MIL-53(Fe)@perylene Diimide All-Organic Heterojunctions for the Enhanced Photocatalytic Removal of Pollutants and Selective Oxidation of Benzyl Alcohol
Abstract
1. Introduction
2. Results and Discussion
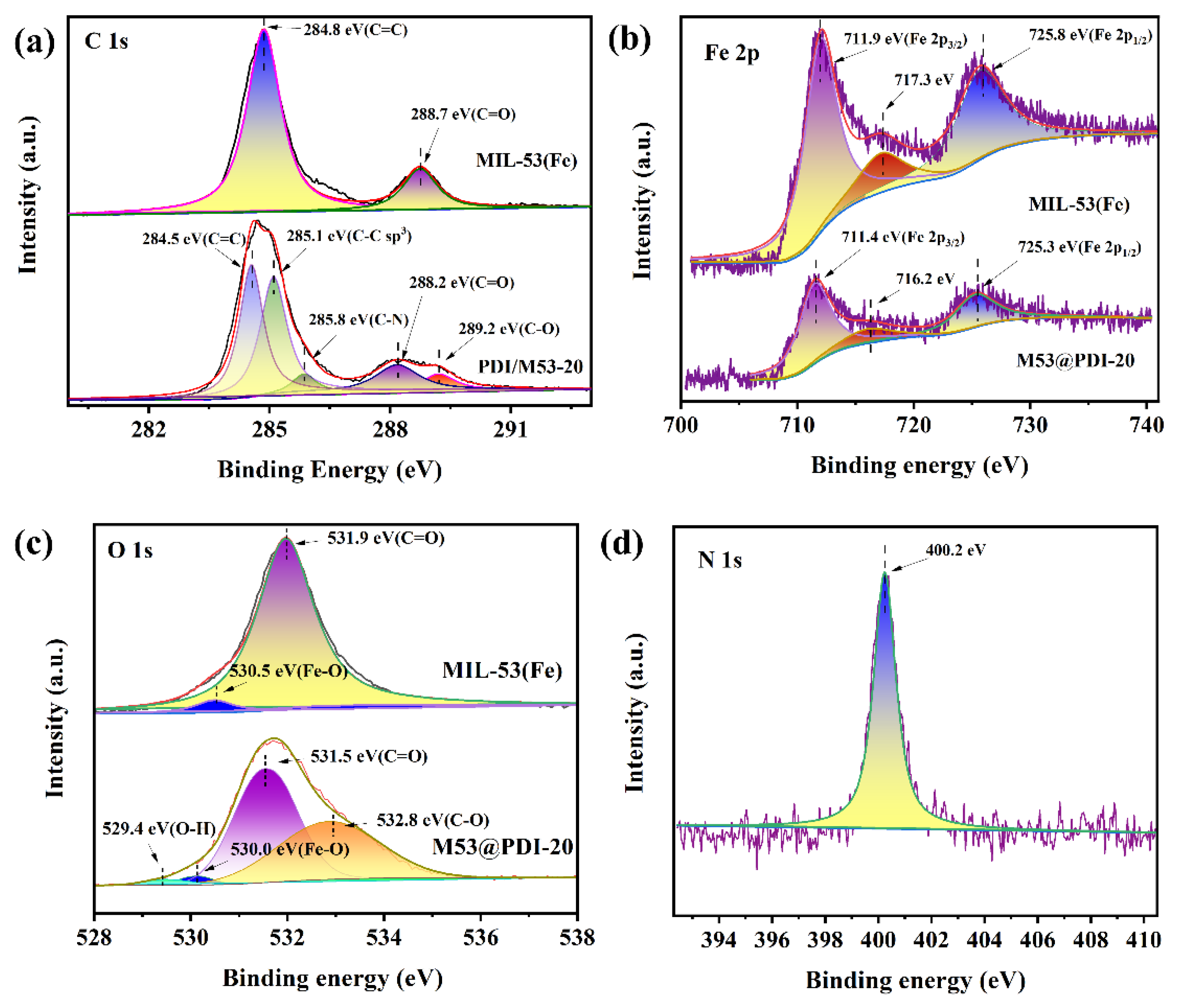
2.1. Structural Characterizations
2.2. Morphological Analysis
2.3. UV–Vis DRS Analysis
2.4. Photocatalytic Performances
2.4.1. Photocatalytic Degradation of RhB and Cr(VI) Pollutants
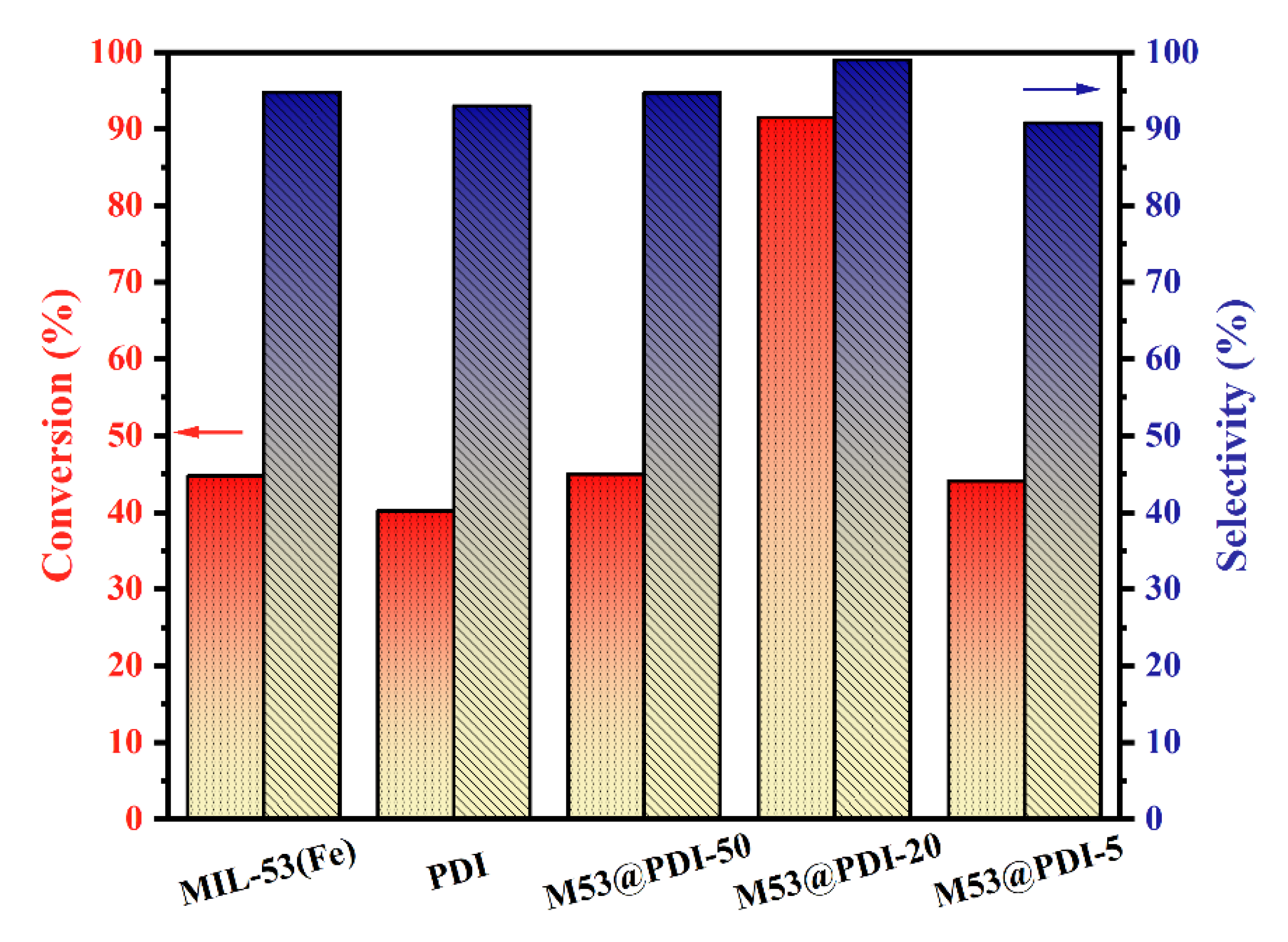
2.4.2. Evaluation of the Selective Oxidation of Benzyl Alcohol
2.5. Photoelectrochemical and Optical Properties
2.6. Contact Angle Analysis
2.7. Active Species Capturing Experiments and Possible Mechanism
3. Experimental Section
3.1. Synthesis of MIL-53(Fe)
3.2. Synthesis of PDI
3.3. Synthesis of PDI/MIL-53(Fe) all-Organic Heterojunctions
3.4. Photocatalytic Performances
3.4.1. Photocatalytic Degradations of the Simulated Pollutants
3.4.2. Photocatalytic Cr(VI) Reduction
3.4.3. Photocatalytic Selective Oxidation of Benzyl Alcohol
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Murdoch, M.; Waterhouse, G.I.; Nadeem, M.A.; Metson, J.B.; Keane, M.A.; Howe, R.F.; Llorca, J.; Idriss, H. The Effect of Gold Loading and Particle Size on Photocatalytic Hydrogen Production from Ethanol over Au/TiO2 Nanoparticles. Nat. Chem. 2011, 3, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Murgolo, S.; Franz, S.; Arab, H.; Bestetti, M.; Falletta, E.; Mascolo, G. Degradation of Emerging Organic Pollutants in Wastewater Effluents by Electrochemical Photocatalysis on Nanostructured TiO2 Meshes. Water Res. 2019, 164, 114920. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xu, X.; Liang, X.; Lei, C.; Wei, Y.; He, P.; Lv, B.; Ma, H.; Lei, Z. MIL-53(Fe)-Graphene Nanocomposites: Efficient Visible-Light Photocatalysts for the Selective Oxidation of Alcohols. Appl. Catal. B Environ. 2016, 198, 112–123. [Google Scholar] [CrossRef]
- Tayebi, M.; Masoumi, Z.; Kolaei, M.; Tayyebi, A.; Tayebi, M.; Seo, B.; Lim, C.-S.; Kim, H.-G.; Lee, B.-K. Highly Efficient and Stable WO3/MoS2-MoOX Photoanode for Photoelectrochemical Hydrogen Production; a Collaborative Approach of Facet Engineering and P-N Junction. Chem. Eng. J. 2022, 446, 136830. [Google Scholar] [CrossRef]
- Chen, F.; Huang, H.; Guo, L.; Zhang, Y.; Ma, T. The Role of Polarization in Photocatalysis. Angew. Chem. Int. Ed. 2019, 58, 10061–10073. [Google Scholar] [CrossRef]
- Masoumi, Z.; Tayebi, M.; Kolaei, M.; Lee, B.-K. Improvement of Surface Light Absorption of ZnO Photoanode Using a Double Heterojunction with α–Fe2O3/g–C3N4 Composite to Enhance Photoelectrochemical Water Splitting. Appl. Surf. Sci. 2023, 608, 154915. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Wu, C.; Gao, J.; Li, M.; Xing, Z.; Li, Z.; Zhou, W. Hollow Nanoboxes Cu2-xS@ZnIn2S4 Core-Shell S-Scheme Heterojunction with Broad-Spectrum Response and Enhanced Photothermal-Photocatalytic Performance. Small 2022, 18, e2202544. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, L.; Luo, B.; Lyu, M.; Wang, Z.; Huang, H.; Wang, S.; Du, A.; Wang, L. Molten-Salt-Mediated Synthesis of an Atomic Nickel Co-Catalyst on TiO2 for Improved Photocatalytic H2 Evolution. Angew. Chem. Int. Ed. 2020, 59, 7230–7234. [Google Scholar] [CrossRef]
- Peng, X.; Wu, J.; Zhao, Z.; Wang, X.; Dai, H.; Wei, Y.; Xu, G.; Hu, F. Activation of Peroxymonosulfate by Single Atom Co-N-C Catalysts for High-Efficient Removal of Chloroquine Phosphate via Non-Radical Pathways: Electron-Transfer Mechanism. Chem. Eng. J. 2022, 429, 132245. [Google Scholar] [CrossRef]
- Dai, W.; Jiang, L.; Wang, J.; Pu, Y.; Zhu, Y.; Wang, Y.; Xiao, B. Efficient and Stable Photocatalytic Degradation of Tetracycline Wastewater by 3D Polyaniline/Perylene Diimide Organic Heterojunction under Visible Light Irradiation. Chem. Eng. J. 2020, 397, 125476. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Y.; Wu, Y.; Jiang, Y.; Wang, Q.; Lin, X.; Song, G.; Huang, K.; Yao, Z. An Efficient Approach for Rapid Detection of Polymyxins B Based on the Optically Active Supramolecular Aggregates of Water-Soluble Perylene Diimide. Sens. Actuators B Chem. 2020, 321, 128594. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Liu, D. Recent Developments of Perylene Diimide (PDI) Supramolecular Photocatalysts: A Review. J. Photochem. Photobiol. C Photochem. Rev. 2021, 48, 100436. [Google Scholar] [CrossRef]
- Liu, D.; Wang, J.; Bai, X.; Zong, R.; Zhu, Y. Self-Assembled PDINH Supramolecular System for Photocatalysis under Visible Light. Adv. Mater. 2016, 28, 7284–7290. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, W.; Liu, D.; Zhang, Z.; Zhu, Y.; Wang, D. Supramolecular Organic Nanofibers with Highly Efficient and Stable Visible Light Photooxidation Performance. Appl. Catal. B Environ. 2017, 202, 289–297. [Google Scholar] [CrossRef]
- Gerdes, R.; Woehrle, D.; Spiller, W.; Schneider, G.; Schnurpfeil, G.; Schulz-Ekloff, G. Photo-Oxidation of Phenol and Monochlorophenols in Oxygen-Saturated Aqueous Solutions by Different Photosensitizers. J. Photochem. Photobiol. A 1997, 111, 65–74. [Google Scholar] [CrossRef]
- Sheng, Y.; Li, W.; Xu, L.; Zhu, Y. High Photocatalytic Oxygen Evolution via Strong Built-In Electric Field Induced by High Crystallinity of Perylene Imide Supramolecule. Adv. Mater. 2022, 34, e2102354. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Ma, M.; Li, W.; Yang, J.; Miao, H.; Zhang, Z.; Zhu, Y. Enhanced Photocatalytic Activity of PTCDI-C60 via π–π Interaction. Appl. Catal. B Environ. 2018, 238, 302–308. [Google Scholar] [CrossRef]
- Zeng, W.; Cai, T.; Liu, Y.; Wang, L.; Dong, W.; Chen, H.; Xia, X. An Artificial Organic-Inorganic Z-Scheme Photocatalyst WO3@Cu@PDI Supramolecular with Excellent Visible Light Absorption and Photocatalytic Activity. Chem. Eng. J. 2020, 381, 122691. [Google Scholar] [CrossRef]
- Miao, H.; Yang, J.; Wei, Y.; Li, W.; Zhu, Y. Visible-Light Photocatalysis of PDI Nanowires Enhanced by Plasmonic Effect of the Gold Nanoparticles. Appl. Catal. B Environ. 2018, 239, 61–67. [Google Scholar] [CrossRef]
- Yin, H.; Sui, M.-Y.; Pan, Q.-Q.; Sun, G.-Y.; Geng, Y. A Series of Bowl-Shaped PDI Dimers Designed for Organic Photovoltaic Cells through Engineering N-Annulated Bridge towards Potential Alternatives of PDI Bridged Dimer Acceptors. Dye. Pigment. 2018, 148, 394–404. [Google Scholar] [CrossRef]
- Yang, J.; Jing, J.; Li, W.; Zhu, Y. Electron Donor-Acceptor Interface of TPPS/PDI Boosting Charge Transfer for Efficient Photocatalytic Hydrogen Evolution. Adv. Sci. 2022, 9, e2201134. [Google Scholar] [CrossRef] [PubMed]
- Bavykina, A.; Kolobov, N.; Khan, I.S.; Bau, J.A.; Ramirez, A.; Gascon, J. Metal-Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120, 8468–8535. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, G.; Park, I.H.; Medishetty, R.; Vittal, J.J. Two-Dimensional Metal-Organic Framework Materials: Synthesis, Structures, Properties and Applications. Chem. Rev. 2021, 121, 3751–3891. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, S.; Kumar, R.; Deep, A.; Kurade, M.B.; Ji, S.-W.; Jeon, B.-H. Metal–Organic Frameworks (MOFs) for the Removal of Emerging Contaminants from Aquatic Environments. Coord. Chem. Rev. 2019, 380, 330–352. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, Y.; Li, G. Promotion of Sulfameter Degradation by Coupling Persulfate and Photocatalytic Advanced Oxidation Processes with Fe-Doped MOFs. Sep. Purif. Technol. 2022, 282, 119632. [Google Scholar] [CrossRef]
- George, P.; Chowdhury, P. Enhanced Photocatalytic Performance of Novel S2− Doped MIL-53(Fe) under Visible Light. J. Alloys Compd. 2021, 850, 156578. [Google Scholar] [CrossRef]
- Dinh Du, P.; Ngoc Hoai, P.; Ţălu, Ş. Synthesis of MIL-53(Fe) Metal-Organic Framework Material and Its Application as a Catalyst for Fenton-Type Oxidation of Organic Pollutants. Adv. Mater. Sci. Eng. 2021, 2021, 5540344. [Google Scholar] [CrossRef]
- Feng, X.; Jena, H.S.; Krishnaraj, C.; Leus, K.; Wang, G.; Chen, H.; Jia, C.; Van Der Voort, P. Generating Catalytic Sites in UiO-66 through Defect Engineering. ACS Appl. Mater. Interfaces 2021, 13, 60715–60735. [Google Scholar] [CrossRef]
- Fu, Y. Enhanced Photocatalytic CO2 Reduction over Co-Doped NH2-MIL-125(Ti) under Visible Light. RSC Adv. 2017, 7, 42819–42825. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Ao, D. Construction of Heterostructured ZnIn2S4@NH2-MIL-125(Ti) Nanocomposites for Visible-Light-Driven H2 Production. Appl. Catal. B Environ. 2018, 221, 433–442. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Zhang, J.; Tang, L.; Luo, L.; Zeng, G. Iron Containing Metal-Organic Frameworks: Structure, Synthesis, and Applications in Environmental Remediation. ACS Appl. Mater. Interfaces 2017, 9, 20255–20275. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liang, R.; Zhou, C.; Yan, G.; Wu, L. Carbon Quantum Dots (CQDs)/Noble Metal Co-Decorated MIL-53(Fe) as Difunctional Photocatalysts for the Simultaneous Removal of Cr(VI) and Dyes. Sep. Purif. Technol. 2021, 255, 117725. [Google Scholar] [CrossRef]
- Huang, W.; Liu, N.; Zhang, X.; Wu, M.; Tang, L. Metal Organic Framework G-C3N4/MIL-53(Fe) Heterojunctions with Enhanced Photocatalytic Activity for Cr(VI) Reduction under Visible Light. Appl. Surf. Sci. 2017, 425, 107–116. [Google Scholar] [CrossRef]
- Du, J.-J.; Yuan, Y.-P.; Sun, J.-X.; Peng, F.-M.; Jiang, X.; Qiu, L.-G.; Xie, A.-J.; Shen, Y.-H.; Zhu, J.-F. New Photocatalysts Based on MIL-53 Metal–Organic Frameworks for the Decolorization of Methylene Blue Dye. J. Hazard. Mater. 2011, 190, 945–951. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, W.; Liu, Y.; Dong, W.; Cai, T.; Tang, L.; Li, J.; Li, W. Unique MIL-53(Fe)/PDI Supermolecule Composites: Z-Scheme Heterojunction and Covalent Bonds for Uprating Photocatalytic Performance. ACS Appl. Mater. Interfaces 2021, 13, 16364–16373. [Google Scholar] [CrossRef]
- Yang, J.; Miao, H.; Jing, J.; Zhu, Y.; Choi, W. Photocatalytic Activity Enhancement of PDI Supermolecular via π-π Action and Energy Level Adjusting with Graphene Quantum Dots. Appl. Catal. B Environ. 2021, 281, 119547. [Google Scholar] [CrossRef]
- Ma, Y.; Li, M.; Jiang, J.; Li, T.; Wang, X.; Song, Y.; Dong, S. In-Situ Prepared MIL-53(Fe)/BiOI Photocatalyst for Efficient Degradation of Tetracycline under Visible-Light Driven Photo-Fenton System: Investigation of Performance and Mechanism. J. Alloys Compd. 2021, 870, 159524. [Google Scholar] [CrossRef]
- Cui, Y.; Nengzi, L.; Gou, J.; Huang, Y.; Li, B.; Cheng, X. Fabrication of Dual Z-Scheme MIL-53(Fe)/α-Bi2O3/g-C3N4 Ternary Composite with Enhanced Visible Light Photocatalytic Performance. Sep. Purif. Technol. 2020, 232, 115959. [Google Scholar] [CrossRef]
- Tang, L.; Lv, Z.; Xue, Y.; Xu, L.; Qiu, W.; Zheng, C.; Chen, W.; Wu, M. MIL-53(Fe) Incorporated in the Lamellar BiOBr: Promoting the Visible-Light Catalytic Capability on the Degradation of Rhodamine B and Carbamazepine. Chem. Eng. J. 2019, 374, 975–982. [Google Scholar] [CrossRef]
- Geng, N.; Chen, W.; Xu, H.; Ding, M.; Lin, T.; Wu, Q.; Zhang, L. Insights into the Novel Application of Fe-MOFs in Ultrasound-Assisted Heterogeneous Fenton System: Efficiency, Kinetics and Mechanism. Ultrason. Sonochem. 2021, 72, 105411. [Google Scholar] [CrossRef]
- Wang, X.; Meng, J.; Yang, X.; Hu, A.; Yang, Y.; Guo, Y. Fabrication of a Perylene Tetracarboxylic Diimide–Graphitic Carbon Nitride Heterojunction Photocatalyst for Efficient Degradation of Aqueous Organic Pollutants. ACS Appl. Mater. Interfaces 2019, 11, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Guan, J.; Mu, P.; Yang, K.; Xie, Y.; Li, X.; Zou, L.; Huang, W.; Yu, C.; Dai, W. Visible and Near-Infrared Driven Yb3+/Tm3+ Co-Doped InVO4 Nanosheets for Highly Efficient Photocatalytic Applications. Dalton Trans. 2020, 49, 14030–14045. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Miao, H.; Li, W.; Li, H.; Zhu, Y. Designed Synthesis of a P-Ag2S/n-PDI Self-Assembled Supramolecular Heterojunction for Enhanced Full-Spectrum Photocatalytic Activity. J. Mater. Chem. A 2019, 7, 6482–6490. [Google Scholar] [CrossRef]
- Wang, J.; Liu, D.; Zhu, Y.; Zhou, S.; Guan, S. Supramolecular Packing Dominant Photocatalytic Oxidation and Anticancer Performance of PDI. Appl. Catal. B Environ. 2018, 231, 251–261. [Google Scholar] [CrossRef]
- Masoumi, Z.; Tayebi, M.; Kolaei, M.; Lee, B.-K. Efficient and Stable Core-Shell α–Fe2O3/WS2/WOx Photoanode for Oxygen Evolution Reaction to Enhance Photoelectrochemical Water Splitting. Appl. Catal. B Environ. 2022, 313, 121447. [Google Scholar] [CrossRef]
- Li, X.; Kang, B.; Dong, F.; Zhang, Z.; Luo, X.; Han, L.; Huang, J.; Feng, Z.; Chen, Z.; Xu, J.; et al. Enhanced Photocatalytic Degradation and H2/H2O2 Production Performance of S-PCN/WO2.72 S-Scheme Heterojunction with Appropriate Surface Oxygen Vacancies. Nano Energy 2021, 81, 105671. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, K.; Yu, C.; Lu, K.; Huang, W.; Xu, L.; Zou, L.; Wang, S.; Chen, Z.; Hu, J.; et al. Steering Unit Cell Dipole and Internal Electric Field by Highly Dispersed Er Atoms Embedded into NiO for Efficient CO2 Photoreduction. Adv. Funct. Mater. 2022, 32, 2111999. [Google Scholar] [CrossRef]
- Li, H.; Wang, C.; Bai, X.; Wang, X.; Sun, B.; Li, D.; Zhao, L.; Zong, R.; Hao, D. In-Plane Polarization Induced by the Hydrogen Bonding and π–π Stacking of Functionalized PDI Supramolecules for the Efficient Photocatalytic Degradation of Organic Pollutants. Mater. Chem. Front. 2020, 4, 2673–2687. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, K.; Wang, F.; Li, X.; Huang, W.; Lu, K.-Q.; Yu, C.; Yang, K. MIL-53(Fe)@perylene Diimide All-Organic Heterojunctions for the Enhanced Photocatalytic Removal of Pollutants and Selective Oxidation of Benzyl Alcohol. Catalysts 2023, 13, 471. https://doi.org/10.3390/catal13030471
Shi K, Wang F, Li X, Huang W, Lu K-Q, Yu C, Yang K. MIL-53(Fe)@perylene Diimide All-Organic Heterojunctions for the Enhanced Photocatalytic Removal of Pollutants and Selective Oxidation of Benzyl Alcohol. Catalysts. 2023; 13(3):471. https://doi.org/10.3390/catal13030471
Chicago/Turabian StyleShi, Kaiyang, Fulin Wang, Xiangwei Li, Weiya Huang, Kang-Qiang Lu, Changlin Yu, and Kai Yang. 2023. "MIL-53(Fe)@perylene Diimide All-Organic Heterojunctions for the Enhanced Photocatalytic Removal of Pollutants and Selective Oxidation of Benzyl Alcohol" Catalysts 13, no. 3: 471. https://doi.org/10.3390/catal13030471
APA StyleShi, K., Wang, F., Li, X., Huang, W., Lu, K.-Q., Yu, C., & Yang, K. (2023). MIL-53(Fe)@perylene Diimide All-Organic Heterojunctions for the Enhanced Photocatalytic Removal of Pollutants and Selective Oxidation of Benzyl Alcohol. Catalysts, 13(3), 471. https://doi.org/10.3390/catal13030471







