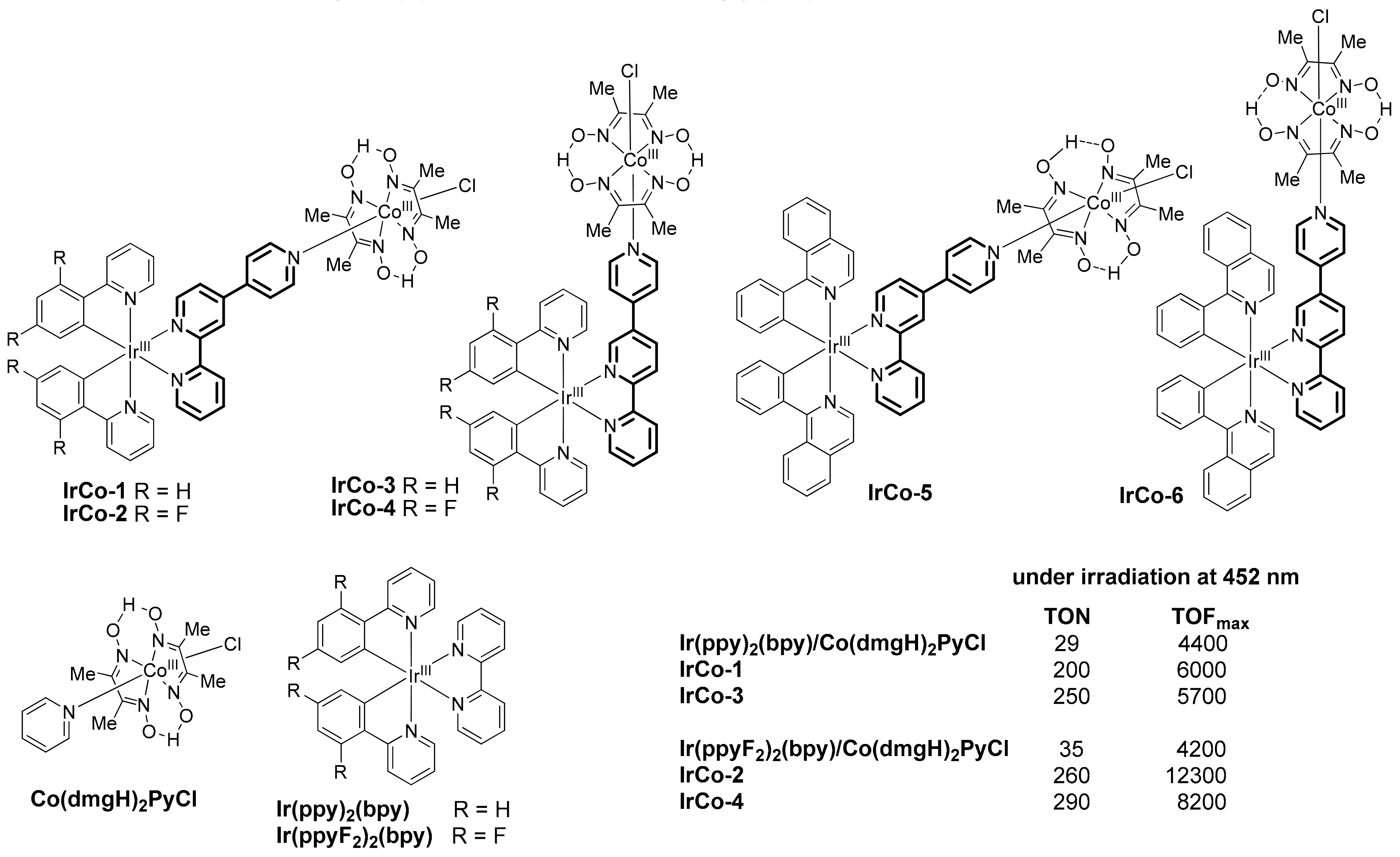
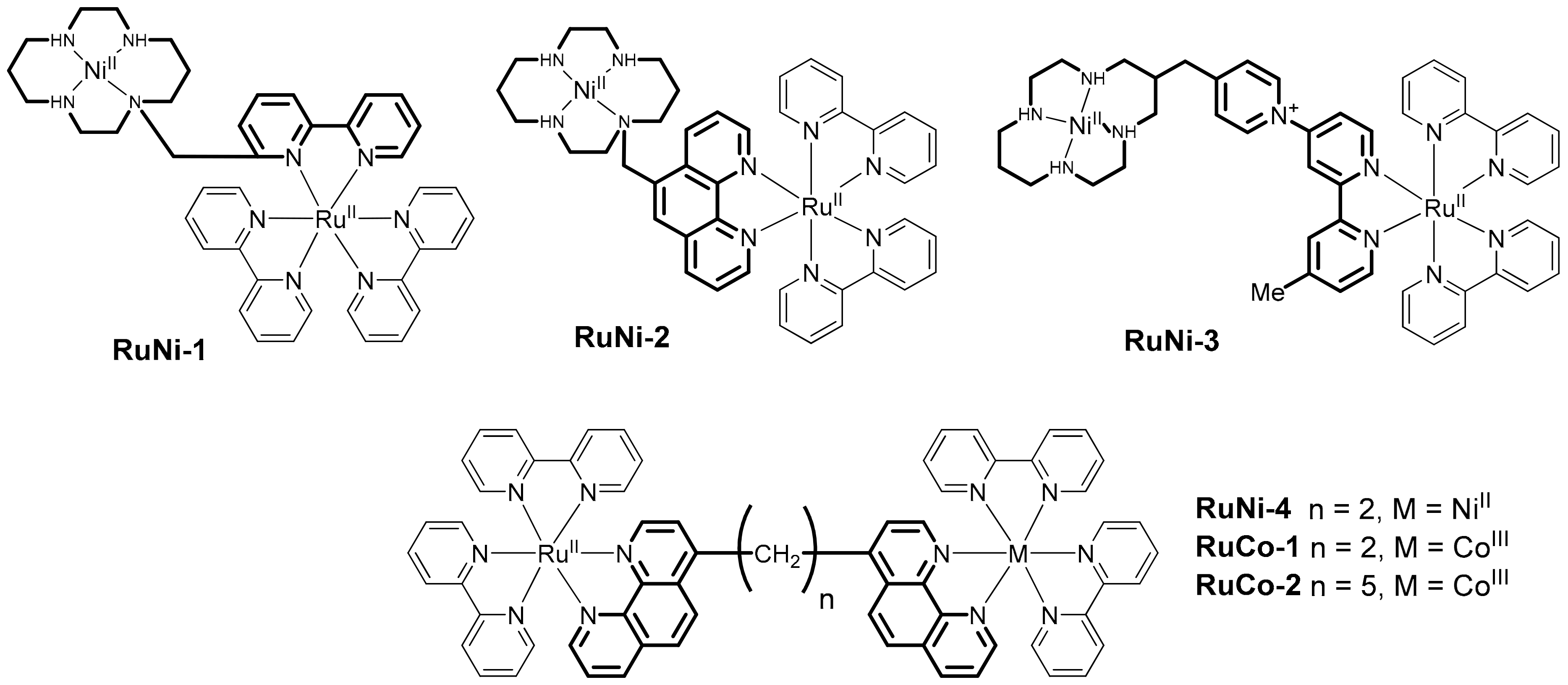
2.1. Heteropolynuclear Complexes for Hydrogen Photogeneration
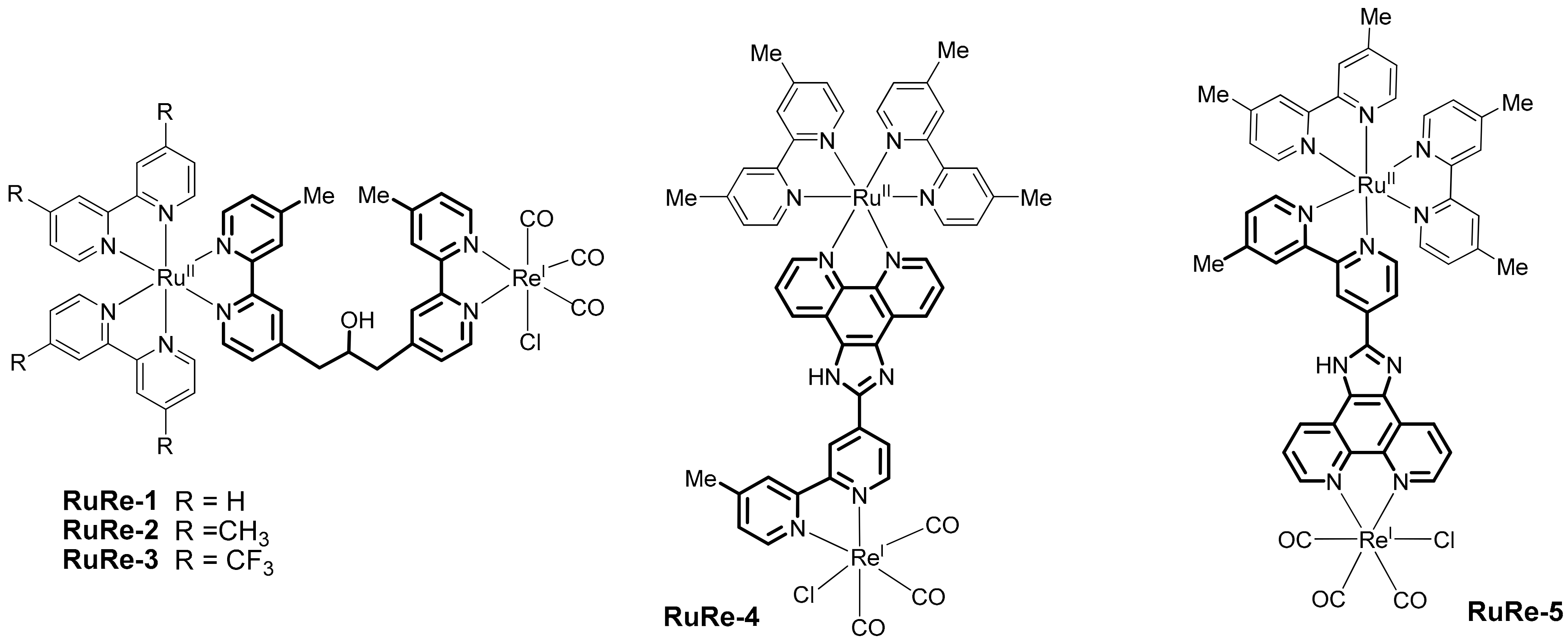
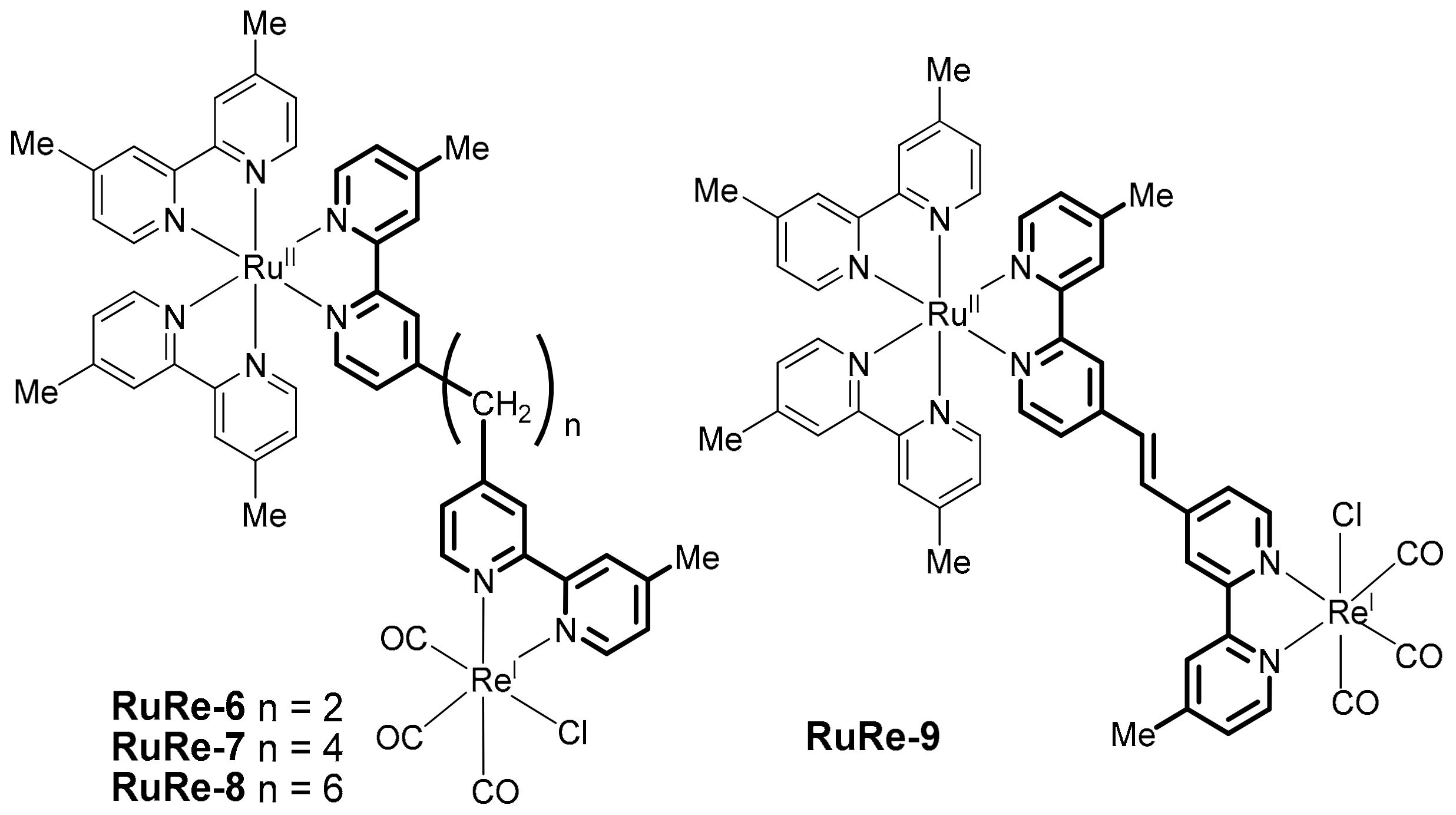
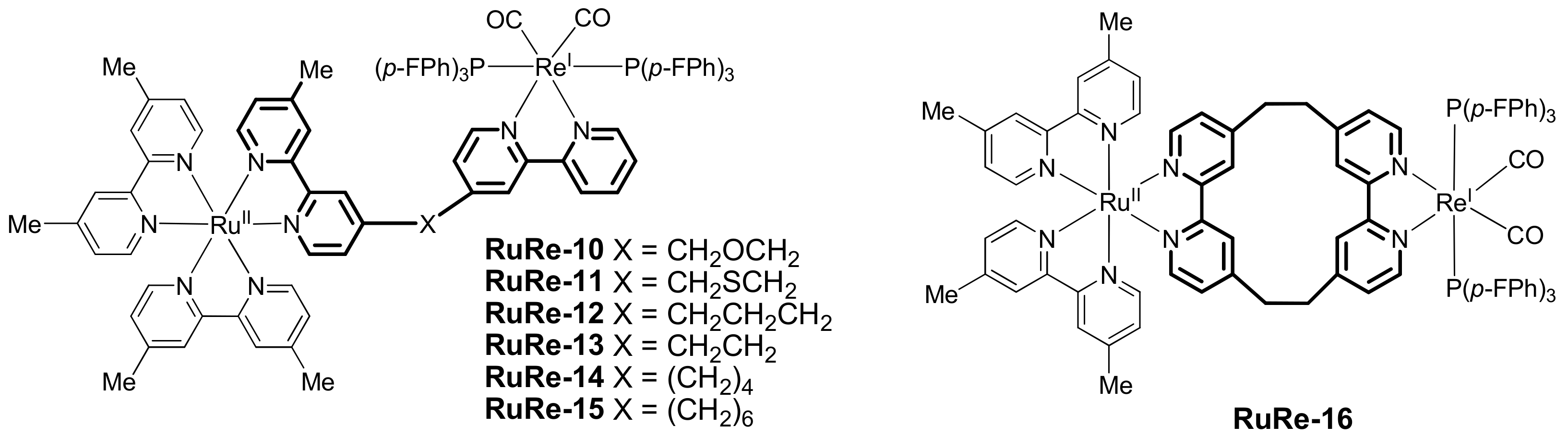
2.2. Heterobinuclear Complexes for Photocaytalytic CO2 Reduction
2.3. Heterobinuclear Photocatalysts in Organic Synthesis
2.3.1. Dimerization of α-Methylstyrene
2.3.2. Other Pd-Catalyzed Reactions
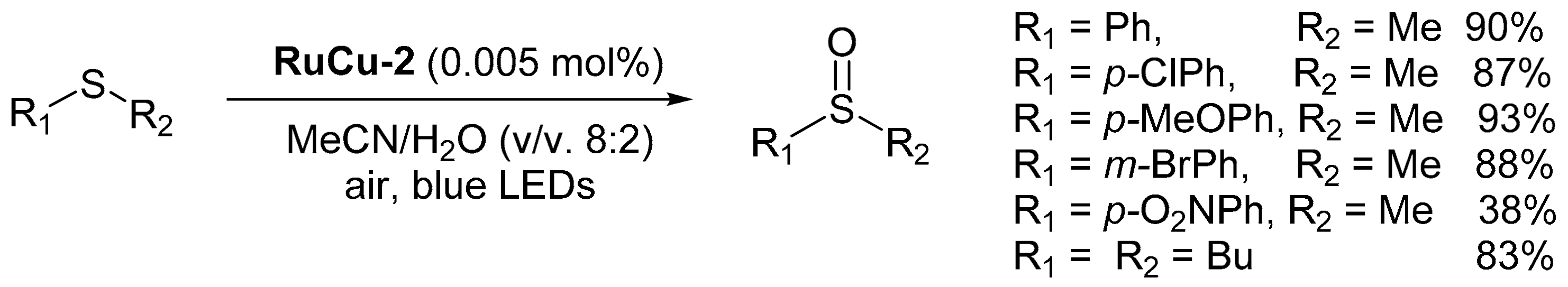
2.3.3. Oxidation Reactions
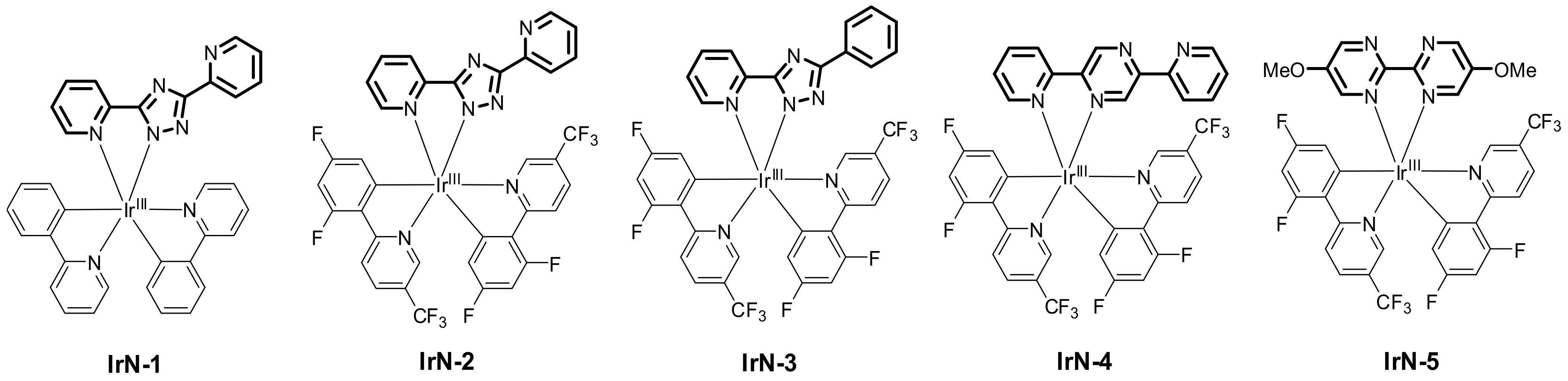
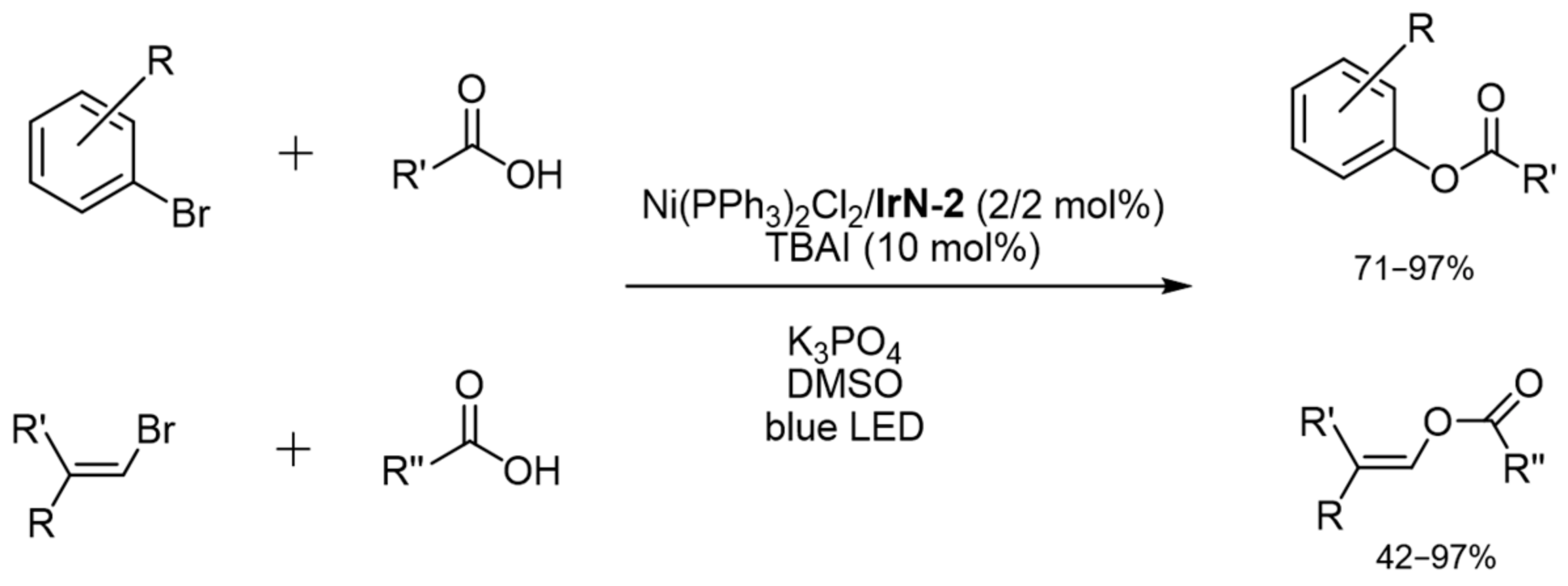
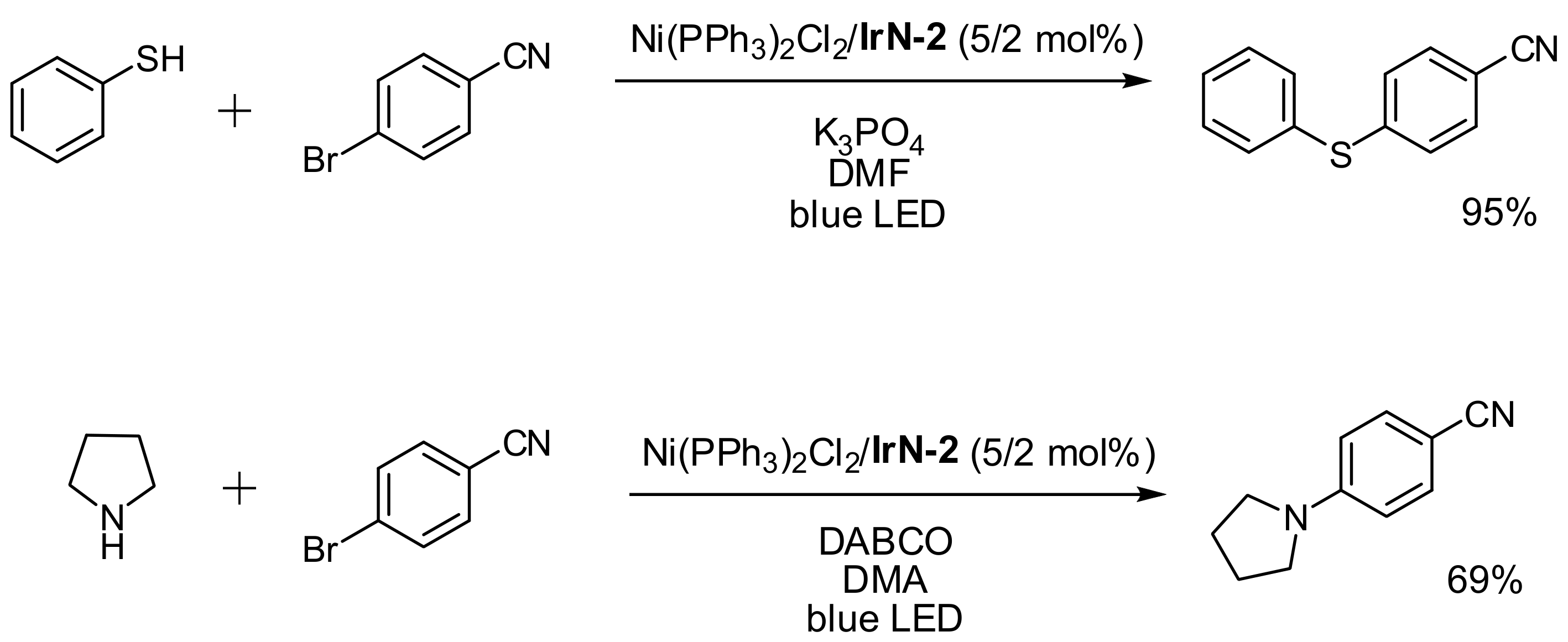
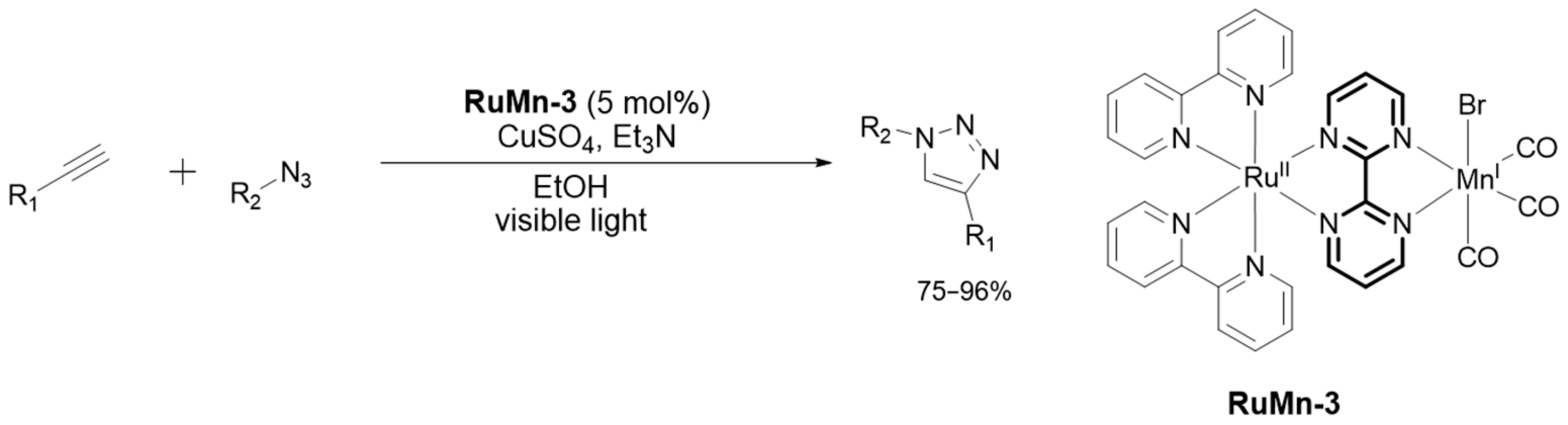
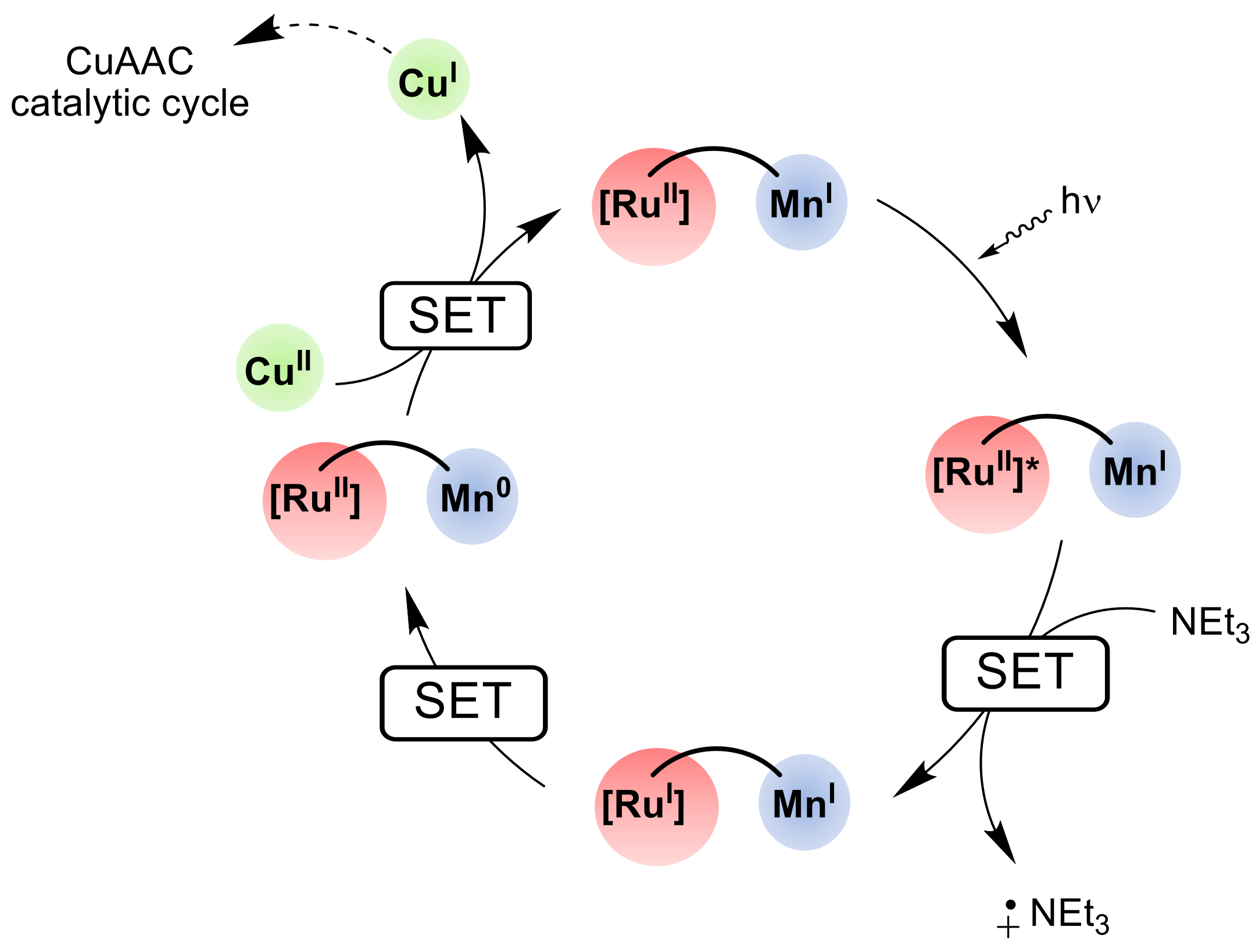
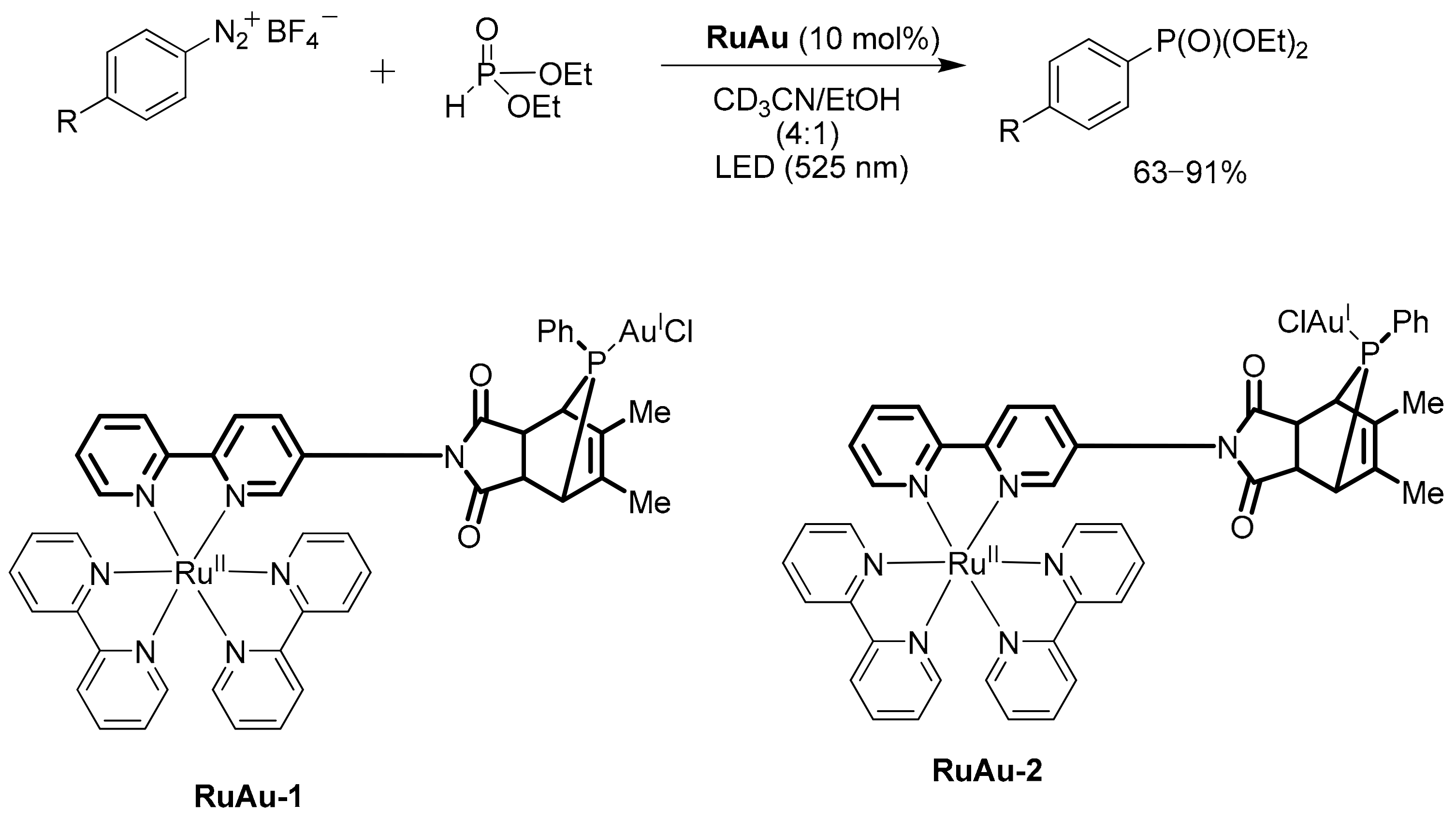
2.3.4. Catalysis by Ni, Mn and Au Containing Complexes
3. Conclusions and Prospect
Author Contributions
Funding
Conflicts of Interest
References
- Yoon, T.P. Visible light photocatalysis: The development of photocatalytic radical ion cycloadditions. ACS Catal. 2013, 3, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, D.; Protti, S.; Fagnoni, M. Carbon–Carbon bond forming reactions via photogenerated intermediates. Chem. Rev. 2016, 116, 9850–9913. [Google Scholar] [CrossRef] [PubMed]
- Ischay, M.A.; Anzovino, M.E.; Du, J.; Yoon, T.P. Efficient visible light photocatalysis of [2+2] enone cycloadditions. J. Am. Chem. Soc. 2008, 130, 12886–12887. [Google Scholar] [CrossRef] [PubMed]
- Nicewicz, D.A.; MacMillan, D.W.C. Merging photoredox catalysis with organocatalysis: The direct asymmetric alkylation of aldehydes. Science 2008, 322, 77–80. [Google Scholar] [CrossRef]
- Corrigan, N.; Shanmugam, S.; Xu, J.; Boyer, C. Photocatalysis in organic and polymer synthesis. Chem. Soc. Rev. 2016, 45, 6165–6212. [Google Scholar] [CrossRef]
- Romero, N.A.; Nicewicz, D.A. Organic photoredox catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible light photoredox catalysis with transition metal complexes: Applications in organic synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef]
- Lang, X.; Zhao, J.; Chen, X. Cooperative photoredox catalysis. Chem. Soc. Rev. 2016, 45, 3026–3038. [Google Scholar] [CrossRef]
- Chan, A.Y.; Perry, I.B.; Bissonnette, N.B.; Buksh, B.F.; Edwards, G.A.; Frye, L.I.; Garry, O.L.; Lavagnino, M.N.; Li, B.X.; Liang, Y.; et al. Metallaphotoredox: The merger of photoredox and transition metal catalysis. Chem. Rev. 2022, 122, 1485–1542. [Google Scholar] [CrossRef]
- Lipp, A.; Badir, S.O.; Molander, G.A. Stereoinduction in metallaphotoredox catalysis. Angew. Chem. Int. Ed. 2021, 60, 1714–1726. [Google Scholar] [CrossRef]
- Zhu, C.; Yue, H.; Jia, J.; Rueping, M. Nickel-catalyzed C-Heteroatom cross-coupling reactions under mild conditions via facilitated reductive elimination. Angew. Chem. Int. Ed. 2021, 60, 17810–17831. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.P.S.; Sarkar, S.; Gevorgyan, V. Visible light-induced transition metal catalysis. Chem. Rev. 2022, 122, 1543–1625. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.W.; Stephenson, C.R.J. Shining light on photoredox catalysis: Theory and synthetic applications. J. Org. Chem. 2012, 77, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, G.; Hammond, C. Catalytic formation of C(sp3)–F bonds via heterogeneous photocatalysis. ACS Catal. 2018, 8, 10321–10330. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Liu, H.; Liu, C.; Wan, Y.; Long, Y.; Cai, Z. Recent advances and applications of semiconductor photocatalytic technology. Appl. Sci. 2019, 9, 2489. [Google Scholar] [CrossRef]
- Lang, X.; Chen, X.; Zhao, J. Heterogeneous visible light photocatalysis for selective organic transformations. Chem. Soc. Rev. 2014, 43, 473–486. [Google Scholar] [CrossRef]
- Chen, J.; Cen, J.; Xu, X.; Li, X. The application of heterogeneous visible light photocatalysts in organic synthesis. Catal. Sci. Technol. 2016, 6, 349–362. [Google Scholar] [CrossRef]
- Pieber, B.; Shalom, M.; Antonietti, M.; Seeberger, P.H.; Gilmore, K. Continuous heterogeneous photocatalysis in serial micro-batch reactors. Angew. Chem. Int. Ed. 2018, 57, 9976–9979. [Google Scholar] [CrossRef]
- Dong, K.; Pezzetta, C.; Chen, Q.-C.; Kaushansky, A.; Agosti, A.; Bergamini, G.; Davidson, R.; Amirav, L. Nanorod photocatalysts for C−O cross-coupling reactions. ChemCatChem 2022, 14, e202200477. [Google Scholar] [CrossRef]
- Easun, T.L.; Alsindi, W.Z.; Towrie, M.; Ronayne, K.L.; Sun, X.-Z.; Ward, M.D.; George, M.W. Photoinduced energy transfer in a conformationally flexible Re(I)/Ru(II) dyad probed by time-resolved infrared spectroscopy: Effects of Conformation and Spatial Localization of Excited States. Inorg. Chem. 2008, 47, 5071–5078. [Google Scholar] [CrossRef]
- Schallenberg, D.; Neubauer, A.; Erdmann, E.; Tänzler, M.; Villinger, A.; Lochbrunner, S.; Seidel, W.W. Dinuclear Ru/Ni, Ir/Ni, and Ir/Pt complexes with bridging phenanthroline-5,6-dithiolate: Synthesis, structure, and electrochemical and photophysical Behavior. Inorg. Chem. 2014, 53, 8859–8873. [Google Scholar] [CrossRef] [PubMed]
- Troian-Gautier, L.; Moucheron, C. RutheniumII complexes bearing fused polycyclic ligands: From fundamental aspects to potential applications. Molecules 2014, 19, 5028–5087. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-J.; Kim, S.-Y.; Choi, C.M.; Kim, N.J.; Kim, C.H.; Cho, D.W.; Son, H.-J.; Pac, C.; Kang, S.O. Photophysics and excited-state properties of cyclometalated iridium(III)–platinum(II) and iridium(III)–iridium(III) bimetallic complexes bridged by dipyridylpyrazine. Inorg. Chem. 2017, 56, 5305–5315. [Google Scholar] [CrossRef] [PubMed]
- Laramée-Milette, B.; Hanan, G.S. Going against the flow: Os(II)-to-Ru(II) energy transfer in rod-like polypyridyl chromophore. Chem. Commun. 2017, 53, 10496–10499. [Google Scholar] [CrossRef] [PubMed]
- Zigler, D.F.; Morseth, Z.A.; White, T.A.; Canterbury, T.R.; Sayre, H.J.; Rodríguez-Corrales, J.Á.; Brennaman, M.K.; Brewer, K.J.; Papanikolas, J.M. Ultrafast kinetics of supramolecules with a Ru(II)- or Os(II)-polypyridyl light absorber, cis-Rh(III)Cl2-polypyridyl electron collector, and 2,3-bis(2-pyridyl)pyrazine bridge. Inorg. Chim. Acta 2017, 454, 266–274. [Google Scholar] [CrossRef]
- Erdmann, E.; Lütgens, M.; Lochbrunner, S.; Seidel, W.W. Ultrafast energy transfer in dinuclear complexes with bridging 1,10-phenanthroline-5,6-dithiolate. Inorg. Chem. 2018, 57, 4849–4863. [Google Scholar] [CrossRef]
- Staniszewska, M.; Kupfer, S.; Guthmuller, J. Effect of the catalytic center on the electron transfer dynamics in hydrogen-evolving ruthenium-based photocatalysts investigated by theoretical calculations. J. Phys. Chem. C 2019, 123, 16003–16013. [Google Scholar] [CrossRef]
- Zedler, L.; Müller, C.; Wintergerst, P.; Mengele, A.K.; Rau, S.; Dietzek-Ivanšić, B. Influence of the linker chemistry on the photoinduced charge-transfer dynamics of hetero-dinuclear photocatalysts. Chem. Eur. J. 2022, 28, e202200490. [Google Scholar] [CrossRef]
- Tamaki, Y.; Ishitani, O. Supramolecular photocatalysts for the reduction of CO2. ACS Catalysis 2017, 7, 3394–3409. [Google Scholar] [CrossRef]
- Kuramochi, Y.; Ishitani, O.; Ishida, H. Reaction mechanisms of catalytic photochemical CO2 reduction using Re(I) and Ru(II) complexes. Coord. Chem. Rev. 2018, 373, 333–356. [Google Scholar] [CrossRef]
- Pirzada, B.M.; Dar, A.H.; Shaikh, M.N.; Qurashi, A. Reticular-chemistry-inspired supramolecule design as a tool to achieve efficient photocatalysts for CO2 reduction. ACS Omega 2021, 6, 29291–29324. [Google Scholar] [CrossRef]
- Halpin, Y.; Pryce, M.T.; Rau, S.; Dini, D.; Vos, J.G. Recent progress in the development of bimetallic photocatalysts for hydrogen generation. Dalton Trans. 2013, 42, 16243–16254. [Google Scholar] [CrossRef] [PubMed]
- Manbeck, G.F.; Brewer, K.J. Photoinitiated electron collection in polyazine chromophores coupled to water reduction catalysts for solar H2 production. Coord. Chem. Rev. 2013, 257, 1660–1675. [Google Scholar] [CrossRef]
- Dini, D.; Pryce, M.T.; Schulz, M.; Vos, J.G. CHAPTER 12 Metallosupramolecular assemblies for application as photocatalysts for the production of solar fuels. In Functional Metallosupramolecular Materials; The Royal Society of Chemistry: London, UK, 2015; pp. 345–396. [Google Scholar]
- Juris, A.; Balzani, V.; Barigelletti, F.; Campagna, S.; Belser, P.; von Zelewsky, A. Ru(II) polypyridine complexes: Photophysics, photochemistry, eletrochemistry, and chemiluminescence. Coord. Chem. Rev. 1988, 84, 85–277. [Google Scholar] [CrossRef]
- Lutz, F.; Lorenzo-Parodi, N.; Schmidt, T.C.; Niemeyer, J. Heteroternary cucurbit [8] uril complexes as supramolecular scaffolds for self-assembled bifunctional photoredoxcatalysts. Chem. Commun. 2021, 57, 2887–2890. [Google Scholar] [CrossRef]
- Frischmann, P.D.; Mahata, K.; Würthner, F. Powering the future of molecular artificial photosynthesis with light-harvesting metallosupramolecular dye assemblies. Chem. Soc. Rev. 2013, 42, 1847–1870. [Google Scholar] [CrossRef]
- Bindra, G.S.; Schulz, M.; Paul, A.; Soman, S.; Groarke, R.; Inglis, J.; Pryce, M.T.; Browne, W.R.; Rau, S.; Maclean, B.J.; et al. The effect of peripheral bipyridine ligands on the photocatalytic hydrogen production activity of Ru/Pd catalysts. Dalton Trans. 2011, 40, 10812–10814. [Google Scholar] [CrossRef]
- Bindra, G.S.; Schulz, M.; Paul, A.; Groarke, R.; Soman, S.; Inglis, J.L.; Browne, W.R.; Pfeffer, M.G.; Rau, S.; MacLean, B.J.; et al. The role of bridging ligand in hydrogen generation by photocatalytic Ru/Pd assemblies. Dalton Trans. 2012, 41, 13050–13059. [Google Scholar] [CrossRef]
- Kowacs, T.; O’Reilly, L.; Pan, Q.; Huijser, A.; Lang, P.; Rau, S.; Browne, W.R.; Pryce, M.T.; Vos, J.G. Subtle changes to peripheral ligands enable high turnover numbers for photocatalytic hydrogen generation with supramolecular photocatalysts. Inorg. Chem. 2016, 55, 2685–2690. [Google Scholar] [CrossRef]
- Pan, Q.; Mecozzi, F.; Korterik, J.P.; Vos, J.G.; Browne, W.R.; Huijser, A. The critical role played by the catalytic moiety in the early-time photodynamics of hydrogen-generating bimetallic photocatalysts. ChemPhysChem 2016, 17, 2654–2659. [Google Scholar] [CrossRef]
- Kowacs, T.; Pan, Q.; Lang, P.; O’Reilly, L.; Rau, S.; Browne, W.R.; Pryce, M.T.; Huijser, A.; Vos, J.G. Supramolecular bimetallic assemblies for photocatalytic hydrogen generation from water. Faraday Discuss. 2015, 185, 143–170. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Manbeck, G.F.; Wimer, D.G.; Brewer, K.J. A new RuIIRhIII bimetallic with a single Rh–Cl bond as a supramolecular photocatalyst for proton reduction. Chem. Commun. 2015, 51, 12966–12969. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.M.; Arachchige, S.M.; Brewer, K.J. Enhancement of solar fuel production schemes by using a Ru,Rh,Ru supramolecular photocatalyst containing hydroxide labile ligands. Chem. Eur. J. 2015, 21, 16948–16954. [Google Scholar] [CrossRef] [PubMed]
- Canterbury, T.R.; Arachchige, S.M.; Brewer, K.J.; Moore, R.B. A new hydrophilic supramolecular photocatalyst for the production of H2 in aerobic aqueous solutions. Chem. Commun. 2016, 52, 8663–8666. [Google Scholar] [CrossRef]
- White, J.K.; Brewer, K.J. A new Ru,Ru,Pt supramolecular architecture for photocatalytic H2 production. Chem. Commun. 2015, 51, 16123–16126. [Google Scholar] [CrossRef] [PubMed]
- Karnahl, M.; Kuhnt, C.; Ma, F.; Yartsev, A.; Schmitt, M.; Dietzek, B.; Rau, S.; Popp, J. Tuning of photocatalytic hydrogen production and photoinduced intramolecular electron transfer rates by regioselective bridging ligand substitution. ChemPhysChem 2011, 12, 2101–2109. [Google Scholar] [CrossRef]
- Pfeffer, M.G.; Kowacs, T.; Wächtler, M.; Guthmuller, J.; Dietzek, B.; Vos, J.G.; Rau, S. Optimization of hydrogen-evolving photochemical molecular devices. Angew. Chem. Int. Ed. 2015, 54, 6627–6631. [Google Scholar] [CrossRef]
- Pfeffer, M.G.; Schäfer, B.; Smolentsev, G.; Uhlig, J.; Nazarenko, E.; Guthmuller, J.; Kuhnt, C.; Wächtler, M.; Dietzek, B.; Sundström, V.; et al. Palladium versus platinum: The metal in the catalytic center of a molecular photocatalyst determines the mechanism of the hydrogen production with visible light. Angew. Chem. Int. Ed. 2015, 54, 5044–5048. [Google Scholar] [CrossRef]
- Mengele, A.K.; Kaufhold, S.; Streb, C.; Rau, S. Generation of a stable supramolecular hydrogen evolving photocatalyst by alteration of the catalytic center. Dalton Trans. 2016, 45, 6612–6618. [Google Scholar] [CrossRef]
- Pfeffer, M.G.; Zedler, L.; Kupfer, S.; Paul, M.; Schwalbe, M.; Peuntinger, K.; Guldi, D.M.; Guthmuller, J.; Popp, J.; Gräfe, S.; et al. Tuning of photocatalytic activity by creating a tridentate coordination sphere for palladium. Dalton Trans. 2014, 43, 11676–11686. [Google Scholar] [CrossRef]
- Kaufhold, S.; Imanbaew, D.; Riehn, C.; Rau, S. Rational in situ tuning of a supramolecular photocatalyst for hydrogen evolution. Sustain. Energy Fuels 2017, 1, 2066–2070. [Google Scholar] [CrossRef]
- Das, N.; Bindra, G.S.; Paul, A.; Vos, J.G.; Schulz, M.; Pryce, M.T. Enhancing photocatalytic hydrogen generation: The impact of the peripheral ligands in Ru/Pd and Ru/Pt complexes. Chem. Eur. J. 2017, 23, 5330–5337. [Google Scholar] [CrossRef] [PubMed]
- Stoll, T.; Gennari, M.; Fortage, J.; Castillo, C.E.; Rebarz, M.; Sliwa, M.; Poizat, O.; Odobel, F.; Deronzier, A.; Collomb, M.-N. An efficient RuII–RhIII–RuII polypyridyl photocatalyst for visible-light-driven hydrogen production in aqueous solution. Angew. Chem. Int. Ed. 2014, 53, 1654–1658. [Google Scholar] [CrossRef] [PubMed]
- Lentz, C.; Schott, O.; Auvray, T.; Hanan, G.S.; Elias, B. Design and photophysical studies of iridium(III)–cobalt(III) dyads and their application for dihydrogen photo-evolution. Dalton Trans. 2019, 48, 15567–15576. [Google Scholar] [CrossRef]
- Zedler, L.; Wintergerst, P.; Mengele, A.K.; Müller, C.; Li, C.; Dietzek-Ivanšić, B.; Rau, S. Outpacing conventional nicotinamide hydrogenation catalysis by a strongly communicating heterodinuclear photocatalyst. Nat. Commun. 2022, 13, 2538. [Google Scholar] [CrossRef]
- Kimura, E.; Wada, S.; Shionoya, M.; Takahashi, T.; Litaka, Y. A novel cyclam–nickel(II) complex appended with a tris-(2,2′-bipyridine) ruthenium(II) complex (cyclam = 1,4,8,11-tetra-azacyclotetradecane). J. Chem. Soc. Chem. Commun. 1990, 5, 397–398. [Google Scholar] [CrossRef]
- Kimura, E.; Bu, X.; Shionoya, M.; Wada, S.; Maruyama, S. A new nickel(II) cyclam (cyclam = 1,4,8,11-tetraazacyclotetradecane) complex covalently attached to tris(1,10-phenanthroline)ruthenium(2+). A new candidate for the catalytic photoreduction of carbon dioxide. Inorg. Chem. 1992, 31, 4542–4546. [Google Scholar] [CrossRef]
- Kimura, E.; Wada, S.; Shionoya, M.; Okazaki, Y. New series of multifunctionalized nickel(II)-cyclam (cyclam = 1,4,8,11-tetraazacyclotetradecane) complexes. application to the photoreduction of carbon dioxide. Inorg. Chem. 1994, 33, 770–778. [Google Scholar] [CrossRef]
- Komatsuzaki, N.; Himeda, Y.; Hirose, T.; Sugihara, H.; Kasuga, K. Synthesis and photochemical properties of ruthenium–cobalt and ruthenium–nickel dinuclear complexes. Bull. Chem. Soc. Jpn. 1999, 72, 725–731. [Google Scholar] [CrossRef]
- Gholamkhass, B.; Mametsuka, H.; Koike, K.; Tanabe, T.; Furue, M.; Ishitani, O. Architecture of supramolecular metal complexes for photocatalytic CO2 reduction: ruthenium−rhenium bi- and tetranuclear complexes. Inorg. Chem. 2005, 44, 2326–2336. [Google Scholar] [CrossRef]
- Koike, K.; Naito, S.; Sato, S.; Tamaki, Y.; Ishitani, O. Architecture of supramolecular metal complexes for photocatalytic CO2 reduction: III: Effects of length of alkyl chain connecting photosensitizer to catalyst. J. Photochem. Photobiol. A Chem. 2009, 207, 109–114. [Google Scholar] [CrossRef]
- Bian, Z.-Y.; Chi, S.-M.; Li, L.; Fu, W. Conjugation effect of the bridging ligand on the CO2 reduction properties in difunctional photocatalysts. Dalton Trans. 2010, 39, 7884–7887. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, Y.; Koike, K.; Morimoto, T.; Ishitani, O. Substantial improvement in the efficiency and durability of a photocatalyst for carbon dioxide reduction using a benzoimidazole derivative as an electron donor. J. Catal. 2013, 304, 22–28. [Google Scholar] [CrossRef]
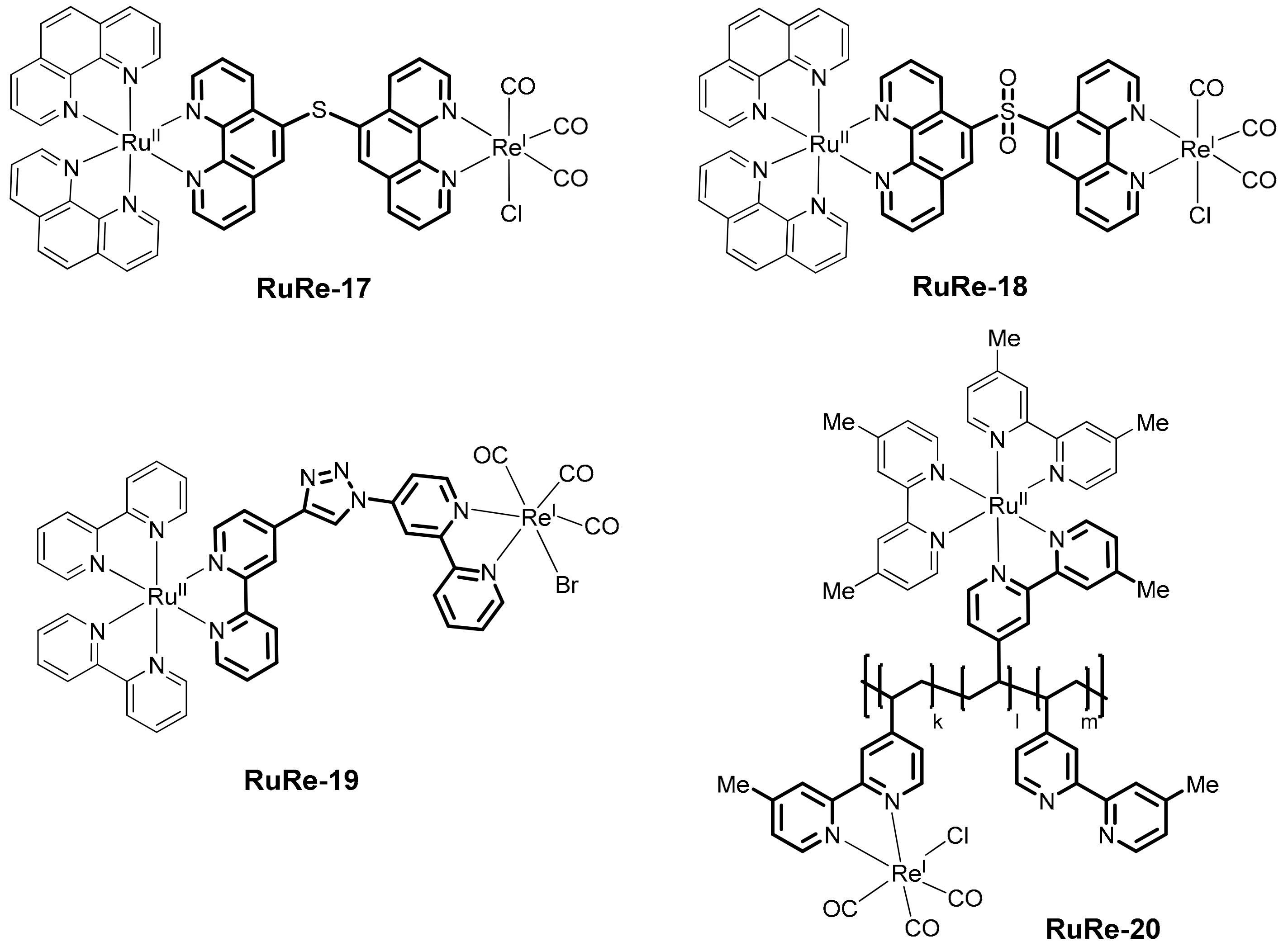
- Kato, E.; Takeda, H.; Koike, K.; Ohkubo, K.; Ishitani, O. Ru(II)–Re(I) binuclear photocatalysts connected by –CH2XCH2– (X = O, S, CH2) for CO2 reduction. Chem. Sci. 2015, 6, 3003–3012. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Ohkubo, K.; Saito, D.; Yatsu, T.; Tamaki, Y.; Tanaka, S.I.; Koike, K.; Onda, K.; Ishitani, O. Kinetics and mechanism of intramolecular electron transfer in Ru(II)–Re(I) supramolecular CO2–reduction photocatalysts: Effects of bridging ligands. Inorg. Chem. 2019, 58, 11480–11492. [Google Scholar] [CrossRef]
- Tamaki, Y.; Watanabe, K.; Koike, K.; Inoue, H.; Morimoto, T.; Ishitani, O. Development of highly efficient supramolecular CO2 reduction photocatalysts with high turnover frequency and durability. Faraday Discuss. 2012, 155, 115–127. [Google Scholar] [CrossRef]
- Ohkubo, K.; Yamazaki, Y.; Nakashima, T.; Tamaki, Y.; Koike, K.; Ishitani, O. Photocatalyses of Ru(II)–Re(I) binuclear complexes connected through two ethylene chains for CO2 reduction. J. Catal. 2016, 343, 278–289. [Google Scholar] [CrossRef]
- Brown, C.M.; Auvray, T.; DeLuca, E.E.; Ezhova, M.B.; Hanan, G.S.; Wolf, M.O. Controlling photocatalytic reduction of CO2 in Ru(II)/Re(I) dyads via linker oxidation state. Chem. Commun. 2020, 56, 10750–10753. [Google Scholar] [CrossRef]
- Gotico, P.; Tran, T.-T.; Baron, A.; Vauzeilles, B.; Lefumeux, C.; Ha-Thi, M.-H.; Pino, T.; Halime, Z.; Quaranta, A.; Leibl, W.; et al. Tracking charge accumulation in a functional triazole-linked ruthenium-rhenium dyad towards photocatalytic carbon dioxide reduction. ChemPhotoChem 2021, 5, 654–664. [Google Scholar] [CrossRef]
- Cerpentier, F.J.R.; Karlsson, J.; Lalrempuia, R.; Brandon, M.P.; Sazanovich, I.V.; Greetham, G.M.; Gibson, E.A.; Pryce, M.T. Ruthenium assemblies for CO2 reduction and H2 generation: Time resolved infrared spectroscopy, spectroelectrochemistry and a photocatalysis study in solution and on NiO. Front. Chem. 2021, 9, 795877. [Google Scholar] [CrossRef]
- Kuttassery, F.; Kumagai, H.; Kamata, R.; Ebato, Y.; Higashi, M.; Suzuki, H.; Abe, R.; Ishitani, O. Supramolecular photocatalysts fixed on the inside of the polypyrrole layer in dye sensitized molecular photocathodes: Application to photocatalytic CO2 reduction coupled with water oxidation. Chem. Sci. 2021, 12, 13216–13232. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.S.; Thomas, C.; Kränzlein, M.; Pehl, T.M.; Rieger, B. Macromolecular rhenium–ruthenium complexes for photocatalytic CO2 conversion: From catalytic Lewis pair polymerization to well-defined poly(vinyl bipyridine)–metal complexes. Macromolecules 2022, 55, 7039–7048. [Google Scholar] [CrossRef]
- Fabry, D.C.; Koizumi, H.; Ghosh, D.; Yamazaki, Y.; Takeda, H.; Tamaki, Y.; Ishitani, O. A Ru(II)–Mn(I) supramolecular photocatalyst for CO2 reduction. Organometallics 2020, 39, 1511–1518. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Takeda, H.; Ohashi, K.; Asatani, T.; Kosumi, D.; Hashimoto, H.; Ishitani, O.; Tamiaki, H. Photochemical reduction of CO2 with red light using synthetic chlorophyll–rhenium bipyridine dyad. Chem. Lett. 2014, 43, 1383–1385. [Google Scholar] [CrossRef]
- Kuramochi, Y.; Fujisawa, Y.; Satake, A. Photocatalytic CO2 reduction mediated by electron transfer via the excited triplet state of Zn(II) porphyrin. J. Am. Chem. Soc. 2020, 142, 705–709. [Google Scholar] [CrossRef]
- Kuramochi, Y.; Satake, A. Photocatalytic CO2 reductions catalyzed by meso-(1,10-phenanthrolin-2-yl)-porphyrins having a rhenium(I) tricarbonyl complex. Chem. Eur. J. 2020, 26, 16365–16373. [Google Scholar] [CrossRef]
- Lang, P.; Pfrunder, M.; Quach, G.; Braun-Cula, B.; Moore, E.G.; Schwalbe, M. Sensitized photochemical CO2 reduction by hetero-Pacman compounds linking a ReI tricarbonyl with a porphyrin unit. Chem. Eur. J. 2019, 25, 4509–4519. [Google Scholar] [CrossRef] [PubMed]
- Matlachowski, C.; Braun, B.; Tschierlei, S.; Schwalbe, M. Photochemical CO2 reduction catalyzed by phenanthroline extended tetramesityl porphyrin complexes linked with a rhenium(I) tricarbonyl unit. Inorg. Chem. 2015, 54, 10351–10360. [Google Scholar] [CrossRef]
- Schneider, J.; Vuong, K.Q.; Calladine, J.A.; Sun, X.-Z.; Whitwood, A.C.; George, M.W.; Perutz, R.N. Photochemistry and photophysics of a Pd(II) metalloporphyrin: Re(I) tricarbonyl bipyridine molecular dyad and its activity toward the photoreduction of CO2 to CO. Inorg. Chem. 2011, 50, 11877–11889. [Google Scholar] [CrossRef] [PubMed]
- Windle, C.D.; Câmpian, M.V.; Duhme-Klair, A.-K.; Gibson, E.A.; Perutz, R.N.; Schneider, J. CO2 photoreduction with long-wavelength light: Dyads and monomers of zinc porphyrin and rhenium bipyridine. Chem. Commun. 2012, 48, 8189–8191. [Google Scholar] [CrossRef]
- Windle, C.D.; George, M.W.; Perutz, R.N.; Summers, P.A.; Sun, X.Z.; Whitwood, A.C. Comparison of rhenium–porphyrin dyads for CO2 photoreduction: Photocatalytic studies and charge separation dynamics studied by time-resolved IR spectroscopy. Chem. Sci. 2015, 6, 6847–6864. [Google Scholar] [CrossRef] [PubMed]
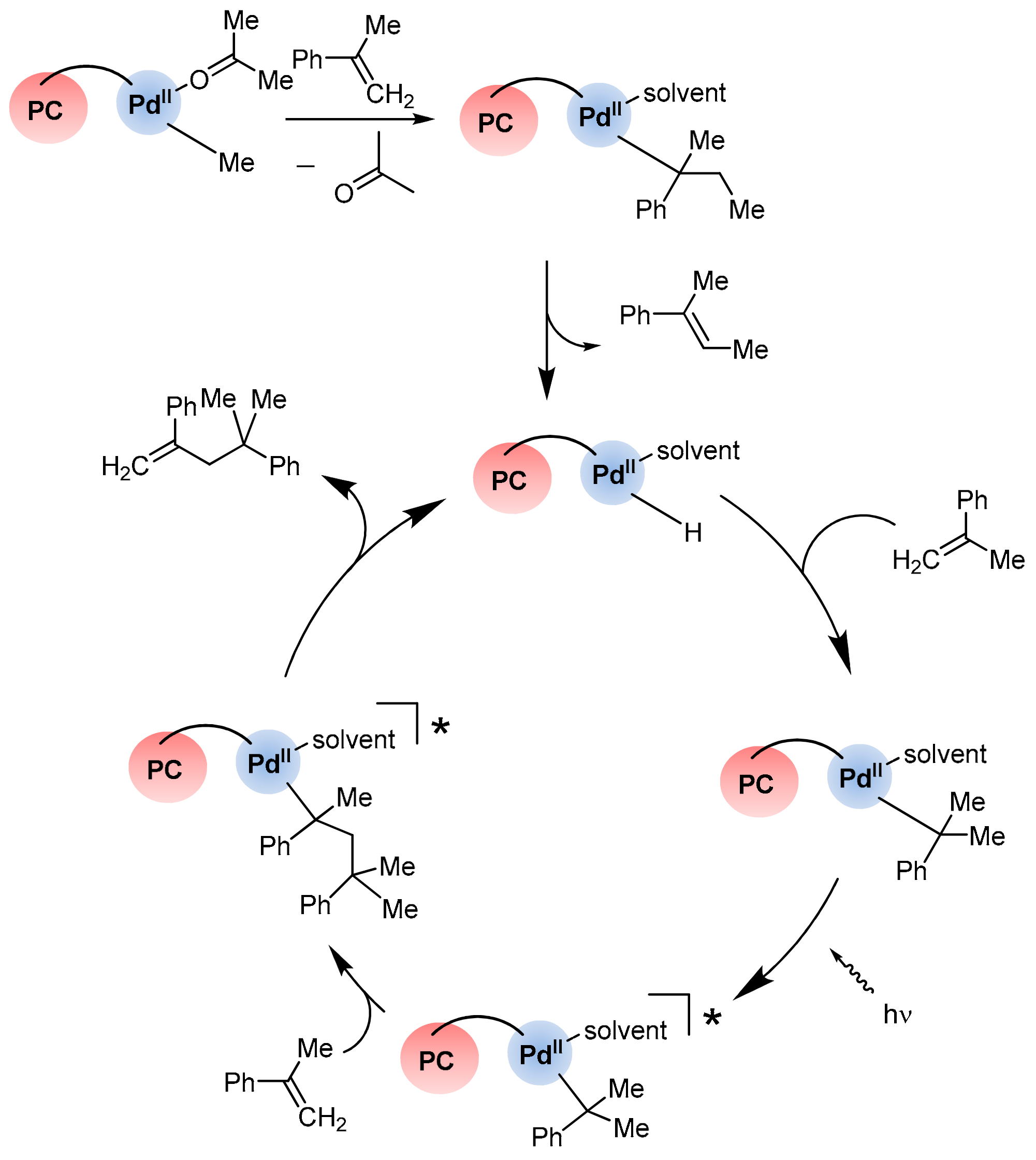
- Inagaki, A.; Edure, S.; Yatsuda, S.; Akita, M. Highly selective photo-catalytic dimerization of α-methylstyrene by a novel palladium complex with photosensitizing ruthenium(II) polypyridyl moiety. Chem. Commun. 2005, 43, 5468–5470. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, A.; Yatsuda, S.; Edure, S.; Suzuki, A.; Takahashi, T.; Akita, M. Synthesis of Pd complexes combined with photosensitizing of a ruthenium(II) polypyridyl moiety through a series of substituted bipyrimidine bridges. Substituent effect of the bridging ligand on the photocatalytic dimerization of α-methylstyrene. Inorg. Chem. 2007, 46, 2432–2445. [Google Scholar] [CrossRef] [PubMed]
- Nitadori, H.; Takahashi, T.; Inagaki, A.; Akita, M. Enhanced photocatalytic activity of α-methylstyrene oligomerization through effective metal-to-ligand charge-transfer localization on the bridging ligand. Inorg. Chem. 2012, 51, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Araki, M.; Inagaki, A.; Akita, M. Syntheses, photophysical properties, and reactivities of novel bichromophoric Pd complexes composed of Ru(II)–polypyridyl and naphthyl moieties. Dalton Trans. 2013, 42, 6989–7001. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Inagaki, A.; Akita, M.; Halet, J.-F.; Costuas, K. Revelation of the photoactive species in the photocatalytic dimerization of α-methylstyrene by a dinuclear ruthenium–palladium complex. Inorg. Chem. 2013, 52, 8030–8039. [Google Scholar] [CrossRef]
- Kikuchi, S.; Saito, K.; Akita, M.; Inagaki, A. Nonradical light-controlled polymerization of styrene and vinyl ethers catalyzed by an iridium-palladium photocatalyst. Organometallics 2018, 37, 359–366. [Google Scholar] [CrossRef]
- Fujiwara, T.; Nomura, K.; Inagaki, A. Cu–Pd dinuclear complexes with earth-abundant Cu photosensitizer: Synthesis and photopolymerization. Organometallics 2020, 39, 2464–2469. [Google Scholar] [CrossRef]
- Armaroli, N. Photoactive mono- and polynuclear Cu(I)–phenanthrolines. A viable alternative to Ru(II)–polypyridines? Chem. Soc. Rev. 2001, 30, 113–124. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Bulger, P.G.; Sarlah, D. Palladium-catalyzed cross-coupling reactions in total synthesis. Angew. Chem. Int. Ed. 2005, 44, 4442–4489. [Google Scholar] [CrossRef]
- Buchwald, S.L. Cross coupling. Acc. Chem. Res. 2008, 41, 1439. [Google Scholar] [CrossRef] [PubMed]
- Johansson Seechurn, C.C.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-Catalyzed cross-coupling: A historical contextual perspective to the 2010 Nobel prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef] [PubMed]
- Devendar, P.; Qu, R.-Y.; Kang, W.-M.; He, B.; Yang, G.-F. Palladium-Catalyzed Cross-Coupling Reactions: A Powerful Tool for the Synthesis of Agrochemicals. J. Agric. Food. Chem. 2018, 66, 8914–8934. [Google Scholar] [CrossRef] [PubMed]
- Osawa, M.; Nagai, H.; Akita, M. Photo-activation of Pd-catalyzed Sonogashira coupling using a Ru/bipyridine complex as energy transfer agent. Dalton Trans. 2007, 8, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, K.C.; Ebukuyo, P.O.; Dhahir, Y.J.; Wheeler, K.; He, H. A BODIPY-functionalized PdII photoredox catalyst for Sonogashira C–C cross-coupling reactions. Chem. Commun. 2019, 55, 4973–4976. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Kawashima, M.; Yamashita, H. Visible-light-enhanced Suzuki–Miyaura coupling reaction by cooperative photocatalysis with an Ru–Pd bimetallic complex. Chem. Commun. 2014, 50, 14501–14503. [Google Scholar] [CrossRef]
- Yao, S.Y.; Cao, M.L.; Zhang, X.L. Photoaccelerated energy transfer catalysis of the Suzuki-Miyaura coupling through ligand regulation on Ir(III)-Pd(II) bimetallic complexes. RSC Adv. 2020, 10, 42874–42882. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-H.; Liu, Y.-H.; Peng, S.-M.; Liu, S.-T. Photoaccelerated Suzuki–Miyaura and Sonogashira coupling reactions catalyzed by an Ir-Pd binuclear complex. Mol. Catal. 2022, 522, 112232. [Google Scholar] [CrossRef]
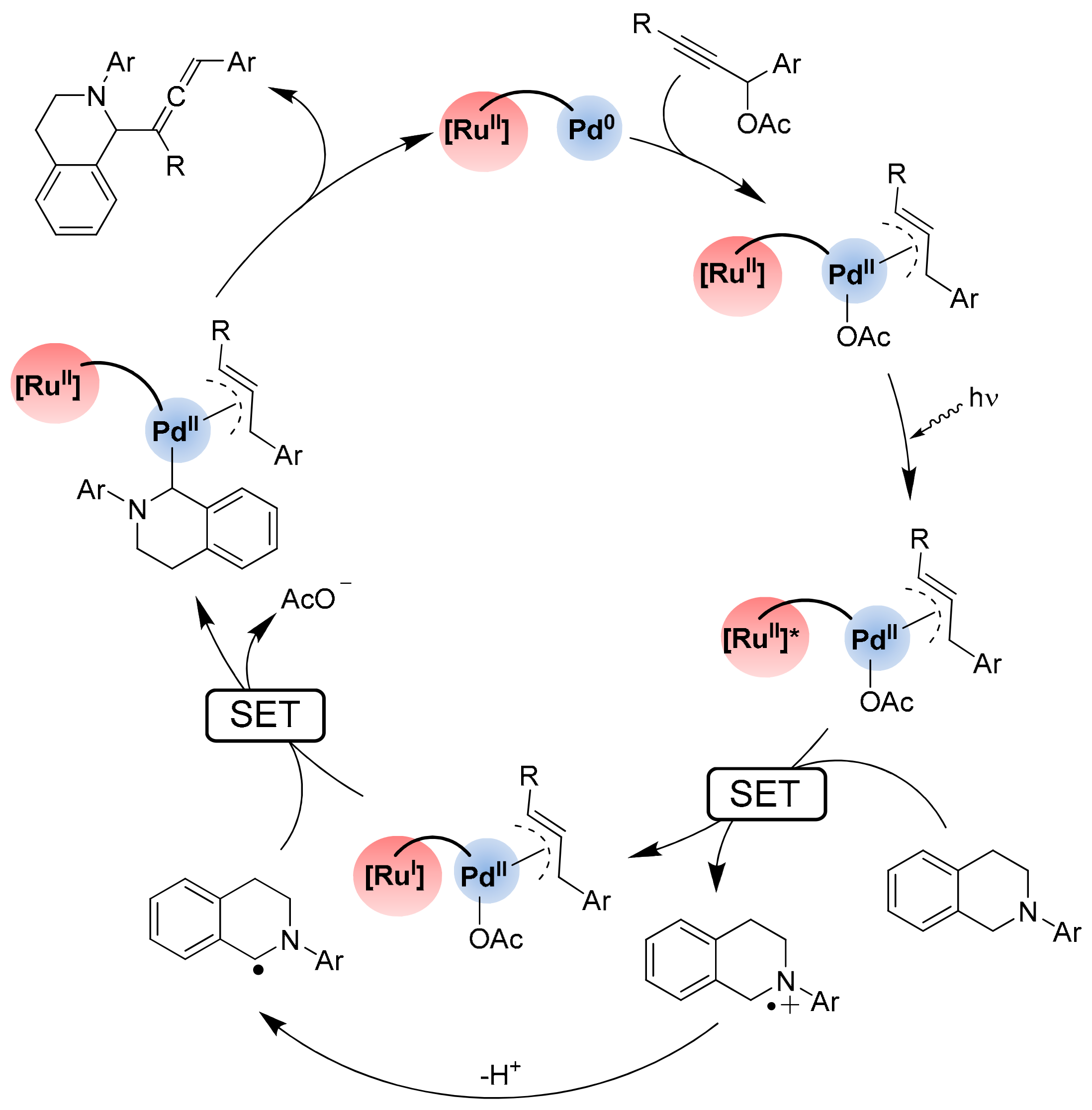
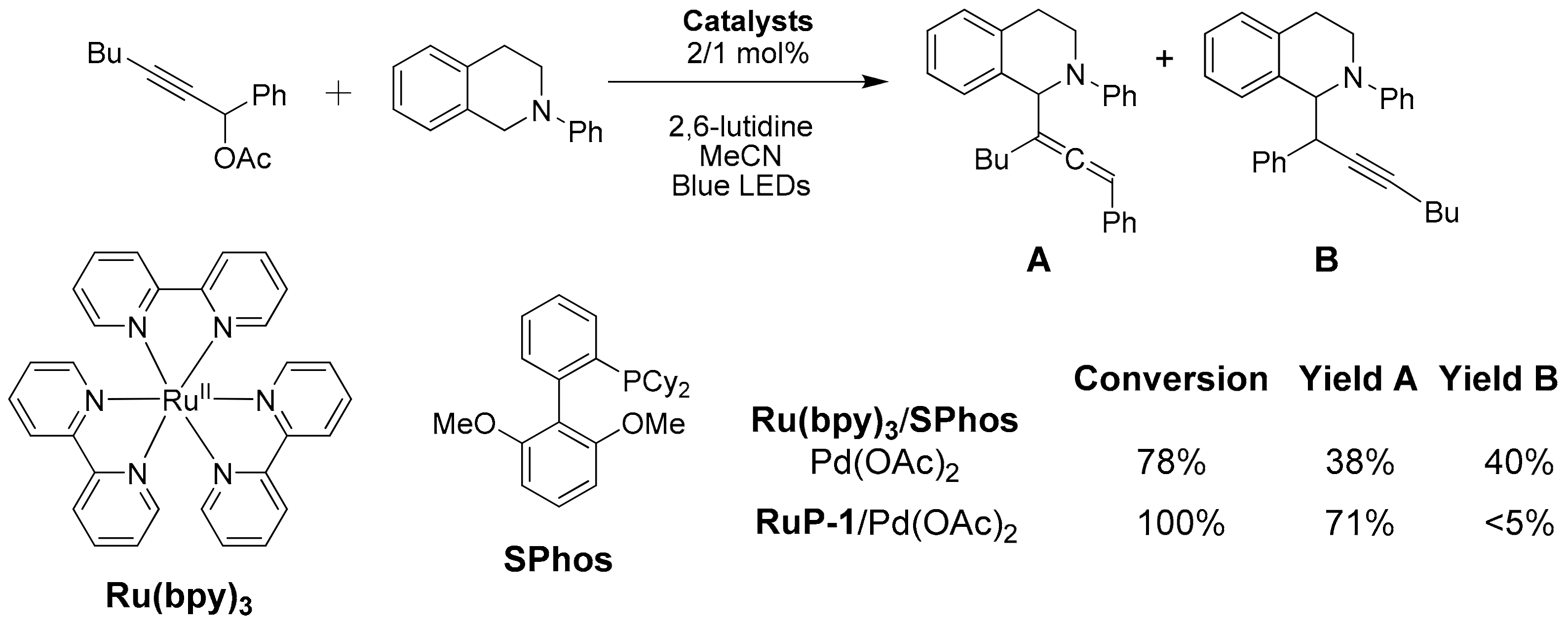
- Li, M.; Chia, X.L.; Zhu, Y. Tethered photocatalyst-directed palladium-catalysed C–H allenylation of N-aryl tetrahydroisoquinolines. Chem. Commun. 2022, 58, 4719–4722. [Google Scholar] [CrossRef]
- Herrero, C.; Quaranta, A.; Fallahpour, R.-A.; Leibl, W.; Aukauloo, A. Identification of the Different Mechanisms of Activation of a [RuII(tpy)(bpy)(OH2)]2+ Catalyst by Modified Ruthenium Sensitizers in Supramolecular Complexes. J. Phys. Chem. C 2013, 117, 9605–9612. [Google Scholar] [CrossRef]
- Herrero, C.; Quaranta, A.; Leibl, W.; Rutherford, A.W.; Aukauloo, A. Artificial photosynthetic systems. Using light and water to provide electrons and protons for the synthesis of a fuel. Energy Environ. Sci. 2011, 4, 2353–2365. [Google Scholar] [CrossRef]
- Nikoloudakis, E.; Alsaleh, A.Z.; Charalambidis, G.; Coutsolelos, A.G.; D’Souza, F. A covalently linked nickel(II) porphyrin–ruthenium(II) tris(bipyridyl) dyad for efficient photocatalytic water oxidation. Chem. Commun. 2022, 58, 12078–12081. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.; Fu, W.-F. Insight into highly selective photocatalytic oxidation of alcohols by a new trinuclear ruthenium complex with visible light. Dalton Trans. 2014, 43, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Herrero, C.; Quaranta, A.; Sircoglou, M.; Sénéchal-David, K.; Baron, A.; Marín, I.M.; Buron, C.; Baltaze, J.-P.; Leibl, W.; Aukauloo, A.; et al. Successive light-induced two electron transfers in a Ru–Fe supramolecular assembly: From Ru–Fe(II)–OH2 to Ru–Fe(IV)–oxo. Chem. Sci. 2015, 6, 2323–2327. [Google Scholar] [CrossRef]
- Skolia, E.; Gkizis, P.L.; Kokotos, C.G. Aerobic photocatalysis: Oxidation of sulfides to sulfoxides. ChemPlusChem 2022, 87, e202200008. [Google Scholar] [CrossRef]
- Baciocchi, E.; Giacco, T.D.; Elisei, F.; Gerini, M.F.; Guerra, M.; Lapi, A.; Liberali, P. Electron transfer and singlet oxygen mechanisms in the photooxygenation of dibutyl sulfide and thioanisole in MeCN Sensitized by N-methylquinolinium tetrafluoborate and 9,10-dicyanoanthracene. The probable involvement of a thiadioxirane intermediate in electron transfer photooxygenations. J. Am. Chem. Soc. 2003, 125, 16444–16454. [Google Scholar] [CrossRef]
- Clennan, E.L. Persulfoxide: Key intermediate in reactions of singlet oxygen with sulfides. Acc. Chem. Res. 2001, 34, 875–884. [Google Scholar] [CrossRef]
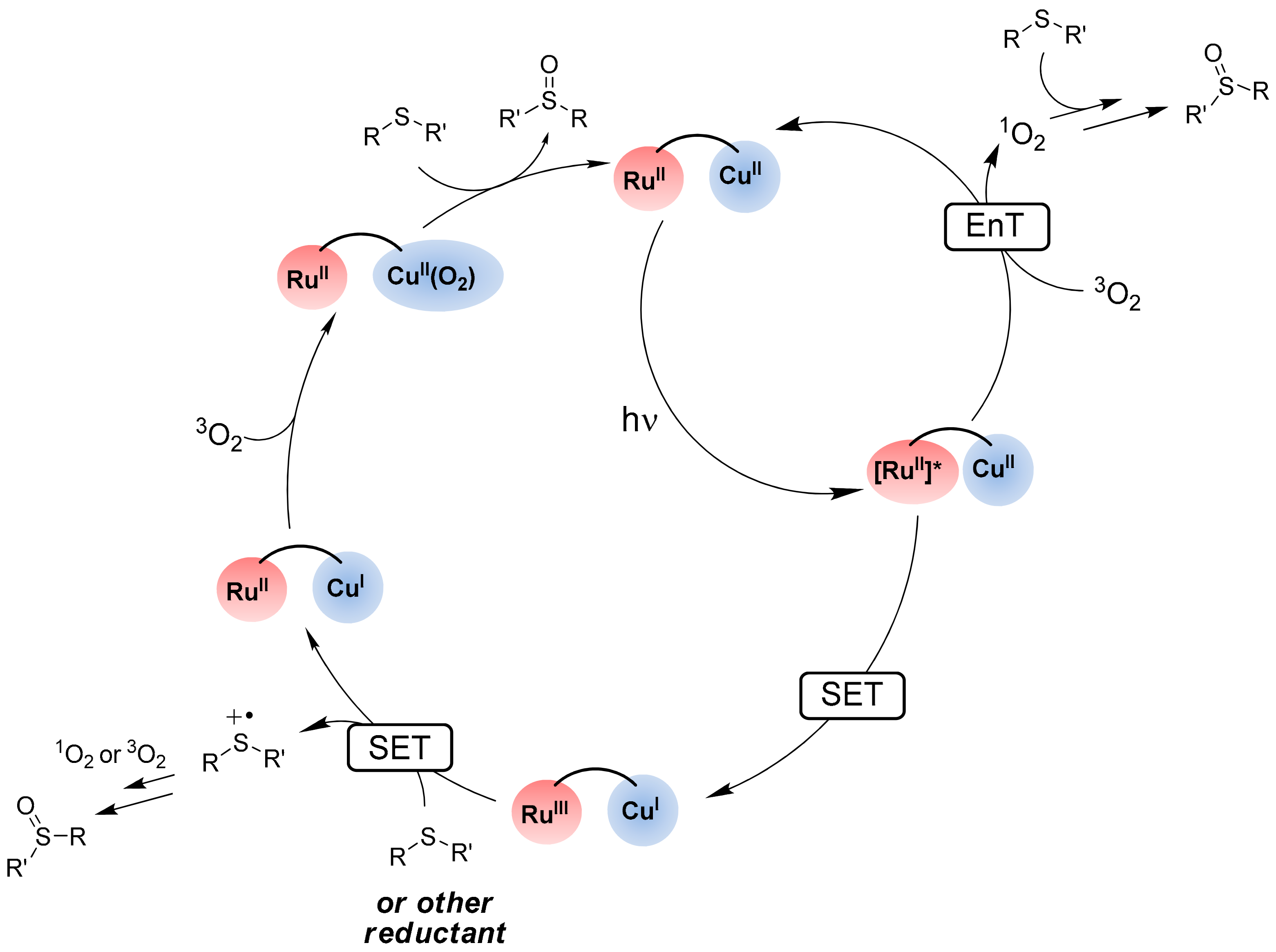
- Iali, W.; Lanoe, P.-H.; Torelli, S.; Jouvenot, D.; Loiseau, F.; Lebrun, C.; Hamelin, O.; Ménage, S. A ruthenium(II)–copper(II) dyad for the photocatalytic oxygenation of organic substrates mediated by dioxygen activation. Angew. Chem. Int. Ed. 2015, 54, 8415–8419. [Google Scholar] [CrossRef]
- Chao, D.; Zhao, M. Robust Cooperative photo-oxidation of sulfides without sacrificial reagent under air using a dinuclear RuII–CuII assembly. ChemSusChem 2017, 10, 3358–3362. [Google Scholar] [CrossRef]
- Ananikov, V.P. Nickel: The “spirited horse” of transition metal catalysis. ACS Catal. 2015, 5, 1964–1971. [Google Scholar] [CrossRef]
- Thoke, M.B.; Sun, G.-J.; Borse, R.A.; Lin, P.; Lin, S.-X. Unimolecular cooperative metallaphotocatalysis with conjugately bridged Ir–Ni complexes and its applications in organic coupling reactions. Org. Chem. Front. 2022, 9, 1797–1807. [Google Scholar] [CrossRef]
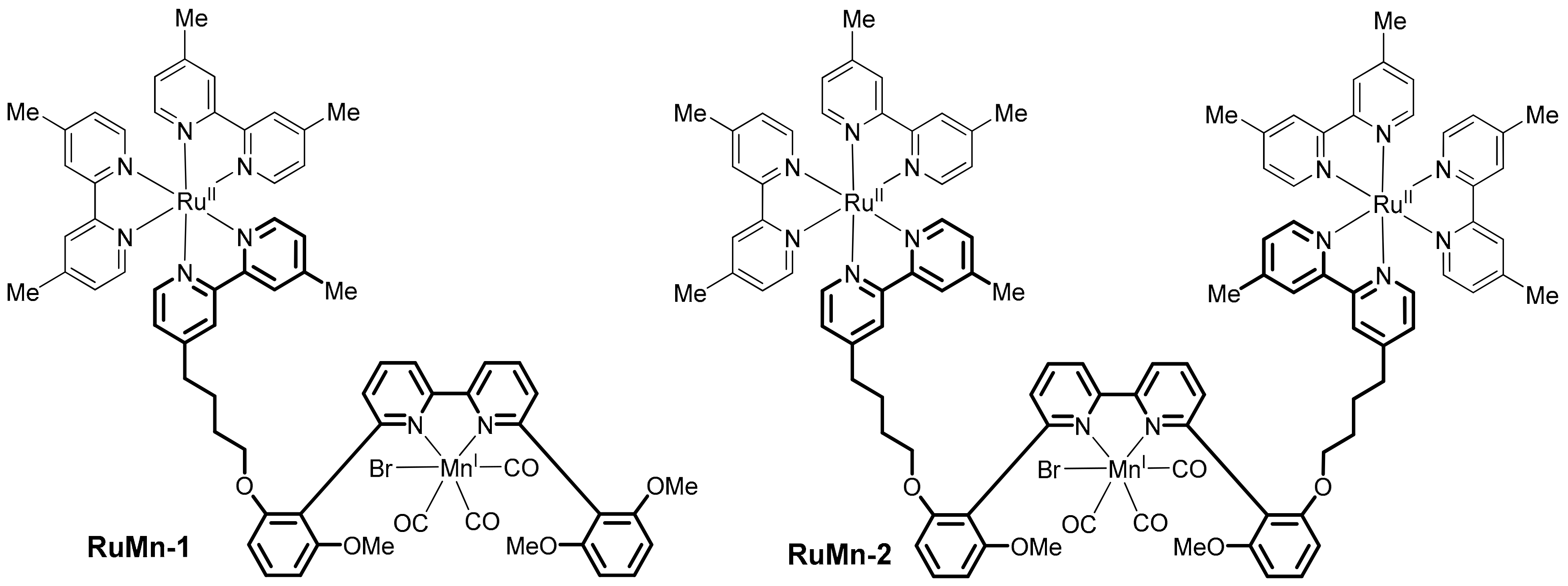
- Kumar, P.; Joshi, C.; Srivastava, A.K.; Gupta, P.; Boukherroub, R.; Jain, S.L. Visible light assisted photocatalytic [3+2] azide–alkyne “Click” reaction for the synthesis of 1,4-substituted 1,2,3-triazoles using a novel bimetallic Ru–Mn complex. ACS Sustain. Chem. Eng. 2016, 4, 69–75. [Google Scholar] [CrossRef]
- Bayer, L.; Birenheide, B.S.; Krämer, F.; Lebedkin, S.; Breher, F. Heterobimetallic gold/ruthenium complexes synthesized via post-functionalization and applied in dual photoredox gold catalysis. Chem. Eur. J. 2022, 28, e202201856. [Google Scholar] [CrossRef] [PubMed]







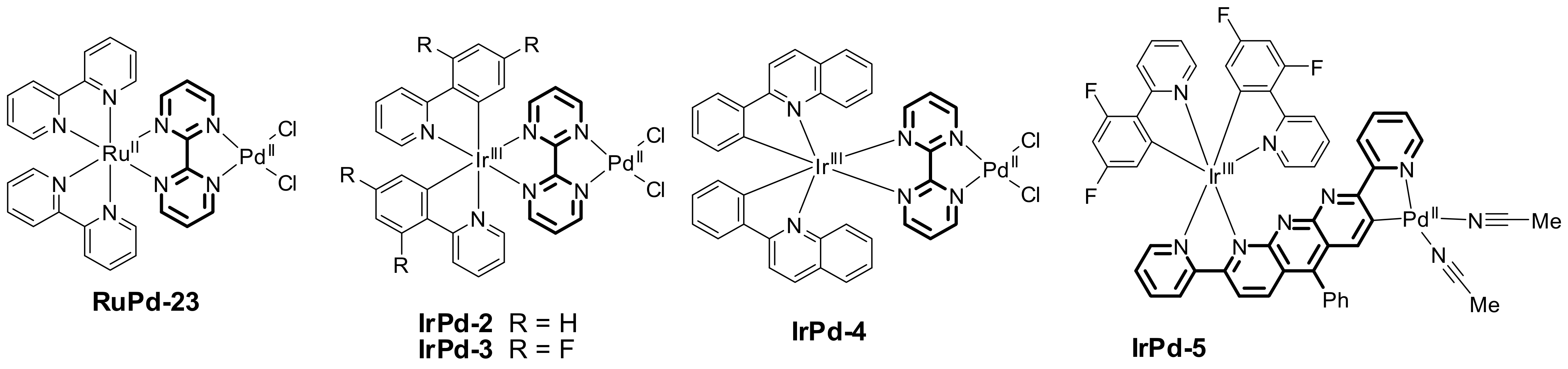
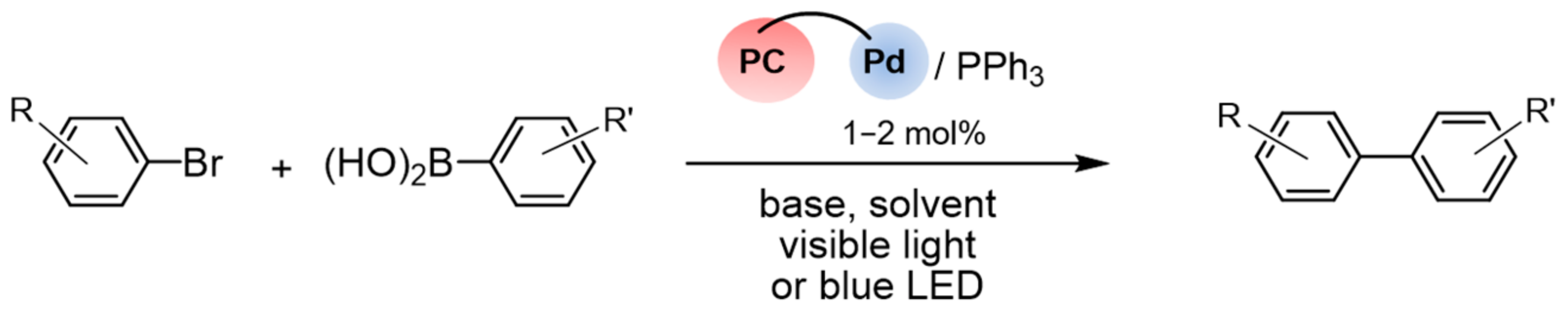










| Entry | R | R’ | Conditions | Yield, % | Reference |
|---|---|---|---|---|---|
| 1 | H | H | RuPd-23/PPh3, (1/2 mol.%) K2CO3, EtOH, r.t., visible light | 10 | [97] |
| 2 | H | H | RuPd-23/PPh3, (1/2 mol.%) K2CO3, EtOH, r.t., dark | 6 | [97] |
| 3 | H | H | Ru(bpy)2(bpm)2+/ Pd(bpy)Cl2/PPh3 (1/1/2 mol.%) K2CO3, EtOH, r.t., visible light | 6 | [97] |
| 4 | H | H | Pd(bpy)Cl2/PPh3 (1/2 mol.%) K2CO3, EtOH, r.t., visible light | 5 | [97] |
| 5 | Me | H | RuPd-23/PPh3, (2.5/5 mol.%) Cs2CO3, DCM-EtOH, r.t., blue LED | 80 | [98] |
| 6 | Me | H | IrPd-2/PPh3, (2.5/5 mol.%) Cs2CO3, DCM-EtOH, r.t., blue LED | 86 | [98] |
| 7 | Me | H | IrPd-3/PPh3, (2.5/5 mol.%) Cs2CO3, DCM-EtOH, r.t., blue LED | 54 | [98] |
| 8 | Me | H | IrPd-4/PPh3, (2.5/5 mol.%)) Cs2CO3, DCM-EtOH, r.t., blue LED | 93 | [98] |
| 9 | Me | H | IrPd-4/PPh3, (2.5/5 mol.%)) Cs2CO3, DCM-EtOH, r.t., dark | 40 | [98] |
| 10 | Me | H | Ir(pq)2(bpy)+/Pd(bpm)Cl2/ PPh3 (2.5/2.5/5 mol.%) Cs2CO3, DCM-EtOH, r.t., blue LED | 75 | [98] |
| 11 | MeC(O) | Me | IrPd-5/PPh3, (1/2 mol.%) Cs2CO3, MeOH, 30 °C, blue LED | 99 | [99] |
| 12 | MeC(O) | Me | Ir(ppy)2L/[Pd(ppy)Cl]2/PPh3 (1/1/2 mol.%) Cs2CO3, MeOH, 30 °C, blue LED | 35 | [99] |
| 13 | Me | Me | IrPd-5/PPh3, (1/2 mol.%) Cs2CO3, MeOH, 30 °C, blue LED | 98 | [99] |
| 14 | CN | Me | IrPd-5/PPh3, (1/2 mol.%) Cs2CO3, MeOH, 30 °C, blue LED | 98 | [99] |
| 15 | OMe | Me | IrPd-5/PPh3, (1/2 mol.%) Cs2CO3, MeOH, 30 °C, blue LED | 78 | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionova, V.A.; Abel, A.S.; Averin, A.D.; Beletskaya, I.P. Heterobinuclear Metallocomplexes as Photocatalysts in Organic Synthesis. Catalysts 2023, 13, 768. https://doi.org/10.3390/catal13040768
Ionova VA, Abel AS, Averin AD, Beletskaya IP. Heterobinuclear Metallocomplexes as Photocatalysts in Organic Synthesis. Catalysts. 2023; 13(4):768. https://doi.org/10.3390/catal13040768
Chicago/Turabian StyleIonova, Violetta A., Anton S. Abel, Alexei D. Averin, and Irina P. Beletskaya. 2023. "Heterobinuclear Metallocomplexes as Photocatalysts in Organic Synthesis" Catalysts 13, no. 4: 768. https://doi.org/10.3390/catal13040768
APA StyleIonova, V. A., Abel, A. S., Averin, A. D., & Beletskaya, I. P. (2023). Heterobinuclear Metallocomplexes as Photocatalysts in Organic Synthesis. Catalysts, 13(4), 768. https://doi.org/10.3390/catal13040768