Efficient Hydrogen Production from the Aqueous-Phase Reforming of Biomass-Derived Oxygenated Hydrocarbons over an Ultrafine Pt Nanocatalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalysts Properties
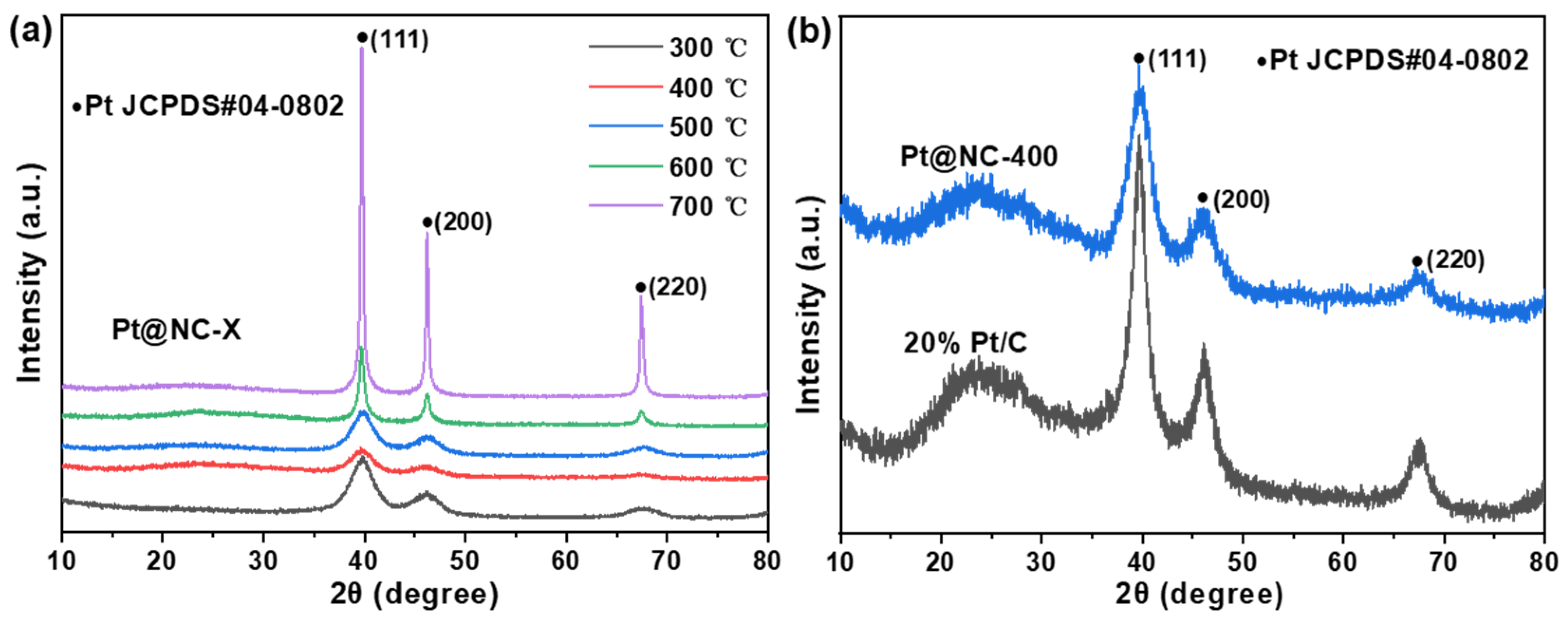
2.1.1. Crystalline Structure of the Pt@NC and 20% Pt/C Catalysts
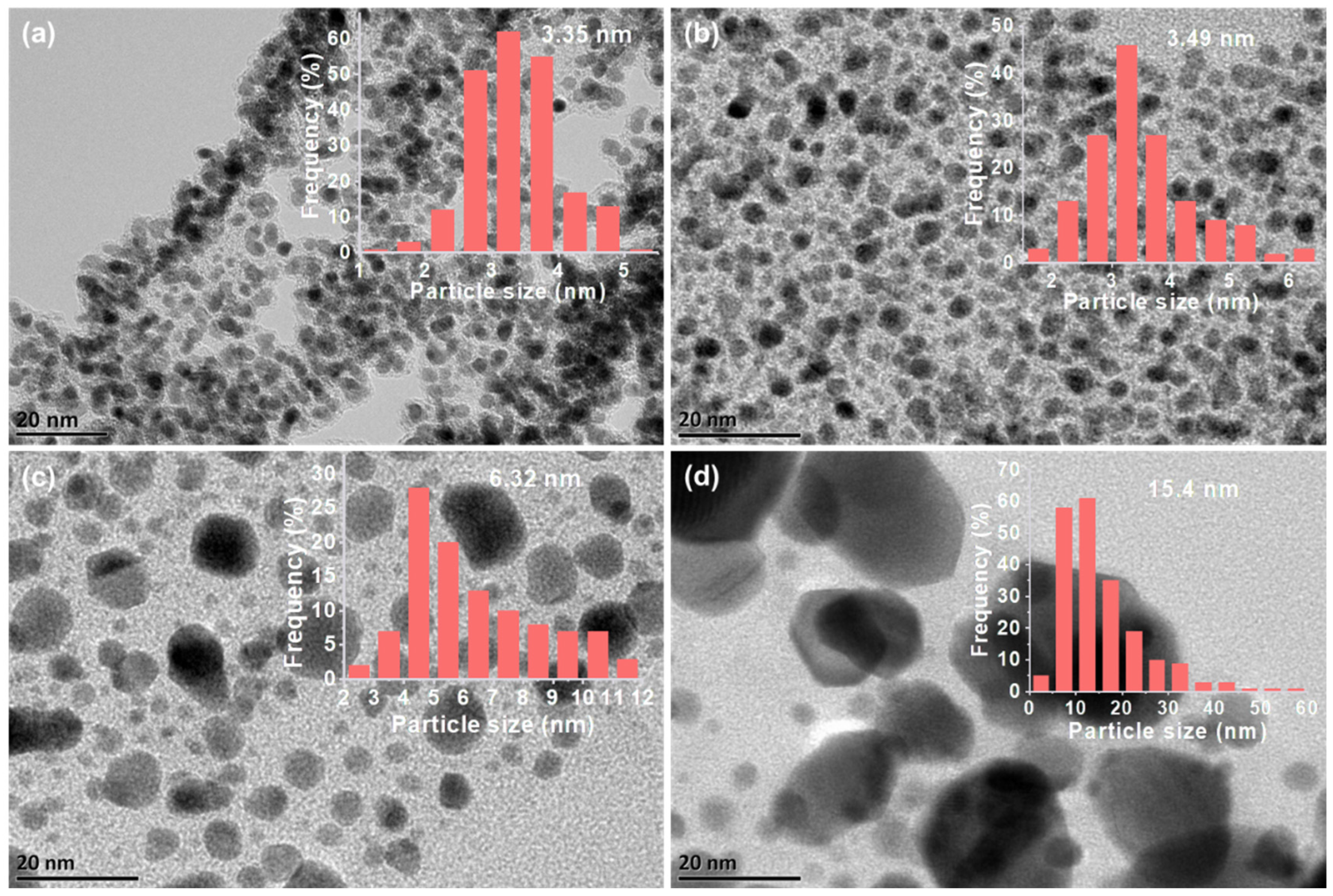
2.1.2. Morphology and Structure of the Pt@NC Catalysts
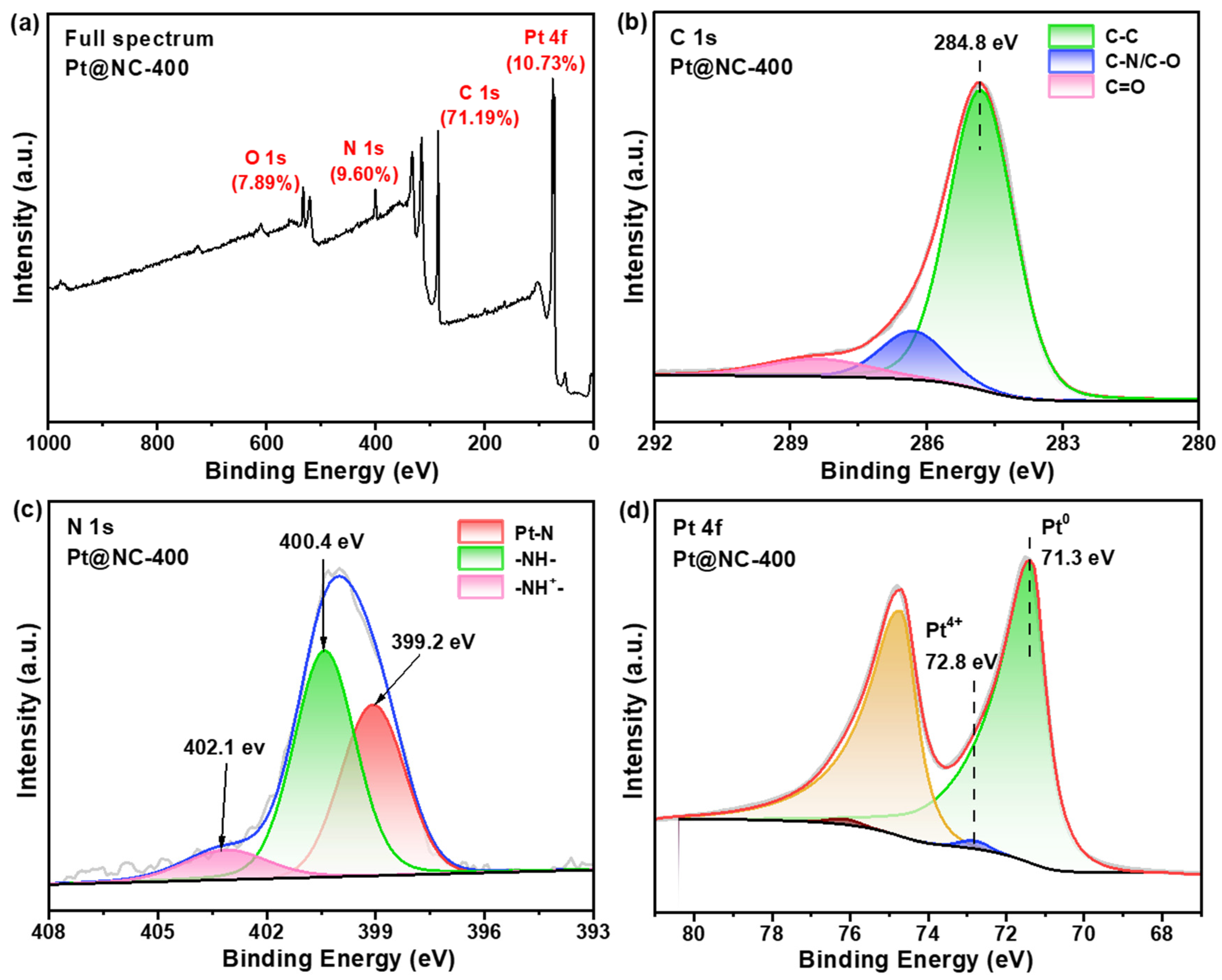
2.1.3. Electronic Properties of the Pt@NC−400 Catalyst
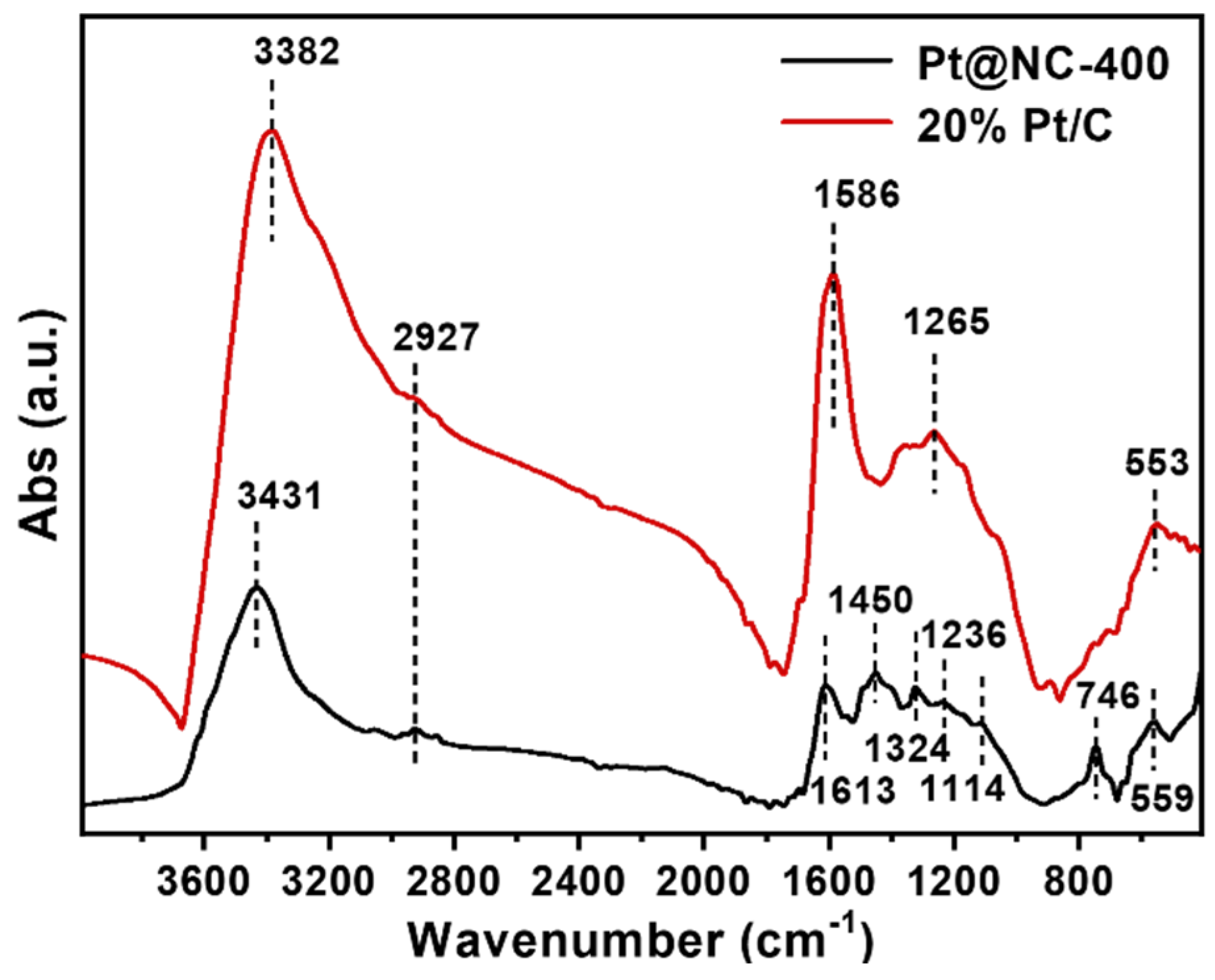
2.1.4. Chemical Structure of Functional Groups within Two Catalysts
2.2. APR Performance and Stability Testing of Different Catalysts
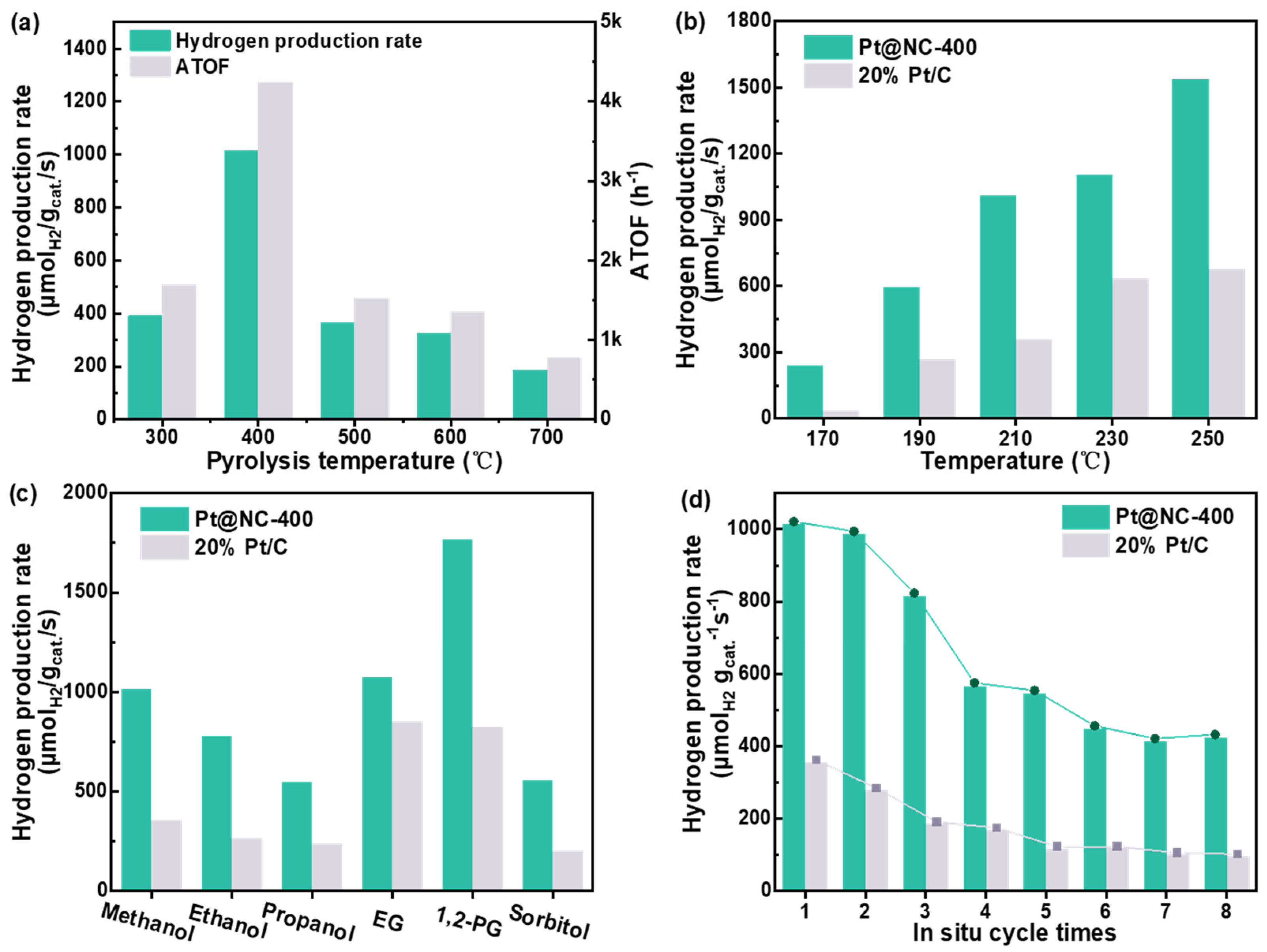
2.2.1. Influence of Pyrolysis Temperature on the Catalytic Activity of Pt@NC
2.2.2. APRM Catalytic Activity of the Pt@NC−400 and 20% Pt/C Catalysts
2.2.3. Evaluation of APR Performance of Different Feedstocks
2.2.4. APRM Reaction Stability Test
3. Experimental Section
3.1. Materials
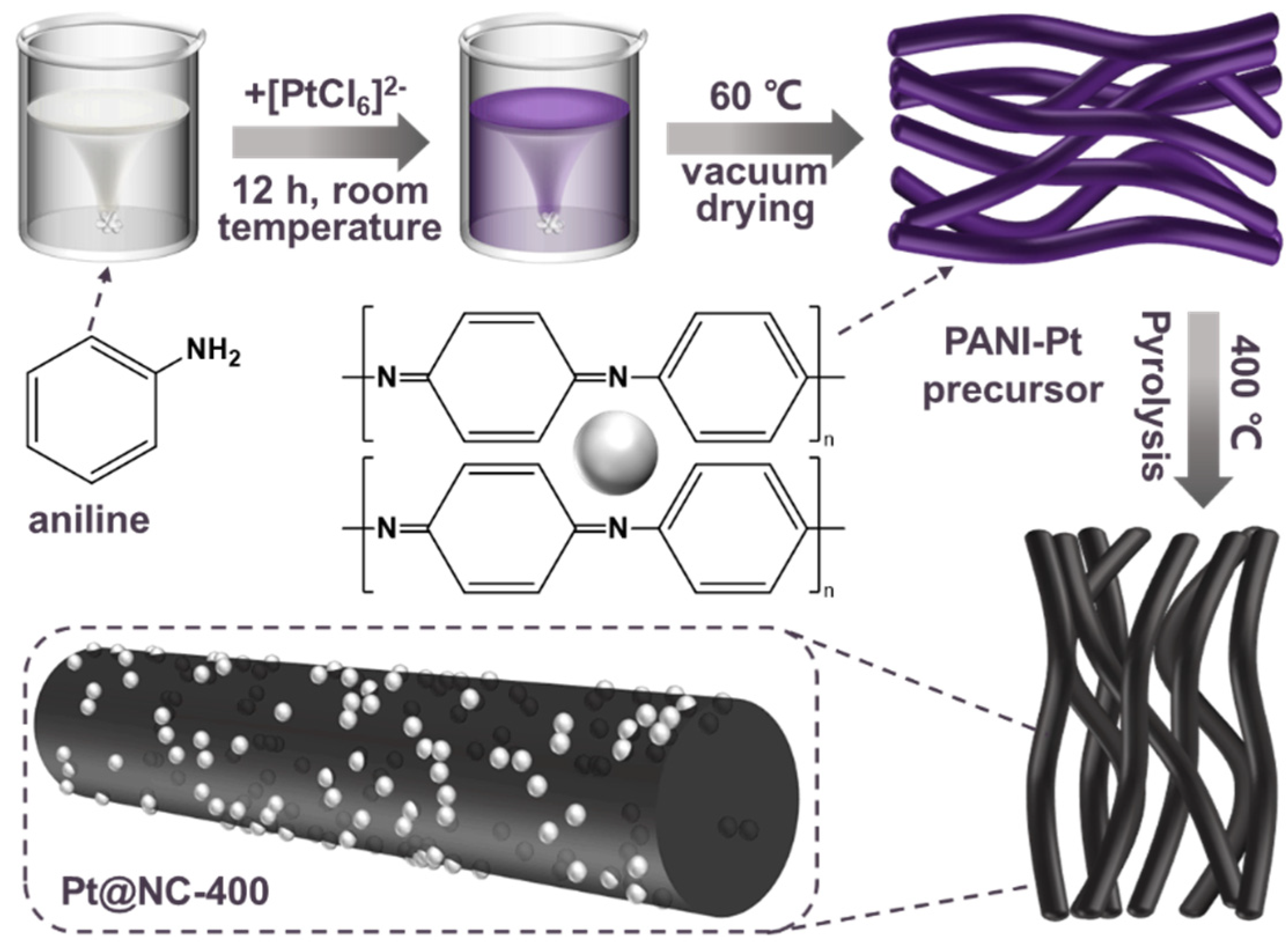
3.2. Catalysts Preparation
3.3. Catalysts Characterizations
3.4. Catalytic Activity Tests
3.4.1. Aqueous-Phase Reforming (APR) Reactions
3.4.2. Catalyst Activity Evaluation Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barreto, L.; Makihira, A.; Riahi, K. The hydrogen economy in the 21st century: A sustainable development scenario. Int. J. Hydrog. Energy 2003, 28, 267–284. [Google Scholar] [CrossRef]
- Olah, G.A. Towards Oil Independence through Renewable Methanol Chemistry. Angew. Chem. Int. Ed. 2013, 52, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.R.; Dogutan, D.K.; Reece, S.Y.; Surendranath, Y.; Teets, T.S.; Nocera, D.G. Solar Energy Supply and Storage for the Legacy and Nonlegacy Worlds. Chem. Rev. 2010, 110, 6474–6502. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.S. Powering the Planet. MRS Bull. 2011, 32, 808–820. [Google Scholar] [CrossRef]
- Grätzel, M. Powering the planet. Nature 2000, 403, 363. [Google Scholar] [CrossRef]
- Chen, L.-N.; Hou, K.-P.; Liu, Y.-S.; Qi, Z.-Y.; Zheng, Q.; Lu, Y.-H.; Chen, J.-Y.; Chen, J.-L.; Pao, C.-W.; Wang, S.-B.; et al. Efficient Hydrogen Production from Methanol Using a Single-Site Pt1/CeO2 Catalyst. J. Am. Chem. Soc. 2019, 141, 17995–17999. [Google Scholar] [CrossRef]
- Lin, L.; Zhou, W.; Gao, R.; Yao, S.; Zhang, X.; Xu, W.; Zheng, S.; Jiang, Z.; Yu, Q.; Li, Y.W.; et al. Low-temperature hydrogen production from water and methanol using Pt/alpha-MoC catalysts. Nature 2017, 544, 80–83. [Google Scholar] [CrossRef]
- Schlapbach, L.; Zuttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Cheng, Y.H.; Zhang, J.Y. A Review of High Density Solid Hydrogen Storage Materials by Pyrolysis for Promising Mobile Applications. Ind. Eng. Chem. Res. 2021, 60, 2737–2771. [Google Scholar] [CrossRef]
- Liu, Y.F.; Ren, Z.H.; Zhang, X.; Jian, N.; Yang, Y.X.; Gao, M.X.; Pan, H.G. Development of Catalyst-Enhanced Sodium Alanate as an Advanced Hydrogen-Storage Material for Mobile Applications. Energy Technol.-Ger. 2018, 6, 487–500. [Google Scholar] [CrossRef]
- Wismann, S.T.; Engbaek, J.S.; Vendelbo, S.B.; Bendixen, F.B.; Eriksen, W.L.; Aasberg-Petersen, K.; Frandsen, C.; Chorkendorff, I.; Mortensen, P.M. Electrified methane reforming: A compact approach to greener industrial hydrogen production. Science 2019, 364, 756–759. [Google Scholar] [CrossRef]
- Callison, J.; Subramanian, N.D.; Rogers, S.M.; Chutia, A.; Gianolio, D.; Catlow, C.R.A.; Wells, P.P.; Dimitratos, N. Directed aqueous-phase reforming of glycerol through tailored platinum nanoparticles. Appl. Catal. B-Environ. 2018, 238, 618–628. [Google Scholar] [CrossRef]
- Debek, R.; Motak, M.; Galvez, M.E.; Grzybek, T.; Da Costa, P. Promotion effect of zirconia on Mg(Ni,Al)O mixed oxides derived from hydrotalcites in CO2 methane reforming. Appl. Catal. B-Environ. 2018, 223, 36–46. [Google Scholar] [CrossRef]
- Zou, X.X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.S.; Wen, X.H.; Xu, H.P. Study on isolated boost full bridge converter in FCEV. In Proceedings of the Ipec: 2005 International Power Engineering Conference, Singapore, 29 November–2 December 2005; Volumes 1 and 2, pp. 827–830. [Google Scholar] [CrossRef]
- Pipitone, G.; Zoppi, G.; Pirone, R.; Bensaid, S. A critical review on catalyst design for aqueous phase reforming. Int. J. Hydrog. Energy 2022, 47, 151–180. [Google Scholar] [CrossRef]
- Zoppi, G.; Pipitone, G.; Pirone, R.; Bensaid, S. Aqueous phase reforming process for the valorization of wastewater streams: Application to different industrial scenarios. Catal. Today 2022, 387, 224–236. [Google Scholar] [CrossRef]
- Li, D.D.; Li, Y.; Liu, X.H.; Guo, Y.; Pao, C.W.; Chen, E.L.; Hu, Y.F.; Wang, Y.Q. NiAl2O4 Spinel Supported Pt Catalyst: High Performance and Origin in Aqueous-Phase Reforming of Methanol. Acs Catal. 2019, 9, 9671–9682. [Google Scholar] [CrossRef]
- Kuhn, J.N.; Huang, W.; Tsung, C.-K.; Zhang, Y.; Somorjai, G.A. Structure Sensitivity of Carbon−Nitrogen Ring Opening: Impact of Platinum Particle Size from below 1 to 5 nm upon Pyrrole Hydrogenation Product Selectivity over Monodisperse Platinum Nanoparticles Loaded onto Mesoporous Silica. J. Am. Chem. Soc. 2008, 130, 14026–14027. [Google Scholar] [CrossRef] [PubMed]
- Tsung, C.K.; Kuhn, J.N.; Huang, W.Y.; Aliaga, C.; Hung, L.I.; Somorjai, G.A.; Yang, P.D. Sub-10 nm Platinum Nanocrystals with Size and Shape Control: Catalytic Study for Ethylene and Pyrrole Hydrogenation. J. Am. Chem. Soc. 2009, 131, 5816–5822. [Google Scholar] [CrossRef] [PubMed]
- Frelink, T.; Visscher, W.; van Veen, J.A.R. Particle size effect of carbon-supported platinum catalysts for the electrooxidation of methanol. J. Electroanal. Chem. 1995, 382, 65–72. [Google Scholar] [CrossRef]
- Ravenelle, R.M.; Copeland, J.R.; Kim, W.-G.; Crittenden, J.C.; Sievers, C. Structural Changes of γ-Al2O3-Supported Catalysts in Hot Liquid Water. Acs Catal. 2011, 1, 552–561. [Google Scholar] [CrossRef]
- Shabaker, J.W.; Davda, R.R.; Huber, G.W.; Cortright, R.D.; Dumesic, J.A. Aqueous-phase reforming of methanol and ethylene glycol over alumina-supported platinum catalysts. J. Catal. 2003, 215, 344–352. [Google Scholar] [CrossRef]
- Kim, T.-W.; Kim, H.-D.; Jeong, K.-E.; Chae, H.-J.; Jeong, S.-Y.; Lee, C.-H.; Kim, C.-U. Catalytic production of hydrogen through aqueous-phase reforming over platinum/ordered mesoporous carbon catalysts. Green Chem. 2011, 13, 1718–1728. [Google Scholar] [CrossRef]
- Vikla, A.K.K.; Simakova, I.; Demidova, Y.; Keim, E.G.; Calvo, L.; Gilarranz, M.A.; He, S.; Seshan, K. Tuning Pt characteristics on Pt/C catalyst for aqueous-phase reforming of biomass-derived oxygenates to bio-H2. Appl. Catal. A Gen. 2021, 610, 117963. [Google Scholar] [CrossRef]
- Lemus, J.; Bedia, J.; Calvo, L.; Simakova, I.L.; Murzin, D.Y.; Etzold, B.J.M.; Rodriguez, J.J.; Gilarranz, M.A. Improved synthesis and hydrothermal stability of Pt/C catalysts based on size-controlled nanoparticles. Catal. Sci. Technol. 2016, 6, 5196–5206. [Google Scholar] [CrossRef]
- Liu, J. Catalysis by Supported Single Metal Atoms. Acs Catal. 2016, 7, 34–59. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Zhang, M.; Ma, Y.; Hu, J.; Qu, Y. Sustainable production of hydrogen with high purity from methanol and water at low temperatures. Nat. Commun. 2022, 13, 5527. [Google Scholar] [CrossRef]
- Liu, F.J.; Huang, L.M.; Wen, T.C.; Gopalan, A.; Hung, J.S. Interfacial synthesis of platinum loaded polyaniline nanowires in poly(styrene sulfonic acid). Mater. Lett. 2007, 61, 4400–4405. [Google Scholar] [CrossRef]
- Cho, W.; Park, S.-J.; Kim, S. Effect of monomer concentration on interfacial synthesis of platinum loaded polyaniline nanocomplex using poly(styrene sulfonic acid). Synth. Met. 2011, 161, 2446–2450. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Zhang, L.; Han, J. In situ formation of ultrafine Pt nanoparticles on surfaces of polyaniline nanofibers as efficient heterogeneous catalysts for the hydrogenation reduction of nitrobenzene. Colloid Polym. Sci. 2018, 296, 567–574. [Google Scholar] [CrossRef]
- Xiong, Y.J.; Ma, Y.A.; Zou, L.L.; Han, S.B.; Chen, H.; Wang, S.; Gu, M.; Shen, Y.; Zhang, L.P.; Xia, Z.H.; et al. N-doping induced tensile-strained Pt nanoparticles ensuring an excellent durability of the oxygen reduction reaction. J. Catal. 2020, 382, 247–255. [Google Scholar] [CrossRef]
- Liu, M.Y.; Hu, A.P.; Ma, Y.N.; Wang, G.L.; Zou, L.L.; Chen, X.H.; Yang, H. Nitrogen-doped Pt3Co intermetallic compound nanoparticles: A durable oxygen reduction electrocatalyst. J. Electroanal. Chem. 2020, 871, 114267. [Google Scholar] [CrossRef]
- Chang, A.C.C.; Louh, R.F.; Wong, D.; Tseng, J.; Lee, Y.S. Hydrogen production by aqueous-phase biomass reforming over carbon textile supported Pt–Ru bimetallic catalysts. Int. J. Hydrog. Energy 2011, 36, 8794–8799. [Google Scholar] [CrossRef]
- Meryemoglu, B.; Hesenov, A.; Irmak, S.; Atanur, O.M.; Erbatur, O. Aqueous-phase reforming of biomass using various types of supported precious metal and raney-nickel catalysts for hydrogen production. Int. J. Hydrog. Energy 2010, 35, 12580–12587. [Google Scholar] [CrossRef]
- Davda, R.R.; Dumesic, J.A. Renewable hydrogen by aqueous-phase reforming of glucose. Chem. Commun. 2004, 36–37. [Google Scholar] [CrossRef]
- Shabaker, J.W.; Huber, G.W.; Davda, R.R.; Cortright, R.D.; Dumesic, J.A. Aqueous-phase reforming of ethylene glycol over supported platinum catalysts. Catal. Lett. 2003, 88, 1–8. [Google Scholar] [CrossRef]
- Huber, G.W.; Shabaker, J.W.; Dumesic, J.A. Raney Ni-Sn Catalyst for H2 Production from Biomass-Derived Hydrocarbons. Science 2003, 300, 2075–2077. [Google Scholar] [CrossRef]
- Lin, L.; Yu, Q.; Peng, M.; Li, A.; Yao, S.; Tian, S.; Liu, X.; Li, A.; Jiang, Z.; Gao, R.; et al. Atomically Dispersed Ni/alpha-MoC Catalyst for Hydrogen Production from Methanol/Water. J. Am. Chem. Soc. 2021, 143, 309–317. [Google Scholar] [CrossRef]
- Davda, R.R.; Shabaker, J.W.; Huber, G.W.; Cortright, R.D.; Dumesic, J.A. Aqueous-phase reforming of ethylene glycol on silica-supported metal catalysts. Appl. Catal. B Environ. 2003, 43, 13–26. [Google Scholar] [CrossRef]
- Larimi, A.; Khorasheh, F. Renewable hydrogen production over Pt/Al₂O₃ nano-catalysts: Effect of M-promoting (M=Pd, Rh, Re, Ru, Ir, Cr). Int. J. Hydrog. Energy 2019, 44, 8243–8251. [Google Scholar] [CrossRef]
- Mao, Q.; Guo, Y.; Liu, X.; Shakouri, M.; Hu, Y.; Wang, Y. Identifying the realistic catalyst for aqueous phase reforming of methanol over Pt supported by lanthanum nickel perovskite catalyst. Appl. Catal. B Environ. 2022, 313, 121435. [Google Scholar] [CrossRef]
- Lei, Y.; Lee, S.; Low, K.-B.; Marshall, C.L.; Elam, J.W. Combining Electronic and Geometric Effects of ZnO-Promoted Pt Nanocatalysts for Aqueous Phase Reforming of 1-Propanol. Acs Catal. 2016, 6, 3457–3460. [Google Scholar] [CrossRef]
- Bossola, F.; Pereira-Hernández, X.I.; Evangelisti, C.; Wang, Y.; Dal Santo, V. Investigation of the promoting effect of Mn on a Pt/C catalyst for the steam and aqueous phase reforming of glycerol. J. Catal. 2017, 349, 75–83. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Z.; Lin, X.; Feng, W.; Chen, B.; Meng, Q.; Wang, T. Efficient Hydrogen Production from the Aqueous-Phase Reforming of Biomass-Derived Oxygenated Hydrocarbons over an Ultrafine Pt Nanocatalyst. Catalysts 2023, 13, 1428. https://doi.org/10.3390/catal13111428
Xiao Z, Lin X, Feng W, Chen B, Meng Q, Wang T. Efficient Hydrogen Production from the Aqueous-Phase Reforming of Biomass-Derived Oxygenated Hydrocarbons over an Ultrafine Pt Nanocatalyst. Catalysts. 2023; 13(11):1428. https://doi.org/10.3390/catal13111428
Chicago/Turabian StyleXiao, Ze, Xi Lin, Wenhua Feng, Binyi Chen, Qingwei Meng, and Tiejun Wang. 2023. "Efficient Hydrogen Production from the Aqueous-Phase Reforming of Biomass-Derived Oxygenated Hydrocarbons over an Ultrafine Pt Nanocatalyst" Catalysts 13, no. 11: 1428. https://doi.org/10.3390/catal13111428
APA StyleXiao, Z., Lin, X., Feng, W., Chen, B., Meng, Q., & Wang, T. (2023). Efficient Hydrogen Production from the Aqueous-Phase Reforming of Biomass-Derived Oxygenated Hydrocarbons over an Ultrafine Pt Nanocatalyst. Catalysts, 13(11), 1428. https://doi.org/10.3390/catal13111428





