Preparation of di-[EMIM]CoCl3 Ionic Liquid Catalyst and Coupling with Oxone for Desulfurization at Room Temperature
Abstract
1. Introduction
2. Experimental
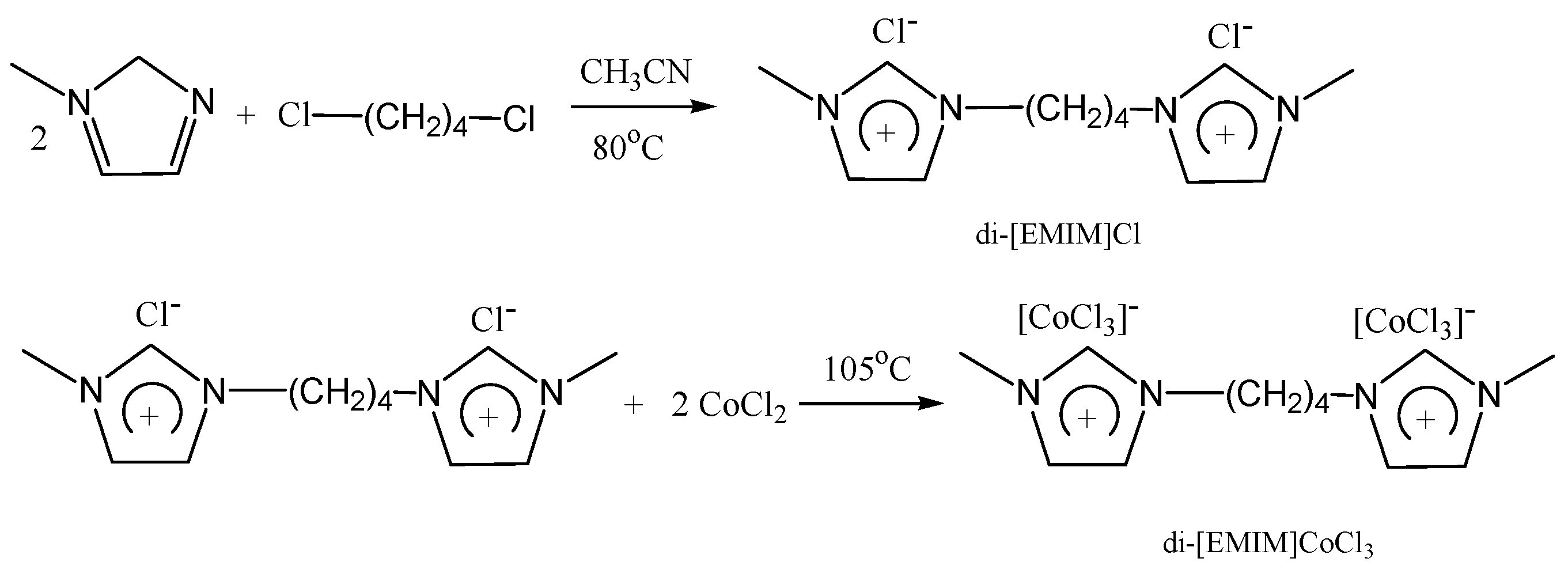
2.1. Preparation of Ionic Liquid Catalyst
2.2. Desulfurization Process
2.3. Characterization
3. Results and Discussions
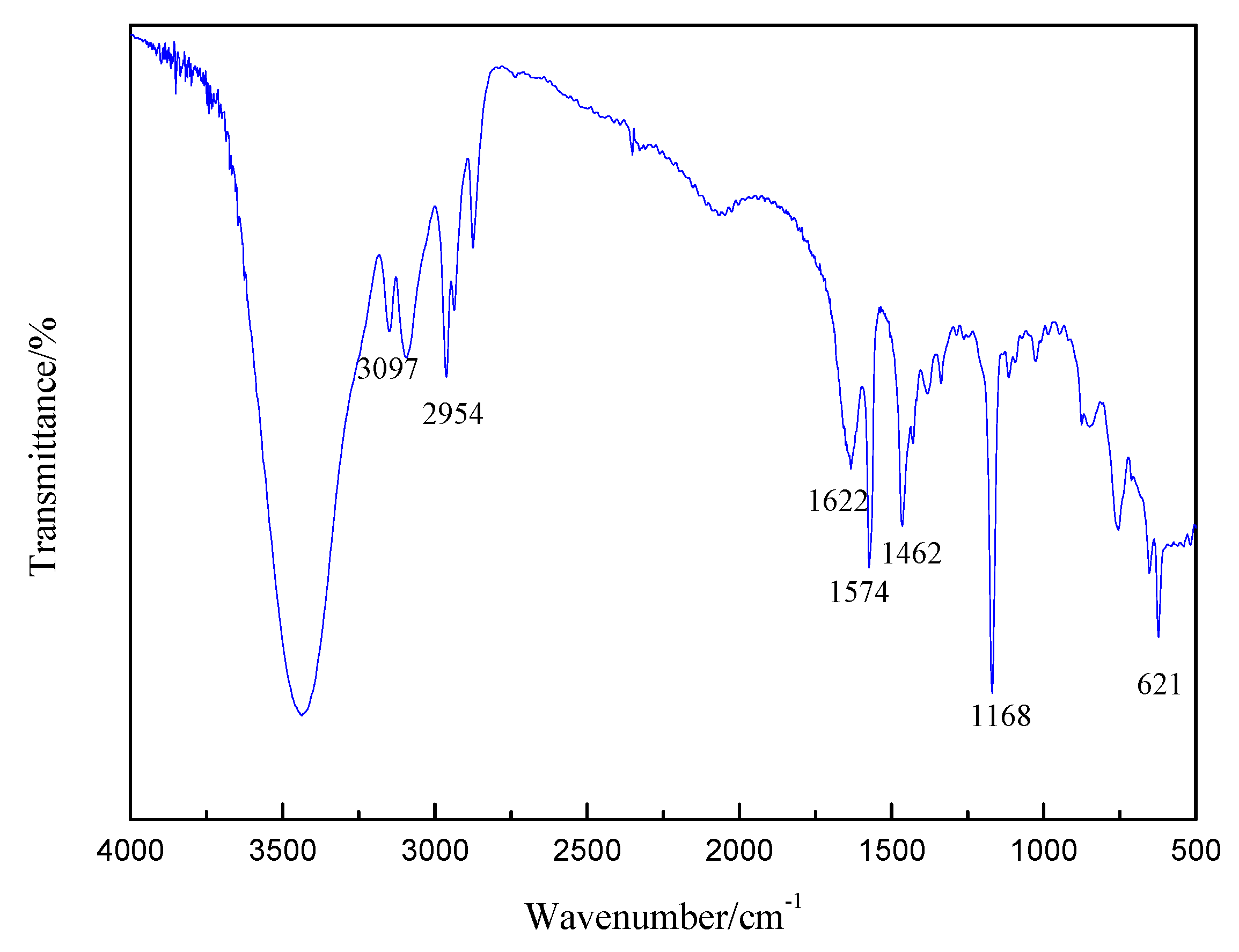
3.1. HNMR and FTIR of di-[EMIM]Cl
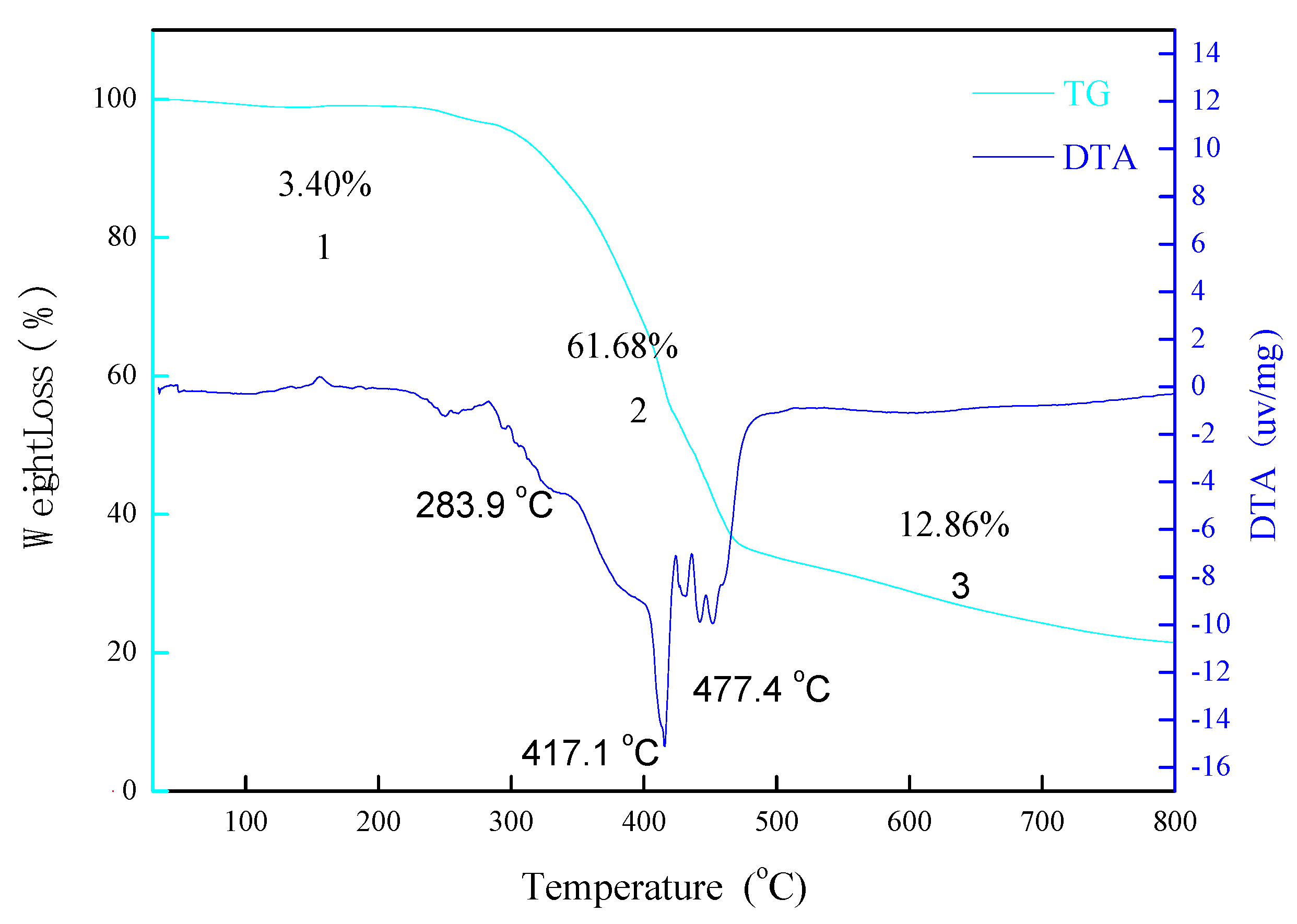
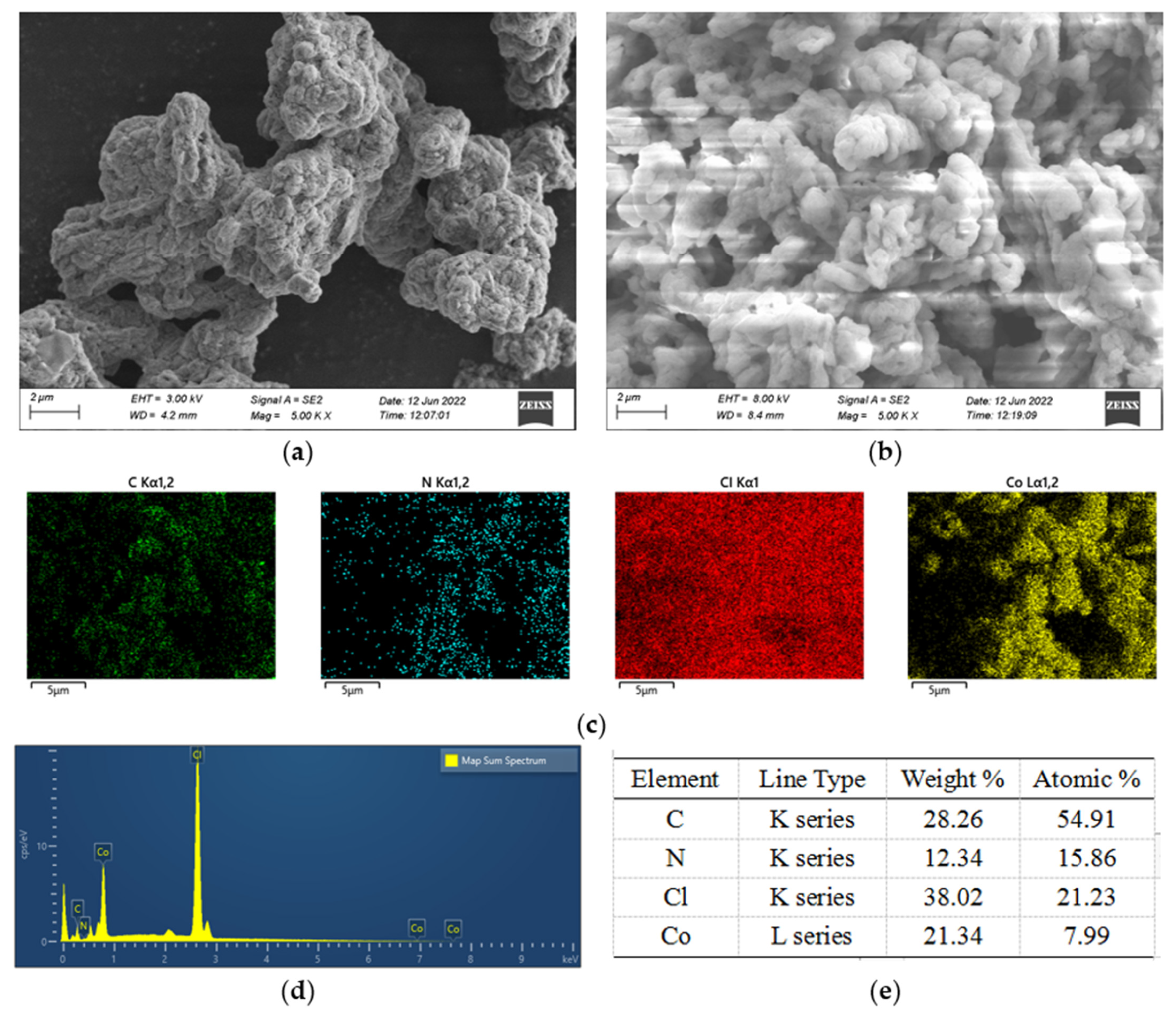
3.2. TG and SEM–EDS of di-[EMIM]CoCl3
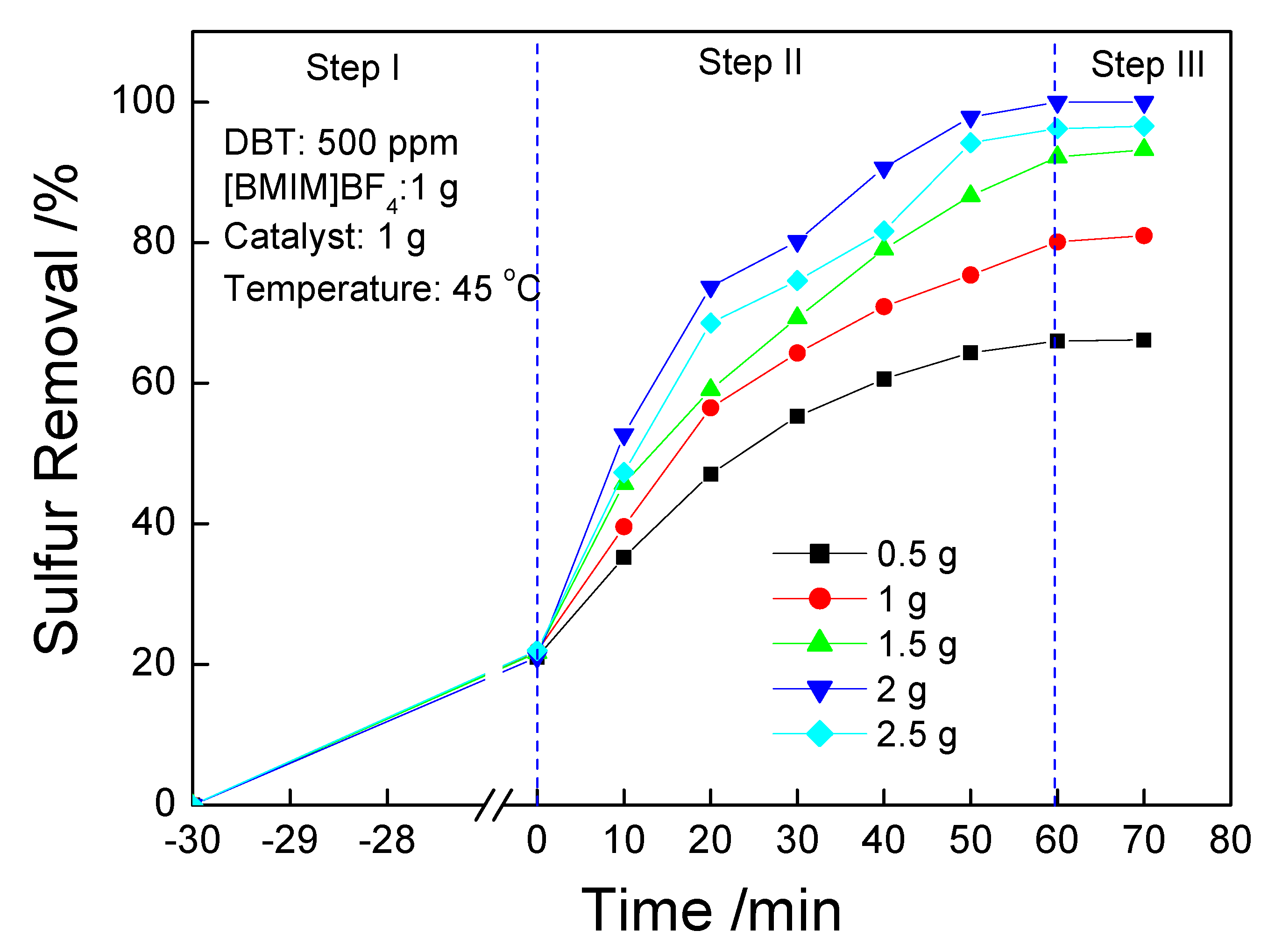
3.3. Effect of di-[EMIM]CoCl3 Dosage
3.4. Effect of Oxone (20wt%) Dosage
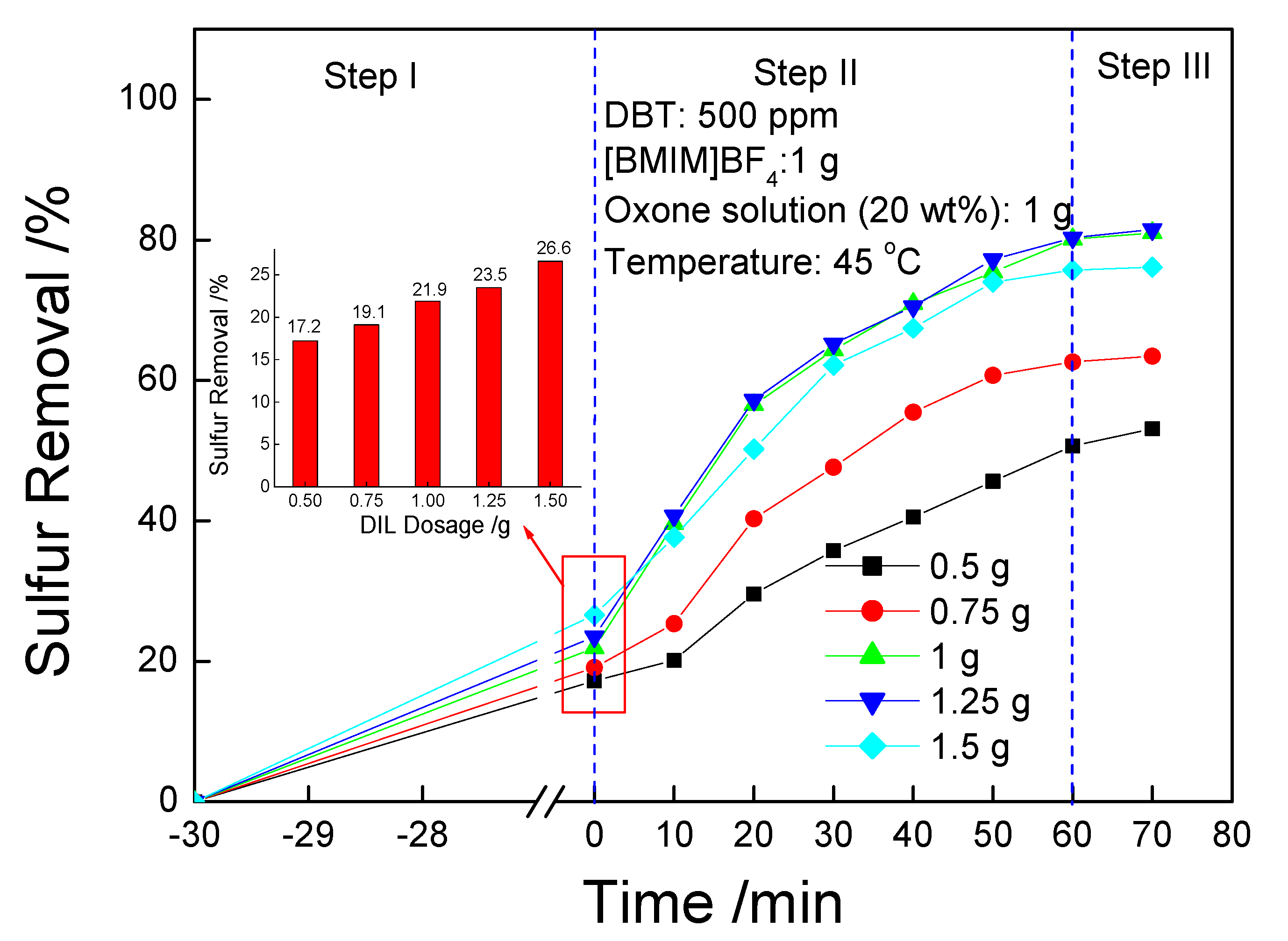
3.5. Effect of [BMIM]BF4 Dosage
3.6. Effect of Temperature
3.7. Effect of Different Sulfur Organic Compounds
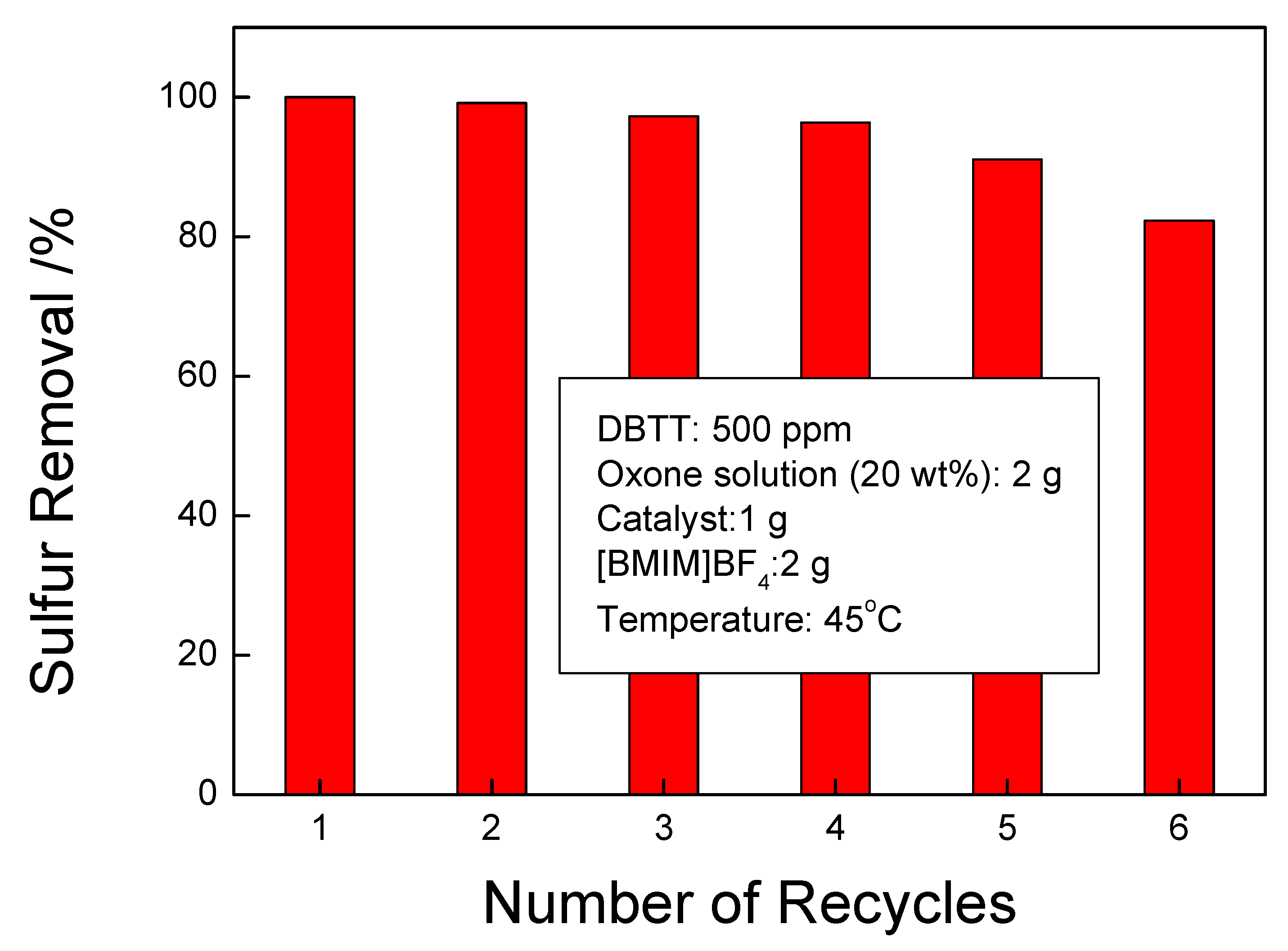
3.8. Recycling Performance of di-[EMIM]CoCl3
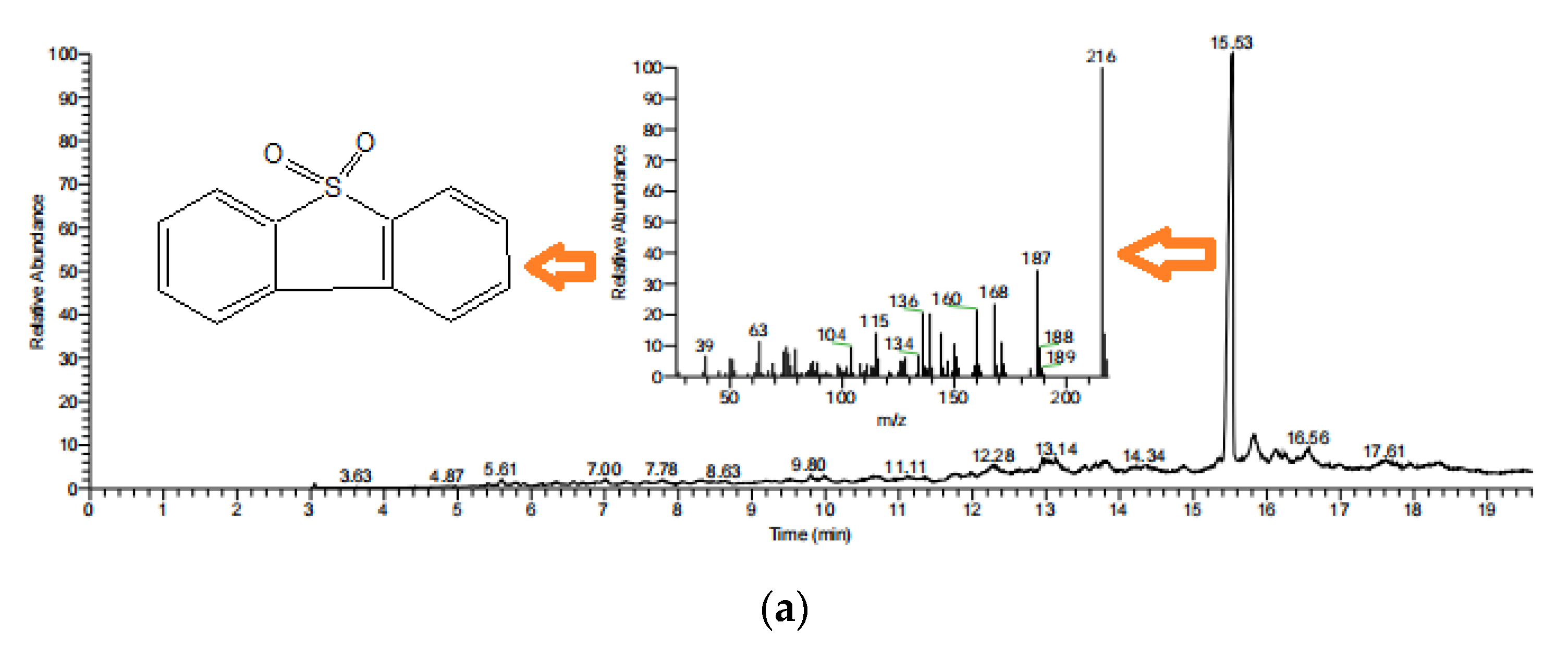
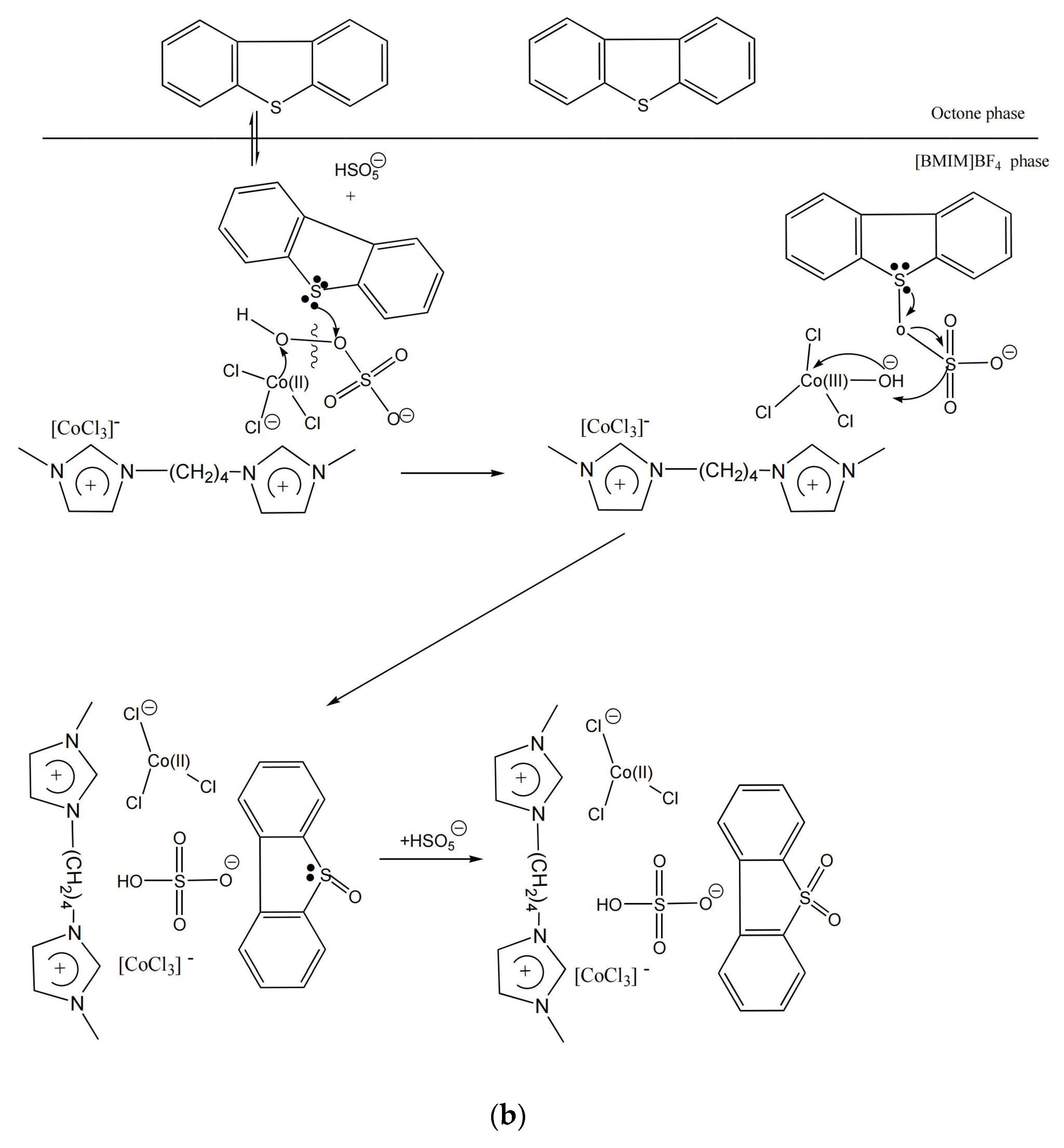
3.9. Desulfurization Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, X.; Zhang, S.; Xing, J.; Wang, Y.; Chen, W.; Ding, D.; Wu, Y.; Wang, S.; Duan, L.; Hao, J. Progress of Air Pollution Control in China and Its Challenges and Opportunities in the Ecological Civilization Era. Engineering 2020, 6, 1423–1431. [Google Scholar] [CrossRef]
- Saha, B.; Vedachalam, S.; Dalai, A.K. Review on recent advances in adsorptive desulfurization. Fuel Process. Technol. 2021, 214, 106685. [Google Scholar] [CrossRef]
- Rajendran, A.; Cui, T.-Y.; Fan, H.-X.; Yang, Z.-F.; Feng, J.; Li, W.-Y. A comprehensive review on oxidative desulfurization catalysts targeting clean energy and environment. J. Mater. Chem. A 2020, 8, 2246–2285. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, Y.; Chen, J.; Xue, H.; Liu, Y. A combined experimental and DFT study on the catalysis performance of a Co-doped MoS2 monolayer for hydrodesulfurization reaction. New J. Chem. 2022, 46, 5065–5077. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, D.; Wu, F.; Wei, X.; Zhang, J. Deep desulfurization of fuels with cobalt chloride-choline chloride/polyethylene glycol metal deep eutectic solvents. Fuel 2018, 225, 104–110. [Google Scholar] [CrossRef]
- Taghiyar, H.; Yadollahi, B. Keggin polyoxometalates encapsulated in molybdenum-iron-type Keplerate nanoball as efficient and cost-effective catalysts in the oxidative desulfurization of sulfides. Sci. Total Environ. 2020, 708, 134860. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, D.; Wu, F. Deep oxidative desulfurization of fuels based on [C4mimCl]CoCl2 ionic liquid oxone solutions at room temperature. Fuel 2017, 208, 508–513. [Google Scholar] [CrossRef]
- Liu, F.; Yu, J.; Qazi, A.B.; Zhang, L.; Liu, X. Metal-Based Ionic Liquids in Oxidative Desulfurization: A Critical Review. Environ. Sci. Technol. 2021, 55, 1419–1435. [Google Scholar] [CrossRef]
- Chen, X.; Song, D.; Asumana, C.; Yu, G. Deep oxidative desulfurization of diesel fuels by Lewis acidic ionic liquids based on 1-n-butyl-3-methylimidazolium metal chloride. J. Mol. Catal. A Chem. 2012, 359, 8–13. [Google Scholar] [CrossRef]
- Zhao, R.-X.; Li, X.-P.; Mao, C.-F.; Hou, L.; Gao, X. [HDMF]Cl-based DES as highly efficient extractants and catalysts for oxidative desulfurization of model oil. RSC Adv. 2019, 9, 14400–14406. [Google Scholar] [CrossRef]
- Alenazi, B.; Alsalme, A.; Alshammari, S.G.; Khan, R.A.; Siddiqui, M.R.H. Ionothermal Synthesis of Metal Oxide-Based Nanocatalysts and Their Application towards the Oxidative Desulfurization of Dibenzothiophene. J. Chem. 2020, 2020, 3894804. [Google Scholar] [CrossRef]
- Li, E.; Zhu, Y.; Xu, Y. Desulfurization of gasoline by [C4, 6, 8mim]Br/FeCl3 ILs collaboration with CTAB. Sep. Sci. Technol. 2021, 56, 310–321. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Li, H.; Wei, Y.; Fu, Y.; Liao, W.; Zhu, L.; Chen, G.; Zhu, W.; Li, H. Tuning the electrophilicity of vanadium-substituted polyoxometalate based ionic liquids for high-efficiency aerobic oxidative desulfurization. Appl. Catal. B Environ. 2020, 271, 118936. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, J.; Zhang, D. Catalytic oxidation desulfurization of silica-gel-supported ionic liquid [Bmim]CoCl3 coupling oxone. Fuel 2021, 288, 119655. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Z.; Xu, P. Dicationic Ionic Liquid @MIL-101 for the Cycloaddition of CO2 and Epoxides under Cocatalyst-free Conditions. Cryst. Growth Des. 2021, 21, 3689–3698. [Google Scholar] [CrossRef]
- Pérez, S.; Montalbán, M.; Carissimi, G.; Licence, P.; Víllora, G. In vitro cytotoxicity assessment of monocationic and dicationic pyridinium-based ionic liquids on HeLa, MCF-7, BGM and EA.hy926 cell lines. J. Hazard. Mater. 2020, 385, 121513. [Google Scholar] [CrossRef]
- Li, J.; Lei, X.-J.; Tang, X.-D.; Zhang, X.-P.; Wang, Z.-Y.; Jiao, S. Acid Dicationic Ionic Liquids as Extractants for Extractive Desulfurization. Energy Fuels 2019, 33, 4079–4088. [Google Scholar] [CrossRef]
- Verma, K.; Sharma, A.; Singh, J.; Badru, R. Ionic liquid mediated carbonylation of amines: Selective carbamate synthesis. Sustain. Chem. Pharm. 2021, 20, 100377. [Google Scholar] [CrossRef]
- Li, J.; Guo, Y.; Tan, J. Polyoxometalate dicationic ionic liquids as catalyst for extractive coupled catalytic oxidative desulfurization. Catalysts 2021, 11, 356–362. [Google Scholar] [CrossRef]
- Nezampour, F.; Ghiaci, M.; Farrokhpour, H. Dicationic ionic liquids/heteropoly acid composites as heterogeneous catalysts for cyclohexene oxidation with molecular oxygen under solvent-free condition: Insights from theory and experiments. Appl. Catal. A-Gen. 2017, 543, 104–114. [Google Scholar] [CrossRef]
- Xu, H.; Wang, S.; Wu, F.; Yuan, Q.; Guo, Y.; Zhang, Y.; Wei, X.; Zhang, J. A fully-organic polymerization carrier calix[4]resorcinarene supported cobalt ionic liquid catalyst with oxone for desulfurization. Fuel 2022, 318, 123670. [Google Scholar] [CrossRef]
- Barman, B.; Rajbanshi, B.; Yasmin, A. Exploring inclusion complexes of ionic liquids with alpha- and beta-cyclodextrin by NMR, IR, Mass, Density, Viscosity, Surface Tension and Conductance Study. J. Mol. Struct. 2018, 1159, 205–215. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Z.; Yang, J.; Wang, L.; Lin, Y.; Wei, Y. Immobilization of room temperature Ionic Liquid (RTIL) on silica gel for adsorption removal of thiophenic sulfur compounds from fuel. Fuel 2013, 107, 394–399. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, F.; Zhang, Z. Polymer-supported ionic liquids: Synthesis, characterization and application in fuel desulfurization. Fuel 2014, 116, 273–280. [Google Scholar] [CrossRef]
- Soliman, S.; Sanad, M.; Shalan, A. Synthesis, characterization and antimicrobial activity applications of grafted copolymer alginate-g-poly(N-Vinyl Imidazole). RSC Adv. 2021, 11, 11541–11548. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Tong, W.; Xie, Y.; Hu, W.; Li, Y.; Zhang, Y.; Wang, Y. Yeast biomass-Induced Co2p/biochar composite for sulfonamide antibiotics degradation through peroxymonosulfate activation. Environ. Pollut. 2021, 268, 115930. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Dong, C.; Kong, D.; Lu, J. New insights into atrazine degradation by cobalt catalyzed peroxymonosulfate oxidation: Kinetics, reaction products and transformation mechanisms. J. Hazard. Mater. 2015, 285, 491–500. [Google Scholar] [CrossRef]
- Zhu, J.; Li, H.; Shan, C.; Wang, S.; Lv, L.; Pan, B. Trace Co2+ coupled with phosphate triggers efficient peroxymonosulfate activation for organic degradation. J. Hazard. Mater. 2021, 409, 124920. [Google Scholar] [CrossRef]
- Yan, S.; Geng, J.; Guo, R. Hydronium jarosite activation of peroxymonosulfate for theoxidation of organic contaminant in an electrochemical reactor driven by microbial fuel Cell. J. Hazard. Mater. 2017, 333, 358–368. [Google Scholar] [CrossRef]
- Jia, J.; Liu, D.; Wang, S.; Li, H.; Ni, J.; Li, X.; Tian, J.; Wang, Q. Visible-light-induced activation of peroxymonosulfate by TiO2 nano-tubes arrays for enhanced degradation of bisphenol. Sep. Purif. Technol. 2020, 253, 117510. [Google Scholar] [CrossRef]
- Xiao, M.; Liu, H.; Gao, H.; Olson, W.; Liang, Z. CO2 capture with hybrid absorbents of low viscosity imidazolium-based ionic liquids and amine. Appl. Energy 2019, 235, 311–319. [Google Scholar] [CrossRef]
- Sadanandhan, A.M.; Khatri, P.K.; Jain, S.L. A novel series of cyclophosphazene derivatives containing imidazolium ionic liquids with variable alkyl groups and their physicochemical properties. J. Mol. Liq. 2019, 295, 111722. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Zhao, R. A facile sol-gel method based on urea-SnCl2 deep eutectic solvents for the synthesis of SnO2/SiO2 with high oxidation desulfurization activity. New J. Chem. 2021, 45, 15901–15911. [Google Scholar] [CrossRef]
- Cui, J.; Wang, G.; Liu, W.; Ke, P.; Tian, Q.; Li, X.; Tian, Y. Synthesis BiVO4 modified by CuO supported onto bentonite for molecular oxygen photocatalytic oxidative desulfurization of fuel under visible light. Fuel 2021, 290, 120066. [Google Scholar] [CrossRef]
- Hao, Y.; Ren, J. Extractive/catalytic oxidative mechanisms over [Hnmp]Cl·xFeCl3 ionic liquids towards the desulfurization of model oils. New J. Chem. 2019, 43, 7725–7732. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Xu, H. Preparation of di-[EMIM]CoCl3 Ionic Liquid Catalyst and Coupling with Oxone for Desulfurization at Room Temperature. Catalysts 2023, 13, 410. https://doi.org/10.3390/catal13020410
Wang S, Xu H. Preparation of di-[EMIM]CoCl3 Ionic Liquid Catalyst and Coupling with Oxone for Desulfurization at Room Temperature. Catalysts. 2023; 13(2):410. https://doi.org/10.3390/catal13020410
Chicago/Turabian StyleWang, Shaokang, and Hang Xu. 2023. "Preparation of di-[EMIM]CoCl3 Ionic Liquid Catalyst and Coupling with Oxone for Desulfurization at Room Temperature" Catalysts 13, no. 2: 410. https://doi.org/10.3390/catal13020410
APA StyleWang, S., & Xu, H. (2023). Preparation of di-[EMIM]CoCl3 Ionic Liquid Catalyst and Coupling with Oxone for Desulfurization at Room Temperature. Catalysts, 13(2), 410. https://doi.org/10.3390/catal13020410





