Abstract
Biodiesel can be produced both in homogeneous and heterogeneous ways. Heterogeneous synthesis allows to easily separate catalyst from esters. In this work, eggshells as a heterogeneous catalyst were used for triglyceride transesterification with 1-butanol. Response surface methodology was used for process optimization. It was obtained that eggshells are a suitable catalyst for transesterification processes. A longer process duration and higher catalyst amount have a high influence on ester yield. However, the amount of 1-butanol should not be maximized. Optimum transesterification reaction conditions were obtained when the process temperature was 110 °C, 1-butanol-to-oil molar ratio 11.3:1, eggshells amount 7.41 wt%, and process duration 11.81 h. Under these conditions, 98.78 wt% of ester yield was obtained.
1. Introduction
The world is going through an energy crisis related to the transition to a wider use of renewable energy resources. The energy situation is not stable due to climate changes, greenhouse gas emissions, and fossil fuel depletion [1]. Despite the global community’s efforts to reduce greenhouse gas emissions, carbon dioxide (CO2) emissions to the Earth’s atmosphere annually increase by an average of 2.2 %, and have done so over the past two decades. We are currently facing problems with obtaining oil due to the war in Ukraine and the situation is becoming more complicated every day. Adopted legal acts and concern about global warming and energy security encourage to pay more and more attention to renewable energy sources, which play an important role in ensuring energy security and sustainable development [2].
The increasing use of fossil fuels in vehicles is harmful to the environment [3,4]. In the countries of the European Union (EU), liquid biofuels are becoming more and more important.
Biodiesel (fatty acid esters) is an attractive fuel that can be used in diesel engines both pure and blended in any ratio with mineral diesel [5,6,7]. As the use and development of biodiesel grows, research related to its production also gains more interest.
The most common method of biodiesel production is the transesterification of oil with methanol and the use of homogeneous alkaline catalysts [8,9]. Since homogeneous catalysts cannot be regenerated, an increased amount of research has recently been conducted using heterogeneous catalysts or biocatalysts [10,11,12]. Calcium oxide, both pure and in mixtures with other metals or their oxides, is used as a heterogeneous alkaline catalyst in biodiesel synthesis [13,14]. Scientists are conducting research to find calcium-rich wastes that can be used as catalysts for oil transesterification [15,16,17]. Large amounts of CaO are found in eggshells, shells of snails, molluscs, crabs, shrimps, and animal bones [18]. One of the waste products with high calcium content is eggshell. The worldwide egg production was 64.185 million tonnes in 2010, 72.125 million tonnes in 2015, and has reached 86.67 million tonnes in 2020 [19]. Since about 10% of the egg’s weight consists of its shell [20], large amounts of this food production waste are generated. Therefore, it is relevant to study the possibilities of their use.
Researchers have conducted biodiesel synthesis studies using eggshell and methanol as a catalyst [20,21,22,23]. No information was found about oil transesterification studies using eggshell as a catalyst and other alcohols than methanol. Therefore, research in this field is original. One of such alcohols is butanol, and renewable resources can become raw material for its production; therefore, butanol use for the synthesis of fatty acid butyl esters is attractive and increases the share of renewable resources. The objective of the study is to investigate the process of transesterification of rapeseed oil with butanol using eggshells as heterogeneous catalysts. The research is relevant because it aims to investigate the production process of exclusively renewable biofuels produced using renewable materials.
2. Results and Discussions
2.1. Eggshell Preparation
It was found that chicken eggshell—which was used as a heterogeneous catalyst—contains 89.01 ± 0.79% of CaO, which means that content of Ca is 63.58%. Khemthong et al. [24] and Nath et al. [25] determined a higher amount of CaO in eggshell: 99.2 wt% and 99.06 wt%, respectively.
Before transesterification tests, is important to prepare the catalyst. Gaide and colleagues [15] studied dolomite as a catalyst for ester synthesis. The optimum fraction size of dolomite was obtained as 0.315–0.1 mm, and the most suitable calcination temperature was 850 °C for 4 h. For the research, snail shells were prepared under the same conditions.
There is no big difference between the calcination conditions which were used in the study and from those recommended by other researchers. Rochat and colleagues confirm that the complete process of decomposition of CaCO3 to CaO occurs at a temperature not lower than 850 °C. [26]. Ramesh et al. recommends to calcinate eggshell at a temperature no lower than 800 °C [27]. Graziottin and colleagues [28] and Goli and colleagues [23] calcined chicken eggshell in a range of temperatures of 200–1100 °C for 3 h; the same calcination temperature (900 °C) as Goli et al. [23] was recommended by other researchers [29,30,31]. Wei et al. [22] studied the calcination process of eggshell by varying the temperature from 200 to 1000 °C with a constant process duration of 2 h; an optimal temperature of 1000 °C was chosen. In our case a lower temperature of 850 °C was used, however, calcination was performed for a longer duration.
2.2. Modeling and Determination of Optimal Reaction Conditions
Many studies show that the optimum biodiesel synthesis temperature depends on the alcohol type. Higher temperature has a positive effect, as it intensifies the activity of the catalyst, and the molecules move faster, leading to a higher rate of collisions [23]. Most of the transesterification reactions have been performed at the boiling point of alcohol. The optimum temperature of 110 °C was found in the transesterification process of rapeseed oil using butanol and dolomite as a catalyst [16]. During the transesterification of Jatropha curcas oil with butanol, it is determined that the reaction takes place most efficiently at a temperature of 105 °C [32]. Urasaki et al. performed conversion of triolein with butanol at a temperature of 120 °C [33]. However, conducting transesterification processes at a higher temperature than an alcohol’s boiling point requires assurance that the alcohol does not evaporate. Therefore, all experiments in this study were conducted under the 110 °C, as 1-butanol’s boiling point under the atmospheric pressure is 117.7 °C.
The experimental design which was planned with ANOVA, predicted, and experimental values are presented Table 1. The highest predicted butyl ester yield was 98.99%, when the 1-butanol-to-oil molar ratio was 12:1, catalyst amount 6 wt%, and the reaction duration was 12.6 h. Under these conditions, the highest experimental butyl ester yield of 98.55 ± 0.85 wt% was obtained.

Table 1.
Experimental plan, and modelled and experimental results.
An F value of 1722.79 and a p value of less than 0.0001 was determined by the quadratic model, which indicates that the model is statistically significant (Table 2). Values that are less than 0.0500 are significant. Insignificant elements were removed. Equation (1) represents the butyl ester yield after the modified model:
where
EY = 83.58 − 2.82A + 6.35B + 19.84C − 0.51AB − 0.49BC − 10.35A2 − 5.45B2 − 5.83C2

Table 2.
Analysis of variation of quadratic model.
- EY—the butyl ester yield (%);
- A—the 1-butanol-to-oil molar ratio;
- B—the catalyst content (wt%);
- C—the reaction duration (h).
A second-order polynomial equation was used to express the relationship between independent variables and response values, where positive coefficient symbols indicate greater interaction with the response (butyl ester yield) and negative coefficient symbols indicate less interaction. According to the given Equation (1), it can be seen that the butyl ester yield can increase when the molar ratio of butanol to oil decreases and the amount of catalyst (%) and reaction time (h) increase.
The adjusted R2 and predicted R2 values are 0.9988 and 0.9973, respectively, which shows that experimental values are in a reasonable agreement with software predicted values and that the model can account for 99.88% of the response‘s variations.
The ratio of signal to noise is indicated by adequate precision. This ratio is desirable to exceed 4. In this case, the value of adequate precision is 143.512, which is clearly higher than the desirable number (Table 3).

Table 3.
Statistical parameters determined by ANOVA.
An interaction of independent variables that influence the butyl ester yield, visualized in graphical figures. The catalyst (eggshell) amount and butanol-to-oil molar ratio influence on butyl ester yield (the process duration is 12 h) is shown in Figure 1. It is clear that a higher catalyst content leads to a higher ester yield. While small amount (<6 wt%) of eggshells is used, the maximum butyl ester yield is not reached. Increasing the 1-butanol-to-oil molar ratio up to around 11:1, the ester yield also increases, however, further increase leads to ester yield decrease.

Figure 1.
The interaction between the catalyst content and the 1-butanol-to-oil molar ratio for the ester yield (the process time is 12 h) presented by a response surface contour plot.
No studies by other researchers analyzing the oil transesterification process using butanol and eggshell as a catalyst could be found. Urasaki et al. [33] analyzed the process of transesterification of triolein with different alcohols (methanol, ethanol, butanol, propanol) using calcium oxide as a catalyst and found that the most effective reaction takes place using methanol, however, the highest amount of calcium enters the product at the same time. Using higher alcohols, the structure of calcium oxide did not change, leaching of calcium was not observed, and triolein conversion reached 100%.
Avhad et al. [34] studied the transesterification process of jojoba oil with 1-butanol using CaO derived from mussel shells as a catalyst and observed similar trends: increasing the 1-butanol-to-oil molar ratio from 6:1 to 12:1 increased the conversion from 39 to 58.93 wt%, while a 14:1 molar ratio lead to the conversion decreasing to 46.02 wt%. The authors of the study believe that the high amount of butanol leads to the dilution of the mixture, and this interferes with the interaction between jojoba oil and butoxide ions. This is also confirmed by the results of this research. Similar trends that the ester content increases up to a certain limit by increasing the molar ratio of alcohol-to-oil were also found by other researchers who analyzed the process of transesterification of oil with methanol using chicken eggshell as a catalyst. Goli and Sahu [23] found that the yield of soybean methyl ester increased with an alcohol-to-oil molar ratio up to 10:1; after that, it starts decreasing (catalyst calcined eggshell). Correia et al. found that in the process of transesterification of sunflower oil, the optimal molar ratio of methanol-to-oil is 10:1, allowing to obtain the maximum yield of the product [35]. Proenca et al. [31] found 10:1 to be the optimal alcohol-to-oil molar ratio in the macauba oil methyl ester production process. A study on the synthesis of soyabean oil methyl ester was conducted by Wei et al. [22]; it was found that the optimal alcohol-to-oil molar ratio is 9:1, and the same optimal molar ratio of methanol-to-oil was determined by Graziottin et al. [28]. Gaide et al. [21] determined that an increase in the yield of rapeseed oil methyl ester is observed until the methanol-to-oil molar ratio increases up to 13:1, and further increase in the methanol amount leads to a decrease in the ester yield. Therefore, a high amount of alcohol is not desirable for the transesterification process as it can increase reversible reaction [35].
The amount of catalyst in our experiments varied from 4 to 8 wt%. It was observed that with the increase in the amount of catalyst, the ester yield also increased. More than 96 wt% of ester yield was obtained when the amount of catalyst was 8 wt%. Other researchers observed the same trends. The optimal amount of eggshell was found to be 6 wt% in the process of soybean oil methyl ester synthesis [22]. The researchers studied the process of methanolysis of rapeseed oil by changing the amount of eggshell from 4 to 10 wt%. The optimal amount was determined to be 6.8 wt% [21]. Wei et al. obtained a very similar optimal amount of catalyst as in this study, although methanol was used in the process [22]. Graziottin et al. state that the optimal amount of catalyst is 3 wt%, however, the maximum obtained methyl ester yield is only 85 wt% [28]. The optimal amount of eggshell—7 wt% was also determined by applying the transesterification of soybean oil using methanol [23]. Other researchers who studied the transesterification of oils with methanol using eggshell as a catalyst determined that the optimal amount of catalyst varied from 2 to 5 wt% [35,36,37]. The higher optimal amount was determined by Avhad et al. [34]. The effect of calcium oxide obtained from mussel shells as a catalyst on the butanolysis process was investigated by varying the amount of catalyst from 6.3 to 17.7 wt%. It was found that the conversion of jojoba oil increases by increasing the amount of catalyst up to 16 wt%; with the amount of catalyst being higher than 16 wt%, the degree of conversion decreases. Authors explain this by the increase in the viscosity of the reaction mixture; external mass transfer resistance can happen as the viscosity of the reaction mixture increases and it interferes with the interaction between the reactionary molecules.
The influence of process duration and the 1-butanol-to-oil molar ratio to the ester yield when the catalyst amount is 8 wt% is presented in Figure 2. The influence of process duration on oil transesterification reaction was tested: as we can see, biodiesel yield increases with time. As it was mentioned earlier, ester yield increases until the 1-butanol-to-oil molar ratio reaches around 11:1, and it reaches more than 98 wt% after 12 h. The impact of the catalyst content and the process duration (when the 1-butanol-to-oil molar ratio is 12:1) on the butyl ester yield is demonstrated in Figure 3. It is clear that a longer process duration and higher catalyst content both have a positive effect on the butyl ester yield.
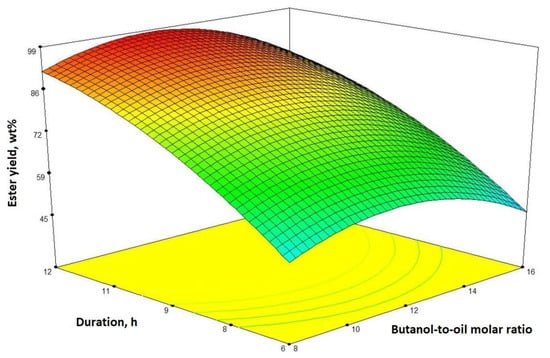
Figure 2.
The interaction between the process time and the 1-butanol-to-oil molar ratio for the ester yield (catalyst content is 8 wt%) presented by a response surface contour plot.
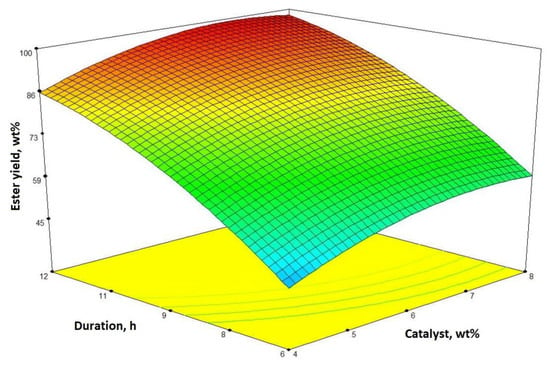
Figure 3.
The interaction between the process time and the catalyst content for the ester yield (the butanol-to-oil molar ratio is 12:1) presented by a response surface contour plot.
Studies of the dependence of the transesterification process on the duration of the process carried out by some scientists confirm the results obtained in this study where the longer the duration, the higher the ester yield. Avhad et al. [34] investigated the process duration influence on jojoba oil conversion using butanol and the CaO from mussel shells as a heterogeneous catalyst. It was found that 96.11 wt% of conversion was achieved after 1800 min (30 h). Controversial results were obtained using eggshell and methanol for transesterification reactions. Piker et al. [38] determined 11 h as the optimal duration of transesterification of soybean oil (97 wt% ester yield), however, the process temperature was only 25 °C. Many other researchers conducted reactions at temperatures close to the alcohol boiling point. Gaide et al. found an ester yield of 97.79 wt% after 9.48 h [21]. Other researchers have found that when using eggshell as a catalyst, ester yield varies from 95 to 99 wt% ester after 1–4 h [22,23,28,33,36,37].
Overall, 96.11 wt% of jojoba oil conversion is achieved when the process duration is 30 h and the amount of calcined mussel shells is 12 wt% [34]. Urasaki and colleagues found that 100 wt% conversion of triolein with butanol is achieved when the amount of CaO is 4 wt% and the process duration is 120 min. [33]. Proenca et al. (2021) used 15 wt% of the catalyst—eggshell—for the transesterification of macauba oil with methanol (process duration—1 h) and obtained a biodiesel yield of 91 wt% [31]. Gaide et al. obtained a yield of 97.79 wt% rapeseed oil methyl ester with a process duration of 9.48 h and 6.8 wt% calcinated eggshell [21].
2.3. Optimization of Fatty Acid Butyl Ester Synthesis Process
In this study, the influence of three independent variables on the yield of rapeseed oil butyl ester was investigated. An optimization step was applied for the process of determining the optimum conditions. Optimum process conditions and predicted and experimental ester yield are presented in Table 4. Optimum conditions when the process temperature is 110 °C and reaction mixing speed is 280 rpm are the following: 1-butanol-to-oil molar ratio 11.3:1, eggshell content 7.41 wt%, and reaction duration 11.81 h. Under determined conditions, the experiment was conducted, and 98.78 ± 0.41 wt% of ester yield was obtained.

Table 4.
Optimal conditions for rapeseed oil butyl ester production, and modelled and experimental ester yield.
Avhad et al. [34] achieved maximum jojoba oil conversion of 96.11 wt% after 30 h (1800 min) of transesterification reaction performed at a temperature of 85 °C using a butanol-to-oil molar ratio of 10:1, mussel shells as heterogeneous catalyst of 12 wt%, and 350 rpm of mixing speed. The process was performed at a lower temperature, but however with a longer process duration of up to 30 h, and more catalyst was required to achieve a higher conversion [34]. A shorter time and a smaller amount of catalyst at a higher temperature are indicated by Urasaki et al. [32]; 100 wt% conversion of triolein was obtained when the butanol-to-oil molar ratio was 6:1, the reaction duration 2 h, the amount of CaO 4 wt%, and the reaction temperature was 120 °C. These differences between the mentioned study and our research can occur due to pure triolein being used in other researcher’s studies, while rapeseed oil was used in our analysis.
Navas et al. studied the transesterification processes of soybean and castor oil with methanol and butanol using heterogeneous catalysts. Better results were obtained using butanol than methanol. Catalysts (MgO/c-Al2O3 and ZnO/c-Al2O3) were used in the transesterification of soybean oil with butanol; a selectivity percentage and FABE yield of 100% and 50% for MgO/c-Al2O3, and 97% and 45% for ZnO/c-Al2O3, respectively, was obtained. Conversion values of 97% and 85% were achieved for MgO/-Al2O3 and ZnO/c-Al2O3, respectively, after 6 h of reaction [39].
Jazie et al. optimized the process of transesterification of rapeseed oil with methanol and calcined eggshell. The predicted biodiesel yield was 96% when the process duration is 3 h, the reaction temperature is 60 °C, the amount of catalysts is 3 wt% [40]. Yasar obtained 95.12% rapeseed oil methyl ester yields, when the catalysts was 4%, reaction time 1 h, and reaction temperature 60 °C [41].
Many authors, after analyzing the process of transesterification of oil with methanol using eggshells as a catalyst, obtained the optimal duration of the process as being 1.5–5 h, however, various oils/fats were used: date seed oil (Phoenix dactylifera L.) [42], triglycerides [22], and high-free-fatty-acid chicken fat [43].
It is difficult to compare the obtained optimal process conditions with the results obtained by other scientists, because other studies used a shorter-chain alcohol—methanol.
2.4. Quality Parameters of Rapeseed Oil Butyl Esters
Physical and chemical properties of rapeseed butyl esters produced at optimal conditions are presented in Table 5.

Table 5.
Physical and chemical parameters of rapeseed oil butyl esters.
It is clear that under determined conditions, obtained rapeseed butyl esters meet the requirements of EN 14214 standard for biodiesel fuel.
3. Materials and Methods
3.1. Catalyst Preparation
Eggshell is a natural material which is mainly composed of calcium compounds. Calcium compounds can be converted to calcium oxide. Eggshells were collected after purchasing eggs in a local supermarket. Eggshell was prepared according to the results of our previous experiments, where dolomite was investigated for biodiesel production, determining the optimum conditions for calcination in a muffle furnace (AB UMEGA SNOL 8.2/1100, Utena, Lithuania) for 5 h at 850 °C [15]. Before calcination, the eggshell was crushed in a laboratory mill. After that, the material was sieved through a sieve and fractions of 0.315–0.1 mm were collected.
3.2. CaO Content Determination in Eggshell
For investigation of CaO content in eggshell decomposition of material, titration using trilon B (EDTA) and calculations were completed according to Gaide et al. [21].
3.3. Transesterification of Rapeseed Oil
Rapeseed oil, which met the national requirement for edible oil, was purchased in a local supermarket. Transesterification reactions with 1-butanol (99.5%, Chempur, Piekary Śląskie, Poland) were conducted in conical flasks, which were connected to reflux condensers in order to reduce te loss of 1-butanol from the reaction mixture. Mixers at 250 rpm and thermometers were used.
Research on the biodiesel production process was carried out under the following conditions: temperature—110 °C; butanol-to-oil molar ratio—from 5 to 16; catalyst content—from 3 to 8%; duration of reactions—from 5 to 12.6 h.
After reaction, the filtration step was applied in order to separate the catalyst from the final product. Purification was conducted by washing the product with 5% H3PO4 solution (10% of the final product) and two times with distilled water (10% of the mixture), followed by the evaporation of residual water at 110 °C using a stirrer with a hot plate.
3.4. Ester Yield Analysis
The ester yield was investigated regarding the glyceride contents (glycerol, monoglycerides, diglycerides, triglycerides) in the samples. Glyceride contents were determined by gas chromatography using a Perkin Elmer Clarus 500 (detector—FID) (Boston, MA, USA) gas chromatograph according to the requirements of the EN 14105 standard. The ester levels were calculated based on the glyceride levels according to the following Equation (2), presented by Bailer et al. (1994) [44].
where
- EY—ester yield, %;
- MG, DG, and TG—the concentrations of monoglyceride, diglyceride, triglycerides, respectively, %;
- 0.2411, 0.1426, and 0.1012—the respective conversion indicators for the glycerides;
- 10.441—the amount of glycerol which obtained from 1 kg of rapeseed oil.
3.5. Response Surface Analysis
The plan for experiment was set and results were analyzed using response surface methodology (RSM). In order to determine the optimal conditions for transesterification of rapeseed oil with 1-butanol and eggshell as a heterogeneous catalyst, Design Expert version 8.01 (Stat-Ease, Minneapolis, MN, USA, 20110) was used.
The rapeseed oil butyl ester yield was optimized in a 4-factorial experiment depending on the amount of butanol and the catalyst and the reaction duration. The transesterification reaction was carried out under different conditions, varying the values of the following parameters: the butanol-to-oil molar ratio (mol:mol) (from 8 to 16), the catalyst amount (from 4 to 8 wt%), and the reaction time (from 6 to 12 h). The central composite design consisted of 20 experimental trials, 14 non-central points, and 6 repeated central points (α = 1.2). [45].
A computer simulation program was used for mathematical model creation. Interaction between the transesterification reaction conditions and the rapeseed oil butyl ester yield was determined by using a quadratic reaction surface model.
The mathematical relationship between factors and the response is shown in Equation (3), as there were three factors involved in the research:
where
- Y—predicted response;
- β0—the offset term;
- βi—the linear coefficients;
- βii and βij—the interaction coefficients;
- xi and xj—the independent variables [45].
After all experiments were performed, results were analyzed, and a final model that best fit was applied. Selected model gave the values of independent variables on which maximum ester yield can be obtained.
4. Conclusions
Calcinated at 850 °C temperature for 4 h, eggshell fractions of 0.315–0.1 mm size contain 89.01 ± 0.79% of CaO, and therefore eggshell can be used as a heterogeneous catalyst for transesterification reactions.
The 1-butanol-to-oil molar ratio, the catalyst content, and the duration of the reaction have influence on the yield of fatty acid butyl ester, and therefore this was investigated. It was determined that the higher catalyst content and the longer process duration have a positive effect on the ester yield. Increasing the 1-butanol amount is useful, to a certain point. Response surface methodology was used to optimize the heterogeneous biodiesel synthesis with butanol. The following optimum process conditions were obtained: 1-butanol-to-oil molar ratio 11.3:1, catalyst amount 7.41 wt%, and a reaction duration of 11.81 h at 110 °C. Under determined conditions, the obtained ester yield meets the EN 14214 standard requirement and reaches 98.78 ± 0.41 wt%.
Author Contributions
Conceptualization, V.M. and E.S.; methodology, I.G., V.M. and M.G.; resources, E.S.; investigation, I.G.; data curation, I.G., V.M. and M.G.; writing—original draft preparation, I.G., V.M. and E.S.; writing—review and editing, I.G., V.M. and E.S.; visualization, I.G. and E.S.; supervision, V.M.; project administration, V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Vytautas Magnus university, grant number P-VDU-22-3.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pillot, B.; Muselli, M.; Poggi, P.; Dias, J.B. Historical trends in global energy policy and renewable power sys-tem issues in Sub-Saharan Africa: The case of solar PV. Energy Policy 2019, 127, 113–124. [Google Scholar] [CrossRef]
- Bastida-Molina, P.; Hurtado-Pérez, E.; Moros Gómez, M.C.; Cárcel-Carrasco, J.; Pérez-Navarro, Á. Energy sustainability evolution in the Mediterranean countries and synergies a global energy scenario for the area. Energy 2022, 252, 124067. [Google Scholar] [CrossRef]
- Nęcka, K.; Knaga, J. Environmental impact assessment for electric vehicles. J. Phys. 2021, 1782, 12023. [Google Scholar] [CrossRef]
- Rajak, U.; Verma, T.N. Effect of emission from ethylic biodiesel of edible and non-edible vegetable oil, animal fats, waste oil and alcohol in CI engine. Energy Convers. Manag. 2018, 166, 704–718. [Google Scholar] [CrossRef]
- Sendzikiene, E.; Makareviciene, V.; Janulis, P. Influence of composition of fatty acid methyl esters on smoke opacity and amount of polycyclic aromatic hydrocarbons in engine emissions. Pol. J. Environ. Stud. 2007, 16, 259–265. [Google Scholar]
- Nabi, M.N.; Akhter, M.S.; Shahadat, M.M.Z. Improvement of engine emissions with conventional diesel fuel and diesel–biodiesel blends. Bioresour. Technol. 2006, 97, 372–378. [Google Scholar] [CrossRef]
- Gonca, G.; Dobrucali, E. Theoretical and experimental study on the performance of a diesel engine fueled with diesel–biodiesel blends. Renew. Energy 2016, 93, 658–666. [Google Scholar] [CrossRef]
- Moraes, P.S.; Engelmann, J.I.; Igansi, A.V.; Sant Anna Cadaval, T.R., Jr.; De Almeida Pinto, L.A. Nile tilapia industrialization waste: Evaluation of the yield, quality and cost of the biodiesel production process. J. Clean. Prod. 2021, 287, 125041. [Google Scholar] [CrossRef]
- Sajjad, N.; Orfali, R.; Perveen, S.; Rehman, S.; Sultan, A.; Akhtar, T.; Iqbal, M. Biodiesel Production from Alkali-Catalyzed Transesterification of Tamarindus indica Seed Oil and Optimization of Process Conditions. Molecules 2022, 27, 3230. [Google Scholar] [CrossRef] [PubMed]
- Makareviciene, V.; Gumbyte, M.; Skorupskaite, V.; Sendzikiene, E. Biodiesel fuel production by enzymatic microalgae oil transesterification with ethanol. J. Renew. Sustain. Energy 2017, 9, 023101. [Google Scholar] [CrossRef]
- Faruque, M.O.; Razzak, S.A.; Hossain, M.M. Application of heterogeneous catalysts for biodiesel production from microalgal oil—A review. Catalysts 2020, 10, 1025. [Google Scholar] [CrossRef]
- Makareviciene, V.; Sendzikiene, E.; Gaide, I. Application of heterogeneous catalysis to biodiesel synthesis using microalgae oil. Front. Environ. Sci. Eng. 2021, 15, 97. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Ma, X.; Wu, Z.; Cui, P.; Lu, W.; Wang, Y. A novel magnetic CaO-based catalyst synthesis and characterization: Enhancing the catalytic activity and stability of CaO for biodiesel production. Chem. Eng. J. 2020, 391, 123549. [Google Scholar] [CrossRef]
- Harsha Hebbar, H.R.; Math, M.C.; Yatish, K.V. Optimization and kinetic study of CaO nano-particles catalyzed biodiesel production from Bombax ceiba oil. Energy 2018, 143, 25–34. [Google Scholar] [CrossRef]
- Gaide, I.; Makareviciene, V.; Sendzikiene, E.; Kazancev, K. Natural rocks–heterogeneous catalysts for oil transesterification in biodiesel synthesis. Catalysts 2021, 11, 384. [Google Scholar] [CrossRef]
- Gaide, I.; Makareviciene, V.; Sendzikiene, E.; Gumbyte, M. Application of dolomite as solid base catalyst for transesterification of rapeseed oil with butanol. Sustain. Energy Technol. Assess. 2022, 52, 102278. [Google Scholar] [CrossRef]
- Shobana, R.; Vijayalakshmi, S.; Deepanraj, B.; Ranjitha, J. Biodiesel production from Capparis spinosa L seed oil using calcium oxide as a heterogeneous catalyst derived from oyster shell. Mater. Today Proc. 2021, in press. [Google Scholar] [CrossRef]
- Takeno, M.L.; Mendonça, I.M.; Barros, S.D.S.; de Sousa Maia, P.J.; Pessoa, W.A.; Souza, M.P.; Soares, E.R.; Bindá, R.D.S.; Calderaro, F.L.; Sá, I.S. S.; et al. A novel CaO-based catalyst obtained from silver croaker (Plagioscion squamosissimus) stone for biodiesel synthesis: Waste valorization and process optimization. Renew. Energy 2021, 172, 1035–1045. [Google Scholar] [CrossRef]
- Shahbandeh Leading Egg Producing Countries Worldwide in 2020 Statista. 2022. Available online: https://www.statista.com/statistics/263971/top-10-countries-worldwide-in-egg-production/ (accessed on 1 October 2022).
- Laca, A.; Laca, A.; Díaz, M. Eggshell waste as catalyst: A review. J. Environ. Manag. 2017, 197, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Gaide, I.; Makareviciene, V.; Sendzikiene, E. Effectiveness of Eggshells as Natural Heterogeneous Catalysts for Transesterification of Rapeseed Oil with Methanol. Catalysts 2022, 12, 246. [Google Scholar] [CrossRef]
- Wei, Z.; Xu, C.; Li, B. Application of waste eggshell as low-cost solid catalyst for biodiesel production. Bioresour. Technol. 2009, 100, 2883–2885. [Google Scholar] [CrossRef] [PubMed]
- Goli, J.; Sahu, O. Development of heterogeneous alkali catalyst from waste chicken eggshell for biodiesel production. Renew. Energy 2018, 128, 142–154. [Google Scholar] [CrossRef]
- Khemthong, P.; Luadthong, C.; Nualpaeng, W.; Changsuwan, P.; Tongprem, P.; Viriya-Empikul, N.; Faungnawakij, K. Industrial eggshell wastes as the heterogeneous catalysts for microwave-assisted biodiesel production. Catal. Today 2012, 190, 112–116. [Google Scholar] [CrossRef]
- Nath, D.; Jangid, K.; Susaniya, A.; Kumar, R.; Vaish, R. Eggshell derived CaO-Portland cement antibacterial composites. Compos. Part C Open Access 2021, 5, 100123. [Google Scholar] [CrossRef]
- Roschat, W.; Siritanon, T.; Yoosuk, B.; Promarak, V. Biodiesel production from palm oil using hydrated lime-derived CaO as a low-cost basic heterogeneous catalyst. Energy Convers. Manag. 2016, 108, 459–467. [Google Scholar] [CrossRef]
- Ramesh, S.; Natasha, A.N.; Tan, C.Y.; Bang, L.T.; Ramesh, S.; Ching, C.Y.; Thambinayagam, C.H. Direct conversion of eggshell to hydroxyapatite ceramic by a sintering method. Ceram. Int. 2016, 42, 7824–7829. [Google Scholar] [CrossRef]
- Graziottin, P.L.; Rosset, M.; Lima, D.S.; Perez-Lopez, O.W. Transesterification of different vegetable oils using eggshells from various sources as catalyst. Vib. Spectrosc. 2020, 109, 103087. [Google Scholar] [CrossRef]
- Gupta, J.; Agarwal, M. Preparation and characterization of CaO nanoparticle for biodiesel production. AIP Conf. Proc. 2016, 1724, 020066. [Google Scholar]
- Buasri, A.; Chaiyut, N.; Loryuenyoung, V.; Wongweang, C.; Khamsrisuk, S. Application of eggshell wastes as a heterogeneous catalyst for biodiesel production: A review. Sustain. Energy 2013, 1, 7–13. [Google Scholar]
- Proenca, B.S.G.; Fioroto, P.O.; Heck, S.C.; Duarte, V.A.; Filho, L.C.; Feihrmann, A.C.; Beneti, S.C. Obtention of methyl esters from macauba oil using egg shell catalyst. Chem. Eng. Res. Des. 2021, 169, 288–296. [Google Scholar] [CrossRef]
- Jha, M.K.; Gupta, A.K.; Kumar, V. Kinetics of Transesterification on Jatropha Curcas Oil to Biodiesel Fuel. In Proceedings of the World Congress on Engineering and Computer Science, London, UK, 2–4 July 2007; pp. 99–102. [Google Scholar]
- Urasaki, K.; Takagi, S.; Mukoyama, T.; Christopher, J.; Urasaki, K.; Kato, S.; Yamasaki, A.; Kojima, T.; Satokawa, S. Effect of the kinds of alcohols on the structure and stability of calcium oxide catalyst in triolein transesterification reaction. Appl. Catal. A. Gen. 2012, 411–412, 44–50. [Google Scholar] [CrossRef]
- Avhad, M.R.; Sanchez, M.; Pena, E.; Bouaid, A.; Martínez, M.; Aracil, J.; Marchetti, J.M. Renewable production of value-added jojobyl alcohols and biodiesel using a naturally-derived heterogeneous green catalyst. Fuel 2016, 179, 332–338. [Google Scholar] [CrossRef]
- Correia, L.M.; Cecilia, J.A.; Rodríguez-Castellón, E.; Cavalcante, C.L.; Vieira, R.S. Relevance of the Physicochemical Properties of Calcined Quail Eggshell (CaO) as a Catalyst for Biodiesel Production. J. Chem. 2017, 5679512. [Google Scholar]
- Correia, L.M.; Saboya, R.M.A.; de Susa Campelo, N.; Cecilia, J.A.; Rodrguez-Castelln, E.; Cavalcante, C.L.; Vieira, M.R.S. Characterization of calcium oxide catalysts from natural sources and their application in the transesterification of sunflower oil. Bioresour. Technol. 2014, 151, 207–213. [Google Scholar] [CrossRef]
- Niju, S.; Begum, M.S.; Anantharaman, N. Modification of egg shell and its application in biodiesel production. J. Saudi Chem. Soc. 2014, 18, 702–706. [Google Scholar] [CrossRef]
- Piker, A.; Tabah, B.; Perkas, N.; Gedanken, A. A green and low-cost room temperature biodiesel production method from waste oil using egg shells as catalyst. Fuel 2016, 182, 34–41. [Google Scholar] [CrossRef]
- Navas, M.B.; Lick, I.D.; Bolla, P.A.; Casella, M.L.; Ruggera, J.F. Transesterification of soybean and castor oil with methanol and butanol using heterogeneous basic catalysts to obtain biodiesel. Chem. Eng. Sci. 2018, 187, 444–454. [Google Scholar] [CrossRef]
- Jazie, A.A.; Pramanik, H.; Sinha, A.S.K.; Jazie, A.A. Egg shell as eco-friendly catalysts for transesterification of rapeseed oil: Optimization for biodiesel production. Int. J. Sustain. Dev. 2013, 2315–4721, 27–32. [Google Scholar]
- Yasar, F. Biodiesel production via waste eggshell as a low-cost heterogeneous catalyst: Its effects on some critical fuel properties and comparison with CaO. Fuel 2019, 255, 115828. [Google Scholar] [CrossRef]
- Farooq, M.; Ramli, A.; Naeem, A.; Mahmood, T.; Ahmad, S.; Humayun, M.; Islam, M.G.U. Biodiesel Production from Date Seed Oil (Phoenix Dactylifera L.) via Egg Shell Derived Heterogeneous Catalyst. Chem. Eng. Res. Des. 2018, 132, 644–651. [Google Scholar] [CrossRef]
- Kirubakaran, M.; Selva, A.M. Eggshell as heterogeneous catalyst for synthesis of biodiesel from high free fatty acid chicken fat and its working characteristics on a CI engine. J. Environ. Chem. Eng. 2018, 6, 4490–4503. [Google Scholar]
- Bailer, J.; Hödl, P.; de Hueber, K.; Mitelbach, M.; Plank, C.; Schindlbauer, H. Handbook of Analytical Methods for Fatty Acid Methyl Esters Used as Biodiesel Fuel Substitutes; Fichte, Research Institute for Chemistry and Technology of Petroleum Products, University of Technology: Vienna, Austria, 1994; pp. 36–38. [Google Scholar]
- Montgomery, D. Design and Analysis of Experiments; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).