A Second-Generation Palladacycle Architecture Bearing a N-Heterocyclic Carbene and Its Catalytic Behavior in Buchwald–Hartwig Amination Catalysis
Abstract
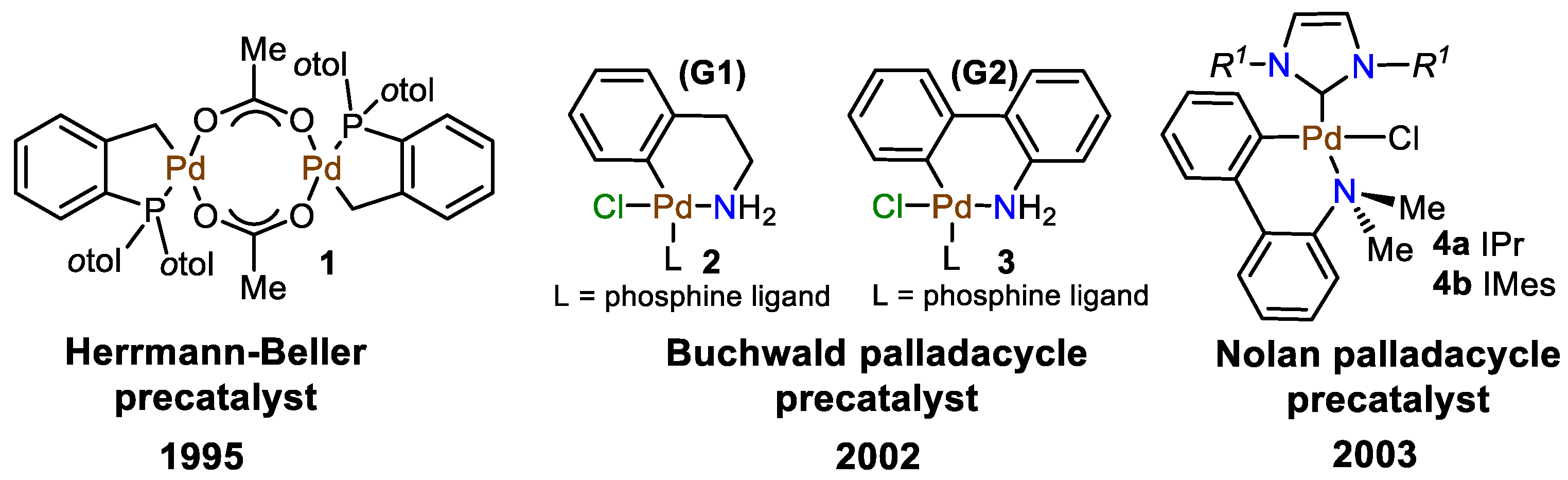
1. Introduction
2. Results and Discussion
2.1. Synthesis of [Pd(NHC)(NH2)(CC)Cl] Palladacycles
2.2. [Pd(NHC)(NH2)(CC)Cl] Palladacycles as Catalysts in the Buchwald–Hartwig Amination Reaction
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. General Procedure for Synthesis of [Pd(NHC)(NH2)(CC)Cl] Complexes (Small Scale)
3.2.2. General Procedure for Synthesis of [Pd(NHC)(NH2)(CC)Cl] Complexes (Larger Scale)
3.2.3. Procedures for the Catalytic Tests Buchwald–Hartwig Reaction
3.2.4. General Procedure for the Buchwald–Hartwig Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marion, N.; Nolan, S.P. Well-Defined N-Heterocyclic Carbenes-Palladium(II) Precatalysts for Cross- Coupling Reactions. Acc. Chem. Res. 2008, 41, 1440–1449. [Google Scholar] [CrossRef]
- Ostrowska, S.; Scattolin, T.; Nolan, S.P. N-Heterocyclic Carbene Complexes Enabling the α-Arylation of Carbonyl Compounds. Chem. Commun. 2021, 57, 4354–4375. [Google Scholar] [CrossRef]
- Nolan, S.P. N-Heterocyclic Carbenes: Effective Tools for Organometallic Synthesis, 1st ed.; Wiley-VCH: Hoboken, NJ, USA, 2014; ISBN 9783527334902. [Google Scholar]
- Maluenda, I.; Navarro, O. Recent Developments in the Suzuki-Miyaura Reaction: 2010–2014. Molecules 2015, 20, 7528–7557. [Google Scholar] [CrossRef]
- Fortman, G.C.; Nolan, S.P. N-Heterocyclic Carbene (NHC) Ligands and Palladium in Homogeneous Cross-Coupling Catalysis: A Perfect Union. Chem. Soc. Rev. 2011, 40, 5151–5169. [Google Scholar] [CrossRef] [PubMed]
- Christmann, U.; Vilar, R. Monoligated Palladium Species as Catalysts in Cross-Coupling Reactions. Angew. Chemie Int. Ed. 2005, 44, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Biscoe, M.R.; Fors, B.P.; Buchwald, S.L. A New Class of Easily Activated Palladium Precatalysts for Facile C-N Cross-Coupling Reactions and the Low Temperature Oxidative Addition of Aryl Chlorides. J. Am. Chem. Soc. 2008, 130, 6686–6687. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Pfeffer, M. Palladacycles; WILEY-VCH GmbH & Co. KGaA: Weinheim, Germany, 2008; ISBN 9783527319527. [Google Scholar]
- Vila, J.M.; Pereira, M.T.; Lucio-Martínez, F.; Reigosa, F. Palladacycles as Efficient Precatalysts for Suzuki-Miyaura Cross-Coupling Reactions. Palladacycles 2019, 1, 1–20. [Google Scholar] [CrossRef]
- Albrecht, M. Cyclometalation Using D-Block Transition Metals: Fundamental Aspects and Recent Trends. Chem. Rev. 2010, 110, 576–623. [Google Scholar] [CrossRef] [PubMed]
- Ghedini, M.; Aiello, I.; Crispini, A.; Golemme, A.; La Deda, M.; Pucci, D. Azobenzenes and Heteroaromatic Nitrogen Cyclopalladated Complexes for Advanced Applications. Coord. Chem. Rev. 2006, 250, 1373–1390. [Google Scholar] [CrossRef]
- Dupont, J.; Consorti, C.S.; Spencer, J. The Potential of Palladacycles: More than Just Precatalysts. Chem. Rev. 2005, 105, 2527–2571. [Google Scholar] [CrossRef]
- Alonso, D.A.; Nájera, C. Oxime-Derived Palladacycles as Source of Palladium Nanoparticles. Chem. Soc. Rev. 2010, 39, 2891–2902. [Google Scholar] [CrossRef]
- Wang, K.; Fan, R.; Wei, X.; Fang, W. Palladacyclic N-Heterocyclic Carbene Precatalysts for Transition Metal Catalysis. Green Synth. Catal. 2022, 3, 327–338. [Google Scholar] [CrossRef]
- Nájera, C. Oxime-Derived Palladacycles: Applications in Catalysis. ChemCatChem 2016, 8, 1865–1881. [Google Scholar] [CrossRef]
- Li, H.; Johansson Seechurn, C.C.C.; Colacot, T.J. Development of Preformed Pd Catalysts for Cross-Coupling Reactions, beyond the 2010 Nobel Prize. ACS Catal. 2012, 2, 1147–1164. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Cheprakov, A.V. Palladacycles in Catalysis—A Critical Survey. J. Organomet. Chem. 2004, 689, 4055–4082. [Google Scholar] [CrossRef]
- Cope, A.C.; Siekman, R.W. Formation of Covalent Bonds from Platinum or Palladium to Carbon by Direct Substitution. J. Am. Chem. Soc. 1965, 87, 3272–3273. [Google Scholar] [CrossRef]
- Cope, A.; Friedrichlb, E. N,N-Dimethylbenzylamines. J. Am. Chem. Soc. 1968, 90, 909–913. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Brossmer, C.; Öfele, K.; Reisinger, C.-P.; Priermeier, T.; Beller, M.; Fischer, H. Palladacycles as Structurally Defined Catalysts for the Heck Olefination of Chloro- and Bromoarenes. Angew. Chemie Int. Ed. Eng. 1995, 34, 1844–1848. [Google Scholar] [CrossRef]
- Bruneau, A.; Roche, M.; Alami, M.; Messaoudi, S. 2-Aminobiphenyl Palladacycles: The “Most Powerful” Precatalysts in C-C and C-Heteroatom Cross-Couplings. ACS Catal. 2015, 5, 1386–1396. [Google Scholar] [CrossRef]
- Molander, G.A.; Barcellos, T.; Traister, K.M. Pd-Catalyzed Cross-Coupling of Potassium Alkenyltrifluoroborates with 2-Chloroacetates and 2-Chloroacetamides. Org. Lett. 2013, 15, 3342–3345. [Google Scholar] [CrossRef] [PubMed]
- Shu, W.; Buchwald, S.L. Use of Precatalysts Greatly Facilitate Palladium-Catalyzed Alkynylations in Batch and Continuous-Flow Conditions. Chem. Sci. 2011, 2, 2321–2325. [Google Scholar] [CrossRef]
- Maiti, D.; Fors, B.P.; Henderson, J.L.; Nakamura, Y.; Buchwald, S.L. Palladium-Catalyzed Coupling of Functionalized Primary and Secondary Amines with Aryl and Heteroaryl Halides: Two Ligands Suffice in Most Cases. Chem. Sci. 2011, 2, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Gorelsky, S.I.; Lapointe, D.; Fagnou, K. Analysis of the Palladium-Catalyzed (Aromatic)C-H Bond Metalation- Deprotonation Mechanism Spanning the Entire Spectrum of Arenes. J. Org. Chem. 2012, 77, 658–668. [Google Scholar] [CrossRef]
- Martin, R.; Buchwald, S.L. Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling Reactions Employing Dialkylbiaryl Phosphine Ligands. Acc. Chem. Res. 2008, 41, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An Overview of N-Heterocyclic Carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Schwarz, J.; Gardiner, M.G. High-Yield Syntheses of Sterically Demanding Bis(N-Heterocyclic Carbene) Complexes of Palladium. Organometallics 1999, 18, 4082–4089. [Google Scholar] [CrossRef]
- Phan, N.T.S.; Van Der Sluys, M.; Jones, C.W. On the Nature of the Active Species in Palladium Catalyzed Mizoroki-Heck and Suzuki-Miyaura Couplings—Homogeneous or Heterogeneous Catalysis, a Critical Review. Adv. Synth. Catal. 2006, 348, 609–679. [Google Scholar] [CrossRef]
- Kapdi, A.R.; Fairlamb, I.J.S. Anti-Cancer Palladium Complexes: A Focus on PdX2L2, Palladacycles and Related Complexes. Chem. Soc. Rev. 2014, 43, 4751–4777. [Google Scholar] [CrossRef]
- Emin Günay, M.; Gümüşada, R.; Özdemir, N.; Dinçer, M.; Çetinkaya, B. Synthesis, X-Ray Structures, and Catalytic Activities of (Κ2-C,N)-Palladacycles Bearing Imidazol-2-Ylidenes. J. Organomet. Chem. 2009, 694, 2343–2349. [Google Scholar] [CrossRef]
- Kilinçarslan, R.; Günay, M.E.; Firinci, R.; Denizalti, S.; Çetinkaya, B. New Palladium(II)-N-Heterocyclic Carbene Complexes Containing Benzimidazole-2-Ylidene Ligand Derived from Menthol: Synthesis, Characterization and Catalytic Activities. Appl. Organomet. Chem. 2016, 30, 268–272. [Google Scholar] [CrossRef]
- Micksch, M.; Tenne, M.; Strassner, T. Cyclometalated 2-Phenylimidazole Palladium Carbene Complexes in the Catalytic Suzuki-Miyaura Cross-Coupling Reaction. Organometallics 2014, 33, 3966–3976. [Google Scholar] [CrossRef]
- Babahan, İ.; Fırıncı, R.; Özdemir, N.; Emin Günay, M. Synthesis, Characterization and Catalytic Activity of N-Heterocyclic Carbene Ligated Schiff Base Palladacycles. Inorg. Chim. Acta 2021, 522, 120360. [Google Scholar] [CrossRef]
- Schroeter, F.; Soellner, J.; Strassner, T. Cyclometalated Palladium NHC Complexes Bearing PEG Chains for Suzuki-Miyaura Cross-Coupling in Water. Organometallics 2018, 37, 4267–4275. [Google Scholar] [CrossRef]
- Deng, Q.; Shen, Y.; Zhu, H.; Tu, T. A Magnetic Nanoparticle-Supported N-Heterocyclic Carbene-Palladacycle: An Efficient and Recyclable Solid Molecular Catalyst for Suzuki-Miyaura Cross-Coupling of 9-Chloroacridine. Chem. Commun. 2017, 53, 13063–13066. [Google Scholar] [CrossRef]
- Deng, Q.; Zheng, Q.; Zuo, B.; Tu, T. Robust NHC-Palladacycles-Catalyzed Suzuki−Miyaura Cross-Coupling of Amides via C-N Activation. Green Synth. Catal. 2020, 1, 75–78. [Google Scholar] [CrossRef]
- Zuo, B.; Lu, Z.; Wu, X.; Fang, W.; Li, W.; Huang, M.; Yang, D.Y.; Deng, Q.; Tu, T. A N-Heterocyclic Carbene-Palladacycle with Constrained Aliphatic Linker: Synthesis, Characterization and Its Catalytic Application towards Suzuki-Miyaura Cross-Coupling. Asian, J. Org. Chem. 2021, 10, 3233–3236. [Google Scholar] [CrossRef]
- Xu, C.; Li, H.M.; Xiao, Z.Q.; Wang, Z.Q.; Tang, S.F.; Ji, B.M.; Hao, X.Q.; Song, M.P. Cyclometalated Pd(Ii) and Ir(Iii) 2-(4-Bromophenyl)Pyridine Complexes with N-Heterocyclic Carbenes (NHCs) and Acetylacetonate (Acac): Synthesis, Structures, Luminescent Properties and Application in One-Pot Oxidation/Suzuki Coupling of Aryl Chlorides Containing Hydroxymethyl. Dalt. Trans. 2014, 43, 10235–10247. [Google Scholar] [CrossRef]
- Serrano, J.L.; Perez, J.; Garcia, L.; Sanchez, G.; Garcia, J.; Lozano, P.; Zende, V.; Kapdi, A. N-Heterocyclic-Carbene Complexes Readily Prepared from Di-μ-Hydroxopalladacycles Catalyze the Suzuki Arylation of 9-Bromophenanthrene. Organometallics 2015, 34, 522–533. [Google Scholar] [CrossRef]
- Xu, C.; Lou, X.H.; Wang, Z.Q.; Fu, W.J. N-Heterocyclic Carbene Adducts of Cyclopalladated Ferrocenylchloropyrimidine: Synthesis, Structural Characterization and Application in the Sonogashira Reaction. Transit. Met. Chem. 2012, 37, 519–523. [Google Scholar] [CrossRef]
- Li, H.M.; Xu, C.; Duan, L.M.; Lou, X.H.; Wang, Z.Q.; Li, Z.; Fan, Y.T. N-Heterocyclic Carbene Adducts of Cyclopalladated Ferrocenylpyridine Containing Chloride or Iodide Anions: Synthesis, Crystal Structures and Application in the Coupling of Terminal Alkynes with Arylboronic Acids. Transit. Met. Chem. 2013, 38, 313–318. [Google Scholar] [CrossRef]
- Doucet, H.; Hierso, J.C. Palladium-Based Catalytic Systems for the Synthesis of Conjugated Enynes by Sonogashira Reactions and Related Alkynylations. Angew. Chemie Int. Ed. 2007, 46, 834–871. [Google Scholar] [CrossRef]
- Kantchev, E.A.B.; Peh, G.R.; Zhang, C.; Ying, J.Y. Practical Heck-Mizoroki Coupling Protocol for Challenging Substrates Mediated by an N-Heterocyclic Carbene-Ligated Palladacycle. Org. Lett. 2008, 10, 3949–3952. [Google Scholar] [CrossRef]
- Green, K.A.; Maragh, P.T.; Abdur-Rashid, K.; Lough, A.J.; Dasgupta, T.P. Benzimidazol-2-Ylidene Ligated Palladacyclic Complexes of N,N-Dimethylbenzylamine—Synthesis and Application to C-C Coupling Reactions. Inorg. Chim. Acta 2016, 449, 38–43. [Google Scholar] [CrossRef]
- Ren, G.; Cui, X.; Yang, E.; Yang, F.; Wu, Y. Study on the Heck Reaction Promoted by Carbene Adduct of Cyclopalladated Ferrocenylimine and the Related Reaction Mechanism. Tetrahedron 2010, 66, 4022–4028. [Google Scholar] [CrossRef]
- Dorel, R.; Grugel, C.P.; Haydl, A.M. The Buchwald–Hartwig Amination After 25 Years. Angew. Chemie Int. Ed. 2019, 58, 17118–17129. [Google Scholar] [CrossRef] [PubMed]
- Marion, N.; Nolan, S.P. N-Heterocyclic Carbenes in Gold Catalysis. Chem. Soc. Rev. 2008, 37, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Kantchev, E.A.B.; Ying, J.Y. Practical One-Pot, Three-Component Synthesis of N-Heterocyclic Carbene (NHC) Ligated Palladacycles Derived from n,n-Dimethylbenzylamine. Organometallics 2009, 28, 289–299. [Google Scholar] [CrossRef]
- Zuo, B.; Shao, H.; Qu, E.; Ma, Y.; Li, W.; Huang, M.; Deng, Q. An Alkoxy Modified N-Heterocyclic Carbene-Palladacycle: Synthesis, Characterization and Application towards Buchwald-Hartwig and Suzuki-Miyaura Coupling Reactions. ChemistrySelect 2021, 6, 10121–10126. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Zhang, C.; Yu, J.; Liu, J.; Xia, C. Phosphine-Free, Efficient Double Carbonylation of Aryl Iodides with Amines Catalyzed by Water-Insoluble and Water-Soluble N-Heterocyclic Carbene-Amine Palladium Complexes. Adv. Synth. Catal. 2014, 356, 2539–2546. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Xia, C. Aryl-Palladium-NHC Complex: Efficient Phosphine-Free Catalyst Precursors for the Carbonylation of Aryl Iodides with Amines or Alkynes. Org. Biomol. Chem. 2014, 12, 9702–9706. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Xia, C. Palladium-N-Heterocyclic Carbene (NHC)-Catalyzed Synthesis of 2-Ynamides via Oxidative Aminocarbonylation of Alkynes with Amines. Catal. Sci. Technol. 2015, 5, 4750–4754. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, S. Palladacycles Bearing COOH-/Ester-Functionalized N-Heterocyclic Carbenes: Divergent Syntheses and Catalytic Applications. Appl. Organomet. Chem. 2019, 33, e4703. [Google Scholar] [CrossRef]
- Niggli, N.E.; Baudoin, O. Design of Chiral NHC-Carboxylates as Potential Ligands for Pd-Catalyzed Enantioselective C−H Activation. Helv. Chim. Acta 2021, 104, e2100015. [Google Scholar] [CrossRef]
- Viciu, M.S.; Kelly, R.A.; Stevens, E.D.; Naud, F.; Studer, M.; Nolan, S.P. Synthesis, Characterization, and Catalytic Activity of N-Heterocyclic Carbene (NHC) Palladacycle Complexes. Org. Lett. 2003, 5, 1479–1482. [Google Scholar] [CrossRef]
- Schnyder, A.; Indolese, A.F.; Studer, M.; Blaser, H.-U. A New Genertion of Air-Stable, Highly Active Pd Comnplexes for C-C and C-N Coupling Reactions with Aryl Chlorides. Angew. Chem. Int. Engl. Ed. 2002, 41, 3668–3671. [Google Scholar] [CrossRef]
- Navarro, O.; Marion, N.; Oonishi, Y.; Kelly, R.A.; Nolan, S.P. Suzuki-Miyaura, α-Ketone Arylation and Dehalogenation Reactions Catalyzed by a Versatile N-Heterocyclic Carbene-Palladacycle Complex. J. Org. Chem. 2006, 71, 685–692. [Google Scholar] [CrossRef]
- Navarro, O.; Kelly, R.A.; Nolan, S.P. A General Method for the Suzuki-Miyaura Cross-Coupling of Sterically Hindered Aryl Chlorides: Synthesis of Di- and Tri-Ortho-Substituted Biaryls in 2-Propanol at Room Temperature. J. Am. Chem. Soc. 2003, 125, 16194–16195. [Google Scholar] [CrossRef]
- Martynova, E.A.; Tzouras, N.V.; Pisanò, G.; Cazin, C.S.J.; Nolan, S.P. The “Weak Base Route” Leading to Transition Metal-N-Heterocyclic Carbene Complexes. Chem. Commun. 2021, 57, 3836–3856. [Google Scholar] [CrossRef]
- Ma, X.; Guillet, S.G.; Peng, M.; Van Hecke, K.; Nolan, S.P. A Simple Synthesis of [RuCl2(NHC)(p-Cymene)] Complexes and Their Use in Olefin Oxidation Catalysis. Dalt. Trans. 2021, 50, 3959–3965. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Guillet, S.G.; Liu, Y.; Cazin, C.S.J.; Nolan, S.P. Simple Synthesis of [Ru(CO3)(NHC)(p-Cymene)] Complexes and Their Use in Transfer Hydrogenation Catalysis. Dalt. Trans. 2021, 50, 13012–13019. [Google Scholar] [CrossRef]
- Liu, Y.; Scattolin, T.; Gobbo, A.; Beliš, M.; Van Hecke, K.; Nolan, S.P.; Cazin, C.S.J. A Simple Synthetic Route to Well-Defined [Pd(NHC)Cl(1-TBu-Indenyl)] Pre-Catalysts for Cross-Coupling Reactions. Eur. J. Inorg. Chem. 2022, 2022, e202100840. [Google Scholar] [CrossRef]
- Liu, Y.; Voloshkin, V.A.; Scattolin, T.; Peng, M.; Van Hecke, K.; Nolan, S.P.; Cazin, C.S.J. Versatile and Highly Efficient Trans[Pd(NHC)Cl2(DMS/THT)] Precatalysts for C−N and C−C Coupling Reactions in Green Solvents. Eur. J. Org. Chem. 2022, 2022, e202200309. [Google Scholar] [CrossRef]
- Zinser, C.M.; Warren, K.G.; Nahra, F.; Al-Majid, A.; Barakat, A.; Islam, M.S.; Nolan, S.P.; Cazin, C.S.J. Palladate Precatalysts for the Formation of C-N and C-C Bonds. Organometallics 2019, 38, 2812–2817. [Google Scholar] [CrossRef]
- Surry, D.S.; Buchwald, S.L. Biaryl Phosphane Ligands in Palladium-Catalyzed Amination. Angew. Chem. Int. Ed. 2008, 47, 6338–6361. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Ryberg, P.; Hartwig, J.F. Reevaluation of the Mechanism of the Amination of Aryl Halides Catalyzed by BINAP-Ligated Palladium Complexes. J. Am. Chem. Soc. 2006, 128, 3584–3591. [Google Scholar] [CrossRef] [PubMed]
- Muci, A.R.; Buchwald, S.L. Practical Palladium Catalysts for C-N and C-O Bond Formation. Top. Curr. Chem. 2002, 219, 131–209. [Google Scholar] [CrossRef]
- Titcomb, R.L.; Caddick, S.; Geoffrey, F.; Cloke, N.; Wilson, D.J.; McKerrecher, D. Unexpected reactivity of two-coordinate palladium–carbene complexes; synthetic and catalytic implications. Chem. Commun. 2001, 15, 1388–1389. [Google Scholar] [CrossRef]
- Meiries, S.; Le Duc, G.; Chartoire, A.; Collado, A.; Speck, K.; Arachchige, K.S.A.; Slawin, A.M.Z.; Nolan, S.P. Large yet Flexible N-Heterocyclic Carbene Ligands for Palladium Catalysis. Chem.-A Eur. J. 2013, 19, 17358–17368. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Z.X. Nickel-Catalyzed Amination of Aryl 2-Pyridyl Ethers via Cleavage of the Carbon–Oxygen Bond. Org. Lett. 2017, 19, 3723–3726. [Google Scholar] [CrossRef]
- Yang, J.; Li, P.; Zhang, Y.; Wang, L. Dinuclear N-heterocyclic carbene palladium(II) complexes as efficient catalysts for the Buchwald–Hartwig amination. J. Organomet. Chem. 2014, 766, 73–78. [Google Scholar] [CrossRef]
- Krinsky, J.L.; Martínez, A.; Godard, C.; Castillón, S.; Claver, C. Modular Synthesis of Functionalisable Alkoxy-Tethered N-Heterocyclic Carbene Ligands and an Active Catalyst for Buchwald–Hartwig Aminations. Adv. Synth. Catal. 2014, 356, 460–474. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Fan, R. One-Pot Synthesis of Diarylamines from Two Aromatic Amines via Oxidative Dearomatization–Imino Exchange–Reductive Aromatization. Org. Lett. 2013, 15, 2018–2021. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Kang, Q.K.; Li, Y.; Wu, W.Q.; Zhu, H.; Shi, H. Catalytic Amination of Phenols with Amines. J. Am. Chem. Soc. 2022, 144, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, X.; Han, J.; Wu, W.; Wang, L. Direct arylation of tertiary amines via aryne intermediates using diaryliodonium salts. Tetrahedron Lett. 2018, 59, 1737–1741. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Xu, Y.T.; Shao, L.X. Synthesis of N-heterocyclic carbene-Pd(II)-5-phenyloxazole complexes and initial studies of their catalytic activity toward the Buchwald-Hartwig amination of aryl chlorides. J. Organomet. Chem. 2021, 940, 121683. [Google Scholar] [CrossRef]
- Cai, L.; Qian, X.; Song, W.; Liu, T.; Tao, X.; Li, W.; Xie, X. Effects of solvent and base on the palladium-catalyzed amination: PdCl2(Ph3P)2/Ph3P-catalyzed selective arylation of primary anilines with aryl bromides. Tetrahedron 2014, 70, 4754–4759. [Google Scholar] [CrossRef]
- Broggi, J.; Clavier, H.; Nolan, S.P. N-Heterocyclic Carbenes (NHCs) Containing N-C-Palladacycle Complexes: Synthesis and Reactivity in Aryl Amination Reactions. Organometallics 2008, 27, 5525–5531. [Google Scholar] [CrossRef]
- Liu, F.; Hu, Y.-Y.; Li, D.; Zhou, Q.; Lu, J.-M. N-Heterocyclic carbene-palladacyclic complexes: Synthesis, characterization and their applications in the C-N coupling and α-arylation of ketones using aryl chlorides. Tetrahedron 2018, 74, 5683–5690. [Google Scholar] [CrossRef]
- Deng, Q.; Zhang, Y.; Zhu, H.; Tu, T. Robust Acenaphthoimidazolylidene Palladacycles: Highly Efficient Catalysts for the Amination of N-Heteroaryl Chlorides. Chem. Asian J. 2017, 12, 2364–2368. [Google Scholar] [CrossRef]
- CrysAlis PRO 1.171.42.75a, Rigaku Oxford Diffraction; Rigaku Corporation (and its Global Subsidiaries): Tokyo, Japan, 2022.
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and Analysis of Crystal Structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]





 | ||||||
| Entry | Solvent | Base | T (°C) | Time (h) | Conv. a (%) | Yield a (%) |
|---|---|---|---|---|---|---|
| 1 | Acetone | K2CO3 | 60 | 2 | >99 | 91 |
| 2 | Acetone | K2CO3 | 40 | 12 | >99 | 82 |
| 3 | Acetone | Et3N | 60 | 24 | 0 | 0 |
| 4 | Acetone | NaOAc | 60 | 6 | >99 | 66 |
| 5 | Toluene | K2CO3 | 40 | 18 | >99 | 52 |
| 6 | Toluene | K2CO3 | 60 | 18 | >99 | 52 |
| 7 | Toluene | K2CO3 | 80 | 6 | >99 | 58 |
| 8 | Toluene | K2CO3 | 100 | 1 | >99 | 90 |
| 9 | EtOAc | K2CO3 | 40 | 3 | >99 | 71 |
| 10 | EtOAc | K2CO3 | 50 | 3 | >99 | 82 |
| 11 | EtOAc | K2CO3 | 60 | 3 | >99 | 75 |
| 12 | EtOH | K2CO3 | 40 | 3 | >99 | 3 |
 | ||||||
| Entry | Loading Cat. [mol%] | Solvent | Base | Temp. [°C] | Time [h] | Conversion [%] a |
|---|---|---|---|---|---|---|
| 1 | 1 | THF | KOtBu | 80 | 4 | 83 |
| 2 | 1 | Me-THF | KOtBu | 80 | 4 | 91 |
| 3 | 1 | 1,4-dioxane | KOtBu | 80 | 4 | 98 |
| 4 | 1 | 1,4-dioxane | KOtBu | 100 | 4 | 100 |
| 5 | 1 | CPME | KOtBu | 100 | 4 | 99 |
| 6 | 1 | CPME | KOtBu | 80 | 4 | 100 |
| 7 | 1 | CPME | KOtBu | 80 | 2 | 100 |
| 8 | 1 | CPME | KOtBu | 80 | 1 | 94 |
| 9 | 1 | CPME | KOtBu | 60 | 24 | NR |
| 10 | 1 | CPME | KOtBu | 70 | 24 | NR |
| 11 | 1 | CPME | K2CO3 | 80 | 24 | NR |
| 12 | 1 | CPME | NaOAc | 80 | 2 | 62 (63) b |
| 13 | 1 | CPME | Cs2CO3 | 80 | 2 | NR |
| 14 | 0.5 | CPME | KOtBu | 80 | 2 | 100 |
| 15 | 0.5 | CPME | KOtBu | 80 | 1 | 98 |
| 16 | 0.2 | CPME | KOtBu | 80 | 2 | 8 (8) b |
| 17 | 0.3 | CPME | KOtBu | 80 | 2 | 14 (15) b |
| 18 | 0.4 | CPME | KOtBu | 80 | 2 | 33 (54) b |
| 19 c | 0.5 | CPME | KOtBu | 80 | 24 | NR |
| 20 d | 0.5 | CPME | KOtBu | 80 | 2 | 84 (84) b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostrowska, S.; Palio, L.; Czapik, A.; Bhandary, S.; Kwit, M.; Van Hecke, K.; Nolan, S.P. A Second-Generation Palladacycle Architecture Bearing a N-Heterocyclic Carbene and Its Catalytic Behavior in Buchwald–Hartwig Amination Catalysis. Catalysts 2023, 13, 559. https://doi.org/10.3390/catal13030559
Ostrowska S, Palio L, Czapik A, Bhandary S, Kwit M, Van Hecke K, Nolan SP. A Second-Generation Palladacycle Architecture Bearing a N-Heterocyclic Carbene and Its Catalytic Behavior in Buchwald–Hartwig Amination Catalysis. Catalysts. 2023; 13(3):559. https://doi.org/10.3390/catal13030559
Chicago/Turabian StyleOstrowska, Sylwia, Lorenzo Palio, Agnieszka Czapik, Subhrajyoti Bhandary, Marcin Kwit, Kristof Van Hecke, and Steven P. Nolan. 2023. "A Second-Generation Palladacycle Architecture Bearing a N-Heterocyclic Carbene and Its Catalytic Behavior in Buchwald–Hartwig Amination Catalysis" Catalysts 13, no. 3: 559. https://doi.org/10.3390/catal13030559
APA StyleOstrowska, S., Palio, L., Czapik, A., Bhandary, S., Kwit, M., Van Hecke, K., & Nolan, S. P. (2023). A Second-Generation Palladacycle Architecture Bearing a N-Heterocyclic Carbene and Its Catalytic Behavior in Buchwald–Hartwig Amination Catalysis. Catalysts, 13(3), 559. https://doi.org/10.3390/catal13030559









