1. Introduction
The consumption of fossil fuels and human activities have accelerated CO
2 emissions, which may lead to a number of issues, such as global warming and ocean acidification [
1,
2]. A feasible strategy to mitigate this issue is CO
2 hydrogenation to value-added chemicals, such as methanol, olefins and aromatics, or liquid fuels using green hydrogen [
3,
4,
5]. Methanation of CO
2 and the reverse water gas shift into CO are two techniques by which CO
2 is converted into various chemicals [
6], while CH
4 and CO are regarded as the CO
2RR species with the highest commercial value [
7]. Olefin synthesis from CO
2 mainly occurs via two different routes, namely the methanol-mediated route and the CO
2 Fischer–Tropsch (CO
2-FT) synthesis route. The former process occurs at temperatures at approximately 400 °C, which results in a very low olefin yield due to high CO selectivity (>50%). CO
2-FT is a multi-step process that combines reverse water gas shift (RWGS) and FTS. CO
2 is initially converted to CO by the RWGS reaction and subsequent hydrogenation of CO to olefins via the FTS reaction [
8,
9]. CO, which is generated by RWGS, is an important intermediate of the CO
2-FT reaction, while competing CO
2 methanation hinders the progress of RWGS reaction and reduces the CO yield [
10]. The RWGS reaction mainly contains two pathways, which are redox and association pathways [
11]. Under reaction conditions, the materials containing reducible oxides mostly follow the redox pathway [
12], while the association pathways are mainly dominated by noble metal nanoparticles (NPs) on reducible support catalysts [
13]. This indicates that the RWGS reaction is highly dependent on the type of catalyst and on reaction conditions [
14]. Iron-based catalysts have emerged as the ideal candidates for CO
2-FT because they have shown exceptional activity for both reaction steps. Typically, the active phase of RWGS, which converts CO
2 to CO, is considered to be Fe
3O
4 [
5,
15], and iron carbides are regarded as active phases in Fischer–Tropsch synthesis reactions [
16,
17].
Under reaction conditions, catalyst precursors, which are normally in the form of bulk iron oxides, are reduced and carburized to form active iron carbides for C–C coupling. The carbides can also be re-oxidized by water and CO2 during the reaction. The transformation of iron precursors to iron carbides is recognized as a complex and dynamic process that involves oxygen removal, carbon diffusion, hydrogenation and surface carbon deposition. The structure and composition of the catalyst strongly depends on the catalyst preparation methods, promoters, pretreatment protocols and reaction conditions.
Pretreatment conditions, in particular the composition of pretreatment gas, have been reported to strongly affect the structure and catalytic performance of the iron-based FT catalysts. Bukur et al. studied the effects of different pretreatment atmospheres on iron-based catalysts and found that CO and syngas pretreatment catalysts were more susceptible to deactivation than hydrogen pretreated catalysts, but were more conducive to the formation of long-chain hydrocarbons [
18,
19,
20]. However, Lee et al. argued that CO-pretreated catalysts contained more iron carbides, which improved the FTS activity and olefin selectivity [
21]. In another study by Li et al., the authors reported a selectivity shift to heavy hydrocarbons upon increasing CO partial pressure in the pretreatment H
2/CO gas mixture [
22]. In the field of electrocatalysis, Bhalothia et al. processed catalysts under different reduction conditions, obtained catalysts with the same composition but different nanostructures, and clarified their structure-dependent ORR performance [
23].
The dynamic transformation of iron-based FT catalysts during activation was studied experimentally using in-situ characterization techniques and theoretically using atomistic thermodynamics. Using in-situ X-ray diffraction, Lu et al. tracked the temperature-dependent evolution of Fe
3O
4, FeO, Fe (0), Fe
5C
2 and Fe
3C under the syngas environment with varying H
2/CO ratios. They further obtained a high-purity (95%) χ-Fe
5C
2 catalyst under the optimal H
2/CO ratio of two. The obtained catalyst showed enhanced FT activity and the selectivity of C
5+ hydrocarbons [
24]. Emiel de Smit et al. correlated the stability and reactivity of various iron carbides (e.g., Ɛ-Fe
2C, χ-Fe
5C
2, and θ-Fe
3C) with carbon chemical potential (µ
c) and reaction temperature. Different gas phase compositions, which have different carbon chemical potentials, and reaction temperature have an impact on the relative thermodynamic stability of iron carbides, thereby regulating the type of iron carbides formed. In particular, high µ
c is beneficial to the formation of χ-Fe
5C
2, and low µ
c is conducive to the formation of θ-Fe
3C. High µ
c at low temperature favors the formation of Ɛ-Fe
2C. Under FTS conditions, the catalysts with crystalline χ-Fe
5C
2 as their major component were quite sensitive to oxidation, whereas those with θ-Fe
3C and amorphous carbide phases exhibited decreased activity and selectivity, primarily due to the accumulation of carbonaceous deposits on the catalyst surface, indicating that the final catalyst performance was significantly influenced by the amorphous phases and the resulting textural properties [
25]. A recent study conducted by Li et al., pointed that the carbonization rate and the equilibrium phase composition iron catalyst is not solely determined by the theoretical μ
C of the pretreatment gas, but also related to kinetic and entropic variables [
26]. Although prominent endeavors have been devoted to study the influence of pretreatment conditions on the chemical composition, structure, and the resulting performance of catalysts, the pretreatment methods to deliver preferential iron phases remain elusive and controversial.
The dynamic evolution of iron catalysts in the FTS reaction was also observed in CO
2 hydrogenation conditions. Han et al. used operando XRD/Raman to study the phase transition of bulk iron-based catalysts in direct CO
2-FT to olefins, and believed that the oxidation of iron carbides was the main reason for the iron catalyst deactivation. The activity and C
2–C
4 olefins selectivity of the catalyst can be greatly restored by CO
2-CO regeneration treatment. Additionally, various iron-based precursors will produce several different iron carbides. While θ-Fe
3C obtained from γ-Fe
2O
3 is more desirable for the formation of C
5+ hydrocarbons, χ-Fe
5C
2 derived from α-Fe
2O
3 is favorable for the generation of lower olefins [
27,
28]. Recently, Guo’s team combined experiments and theoretical calculations to reveal the relationship between the dynamic structure and catalytic performance of iron-based catalysts during CO
2 hydrogenation. The results showed that the change of surface composition depends on the balance of oxidation and carbonization and it is highly sensitive to the operating conditions (e.g., GHSV and H/C), where by-product water is critical [
29]. Andrey S. Skrypnik et al. studied the role of different iron species in CO
2 hydrogenation, and found that the proportion of iron carbides was positively correlated with the selectivity of C
2+ hydrocarbons. Fe
3O
4 and metallic Fe are responsible for CO hydrogenation to CH
4; however, the main function of Fe
3O
4 is to promote CO
2 conversion into CO through the RWGS reaction [
30].
To improve the olefins selectivity and simultaneously suppress the formation of undesired CO and methane, iron catalysts must be promoted with alkali metals and transition metals, such as Zn, Cu, and Mn [
31,
32,
33]. These metals, mainly in their oxide form, can function as an electronic or structural promoter, enhancing the reducibility of the iron oxide precursor, dispersion and stability of resulting iron carbides, and surface basicity. In a previous work, the Fe
2Zn
1 catalyst achieved the selectivity of C
2–C
7 olefins in the gas phase product of 57.8% and C
4+ olefins selectivity in the liquid phase product of 81.9% when the CO
2 conversion was 35%. The catalyst enables RWGS to occur on the formed ZnO and highly dispersed FeO
X, and C–C coupling and olefin formation on FeC
x. The aggregation and interaction of Na and Fe to the catalyst surface during activation inhibits the oxidation of FeC
x, resulting in excellent stability [
34]. In addition, the introduction of Zn promoted CO
2 conversion, olefin desorption and chain growth, and inhibited the secondary hydrogenation of olefins, while changing the balanced ratios of FeC
x/FeO
x during the reaction [
31].
It is challenging to explore the CO2-FT process due to the intricacy of the phase and structural transitions involved in iron-based catalysts and the limitations of characterization techniques. Among them, the effects of the pretreatment atmosphere on iron-based catalysts in the CO2-FT procedure has thus received little consideration. In addition, in situ DRIFTS is a very infrequently used technique within high pressure reactions, despite being a crucial tool for identifying species adsorbed on catalyst surfaces. In this study, we investigated the impacts of pretreating protocols on the phase composition of a Fe-Zn-Na catalyst prepared via a coprecipitation method, and pretreated the catalyst using different activation agents, namely 10% CO, 5% CO/5% H2 and 10% H2. The chemical composition and structure of the catalysts after reductive pretreatment and CO2-FT reaction were investigated using multiple characterizations. Meanwhile, the possible adsorbed species on catalyst, pretreated under different conditions were investigated using high-pressure diffuse reflectance FTIR spectroscopy (DRIFTS). A direct correlation between the reductive pretreatment and the mechanistic pathways were discussed.
2. Results and Discussion
2.1. Catalytic Performance of CO2 Hydrogenation
The CO
2 conversion and the selectivity of CO, CH
4, C
2–C
4 alkenes and alkanes and C
5+ products in the CO
2 hydrogenation of catalysts pretreated with different reductive gases are shown in
Figure 1a and
Figure S1a,b. The hydrocarbon distribution of the liquid products is displayed in
Figure 1b and
Figure S1c,d.
The pretreatment gases showed different impacts on the performance of CO2 hydrogenation. First of all, the performance of the catalysts pretreated by different atmospheres did not show obvious deactivation within 100 h, indicating that the catalyst materials pretreated with different reductive gases remained stable during the CO2-FT reaction. Interestingly, the induction period, i.e., the time needed to establish a stable conversion and product composition, of the catalyst subjected to different pretreatment gases were distinct. For instance, the CO and syngas pretreated catalyst showed a sharp increase in the olefin/paraffin (O/P) ratio within 10 h TOS and subsequently stabilized at 20 h. In contrast, the H2 pretreated catalyst showed a dramatic decrease in the O/P ratio within 50 h TOS.
When comparing the stabilization period data (100 h) of different atmospheres’ pretreatment catalysts, it can be observed that the CO2 conversion of the H2 pretreatment catalyst was approximately 28%, while the CO and syngas pretreatment catalysts were 35% and 32%, respectively. As for product selectivity, the H2-pretreated catalyst formed substantial amounts of C1 products (CO: 61.1%, CH4: 13.2%) and small amounts of C2–C4 olefins (15.9%) and C5+ (6.7%). The lowest CO selectivity (50.6%) and highest selectivity of C2–C4 olefins and C5+ products (22.3% and 10%) were obtained in CO pretreated catalysts. The product distribution of the syngas pretreated catalyst was in between, where the selectivity of C2–C4 olefins and C5+ products were 18.9% and 7.9%, respectively, while the CO selectivity was 55.6%. The selectivity of CH4 and C2–C4 alkanes was not significantly impacted by the pretreatment gases; CH4 had a selectivity of roughly 13%, while C2–C4 alkanes had a selectivity of approximately 3%. For the STY of C2-C7 olefins, the catalysts of different atmospheres’ pretreatment increased over time, and finally remained stable. Among them, the initial STY of the CO pretreatment catalyst was the highest (22 mmol·), while that of syngas and H2 pretreatment was similar (12 mmol· and 14 mmol·). The final CO pretreatment catalyst was stable at 28 mmol·, while that of syngas and H2 was 24 mmol· and 21 mmol·, respectively. The highest O/P of 6.2 was obtained in the CO pretreated catalyst. Both syngas and H2 pretreatment catalysts possess an O/P of 5.1. The O/P of the products in the liquid phase were 2.47 (for CO pretreatment), 2.36 (for syngas pretreatment), and 2.02 (for H2 pretreatment). In short, CO pretreatment catalysts displayed better activity and olefin selectivity compared to H2 and syngas pretreatment catalysts, favoring the production of C2–C4 olefins and C5+ hydrocarbons.
2.2. Structure and Properties of Fresh Catalysts
The elemental composition of the fresh catalyst was 44% for Fe, 25% for Zn and 1.5% for Na measured by ICP.
The XRD pattern of the fresh catalyst is shown in
Figure S2a. The characteristic peaks located at 29.9° and 35.3° were attributed to the spinel ZnFe
2O
4 (JCPDS 89-1012). The Raman spectra (
Figure S2b) of fresh catalysts were in agreement with the XRD. The typical peaks of Raman shift 451 cm
−1 and 647 cm
−1 were attributed to ZnFe
2O
4, suggesting that the catalyst includes only ZnFe
2O
4. The morphology and particle size of the catalyst were investigated by TEM (
Figure S2c). The particle size of the fresh catalyst was 7.9 ± 0.1 nm.
2.3. Structure and Properties of Pretreated Catalysts
To illustrate the effect of different activation atmospheres on the reduction and carbonization processes of the catalysts, we characterized the pretreated and spent catalysts.
The surface phases of the activated catalysts were characterized by Raman and XPS. As shown in
Figure S3, all three activated catalysts showed the Fe-O vibrational peaks at the Raman shift of 660 cm
−1, whereas the CO and syngas activated catalysts showed the distinctive peaks of carbon deposition at the Raman shift of 1350 cm
−1 and 1590 cm
−1, which were ascribed to the amorphous carbon (denoted as D peak) and graphitic carbon (G peak) [
27,
35,
36]. Typically, the intensity ratio of the D to G peaks is an indicator of the disorder degree [
37,
38]. The CO-activated catalyst had a lower I
D/I
G than the syngas-activated catalyst, which implies that it produced more graphitized carbon species. More graphitized carbon species would facilitate the carburization of iron species [
37], which would improve the generation of FeC
x. The strength of the Fe-O vibration peak diminishes when CO was added to the activated atmosphere and its concentration rises, while the intensity of the D and G peaks increases, which indicates that CO encourages the reduction of FeO
x and the penetration of carbon.
In the Fe 2p spectra (
Figure 2a), the activated catalyst both showed the peaks at the binding energies of 711 eV and 725 eV [
39], which were attributed to Fe
3O
4. Additionally, the catalysts pretreated with the two CO-bearing gases displayed distinctive peaks ascribed to FeC
x with binding energies of 707 eV and 720 eV [
39]. It is worth noting that the peak of metallic iron did not appear in the H
2 pretreated catalyst, which may be due to the oxidation of metallic iron on the surface of the catalyst during the passivation process. In the C 1s spectra (
Figure 2b), the Fe–C bond is responsible for the binding energy at 283.58 eV [
40]. The carbon content in the Fe–C bond of the CO-activated catalyst was larger (47%) than that of the syngas-activated catalyst (42.9%), in terms of all carbon species. Graphitic carbon is responsible for the binding energy at 284.58 eV [
40], which is somewhat higher in the CO-activated catalyst (30.4%) than in the syngas-activated catalyst (28%). The graphitic carbon peak shifted by 1 eV identified as sp
3-hybridized C [
40]. The peak with the highest binding energy (288.6 eV) was associated with carbonates surface species [
41].
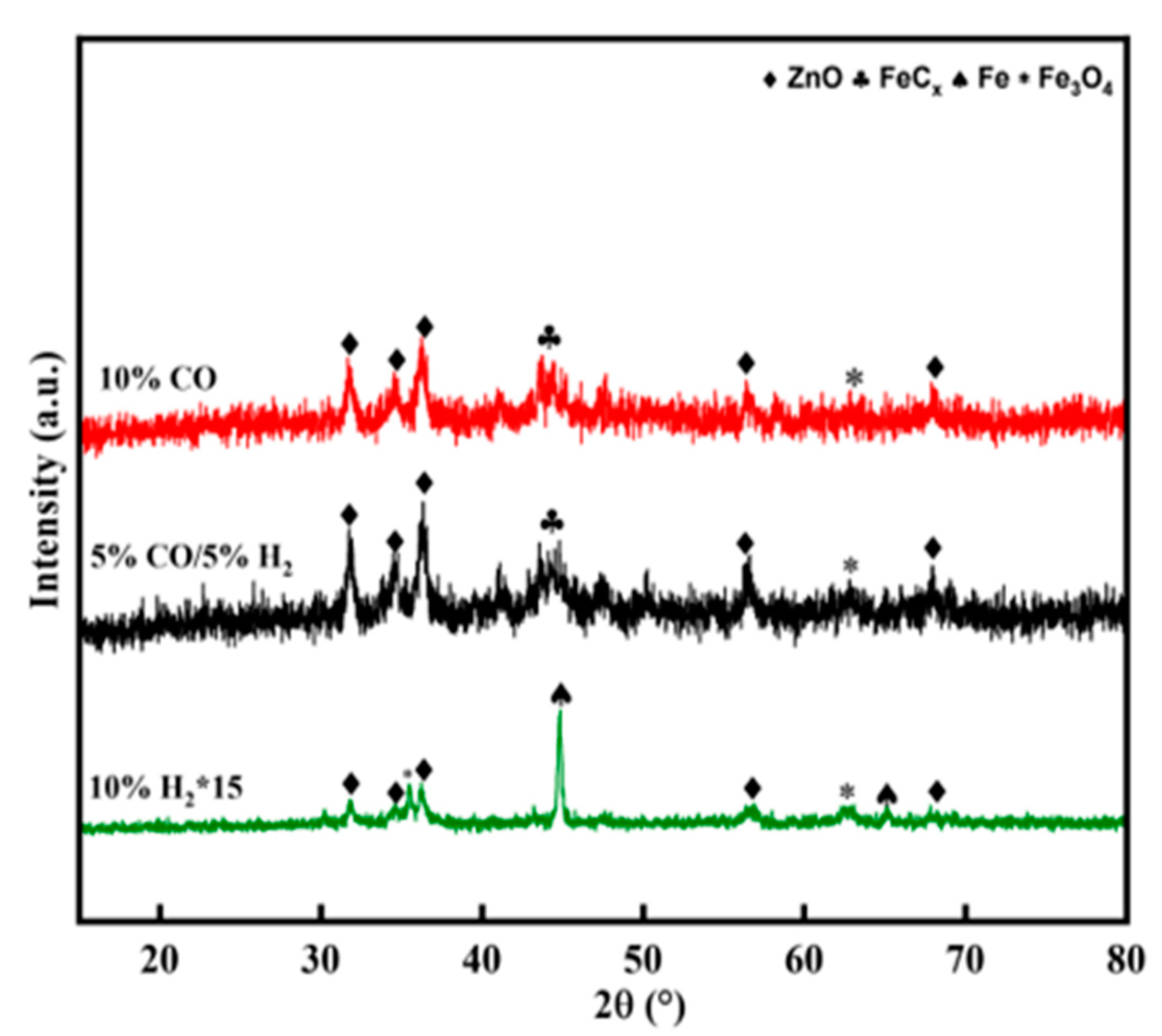
The XRD pattern of the activated catalyst is exhibited in
Figure 3, and was consistent with the Raman and XPS. All catalysts showed characteristic diffraction peaks of ZnO (JCPDS 79-0207), which originated from the decomposition of ZnFe
2O
4. When the activating atmosphere was H
2, the diffraction peaks at 44.7°, 65.1°, and 82.5° were assigned to metallic iron (JCPDS 87-0722), whereas those at 35.4° and 62.5° were attributed to Fe
3O
4 (JCPDS 87-2334). However, for CO and syngas-activated catalysts, in addition to the characteristic peaks of ZnO and Fe
3O
4, the diffraction peaks occurring between 41° and 48° were assigned to iron carbide. However, because of the poor resolution and overlapping patterns of the iron carbide peaks, it was challenging to identify which iron carbide phase was present. Therefore, the specific phase compositions were further determined by MES.
As shown in
Figure S4 and
Table 1, according to the MES, the activated catalyst contained several doublets and sextets. The doublets were assigned to the superparamagnetic Fe
3+ or Fe
2+. The sextets with the Hhf values of 215, 176 and 111 kOe were related to three different sites of stoichiometric χ-Fe
5C
2 [
34], and the sextets with the Hhf values of 190 and 329 kOe corresponded to FeC
3 and metallic Fe [
15,
42]. For the Hhf values of 480 and 447 kOe, which were assigned to the Fe
3O
4 [
42], the primary phase in CO and syngas-activated catalysts was FeC
x, which also contains trace amounts of Fe
2+ or Fe
3+. The content of Fe
5C
2 and Fe
3C in CO-activated catalysts were 67.9% and 21%, respectively, while the content of Fe
5C
2 in syngas-activated catalysts and Fe
3C were 77.5% and 9.8%, respectively, which indicates that the iron phase mainly exists in the form of Fe
5C
2. On the contrary, the H
2-activated catalyst had the highest proportion of metallic iron, which is the main form of iron, with a content of 50.2%, followed by Fe
3O
4 with a content of 30.6%.
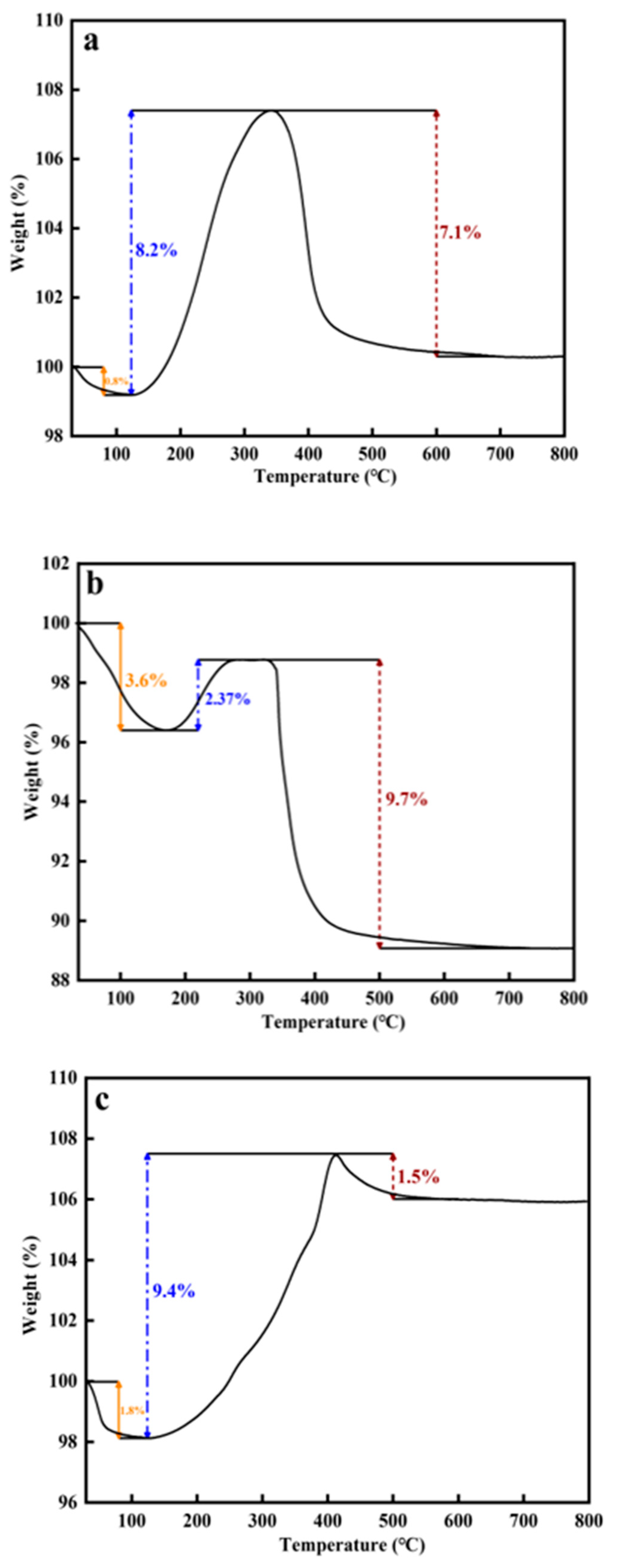
The surface carbon deposits of the pretreated catalysts were analyzed using a thermogravimetric analyzer (TGA). The TGA curve can be divided into three stages. As shown in
Figure 4a,b, the weight loss in the first stage was the removal of bound water, FeC
x oxidation causes the weight growth in the second stage, and the oxidation of carbon deposits causes the final weight loss. The weight gain ratio of the catalyst after CO activation in the second stage (12.9%) was significantly higher than that of the catalyst after syngas activation (10.2%), which indicates that the content of FeC
x species in the catalyst after CO activation was more than that of the catalyst after syngas activation. It is worth noting that the CO-activated catalyst had less weight loss (6.4%) at lower combustion temperature (354.7 °C) (
Figure S5a), while the syngas-activated catalyst had more severe carbon deposition (7.1%) and correspondingly higher combustion temperature (358.5 °C) (
Figure S5b). This indicates that the CO-activated catalyst better maintains the CO dissociation and carburization process.
The average particle size and morphology of the activated catalyst were observed by TEM. As depicted in
Figure 5a–c, compared with the fresh catalyst, the average particle size of the activated catalyst was significantly larger, among which, the average particle size of the H
2-activated catalyst was the largest, while the average particle size of the CO and syngas-activated catalysts was relatively high. This may be due to the fact that H
2 promotes particle agglomeration and sintering. Additionally, because Fe
3O
4 has a lower density than FeC
x, which allows carbides from the splitting of Fe
3O
4, it is also likely that the particle size of FeC
x was less than that of Fe
3O
4 [
43,
44]. As shown in
Figure S6 of the Supplementary Materials, for the CO-activated particle, the lattice spacing is 0.192 nm, consistent with the (221) facet of Fe
5C
2. For the syngas-activated particle, the lattice spacing is 0.214 nm, consistent with the (202) facet of Fe
5C
2. The CO-activated and syngas-activated catalysts display the lattice fringe of 0.246 nm, corresponding to the (121) plane of Fe
3C. For the H
2-activated particle, the lattice spacing is 0.248 nm and 0.21 nm, consistent with the (311) facet of Fe
3O
4 and the (100) plane of Fe. The results from XRD and MES are in accordance with the observations.
2.4. Structure and Properties of Spent Catalysts
Taking into account the differences between catalysts pretreated with different atmospheres, the structure and phase compositions of the spent catalysts were further examined by Raman, XPS, XRD, and MES. As illustrated in
Figure 6a, the spent catalysts pretreated with H
2 also have vibrational peaks at 1350 cm
−1 and 1590 cm
−1 ascribed to carbon deposition, which indicates that carburization occurred during the reaction and FeC
x was generated.
The XPS results are displayed in
Figure 6b. The Fe 2p
3/2 peak at the binding energy of 707.3 eV refers to FeC
x, and the locations at 710.1 eV and 712.1 eV correspond to Fe
2+ and Fe
3+ in iron oxides, respectively [
45,
46]. A
FeCx/A
total represents the proportion of FeC
x to total iron species on the catalyst surface. The A
FeCx/A
total of the catalyst for the syngas pretreatment was the lowest (0.03), and the A
FeCx/A
total of the catalyst for the H
2 pretreatment was the highest (0.15). It is possible that the syngas pretreatment catalyst’s surface carbon was relatively severe after the reaction, which interferes with the ability to identify FeC
x species. Furthermore, metallic iron might be more conducive to the dissociation of CO
2 and the penetration of carbon than FeO
X, thereby increasing the content of FeC
X. The A
FeCx/A
total of the catalyst for the syngas pretreatment and the CO pretreatment catalyst (0.11) were essentially equivalent.
As depicted in
Figure 7, for the spent catalyst of H
2 pretreatment, the diffraction peaks appeared between 41° and 48°, which were assigned to FeC
x, while metallic iron diffraction peaks vanished, indicating that metallic iron was transformed into FeC
x during the reaction. In contrast to the following activation, the catalyst phase for CO and syngas pretreatment catalysts remained mostly unchanged after the reaction. The composition of the particular phase should be further determined by MES.
As illustrated in
Figure S7 and
Table 2, the spent catalysts with varied pretreatment atmospheres contained one doublet and four sextets. The superparamagnetic (spm) Fe
2+ was responsible for the doublet with IS values of 0.4, 0.52, and 0.64 mm·s
−1, which was increased in comparison to the activated catalyst of CO and syngas pretreatment catalysts. The proportion of FeC
x dropped, indicating that the reaction caused FeC
x to be oxidized. The contents of Fe
5C
2 and FeC
3 for the spent catalyst of CO pretreated were 64.6% and 19%, 76.3% and 8.2% for the syngas pretreated, and 78.9% and 12.2% for the H
2 pretreated, respectively.
The thermogravimetric results of the spent catalysts are depicted in
Figure 8a–c and
Figure S8a–c. Carbon deposition was the most severe for syngas pretreatment catalysts (9.7%) and mildest for hydrogen pretreatment catalysts (1.5%), possibly due to the faster carburization rate of metallic iron compared to iron oxides [
47], accelerating the consumption of carbon species. The oxidation of FeC
X was responsible for the weight gain at the second stage of the curve, and the order was H
2-pretreated > CO-pretreated > syngas-pretreated, proving that metallic iron has a greater carburizing ability. This may be because the removal of O during the carburization of FeO
X slowed the rate of carbonization, whereas the carburization of metallic iron was a comparatively simple process.
In situ DRIFTS was employed to investigate the adsorbed species and reaction intermediates on the catalyst surface in reaction conditions after different atmospheres’ pretreatments (
Figure 9a–c). After reaching the reaction temperature, the bands attributed to bicarbonate species (HCO
3*, ca. 1395 and 1611 cm
−1), gaseous CO (ca. 2100 and 2170 cm
−1) and the vibration of C-H in CH
4 (ca. 3009 cm
−1) were detected in all catalysts [
15,
34]. Only the CO pretreated and syngas pretreated catalysts showed the peaks at 2861 cm
−1 and 2927 cm
−1, which were attributed to -CH
2 group, and the band at 2958 cm
−1, that was ascribed to C-H in -CH
3 group [
48,
49,
50]. Compared to syngas pretreatment catalysts, the peak intensity at these positions was noticeably stronger for CO pretreatment catalysts due to the fact that -CH
2 and -CH
3 groups were important species for C–C coupling to generate C
2+ hydrocarbons. This was in accordance with the catalyst for CO pretreatment having the greatest selectivity towards C
5+ hydrocarbons. In addition, only the CO-pretreated and syngas-pretreated catalysts showed the C=C band of π-bonded CH
2=CH
2 at 1710 cm
−1 and the C-H stretching of alkenes [
51,
52,
53], and these bands in the CO pretreated catalysts were stronger. This may account for the highest C
2–C
4 olefin selectivity and O/P of the CO pretreatment catalyst.
It is worth noting that the initial O/P of the H2 pretreated catalyst was high and then gradually decreased, while the trend of CO and syngas pretreated catalyst was the opposite. This may be due to the fact that the initial phases of the H2 pretreated catalyst were mainly Fe and Fe3O4, which have a relatively strong dissociation ability for CO2, but a weak dissociation ability for H2, consistent with the absence of C-H peaks in the in situ DRIFTS. At the same time, the low H concentration on the surface was not conducive to the secondary hydrogenation, so the initial O/P was high. With the formation of FeCX, the dissociation of H2 gradually increased, so the O/P gradually decreased. On the contrary, the initial phases of the catalyst for CO and syngas pretreated contained a large amount of FeCX, which has a strong dissociation ability for CO2 and H2, and the H concentration on the surface was higher, so the initial O/P was lower. As the reaction proceeded, FeCX was oxidized to Fe3O4, which weakened the dissociation of H2, and O/P gradually increased.
The absence of an olefin signal in the DRIFTS analysis of the H
2-pretreated catalyst can be ascribed to the lack of FeCx phases during the initial stage of the reaction. Furthermore, the greater particle size of the H
2-pretreated catalyst (as seen in
Figure 5) compared to CO- or syngas-pretreated catalysts is another reason for its low initial activity.
As illustrated in
Figure S9a,b, C
2H
4 and C
3H
6 were employed as model olefins to investigate olefin desorption on the spent catalyst. There are two desorption peaks for C
3H
6-TPD, the peak centers at 90, 100, and 148 °C were physical adsorption [
31]. The peaks above 460 °C were chemisorption [
31], which dropped from 479 °C to 466 °C, indicating that the CO pretreatment catalyst was more conducive to C
3H
6 desorption. Only chemisorption with a desorption temperature above 480 °C arose in C
2H
4-TPD, and the peak centers were located at 482, 484, and 489 °C [
54], with a trend of change consistent with that of C
3H
6-TPD. This indicates that the CO pretreatment catalyst was more beneficial to olefin desorption, thereby enhancing olefin selectivity.
2.5. Structure-Performance Relationship
A series of characterization and performance tests on activated and spent catalysts demonstrated that pretreatment atmospheres have a significant impact on catalyst performance in CO
2 hydrogenation, which can be briefly summarized as follows (
Figure S10). In the H
2-pretreated catalyst, the iron phase was mostly reduced to metallic iron, while particle aggregation and sintering occurred. The fresh catalyst was pretreated with CO and syngas, iron carbides appeared, mainly Fe
5C
2 and Fe
3C, and the content of Fe
5C
2 was higher than that of Fe
3C. The content of Fe
5C
2 in the syngas-pretreated catalyst was higher than the CO-pretreated catalyst, which could be due to the addition of H
2 to promote CO dissociation and increase the carbonization rate, which in turn promoted the formation of carbon-rich Fe
5C
2 [
55], but the content of Fe
3C in the CO-pretreated catalyst was higher. Fe
3C as the active phase was beneficial to the formation of C
2–C
4= and C
5+ hydrocarbons [
56,
57,
58], which was also proved by the in situ DRIFTS.
According to the Raman analysis, the surface of the H2 pretreatment catalyst primarily consisted of FeOX, whereas that of the CO and syngas pretreatment catalyst typically consisted of FeOX and carbon deposition. The results of the XPS confirmed the existence of FeOX on the surface of three different atmosphere pretreatment catalysts, and Fe3O4 was the main form. At the same time, FeCx also appeared on the CO and syngas pretreatment catalysts, which corresponded to the carbon deposition in the Raman results, indicating that the catalysts had experienced carburization. The MES results further confirmed the existence of the iron phase form, and clarified that the FeCx in CO and syngas pretreatment catalysts were mainly Fe3C and Fe5C2, between which Fe3C was conducive to the formation of C2–C4= and C5+ hydrocarbons, also consistent with the optimal performance of the CO-pretreated spent catalyst with the highest Fe3C content.
The H
2-pretreated catalyst demonstrated a high O/P ratio at the beginning of the reaction and then flattened out with time-on-stream, manifesting a significant phase transition in the initial stage of the reaction. The phase transition can be explained as the variation in surface hydrogen/carbon balance. The H
2 pretreated catalyst had a high hydrogen coverage and a low carbon coverage at the initial stage, which favored the hydrogen-assisted CO
2 adsorption to generate bicarbonate species as evidenced in the in situ DRIFTS study (
Figure 9). These bicarbonate species can further undergo step hydrogenation to form CO*, which can be dissociated on the catalyst surface to generate free carbons to drive carburization and CH
x species to initiate C–C coupling reaction. As the reaction proceeds, the hydrogen coverage decreases rapidly, which may retard hydrogenation reaction since the adsorption of gaseous hydrogen is required. On the other hand, the sluggish hydrogenation rate may shift the surface H/C balance towards a carbon-rich surface, thus enhancing the carburization process. The carburization reaction involves the permeation of free carbons into the bulk iron, which is expected to occur at a much slower rate than the surface C–C coupling reactions. As the reaction continues, the formation of free CH
x species is slowed due to rapid consumption of adsorbed hydrogen, while the carbon accumulation varies in the opposite way. The observed trend for O/P ratio suggests a dynamic balance of carburization and hydrogenation.
In contrast to the H2-pretreated catalyst, the surface free carbon concentration of CO and syngas-pretreated catalysts was higher, resulting in a faster dissociation rate of H2 in reaction gas than CO2 dissociation rate at the initial reaction stage. The relatively high hydrogen coverage on the surface of the catalyst was conducive to the formation of -CH2 and -CH3 intermediates, which further undergo C–C coupling to generate long-chain hydrocarbons. The bulk phase of CO and syngas-pretreated catalysts consisted mostly of iron carbide, and the content of Fe3O4 was very low, which was not conducive to the RWGS reaction. As the reaction continued, the free carbon on the surface either combined with hydrogen or penetrated into the bulk phase and combined with iron, and was gradually consumed. At the same time, the oxidation of FeCx by the water and CO2 increased the iron oxide content, which further accelerated the RWGS reaction.
When compared to the activated catalyst, the FeCX content of the CO-pretreated and syngas-pretreated spent catalysts decreased while the FeOX content increased, and carbon deposition was exacerbated, indicating that carbonization and oxidation happened parallelly during CO2 hydrogenation. The lowest activity of the H2-pretreated spent catalyst may be due to the relatively small FeOX content, which was not conducive to the occurrence of the RWGS. The CO-pretreated spent catalyst had the highest FeOX and Fe3C contents, and the carbon deposition was relatively mild, which was conducive to maintaining the oxidation and carbonization balance. The high FeOX content was beneficial to the occurrence of RWGS. FeC3 promotes Fischer–Tropsch synthesis (FTS) and also drives the progression of RWGS. Thus, the CO-pretreated spent catalyst had the highest activity, which favors the formation of olefins and C5+ hydrocarbons.
3. Materials and Methods
3.1. Catalyst Preparation
Bulk Fe-Zn-Na (molar ratio of Fe:Zn = 2:1) catalyst was prepared via a co-precipitation at room temperature, as described in our previous work [
34]. Briefly, a certain amount of FeCl
3•6H
2O (Analytical Reagent) and ZnCl
2 (Analytical Reagent), which obtained from Sinopharm Chemical Reagent (Shanghai, China), was weighed and dissolved in ethylene glycol to create a mixed solution. The solution was then transferred to a beaker and agitated for 30 min at room temperature. A sodium carbonate solution was slowly added dropwise to the Fe-Zn precursor solution while stirring. The resultant precipitate was recovered by centrifugation and washed with deionized water multiple times. The resultant slurry was dried at 60 °C overnight, and calcined at 450 °C (heating rate 2 °C/min) for 4 h in a muffle furnace (
Figure S11).
3.2. Pretreatment and Evaluation of Catalyst
The CO
2 hydrogenation was carried out in a stainless-steel fixed bed reactor with an internal diameter of 6 mm and a length of 400 mm. A 25 mg sample diluted with 75 mg of silicon carbide was then placed in the isothermal zone of the tubular reactor. The catalyst was temperature-programed heated under three different reductive gases (10% CO/90% Ar, 5% CO/5% H
2/90% Ar or 10% H
2/90% Ar) at 350 °C (heating rate 2 °C/min) with a gas flow rate of 25 mL/min under atmospheric pressure for 5 h. Then, the reactor was cooled down to 330 °C and pressurized to 1.5 MPa with the reactant gas (20% CO
2/60% H
2/20% N
2, GHSV = 60,000 mL∙g
cat−1∙h
−1) The gaseous products were analyzed by an online gas chromatograph (GC, Clarus 580, PerkinElmer, Waltham, MA, USA) equipped with a thermal conductivity detector (TCD) and a flame ionization detector (FID). H
2, N
2, CO, and CO
2 were analyzed by a TDX-01 column connected to TCD and the hydrocarbons were analyzed by a capillary column (Agilent HP-Plot Q) connected to FID. The liquid products were collected in a cold trap and analyzed offline using an GC-MS (GC(7890A)-MS(5975C), Agilent Technologies Inc., Santa Clara, CA, USA) equipped with a PONA column. A carbon balance of over 92% was established for all catalytic performance tests. The catalyst samples after reductive pretreatment and reaction were carefully collected without air exposure and then passivated with 1%O
2/99%Ar at room temperature for 15 min [
59].
CO
2 conversion (
) was calculated by Equation (1):
where CO
2,in and CO
2,out represent the moles of CO
2 inlet and outlet, respectively.
The selectivity of hydrocarbon (
) in gaseous products was calculated by Equation (2):
CO selectivity (
) was calculated according to Equation (3):
The selectivity of hydrocarbon (
) in liquid products was calculated by Equation (4):
The space time yield (STY) was calculated by Equation (5):
where
is the total volume flow rate of the inlet reactant gas, mL/h.
is the proportion of CO
2 in the inlet reactant gas, and V
m represents the molar volume of an ideal gas at standard temperature and pressure, V
m = 22.4 mL/mmol.
3.3. Characterization Methods
The elemental composition of the catalyst was analyzed by inductively coupled plasma optical emission spectrometer (ICP-OES, 725, Agilent Technologies Inc., Santa Clara, CA, USA).
The Raman spectra were recorded on a confocal Raman spectrometer (LabRAM HR, Horiba J.Y., Paris, France) equipped with a visible 633 nm Ar+ laser and 325 nm Ar+ laser and high-grade Leica microscopes (long working distance objective 50× and 20×, respectively). The Raman peak of single-crystal silicon at 520.7 cm−1 was used for calibration. For all samples, the test conditions were set to 200 μm confocal pore size, 20 s exposure time, l mW irradiation intensity, and attenuated by a factor of 10 to avoid damaging samples. The signal was collected at 180° (back scattering plane) by a CCD array detector (1024 × 256 pixels, 26 mm in size).
The bulk crystalline structures of all samples were determined by XRD (D8 Advance, Bruker, Karlsruhe, Germany) using a Cu Kα monochromatized radiation source (k = 1.5418 Å) at 40 kV and 40 mA. The scanning speed was 5° min−1 in a 2θ angle range of 5–90°.
The 57Fe Mössbauer spectroscopy (MES) was performed on an electromechanical spectrometer (Wissel 1550, Wissenschaftliche Elektronik GmbH, Starnberg, Germany) equipped with a 57Co/Pd irradiation source at room temperature. The velocity was calibrated by a 25 μm-thick α-Fe foil, and the isomer shift (IS) was referenced to α-Fe at room temperature.
The particle size and morphology were determined by a transmission electron microscope (JEOL JEM 2100F, JEOL, Akishima, Japan) (accelerating voltage = 200 kV). The average particle size was determined by measuring more than 200 nanoparticles.
Temperature-programmed desorption of C2H4, C3H6 (C2H4, C3H6-TPD) was conducted in a micro fixed-bed reactor connected to a mass spectrometer (HPR-20, Hiden Analytical, Britain). The catalyst was first treated at 250 °C for 2 h to remove the bound water from the sample under pure Ar. After lowering to room temperature, the surface oxide layer was removed by treatment with 10% CO at 350 °C for 2 h. Subsequently, the temperature was reduced to room temperature while the residual CO was purged by pure Ar, and then the adsorption species were switched in the reactor for 2 h. After that, a stable baseline was obtained on the MS by pure Ar purging. The catalysts were heated from room temperature to 800 °C at 10 °C/min. Mass of m/z = 26 (C2H4), 41 (C3H6) was monitored.
Thermogravimetric (TG) analysis was performed on a TG analyzer (Rigaku DTA8122, Rigaku, Tokyo, Japan) with a heating rate of 10 °C/min from room temperature to 800 °C in air condition.
In situ DRIFT spectra were recorded on a Frontier spectrometer (Spectrum 100, PerkinElmer, Waltham, MA, USA) equipped with a Harrick Praying Mantis diffuse reflection cell and a mercury cadmium telluride (MCT) detector cooled by liquid nitrogen. The catalyst sample was pretreated using different reductive gases at 350 °C and 1 bar for 5 h. Then, the temperature was lowered to 330 °C and the pressure was set to 1.5 MPa under Ar flow and a background spectrum was recorded. The pretreated sample was next exposed to a reactant gas mixture containing 20 vol. % CO2, 60 vol. % H2, and 20 vol. % Ar with a total gas flow of 25 mL/min for 30 min. The DRIFT spectra were collected at 4 cm−1 resolution and 64 scans.