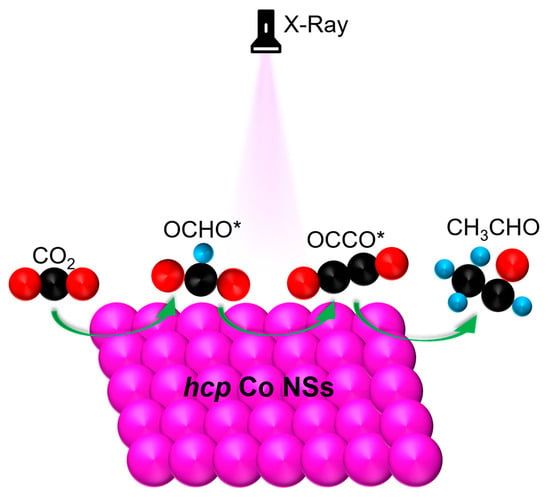
The Evolution of Hexagonal Cobalt Nanosheets for CO2 Electrochemical Reduction Reaction
Abstract
:1. Introduction
2. Results
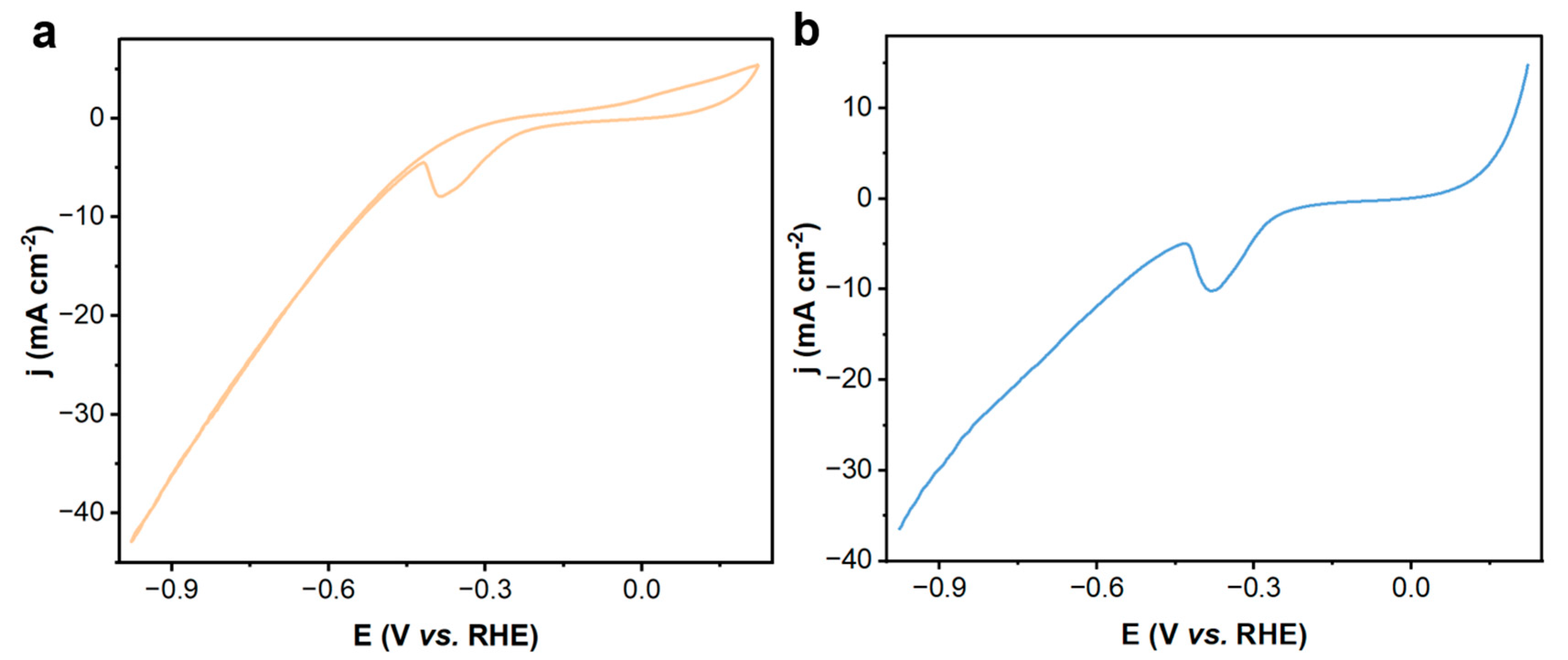
2.1. The Catalytic Properties
2.2. In Situ Raman Spectra
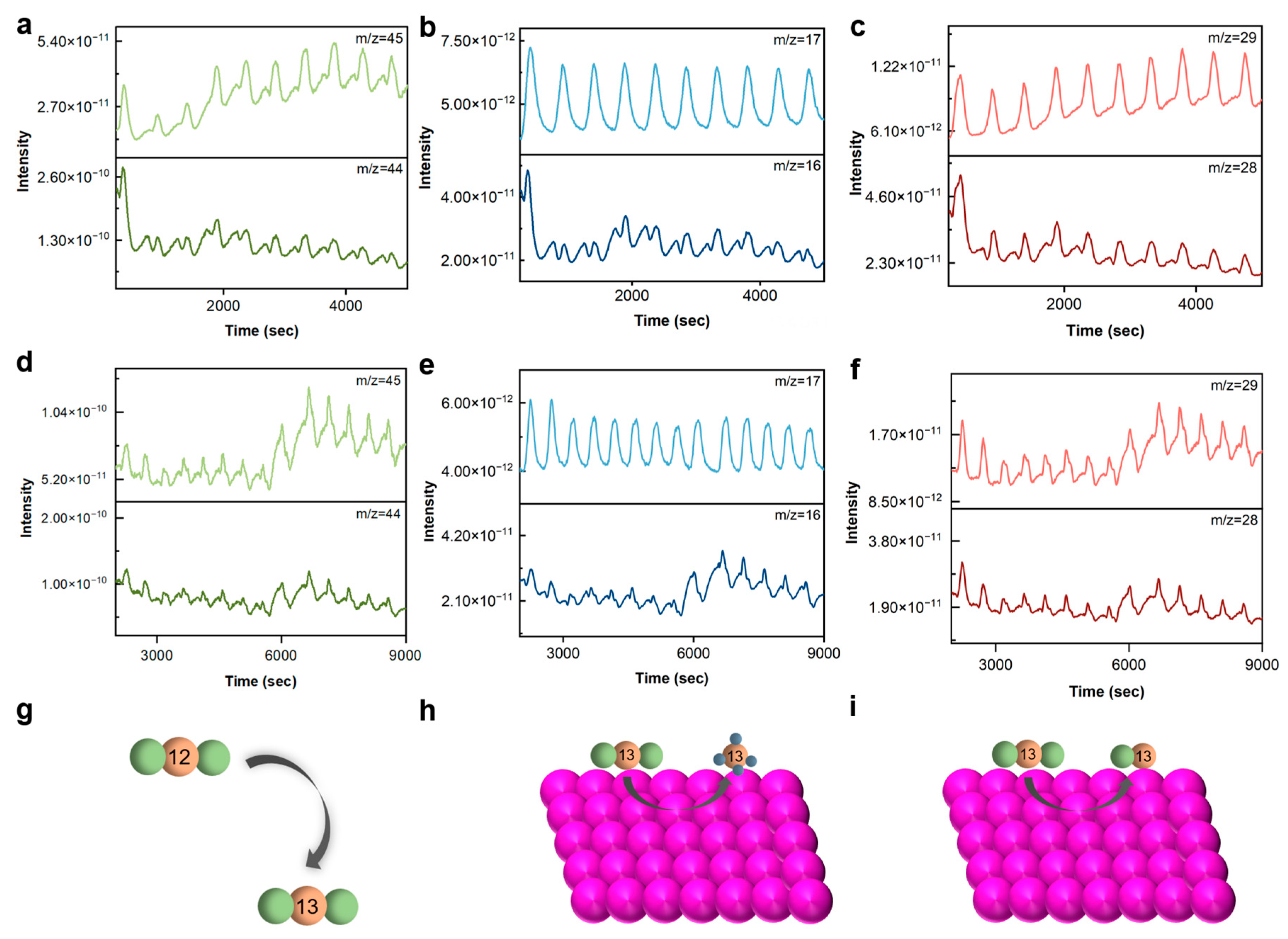
2.3. Differential Electrochemical Mass Spectrometry (DEMS)
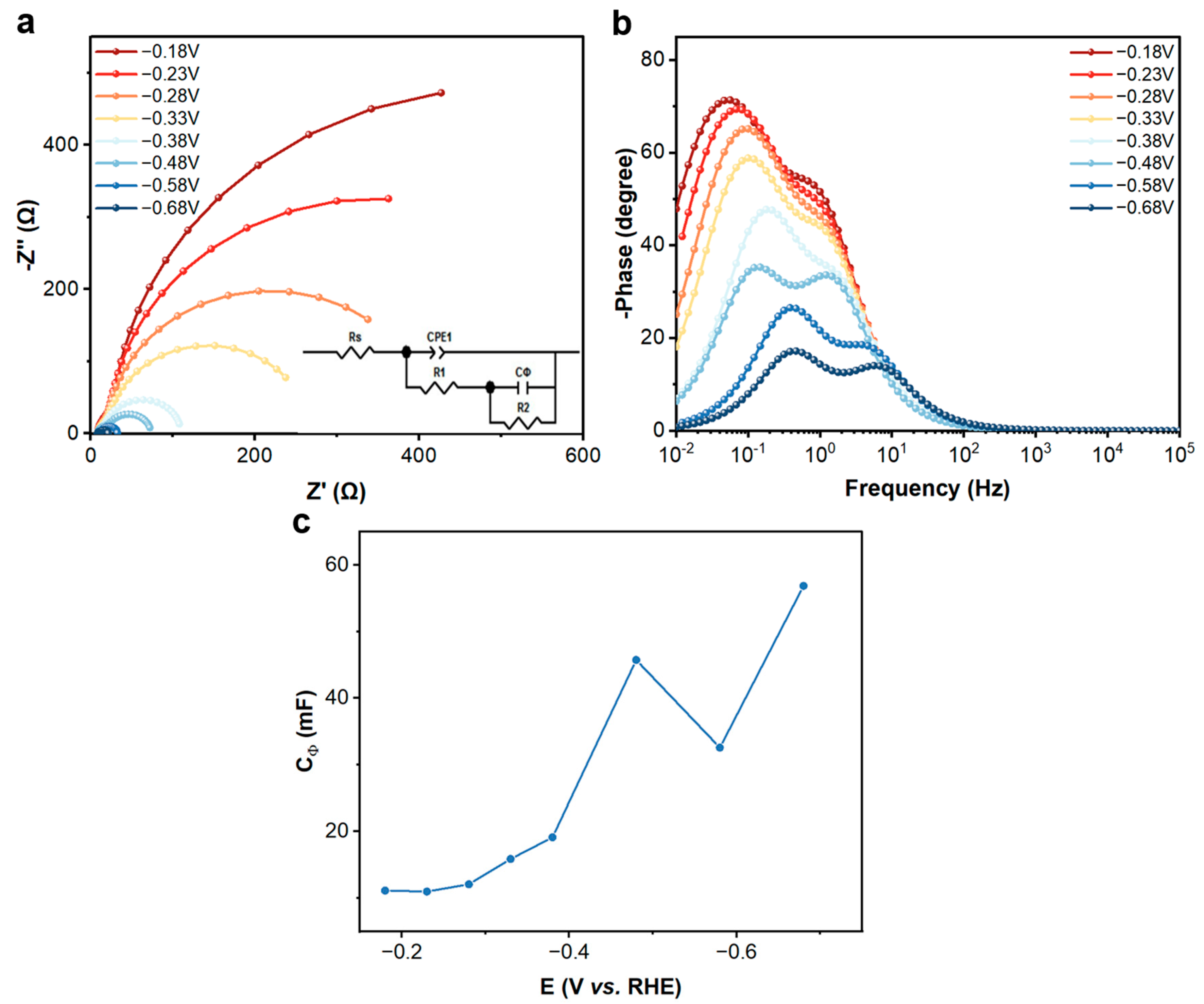
2.4. In Situ Electrochemical Impedance Spectroscopy (EIS)
2.5. Quasa In Situ Transmission Electronic Microscopy (TEM)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Materials
4.2.1. Synthesis of hcp Co NSs
4.2.2. Preparation of Working Electrodes
4.3. Electrochemical Measurements
4.4. In-Situ Raman Spectra Measurements
4.5. Differential Electrochemical Mass Spectrometry (DEMS) Measurement
4.6. Quasa In Situ Transmission Electronic Microscopy (TEM) Measurement
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, D.D.; Liu, J.L.; Qiao, S.Z. Recent Advances in Inorganic Heterogeneous Electrocatalysts for Reduction of Carbon Dioxide. Adv. Mater. 2016, 28, 3423–3452. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Xu, J.; Wei, Z.; Ma, J.; Guo, S.; Wang, S.; Liu, H.; Dou, S. Metal-Free Carbon Materials for CO2 Electrochemical Reduction. Adv. Mater. 2017, 29, 1701784. [Google Scholar] [CrossRef] [PubMed]
- Kerimkulova, A.R.; Azat, S.; Velasco, L.; Mansurov, Z.A.; Lodewyckx, P.; Tulepov, M.I.; Makpal, R.; Berezovskaya, B.; Imangazy, A. Granular rice husk-based sorbents for sorption of vapors of organic and inorganic matters. J. Chem. Technol. Metall. 2019, 54, 578–584. [Google Scholar]
- Wang, G.; Chen, J.; Ding, Y.; Cai, P.; Yi, L.; Li, Y.; Tu, C.; Hou, Y.; Wen, Z.; Dai, L. Electrocatalysis for CO2 conversion: From fundamentals to value-added products. Chem. Soc. Rev. 2021, 50, 4993–5061. [Google Scholar] [CrossRef]
- Ma, W.; He, X.; Wang, W.; Xie, S.; Zhang, Q.; Wang, Y. Electrocatalytic reduction of CO2 and CO to multi-carbon compounds over Cu-based catalysts. Chem. Soc. Rev. 2021, 50, 12897–12914. [Google Scholar] [CrossRef]
- Tan, X.; Yu, C.; Ren, Y.; Cui, S.; Li, W.; Qiu, J. Recent advances in innovative strategies for the CO2 electroreduction reaction. Energy Environ. Sci. 2021, 14, 765–780. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, Y.; Gao, M.; Ma, T.; Sun, W.; Pan, H. Recent progress on hybrid electrocatalysts for efficient electrochemical CO2 reduction. Nano Energy 2021, 80, 105504. [Google Scholar] [CrossRef]
- Kortlever, R.; Shen, J.; Schouten, K.J.P.; Calle-Vallejo, F.; Koper, M.T.M. Catalysts and Reaction Pathways for the Electrochemical Reduction of Carbon Dioxide. J. Phys. Chem. Lett. 2015, 6, 4073–4082. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Wang, Y.; Al-Enizi, A.M.; Zheng, G. Tuning of CO2 Reduction Selectivity on Metal Electrocatalysts. Small 2017, 13, 1701809. [Google Scholar] [CrossRef]
- Jouny, M.; Luc, W.; Jiao, F. General Techno-Economic Analysis of CO2 Electrolysis Systems. Ind. Eng. Chem. Res. 2018, 57, 2165–2177. [Google Scholar] [CrossRef]
- Shin, H.; Hansen, K.U.; Jiao, F. Techno-economic assessment of low-temperature carbon dioxide electrolysis. Nat. Sustain. 2021, 4, 911–919. [Google Scholar] [CrossRef]
- Yin, J.; Yin, Z.; Jin, J.; Sun, M.; Huang, B.; Lin, H.; Ma, Z.; Muzzio, M.; Shen, M.; Yu, C.; et al. A New Hexagonal Cobalt Nanosheet Catalyst for Selective CO2 Conversion to Ethanal. J. Am. Chem. Soc. 2021, 143, 15335–15343. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.J.; Yin, R.; Zhou, L.; Li, J.; Kikas, R.; Xu, T.; Wang, Z.J.; Jin, H.; Wang, X.; Wang, S. Microenvironment Engineering for the Electrocatalytic CO2 Reduction Reaction. Angew. Chem. Int. Ed. 2022, 61, e202207252. [Google Scholar] [CrossRef]
- Hess, C. New advances in using Raman spectroscopy for the characterization of catalysts and catalytic reactions. Chem. Soc. Rev. 2021, 50, 3519–3564. [Google Scholar] [CrossRef]
- Li, H.; Wei, P.; Gao, D.; Wang, G. In situ Raman spectroscopy studies for electrochemical CO2 reduction over Cu catalysts. Curr. Opin. Green Sustain. Chem. 2022, 34, 100589. [Google Scholar] [CrossRef]
- Chen, L.D.; Urushihara, M.; Chan, K.; Nørskov, J.K. Electric Field Effects in Electrochemical CO2 Reduction. ACS Catal. 2016, 6, 7133–7139. [Google Scholar] [CrossRef]
- Zhi, X.; Vasileff, A.; Zheng, Y.; Jiao, Y.; Qiao, S.-Z. Role of oxygen-bound reaction intermediates in selective electrochemical CO2 reduction. Energy Environ. Sci. 2021, 14, 3912–3930. [Google Scholar] [CrossRef]
- Yoon, Y.; Hall, A.S.; Surendranath, Y. Tuning of Silver Catalyst Mesostructure Promotes Selective Carbon Dioxide Conversion into Fuels. Angew. Chem. Int. Ed. 2016, 55, 15282–15286. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.S.; Yoon, Y.; Wuttig, A.; Surendranath, Y. Mesostructure-Induced Selectivity in CO2 Reduction Catalysis. J. Am. Chem. Soc. 2015, 137, 14834–14837. [Google Scholar] [CrossRef]
- Varela, A.S.; Kroschel, M.; Reier, T.; Strasser, P. Controlling the selectivity of CO2 electroreduction on copper: The effect of the electrolyte concentration and the importance of the local pH. Catal. Today 2016, 260, 8–13. [Google Scholar] [CrossRef]
- Zhao, Y.; Zu, X.; Chen, R.; Li, X.; Jiang, Y.; Wang, Z.; Wang, S.; Wu, Y.; Sun, Y.; Xie, Y. Industrial-Current-Density CO2-to-C2+ Electroreduction by Anti-swelling Anion-Exchange Ionomer-Modified Oxide-Derived Cu Nanosheets. J. Am. Chem. Soc. 2022, 144, 10446–10454. [Google Scholar] [CrossRef]
- Goyal, A.; Marcandalli, G.; Mints, V.A.; Koper, M.T.M. Competition between CO2 Reduction and Hydrogen Evolution on a Gold Electrode under Well-Defined Mass Transport Conditions. J. Am. Chem. Soc. 2020, 142, 4154–4161. [Google Scholar] [CrossRef]
- Zhang, F.; Co, A.C. Direct Evidence of Local pH Change and the Role of Alkali Cation during CO2 Electroreduction in Aqueous Media. Angew. Chem. Int. Ed. 2020, 59, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Mora-Hernandez, J.M.; González-Suárez, W.I.; Manzo-Robledo, A.; Luna-Trujillo, M. A comparative differential electrochemical mass spectrometry (DEMS) study towards the CO2 reduction on Pd, Cu, and Sn -based electrocatalyst. J. CO2 Util. 2021, 47, 101504. [Google Scholar] [CrossRef]
- Reitz, W. A Review of: “Impedance Spectroscopy, Theory, Experiment, and Applications”. Mater. Manuf. Process. 2006, 21, 425. [Google Scholar] [CrossRef]
- Ampelli, C.; Tavella, F.; Giusi, D.; Ronsisvalle, A.M.; Perathoner, S.; Centi, G. Electrode and cell design for CO2 reduction: A viewpoint. Catal. Today 2023, 421, 114217. [Google Scholar] [CrossRef]
- Bienen, F.; Kopljar, D.; Geiger, S.; Wagner, N.; Friedrich, K.A. Investigation of CO2 Electrolysis on Tin Foil by Electrochemical Impedance Spectroscopy. ACS Sustain. Chem. Eng. 2020, 8, 5192–5199. [Google Scholar] [CrossRef]
- Bienen, F.; Kopljar, D.; Löwe, A.; Geiger, S.; Wagner, N.; Klemm, E.; Friedrich, K.A. Revealing Mechanistic Processes in Gas-Diffusion Electrodes During CO2 Reduction via Impedance Spectroscopy. ACS Sustain. Chem. Eng. 2020, 8, 13759–13768. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.-X.; Gou, W.; Zhang, M.; Xia, Z.; Zhang, S.; Chang, C.-R.; Ma, Y.; Qu, Y. Ethylene-glycol ligand environment facilitates highly efficient hydrogen evolution of Pt/CoP through proton concentration and hydrogen spillover. Energy Environ. Sci. 2019, 12, 2298–2304. [Google Scholar] [CrossRef]
- Damian, A.; Omanovic, S. Ni and NiMo hydrogen evolution electrocatalysts electrodeposited in a polyaniline matrix. J. Power Sources 2006, 158, 464–476. [Google Scholar] [CrossRef]
- Krstajić, N.V.; Grgur, B.N.; Mladenović, N.S.; Vojnović, M.V.; Jakšić, M.M. The determination of kinetics parameters of the hydrogen evolution on TiNi alloys by ac impedance. Electrochim. Acta 1997, 42, 323–330. [Google Scholar] [CrossRef]
- Chen, S.; Wang, S.; Hao, P.; Li, M.; Zhang, Y.; Guo, J.; Ding, W.; Liu, M.; Wang, J.; Guo, X. N,O-C Nanocage-mediated high-efficient hydrogen evolution reaction on IrNi@N,O-C electrocatalyst. Appl. Catal. B Environ. 2022, 304, 120996. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Kao, C.W.; Luo, T.; Chen, K.; Fu, J.; Ma, C.; Li, H.; Li, M.; Chan, T.S.; et al. Unveiling the Proton-Feeding Effect in Sulfur-Doped Fe-N-C Single-Atom Catalyst for Enhanced CO2 Electroreduction. Angew. Chem. Int. Ed. 2022, 61, e202206233. [Google Scholar] [CrossRef]
- Ge, W.; Chen, Y.; Fan, Y.; Zhu, Y.; Liu, H.; Song, L.; Liu, Z.; Lian, C.; Jiang, H.; Li, C. Dynamically Formed Surfactant Assembly at the Electrified Electrode–Electrolyte Interface Boosting CO2 Electroreduction. J. Am. Chem. Soc. 2022, 144, 6613–6622. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Cheng, H.; Feng, M.; Wu, X.; Fan, Z.; Pan, D.; He, G. Catalytic hydrogenation performance of ZIF-8 carbide for electrochemical reduction of carbon dioxide. Chin. J. Chem. Eng. 2021, 39, 144–153. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Hou, Y.; Yin, J.; Xi, P. The Evolution of Hexagonal Cobalt Nanosheets for CO2 Electrochemical Reduction Reaction. Catalysts 2023, 13, 1384. https://doi.org/10.3390/catal13101384
Li Q, Hou Y, Yin J, Xi P. The Evolution of Hexagonal Cobalt Nanosheets for CO2 Electrochemical Reduction Reaction. Catalysts. 2023; 13(10):1384. https://doi.org/10.3390/catal13101384
Chicago/Turabian StyleLi, Qingyu, Yichao Hou, Jie Yin, and Pinxian Xi. 2023. "The Evolution of Hexagonal Cobalt Nanosheets for CO2 Electrochemical Reduction Reaction" Catalysts 13, no. 10: 1384. https://doi.org/10.3390/catal13101384
APA StyleLi, Q., Hou, Y., Yin, J., & Xi, P. (2023). The Evolution of Hexagonal Cobalt Nanosheets for CO2 Electrochemical Reduction Reaction. Catalysts, 13(10), 1384. https://doi.org/10.3390/catal13101384