Performance of Eversa Transform 2.0 Lipase in Ester Production Using Babassu Oil (Orbignya sp.) and Tucuman Oil (Astrocaryum vulgar): A Comparative Study between Liquid and Immobilized Forms in Fe3O4 Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
2.1. Densities of Hydrolyzed Oils
2.2. Saponification Index (Is)
2.3. Esterification Reaction
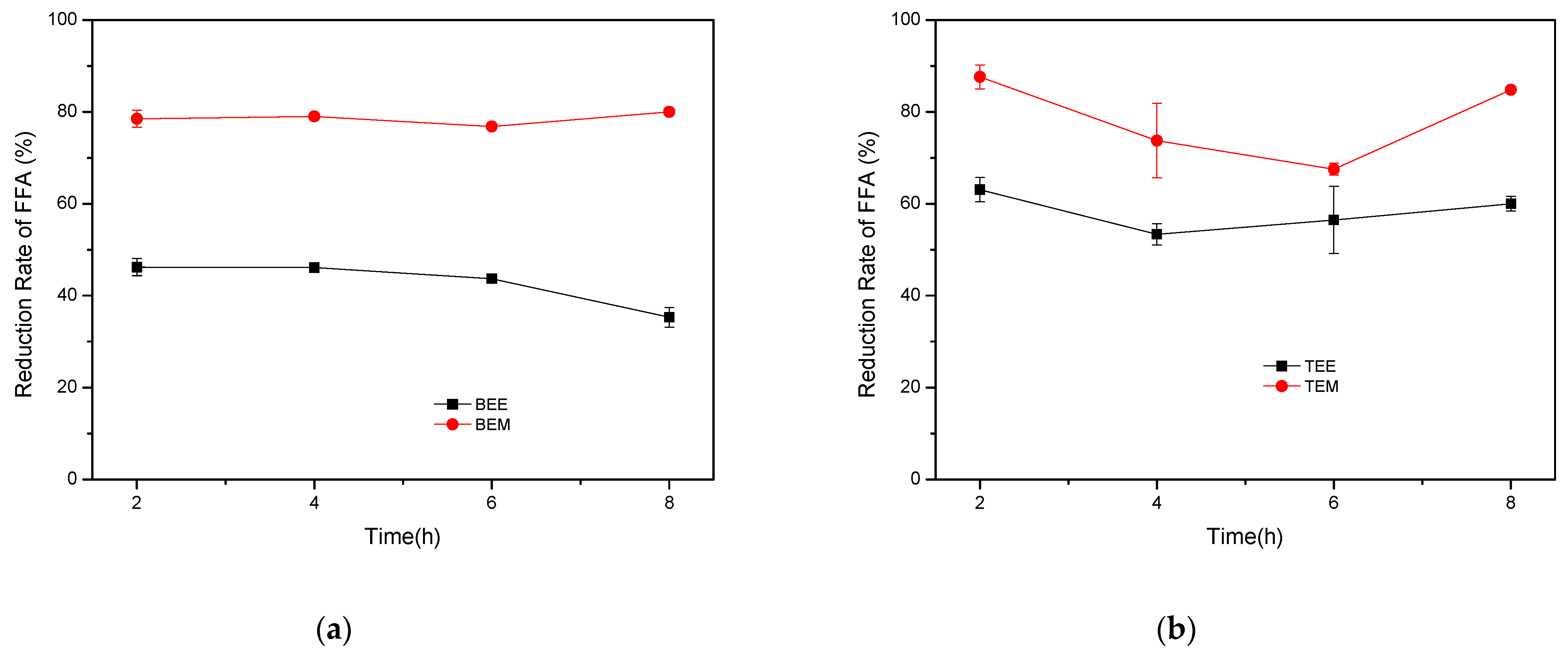
2.3.1. Homogeneous Catalysts
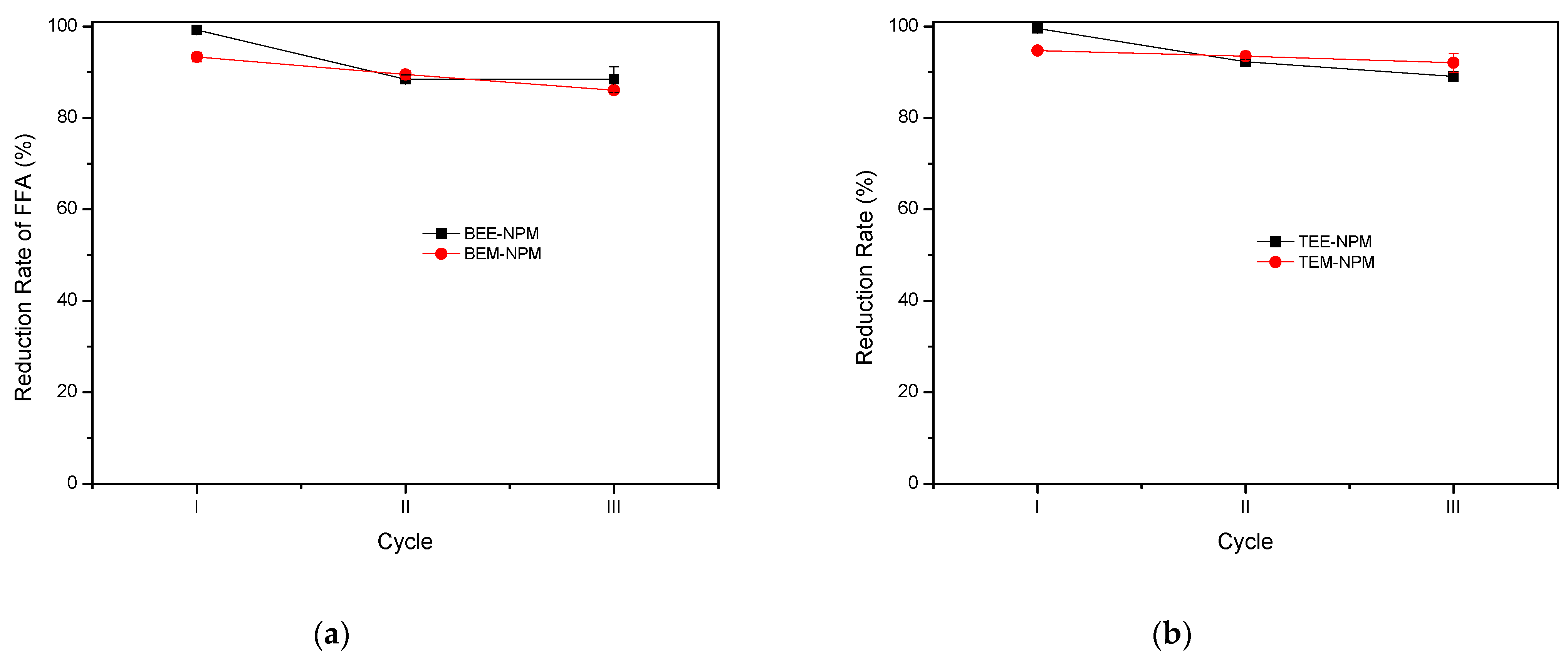
2.3.2. Heterogeneous Catalysts
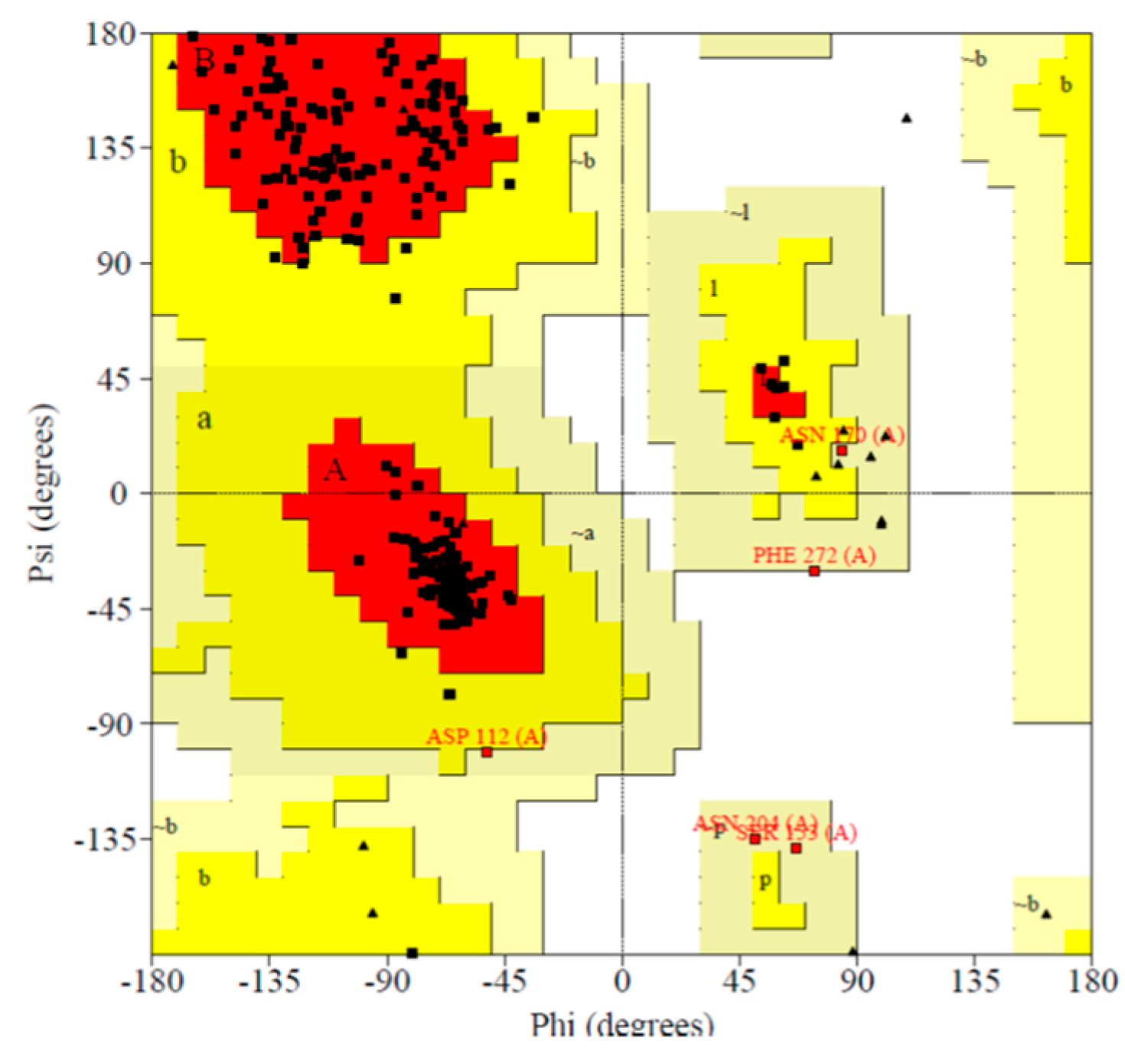
2.4. Protein Modeling
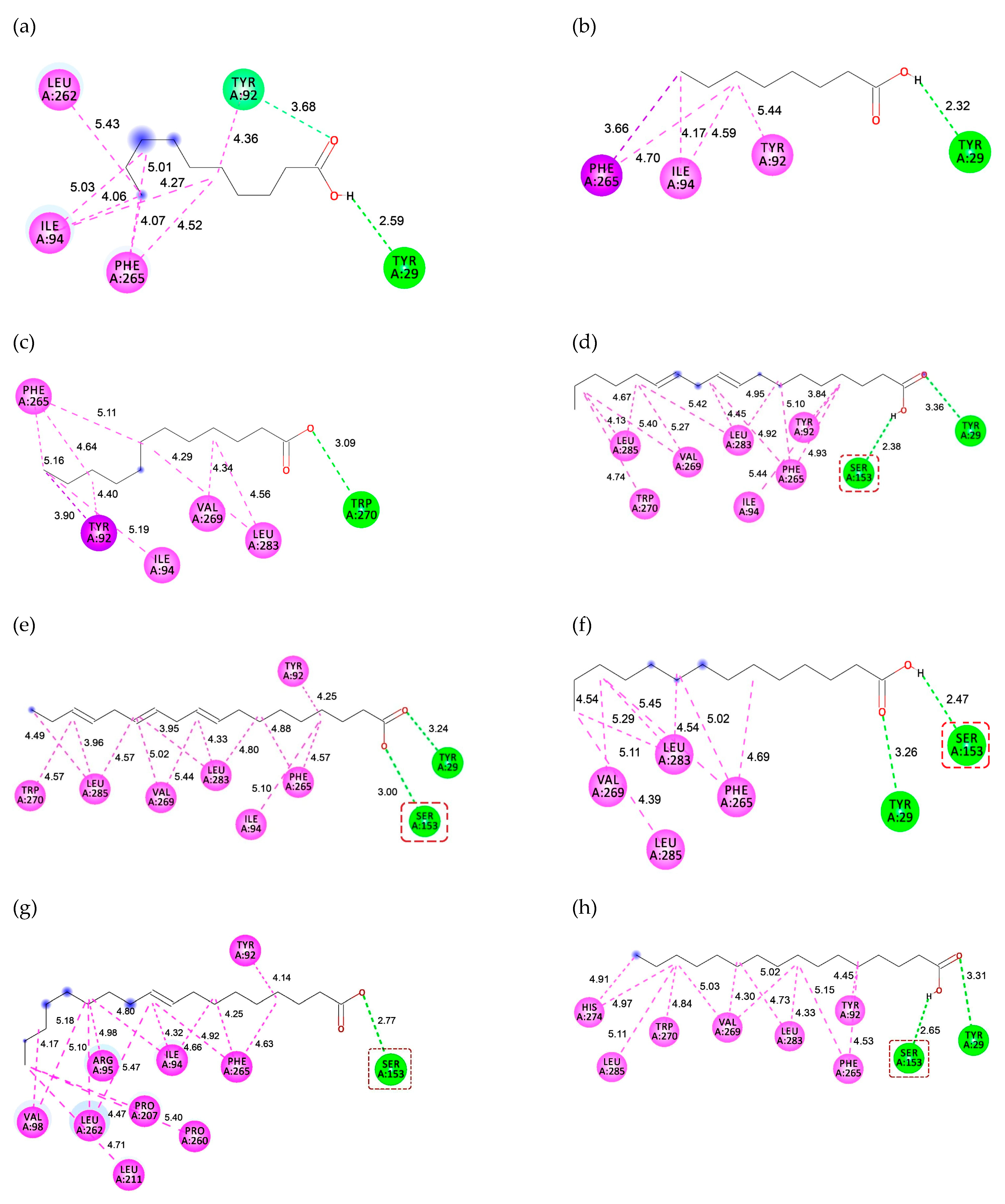
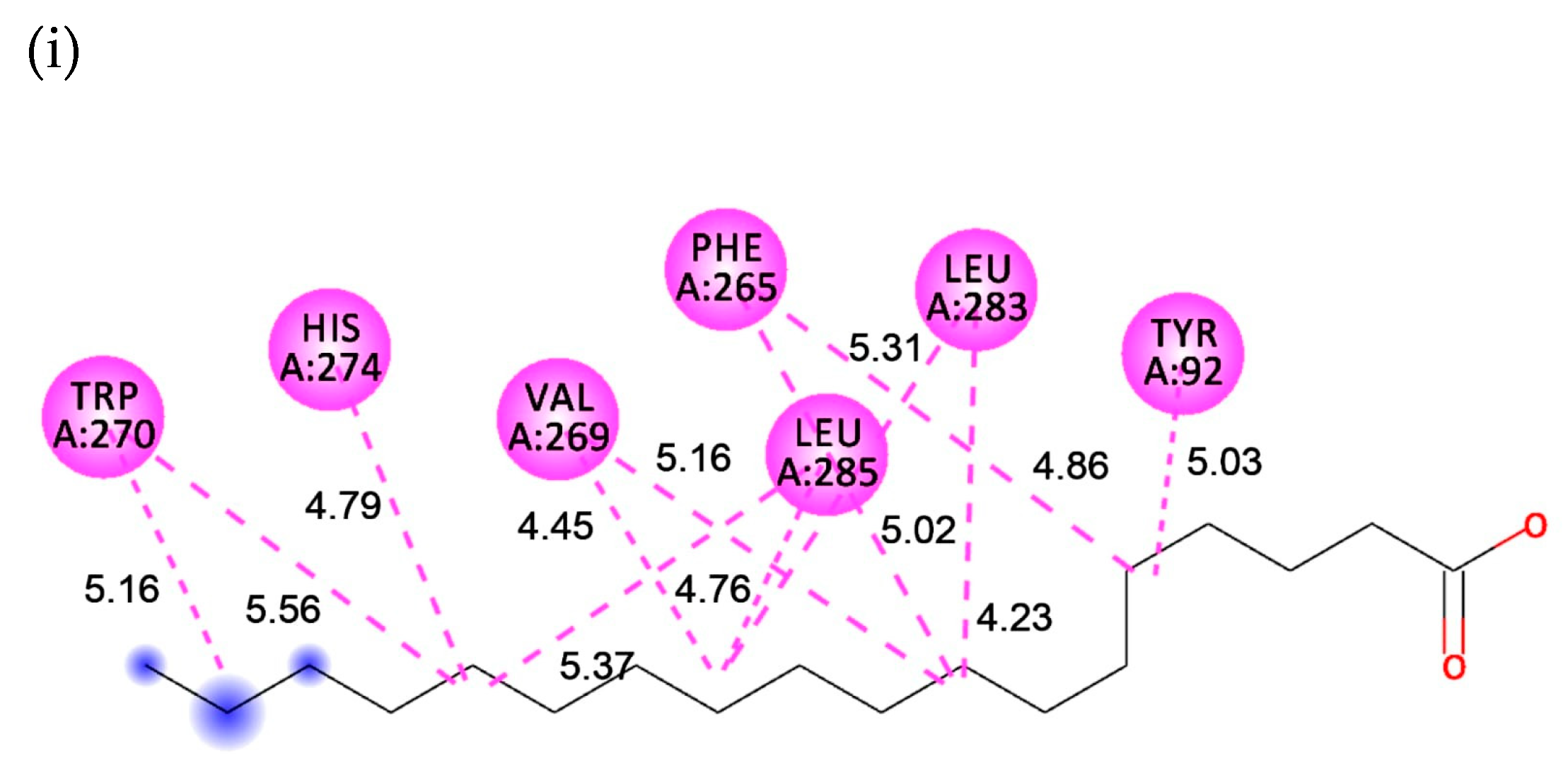
2.5. Interaction between Substrate and Lipase
3. Materials and Methods
3.1. Materials
3.1.1. Babassu (Attallea spp.)
3.1.2. Tucuman (Astrocaryum spp.)
3.1.3. Origin of Materials
3.2. Methods
3.2.1. Preparation of Magnetite Nanoparticles
3.2.2. Treatment of g-Aminopropiltriethoxysilane (APTES) Support
3.2.3. Cross-Linking with Glutaraldehyde Solution (GLU)
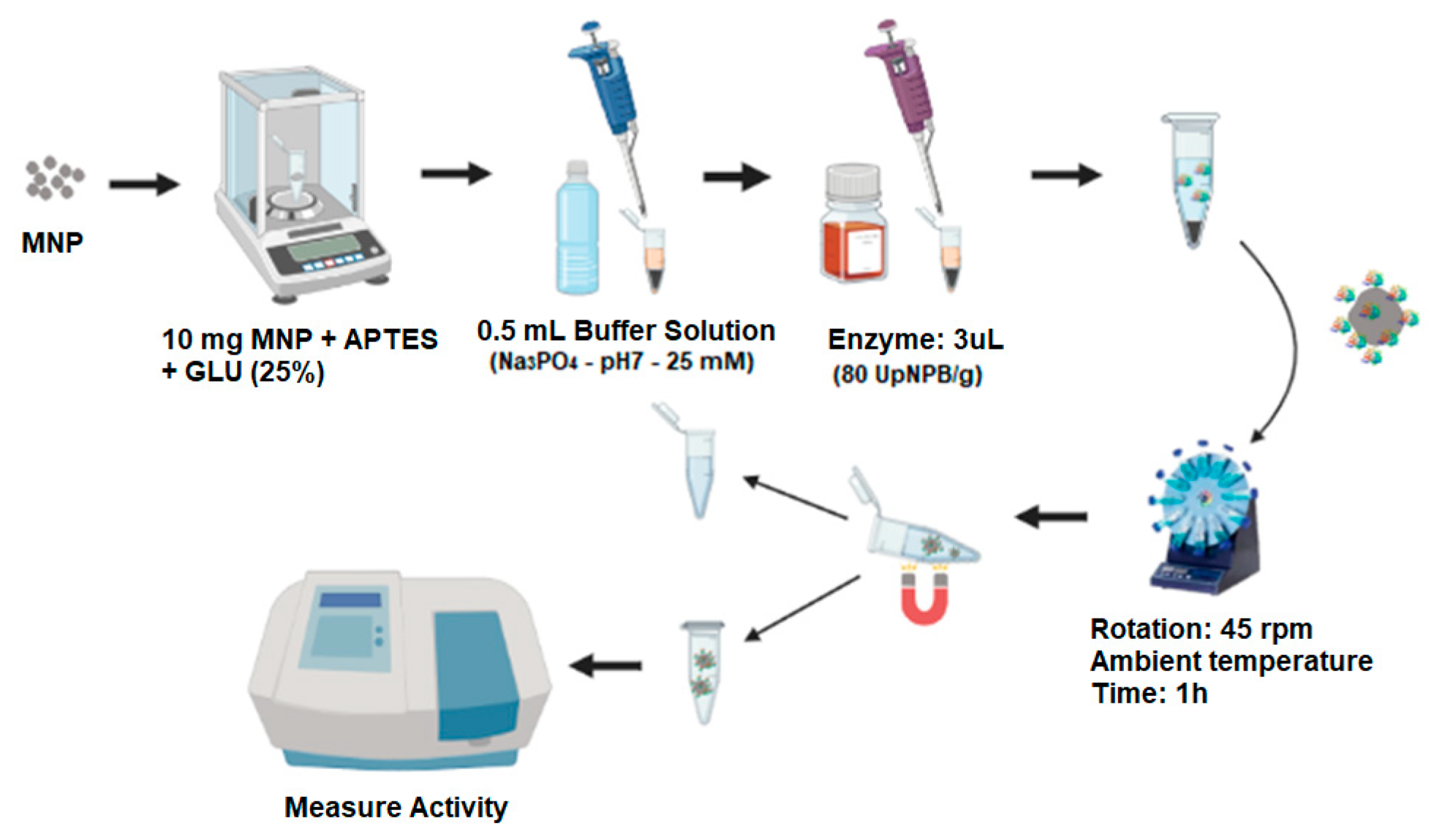
3.2.4. Immobilization of Enzymes
3.2.5. Determination of Enzyme Activity
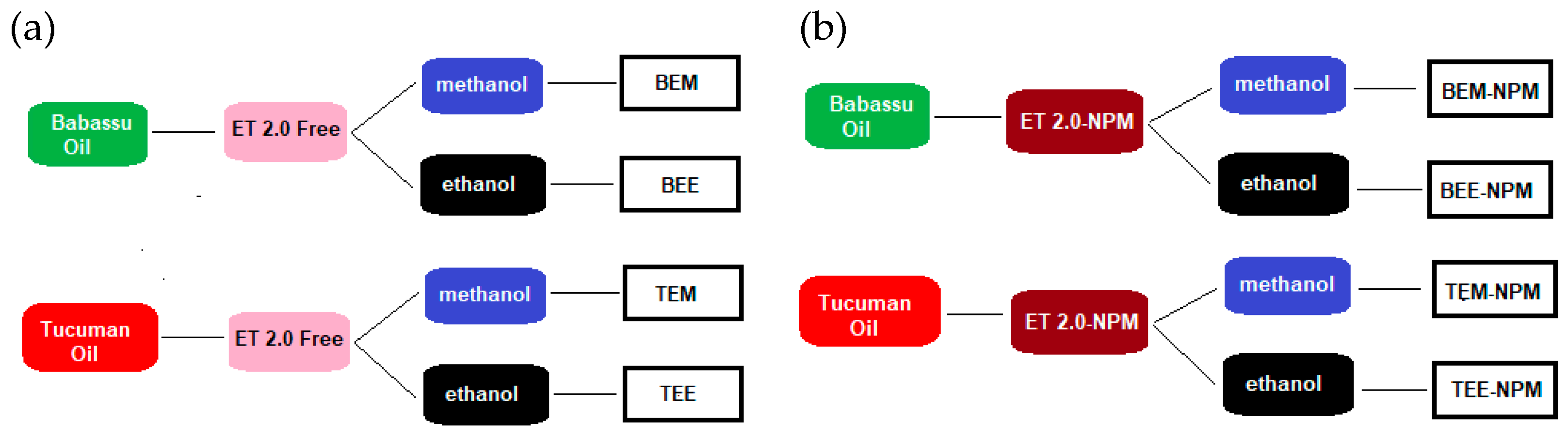
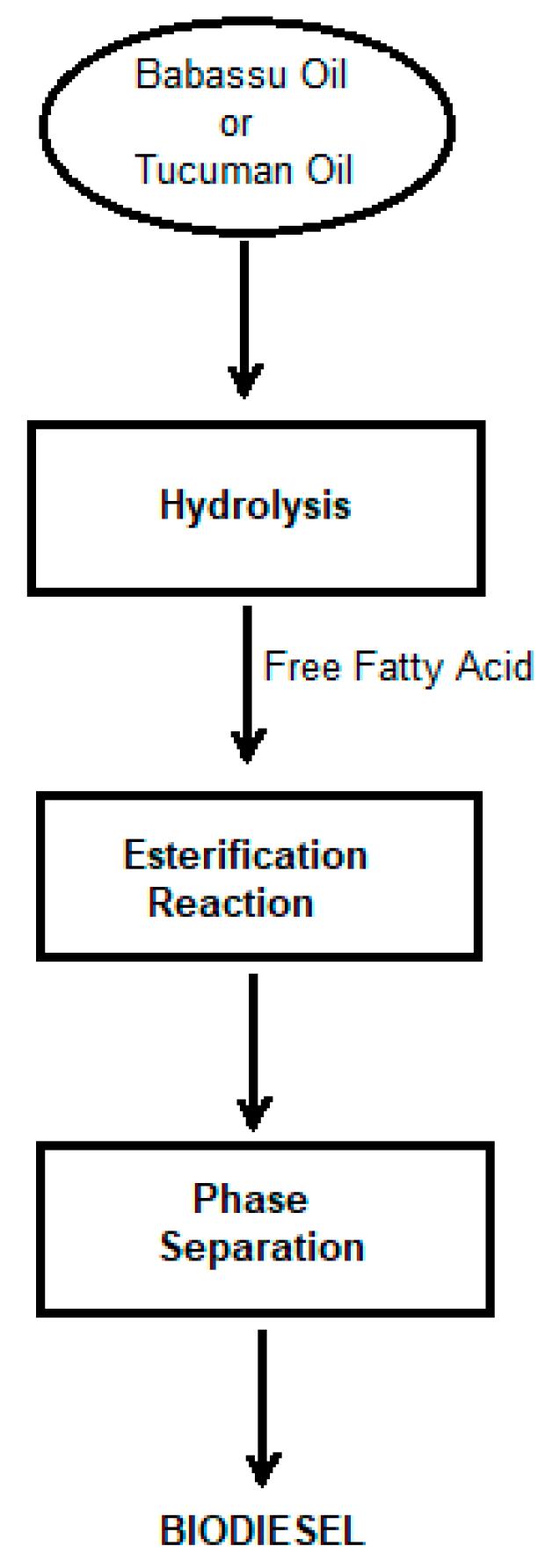
3.2.6. Enzymatic Esterification
Homogeneous
Heterogeneous
3.2.7. Hydrolysis of Vegetable Oils
3.2.8. Characterization of Hydrolyzed Vegetable Oils
Determination of Acidity Index (AI)
Determination of Free Fatty Acid (FFA) Content
Determination of Moisture Content
Saponification Index (Is) Determination
Density Determination
3.2.9. Ester Content Formed
3.3. Homology Modeling
3.3.1. Identification and Selection of Protein-Fold
3.3.2. Alignment of Target and Mold Sequences
3.3.3. Model Construction and Optimization
3.3.4. Protein Validation
3.4. Protein Preparation
3.5. Obtaining the Ligand
3.6. Molecular Docking and Visualization of Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Cerqueira Leite, R.C.; Leal, M.R.L.V. O biocombustível no Brasil. Novos Estud. CEBRAP 2007, 78, 15–21. [Google Scholar] [CrossRef]
- Duarte, V.H.; Valentini, M.H.K.; Santos, G.B.; Nadaletti, W.C.; Vieira, B. Biocombustíveis: Uma revisão sobre o panorama histórico, produção e aplicações do biodiesel. Open J. Syst. Meio Ambiente 2022, 68, 50–68. [Google Scholar]
- Torroba, A. Atlas dos Biocombustíveis Líquidos; Instituto Interamericano de Cooperação para a Agricultura (IICA): São José, Costa Rica, 2021. [Google Scholar]
- Biodieselbr. Brasil Produziu 6,76 bi de Litros de Biodiesel em 2021. 2022. Available online: https://www.biodieselbr.com/noticias/usinas/producao/brasil-produziu-6-76-bi-de-litros-de-biodiesel-em-2021-010222 (accessed on 1 March 2023).
- Biodieselbr. Matéria-Prima Para Biodiesel. 2014. Available online: https://www.biodieselbr.com/plantas/oleaginosas/index (accessed on 27 February 2023).
- Ubrabio. PNPB-Programa Nacional de Produção e Uso do Biodiesel-Ubrabio. 2023. Available online: https://ubrabio.com.br/pnpb/ (accessed on 27 February 2023).
- de Oliveira, V.F.; Parente, E.J.; Manrique-Rueda, E.D.; Cavalcante, C.L.; Luna, F.M.T. Fatty acid alkyl esters obtained from babassu oil using C1–C8 alcohols and process integration into a typical biodiesel plant. Chem. Eng. Res. Des. 2020, 160, 224–232. [Google Scholar] [CrossRef]
- Chang, M.Y.; Chan, E.-S.; Song, C.P. Biodiesel production catalysed by low-cost liquid enzyme Eversa® Transform 2.0: Effect of free fatty acid content on lipase methanol tolerance and kinetic model. Fuel 2020, 283, 119266. [Google Scholar] [CrossRef]
- de Sousa, I.G.; Chaves, A.V.; de Oliveira, A.L.B.; Moreira, K.d.S.; Junior, P.G.d.S.; Neto, F.S.; de Carvalho, S.C.F.; Valério, R.B.R.; Lima, G.V.; Lopes, A.A.S.; et al. A novel hybrid biocatalyst from immobilized Eversa® Transform 2.0 lipase and its application in biolubricant synthesis. Biocatal. Biotransformation 2022, 41, 1–22. [Google Scholar] [CrossRef]
- Wancura, J.H.C.; Rosset, D.V.; Ugalde, G.A.; Oliveira, J.V.; Mazutti, M.A.; Tres, M.V.; Jahn, S.L. Feeding strategies of methanol and lipase on eversa® transform-mediated hydroesterification for FAME production. Can. J. Chem. Eng. 2019, 97, 1332–1339. [Google Scholar] [CrossRef]
- Parente, E.J.d.S. Biodiesel: Uma Aventura Num País Engraçado; Tecbio: Fortaleza, Brazil, 2003. [Google Scholar]
- Mibielli, G.M.; Fagundes, A.P.; Bender, J.P.; Oliveira, J.V. Lab and pilot plant FAME production through enzyme-catalyzed reaction of low-cost feedstocks. Bioresour. Technol. Rep. 2019, 5, 150–156. [Google Scholar] [CrossRef]
- César, A.D.S.; Conejero, M.A.; Ribeiro, E.C.B.; Batalha, M.O. Competitiveness analysis of “social soybeans” in biodiesel production in Brazil. Renew. Energy 2019, 133, 1147–1157. [Google Scholar] [CrossRef]
- Lodi, A.L. Produção de Biodiesel Avança 9% em 2020, Mesmo Com Pandemia e Reduções Temporárias da Mistura Obrigatória -Mercados Agrícolas; StoneX: New York, NY, USA, 2021; Available online: https://www.mercadosagricolas.com.br/oleos-vegetais/producao-de-biodiesel-avanca-9-em-2020-mesmo-com-pandemia-e-reducoes-temporarias-da-mistura-obrigatoria/ (accessed on 7 August 2022).
- Sun, S.; Guo, J.; Chen, X. Biodiesel preparation from Semen Abutili (Abutilon theophrasti Medic.) seed oil using low-cost liquid lipase Eversa® transform 2.0 as a catalyst. Ind. Crop. Prod. 2021, 169, 113643. [Google Scholar] [CrossRef]
- Suarez, P.A.Z.; Santos, A.L.F.; Rodrigues, J.P.; Alves, M.B. Biocombustíveis a partir de óleos e gorduras: Desafios tecnológicos para viabilizá-los. Química Nova 2009, 32, 768–775. [Google Scholar] [CrossRef]
- Saraiva, A.F.d.S.; de Oliveira, N.M.; Filho, M.X.P.; Lopes, W.S. Cadeia produtiva do babaçu: Uma análise através das dimensões da abordagem da cadeia global de valor. In Cadenas Globales de Valor, 1st ed.; Senhoras, E.M., Bermúdez, L.E.R., Boa Vista, R.R., Eds.; Editora Ioles: Boa Vista, Brazil, 2022; pp. 291–314. [Google Scholar]
- Neto, P.R.C.; Rossi, L.F.S.; Zagonel, G.F.; Ramos, L.P. Produção de Biocombustível Alternativo ao Óleo Diesel Através da Transesterificação de Óleo de Soja Usado em Frituras. Quim. Nova 2000, 23, 531–537. [Google Scholar] [CrossRef]
- Machado, G.C.; Chaves, J.B.P.; Antoniassi, e.R. Physical and chimical characterization and fatty acid composition of babassu oil. Rev. Ceres 2006, 53, 463–470. [Google Scholar]
- Cavalcante, F.T.T.; Neto, F.S.; Falcão, I.R.D.A.; Souza, J.E.D.S.; Junior, L.S.D.M.; Sousa, P.D.S.; Rocha, T.G.; de Sousa, I.G.; Gomes, P.H.D.L.; de Souza, M.C.M.; et al. Opportunities for improving biodiesel production via lipase catalysis. Fuel 2021, 288, 119577. [Google Scholar] [CrossRef]
- Taher, H.; Al-Zuhair, S. The use of alternative solvents in enzymatic biodiesel production: A review. Biofuels Bioprod. Biorefining 2017, 11, 168–194. [Google Scholar] [CrossRef]
- Moreira, K.S.; Júnior, L.S.M.; Monteiro, R.R.C.; De Oliveira, A.L.B.; Valle, C.P.; Freire, T.M.; Fechine, P.B.A.; De Souza, M.C.M.; Fernandez-Lorente, G.; Guisan, J.M.; et al. Optimization of the Production of Enzymatic Biodiesel from Residual Babassu Oil (Orbignya sp.) via RSM. Catalysts 2020, 10, 414. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- Zhong, L.; Feng, Y.; Wang, G.; Wang, Z.; Bilal, M.; Lv, H.; Jia, S.; Cui, J. Production and use of immobilized lipases in/on nanomaterials: A review from the waste to biodiesel production. Int. J. Biol. Macromol. 2020, 152, 207–222. [Google Scholar] [CrossRef]
- Costantini, A.; Califano, V. Lipase Immobilization in Mesoporous Silica Nanoparticles for Biofuel Production. Catalysts 2021, 11, 629. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization–purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef]
- Aguieiras, E.C.G.; Ribeiro, D.S.; Couteiro, P.P.; Bastos, C.M.B.; de Queiroz, D.S.; Parreira, J.M.; Langone, M.A.P. Investigation of the Reuse of Immobilized Lipases in Biodiesel Synthesis: Influence of Different Solvents in Lipase Activity. Appl. Biochem. Biotechnol. 2016, 179, 485–496. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Vakhshiteh, F.; Barkhi, M.; Baharifar, H.; Poor-Akbar, E.; Zari, N.; Stamatis, H.; Bordbar, A.-K. Immobilization of cellulase enzyme onto magnetic nanoparticles: Applications and recent advances. Mol. Catal. 2017, 442, 66–73. [Google Scholar] [CrossRef]
- Suo, H.; Xu, L.; Xue, Y.; Qiu, X.; Huang, H.; Hu, Y. Ionic liquids-modified cellulose coated magnetic nanoparticles for enzyme immobilization: Improvement of catalytic performance. Carbohydr. Polym. 2020, 234, 115914. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.; Rathod, V.K. Magnetic-metal organic framework (magnetic-MOF): A novel platform for enzyme immobilization and nanozyme applications. Int. J. Biol. Macromol. 2018, 120, 2293–2302. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Jenkins, B.; Stroeve, P. Multi-objective optimization of transesterifi cation in biodiesel production catalyzed by immobilized lipase. Biofuels Bioprod. Biorefining 2016, 10, 804–818. [Google Scholar] [CrossRef]
- Xie, W.; Ma, N. Enzymatic transesterification of soybean oil by using immobilized lipase on magnetic nano-particles. Biomass Bioenergy 2010, 34, 890–896. [Google Scholar] [CrossRef]
- MAPA-Ministério da Agricultura. Sistema integrado de legislação. In MAPA-Ministério da Agricultura, Pecuária e Abatecimento; MAPA-Ministério da Agricultura: Madrid, Spain, 2006. Available online: https://sistemasweb.agricultura.gov.br/sislegis/action/detalhaAto.do?method=visualizarAtoPortalMapa&chave=643062246 (accessed on 15 July 2022).
- Grunwald, P. Industrial Biocatalysis, 1st ed.; Jenny Stanford Publishing: New York, NY, USA, 2014. [Google Scholar]
- Remonatto, D.; de Oliveira, J.V.; Guisan, J.M.; de Oliveira, D.; Ninow, J.; Fernandez-Lorente, G. Production of FAME and FAEE via Alcoholysis of Sunflower Oil by Eversa Lipases Immobilized on Hydrophobic Supports. Appl. Biochem. Biotechnol. 2018, 185, 705–716. [Google Scholar] [CrossRef]
- Facin, B.R.; Valério, A.; de Oliveira, D.; Oliveira, J.V. Developing an immobilized low-cost biocatalyst for FAME synthesis. Biocatal. Agric. Biotechnol. 2020, 29, 101752. [Google Scholar] [CrossRef]
- Meneses, C.H.S.G.; Rouws, A.J.L.S.; Vidal, M.S.; Baldani, J.I. Modelagem molecular de proteínas: O caso de uma glucoronosiltransferase (GumK) de Gluconacetobacter diazotrophicus PAL5. J. Biol. Pharm. Agric. Manag. 2014, 141, 10. [Google Scholar]
- Barbany, M.; Meyer, T.; Hospital, A.; Faustino, I.; D’Abramo, M.; Morata, J.; Orozco, M.; de la Cruz, X. Molecular Dynamics Study of Naturally Existing Cavity Couplings in Proteins. PLoS ONE 2015, 10, e0119978. [Google Scholar] [CrossRef]
- Chaturvedi, S.K.; Zaidi, N.; Alam, P.; Khan, J.M.; Qadeer, A.; Siddique, I.A.; Asmat, S.; Zaidi, Y.; Khan, R.H. Unraveling Comparative Anti-Amyloidogenic Behavior of Pyrazinamide and D-Cycloserine: A Mechanistic Biophysical Insight. PLoS ONE 2015, 10, e0136528. [Google Scholar] [CrossRef]
- Silvestrini, L.; Cianci, M. Principles of lipid–enzyme interactions in the limbus region of the catalytic site of Candida antarctica Lipase B. Int. J. Biol. Macromol. 2020, 158, 358–363. [Google Scholar] [CrossRef]
- Cen, Y.; Singh, W.; Arkin, M.; Moody, T.S.; Huang, M.; Zhou, J.; Wu, Q.; Reetz, M.T. Artificial cysteine-lipases with high activity and altered catalytic mechanism created by laboratory evolution. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ben Hlima, H.; Dammak, M.; Karray, A.; Drira, M.; Michaud, P.; Fendri, I.; Abdelkafi, S. Molecular and Structural Characterizations of Lipases from Chlorella by Functional Genomics. Mar. Drugs 2021, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.H.; Karanth, N.G. Lipases and lipase-catalyzed esterification reactions in nonaqueous media. Catal. Rev. 2002, 44, 499–591. [Google Scholar] [CrossRef]
- Stergiou, P.-Y.; Foukis, A.; Filippou, M.; Koukouritaki, M.; Parapouli, M.; Theodorou, L.G.; Hatziloukas, E.; Afendra, A.; Pandey, A.; Papamichael, E.M. Advances in lipase-catalyzed esterification reactions. Biotechnol. Adv. 2013, 31, 1846–1859. [Google Scholar] [CrossRef]
- Porro, R. A economia invisível do babaçu e sua importância para meios de vida em comunidades agroextrativistas. Ciências Hum. 2019, 14, 169–188. [Google Scholar] [CrossRef]
- da Mata, L.R. Caracterização Molecular e Anatômica do Complexo Babaçu (Attalea spp., Arecaceae) Lorena; UnB: Brasília, Brazil, 2016. [Google Scholar]
- Carrazza, L.R.; Ávila, J.C.C.; Silva, M.L. Manual Tecnológico de Aproveitamento Integral do Fruto do Babaçu, 2nd ed.; ISPN: Brasília, Brazil, 2012. [Google Scholar]
- Cremonez, P.A.; Feroldi, M.; de Araújo, A.V.; Borges, M.N.; Meier, T.W.; Feiden, A.; Teleken, J.G. Biofuels in Brazilian aviation: Current scenario and prospects. Renew. Sustain. Energy Rev. 2015, 43, 1063–1072. [Google Scholar] [CrossRef]
- de Oliveira Lima, J.R.; da Silva, R.B.; da Silva, C.C.M.; Santos, L.S.S.; dos Santos, J.R., Jr.; Moura, E.M.; de Moura, C.V.R. Biodiesel de babaçu (Orbignya sp.) obtido por via etanólica. Quim. Nova 2007, 30, 600–603. [Google Scholar] [CrossRef]
- Bacelar-Lima, C.G.; de Mendonça, M.S.; Barbosa, T.C.T.S. Morfologia Floral de uma População de Tucumã. Acta Amaz. 2006, 36, 407–412. [Google Scholar] [CrossRef]
- de Souza Ferreira, E.; Lucien, V.G.; Amaral, A.S.; da Silva Silveira, C. Caracterização físico-química do fruto e do óleo extraído de tucumã (astrocaryum vulgare mart). Aliment. Nutr. Araraquara 2008, 19, 427–433. Available online: http://serv-bib.fcfar.unesp.br/seer/index.php/alimentos/article/viewFile/652/548 (accessed on 10 August 2021).
- Pesce, C. Oleaginosas da Amazônia, 2nd ed.; Museu Paraense Emílio Goeldi: Belém, Brazil, 2009. [Google Scholar]
- Morais, L.R.B.; Gutjahr, E. Química De Oleaginosas Chemistry of Vegetable Oils; Câmara Brasileira do Livro: Belém, Brazil, 2012. [Google Scholar]
- Barbosa, B.S.; Koolen, H.H.F.; Barreto, A.C.; Da Silva, J.D.; Figliuolo, R.; Nunomura, S. Aproveitamento do Óleo das Amêndoas de Tucumã do Amazonas na Produção de Biodiesel. Acta Amaz. 2009, 39, 371–376. [Google Scholar] [CrossRef]
- Araújo, V.; Petry, A.; Echeverria, R.; Fernandes, E.; Pastore, F., Jr. The use of tucumã of amazonas kernel oil in the biodiesel production. In Project ITTO PD 31/99 Rev.3; UnB: Brasília, Brazil, 2007; p. 244. Available online: http://www.itto.int/files/itto_project_db_input/2202/Technical/2.2PlantasdaAmazôniaparaproduçãocosmética.pdf (accessed on 14 February 2023).
- Bora, P.S.; Narain, N.; Rocha, R.V.M.; De Oliveira Monteiro, A.C.; De Azevedo Moreira, R. Caracterización de las fracciones proteicas y lípídicas de pulpa y semillas de tucuma (Astrocaryum Vulgare Mart.). Cienc. Tecnol. Aliment. 2001, 3, 111–116. Available online: http://www.redalyc.org/articulo.oa?id=72430106 (accessed on 14 February 2023). [CrossRef]
- Guedes, M.M. Estudo da Extração de Óleo da Polpa de Tucumã por CO2 Supercrítico; Universidade Federal do Pará: Belém, Brazil, 2006; p. 80. [Google Scholar]
- Barreto, A.C.H.; Maia, F.J.N.; Santiago, V.R.; Ribeiro, V.G.P.; DeNardin, J.C.; Mele, G.; Carbone, L.; Lomonaco, D.; Mazzetto, S.E.; Fechine, P.B.A. Novel ferrofluids coated with a renewable material obtained from cashew nut shell liquid. Microfluid. Nanofluidics 2011, 12, 677–686. [Google Scholar] [CrossRef]
- de Souza, A.T. Síntese e Caracterização de Nanopartículas Magnéticas de Óxido de Ferro Para Aplicações Biomédicas—Um Estudo Citotóxico em Linhagem Celular de Carcinoma Cervical Humano (Células HeLa); Universidade Estadual Paulista: São José do Rio Preto, Brazil, 2011. [Google Scholar]
- Karimi, M. Immobilization of lipase onto mesoporous magnetic nanoparticles for enzymatic synthesis of biodiesel. Biocatal. Agric. Biotechnol. 2016, 8, 182–188. [Google Scholar] [CrossRef]
- Xu, J.; Sun, J.; Wang, Y.; Sheng, J.; Wang, F.; Sun, M. Application of Iron Magnetic Nanoparticles in Protein Immobilization. Molecules 2014, 19, 11465–11486. [Google Scholar] [CrossRef]
- Ribeiro, V.G.P.; Mota, J.P.F.; Júnior, A.E.C.; Lima, N.M.A.; Fechine, P.B.A.; Denardin, J.C.; Carbone, L.; Bloise, E.; Mele, G.; Mazzetto, S.E. Nanomaterials Based on Fe3O4 and Phthalocyanines Derived from Cashew Nut Shell Liquid. Molecules 2019, 24, 3284. [Google Scholar] [CrossRef]
- de Souza, M.C.M. Imobilização de Lipase de Candida Antarctica do Tipo B em Nanopartículas Magnéticas Visando a Aplicação na Síntese de Ésteres; Vasa: Richmond, BC, Canada, 2013; p. 88. [Google Scholar]
- Touqeer, T.; Mumtaz, M.W.; Mukhtar, H. Lipase-Immobilized Magnetic Nanoparticles: Promising Nanobiocatalysts for Biodiesel Production; Wiley Online Library: Hoboken, NJ, USA, 2021; pp. 295–312. [Google Scholar] [CrossRef]
- Wang, W.; Guo, N.; Huang, W.; Zhang, Z.; Mao, X. Immobilization of Chitosanases onto Magnetic Nanoparticles to Enhance Enzyme Performance. Catalysts 2018, 8, 401. [Google Scholar] [CrossRef]
- De Souza, M.C.M.; Dos Santos, K.P.; Freire, R.M.; Barreto, A.C.H.; Fechine, P.B.A.; Gonçalves, L.R.B. Production of flavor esters catalyzed by lipase B from Candida antarctica immobilized on magnetic nanoparticles. Braz. J. Chem. Eng. 2017, 34, 681–690. [Google Scholar] [CrossRef]
- Hu, B.; Pan, J.; Yu, H.-L.; Liu, J.-W.; Xu, J.-H. Immobilization of Serratia marcescens lipase onto amino-functionalized magnetic nanoparticles for repeated use in enzymatic synthesis of Diltiazem intermediate. Process. Biochem. 2009, 44, 1019–1024. [Google Scholar] [CrossRef]
- Christopher, L.P.; Kumar, H.; Zambare, V.P. Enzymatic biodiesel: Challenges and opportunities. Appl. Energy 2014, 119, 497–520. [Google Scholar] [CrossRef]
- Wancura, J.H.; Fantinel, A.L.; Ugalde, G.A.; Donato, F.F.; de Oliveira, J.V.; Tres, M.V.; Jahn, S.L. Semi-continuous production of biodiesel on pilot scale via enzymatic hydroesterification of waste material: Process and economics considerations. J. Clean. Prod. 2020, 285, 124838. [Google Scholar] [CrossRef]
- Vieira, J.S.C.; Sousa, T.L.; Rosas, L.S.; Lima, A.L.; Ronconi, C.M.; Mota, C.J.A. Esterificação e transesterificação homogênea de óleos vegetais contendo alto teor de ácidos graxos livres. Quim. Nova 2018, 41, 10–16. [Google Scholar] [CrossRef]
- Clarke, J.R.P.; Fredricks, K.M. Gas Chromatography of Free Fatty Acids. J. Chromatogr. Sci. 1967, 5, 99–102. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.; Sali, A. Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc. Bioinform. 2016, 54, 5.6.1–5.6.37. [Google Scholar] [CrossRef]
- Bedoya, O.F.; Tischer, I. Detección de homología remota de proteínas usando modelos 3D enriquecidos con propiedades fisicoquímicas. Ing. Y Compet. 2015, 17, 75–84. [Google Scholar] [CrossRef]
- Ramachandran, G.; Ramakrishnan, C.; Sasisekharan, V. Stereochemistry of polypeptide chain configurations. J. Mol. Biol. 1963, 7, 95–99. [Google Scholar] [CrossRef]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Ahmadi, M.; Jahed Motlagh, M.; Rahmani, A.T.; Zolfagharzadeh, M.M.; Shariatpanahi, P.; Chermack, T.J.; Coons, L.M.; Cotter, J.; Eyiah-Donkor, E.; Poti, V.; et al. Chem3D 15.0 User Guide. Macromolecules 2005, 24, 1–61. [Google Scholar]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- DeLano, W.L. The PyMOL Molecular Graphics System; Schrödinger LLC: New York, NY, USA, 2002. [Google Scholar] [CrossRef]

| Oil | Density * (g/mL), 25 °C | Is * (mg KOH/g Oil) | Moisture Content ** (%) |
|---|---|---|---|
| Babassu | 0.918–0.919 | 135.8 | ≤0.1 |
| Tucuman | 0.913–0.914 | 137.6 | ≤0.1 |
| Oil | Alcohol | Code | Consumption Rate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reaction Time (h) | ||||||||||
| 2 | 4 | 6 | 8 | |||||||
| R (%) | E (%) | R (%) | E (%) | R (%) | E (%) | R (%) | E (%) | |||
| Babassu | ethanol | BEE | 46.2 | ±1.85 | 46.1 | ±0.03 | 43.7 | ±0.69 | 35.3 | ±2.13 |
| methanol | BEM | 78.5 | ±1.85 | 79.0 | ±0.07 | 76.8 | ±0.11 | 80.0 | ±0.09 | |
| Tucuman | ethanol | TEE | 63.1 | ±2.62 | 53.4 | ±2.31 | 56.5 | ±7.32 | 60.0 | ±1.60 |
| methanol | TEM | 87.6 | ±2.62 | 73.8 | ±8.09 | 67.5 | ±1.31 | 84.8 | ±0.03 | |
| Oil | Alcohol | Code | Sample | Reaction Time (h) | Content (%) |
|---|---|---|---|---|---|
| Babassu | ethanol | BEE | B1 | 2 | 93.4 |
| methanol | BEM | B2 | 2 | 96.7 | |
| Tucuman | ethanol | TEE | B3 | 2 | 57.5 |
| methanol | TEM | B4 | 2 | 52.6 |
| Oil | Alcohol | Code | Consumption Rate | |||||
|---|---|---|---|---|---|---|---|---|
| Cycle | ||||||||
| I | II | III | ||||||
| R (%) | E (%) | R (%) | E (%) | R (%) | E (%) | |||
| Babassu | ethanol | BEE-NPM | 99.2 | 0.27 | 88.4 | 0.93 | 88.4 | 2.78 |
| methanol | BEM-NPM | 93.4 | 1.06 | 89.5 | 0.97 | 86.1 | 0.10 | |
| Tucuman | ethanol | TEE-NPM | 99.6 | 0.07 | 92.3 | 0.44 | 89.1 | 0.21 |
| methanol | TEM-NPM | 94.7 | 0.11 | 93.5 | 0.12 | 92.1 | 2.11 | |
| Oil | Enzyme | Alcohol | Code | Sample | Ester Content (%) |
|---|---|---|---|---|---|
| Babassu | ET 2.0-NPM | ethanol | BEE-NPM | B5 | 82.2 |
| methanol | BEM-NPM | B6 | 68.7 | ||
| Tucuman | ET 2.0-NPM | ethanol | TEE-NPM | B7 | 86.0 |
| methanol | TEM-NPM | B8 | 32.5 |
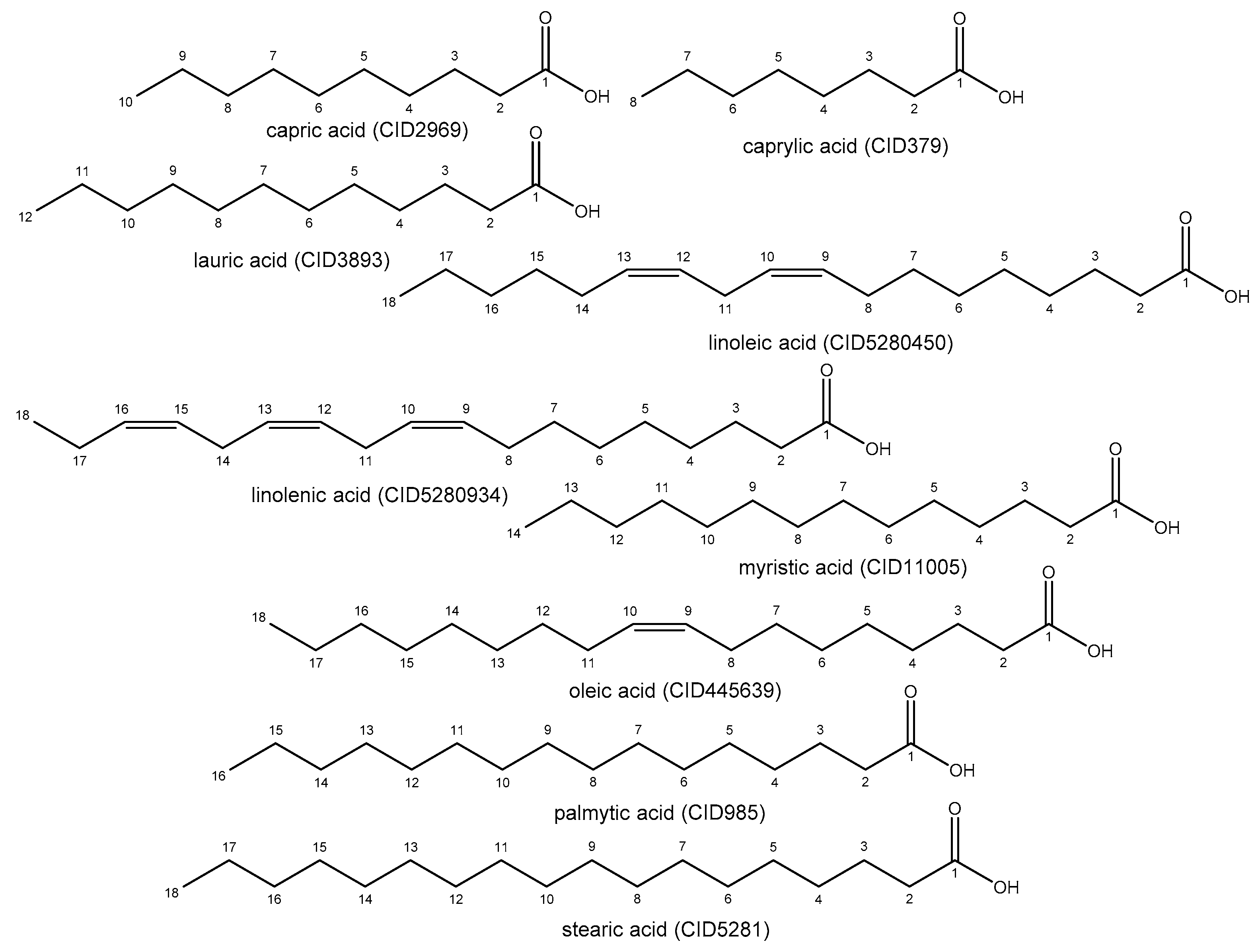
| Compounds | Energy (kcal/mol) | RMSD (Å) |
|---|---|---|
| capric acid (CID2969) | −4.7 | 0.84 |
| caprylic acid (CID379) | −4.6 | 1.39 |
| lauric acid (CID3893) | −5.0 | 0.96 |
| linoleic acid (CID5280450) | −6.5 | 1.68 |
| linolenic acid (CID5280934) | −6.3 | 1.84 |
| myristic acid (CID11005) | −5.5 | 1.54 |
| oleic acid (CID445639) | −5.8 | 2.00 |
| palmitic acid (CID985) | −5.7 | 2.00 |
| stearic acid (CID5281) | −5.1 | 2.00 |
| Fatty Acid | IUPAC Term | Structure | Formula | Content (%) |
|---|---|---|---|---|
| caprylic acid | octanoic acid | C8:0 | C8H16O2 | 3.79% |
| capric acid | decanoic acid | C10:0 | C10H20O2 | 5.42% |
| lauric acid | dodecanoic acid | C12:0 | C12H24O2 | 47.75% |
| myristic acid | tetradecainoic acid | C14:0 | C14H28O2 | 16.54% |
| palmitic acid | hexadecanoic acid | C16:0 | C16H32O2 | 8.58% |
| stearic acid | octadecanoic acid | C18:0 | C18H36O2 | 3.45% |
| oleic acid | octadec-9-enoic acid | C18:1n9c | C18H34O2 | 12.0% |
| linoleic acid | cis, cis-9,12-octadecadienoic acid | C18:2n6c | C18H32O2 | 2.46% |
| Species | C8:0 | C10:0 | C12:0 | C14:0 | C16:0 | C18:0 | C18:1 | C18:2 | C18:3 | C19:0 | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | % | % | % | % | % | ||
| Tucuman-do-Amazonas | 2.0 | 1.8 | 51.4 | 26.1 | 5.6 | 2.7 | 6.0 | 2.1 | - | - | [54] |
| Tucuman-do-Amazonas | 1.3 | 4.4 | 48.9 | 21.6 | 6.4 | 1.7 | 13.2 | 2.5 | - | - | [55] |
| Tucuman-do-Pará | - | - | - | - | 13.9 | 9.8 | 46.8 | 26.1 | 0.9 | - | [56] |
| Tucuman-do-Pará | - | - | - | - | 29.6 | 3.0 | 58.5 | 3.8 | 5.5 | - | [57] |
| Tucuman-do-Pará | - | 0.8 | - | - | 22.9 | 3.0 | 67.6 | 1.2 | - | 2.6 | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandão Júnior, J.; Andrade do Nascimento, J.G.; França Silva, M.P.; Lima Brandão, E.d.A.; de Castro Bizerra, V.; dos Santos, K.M.; Serpa, J.d.F.; Santos, J.C.S.d.; da Fonseca, A.M.; Vasconcelos de Oliveira, D.L.; et al. Performance of Eversa Transform 2.0 Lipase in Ester Production Using Babassu Oil (Orbignya sp.) and Tucuman Oil (Astrocaryum vulgar): A Comparative Study between Liquid and Immobilized Forms in Fe3O4 Nanoparticles. Catalysts 2023, 13, 571. https://doi.org/10.3390/catal13030571
Brandão Júnior J, Andrade do Nascimento JG, França Silva MP, Lima Brandão EdA, de Castro Bizerra V, dos Santos KM, Serpa JdF, Santos JCSd, da Fonseca AM, Vasconcelos de Oliveira DL, et al. Performance of Eversa Transform 2.0 Lipase in Ester Production Using Babassu Oil (Orbignya sp.) and Tucuman Oil (Astrocaryum vulgar): A Comparative Study between Liquid and Immobilized Forms in Fe3O4 Nanoparticles. Catalysts. 2023; 13(3):571. https://doi.org/10.3390/catal13030571
Chicago/Turabian StyleBrandão Júnior, João, Jean Gleison Andrade do Nascimento, Michael Pablo França Silva, Eliane de Aquino Lima Brandão, Viviane de Castro Bizerra, Kaiany Moreira dos Santos, Juliana de França Serpa, José Cleiton Sousa dos Santos, Aluísio Marques da Fonseca, Diego Lomonaco Vasconcelos de Oliveira, and et al. 2023. "Performance of Eversa Transform 2.0 Lipase in Ester Production Using Babassu Oil (Orbignya sp.) and Tucuman Oil (Astrocaryum vulgar): A Comparative Study between Liquid and Immobilized Forms in Fe3O4 Nanoparticles" Catalysts 13, no. 3: 571. https://doi.org/10.3390/catal13030571
APA StyleBrandão Júnior, J., Andrade do Nascimento, J. G., França Silva, M. P., Lima Brandão, E. d. A., de Castro Bizerra, V., dos Santos, K. M., Serpa, J. d. F., Santos, J. C. S. d., da Fonseca, A. M., Vasconcelos de Oliveira, D. L., & Souza, M. C. M. d. (2023). Performance of Eversa Transform 2.0 Lipase in Ester Production Using Babassu Oil (Orbignya sp.) and Tucuman Oil (Astrocaryum vulgar): A Comparative Study between Liquid and Immobilized Forms in Fe3O4 Nanoparticles. Catalysts, 13(3), 571. https://doi.org/10.3390/catal13030571







