A One-Pot Hydrothermal Preparation of High Loading Ni/La2O3 Catalyst for Efficient Hydrogenation of Cinnamaldehyde
Abstract
1. Introduction
2. Results and Discussion
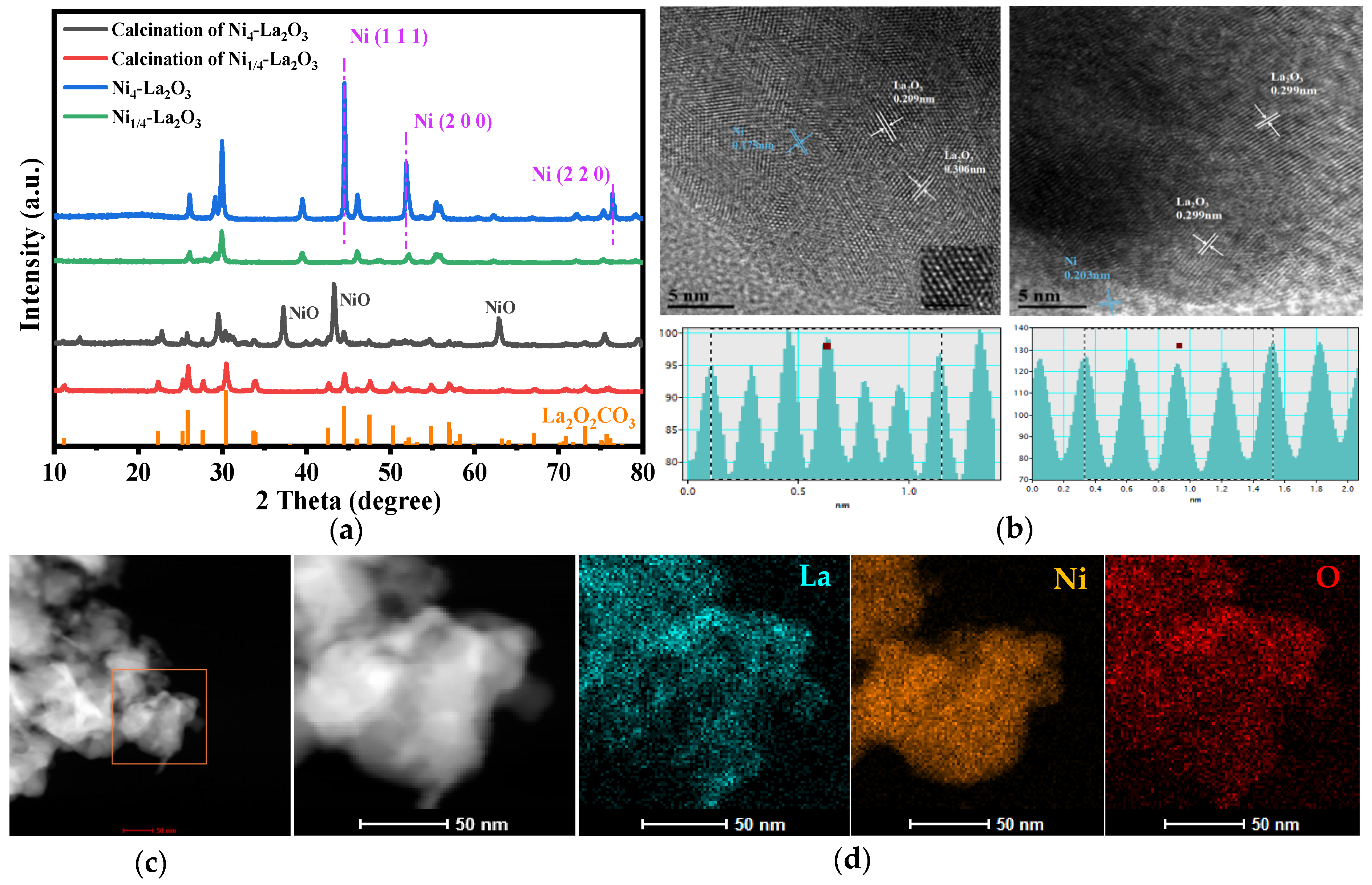
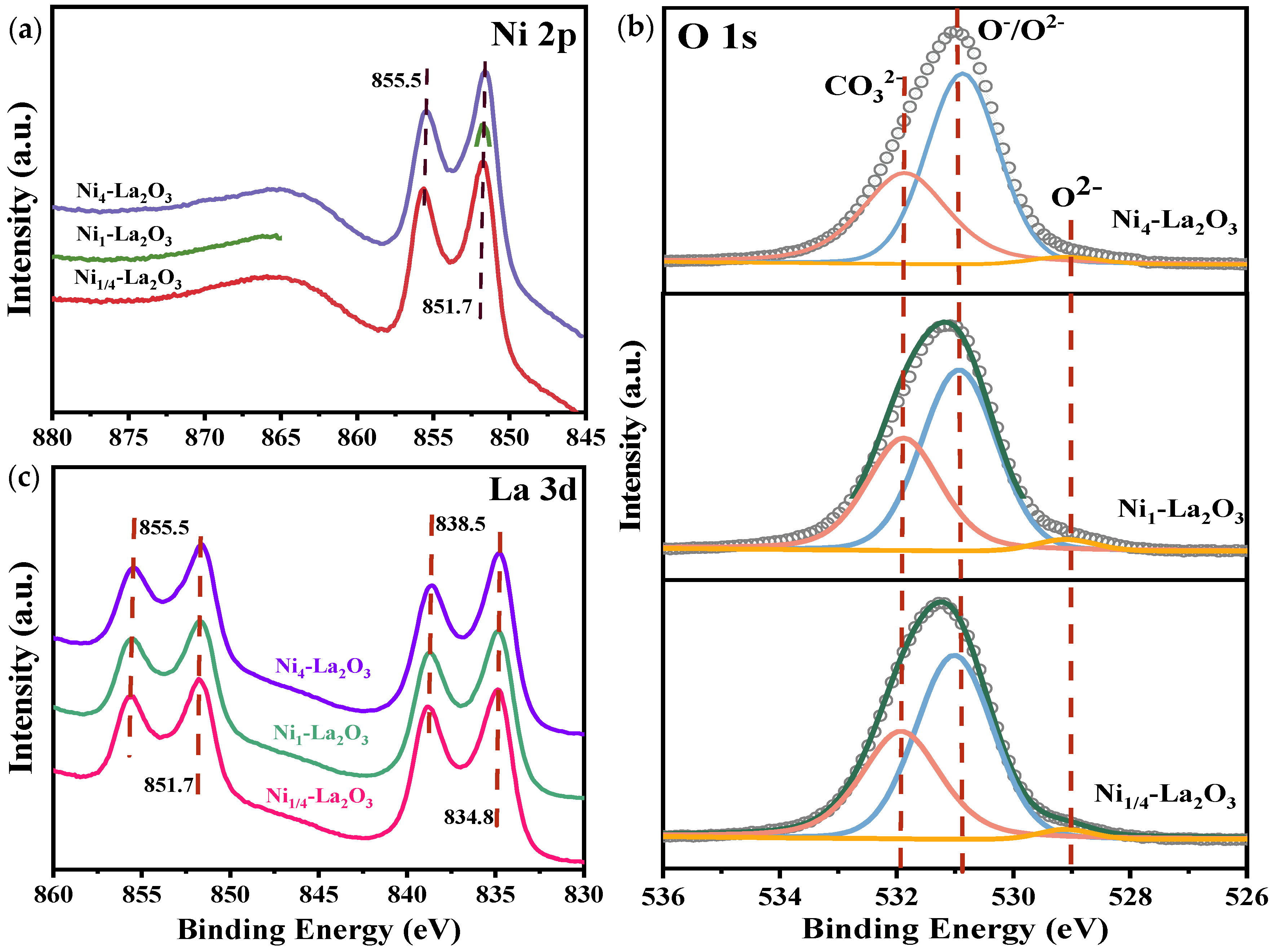
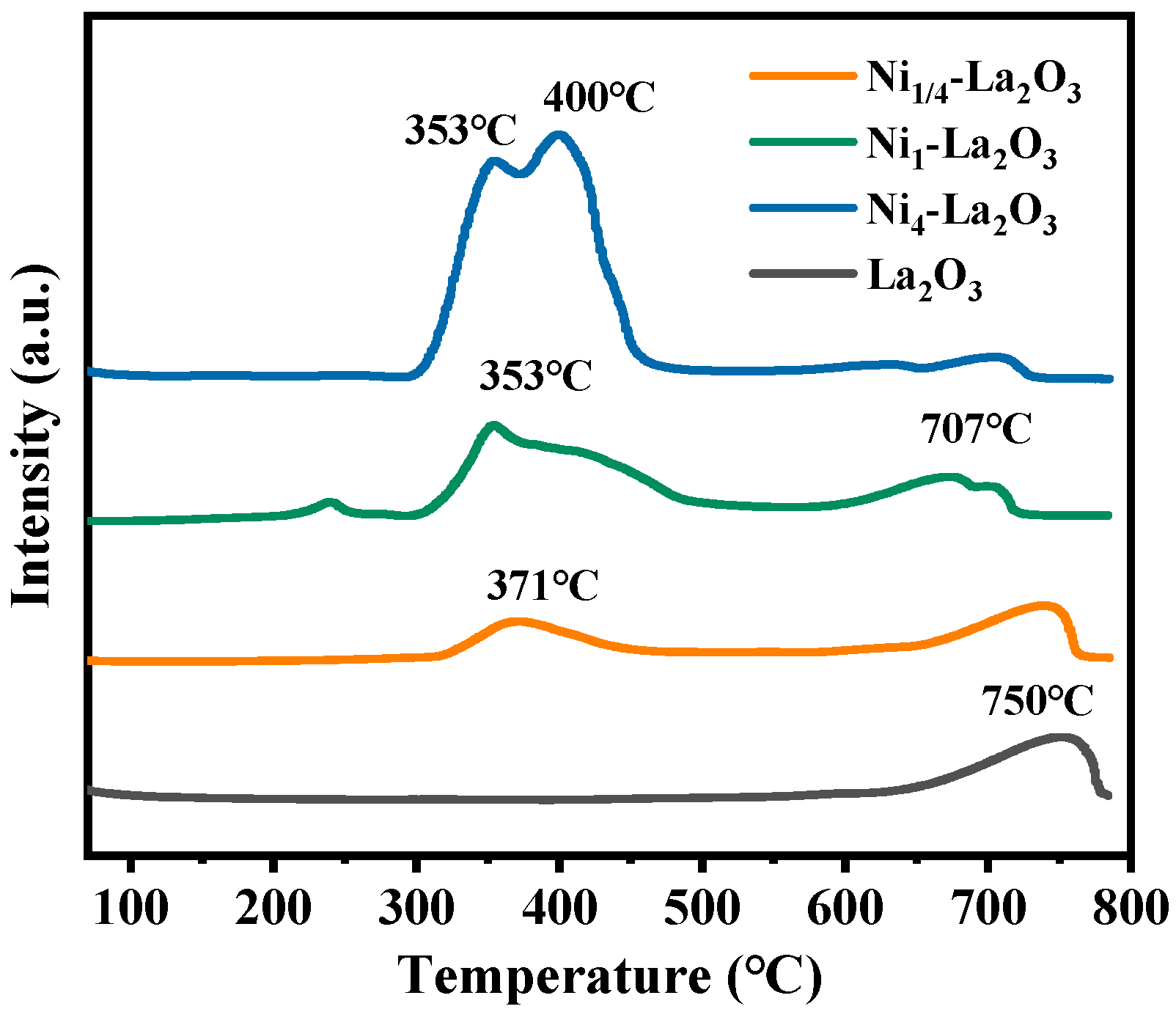
2.1. Characterization of the Catalysts
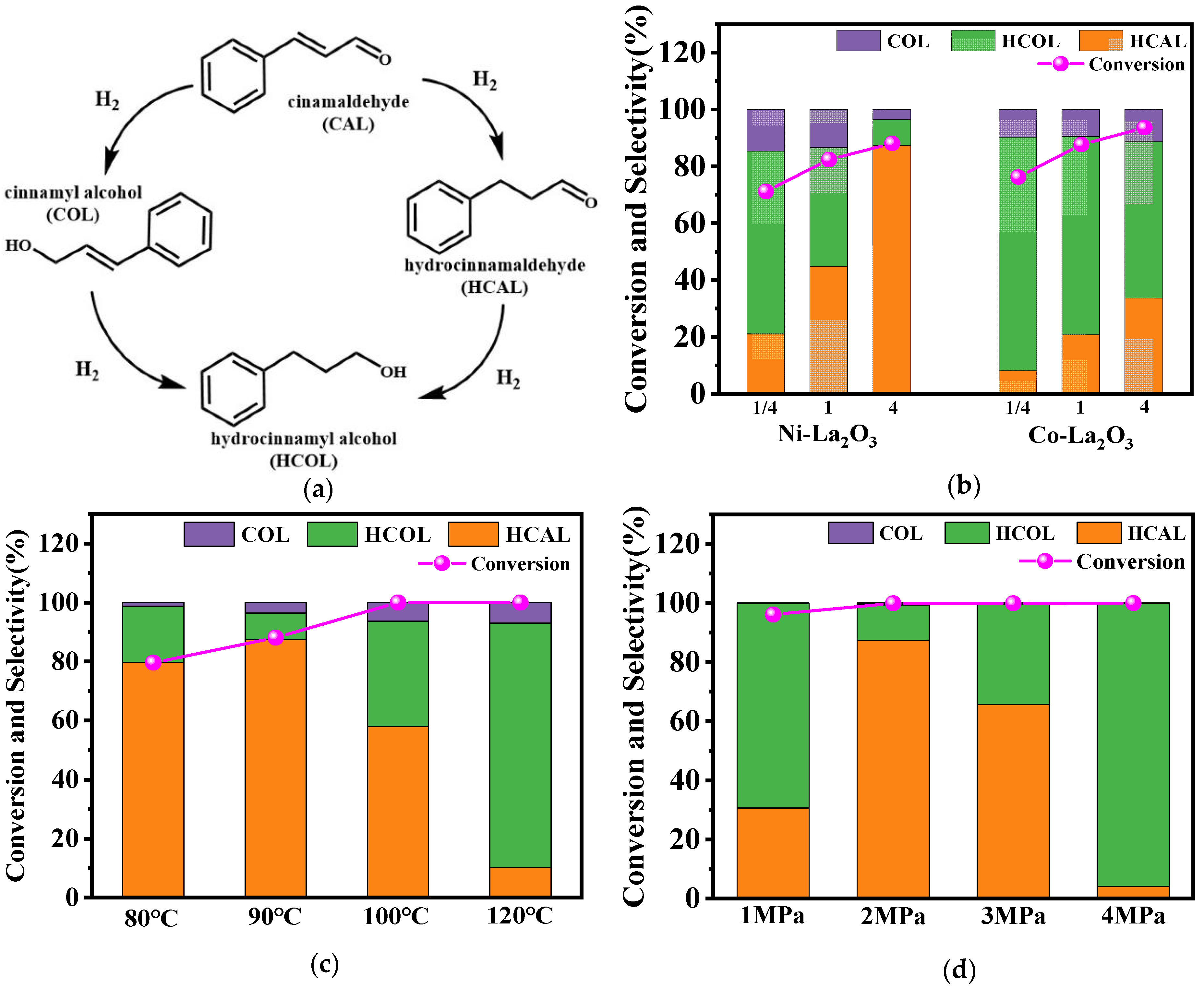
2.2. Catalytic Selective Hydrogenation of Cinnamaldehyde
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Nix-La2O3
3.3. Catalyst Characterization
3.4. Catalytic Reaction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Farrar-Tobar, R.A.; Dell’Acqua, A.; Tin, S.; de Vries, J.G. Metal-catalysed selective transfer hydrogenation of α, β-unsaturated carbonyl compounds to allylic alcohols. Green Chem. 2020, 22, 3323–3357. [Google Scholar] [CrossRef]
- Zahid, M.; Li, J.; Ismail, A.; Zaera, F.; Zhu, Y. Platinum and cobalt intermetallic nanoparticles confined within MIL-101 (Cr) for enhanced selective hydrogenation of the carbonyl bond in α, β-unsaturated aldehydes: Synergistic effects of electronically modified Pt sites and Lewis acid sites. Catal. Sci. Technol. 2021, 11, 2433–2445. [Google Scholar] [CrossRef]
- Iqbal, Z.; Sadiq, M.; Sadiq, S.; Saeed, K. Selective hydrogenation of cinnamaldehyde to cinnamyl alcohol over palladium/zirconia in microwave protocol. Catal. Today 2022, 397, 389–396. [Google Scholar] [CrossRef]
- Sreenavya, A.; Mallannavar, C.N.; Sakthivel, A. Functional group hydrogenation of cinnamaldehyde to hydrocinnamyl alcohol over nickel-ruthenium containing hydrotalcite. Mater. Today Proc. 2021, 46, 3152–3157. [Google Scholar] [CrossRef]
- Zhao, J.; Yuan, H.; Gui, Y.; Li, X.; Qin, X.; Wei, C.; Liu, Y.; Wang, G.; Zhou, L.; Fang, S. Engineering the interface of Au nanocatalysts with FeOx for enhanced selective hydrogenation of cinnamaldehyde. J. Mater. Sci. 2021, 56, 5760–5771. [Google Scholar] [CrossRef]
- Li, Z.J.; Wei, W.; Li, H.H.; Li, S.H.; Leng, L.P.; Zhang, M.Y.; Horton, J.H.; Wang, D.S.; Sun, W.W.; Guo, C.M.; et al. Low-Temperature Synthesis of Single Palladium Atoms Supported on Defective Hexagonal Boron Nitride Nanosheet for Chemoselective Hydrogenation of Cinnamaldehyde. ACS Nano 2021, 15, 10175–10184. [Google Scholar] [CrossRef] [PubMed]
- Das, T.K.; Remanan, S.; Ghosh, S.; Das, N.C. An environment friendly free-standing cellulose membrane derived for catalytic reduction of 4-nitrophenol: A sustainable approach. J. Environ. Chem. Eng. 2021, 9, 104596. [Google Scholar] [CrossRef]
- Das, T.K.; Das, N.C. Advances on catalytic reduction of 4-nitrophenol by nanostructured materials as benchmark reaction. Int. Nano Lett. 2022, 23, 1–20. [Google Scholar] [CrossRef]
- Tang, Y.; Li, H.K.; Cui, K.C.; Xia, Y.D.; Yuan, G.Y.; Feng, J.; Xiong, W. Chemoselective hydrogenation of cinnamaldehyde over amorphous coordination polymer supported Pt-Co bimetallic nanocatalyst. Chem. Phys. Lett. 2022, 801, 8. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, J.F.; Chen, J.N.; Dai, C.S.; Zhang, B.S. Insight into the role of iron in platinum-based bimetallic catalysts for selective hydrogenation of cinnamaldehyde. Chin. Chem. Lett. 2022, 33, 3757–3761. [Google Scholar] [CrossRef]
- Alfilfil, L.; Ran, J.S.; Chen, C.L.; Dong, X.L.; Wang, J.J.; Han, Y. Highly dispersed Pd nanoparticles confined in ZSM-5 zeolite crystals for selective hydrogenation of cinnamaldehyde. Microporous. Mesoporous. Mat. 2022, 330, 5. [Google Scholar] [CrossRef]
- Zhang, B.; Li, M.; Lei, H.; Chen, J.; Wang, S.; Chen, C. Combining shell confinement and outside decoration to boost the selectivity of Pt nanocatalysts in hollow-flower like Zr-MOFs double shell micro-reactor for hydrogenation cinnamaldehyde to unsaturated alcohol. Appl. Surf. Sci. 2022, 599, 153899. [Google Scholar] [CrossRef]
- Wei, X.J.; Rang, X.D.; Zhu, W.H.; Xiang, M.; Deng, Y.Y.; Jiang, F.H.; Mao, R.; Zhang, Z.W.; Kong, X.Q.; Wang, F. Morphology effect of CeO2 on Ni/CeO2 catalysts for selective hydrogenation of cinnamaldehyde. Chem. Phys. 2021, 542, 6. [Google Scholar] [CrossRef]
- Bustamante, T.M.; Fraga, M.A.; Fierro, J.L.G.; Campos, C.H.; Pecchi, G. Cobalt SiO2 core-shell catalysts for chemoselective hydrogenation of cinnamaldehyde. Catal. Today 2020, 356, 330–338. [Google Scholar] [CrossRef]
- Patil, K.N.; Manikanta, P.; Srinivasappa, P.M.; Jadhav, A.H.; Nagaraja, B.M. Exploring the confined space and active sites of Ni@ OCNTs catalyst for chemoselective hydrogenation of cinnamaldehyde to hydrocinnamaldehyde. J. Environ. Chem. Eng. 2022, 10, 108208. [Google Scholar] [CrossRef]
- Gryglewicz, S.; Sliwak, A.; Cwikla, J.; Gryglewicz, G. Performance of Carbon Nanofiber and Activated Carbon Supported Nickel Catalysts for Liquid-Phase Hydrogenation of Cinnamaldehyde into Hydrocinnamaldehyde. Catal. Lett. 2014, 144, 62–69. [Google Scholar] [CrossRef]
- Pei, A.; Ruan, L.N.; Liao, J.H.; Fu, H.; Zeng, L.; Liu, J.; Li, M.; Chen, B.H.; Zhu, L.H. Platinum Island-on-Copper-Nickel Alloy Nanoparticle/Carbon Trimetallic Nanocatalyst for Selective Hydrogenation of Cinnamaldehyde. Catal. Lett. 2021, 151, 559–572. [Google Scholar] [CrossRef]
- Jia, A.Z.; Yao, X.F.; Feng, L.; Ma, Z.X.; Li, F.; Wang, Y.J. Synthesis of Hierarchically Porous Amorphous Alloy Hollow Sphere with High Surface Area as Effective and Selective Catalysts for Cinnamaldehyde Hydrogenation. Eur. J. Inorg. Chem. 2020, 2020, 1184–1191. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, Y.L.; Zhou, L.; Zhang, W.H.; Li, Y.X. Amorphous Nickel Phosphide Nanoparticles for Selective Hydrogenation of Cinnamaldehyde. Catal. Lett. 2020, 150, 2695–2702. [Google Scholar] [CrossRef]
- Li, Z.; Dong, X.; Zhang, M.; Leng, L.; Chen, W.; Horton, J.H.; Wang, J.; Li, Z.; Wu, W. Selective hydrogenation on a highly active single-atom catalyst of palladium dispersed on ceria nanorods by defect engineering. ACS Appl. Mater. Interfaces 2020, 12, 57569–57577. [Google Scholar] [CrossRef]
- Wang, H.; Shu, Y.; Zheng, M.; Zhang, T. Selective hydrogenation of cinnamaldehyde to hydrocinnamaldehyde over SiO2 supported nickel phosphide catalysts. Catal. Lett. 2008, 124, 219–225. [Google Scholar] [CrossRef]
- Wang, H.; Liu, B.; Liu, F.; Wang, Y.; Lan, X.; Wang, S.; Ali, B.; Wang, T. Transfer hydrogenation of cinnamaldehyde catalyzed by Al2O3 using ethanol as a solvent and hydrogen donor. ACS Sustain. Chem. Eng. 2020, 8, 8195–8205. [Google Scholar] [CrossRef]
- Cifuentes, B.; Valero, M.F.; Conesa, J.A.; Cobo, M. Hydrogen production by steam reforming of ethanol on Rh-Pt catalysts: Influence of CeO2, ZrO2, and La2O3 as supports. Catalysts 2015, 5, 1872–1896. [Google Scholar] [CrossRef]
- Dragoi, B.; Mazilu, I.; Chirieac, A.; Ciotonea, C.; Ungureanu, A.; Marceau, E.; Dumitriu, E.; Royer, S. Highly dispersed copper (oxide) nanoparticles prepared on SBA-15 partially occluded with the P123 surfactant: Toward the design of active hydrogenation catalysts. Catal. Sci. Technol. 2017, 7, 5376–5385. [Google Scholar] [CrossRef]
- Wu, C.; Lin, L.; Liu, J.; Zhang, J.; Zhang, F.; Zhou, T.; Rui, N.; Yao, S.; Deng, Y.; Yang, F.; et al. Inverse ZrO2/Cu as a highly efficient methanol synthesis catalyst from CO2 hydrogenation. Nat. Commun. 2020, 11, 5767. [Google Scholar] [CrossRef]
- Dai, Y.H.; Xu, M.; Wang, Q.J.; Huang, R.; Jin, Y.Y.; Bian, B.; Tumurbaatar, C.; Ishtsog, B.; Bold, T.; Yang, Y.H. Enhanced activity and stability of Ni/La2O2CO3 catalyst for CO2 methanation by metal-carbonate interaction. Appl. Catal. B-Environ. 2020, 277, 10. [Google Scholar] [CrossRef]
- Shafiee, P.; Alavi, S.M.; Rezaei, M. Mechanochemical synthesis method for the preparation of mesoporous Ni–Al2O3 catalysts for hydrogen purification via CO2 methanation. J. Energy Inst. 2021, 96, 1–10. [Google Scholar] [CrossRef]
- Wang, N.; Yu, X.; Shen, K.; Chu, W.; Qian, W. Synthesis, characterization and catalytic performance of MgO-coated Ni/SBA-15 catalysts for methane dry reforming to syngas and hydrogen. Int. J. Hydrog. Energy 2013, 38, 9718–9731. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, Z.; Wang, S.; Wang, G.; Gao, X.; Zhang, B.; Xing, S.; Zhao, S.; Qin, Y. Origin of synergistic effects in bicomponent cobalt oxide-platinum catalysts for selective hydrogenation reaction. Nat. Commun. 2019, 10, 4166. [Google Scholar] [CrossRef]
- Niu, L.; An, Y.; Yang, X.; Bian, G.; Wu, Q.; Xia, Z.; Bai, G. Highly dispersed Ni nanoparticles encapsulated in hollow mesoporous silica spheres as an efficient catalyst for quinoline hydrogenation. Mol. Catal. 2021, 514, 111855. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Kumar, A.; Khraisheh, M. Combustion synthesis of lanthanum oxide supported Cu, Ni, and CuNi nanoparticles for CO2 conversion reaction. Int. J. Hydrog. Energy, 2022; in press. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Li, Y. Chemoselective hydrogenation of cinnamaldehyde over a Pt-Lewis acid collaborative catalyst under ambient conditions. Ind. Eng. Chem. Res. 2015, 54, 1487–1497. [Google Scholar] [CrossRef]
- Hao, C.-H.; Guo, X.-N.; Pan, Y.-T.; Chen, S.; Jiao, Z.-F.; Yang, H.; Guo, X.-Y. Visible-light-driven selective photocatalytic hydrogenation of cinnamaldehyde over Au/SiC catalysts. J. Am. Chem. Soc. 2016, 138, 9361–9364. [Google Scholar] [CrossRef]

| Entry | Sample | Time (h) | Solvent | Conv. (%) | Selectivity (%) | ||
|---|---|---|---|---|---|---|---|
| COL | HCAL | HCOL | |||||
| entry 1 1 | Blank | 2 | Ethanol | 0 | 0 | 0 | 0 |
| entry 2 1 | Ni4-La2O3 | 1 | Ethanol | 79.72 | 0.51 | 83.16 | 16.33 |
| 3 | 99.92 | 0.51 | 14.95 | 84.54 | |||
| 4 | 99.28 | 0.4 | 3.8 | 95.8 | |||
| entry 3 1 | Ni4-La2O3 | 2 | Methanol | 77.65 | 0.28 | 39.76 | 59.96 |
| Isopropanol | 47.07 | 1.27 | 80.16 | 18.57 | |||
| N-hexane | 99.62 | 0.06 | 37.35 | 62.59 | |||
| entry 4 1 | 10% Pd/C | 2 | Ethanol | 100 | 4.0 | 69.2 | 26.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Ren, Y.; Zhang, R.; Chang, F.; Wei, Q.; Xu, J. A One-Pot Hydrothermal Preparation of High Loading Ni/La2O3 Catalyst for Efficient Hydrogenation of Cinnamaldehyde. Catalysts 2023, 13, 298. https://doi.org/10.3390/catal13020298
Yan H, Ren Y, Zhang R, Chang F, Wei Q, Xu J. A One-Pot Hydrothermal Preparation of High Loading Ni/La2O3 Catalyst for Efficient Hydrogenation of Cinnamaldehyde. Catalysts. 2023; 13(2):298. https://doi.org/10.3390/catal13020298
Chicago/Turabian StyleYan, Haoting, Yongwang Ren, Renkun Zhang, Feixiang Chang, Qinhong Wei, and Jing Xu. 2023. "A One-Pot Hydrothermal Preparation of High Loading Ni/La2O3 Catalyst for Efficient Hydrogenation of Cinnamaldehyde" Catalysts 13, no. 2: 298. https://doi.org/10.3390/catal13020298
APA StyleYan, H., Ren, Y., Zhang, R., Chang, F., Wei, Q., & Xu, J. (2023). A One-Pot Hydrothermal Preparation of High Loading Ni/La2O3 Catalyst for Efficient Hydrogenation of Cinnamaldehyde. Catalysts, 13(2), 298. https://doi.org/10.3390/catal13020298





