Correlation between Key Steps and Hydricity in CO2 Hydrogenation Catalysed by Non-Noble Metal PNP-Pincer Complexes
Abstract
1. Introduction
2. Computational Details
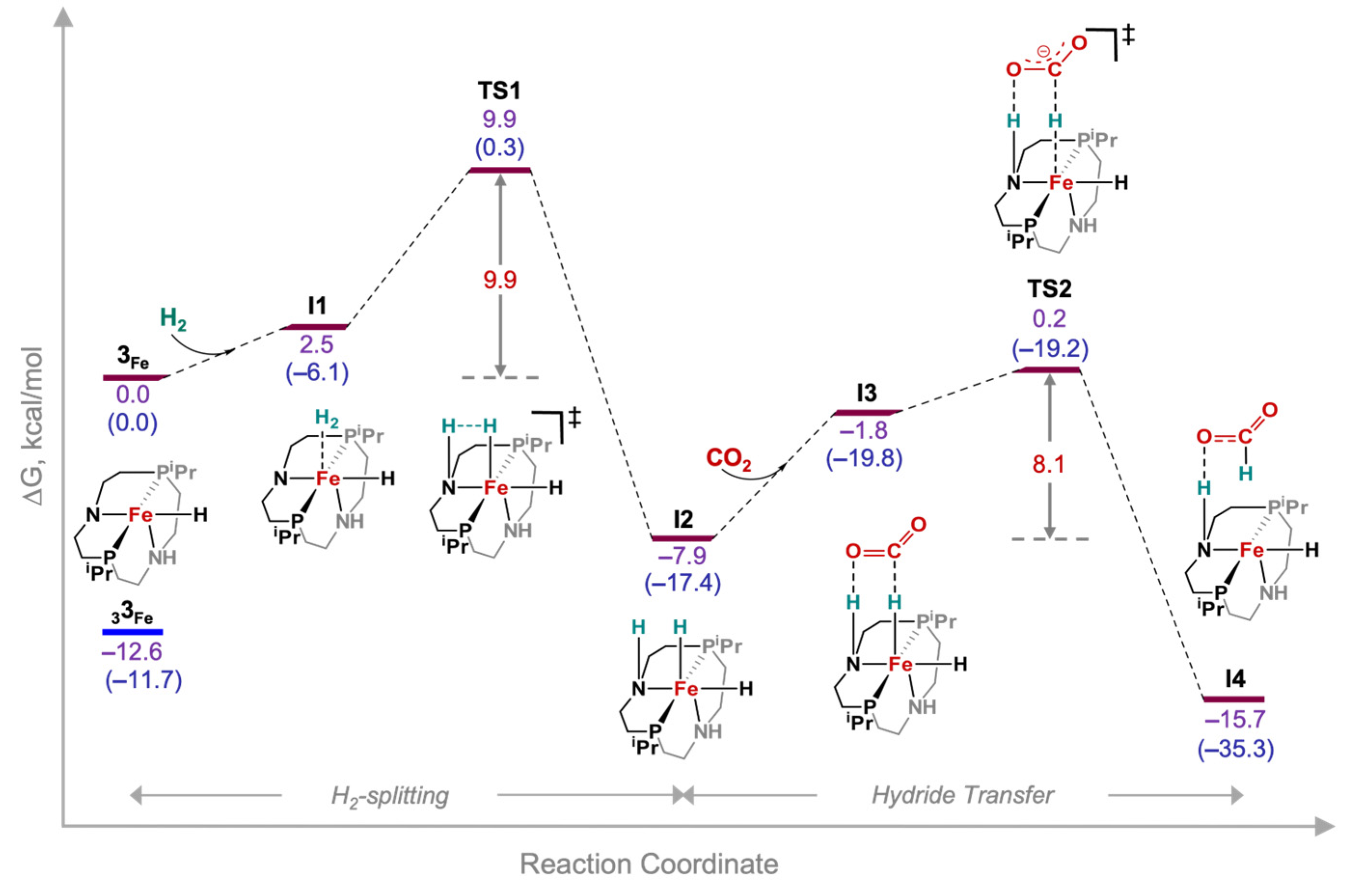
3. Results and Discussion
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solomon, S.; Plattner, G.-K.; Knutti, R.; Friedlingstein, P. Irreversible Climate Change Due to Carbon Dioxide Emissions. Proc. Natl. Acad. Sci. USA 2009, 106, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.J.; Caldeira, K.; Matthews, H.D. Future CO2 Emissions and Climate Change from Existing Energy Infrastructure. Science 2010, 329, 1330–1333. [Google Scholar] [CrossRef]
- Bach, W. Impact of Increasing Atmospheric CO2 Concentrations on Global Climate: Potential Consequences and Corrective Measures. Environ. Int. 1979, 2, 215–228. [Google Scholar] [CrossRef]
- Lüthi, D.; Le Floch, M.; Bereiter, B.; Blunier, T.; Barnola, J.M.; Siegenthaler, U.; Raynaud, D.; Jouzel, J.; Fischer, H.; Kawamura, K.; et al. High-Resolution Carbon Dioxide Concentration Record 650,000–800,000 Years before Present. Nature 2008, 453, 379–382. [Google Scholar] [CrossRef]
- Penuelas, J.; Fernández-Martínez, M.; Vallicrosa, H.; Maspons, J.; Zuccarini, P.; Carnicer, J.; Sanders, T.G.M.; Krüger, I.; Obersteiner, M.; Janssens, I.A.; et al. Increasing Atmospheric CO2 Concentrations Correlate with Declining Nutritional Status of European Forests. Commun. Biol. 2020, 3, 1–11. [Google Scholar] [CrossRef]
- Dincer, I. Environmental Impacts of Energy. Energy Policy 1999, 27, 845–854. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Angelini, A. Catalysis for the Valorization of Exhaust Carbon: From CO2 to Chemicals, Materials, and Fuels. Technological Use of CO2. Chem. Rev. 2014, 114, 1709–1742. [Google Scholar] [CrossRef]
- Xiaoding, X.; Moulijn, J.A. Mitigation of CO2 by Chemical Conversion: Plausible Chemical Reactions and Promising Products. Energy Fuels 1996, 10, 305–325. [Google Scholar] [CrossRef]
- Appel, A.M.; Bercaw, J.E.; Bocarsly, A.B.; Dobbek, H.; Dubois, D.L.; Dupuis, M.; Ferry, J.G.; Fujita, E.; Hille, R.; Kenis, P.J.A.; et al. Frontiers, Opportunities, and Challenges in Biochemical and Chemical Catalysis of CO2 Fixation. Chem. Rev. 2013, 113, 6621–6658. [Google Scholar] [CrossRef] [PubMed]
- Darensbourg, D.J. Chemistry of Carbon Dioxide Relevant to Its Utilization: A Personal Perspective. Inorg. Chem. 2010, 49, 10765–10780. [Google Scholar] [CrossRef]
- Mondal, B.; Song, J.; Neese, F.; Ye, S. Bio-Inspired Mechanistic Insights into CO2 Reduction. Curr. Opin. Chem. Biol. 2015, 25, 103–109. [Google Scholar] [CrossRef]
- Grice, K.A. Carbon Dioxide Reduction with Homogenous Early Transition Metal Complexes: Opportunities and Challenges for Developing CO2 Catalysis. Coord. Chem. Rev. 2017, 336, 78–95. [Google Scholar] [CrossRef]
- Cokoja, M.; Bruckmeier, C.; Rieger, B.; Herrmann, W.A.; Kühn, F.E. Transformation of Carbon Dioxide with Homogeneous Transition-Metal Catalysts: A Molecular Solution to a Global Challenge? Angew. Chem. Int. Ed. 2011, 50, 8510–8537. [Google Scholar] [CrossRef]
- Sakakura, T.; Choi, J.C.; Yasuda, H. Transformation of Carbon Dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef]
- Riduan, S.N.; Zhang, Y. Recent Developments in Carbon Dioxide Utilization under Mild Conditions. Dalton Trans. 2010, 39, 3347–3357. [Google Scholar] [CrossRef]
- Benson, E.E.; Kubiak, C.P.; Sathrum, A.J.; Smieja, J.M. Electrocatalytic and Homogeneous Approaches to Conversion of CO2 to Liquid Fuels. Chem. Soc. Rev. 2009, 38, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent Advances in Catalytic Hydrogenation of Carbon Dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Kothandaraman, J.; Goeppert, A.; Prakash, G.K.S. Advances in Catalytic Homogeneous Hydrogenation of Carbon Dioxide to Methanol. J. CO2 Util. 2018, 23, 212–218. [Google Scholar] [CrossRef]
- Sordakis, K.; Tang, C.; Vogt, L.K.; Junge, H.; Dyson, P.J.; Beller, M.; Laurenczy, G. Homogeneous Catalysis for Sustainable Hydrogen Storage in Formic Acid and Alcohols. Chem. Rev. 2018, 118, 372–433. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Sun, C.; Shi, Z. Transition-Metal-Catalyzed C–C Bond Formation through the Fixation of Carbon Dioxide. Chem. Soc. Rev. 2011, 40, 2435–2452. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, L.; Jackstell, R.; Beller, M. Using Carbon Dioxide as a Building Block in Organic Synthesis. Nat. Commun. 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Federsel, C.; Jackstell, R.; Beller, M. State-of-the-Art Catalysts for Hydrogenation of Carbon Dioxide. Angew. Chem. Int. Ed. 2010, 49, 6254–6257. [Google Scholar] [CrossRef] [PubMed]
- Jessop, P.G.; Ikariya, T.; Noyori, R. Homogeneous Hydrogenation of Carbon Dioxide. Chem. Rev. 1995, 95, 259–272. [Google Scholar] [CrossRef]
- Jessop, P.G.; Joó, F.; Tai, C.-C. Recent Advances in the Homogeneous Hydrogenation of Carbon Dioxide. Coord. Chem. Rev. 2004, 248, 2425–2442. [Google Scholar] [CrossRef]
- Rohmann, K.; Kothe, J.; Haenel, M.W.; Englert, U.; Hölscher, M.; Leitner, W. Hydrogenation of CO 2 to Formic Acid with a Highly Active Ruthenium Acriphos Complex in DMSO and DMSO/Water. Angew. Chem. Int. Ed. 2016, 55, 8966–8969. [Google Scholar] [CrossRef] [PubMed]
- Klankermayer, J.; Wesselbaum, S.; Beydoun, K.; Leitner, W. Selective Catalytic Synthesis Using the Combination of Carbon Dioxide and Hydrogen: Catalytic Chess at the Interface of Energy and Chemistry. Angew. Chem. Int. Ed. 2016, 55, 7296–7343. [Google Scholar] [CrossRef]
- Inoue, Y.; Izumida, H.; Sasaki, Y.; Hashimoto, H. Catalytic Fixation of Carbon Dioxide to Formic Acid by Transition-Metal Complexes Under Mild Conditions. Chem. Lett. 1976, 5, 863–864. [Google Scholar] [CrossRef]
- Tanaka, R.; Yamashita, M.; Nozaki, K. Catalytic Hydrogenation of Carbon Dioxide Using Ir(III)-Pincer Complexes. J. Am. Chem. Soc. 2009, 131, 14168–14169. [Google Scholar] [CrossRef] [PubMed]
- Jeletic, M.S.; Mock, M.T.; Appel, A.M.; Linehan, J.C. A Cobalt-Based Catalyst for the Hydrogenation of CO2 under Ambient Conditions. J. Am. Chem. Soc. 2013, 135, 11533–11536. [Google Scholar] [CrossRef] [PubMed]
- Roy, L.; Mondal, B.; Ye, S. Computational Mechanistic Insights into Non-Noble-Metal-Catalysed CO2 conversion. Dalt. Trans. 2020, 49, 16608–16616. [Google Scholar] [CrossRef] [PubMed]
- Ahlquist, M.S.G. Iridium Catalyzed Hydrogenation of CO2 under Basic Conditions—Mechanistic Insight from Theory. J. Mol. Catal. A Chem. 2010, 324, 3–8. [Google Scholar] [CrossRef]
- Yang, X. Hydrogenation of Carbon Dioxide Catalyzed by PNP Pincer Iridium, Iron, and Cobalt Complexes: A Computational Design of Base Metal Catalysts. ACS Catal. 2011, 1, 849–854. [Google Scholar] [CrossRef]
- Hou, C.; Jiang, J.; Zhang, S.; Wang, G.; Zhang, Z.; Ke, Z.; Zhao, C. Hydrogenation of Carbon Dioxide Using Half-Sandwich Cobalt, Rhodium, and Iridium Complexes: DFT Study on the Mechanism and Metal Effect. ACS Catal. 2014, 4, 2990–2997. [Google Scholar] [CrossRef]
- Jeletic, M.S.; Helm, M.L.; Hulley, E.B.; Mock, M.T.; Appel, A.M.; Linehan, J.C. A Cobalt Hydride Catalyst for the Hydrogenation of CO2: Pathways for Catalysis and Deactivation. ACS Catal. 2014, 4, 3755–3762. [Google Scholar] [CrossRef]
- Ogo, S.; Kabe, R.; Hayashi, H.; Harada, R.; Fukuzumi, S. Mechanistic Investigation of CO2 Hydrogenation by Ru(II) and Ir(III) Aqua Complexes under Acidic Conditions: Two Catalytic Systems Differing in the Nature of the Rate Determining Step. Dalt. Trans. 2006, 39, 4657–4663. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B.; Neese, F.; Ye, S. Control in the Rate-Determining Step Provides a Promising Strategy to Develop New Catalysts for CO2 Hydrogenation: A Local Pair Natural Orbital Coupled Cluster Theory Study. Inorg. Chem. 2015, 54, 7192–7198. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B.; Neese, F.; Ye, S. Toward Rational Design of 3d Transition Metal Catalysts for CO2 Hydrogenation Based on Insights into Hydricity-Controlled Rate-Determining Steps. Inorg. Chem. 2016, 55, 5438–5444. [Google Scholar] [CrossRef] [PubMed]
- Erken, C.; Kaithal, A.; Sen, S.; Weyhermüller, T.; Hölscher, M.; Werlé, C.; Leitner, W. Manganese-Catalyzed Hydroboration of Carbon Dioxide and Other Challenging Carbonyl Groups. Nat. Commun. 2018, 9, 4521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pu, M.; Lei, M. Hydrogenation of CO2 to Methanol Catalyzed by a Manganese Pincer Complex: Insights into the Mechanism and Solvent Effect. Dalt. Trans. 2021, 50, 7348–7355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; MacIntosh, A.D.; Wong, J.L.; Bielinski, E.A.; Williard, P.G.; Mercado, B.Q.; Hazari, N.; Bernskoetter, W.H. Iron Catalyzed CO2 Hydrogenation to Formate Enhanced by Lewis Acid Co-Catalysts. Chem. Sci. 2015, 6, 4291–4299. [Google Scholar] [CrossRef] [PubMed]
- Lane, E.M.; Zhang, Y.; Hazari, N.; Bernskoetter, W.H. Sequential Hydrogenation of CO2 to Methanol Using a Pincer Iron Catalyst. Organometallics 2019, 38, 3084–3091. [Google Scholar] [CrossRef]
- Rong, H.; Zhang, Y.; Ai, X.; Li, W.; Cao, F.; Li, L. Theoretical Study on the Hydrogenolysis of Polyurethanes to Improve the Catalytic Activities. Inorg. Chem. 2022, 61, 14662–14672. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Muckerman, J.T.; Achord, P.; Creutz, C.; Polyansky, D.E.; Fujita, E. Calculation of Thermodynamic Hydricities and the Design of Hydride Donors for CO2 Reduction. Proc. Natl. Acad. Sci. USA 2012, 109, 15657–15662. [Google Scholar] [CrossRef]
- Zhang, X.M.; Bruno, J.W.; Enyinnaya, E. Hydride Affinities of Arylcarbenium Ions and Iminium Ions in Dimethyl Sulfoxide and Acetonitrile. J. Org. Chem. 1998, 63, 4671–4678. [Google Scholar] [CrossRef]
- Ellis, W.W.; Raebiger, J.W.; Curtis, C.J.; Bruno, J.W.; DuBois, D.L. Hydricities of BzNADH, C5H5Mo(PMe3)(CO)2H, and C5Me5Mo(PMe3)(CO)2H in Acetonitrile. J. Am. Chem. Soc. 2004, 126, 2738–2743. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.J.; Miedaner, A.; Ellis, W.W.; DuBois, D.L. Measurement of the Hydride Donor Abilities of [HM(Diphosphine)2]+ Complexes (M = Ni, Pt) by Heterolytic Activation of Hydrogen. J. Am. Chem. Soc. 2002, 124, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Fong, H.; Peters, J.C. Hydricity of an Fe-H Species and Catalytic CO2 Hydrogenation. Inorg. Chem. 2015, 54, 5124–5135. [Google Scholar] [CrossRef]
- Fang, H.; Jing, H.; Ge, H.; Brothers, P.J.; Fu, X.; Ye, S. The Mechanism of E-H (E = N, O) Bond Activation by a Germanium Corrole Complex: A Combined Experimental and Computational Study. J. Am. Chem. Soc. 2015, 137, 7122–7127. [Google Scholar] [CrossRef] [PubMed]
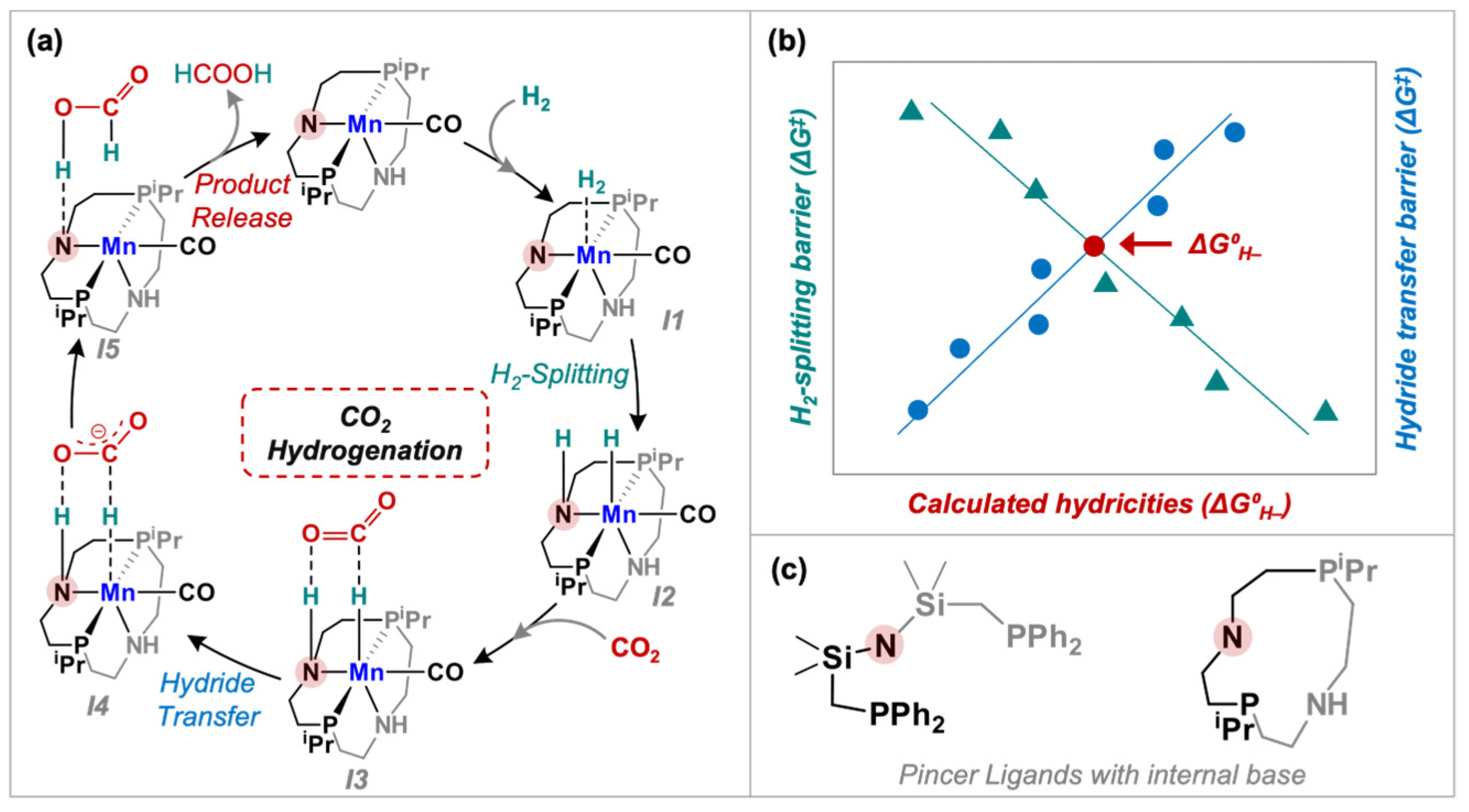
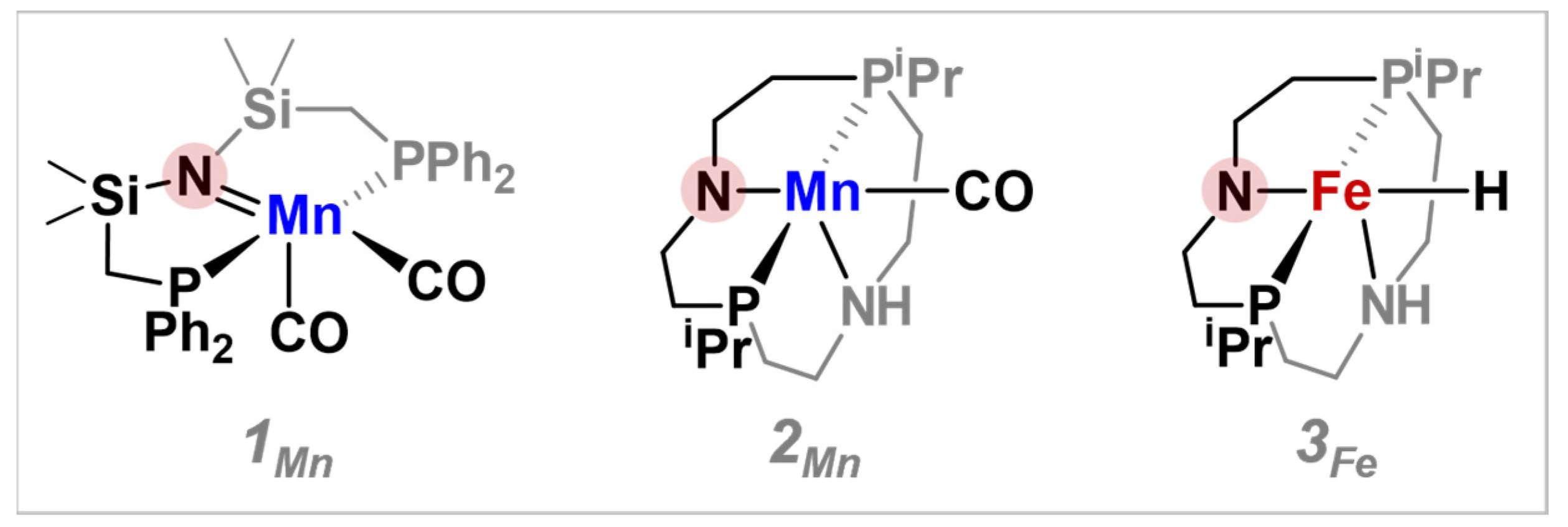
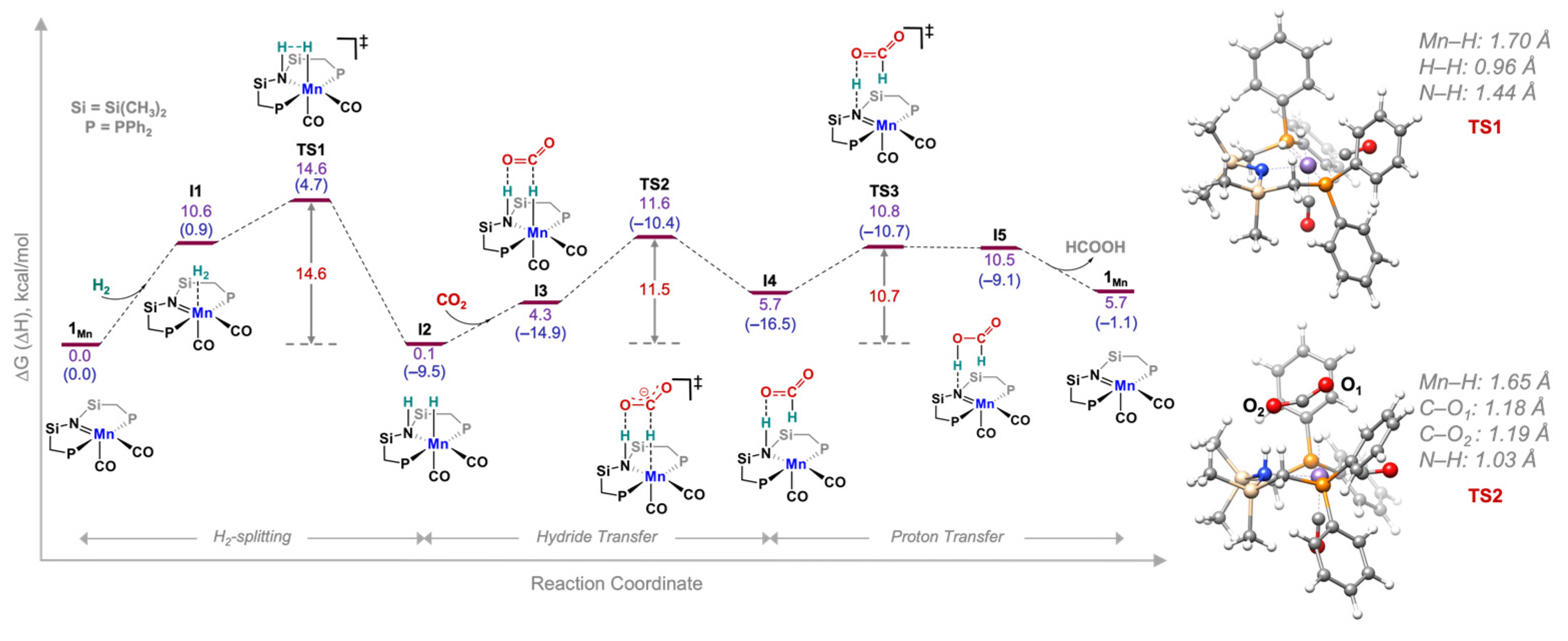
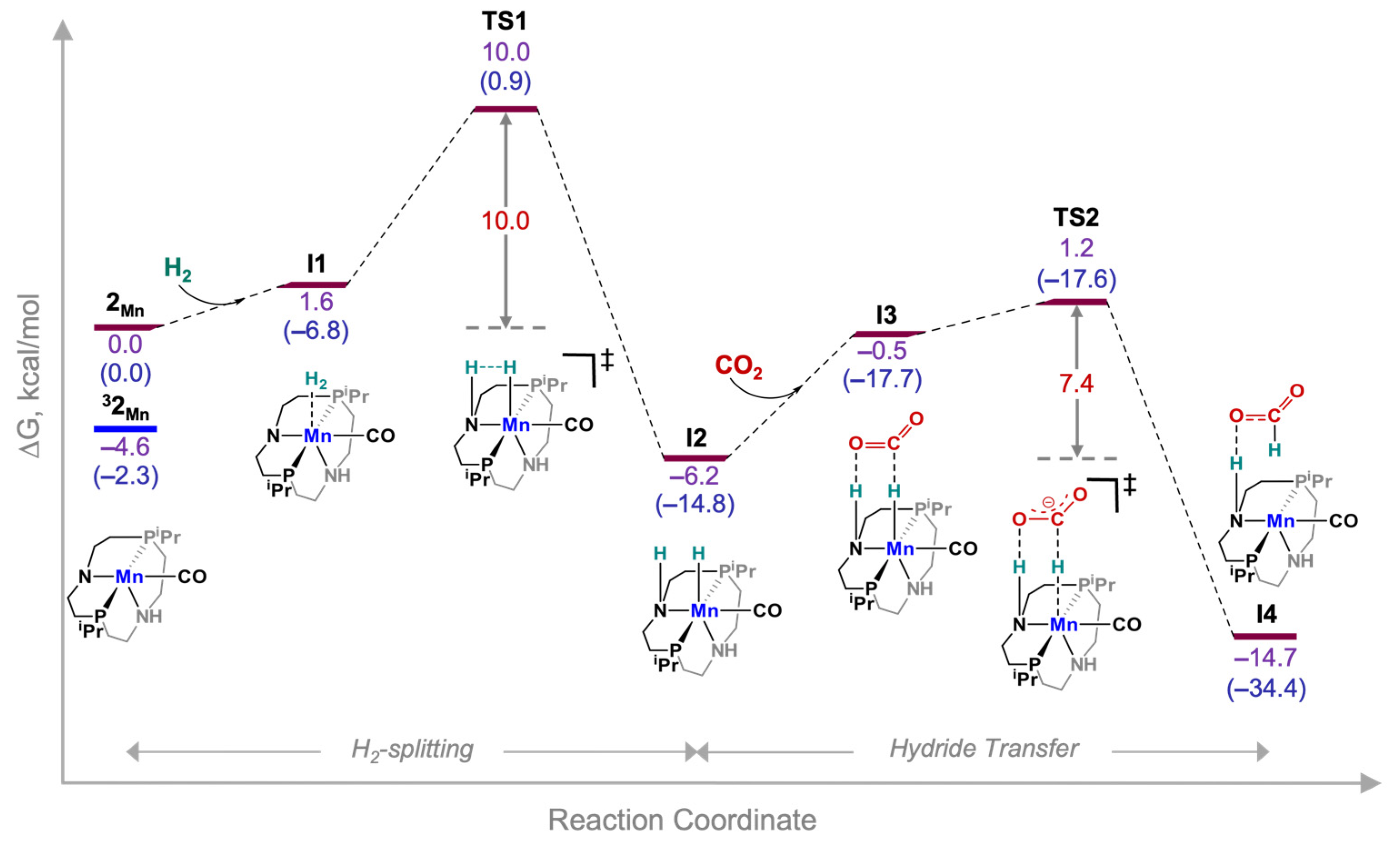

| Species | Calculated Hydricity | Intrinsic H2-Splitting Barrier (ΔG‡, kcal/mol) | Intrinsic Hydride Transfer Barrier (ΔG‡, kcal/mol) |
|---|---|---|---|
| 1Mn | 55.2 | 4.0 | 7.3 |
| 2Mn | 47.1 | 8.4 | 1.7 |
| 3Fe | 48.7 | 7.4 | 2.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moni, S.; Mondal, B. Correlation between Key Steps and Hydricity in CO2 Hydrogenation Catalysed by Non-Noble Metal PNP-Pincer Complexes. Catalysts 2023, 13, 592. https://doi.org/10.3390/catal13030592
Moni S, Mondal B. Correlation between Key Steps and Hydricity in CO2 Hydrogenation Catalysed by Non-Noble Metal PNP-Pincer Complexes. Catalysts. 2023; 13(3):592. https://doi.org/10.3390/catal13030592
Chicago/Turabian StyleMoni, Snehasis, and Bhaskar Mondal. 2023. "Correlation between Key Steps and Hydricity in CO2 Hydrogenation Catalysed by Non-Noble Metal PNP-Pincer Complexes" Catalysts 13, no. 3: 592. https://doi.org/10.3390/catal13030592
APA StyleMoni, S., & Mondal, B. (2023). Correlation between Key Steps and Hydricity in CO2 Hydrogenation Catalysed by Non-Noble Metal PNP-Pincer Complexes. Catalysts, 13(3), 592. https://doi.org/10.3390/catal13030592






