Computational Modelling of Pyrrolic MN4 Motifs Embedded in Graphene for Catalyst Design
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
3.1. Structural Model Comparison
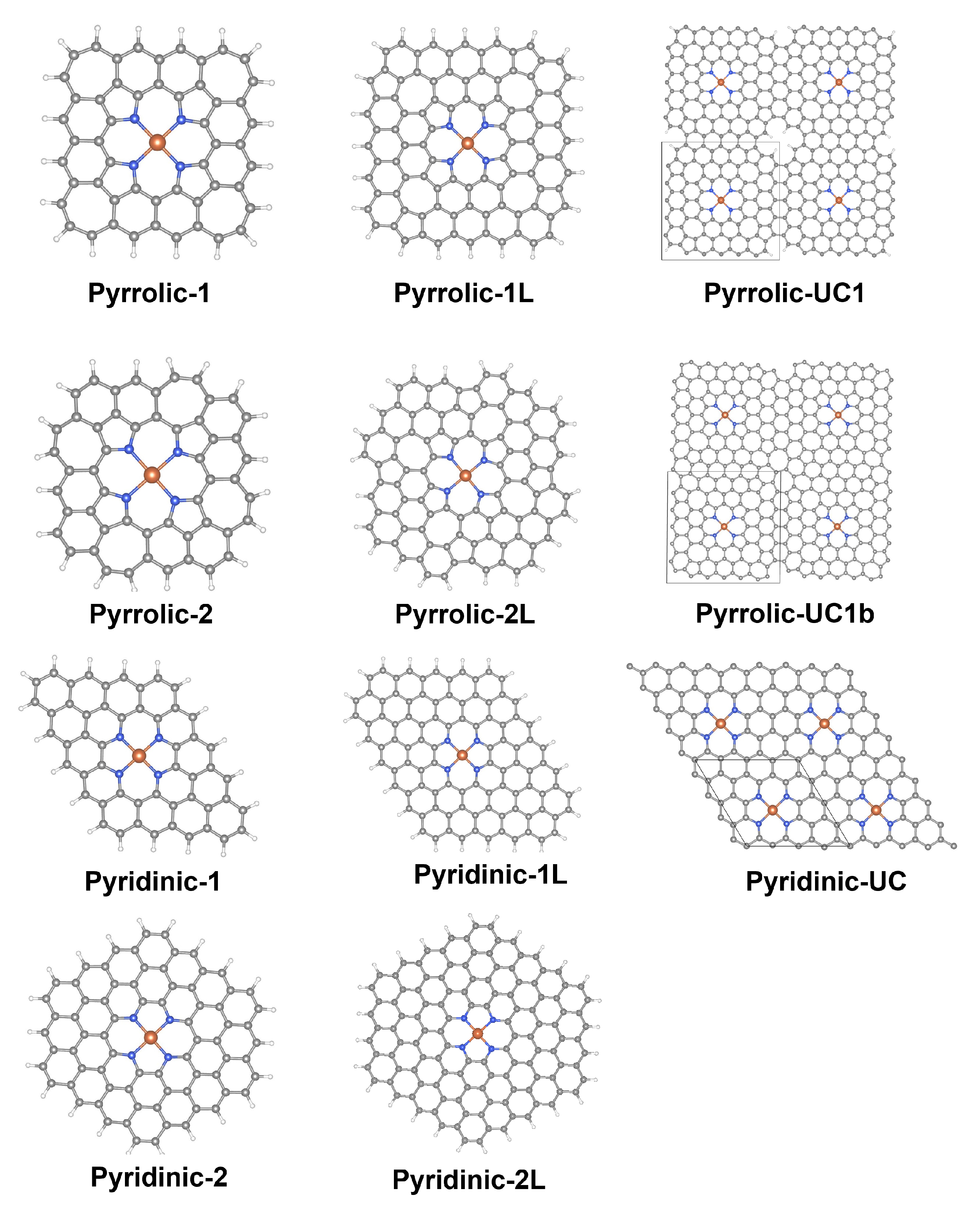
3.2. Binding of Metals in N
3.2.1. Binding Geometry
3.2.2. Binding Energy
3.2.3. Affinity towards Pyrrolic Motifs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| M-N-Cs | Metal- and nitrogen-doped carbons |
| ORR | Oxygen reduction reaction |
| CORR | CO reduction reaction |
| DFT | Density functional theory |
| GGA | Generalized gradient approximation |
| RMM-DIIS | Residual minimization–direct inversion in the iterative subspace |
| NBO | Natural bond orbital |
| FMO | Frontier molecular orbitals |
References
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B Environ. 2005, 56, 9–35. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.P.; Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y. Electrochemical CO2 reduction on metal electrodes. Mod. Asp. Electrochem. 2008, 89–189. [Google Scholar] [CrossRef]
- Kortlever, R.; Shen, J.; Schouten, K.J.P.; Calle-Vallejo, F.; Koper, M.T. Catalysts and reaction pathways for the electrochemical reduction of carbon dioxide. J. Phys. Chem. Lett. 2015, 6, 4073–4082. [Google Scholar] [CrossRef]
- Lefèvre, M.; Proietti, E.; Jaouen, F.; Dodelet, J.P. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science 2009, 324, 71–74. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, X.; Peng, L.; Li, L.; Qiao, J.; Huang, H.; Shi, J. Metal–nitrogen–carbon catalysts of specifically coordinated configurations toward typical electrochemical redox reactions. Adv. Mater. 2021, 33, 2100997. [Google Scholar] [CrossRef]
- Jaouen, F.; Jones, D.; Coutard, N.; Artero, V.; Strasser, P.; Kucernak, A. Toward platinum group metal-free catalysts for hydrogen/air proton-exchange membrane fuel cells. Johns. Matthey Technol. Rev. 2018, 62, 231–255. [Google Scholar] [CrossRef]
- Menga, D.; Low, J.L.; Li, Y.S.; Arčon, I.; Koyutürk, B.; Wagner, F.; Ruiz-Zepeda, F.; Gaberšček, M.; Paulus, B.; Fellinger, T.P. Resolving the dilemma of Fe–N–C catalysts by the selective synthesis of tetrapyrrolic active sites via an imprinting strategy. J. Am. Chem. Soc. 2021, 143, 18010–18019. [Google Scholar] [CrossRef]
- Zitolo, A.; Goellner, V.; Armel, V.; Sougrati, M.T.; Mineva, T.; Stievano, L.; Fonda, E.; Jaouen, F. Identification of catalytic sites for oxygen reduction in iron-and nitrogen-doped graphene materials. Nat. Mater. 2015, 14, 937–942. [Google Scholar] [CrossRef]
- Ni, L.; Gallenkamp, C.; Wagner, S.; Bill, E.; Krewald, V.; Kramm, U.I. Identification of the catalytically dominant iron environment in iron-and nitrogen-doped carbon catalysts for the oxygen reduction reaction. J. Am. Chem. Soc. 2022, 144, 16827–16840. [Google Scholar] [CrossRef]
- Hu, X.; Chen, S.; Chen, L.; Tian, Y.; Yao, S.; Lu, Z.; Zhang, X.; Zhou, Z. What is the Real Origin of the Activity of Fe–N–C Electrocatalysts in the O2 Reduction Reaction? Critical Roles of Coordinating Pyrrolic N and Axially Adsorbing Species. J. Am. Chem. Soc. 2022, 144, 18144–18152. [Google Scholar] [CrossRef]
- Koshy, D.M.; Chen, S.; Lee, D.U.; Stevens, M.B.; Abdellah, A.M.; Dull, S.M.; Chen, G.; Nordlund, D.; Gallo, A.; Hahn, C.; et al. Understanding the origin of highly selective CO2 electroreduction to CO on Ni, N-doped carbon catalysts. Angew. Chem. Int. Ed. 2020, 59, 4043–4050. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhou, T.; Chen, M.; Feng, H.; Yuan, R.; Yan, W.; Tian, Y.; Wu, X.; Chu, W.; Wu, C.; et al. High-purity pyrrole-type FeN 4 sites as a superior oxygen reduction electrocatalyst. Energy Environ. Sci. 2020, 13, 111–118. [Google Scholar] [CrossRef]
- Menga, D.; Ruiz-Zepeda, F.; Moriau, L.; Šala, M.; Wagner, F.; Koyutürk, B.; Bele, M.; Petek, U.; Hodnik, N.; Gaberšček, M.; et al. Active-site imprinting: Preparation of Fe–N–C catalysts from zinc ion–templated ionothermal nitrogen-doped carbons. Adv. Energy Mater. 2019, 9, 1902412. [Google Scholar] [CrossRef]
- Jiao, L.; Li, J.; Richard, L.L.; Sun, Q.; Stracensky, T.; Liu, E.; Sougrati, M.T.; Zhao, Z.; Yang, F.; Zhong, S.; et al. Chemical vapour deposition of Fe–N–C oxygen reduction catalysts with full utilization of dense Fe–N4 sites. Nat. Mater. 2021, 20, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, A.; Gong, M.; Jaouen, F.; Roy, A.; Zitolo, A.; Khan, A.; Sougrati, M.T.; Primbs, M.; Bonastre, A.M.; Fongalland, D.; et al. High loading of single atomic iron sites in Fe–NC oxygen reduction catalysts for proton exchange membrane fuel cells. Nat. Catal. 2022, 5, 311–323. [Google Scholar] [CrossRef]
- Mehmood, A.; Pampel, J.; Ali, G.; Ha, H.Y.; Ruiz-Zepeda, F.; Fellinger, T.P. Facile metal coordination of active site imprinted nitrogen doped carbons for the conservative preparation of non-noble metal oxygen reduction electrocatalysts. Adv. Energy Mater. 2018, 8, 1701771. [Google Scholar] [CrossRef]
- Fellinger, T.P.; Menga, D.; Buzanich, A.G.; Wagner, F. Evaluation of the Specific Activity of M-N-Cs and the Intrinsic Activity of Tetrapyrrolic Fe-N4 Sites for the Oxygen Reduction Reaction. Angew. Chem. 2022, e202207089. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ernzerhof, M.; Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 1996, 105, 9982–9985. [Google Scholar] [CrossRef]
- Ernzerhof, M.; Scuseria, G.E. Assessment of the Perdew–Burke–Ernzerhof exchange-correlation functional. J. Chem. Phys. 1999, 110, 5029–5036. [Google Scholar] [CrossRef]
- Tolba, S.A.; Gameel, K.M.; Ali, B.A.; Almossalami, H.A.; Allam, N.K. The DFT+U: Approaches, Accuracy, and Applications. In Density Functional Calculations; Yang, G., Ed.; IntechOpen: Rijeka, Croatia, 2018; Chapter 1. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Ahlrichs, R.; Bär, M.; Häser, M.; Horn, H.; Kölmel, C. Electronic structure calculations on workstation computers: The program system turbomole. Chem. Phys. Lett. 1989, 162, 165–169. [Google Scholar] [CrossRef]
- Khosravi, A.; Vessally, E.; Oftadeh, M.; Behjatmanesh-Ardakani, R. Ammonia capture by MN4 (M= Fe and Ni) clusters embedded in graphene. J. Coord. Chem. 2018, 71, 3476–3486. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Iwase, K.; Ebner, K.; Diercks, J.S.; Saveleva, V.A.; Unsal, S.; Krumeich, F.; Harada, T.; Honma, I.; Nakanishi, S.; Kamiya, K.; et al. Effect of cobalt speciation and the graphitization of the carbon matrix on the CO2 electroreduction activity of Co/N-doped carbon materials. ACS Appl. Mater. Interfaces 2021, 13, 15122–15131. [Google Scholar] [CrossRef] [PubMed]
- Yudasaka, M.; Kikuchi, R. Graphitization of carbonaceous materials by Ni, Co and Fe. In Supercarbon; Springer: Berlin/Heidelberg, Germany, 1998; pp. 99–105. [Google Scholar]
- Schaftenaar, G.; Noordik, J.H. Molden: A pre-and post-processing program for molecular and electronic structures. J. Comput. Aided Mol. Des. 2000, 14, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Schaftenaar, G.; Vlieg, E.; Vriend, G. Molden 2.0: Quantum chemistry meets proteins. J. Comput. Aided Mol. Des. 2017, 31, 789–800. [Google Scholar]
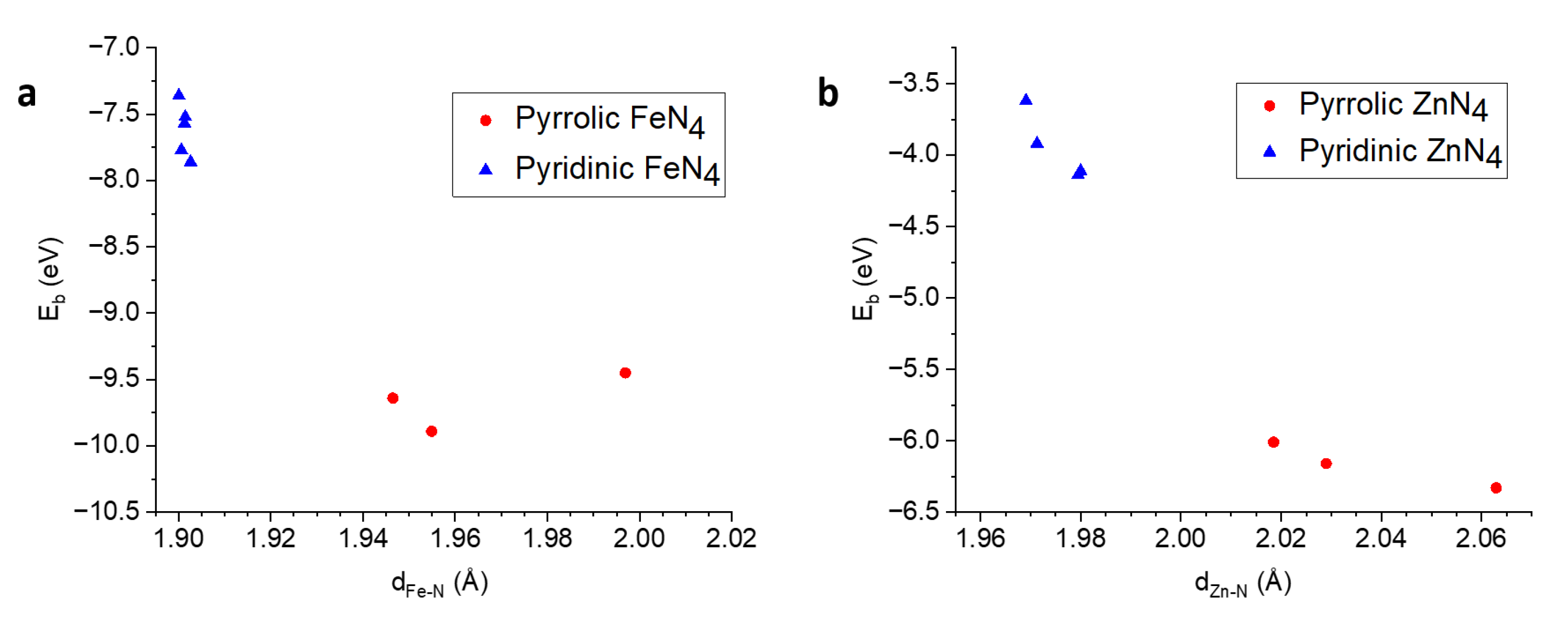
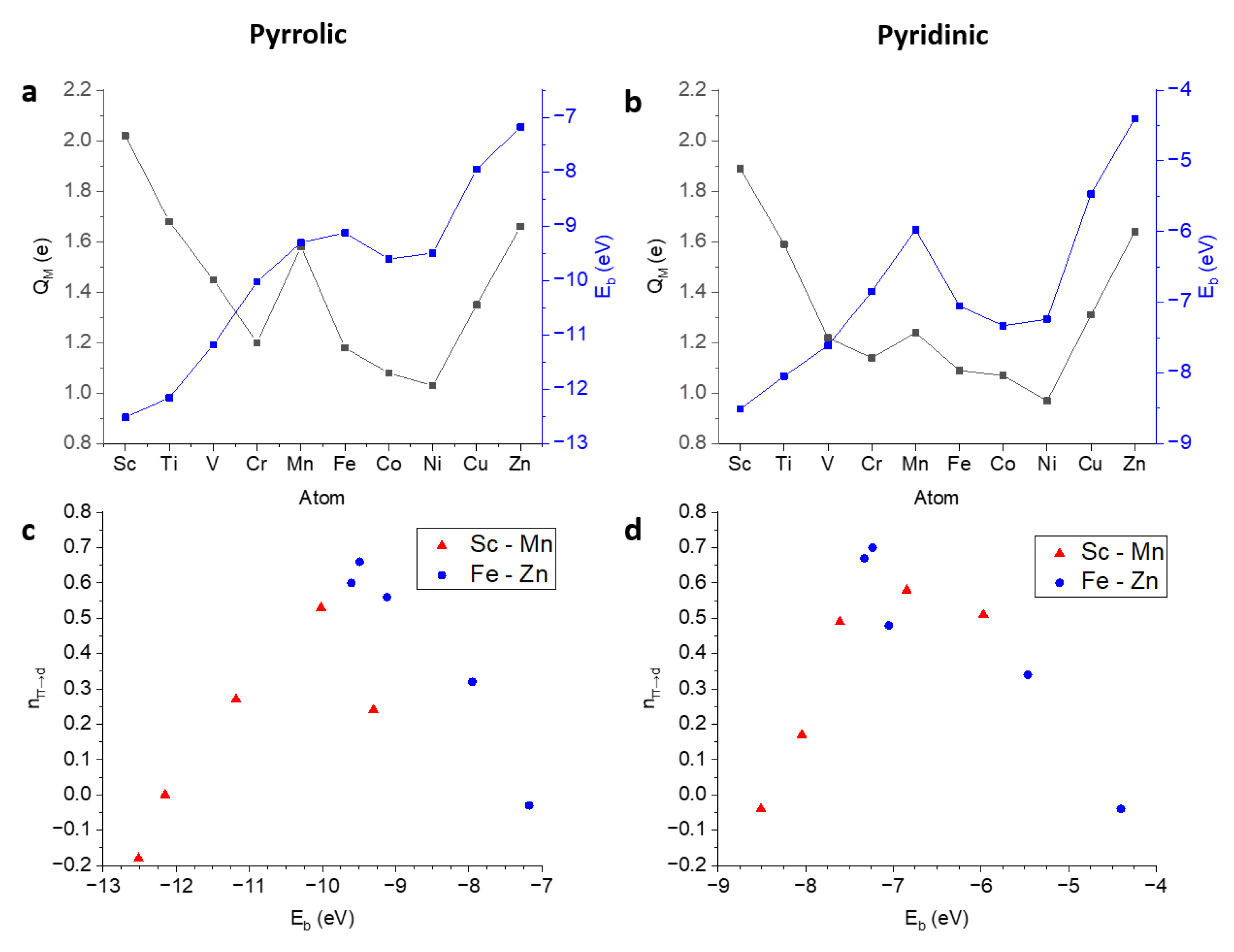
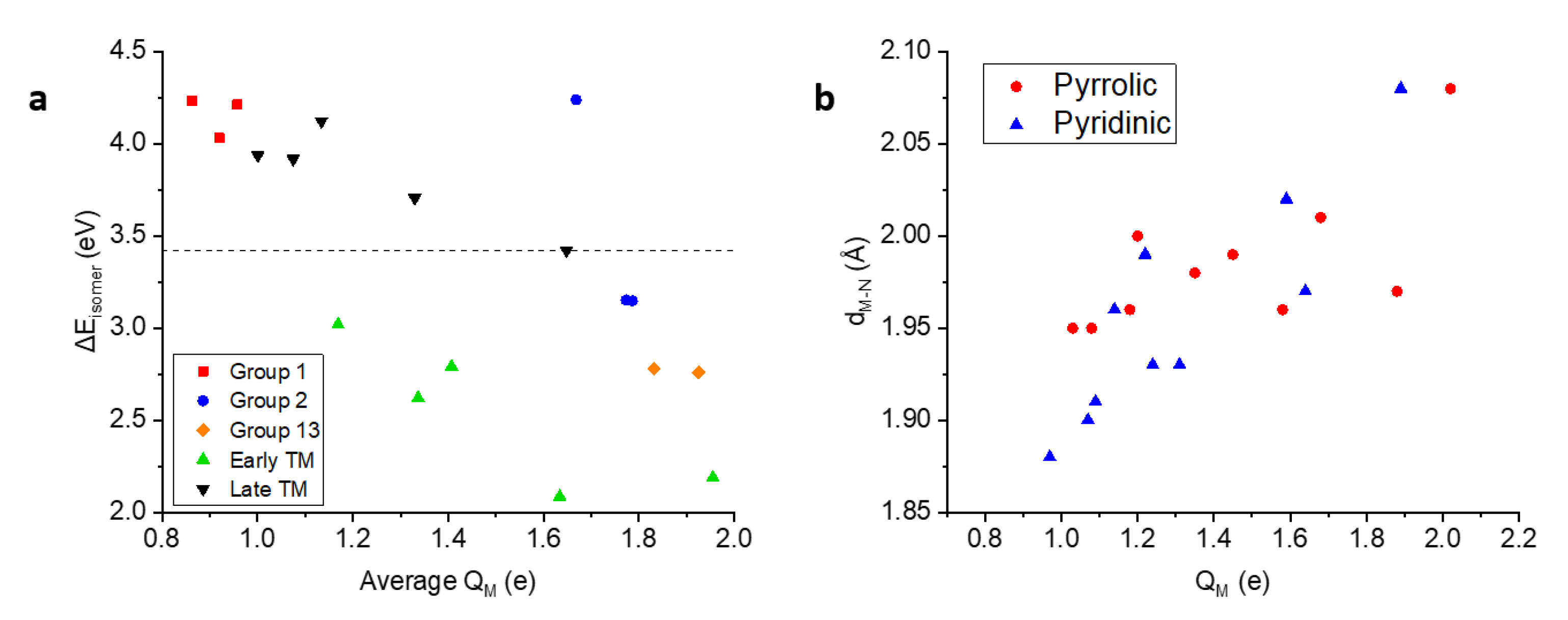
| Species | Model | (eV) | (Å) |
|---|---|---|---|
| Fe | Pyrrolic-1 | −9.64 | 1.95 |
| Pyrrolic-1L | −9.89 | 1.95 | |
| Pyrrolic-UC1 | −9.87 | 1.95 | |
| Pyrrolic-UC1b | −10.11 | 1.93, 1.96 | |
| Pyrrolic-2 | −8.53 | 1.97 | |
| Pyrrolic-2L | −9.45 | 2.00 | |
| Pyridinic-1 | −7.86 | 1.90, 1.91 | |
| Pyridinic-1L | −7.77 | 1.90, 1.91 | |
| Pyridinic-UC | −7.74 | 1.89 | |
| Pyridinic-2 | −7.57 | 1.90 | |
| Pyridinic-2L | −7.36 | 1.90 | |
| Zn | Pyrrolic-1 | −6.01 | 2.02 |
| Pyrrolic-1L | −6.15 | 2.03 | |
| Pyrrolic-UC1 | −5.94 | 2.02 | |
| Pyrrolic-UC1b | −6.11 | 1.99, 2.03 | |
| Pyrrolic-2 | −5.43 | 2.06 | |
| Pyrrolic-2L | −6.34 | 2.06 | |
| Pyridinic-1 | −4.14 | 1.96, 2.00 | |
| Pyridinic-1L | −4.11 | 1.96, 1.99 | |
| Pyridinic-UC | −3.79 | 1.96 | |
| Pyridinic-2 | −3.92 | 1.97 | |
| Pyridinic-2L | −3.62 | 1.97 |
| Metal | (eV) | (Å) | (e) | ||||
|---|---|---|---|---|---|---|---|
| Pyrrolic | Pyridinic | Pyrrolic | Pyridinic | Pyrrolic | Pyridinic | (eV) | |
| empty | - | - | 2.01 ** | 1.92 ** | - | - | 6.19 |
| Li | −7.27 | −5.31 | 2.00 | 1.94 | +0.86 | +0.86 | 4.23 |
| Na * | −5.66 | −3.49 | 2.25 | 2.27 | +0.91 | +0.93 | 4.03 |
| K * | −5.14 | −3.16 | 2.63 | 2.66 | +0.95 | +0.97 | 4.21 |
| Be | −10.31 | −8.35 | 1.91 | 1.84 | +1.67 | +1.67 | 4.24 |
| Mg | −9.13 | −6.09 | 2.02 | 1.97 | +1.78 | +1.77 | 3.15 |
| Ca * | −9.25 | −6.20 | 2.28 | 2.27 | +1.79 | +1.78 | 3.15 |
| Sc * | −12.51 | −8.51 | 2.08 | 2.08 | +2.02 | +1.89 | 2.19 |
| Ti * | −12.15 | −8.04 | 2.01 | 2.02 | +1.68 | +1.59 | 2.09 |
| V * | −11.18 | −7.61 | 1.99 | 1.99 | +1.45 | +1.22 | 2.62 |
| Cr | −10.02 | −6.84 | 2.00 | 1.96 | +1.20 | +1.14 | 3.02 |
| Mn | −9.35 | −5.97 | 1.96 | 1.94 | +1.58 | +1.24 | 2.81 |
| Fe | −9.12 | −7.05 | 1.96 | 1.91 | +1.18 | +1.09 | 4.12 |
| Co | −9.61 | −7.33 | 1.95 | 1.90 | +1.08 | +1.07 | 3.92 |
| Ni | −9.49 | −7.24 | 1.95 | 1.88 | +1.03 | +0.97 | 3.94 |
| Cu | −7.95 | −5.47 | 1.98 | 1.93 | +1.35 | +1.31 | 3.71 |
| Zn | −7.17 | −4.40 | 2.01 | 1.97 | +1.66 | +1.64 | 3.42 |
| Al | −11.89 | −8.46 | 1.94 | 1.89 | +1.97 | +1.88 | 2.76 |
| Ga | −9.45 | −6.03 | 1.97 | 1.93 | +1.88 | +1.79 | 2.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Low, J.L.; Paulus, B. Computational Modelling of Pyrrolic MN4 Motifs Embedded in Graphene for Catalyst Design. Catalysts 2023, 13, 566. https://doi.org/10.3390/catal13030566
Low JL, Paulus B. Computational Modelling of Pyrrolic MN4 Motifs Embedded in Graphene for Catalyst Design. Catalysts. 2023; 13(3):566. https://doi.org/10.3390/catal13030566
Chicago/Turabian StyleLow, Jian Liang, and Beate Paulus. 2023. "Computational Modelling of Pyrrolic MN4 Motifs Embedded in Graphene for Catalyst Design" Catalysts 13, no. 3: 566. https://doi.org/10.3390/catal13030566
APA StyleLow, J. L., & Paulus, B. (2023). Computational Modelling of Pyrrolic MN4 Motifs Embedded in Graphene for Catalyst Design. Catalysts, 13(3), 566. https://doi.org/10.3390/catal13030566






