Abstract
Metal oxide clusters composed of group 5 metal ions, such as Nb and Ta, exhibit catalytic activities for CO2 fixation to styrene oxide (SO) due to the highly negative natural bonding charge of the terminal O atoms that could work as CO2 activation sites. In this study, tetrabutylammonium (TBA) salts of [TaxNb6−xO19]8− (TBA-TaxNb6−x, x = 0–6) were prepared and Ta-substitution effect on the catalytic properties of TBA-TaxNb6−x for CO2 fixation to SO was investigated. We found that TBA-Ta1Nb5 shows the highest styrene carbonate (SC) selectivity (95%) among TBA-TaxNb6−x, although the SO conversion monotonously increases with the incremental Ta substitution amount. The CO2 fixation to SO under various conditions and in situ X-ray absorption fine structure measurements reveal that CO2 is activated on both terminal O sites coordinated to the Ta (terminal OTa) and Nb (terminal ONb) sites, whereas the activation of SO proceeds on the terminal OTa and/or bridge O sites that are connected to Ta. Density functional theory (DFT) calculations reveal that the terminal OTa of TBA-Ta1Nb5 preferentially adsorbs CO2 compared with other ONb base sites. We conclude that the selective CO2 activation at terminal OTa of TBA-Ta1Nb5 without SO activation is a crucial factor for high SC selectivity in the CO2 fixation to SO.
1. Introduction
Several CO2 usage approaches have been implemented to reduce the impact of emitted greenhouse gases and to achieve a carbon neutral level. CO2 utilization in chemical production gained significant attention in replacing traditionally used C1 sources such as phosgene or CO, which post more toxicity. However, the activation of CO2 is challenging due to its thermodynamic stability, which requires appropriate catalysts, such as solid base catalysts, for instance, alkaline earth metal oxides. One of the useful reactions is CO2 cycloaddition to epoxides, which form cyclic carbonates. The resultant cyclic carbonates could be applied as solvents [1,2,3,4,5], monomers [6,7,8,9], electrolytes [10,11,12,13], and pharmaceuticals [14,15].
Recent applications of metal oxide clusters, namely, polyoxometalates, as base catalysts have been reported [16,17,18,19,20,21,22]. One of the advantages of utilizing metal oxide clusters over bulk solid base catalysts is that it does not require the surface activation of catalysts [23]. Up to now, the basicity of metal oxide clusters depends on the structures and the type of metal ions. The Lindqvist-type polyoxotungstate [W6O19]2− shows the basicity with pKa value of 11.1 and defective Keggin-type Ge-incorporated polyoxotungstate [γ-H2GeW10O36]6− exhibits high basicity with a pKa value of 21.9 [24]. The series of group 5 metal polyoxometalates exhibit superior basicity to those of group 6 metal polyoxometalates, and the basicities of [Nb10O28]6−, [Nb6O19]8−, and [Ta6O19]8− increase to a pKa value of 23.8 [17,18]. Recently, Uchida’s group reported that porous ionic crystals containing Nb/Ta were applied to Knoevenagel condensation reactions as base catalyst [19]. Density functional theory (DFT) calculations reveal that the base strength of the clusters is related to the natural bond orbital (NBO) charges of the surface O atoms and the higher negativity of the NBO charges leads to stronger basicity [16,18]. We reported that Lindqvist-type polyoxometalates with group 5 metal ions (Nb, Ta) had higher negative NBO charges compared to group 6 metal ions (Mo, W) and [Nb6O19]8− and [Ta6O19]8− could activate CO2 and worked as catalysts for CO2 fixation and conversion reactions [18,25]. The CO2 was activated on the terminal O sites of metal oxide clusters, which are Lewis base sites, and activated CO2 reacts with epoxides to form carbonates [17,18,21]. The cycloaddition of CO2 to epichlorohydrin proceeded on Keggin-type Na16[SiNb12O40] [21]. In the case of Lindqvist-type [M6O19]8− (M = Nb, Ta), [Ta6O19]8− showed higher activity for CO2 fixation to styrene oxide (SO) at 403 K than [Nb6O19]8− [18]. However, the styrene carbonate (SC) selectivity of [Ta6O19]8− was lower than 90% and byproducts were formed. In our previous study, Brønsted basicity was investigated using sodium salts of [TaxNb6−xO19]8− as solid base catalyst in Knoevenagel condensation reactions and local symmetry of NbO6 and TaO6 units in the clusters affected base catalytic properties [23]. In this study, tetrabutylammonium (TBA) salts of mixed metal oxide clusters [TaxNb6−xO19]8− (TBA-TaxNb6−x, x = 0–6) were prepared and applied to CO2 fixation to SO to elucidate the Ta-substitution effect on the catalytic activities and selectivity. It was found that single-Ta-substituted TBA-Ta1Nb5 exhibited the highest SC selectivity among TBA-TaxNb6−x. We demonstrated that the high SC selectivity was achieved by the selective adsorption of CO2 on the terminal OTa without SO activation under reaction conditions.
2. Results
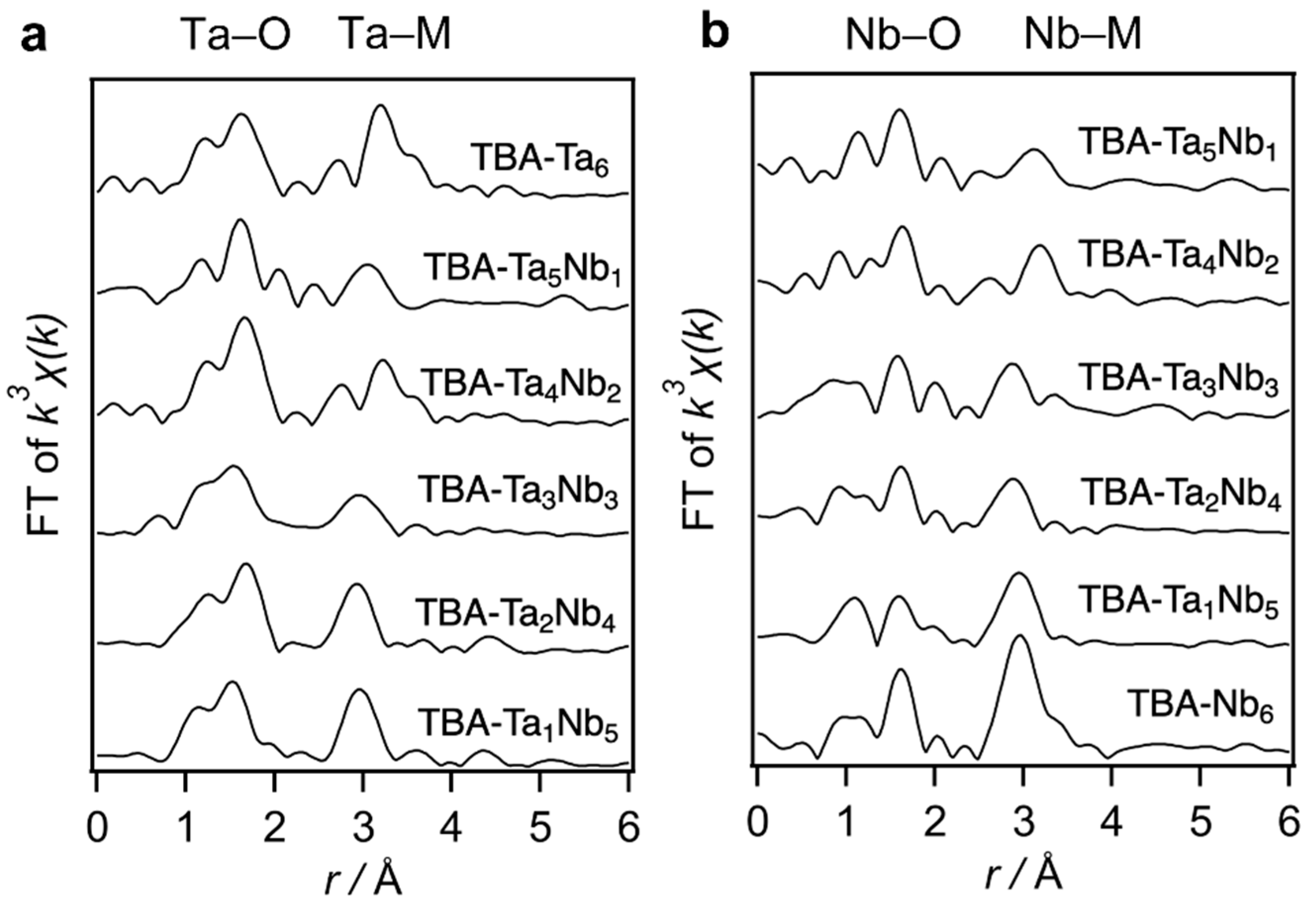
The fabricated TBA-TaxNb6−x were characterized by X-ray absorption spectroscopy (XAS), electrospray ionization mass spectrometry (ESI–MS), Fourier-transformed infrared (FT-IR) in attenuated total reflectance (ATR) mode, and elemental analysis (Figure 1, Figure S1 and Figure S2, and Table S1, respectively). ESI–MS suggests that the various components of Ta–Nb mixed metal oxide clusters are contained in the TBA-TaxNb6−x. Ta L3-edge Fourier-transformed extended X-ray absorption fine structure (FT-EXAFS) of TBA-TaxNb6−x indicates that peaks of Ta–M (M = Nb or Ta) shift to a longer length with increasing Ta content (Figure 1a). A similar peak shift of Nb–M (M = Nb or Ta) is observed in the Nb K-edge FT-EXAFS spectra (Figure 1b). Those indicate the Ta-substitution to Nb sites in [TaxNb6−xO19]8−. Elemental analysis reveals that TBA/[TaxNb6−xO19]8− ratio is 5~6.
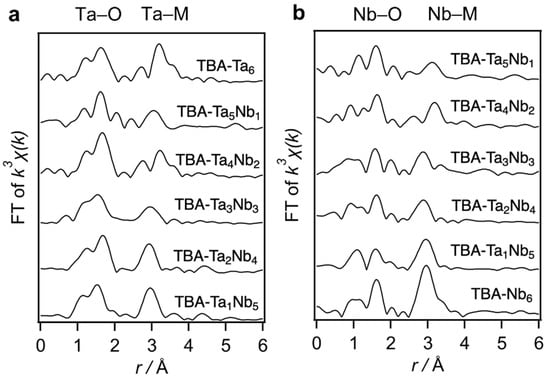
Figure 1.
(a) Ta L3-edge and (b) Nb K-edge FT-EXAFS spectra of TBA-TaxNb6−x. The Ta–O (Nb–O) and Ta–M (Nb–M) are shown in r ranges of 1.2–2.0 Å and 2.5–3.4 Å, respectively.
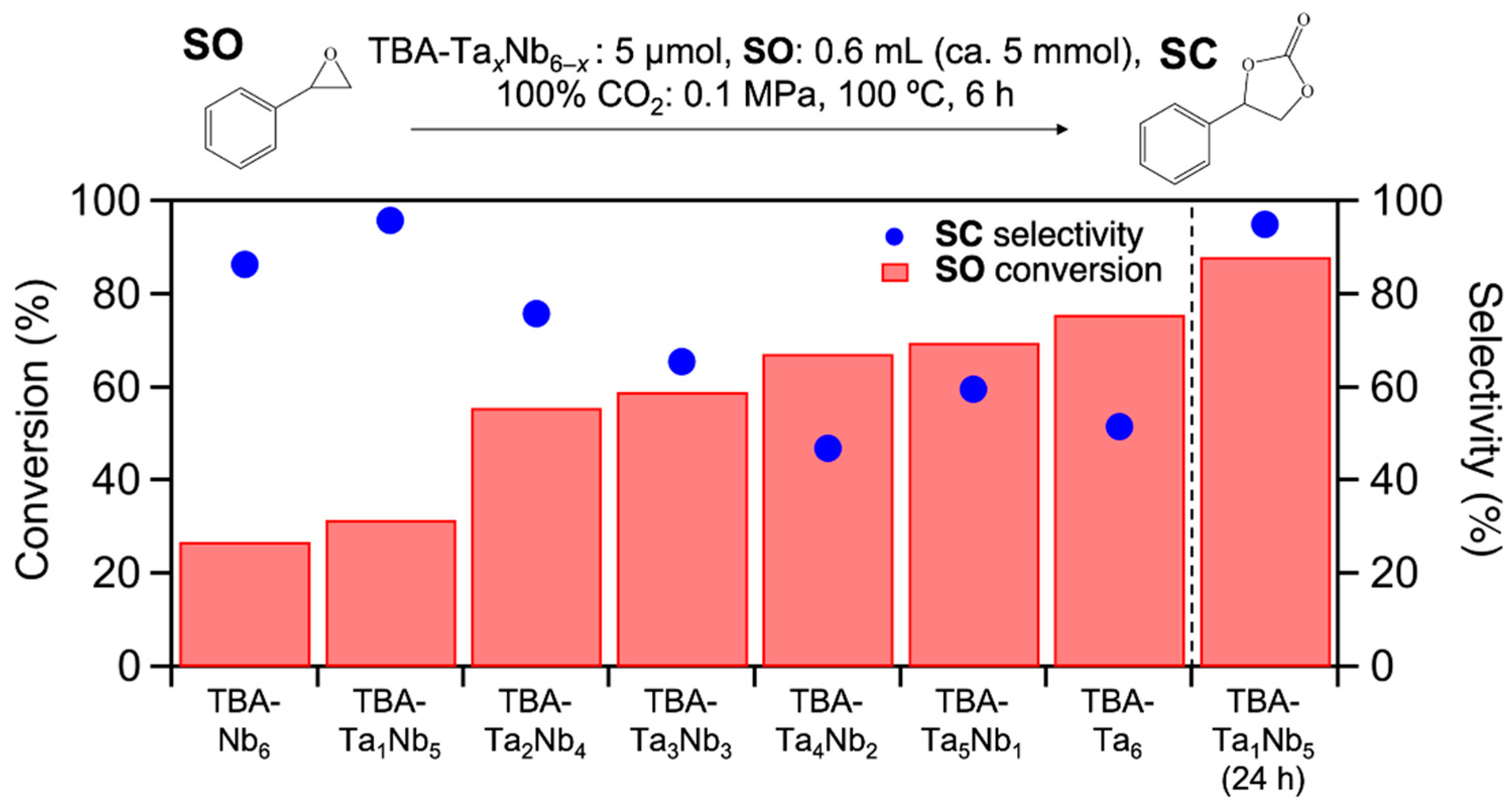
The prepared clusters were employed in the catalytic CO2 fixation to SO. Figure 2 shows the SO conversion and SC selectivity for TBA-TaxNb6−x catalysts. The SO conversion gradually increases with incremental addition of Ta content and TBA-Ta6 exhibits the highest SO conversion among them. On the other hand, the trend of SC selectivity for the composition of clusters differs from that of SO conversation (Figure 2). Interestingly, single-Ta-substituted TBA-Ta1Nb5 provides the highest SC selectivity (95%) among the mixed metal oxide clusters. Further Ta-substitution decreases SC selectivity and the SC selectivity becomes constant in Ta-rich TBA-TaxNb6−x catalysts (x = 4–6). The number of TBA counteractions has a negligible impact on this reaction, although TBA/[TaxNb6−xO19]8− ratio varies with the composition [17]. The byproducts in this reaction over TBA-Ta6 are polymers derived from the polymerization of SO, because the SO conversion is found for TBA-Ta6 at 100 °C under N2 atmosphere without CO2 despite negligible SO conversion for TBA-Nb6, as shown in Figure S3. The high SC selectivity (95%) of TBA-Ta1Nb5 maintains a high SO conversion (88%) for a 24 h reaction (Figure 2). In addition, the >95% SC selectivity is only achieved by TBA-Ta1Nb5 among the TBA-TaxNb6−x catalysts at >80% conversion (Figure S4). Thus, the selective CO2 fixation to SO is achieved by the single Ta substitution to TBA-Nb6.
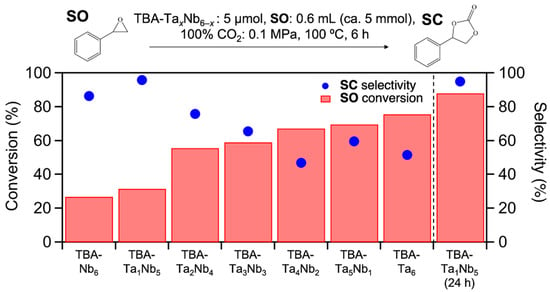
Figure 2.
Results of CO2 fixation to styrene oxide over TBA-TaxNb6−x. Bars represent conversion of SO, blue dots represent selectivity of SC. Reaction condition: catalyst loading = 5 µmol, SO = 0.6 mL (ca. 5 mmol), 100% CO2 = 0.1 MPa, temperature = 100 °C, reaction time = 6 h.
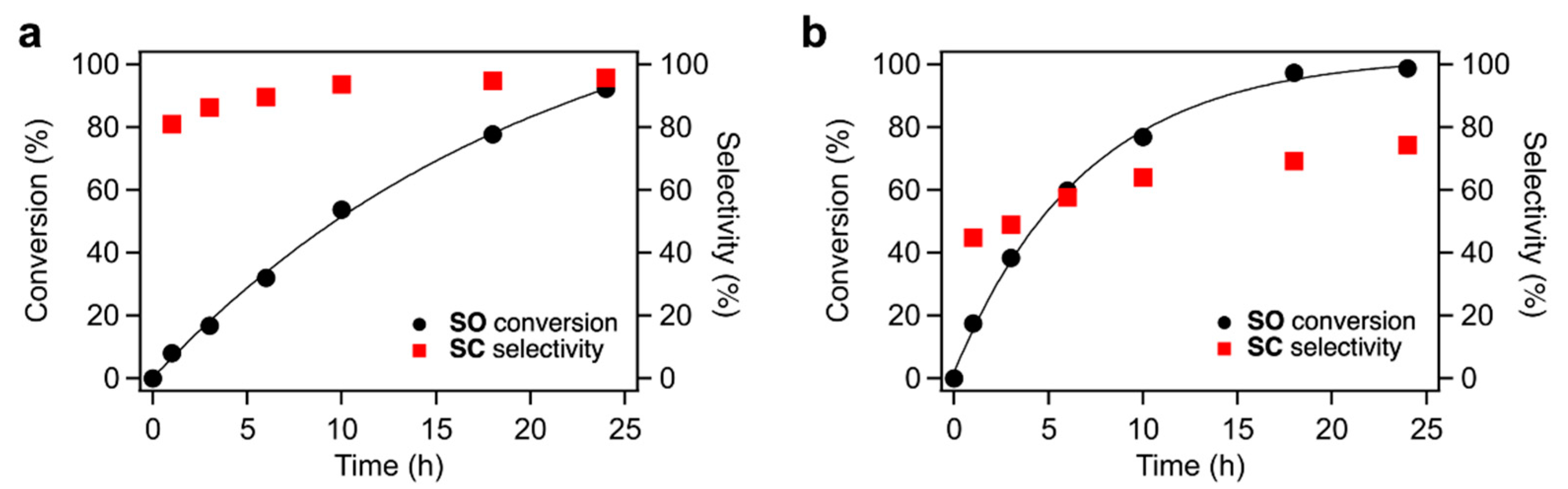
The time courses of CO2 fixation to SO over TBA-Ta6, TBA-Ta1Nb5, and TBA-Nb6 are shown in Figure 3 and Figure S5. The trends of conversion and selectivity depend on the composition of the clusters. TBA-Ta6 exhibits the highest reaction rate among them, and SC selectivity increases with reaction time. TBA-Ta1Nb5 and TBA-Nb6 show high SC selectivity at the initial stage of the reaction and the SC selectivity is maintained at a high SO conversion. Thus, TBA-Nb6 and single-Ta-substituted TBA-Ta1Nb5 have the specific active sites for selective SC formation. The increment in the SC selectivity over TBA-Ta6 is due to the consumption of SO and suppression of undesired reactions during the reaction.
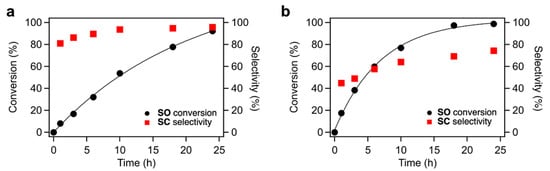
Figure 3.
Time courses of SO conversion in CO2 fixation to SO using (a) TBA-Ta1Nb5 and (b) TBA-Ta6 as the catalysts. Reaction condition: catalyst loading = 10 µmol, SO = 1.2 mL (ca. 10 mmol), 100% CO2 = 0.1 MPa, temperature = 100 °C.
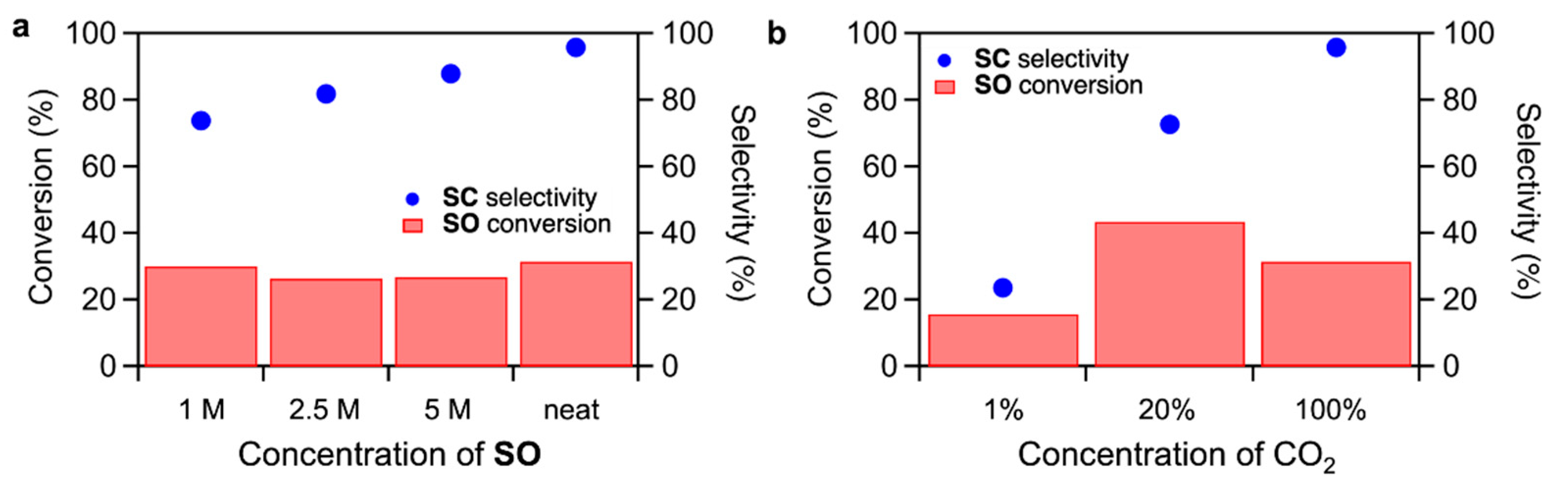
Effects of SO concentration and CO2 concentration on the CO2 fixation to SO were studied for TBA-Ta1Nb5 (Figure 4). When increasing the SO concentration in the dimethyl sulfoxide (DMSO) solution, the SC selectivity slightly increases while maintaining the SO conversion. On the other hand, the SC selectivity dramatically decreases with decreasing CO2 concentration (Figure 4b), suggesting the CO2 activation is a key step in the CO2 fixation to SO. We reported that CO2 fixation to SO proceeds due to the fact that the CO2 is activated on the terminal O sites and activated CO2 reacts with SO to form SC [17,18]. The rate determining step of CO2 fixation to SO is the nucleophilic attack of activated CO2 to SO. The above results could be explained by the reaction mechanism. The decrease in the SC yield by reducing SO concentration, as shown in Figure 4a, is due to the inhibition of the reaction of activated CO2 with SO by a low SO concentration. There are two reasons for the drastic decrease in SC selectivity by reducing CO2 concentration. One is the decrease in the amount of activated CO2. The other is that SO can be activated on TBA-Ta1Nb5 in low CO2 concentration conditions. In fact, the SO conversion of TBA-Ta1Nb5 under N2 atmosphere without CO2 is higher than that of TBA-Nb6, as shown in Figure S3, suggesting that the SO activation occurs by single Ta-substitution at a low CO2 concentration.
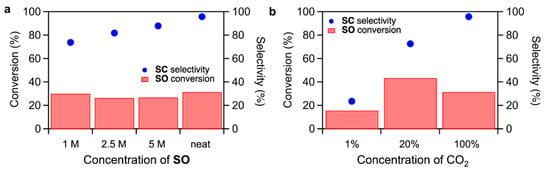
Figure 4.
Effect of (a) SO concentration and (b) CO2 concentration with N2 balance. Reaction conditions: (a) TBA-Ta1Nb5 = 5 µmol, DMSO = 1 mL, SO = 0.6 mL (ca. 5 mmol for neat condition), 100% CO2 = 0.1 MPa, temperature = 100 °C, reaction time = 6 h; (b) TBA-Ta1Nb5 = 5 µmol, SO = 0.6 mL (ca. 5 mmol), neat, CO2 (0.1 MPa), temperature = 100 °C, reaction time = 6 h.
3. Discussion
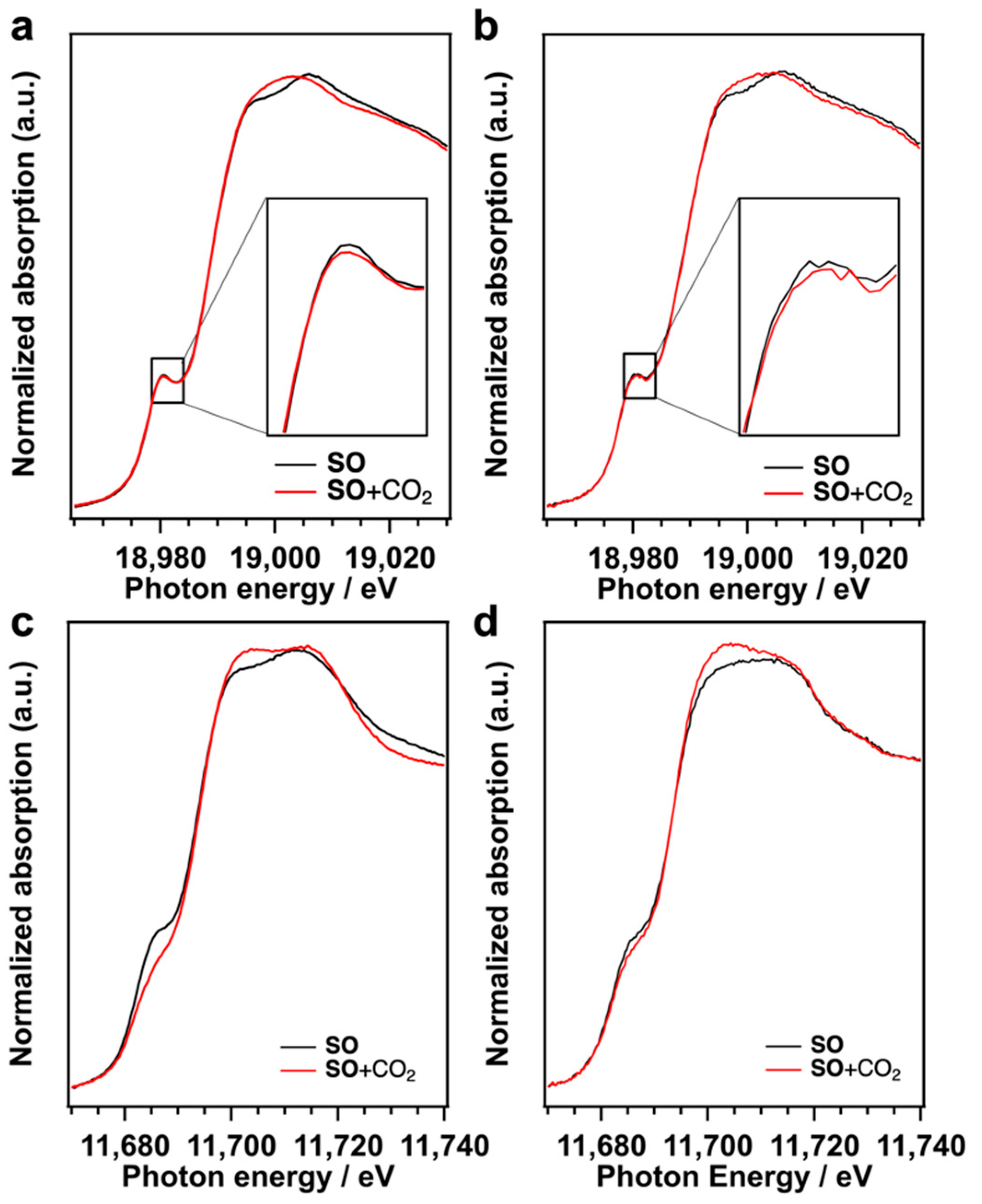
We reported that the CO2 fixation to SO proceeded on the terminal O sites of TBA-Ta6 because the terminal O sites, which have the negatively charged O, work as Lewis base sites [18]. The CO2 is activated on the terminal O sites and the activated CO2 reacts with SO to form SC. The CO2 adsorption on TBA-Nb6, TBA-Ta1Nb5, and TBA-Ta6 in SO was examined by in situ XAFS measurements (Figure 5). Nb K-edge XANES spectrum of TBA-Nb6 in SO exhibits a pre-edge peak at 18,980.5 eV assigned to electron excitation from 1s to hybridized 4d−5p [26,27]. This pre-edge peak intensity gives us the information on the distortion from NbO6 octahedral (Oh) symmetry. The pre-edge peak intensity decreases with the CO2 introduction to TBA-Nb6 in SO, which indicates that the Oh symmetry of NbO6 in TBA-Nb6 is improved by the CO2 addition. Similar results were obtained for TBA-Ta1Nb5, as shown in Figure 5b. Ta L1-edge XANES spectra indicate that the pre-edge peak at 11,686 eV, which is assigned to electron transition from Ta 2s orbitals to hybridized 5d−6p orbitals [26,27], decreases with the introduction of CO2. This change of pre-edge peak intensity reveals that TaO6 Oh symmetry is also improved by the CO2 addition to TBA-Ta6 and TBA-Ta1Nb5. These results suggest that the Oh symmetry of TaO6 unit increases, while NbO6 symmetry is slightly improved in TBA-Ta1Nb5 by CO2 adsorption. Actually, the optimized structure of [Ta1Nb5O19]8− with CO2 adsorbed on the terminal OTa site has highly Oh symmetric TaO6 units compared to the bare [Ta1Nb5O19]8− (Figure S6) CO2 is also adsorbed on TBA-TaxNb6−x in SO solution. This structural change induced by CO2 adsorption is also observed in FT-IR (Figure S7). FT-IR spectra of TBA-Ta1Nb5 in DMSO solvent show the characteristic absorption band assignable to the stretching vibration between the metal and terminal O atoms in MO6 units (M=O bond). The absorption band shifts to high energy, owing to the slight shrink in the O=Nb bond in NbO6 units. Those results indicate that CO2 is preferentially adsorbed on terminal O sites of TaO6 unit and induces the structure change in TBA-TaxNb6−x.
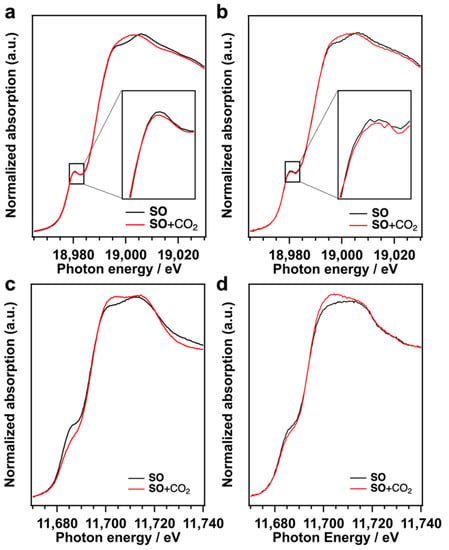
Figure 5.
(a) Nb K-edge of TBA-Nb6 and (b) TBA-Ta1Nb5, and (c) Ta L1-edge XANES spectra of TBA-Ta6 and (d) TBA-Ta1Nb5 in SO solution before (black line) and after (red line) introduction of CO2.
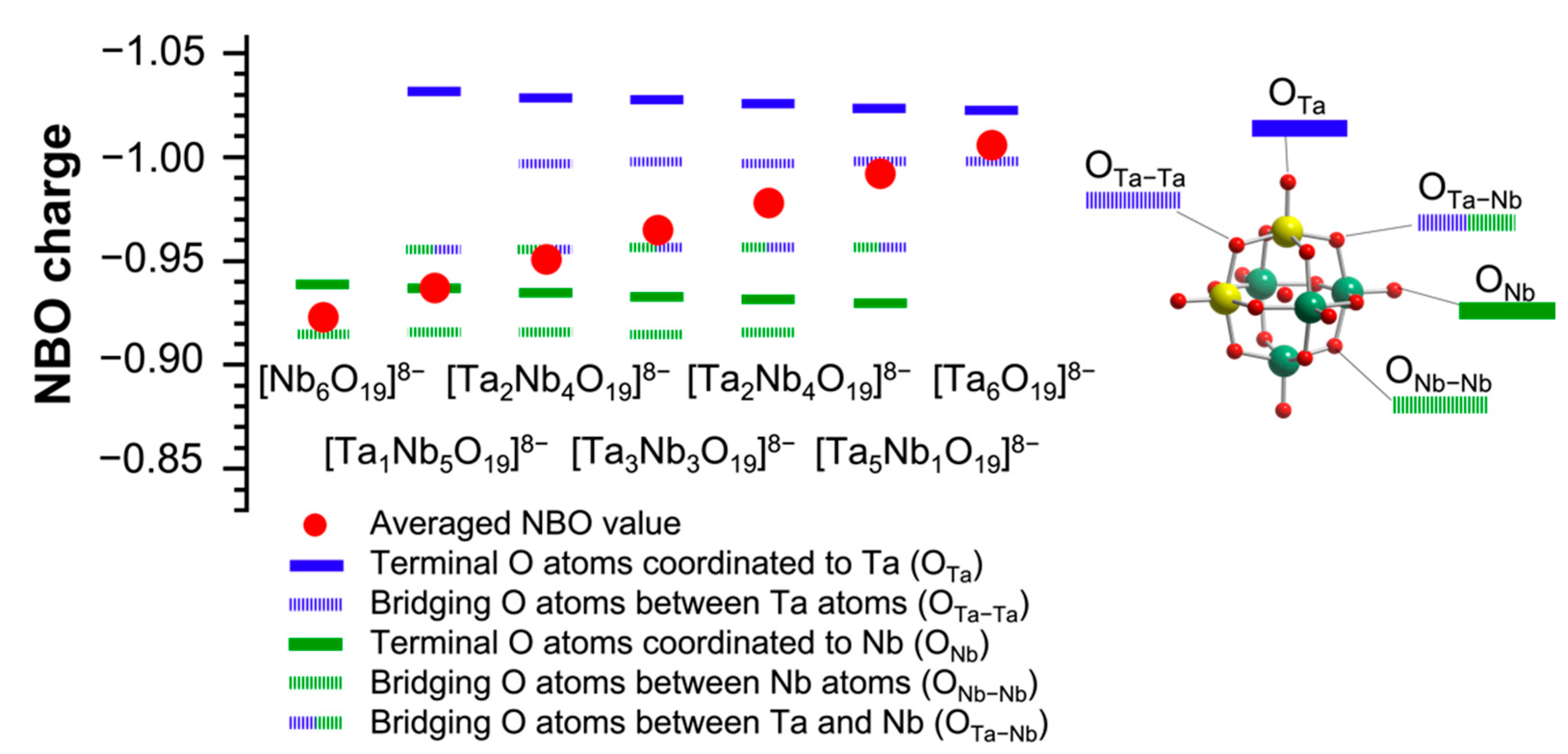
Next, NBO charges of the surface O atoms of TBA-TaxNb6−x were calculated to elucidate the catalytic activity and selectivity of TBA-TaxNb6−x for CO2 fixation to SO (Figure 6). The average NBO charge was also evaluated in Figure 6. The terminal O atoms coordinated to Ta (terminal OTa) have the most negative NBO charges (highest basicity) followed by the bridge O atom between Ta ions (bridge OTa–Ta) in TBA-TaxNb6−x. The values of NBO charge of terminal OTa hardly change with the Ta content. On the other hand, the NBO charge of the terminal O connecting to Nb (terminal ONb) has lower negativity than that of terminal OTa. The order of NBO charges in TBA-TaxNb6−x is terminal OTa, bridge OTa–Ta, bridge OTa–Nb (Ta–O–Nb), terminal ONb, and bridge ONb–Nb (Nb–O–Nb). As a result, the average NBO charges of TBA-TaxNb6−x gradually increase with increasing the Ta content. The catalytic activities of TBA-TaxNb6−x, which increase with incremental addition of Ta content in Figure 2, could be explained by the average NBO charges, indicating the increase in the active sites of terminal OTa by Ta substitution.
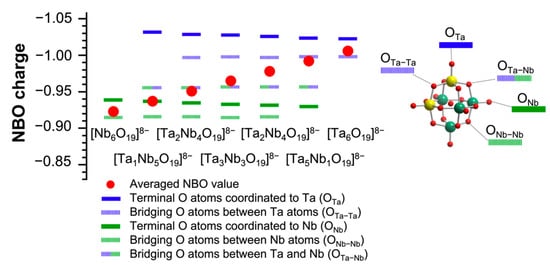
Figure 6.
NBO charge of surface O sites of [TaxNb6−xO19]8−. Color codes: yellow atom, Ta; green atom, Nb; blue line, Ta-coordinated O atoms; green line, Nb-coordinated O atoms; red, total O atoms. Red circle represents the averaged value. Terminal and bridging O atoms represent solid and dashed lines, respectively.
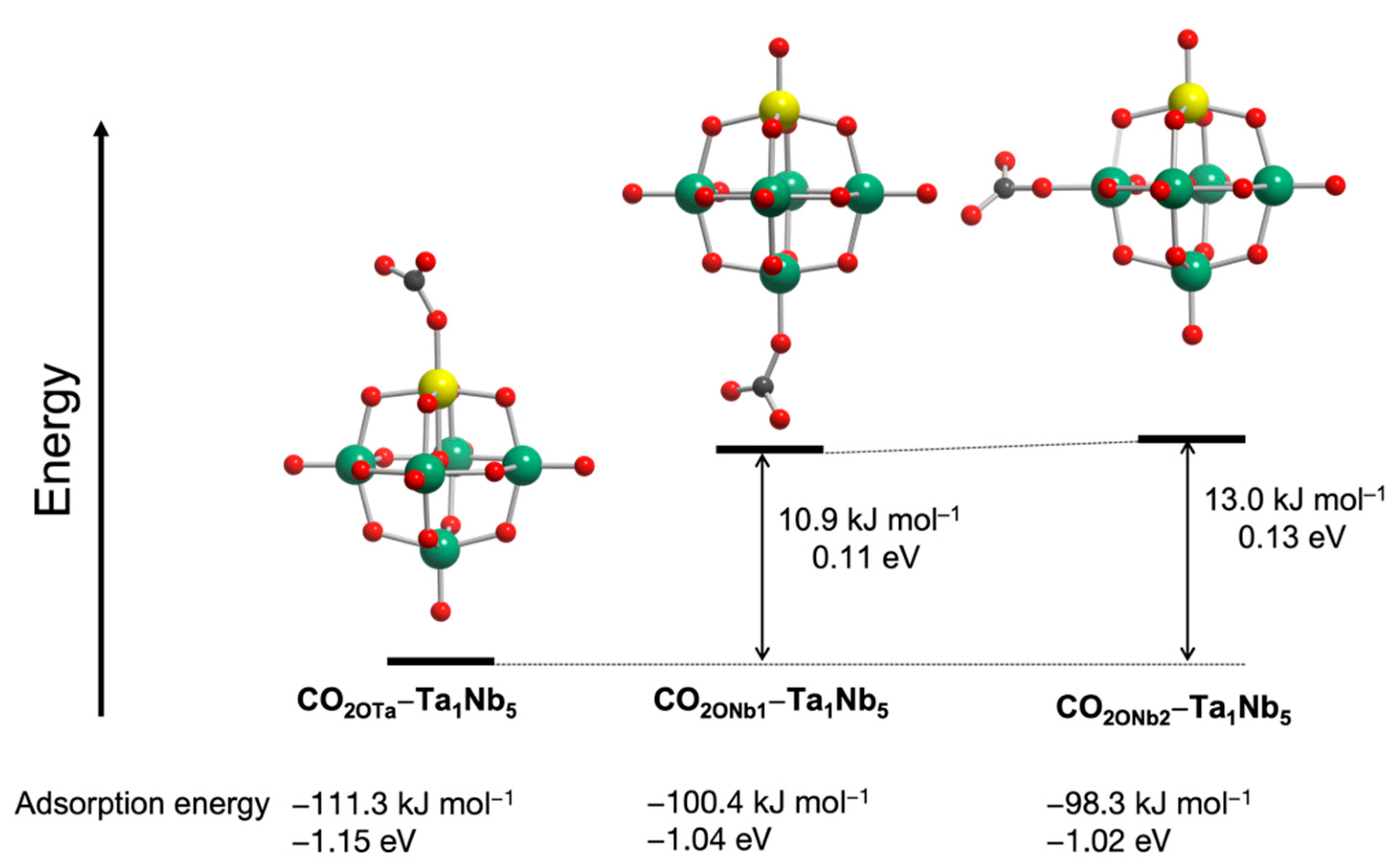
Finally, the CO2 adsorption sites of [Ta1Nb5O19]8− were also predicted by DFT calculations. We reported that CO2 was preferentially adsorbed on terminal O sites rather than bridge O sites [18]. [Ta1Nb5O19]8− has three terminal O sites (see Figure 7). To determine the CO2 activation sites of [Ta1Nb5O19]8−, the CO2 adsorption energy was calculated using three possible configurations (Figure 7). Among the three structures, the lowest energy is found in a structure with CO2 adsorbed on the terminal OTa site, which has the highest negative NBO charge among the surface oxygen atoms in [Ta1Nb5O19]8−. This result indicates that the CO2 is preferentially adsorbed and activated on the terminal OTa. The SO adsorption energy was also calculated to gain the insight into SO activation sites (Figure S8). The adsorption energy of SO on OTa is lower than that of CO2 on OTa in [Ta1Nb5O19]8−. In addition, adsorption energies reveal that SO is more likely to be activated on OTa than ONb. These results suggest that CO2 preferentially adsorbs on OTa site and it is unlikely that SO activation occurs on ONb in TBA-Ta1Nb5. Therefore, high SC selectivity is achieved in TBA-Ta1Nb5. The low SC selectivity in Ta-rich TBA-TaxNb6−x is explained that not only by the fact that CO2 but also SO is activated on OTa sites by competitive adsorption due to the large number of OTa adsorption sites.

Figure 7.
Total energy of CO2-adsorbed [Ta1Nb5O19]8− at different base sites.
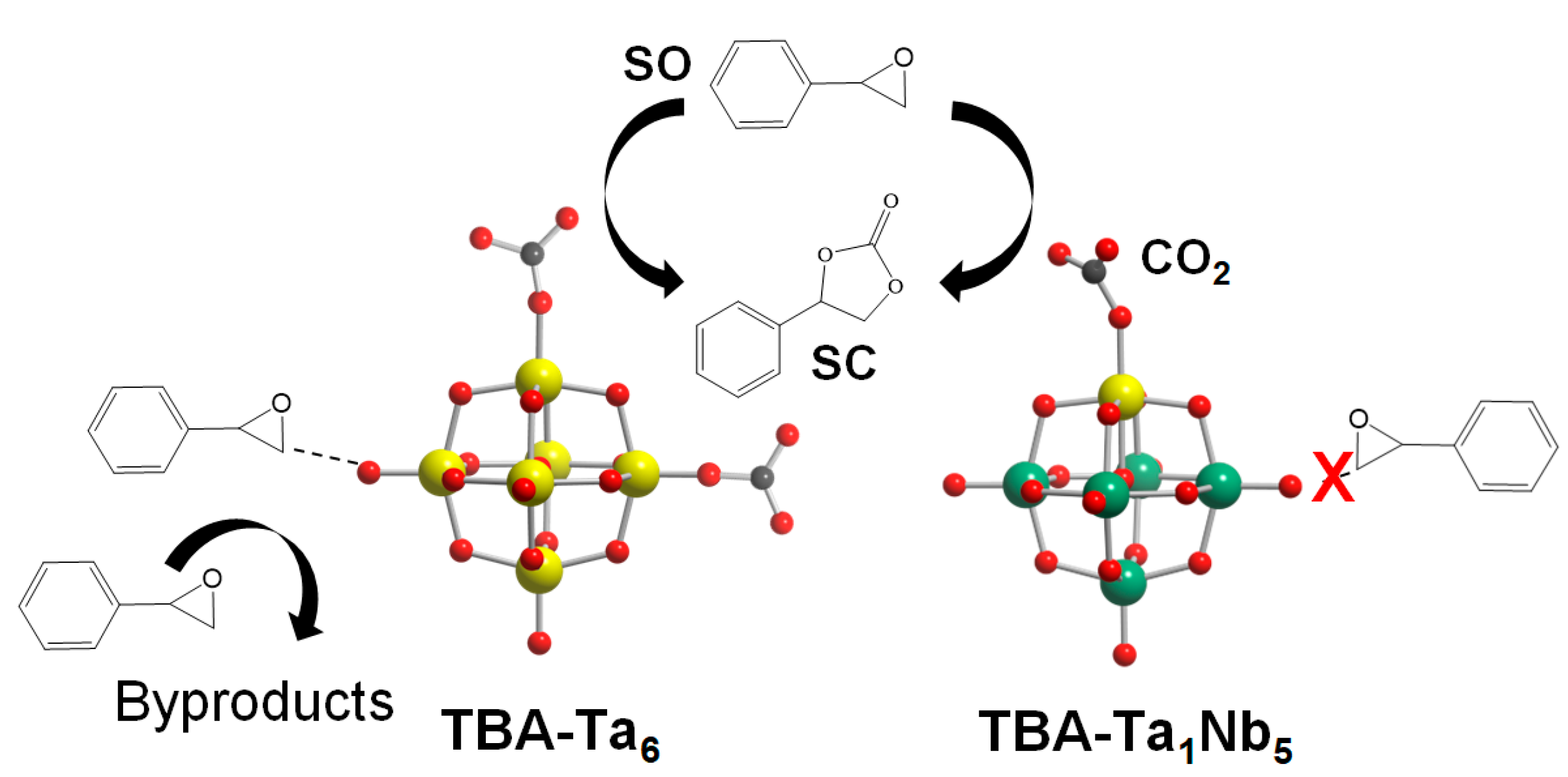
The reaction mechanism of TBA-TaxNb6−x for CO2 fixation to SO is discussed. In the case of TBA-Nb6, CO2 is adsorbed on the terminal ONb sites and the activated CO2 reacts nucleophilically with SO to form SC. The low catalytic activity of TBA-Nb6 for CO2 fixation to SO is due to the weak Lewis base strength (low negativity in NBO charges) of terminal ONb compared with terminal OTa of other TBA-TaxNb6−x. The SO activation hardly occurs on TBA-Nb6, as shown in Figure S3, which is one of the reasons why TBA-Nb6 shows high SC selectivity. The SO conversion gradually increases with Ta substitution amount, as shown in Figure 2. This can be explained by the increase in active terminal OTa sites. On the other hand, the SC selectivity decreases for high Ta content of TBA-TaxNb6−x (x ≥ 2). The low SC selectivity is due to the SO activation on the surface of TBA-TaxNb6−x (x ≥ 2), as shown in Scheme 1. In fact, TBA-Ta6 exhibits the highest SO conversion among TBA-Ta6, TBA-Ta1Nb5, and TBA-Nb6 in the absence of CO2 accompanied with a viscosity increase (Figure S3). The sharp contrast in SO conversion in the absence of CO2 conditions for TBA-Ta6 and TBA-Nb6 clearly indicates that terminal OTa and/or bridge OTa−Ta can activate SO. On the other hand, TBA-Ta1Nb5 exhibits the highest SC selectivity among TBA-TaxNb6−x despite having a terminal OTa site. The DFT calculation (Figure 7) and CO2 concentration dependence on CO2 fixation to SO (Figure 4b) reveal that the single terminal OTa in [Ta1Nb5O19]8− preferentially adsorbs CO2 at 100% CO2 conditions and SO is not activated on terminal ONb, bridge ONb−Nb, and bridge OTa−Nb (Scheme 1). We conclude that the selective CO2 activation at the terminal OTa in TBA-Ta1Nb5 without SO activation is a crucial factor for high SC selectivity in the CO2 fixation to SO.
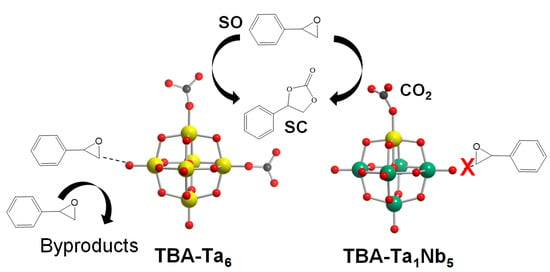
Scheme 1.
Activation of CO2 on OTa leads to a reaction with SO to form SC, while adsorption of SO on OTa leads to the formation of byproducts observed in reaction promoted by TBA-Ta6. In the case of TBA-Ta1Nb5, SO is not activated on ONb and preferential adsorption of CO2 on OTa results in high selectivity of SC.
4. Materials and Methods
TBA salts of [TaxNb6−xO19]8− (TBA-TaxNb6−x, x = 0–6) were prepared by microwave-assisted hydrothermal synthesis (Biotage Initiator+ 400 W) using Ta2(x/6)Nb2(1−x/6)O5·nH2O as the precursors. First, Na3Tax/6Nb1−x/6O4 were prepared by modified solid-state reaction method according to the reported procedures [23,28]. M2O5 (M = Ta or Nb), Na2C2O4, and (NH2)2CO at a molar ratio between (Ta + Nb):Na:(NH2)2CO of 1:1:4 was ground to fine powder prior to calcination at 773 K for 4 h to obtain NaTax/6Nb1−x/6O3. NaTax/6Nb1−x/6O3 was mixed with Na2C2O4, and (NH2)2CO at a molar ratio between NaTax/6Nb1−x/6O3:Na:(NH2)2CO of 1:1:3 followed by calcination at 1173 K for 4 h. The resulting powder, Na3Tax/6Nb1−x/6O4, was characterized by XRD (Rigaku Miniflex) having diffraction patterns corresponding to the references (Figure S9). Na3Tax/6Nb1−x/6O4 was dissolved in water and 1 M HCl was added until pH of the supernatant reached 1 or less. The white precipitate was collected by centrifugation and washed with pure water until the pH of the supernatant became neutral. After drying in vacuum and oven, the Ta2(x/6)Nb2(1−x/6)O5·nH2O was obtained. Then, 10% tetrabutylammonium hydroxide (TBAOH) aqueous solution was added to Ta2(x/6)Nb2(1−x/6)O5·nH2O. The mixture was reacted using microwave-assisted hydrothermal synthesis at 180 °C for 5−15 min. The resultant product was washed with hexane to obtain TBA6H2[TaxNb6−xO19]. The fabricated clusters were characterized by ESI–MS (Figure S1) (Bruker, MicroOTOFII-ST1), Fourier-transformed infrared spectrometry (JASCO, FT/IR-4700) equipped with attenuated total reflectance-infrared spectroscopy (JASCO, ATR-PRO ONE) (Figure S2), elemental analysis (Table S1), and X-ray absorption fine structure (XAFS) analysis (BL01B1, SPring-8) (Figure 1). XAFS spectra were recorded in transmittance mode using ionization chambers as detectors at room temperature. Si(111) double-crystal monochromator was used to obtain the incident X-ray beam for Ta L1- and L3-edges XAFS. In the case of Nb K-edge XAFS measurements, Si(311) double-crystal monochromator was employed. The data were analyzed using xTunes software [29]. The XANES spectra were extracted as the extended X-ray absorption fine structure (EXAFS) after normalization at edge height. The EXAFS spectra in the k range 3.0–14.0 Å−1 were Fourier-transformed into r space to obtain FT-EXAFS spectra. The illustrations of TaxNb6−xO19 were computed using VESTA [30].
In general, CO2 fixation to SO over TBA-TaxNb6−x were carried out using 5 µmol of catalyst, SO (0.6 mL, ca. 5 mmol), 100% CO2 (0.1 MPa) at 100 °C for 6 h using biphenyl as an internal standard. The product solutions were analyzed using gas chromatography equipped with flame ionization detector (GC-FID, Shimadzu, GC-2014 with column Restex, Rtx-1) and gas chromatography–mass spectrometry (GC–MS, Shimadzu, GCMS-QP2010 SE with column Agilent, DB-1MS). Time course of CO2 fixation to SO reactions were carried out using 10 µmol of catalyst, SO (1.2 mL, ca. 10 mmol), 100% CO2 (0.1 MPa) at 100 °C for 24 h. Small amount of solution (ca. 20 µL) was drawn to measure at specified reaction times. The peak areas from GC-FID chromatograms were used to calculate with this formula:
where Sub. = substrate (SO), IS = internal standard (biphenyl), Pro. = product (SC), ECN = equivalent carbon number, subscripted 0 = initial value before reaction.
The DFT calculations were conducted using Gaussian 16 program as previously reported [18]. The structural optimization for [TaxNb6−xO19]8− was performed by B3LYP with the solvation effect of DMSO using PCM (dielectric constant = 46.826). LanL2DZ basis sets were employed for Ta and Nb atoms and 6−31 + G(d) basis sets for O and C atoms to investigate the effect of the composition of the clusters on the NBO charge of O atoms and the adsorption energies of CO2 on [Ta1Nb5O19]8−.
5. Conclusions
In conclusion, TBA-TaxNb6−x (x = 0–6) were prepared by microwave-assisted hydrothermal reaction and were used as catalysts for CO2 fixation to SO to produce SC. Among TBA-TaxNb6−x, TBA-Ta1Nb5 shows the highest selectivity toward SC, whereas the SO conversion increases with the Ta content in the clusters. The effects of SO concentration and CO2 concentration for CO2 fixation to SO indicate that high SO concentration and 100% CO2 atmosphere are required to obtain high SC selectivity for TBA-Ta1Nb5 because the rate-determining step of CO2 fixation to SO is the reaction of the activated CO2 with SO. The SO conversions in the absence of CO2 suggest that the SO activation hardly occurs in TBA-Nb6. DFT calculations reveal that the increase in the SO conversion with Ta content is the increment of the active terminal OTa sites that have the highest negative NBO charges. In addition, [Ta1Nb5O19]8− preferentially adsorbs CO2 at terminal OTa sites compared to other ONb sites. In conclusion, the selective CO2 activation at terminal OTa in TBA-Ta1Nb5 without SO activation is a crucial factor for high SC selectivity in the CO2 fixation to SO.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13020442/s1, Table S1: Elemental analysis results of the synthesized TBA6H2[TaxNb6−xO19]; Figure S1: ESI–MS (negative ion mode) spectra of TBAmHn[TaxNb6−xO19]8−m−n measured in aqueous solutions; Figure S2: FT-IR spectra (ATR mode) of (a) TBA-Ta6, (b) TBA-Ta5Nb1, (c) TBA-Ta4Nb2, (d) TBA-Ta3Nb3, (e) TBA-Ta2Nb4, (f) TBA-Ta1Nb5, and (g) TBA-Nb6; Figure S2: FT-IR spectra (ATR mode); Figure S3: CO2 fixation to SO promoted by TBA-TaxNb6−x under N2 atmosphere under N2 atmosphere; Figure S4: Results of CO2 fixation to SO over TBA-TaxNb6−x; Figure S5: Time course of CO2 fixation to SO and selectivity of SC over TBA-Nb6; Figure S6: Optimized structure of [Ta1Nb5O19]8− and CO2-adsorbed [Ta1Nb5O19]8−; Figure S7: In situ FT-IR spectra (ATR mode) of TBA-Ta1Nb5 in DMSO before (black line) and after CO2 adsorption (red line). (b) Optimized structure of [Ta1Nb5O19]8− and CO2-adsorbed [Ta1Nb5O19]8−. Figure S8: Total energy of SO-adsorbed [Ta1Nb5O19]8− at different base sites. Figure S9: XRD patterns of Na3Tax/6Nb6−x/6O4.
Author Contributions
S.K. and S.Y. designed this study. V.C., M.T. and J.H. synthesized and characterized catalysts and carried out the catalytic tests. H.N. and N.N. carried out the DFT calculations and analyzed the NBO charges of the catalysts. T.Y. measured and analyzed the XRD. V.C., S.K., M.T. and S.Y. measured and analyzed the XAFS spectra. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by NEDO (JPNP14004), JSPS KAKENHI (No. 20K22467, 21H01718, and 22K14543), Tokyo Human Resources Fund for City Diplomacy, Tokyo Metropolitan University Research Fund for Young Scientists, Tokyo Metropolitan Government Advanced Research (R3-1), and Yazaki Memorial Foundation for Science and Technology.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The synchrotron radiation experiment was performed at BL01B1 in SPring-8 under the approval of the Japan Synchrotron Radiation Research Institute (JASRI) as 2020A1068, 2021B1380, 2021A1406, 2021B1535, 2022A1532, 2022A1627, 2022B1684, and 2022B1911.
Conflicts of Interest
The authors declare no conflict of interest.
References
- North, M.; Omedes-Pujol, M. Catalytic, Asymmetric Cyanohydrin Synthesis in Propylene Carbonate. Tetrahedron Lett. 2009, 50, 4452–4454. [Google Scholar] [CrossRef]
- Clegg, W.; Harrington, R.W.; North, M.; Pizzato, F.; Villuendas, P. Cyclic Carbonates as Sustainable Solvents for Proline-Catalysed Aldol Reactions. Tetrahedron Asymmetry 2010, 21, 1262–1271. [Google Scholar] [CrossRef]
- Jessop, P.G. Searching for Green Solvents. Green Chem. 2011, 13, 1391. [Google Scholar] [CrossRef]
- Forero, J.S.B.; Muñoz, J.A.H.; Junior, J.J.; Da Silva, F.M. Propylene Carbonate in Organic Synthesis: Exploring Its Potential as a Green Solvent. Curr. Org. Synth. 2016, 13, 834–846. [Google Scholar] [CrossRef]
- Pescarmona, P.P. Cyclic Carbonates Synthesised from CO2: Applications, Challenges and Recent Research Trends. Curr. Opin. Green Sustain. Chem. 2021, 29, 100457. [Google Scholar] [CrossRef]
- Fukuoka, S.; Fukawa, I.; Tojo, M.; Oonishi, K.; Hachiya, H.; Aminaka, M.; Hasegawa, K.; Komiya, K. A Novel Non-Phosgene Process for Polycarbonate Production from CO2: Green and Sustainable Chemistry in Practice. Catal. Surv. Asia 2010, 14, 146–163. [Google Scholar] [CrossRef]
- Fleischer, M.; Blattmann, H.; Mülhaupt, R. Glycerol-, Pentaerythritol- and Trimethylolpropane-Based Polyurethanes and Their Cellulose Carbonate Composites Prepared via the Non-Isocyanate Route with Catalytic Carbon Dioxide Fixation. Green Chem. 2013, 15, 934. [Google Scholar] [CrossRef]
- Rokicki, G. Aliphatic Cyclic Carbonates and Spiroorthocarbonates as Monomers. Prog. Polym. Sci. 2000, 25, 259–342. [Google Scholar] [CrossRef]
- Yu, W.; Maynard, E.; Chiaradia, V.; Arno, M.C.; Dove, A.P. Aliphatic Polycarbonates from Cyclic Carbonate Monomers and Their Application as Biomaterials. Chem. Rev. 2021, 121, 10865–10907. [Google Scholar] [CrossRef]
- Zhang, S.S. A Review on Electrolyte Additives for Lithium-Ion Batteries. J. Power Sources 2006, 162, 1379–1394. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef]
- Tillmann, S.D.; Isken, P.; Lex-Balducci, A. Gel Polymer Electrolyte for Lithium-Ion Batteries Comprising Cyclic Carbonate Moieties. J. Power Sources 2014, 271, 239–244. [Google Scholar] [CrossRef]
- Yun, J.; Zhang, L.; Qu, Q.; Liu, H.; Zhang, X.; Shen, M.; Zheng, H. A Binary Cyclic Carbonates-Based Electrolyte Containing Propylene Carbonate and Trifluoropropylene Carbonate for 5V Lithium-Ion Batteries. Electrochim. Acta 2015, 167, 151–159. [Google Scholar] [CrossRef]
- Kawai, H.; Sakamoto, F.; Taguchi, M.; Kitamura, M.; Sotomura, M.; Tsukamoto, G. 2-Oxo-1,3-Dioxoles as Specific Substrates for Measurement of Arylesterase Activity. Chem. Pharm. Bull. 1991, 39, 1422–1425. [Google Scholar] [CrossRef]
- Cascio, G.; Manghisi, E.; Porta, R.; Fregnan, G. N-Phenylpiperazine Derivatives with Hypocholesterolemic Activity. J. Med. Chem. 1985, 28, 815–818. [Google Scholar] [CrossRef]
- Kamata, K.; Sugahara, K. Base Catalysis by Mono- and Polyoxometalates. Catalysts 2017, 7, 345. [Google Scholar] [CrossRef]
- Hayashi, S.; Yamazoe, S.; Koyasu, K.; Tsukuda, T. Lewis Base Catalytic Properties of [Nb10O28]6− for CO2 Fixation to Epoxide: Kinetic and Theoretical Studies. Chem. Asian J. 2017, 12, 1635–1640. [Google Scholar] [CrossRef]
- Hayashi, S.; Sasaki, N.; Yamazoe, S.; Tsukuda, T. Superior Base Catalysis of Group 5 Hexametalates [M6O19]8− (M = Ta, Nb) over Group 6 Hexametalates [M6O19]2− (M = Mo, W). J. Phys. Chem. C 2018, 122, 29398–29404. [Google Scholar] [CrossRef]
- Weng, Z.; Ogiwara, N.; Kitao, T.; Kikukawa, Y.; Gao, Y.; Yan, L.; Uchida, S. Incorporating Highly Basic Polyoxometalate Anions Comprising Nb or Ta into Nanoscale Reaction Fields of Porous Ionic Crystals. Nanoscale 2021, 13, 18451–18457. [Google Scholar] [CrossRef]
- Gutierrez, L.F.; Nope, E.; Rojas, H.A.; Cubillos, J.A.; Sathicq, Á.G.; Romanelli, G.P.; Martínez, J.J. New Application of Decaniobate Salt as Basic Solid in the Synthesis of 4H-Pyrans by Microwave Assisted Multicomponent Reactions. Res. Chem. Intermed. 2018, 44, 5559–5568. [Google Scholar] [CrossRef]
- Ge, W.; Wang, X.; Zhang, L.; Du, L.; Zhou, Y.; Wang, J. Fully-Occupied Keggin Type Polyoxometalate as Solid Base for Catalyzing CO2 Cycloaddition and Knoevenagel Condensation. Catal. Sci. Technol. 2016, 6, 460–467. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, Y.; Song, Y.-F. Tri-Lacunary Polyoxometalates of Na8H[PW9O34] as Heterogeneous Lewis Base Catalysts for Knoevenagel Condensation, Cyanosilylation and the Synthesis of Benzoxazole Derivatives. Appl. Catal. Gen. 2014, 475, 140–146. [Google Scholar] [CrossRef]
- Kikkawa, S.; Tsukada, M.; Shibata, K.; Fujiki, Y.; Shibusawa, K.; Hirayama, J.; Nakatani, N.; Yamamoto, T.; Yamazoe, S. Base Catalysis of Sodium Salts of [Ta6−xNbxO19]8− Mixed-Oxide Clusters. Symmetry 2021, 13, 1267. [Google Scholar] [CrossRef]
- Sugahara, K.; Kimura, T.; Kamata, K.; Yamaguchi, K.; Mizuno, N. A Highly Negatively Charged γ-Keggin Germanodecatungstate Efficient for Knoevenagel Condensation. Chem. Commun. 2012, 48, 8422–8424. [Google Scholar] [CrossRef]
- Chudatemiya, V.; Kikkawa, S.; Hirayama, J.; Takahata, R.; Teranishi, T.; Tamura, M.; Yamazoe, S. Bifunctional Platinum-Incorporated Polyoxoniobate Derived Catalyst for N-formylation of Piperidine Using CO2. Asian J. Org. Chem. 2022, e202200521. [Google Scholar] [CrossRef]
- Asakura, H.; Shishido, T.; Yamazoe, S.; Teramura, K.; Tanaka, T. Structural Analysis of Group V, VI, and VII Metal Compounds by XAFS. J. Phys. Chem. C 2011, 115, 23653–23663. [Google Scholar] [CrossRef]
- Yamazoe, S.; Hitomi, Y.; Shishido, T.; Tanaka, T. XAFS Study of Tungsten L1- and L3-Edges: Structural Analysis of WO3 Species Loaded on TiO2 as a Catalyst for Photo-Oxidation of NH3. J. Phys. Chem. C 2008, 112, 6869–6879. [Google Scholar] [CrossRef]
- Fukada, M.; Shibata, K.; Imai, T.; Yamazoe, S.; Hosokawa, S.; Wada, T. Fabrication of Lead-Free Piezoelectric NaNbO3 Ceramics at Low Temperature Using NaNbO3 Nanoparticles Synthesized by Solvothermal Method. J. Ceram. Soc. Jpn. 2013, 121, 116–119. [Google Scholar] [CrossRef]
- Asakura, H.; Yamazoe, S.; Misumi, T.; Fujita, A.; Tsukuda, T.; Tanaka, T. xTunes: A New XAS Processing Tool for Detailed and on-the-Fly Analysis. Radiat. Phys. Chem. 2020, 175, 108270. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).