Abstract
This research reports a simple, innovative, and low-cost doping method of TiO2 nanoparticles presenting the effects of calcination and the weight ratio of TiO2:FeCl3 (1:0.33–1:4.5). The photocatalytic activity of the nanomaterials was investigated by decolorizing Rhodamine B (RhB) dye in an aqueous solution. The main results showed that there is anatase-to-rutile transformation after the calcination process. The Fe-doped process modified the TiO2 spectrum and showed a connection in the Ti–O–Fe vibration. The particle size is within the nanometer range, between 20–51 nm, except for calcined TiO2. The inclusion of Fe in TiO2 decreased the band gap energy from 3.16 (reference) up to 2.06 eV (1:3). Additionally, after the calcination, there was a decrease in this value from 3.03 eV (reference) up to 1.95 eV (1:1.6). The TiO2, with a ratio of (1:1.6), showed the highest activity in the photocatalytic degradation of RhB with an efficiency of 93.8% after 3 h of irradiation.
1. Introduction
Titanium dioxide (TiO2) is a widely studied semiconductor because of its optical and electronic properties, good chemical stability, availability, and low-cost [1,2,3,4,5,6,7]. These characteristics cause TiO2 to be one of the most promising photocatalysts for treating various environmental pollutants. In large-scale and practical applications, the use limitations of TiO2 as a material for the photocatalytic process [5] are mainly 3: TiO2 absorbs only a small part of sunlight in the visible region, making photocatalytic activity at wavelengths less than 400 nm [5,8], the low quantum yield is due to the easy matching of photogenerated electron pairs, and its band gap energy (Eg) range of 3.0–3.2 eV, which is quite large [9,10,11].
TiO2 is an extrinsic n-type semiconductor in three crystallographic structures: anatase, rutile, and brookite. The anatase and rutile phases are the most frequently used in photocatalytic processes. The low recombination rate of its photogenerated electrons and holes on the TiO2 surface causes the anatase phase to show better photoactivity [12,13,14].
An acceleration of the chemical reaction is required to occur photocatalysis. Their shortened band gap energy and distinct electronic structure cause semiconductor materials such as TiO2, ZnO, CeO2, Cds, ZnS, Fe2O3, and others to be chosen as photocatalysts. When these semiconductors are stimulated by solar irradiation, energy levels higher than their band gap energy must be reached [6,15].
Doping in TiO2 nanoparticles changes the electronic structure of TiO2 by modifying their chemical composition and optical properties [16]. To decrease the band gap energy down to wavelengths of visible light, doping with metal ions, such as iron, nickel, chromium, zinc, platinum, and manganese, among others, has been studied. Research aimed at decreasing the recombination rate of the e−/h+ pair and thus increasing the yield of the photocatalytic process by modifying the the semiconductor surfaces with the addition of metal ions [5,9,14,16,17,18,19,20,21,22]. Doping with metal ions increases the recombination time of the e−/h+ pair making the holes accessible for forming •OH radicals [15].
Sunlight comprises visible and infrared photons, with only 3–5% of the ultraviolet (UV) range. More research is underway on doping TiO2 nanoparticles to increase photocatalytic efficiency [4,5,6,23,24] which will explain how the physical and chemical properties of TiO2 determine the photocatalytic activity and develop affordable and appropriate synthesis methods to obtain TiO2 with superior photocatalytic efficiency [5,14,17,25].
The inclusion of iron (Fe3+) allows a broad absorption of the solar spectrum in the visible region. Consequently, the potential for photocatalytic activity is increased since the radius of Fe3+ (0.64° A) and Ti4+ (0.68° A) are very close in terms of size [8,16,26]. Thus, doped-TiO2 is essential for outdoor applications to maximize photocatalytic efficiency [4,27,28]. For synthesizing Fe-doped TiO2, different methods are currently used, such as sol-gel [29,30], ultrasonic radiation-assisted hydrothermal [31], molten salt [6], wet-chemical synthesis [32], and co-precipitation [18], among others. For example, Ghorbanpour et al. (2019) synthesized and characterized Fe-doped TiO2 nanoparticles prepared by the molten salt method and calcination process. The band gap energy of the Fe-doped TiO2 samples decreased with increasing Fe concentration from 3.1 eV for pure TiO2 to 3.02–2.80 eV for Fe-doped TiO2. Thus, the photocatalytic activity was higher for the Fe-doping content of 0.5 wt.% than for pure TiO2 [6]. Another process was addressed by Carneiro et al. (2014), who investigated the incorporation of Fe into TiO2 nanoparticles through a ball milling process using stainless steel balls. They concluded that there are improvements in photocatalytic activity (over 60% at about 120 min) and higher rotational speeds [5].
Moreover, Ganesh et al. (2012) studied different amounts of Fe-doped TiO2 by the co-precipitation method. They found that small amounts of Fe (0.1 wt.%) are sufficient to transform TiO2 from the rutile phase to the full anatase phase and achieve higher photocatalytic activity [18]. From another approach, Adamek et al. (2012) compared the results of photocatalytic degradation with the presence of TiO2 or Fe (III) salts as well as a mixture of TiO2 with Fe (III), more properly with FeCl3. They concluded that the photodegradation rate constant (k) of TiO2/FeCl3 (pH = 3) is approximately four times the rate of the undoped TiO2. Consequently, adding FeCl3 to TiO2 nanoparticles significantly increases the photocatalytic activity by UVA irradiation in the 400–320 nm range after 120 min of stirring [8].
This paper investigated the process of TiO2 doping with different concentrations of iron chloride (FeCl3) via the co-precipitation method. This study is relevant as it aims to improve on and innovate the photocatalysis processes since different percentages of FeCl3 in TiO2 are studied using a simple method. Additionally, this method is low-cost in terms of doped TiO2 production that will used as an efficient photocatalyst when activated under UV radiation and visible light, and will therefore be used to, for example, capture pollutants. Hereafter, the optical, structural, chemical, morphological, and photocatalytic properties of Fe-doped TiO2 are presented and explored before and after calcination.
2. Results and Discussion
2.1. Fourier-Transform Infrared Spectroscopy (FTIR)
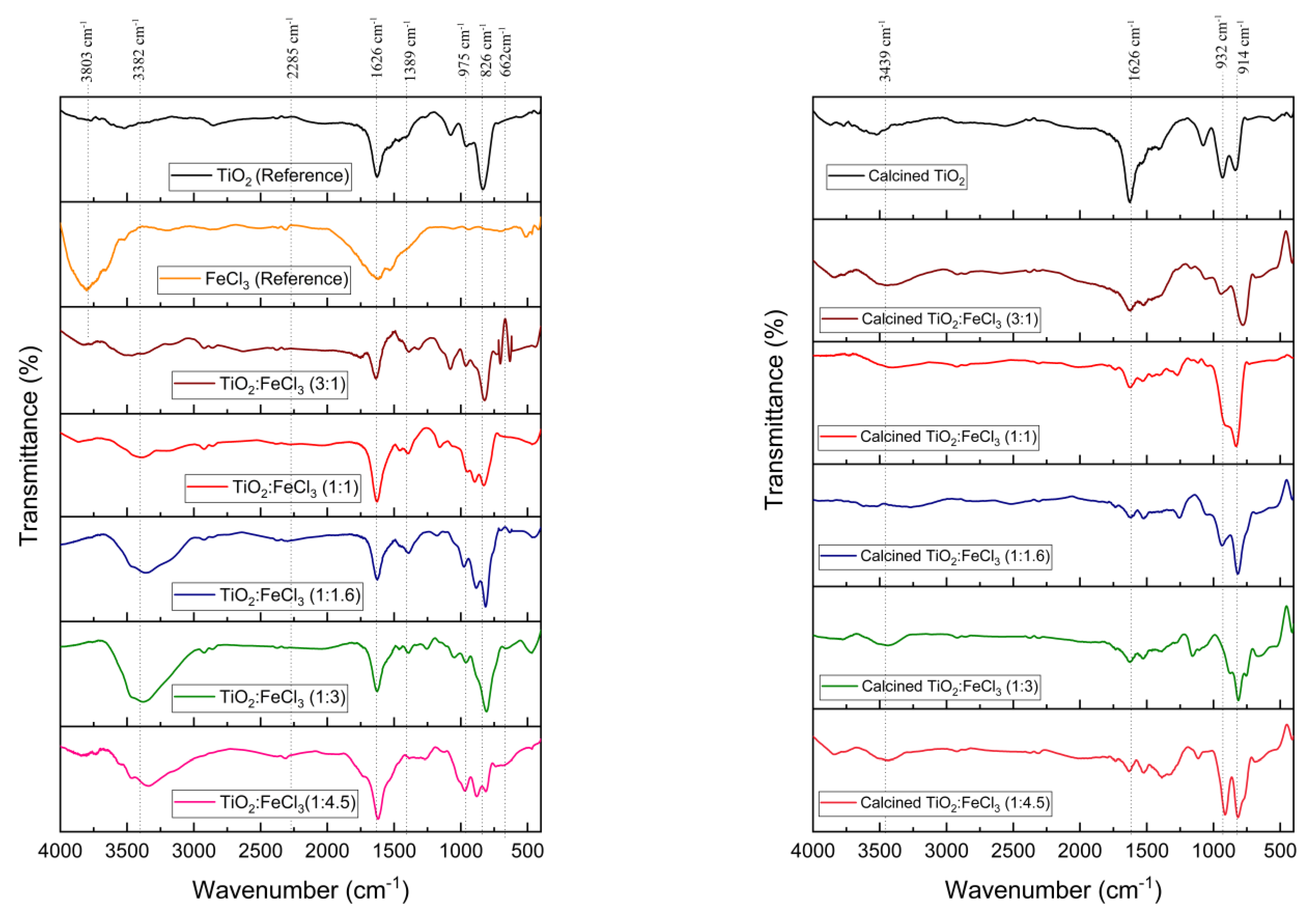
The FTIR spectra of the undoped TiO2 (reference), FeCl3 (reference), and TiO2 doped with various concentrations of FeCl3 before and after the calcination process are shown in Figure 1. The H–O–H vibrational bonds are related to the peaks at 3803, 3382, and 3439 cm−1, and the H2O vibrational bonds are indexed to the peaks at 1626 cm−1 [33]. These indicate the presence of absorbed hydroxyl groups in the samples [34]. In the photocatalytic process, all oxygen-containing groups play an influential role in the photocatalytic activity and are also capable of generating more hydroxyl radicals [35]. The Ti–O–Fe vibration is present at the peak of 2285 cm−1, which is a bond from the doping process [36]. The peaks observed in the range 932–826 cm−1 are due to the Ti–O group [37].
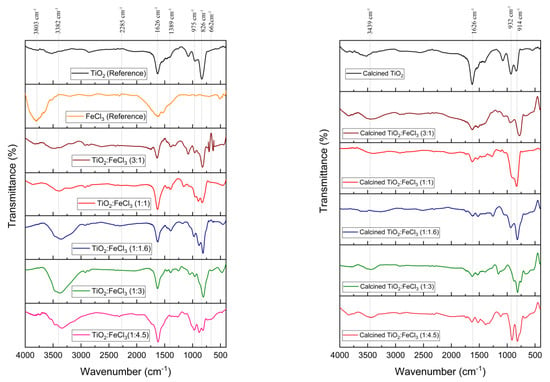
Figure 1.
FTIR spectra for the TiO2 (reference), FeCl3 (reference), and the samples synthesized before calcination (left) and after calcination (right).
Concerning the TiO2 reference spectrum, the Ti–O–Ti bond and the Ti–O stretching vibration correspond to the 800–1200 cm−1 peaks. Regarding the Fe-doped TiO2 peaks, the O–Ti–O vibration and the Fe–O–Fe symmetric stretching vibration are at 1389 cm−1. The peak observed at 662 cm−1 proves the existence of iron oxyhydroxide Fe–O–OH. The similarity between the peak values of the chemical bonds below 1000 cm−1 means that the Fe-O and Ti–O bending frequency overlap may occur.
Thus, for the different TiO2:FeCl3 ratios, there are no significant differences in the material chemical bonds either before or after the calcination process. Nevertheless, the Ti–O–Fe vibration and the hydroxyl groups decrease with the calcination process.
2.2. X-ray Diffraction (XRD)
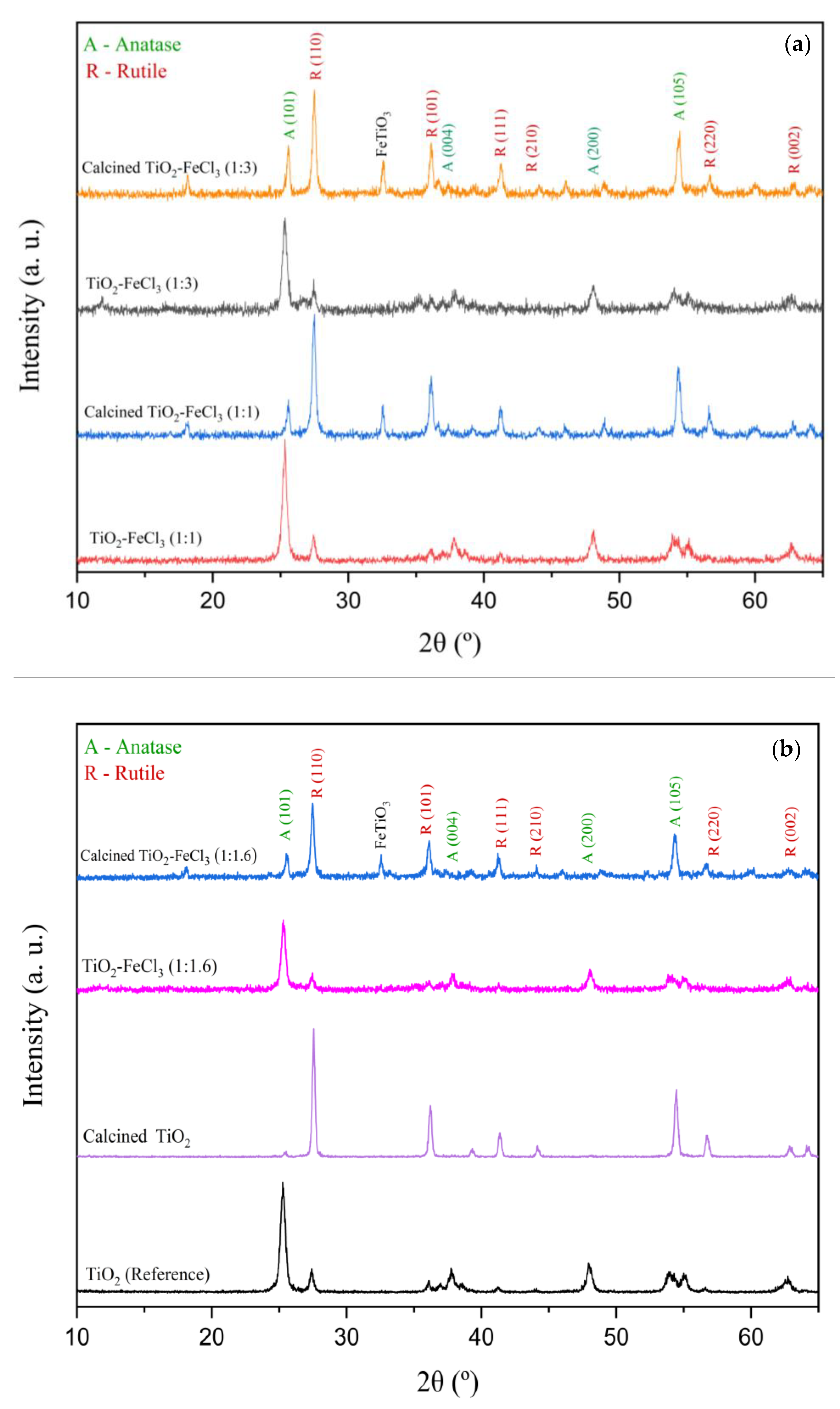
Figure 2 shows the XRD patterns of the doped TiO2 nanoparticles before and after calcination. For each of the samples synthesized, there are no significant differences between the XRD patterns and TiO2 nanoparticles (reference). This means that the fraction of the anatase phase in the doped TiO2 nanoparticles has the same magnitude as TiO2 (reference). Therefore, the doping process has no relevant influence on anatase-to-rutile transformation (ART), but after calcination, there is an ART.
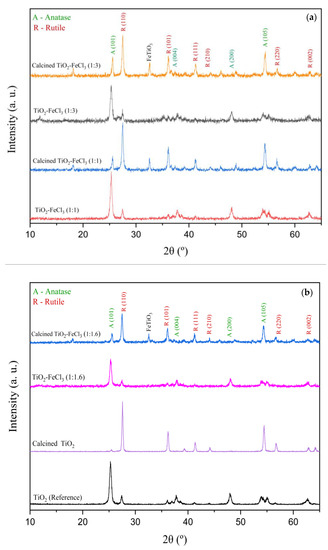
Figure 2.
X-ray diffraction patterns of doped and undoped TiO2 before and after calcination: (a) 1:3 and 1:1 and (b) 1:1.6 and reference.
It is generally observed that the diffraction peaks become slightly broader after the calcination of Fe-doped TiO2. Their relative intensities decrease for the anatase phase and increase for the rutile phase. The XRD spectrum peaks of the anatase phase, crystal planes (101), (105), and (200), and the diffraction planes (110) and (101) of the rutile phase were chosen to determine the average crystallite size, lattice parameters and weight fraction of anatase phase in the doped and calcined powders.
After the calcination process, the new peak in the XRD spectrum at 2θ = 32.56° refers to FeTiO3 (ilmenite). The intensity of this peak is the same for the increasing concentration of FeCl3 in TiO2, as observed by Ganesh et al. (2007). These researchers prepared Fe-doped TiO2 powders by the co-precipitation method and cited the appearance of small amounts of secondary phases α-Fe2O3 and FeTiO3 in their doped samples [18]. In the present study, only FeTiO3 appeared in the calcination process after TiO2 doping. On the contrary, Ghorbanpour et al. (2019) studied iron-doped TiO2 at various concentrations with a calcination process. They reported that no iron-related peaks appeared, i.e., there was no solid iron-titanium solution [6]. The reason for this behavior and the appearance of these new peaks are yet to be fully understood.
As shown in Table 1, before the calcination process, relative to the anatase phase, the crystallite size of TiO2 (reference) nanoparticles is about 18.49 nm. The Fe-doped TiO2 nanoparticles′ sizes were 19.40 nm, 19.85 nm, and 19.68 nm, i.e., for the ratios of TiO2-FeCl3 at (1:1), (1:1.6), and (1:3), respectively. For the rutile phase, the crystallite size of TiO2 nanoparticles (reference) is about 29.75 nm. The increasing Fe-doped concentration ranges from 22.05 nm to 25.74 nm, respectively. After the calcination process, the crystallite size of the calcined TiO2 nanoparticles was about 18.53 nm, i.e., there was no significant difference between the TiO2 (reference) and the calcined TiO2.

Table 1.
Influence of doped material before and after calcination as to the on average crystallite size, lattice parameters, unit cell volume, and anatase phase fraction for doped TiO2 nanoparticles.
With the addition of Fe-doped, the crystallite size increased with increasing concentration, from 19.40 nm to 29.93 nm. Thus, for the anatase phase, there is a significant difference between the crystallite size for the doped material with the material after calcination. However, for the rutile phase, crystallite size increases compared to the doped material with the calcined one. In the rutile phase, the TiO2 doped nanoparticles after the calcination process were about 32.28 nm. With the increase of FeCl3 concentration, the crystallite size changed between 26.36 nm to 27.02 nm. Compared to the calcined TiO2, the crystallite size is smaller. Thus, there was an anatase-to-rutile transformation (ART) for the calcination process. Since the ionic radii of titanium and iron are close (Ti4+ (0.68 Å) and Fe3+ (0.64 Å)), the incorporation of the iron ion into the crystal structure of TiO2 in the anatase phase may occur [6,18].
The lattice parameters were calculated and compared with the crystallographic data of reference TiO2 collected from the JCPDS International Tables for Crystallographers No. 21-1272 for the anatase phase and No. 21-1276 for the rutile phase. Thus, for the anatase phase, parameter a is 3.7852 Å, and parameter c is 9.5139 Å; for the rutile phase, the parameter a is 4.5933 Å, and the parameter c is 2.9592 Å.
For the set of samples studied, doped and after calcination, the values obtained for parameter a in the anatase phase range from 3.7424 Å to 3.7907 Å, and for the lattice parameter c, range from 9.3839 Å to 9.7596 Å. These parameters differ slightly from the theoretical values. The lattice parameter a ranges from a maximum decrease of 1.13% to an increase of 0.15%. The lattice parameter c ranges from a maximum decrease of 1.37% to a maximum increase of 2.58%. Regarding the rutile phase, parameter a ranges from 4.5717 Å to 4.5946 Å, and parameter c from 2.9288 Å to 2.9644 Å. Similarly, the lattice parameter a range from a maximum decrease of 0.47% to a maximum increase of 0.03%. The parameter c ranges from a maximum reduction of 1.03% to a maximum increase of 0.07%. This may refer to the displacement of atoms from their ideal locations [38]. For each sample, there is no significant difference in the lattice parameters, anatase and rutile phases, and unit cell volume (Å3).
Finally, regarding the fraction of the anatase phase before the calcination process, TiO2 (reference) showed a fraction of 80.82%. In the Fe-doped synthesized samples, the ratios (1:1), (1:1.16), and (1:3) showed a fraction of 87.95%, 92.90%, and 60.27%, respectively. After the calcination process, there is a marked decrease in calcined TiO2 (37.31%), compared to TiO2 (reference), and for the different ratios of (1:1), (1.16) and, i.e., 37.16%,42.30%, and 41.69%.
Before the calcination process, the different TiO2:FeCl3 ratios show similar behavior for the crystallite size in the anatase phase and a slight difference for the rutile phase. Additionally, they show no significant differences in the lattice parameters and unit cell volume compared to TiO2 (reference). After the calcination process, the different ratios TiO2:FeCl3 show an increase in crystallite size, a decrease in unit cell volume, and a sharp decrease for the anatase phase fraction, with XA of about 37–41%.
2.3. Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS)
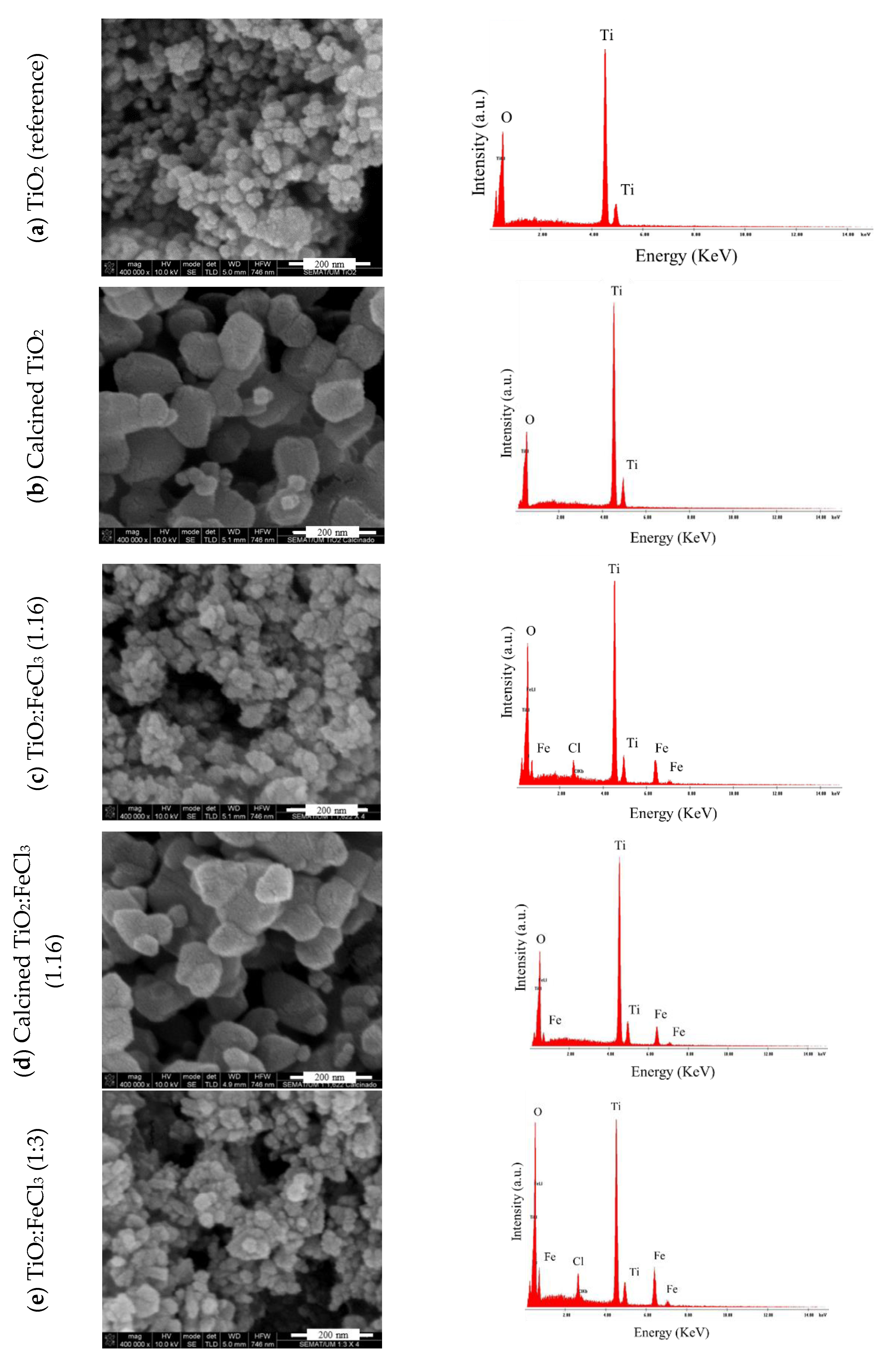
SEM was employed to investigate the homogenization and dispersion of the samples. EDS was performed to analyze their chemical characterization, i.e., elemental analysis of each material. For the analysis, the following samples were selected: (a) TiO2 (reference), (b) calcined TiO2, and in the different ratios of TiO2:FeCl3 doped (c) (1.16), (d) calcined (1:1.6), and (e) (1:3). Figure 3 presents the SEM microscopy representations and the EDS spectrum.
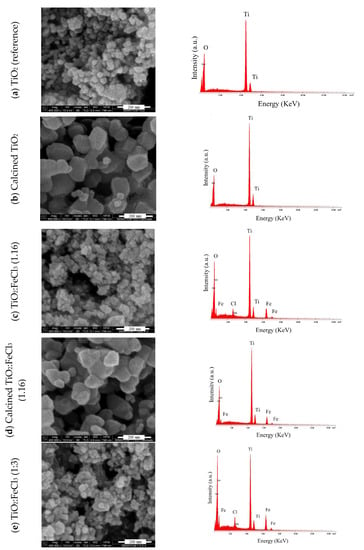
Figure 3.
SEM micrograph representation (left) and EDS spectrum (right) of: (a) reference TiO2, (b) calcined TiO2, (c) TiO2-FeCl3 (1:1.6), (d) calcined TiO2-FeCl3 (1:1.6) and (e) TiO2-FeCl3 (1:3).
For the reference TiO2 (Figure 3a) it is possible to verify the nanometer scale size nanoparticles of TiO2, ranging from 20 to 30 nanometers; for the reference TiO2, after the calcination process, the size of the particles is approximately 50 nm (Figure 3b), indicating that the particles have practically doubled their diameter. Additionally, the surface of the particles became smoother and more regular before the calcination process.
Concerning the TiO2 doped with FeCl3 at the ratio of (1:1.6), it can be inferred that iron is present in the sample, and the positions of the spectrum’s peaks confirm it. Furthermore, the particle size remains similar to the reference TiO2 (Figure 3c). Since the different particles of TiO2 and Fe cannot be observed separately, there are indications of a chemical reaction between them in the doping process; they become single particles, as indicated. Iron is not distributed heterogeneously into the TiO2 nanoparticles. It is also noticed that there are Cl residues in the spectrum of this sample. After the calcination process (Figure 3d) the same magnitude of particle size follows as the calcined TiO2. A change in surface appearance to smoother and more regular than before the calcination process was observed. The disappearance of residual Cl occurred, which may be caused by the washing step of the doping process.
Finally, the (1:3) ratio of Fe-doped TiO2 was analyzed before the calcination process (Figure 3e). It can be observed that the particle dimensions remain with the same size magnitude as the non-calcined reference TiO2 particles, and there is a residual amount of Cl remaining from the washing step of the doping process. In this case, there are also indications of the chemical reaction between TiO2 and Fe.
To summarize the results, Table 2 presents each sample’s chemical composition and particle size. The particle sizes show some variation, as observed in the XRD. The TiO2 (reference) has a particle size between 20–30 nm and is composed of 65.49% titanium and 34.51% oxygen. With respect to calcined TiO2, the size increases, i.e., ranging between 50–108 nm, but the chemical composition is similar to TiO2 (reference). Regarding the different TiO2:FeCl3 ratios, the particle size varies between 23–27 nm, and 27–38 nm, for ratios (1:1.16) and (1:3), respectively. After the calcination process at the ratio (1:1.6), the size also increased (49–51 nm). In the different proportions, there is the appearance of iron (Fe) with 14.00 to 17.95% and chlorine (Cl) with 1.85 to 2.48%. In the proportion (1:1.6) after the calcination process, chlorine is no longer present in the chemical composition.

Table 2.
Particle size variation and characterization of each sample studied.
Thus, before the calcination process, all TiO2:FeCl3 ratios show a similar particle size. After the calcination process, an increase in particle size occurs that is caused by the anatase-to-rutile transformation (ART), as previously discussed in XRD test results section.
2.4. Diffuse Reflectance Spectroscopy (DRS)
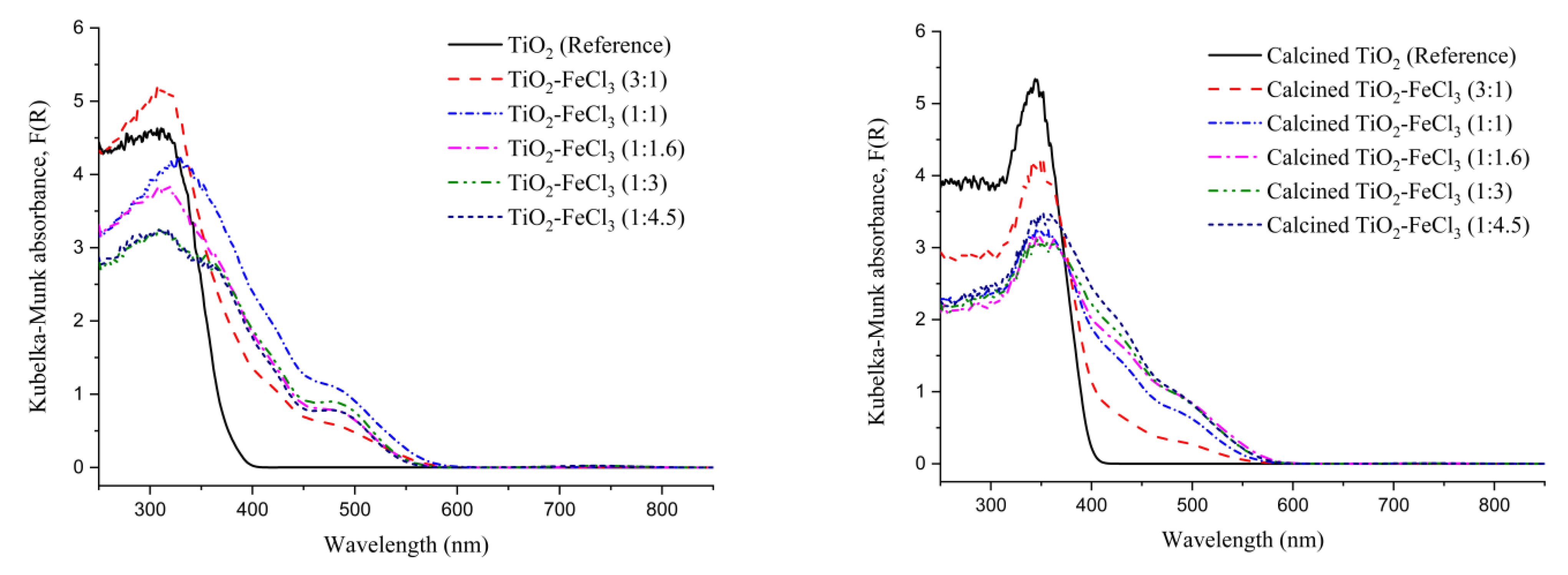
To study the effectiveness of TiO2 doping with FeCl3, the band gap of the semiconductor nanoparticles was analyzed using diffuse reflectance spectroscopy (DRS) and the Kubelka–Munk transform. The measurement of the light absorption of each material doped with different concentrations of FeCl3 and TiO2 (reference) before and after the calcination process (Figure 4) was obtained using the Kubelka–Munk function. The results show that both materials, calcined or uncalcined reference TiO2, depicts strong photoabsorption at wavelengths below 400 nm, i.e., in the ultraviolet (UV) and visible regions. For all concentrations, before and after calcination, the doped TiO2 shows a shift of the absorption edge that is caused by the effect of iron (i.e., dashed curves). However, as intended, changes in the optical properties of the material occur in the process of doping with Fe.
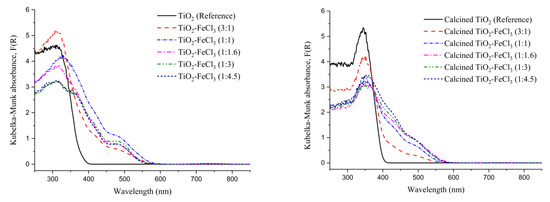
Figure 4.
Kubelka–Munk function versus wavelength for the undoped TiO2 (reference) and the samples synthesized before calcination (left) and after calcination (right).
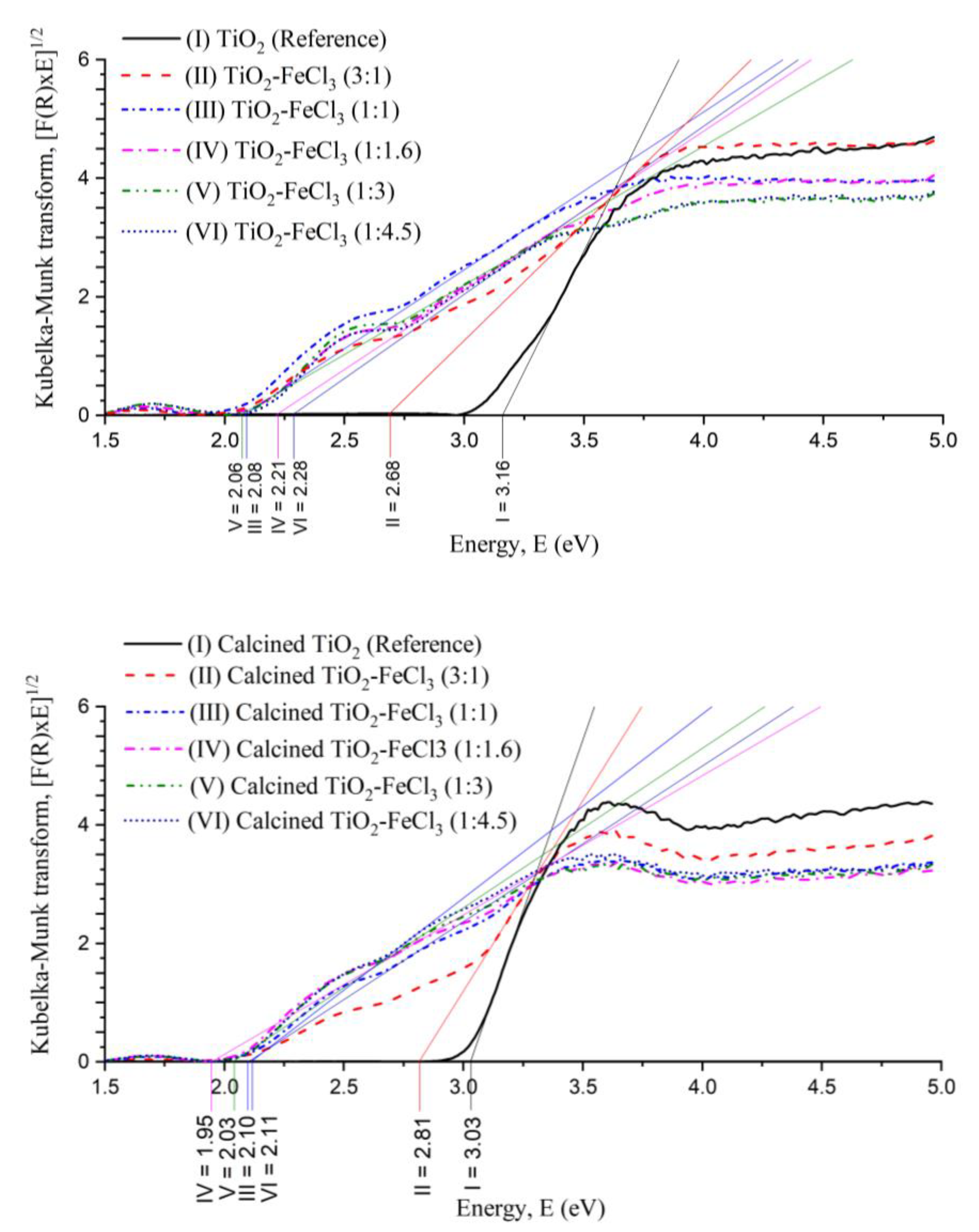
These shifts of the absorption edge to a longer wavelength indicate the band gap energy (Eg) of TiO2 and an increase of the absorption into the visible light region, which is less energetic than the UV region. The values of the Kubelka–Munk transformation versus Energy (eV) can be seen in Figure 5. A tangent line to the inflection point was drawn on the curve to obtain the value of Eg.
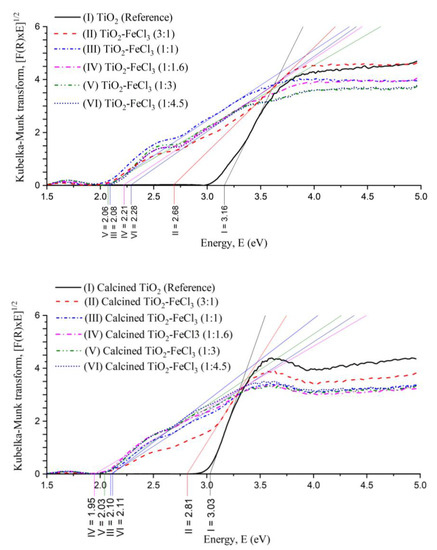
Figure 5.
Plot of Kubelka–Munk transform versus the Energy of the light absorbed for the undoped TiO2 (reference) and the samples synthesized before calcination (top) and after calcination (bottom).
It can be observed that the Eg values for the undoped TiO2 (reference) non-calcined (3.16 eV) and calcined (3.03 eV) are higher if compared to the doped TiO2 with FeCl3 in different concentrations. For both calcined or not calcined situations, the Eg values were always the lowest for the concentrations (1:1), (1:1.6), and (1:3). After the calcination process, the Eg values tend to decrease, except for the situation (3:1). Before calcination, the concentrations (1:1), (1:1.6), and (1:3) presented Eg values of 2.08, 2.21, and 2.06 eV, respectively. After calcination, the Eg of these situations became 2.10, 2.11, and 2.03 eV, respectively. After the calcination process, there is a strong tendency for Eg to decrease, which may be related to the ART, i.e., Eg (rutile) < Eg (anatase), to the formation of a dopant energy level (Fe4+/Fe3+), which comes in the excitation of Fe3+ and also due to the increase in particle size.
Therefore, the incorporation of Fe3+ into the TiO2 lattice causes a decrease in the energy band gaps. This effect is observed comparing the reference TiO2 with the TiO2-FeCl3 ratio (1:1). Its bang gap energy decreases from 3.16 eV to 2.08 eV.
2.5. Evaluation of the Photocatalytic Activity of the Doped Material
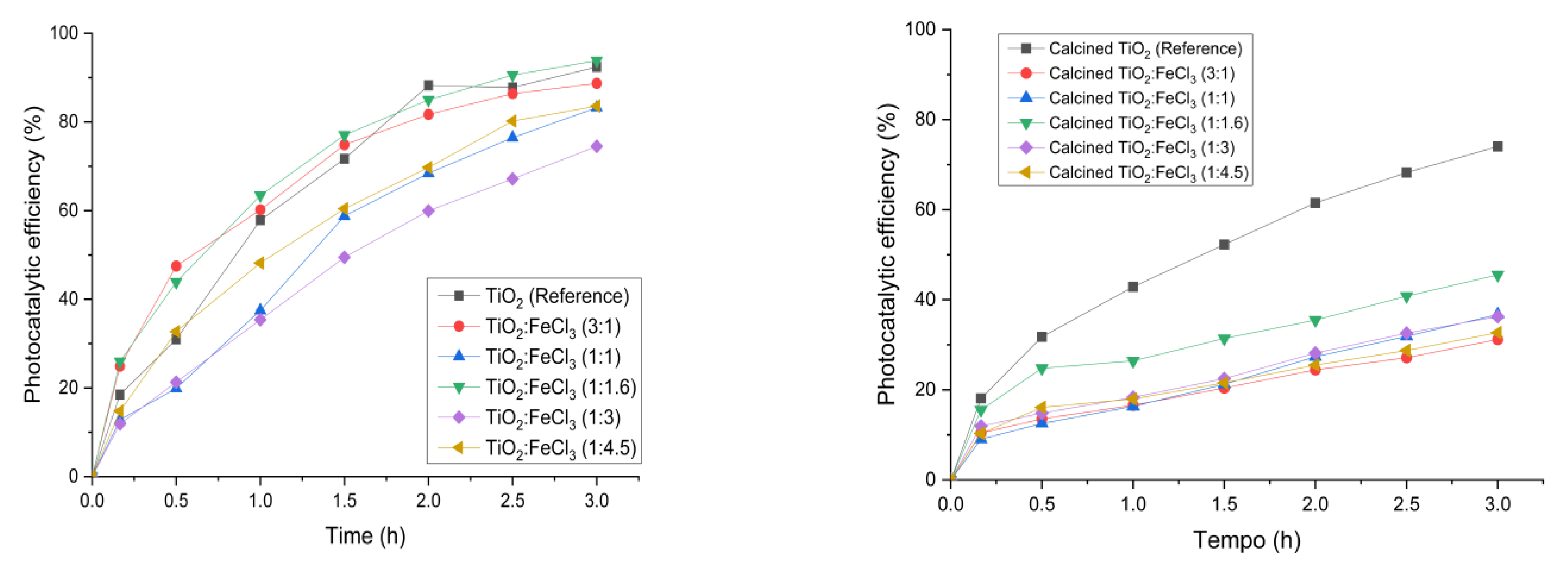
Figure 6 presents the results of photocatalytic efficiency before and after the calcination process. After 3 h of irradiation, the samples after calcination showed a photocatalytic efficiency of less than 70%, except for the calcined TiO2 (74.0%). Regarding the samples synthesized after the calcination process, the different ratios of TiO2:FeCl3 (3:1), (1:1.1), (1:1.16), (1:3), and (1:4.5) showed lower photocatalytic efficiency, i.e., 31.1%, 36.7%, 45.5%, 36.3%, 32.6%, respectively. In contrast, all the samples synthesized before the calcination process show a photocatalytic efficiency higher than 70%. The synthesized sample with the best photocatalytic efficiency was TiO2:FeCl3 (1:16), with a percentage of 93.8%. The reference TiO2 showed a similar photocatalytic efficiency, i.e., 92.43%. The remaining samples, TiO2:FeCl3 (3:1), (1:1), (1:3), and (1:4.5), showed 88.7%, 83.2%, 74.5%, and 83.6%, respectively.
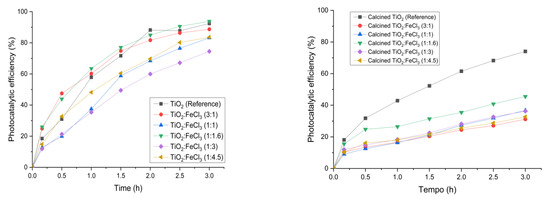
Figure 6.
Photocatalytic efficiency for TiO2 (reference), calcined TiO2, and the synthesized samples before calcination (left) and after calcination (right).
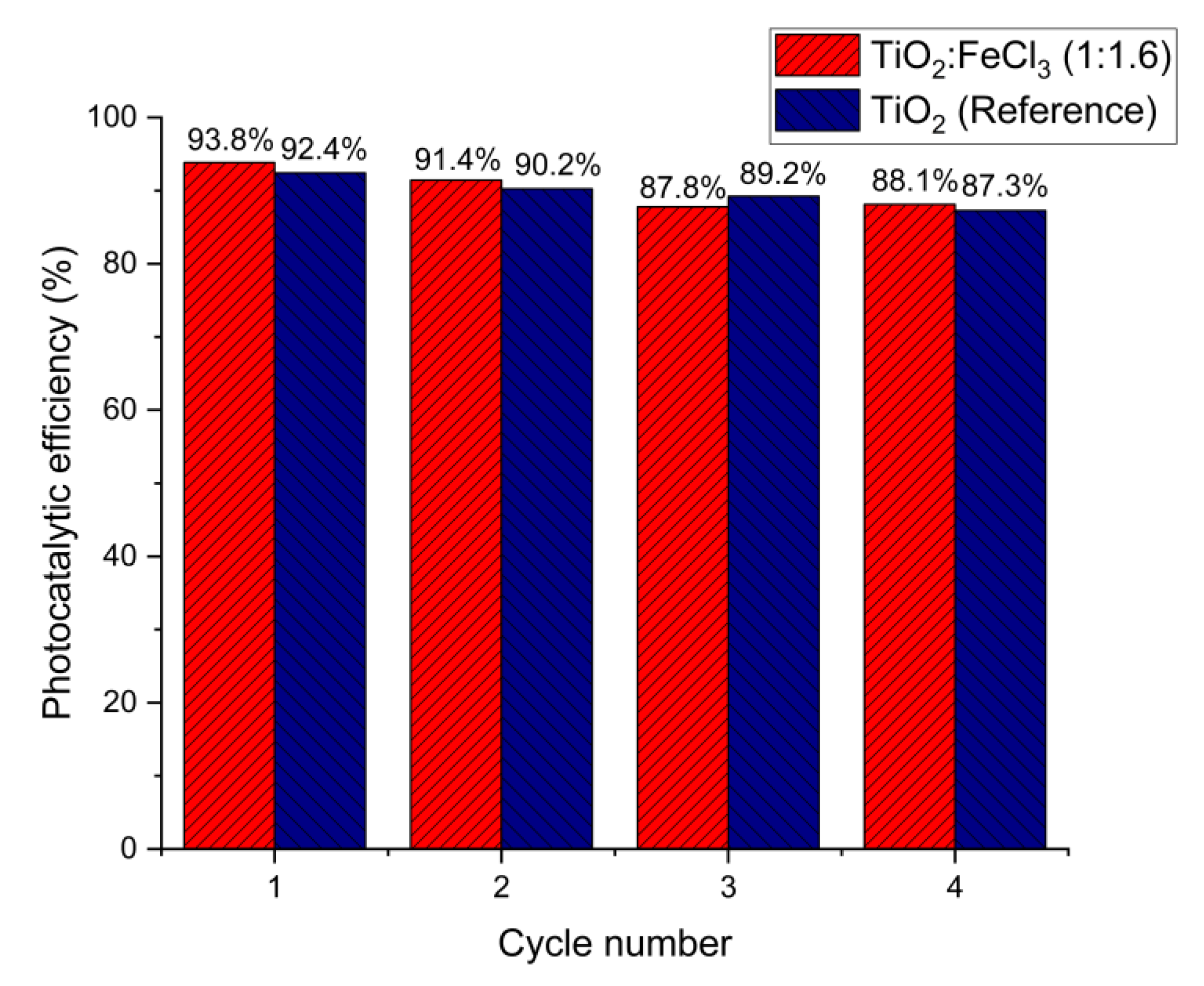
The subsequent use of photocatalysts to determine photocatalytic efficiency is a performance measure. For this purpose, the TiO2:FeCl3 (1:1.16), as a better catalyst, and the TiO2 (reference) samples were submitted to four recycling cycles. As shown in Figure 7, the RhB degradation occurred in all cycles, indicating that both samples were still active during the final cycle. About 10%, 15%, and 20% of the photocatalyst were lost during the filtration and cleaning processes after cycles 1, 2, and 3, respectively. Thus, the loss of the photocatalyst may have contributed to the degradation rate decrease, from 93.8% to 88.1% and from 92.4% to 87.3% for TiO2:FeCl3 (1:1.16) and TiO2 (reference), respectively.
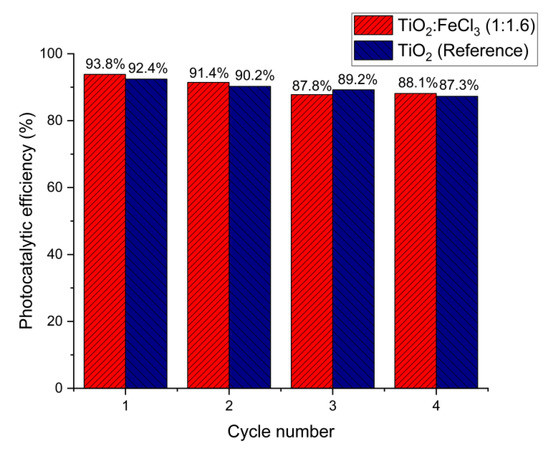
Figure 7.
Recycling of TiO2:FeCl3 (1:1.6) and TiO2 (reference) for degradation of RhB dye.
Through XRD it was observed that there is an ART after the calcination process, which may influence the photocatalytic efficiency. In SEM analyses, a decrease in the surface area was observed, which also influences the photocatalytic efficiency. According to Kim et al. (2014), this occurs because the diffusion radius of the •OH radical is generated in the anatase phase and not in the rutile phase. Consequently, photocatalytic oxidation in the rutile phase is restricted since the reaction zone of the •OH radical is restricted to the surface in the rutile phase, and in the anatase phase, the diffusion radius of the •OH radical is larger. Thus, the anatase phase has a higher photocatalytic activity than the rutile phase [39].
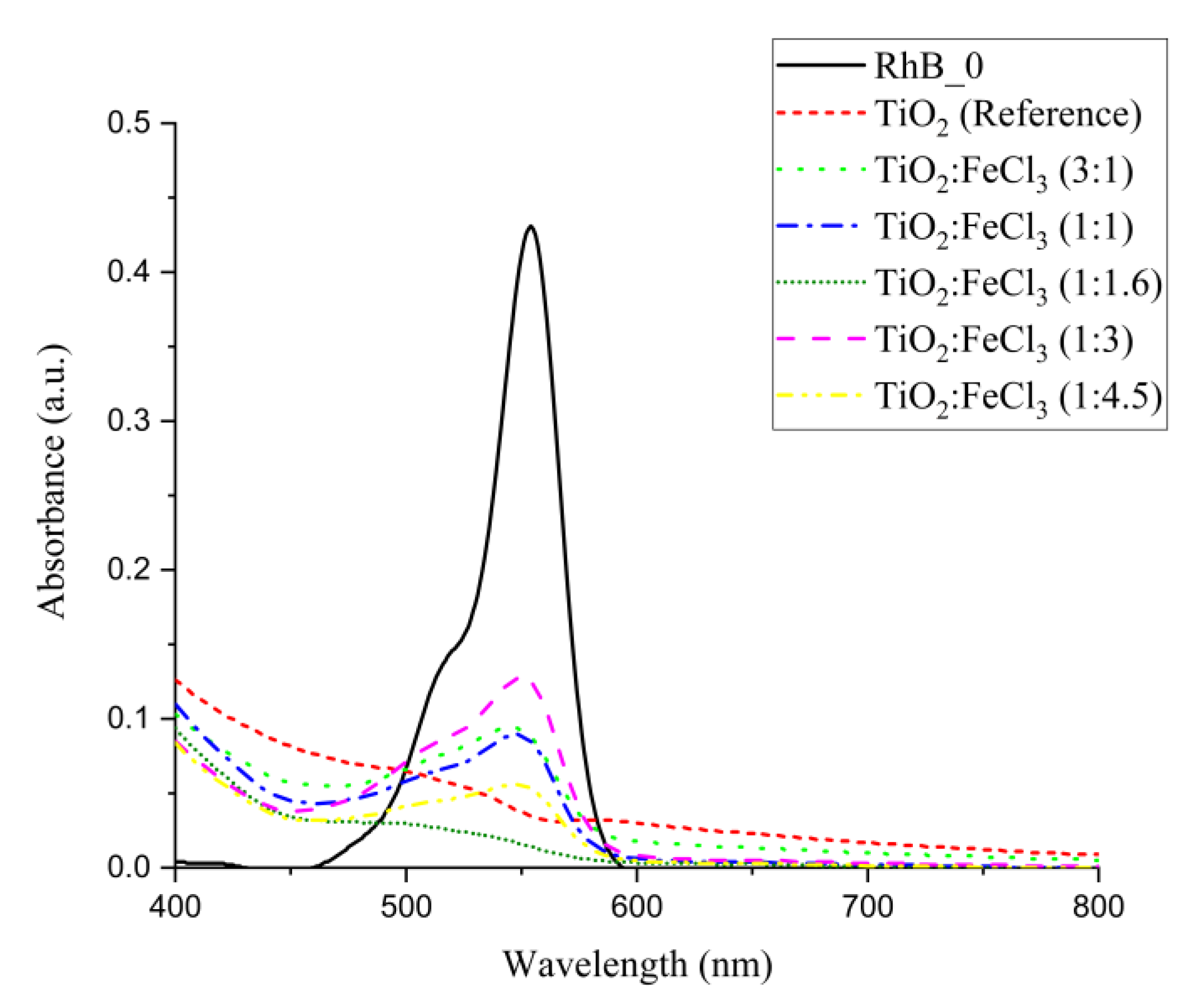
Although some samples showed similar photocatalytic efficiency during the 3 h period, their absorption spectra are presented in Figure 8. In fact, the TiO2 (reference) and doped TiO2-FeCl3 (1:1.16) ratio material showed a photocatalytic efficiency of 92.4% and 93.8%, respectively. Through the absorption curves, it can be seen that the RhB molecules are likely to have undergone a more advanced degradation process for the TiO2-FeCl3 (1:1.16) sample than for TiO2 (reference). Thus, it can be concluded that at the ratio (1:1.16), a higher and better photocatalytic efficiency occurs.
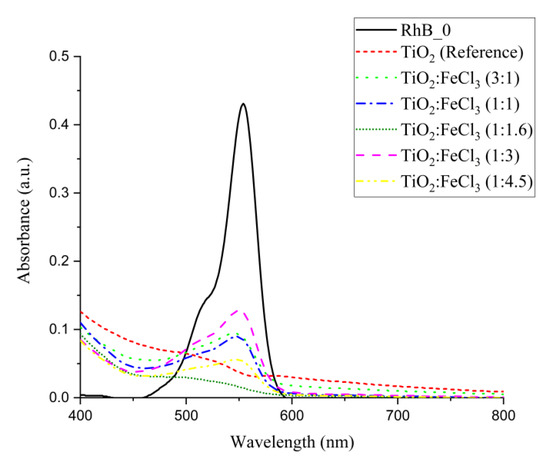
Figure 8.
Absorbance degradation curves of RhB solution after 3 h of exposure to solar irradiation for the different ratios of doped TiO2:FeCl3 and TiO2 (reference). Where RhB_0 is the RhB solution (2 ppm) at time 0 not irradiated.
3. Materials and Methods
The materials used were TiO2 semiconductor nanoparticles (Aeroxide TiO2 P25) purchased from Quimidroga (Barcelona, Spain), and their main properties were a purity >99.5%. The Iron (III) chloride (FeCl3) and Rhodamine B were purchased from Sigma-Aldrich (Lisboa, Portugal). Distilled water was used as a solvent.
An iron (Fe3+) doping process was carried out using semiconductor nanoparticles. First, solutions containing a suspension of TiO2 in distilled water (0.01 g/L) were prepared, and aqueous solutions containing different concentrations of FeCl3 were based on the concentration of TiO2. Subsequently, both solutions were mixed and stirred at 70 °C for 150 min, and then they were filtered and washed with distilled water. Finally, the samples were dried at 60 °C to obtain a solid material [10].
The TiO2:FeCl3 concentrations selected to reach the lowest band gap energy reduction were (3:1), (1:1), (1:1.6), (1:3), and (1:4.5). For example, the ratio (1:3) means that the sample was made with a concentration of 0.01 g/mL of TiO2 and 0.03 g/mL of FeCl3. The particle identification (TiO2:FeCl3) was added to their concentration ratio to identify the testing samples. Subsequently, the powders (doped and undoped TiO2 nanoparticles) were subjected to a calcination process (3 h at 700 °C) [6].
The band gap of the semiconductor nanoparticles was initially analyzed using diffuse reflectance spectroscopy (DRS) and Kubelka–Munk transform to study the effectiveness of doping and calcination. Subsequently, x-ray diffraction (XRD) was used to identify the crystalline phase of each material, as well as lattice parameters, crystal cell volume, and anatase phase fraction. Fourier transform infrared spectroscopy (FTIR) was performed to analyze the chemical composition of the doped materials. Scanning electron microscopy (SEM) was performed to analyze the homogenization and particle size of the samples. Energy dispersive spectroscopy (EDS) was performed to obtain the chemical composition of the doped materials. The doped semiconductor nanoparticles were subsequently immersed in RhB solution to analyze the photocatalytic activity under a sunlight simulator.
3.1. Diffuse Reflectance Spectroscopy (DRS) and Kubelka–Munk Transform
In the first stage, to determine the band gap energy of the reference concentration of TiO2 (undoped) and the various concentrations of doped TiO2-FeCl3 before and after the calcination process, Ultraviolet-Visible diffuse reflectance spectroscopy absorption measurements were performed. This analytical method is similar to the usual UV-vis spectroscopy, except that it is reflected into an integrating sphere and collected instead of transmitting light. Parallel to transmittance for liquids, reflectance, R, is advantageous for quantifying the amount of light reflected on solid surfaces.
The parameter called Kubelka–Munk function, F(R), was established to determine the light absorption [40]. Afterward, using the Kubelka–Munk transform, it was possible to calculate the band gap energy (Eg) [41,42].
3.2. X-ray Diffraction (XRD)
XRD was used to determine the crystallite phase of TiO2-doped materials using x-ray diffraction with a CuKα source from a Philips PW 1710 X-ray diffractometer (Billerica, MA, USA).
The Debye–Scherrer equation was employed to calculate the crystallite size of the doped particles before and after the calcination process [43]. The value of the lattice spacing for each of the selected peaks in the XRD spectrum was estimated from Bragg’s law [5]. To determine the fraction of the anatase phase in TiO2 doped powders, the intensity of the first and most intense XRD peaks of each sample containing the mixture of the two phases were used according to [44].
3.3. Fourier-Transform Infrared Spectroscopy (FTIR)
To check the chemical composition of the doped materials, i.e., to identify the chemical bonds existing in the doping of TiO2 with FeCl3 in the spectral range from 400 cm−1 to 4000 cm−1, Shimadzu IR-Prestige-21 spectrometer (Kyoto, Japan) was used. The most relevant elements, the chemical bonds in undoped TiO2 (reference) and different concentrations of TiO2-FeCl3 after the calcination process, were pointed out.
3.4. Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS)
Scanning electron microscopy (SEM) (Houston, TX, USA) was used to analyze the homogenization and dispersion of the doped materials. Energy dispersive spectroscopy (EDS) (Austin, TX, USA) was used to verify their chemical characterization, i.e., what chemical elements and their concentrations in the sample. These analyzes had the main objective of ascertaining the incorporation of FeCl3 in TiO2 nanoparticles in the doping process.
3.5. Photocatalytic Activity
The photocatalytic efficiency of the doped material was analyzed by Rhodamine B (RhB) degradation. First, 50 mg of nanoparticles were immersed in 50 mL of 2 ppm aqueous RhB solution, and then they were placed in a box 25 cm below a sunlight simulation lamp with a power intensity of 11 W/m2. The samples were conditioned in the dark for 80 min and then exposed to light for 3 h. Therefore, the initial adsorption and photocatalysis were split, and this phenomenon could be accurately analyzed. To prevent evaporation of the RhB solution, the systems were covered with a transparent cling film with less than 10% absorbance and reflectance (between 292 and 900 nm), allowing the almost total light transmission to the samples [45]. The photocatalytic degradation of the RhB solutions was monitored through their maximum absorbance values obtained at different time intervals [5,46,47]. During the test, aliquots of 5 mL were withdrawn from the systems and then centrifuged at 6000 rpm for 30 min to obtain decantation of the doped material. Subsequently, aliquots of 5 mL were taken from the centrifuged dispersions, and their absorbance was then measured using a spectrophotometer (SanSpecUV-Vis) in a wavelength range of 400 to 800 nm. The samples’ photocatalytic efficiency was calculated over time to assess their performance and select the best concentration of TiO2-FeCl3 regarding photodegradation.
To calculate the photocatalytic efficiency, the maximum absorbance (554 nm) of the dye (RhB) was monitored as a function of time (using a Shimadzu 3101 PC) and determined according to [48]. The best samples considering the photocatalytic efficiency will be submitted to four cycling experiments at the same conditions as the initial tests in order to analyze the stability of the prepared photocatalysts.
4. Conclusions
The main objective of this research was to assess doped TiO2 semiconductor nanoparticles with different contents of FeCl3, before and after calcination, through their optical, structural, morphological, chemical, and photocatalytic properties. The following conclusions can be drawn from the results obtained:
- The process of doping TiO2 with FeCl3 provided changes in the optical properties of the material and a decrease in the Eg of TiO2, also after the calcination process. The doping concentrations that presented the lowest Eg values were (1:1), (1:1.6), and (1:3).
- Contrarily to the effect after calcination, in the doping process the nanoparticles have no significant influence on the anatase-to-rutile transformation (ART) compared to the reference TiO2.
- The Fe-doping process modified the reference TiO2 spectrum with higher intensity of hydroxyl bonds and vibration of the Ti–O–Fe bond. After the calcination process, a drastic reduction of these bonds occurred.
- The particles are within the nanometer scale, and there are indications of the chemical reaction between TiO2 and Fe. The calcination process causes a relevant increase in particle size and surface smoothing.
- The ratio (1:1.6) of TiO2:FeCl3 showed the highest activity in the photocatalytic degradation of RhB with an efficiency of 93.8% after 3 h of irradiation.
In conclusion: doping TiO2 nanoparticles using metal ions (e.g., Fe3+) will improve photocatalytic activity under visible light irradiation, becoming a promising material for low-cost photocatalytic processes. However, further investigations for industrial-scale applications are needed to characterize the material’s durability in photocatalytic processes. The doped TiO2 will be applied in different materials for self-cleaning processes, for air purification, e.g., in polymeric materials, ceramics, textiles, and also in civil engineering (e.g., in cementitious materials and asphalt mixtures).
Author Contributions
Conceptualization, C.A., O.L.J. and I.R.S.; methodology, I.R.S., S.L.J., E.F., M.P. and J.C.; validation, I.R.S., S.L.J. and M.P.; formal analysis, C.A., O.L.J., N.H. and I.R.S.; investigation, C.A., I.R.S., S.L.J. and J.C.; resources, J.C., M.F.M.C. and E.F.; data curation, S.L.J. and I.R.S.; writing—original draft preparation, C.A., O.L.J., I.R.S. and S.L.J.; writing—review and editing, É.M., N.H., E.F., M.F.M.C. and J.C.; visualization, I.R.S., S.L.J. and O.L.J.; supervision, I.R.S., E.F. and J.C.; project administration, E.F. and J.C.; funding acquisition, E.F., M.F.M.C. and J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the Portuguese Foundation for Science and Technology (FCT), NanoAir PTDC/FISMAC/6606/2020, MicroCoolPav EXPL/EQU-EQU/1110/2021, UIDB/04650/2020, and UIDB/04029/2020. This research was supported by the doctoral Grant PRT/BD/154269/2022 financed by Portuguese Foundation for Science and Technology (FCT), and with funds from POR Norte - Portugal 2020 and State Budget, under MIT Portugal Program. The third author would like to acknowledge the FCT for funding (2022.00763.CEECIND).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rocha Segundo, I.; Freitas, E.; Branco, V.T.F.C.; Landi, S.; Costa, M.F.; Carneiro, J.O. Review and analysis of advances in functionalized, smart, and multifunctional asphalt mixtures. Renew. Sustain. Energy Rev. 2021, 151, 111552. [Google Scholar] [CrossRef]
- Zabihi-Mobarakeh, H.; Nezamzadeh-Ejhieh, A. Application of supported TiO2 onto Iranian clinoptilolite nanoparticles in the photodegradation of mixture of aniline and 2,4-dinitroaniline aqueous solution. J. Ind. Eng. Chem. 2015, 26, 315–321. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Bahrami, M. Investigation of the photocatalytic activity of supported ZnO–TiO2 on clinoptilolite nano-particles towards photodegradation of wastewater-contained phenol. Desalin. Water Treat. 2015, 55, 1096–1104. [Google Scholar] [CrossRef]
- Rocha Segundo, I.; Freitas, E.; Landi, S., Jr.; Costa, M.F.M.; Carneiro, J.O. Smart, Photocatalytic and Self-Cleaning Asphalt Mixtures: A Literature Review. Coatings 2019, 9, 696. [Google Scholar] [CrossRef]
- Carneiro, J.O.; Azevedo, S.; Fernandes, F.; Freitas, E.; Pereira, M.; Tavares, C.J.; Lanceros-Méndez, S.; Teixeira, V. Synthesis of iron-doped TiO2 nanoparticles by ball-milling process: The influence of process parameters on the structural, optical, magnetic, and photocatalytic properties. J. Mater. Sci. 2014, 49, 7476–7488. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Feizi, A. Iron-doped TiO2 Catalysts with Photocatalytic Activity. J. Water Environ. Nanotechnol. 2019, 4, 60–66. [Google Scholar] [CrossRef]
- Zahabizadeh, B.; Segundo, I.R.; Pereira, J.; Freitas, E.; Camões, A.; Tavares, C.J.; Teixeira, V.; Cunha, V.M.C.F.; Costa, M.F.M.; Carneiro, J.O. Development of Photocatalytic 3D-Printed Cementitious Mortars: Influence of the Curing, Spraying Time Gaps and TiO2 Coating Rates. Buildings 2021, 11, 381. [Google Scholar] [CrossRef]
- Adamek, E.; Baran, W.; Ziemiańska, Justyna Makowski, A.; Sobczak, A. Use of a TiO2/FeCl3 mixture in environmental cleaning technology. Proc. ECOpole 2012, 6. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Zhao, D.; Liang, Y.; Wang, H.; Wang, N.; Jiang, W.; Liu, S.; Liu, C.; Ding, W.; et al. Preparation, structural and photocatalytic activity of Sn/Fe co-doped TiO2 nanoparticles by sol-gel method. Ceram. Int. 2022, 48, 8297–8305. [Google Scholar] [CrossRef]
- Lucas, S.S.; Ferreira, V.M.; de Aguiar, J.L.B. Incorporation of titanium dioxide nanoparticles in mortars—Influence of microstructure in the hardened state properties and photocatalytic activity. Cem. Concr. Res. 2013, 43, 112–120. [Google Scholar] [CrossRef]
- Di Valentin, C.; Finazzi, E.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Paganini, M.C.; Giamello, E. N-doped TiO2: Theory and experiment. Chem. Phys. 2007, 339, 44–56. [Google Scholar] [CrossRef]
- Alkorbi, A.S.; Muhammad Asif Javed, H.; Hussain, S.; Latif, S.; Mahr, M.S.; Mustafa, M.S.; Alsaiari, R.; Alhemiary, N.A. Solar light-driven photocatalytic degradation of methyl blue by carbon-doped TiO2 nanoparticles. Opt. Mater. 2022, 127, 112259. [Google Scholar] [CrossRef]
- Barakat, M.A.; Schaeffer, H.; Hayes, G.; Ismat-Shah, S. Photocatalytic degradation of 2-chlorophenol by Co-doped TiO2 nanoparticles. Appl. Catal. B Environ. 2005, 57, 23–30. [Google Scholar] [CrossRef]
- Cao, X.; Yang, X.; Li, H.; Huang, W.; Liu, X. Investigation of Ce-TiO2 photocatalyst and its application in asphalt-based specimens for NO degradation. Constr. Build. Mater. 2017, 148, 824–832. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Eadi, S.B.; Kim, S.; Jeong, S.W.; Jeon, H.W. Novel Preparation of Fe Doped TiO2 Nanoparticles and Their Application for Gas Sensor and Photocatalytic Degradation. Adv. Mater. Sci. Eng. 2017, 2017, 2191659. [Google Scholar] [CrossRef]
- Zaleska, A. Doped-TiO2: A Review. Recent Patents Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Ganesh, I.; Kumar, P.; Gupta, A.; Sekhar, P.; Radha, K.; Padmanabham, G.; Sundararajan, G. Preparation and characterization of Fe-doped TiO2 powders for solar light response and photocatalytic applications. Process. Appl. Ceram. 2012, 6, 21–36. [Google Scholar] [CrossRef]
- Piątkowska, A.; Janus, M.; Szymański, K.; Mozia, S. C-,N- and S-Doped TiO2 Photocatalysts: A Review. Catalysts 2021, 11, 144. [Google Scholar] [CrossRef]
- Landi, S.; Carneiro, J.; Soares, O.S.G.P.; Pereira, M.F.R.; Gomes, A.C.; Ribeiro, A.; Fonseca, A.M.; Parpot, P.; Neves, I.C. Photocatalytic performance of N-doped TiO2nano-SiO2-HY nanocomposites immobilized over cotton fabrics. J. Mater. Res. Technol. 2019, 8, 1933–1943. [Google Scholar] [CrossRef]
- Liu, C.; Mao, S.; Shi, M.; Wang, F.; Xia, M.; Chen, Q.; Ju, X. Peroxymonosulfate activation through 2D/2D Z-scheme CoAl-LDH/BiOBr photocatalyst under visible light for ciprofloxacin degradation. J. Hazard. Mater. 2021, 420, 126613. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Mao, S.; Shi, M.; Hong, X.; Wang, D.; Wang, F.; Xia, M.; Chen, Q. Enhanced photocatalytic degradation performance of BiVO4/BiOBr through combining Fermi level alteration and oxygen defect engineering. Chem. Eng. J. 2022, 449, 137757. [Google Scholar] [CrossRef]
- Venturini, L.; Bacchi, I. Research, Design and Development of a Photocatalytic Asphalt Pavement. In Proceedings of the 2nd International Conference on Environmentally Friendly Roads, ENVIROAD 2009, Warsaw, Poland, 15–16 October 2009; pp. 1–16. [Google Scholar]
- Cano-Casanova, L.; Ansón-Casaos, A.; Hernández-Ferrer, J.; Benito, A.M.; Maser, W.K.; Garro, N.; Lillo-Ródenas, M.A.; Román-Martínez, M.C. Surface-Enriched Boron-Doped TiO2 Nanoparticles as Photocatalysts for Propene Oxidation. ACS Appl. Nano Mater. 2022, 5, 12527–12539. [Google Scholar] [CrossRef] [PubMed]
- Cano-Casanova, L.; Amorós-Pérez, A.; Ouzzine, M.; Lillo-Ródenas, M.A.; Román-Martínez, M.C. One step hydrothermal synthesis of TiO2 with variable HCl concentration: Detailed characterization and photocatalytic activity in propene oxidation. Appl. Catal. B Environ. 2018, 220, 645–653. [Google Scholar] [CrossRef]
- Qin, Q.; Wang, J.; Xia, Y.; Yang, D.; Zhou, Q.; Zhu, X.; Feng, W. Synthesis and Characterization of Sn/Ni Single Doped and Co–Doped Anatase/Rutile Mixed–Crystal Nanomaterials and Their Photocatalytic Performance under UV–Visible Light. Catalysts 2021, 11, 1341. [Google Scholar] [CrossRef]
- Fan, W.; Chan, K.Y.; Zhang, C.; Zhang, K.; Ning, Z.; Leung, M.K.H. Solar photocatalytic asphalt for removal of vehicular NOx: A feasibility study. Appl. Energy 2018, 225, 535–541. [Google Scholar] [CrossRef]
- Tang, B.; Liu, X.; Huang, W.; Cao, X. Preparation of La-doped nanometer TiO2 and its application for NO removal on asphalt concrete. Road Mater. Pavement Des. 2017, 18, 43–53. [Google Scholar] [CrossRef]
- Yeganeh, M.; Shahtahmasebi, N.; Kompany, A.; Karimipour, M.; Razavi, F.; Nasralla, N.H.S.; Šiller, L. The magnetic characterization of Fe doped TiO2 semiconducting oxide nanoparticles synthesized by sol–gel method. Phys. B Condens. Matter 2017, 511, 89–98. [Google Scholar] [CrossRef][Green Version]
- Ali, T.; Tripathi, P.; Azam, A.; Raza, W.; Ahmed, A.S.; Ahmed, A.; Muneer, M. Photocatalytic performance of Fe-doped TiO2 nanoparticles under visible-light irradiation. Mater. Res. Express 2017, 4, 015022. [Google Scholar] [CrossRef]
- Sood, S.; Umar, A.; Mehta, S.K.; Kansal, S.K. Highly effective Fe-doped TiO2 nanoparticles photocatalysts for visible-light driven photocatalytic degradation of toxic organic compounds. J. Colloid Interface Sci. 2015, 450, 213–223. [Google Scholar] [CrossRef]
- Xiang, L.; Zhao, X. Wet-Chemical Preparation of TiO2-Based Composites with Different Morphologies and Photocatalytic Properties. Nanomaterials 2017, 7, 310. [Google Scholar] [CrossRef] [PubMed]
- Kutuzova, A.; Dontsova, T. Synthesis, characterization and properties of titanium dioxide obtained by hydrolytic method. In Proceedings of the 2017 IEEE 7th International Conference Nanomaterials: Application & Properties (NAP), Odessa, Ukraine, 10–15 September 2017; IEEE: Hoboken, NJ, USA, 2017; pp. 01NNPT02-1–01NNPT02-5. [Google Scholar]
- Homem, N.C.; Yamaguchi, N.U.; Vieira, M.F.; Amorim, M.T.S.P.; Bergamasco, R. Surface modification of microfiltration membrane with GO nanosheets for dyes removal from aqueous solutions. Chem. Eng. Trans. 2017, 60, 259–264. [Google Scholar] [CrossRef]
- Ribao, P.; Corredor, J.; Rivero, M.J.; Ortiz, I. Role of reactive oxygen species on the activity of noble metal-doped TiO2 photocatalysts. J. Hazard. Mater. 2019, 372, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Shahmoradi, B.; Maleki, A.; Byrappa, K. Removal of Disperse Orange 25 using in situ surface-modified iron-doped TiO2 nanoparticles. Desalin. Water Treat. 2015, 53, 3615–3622. [Google Scholar] [CrossRef]
- Marami, M.B.; Farahmandjou, M.; Khoshnevisan, B. Sol–Gel Synthesis of Fe-Doped TiO2 Nanocrystals. J. Electron. Mater. 2018, 47, 3741–3748. [Google Scholar] [CrossRef]
- Tonejc, A..; Djerdj, I.; Tonejc, A. An analysis of evolution of grain size-lattice parameters dependence in nanocrystalline TiO2 anatase. Mater. Sci. Eng. C 2002, 19, 85–89. [Google Scholar] [CrossRef]
- Kim, W.; Tachikawa, T.; Moon, G.; Majima, T.; Choi, W. Molecular-Level Understanding of the Photocatalytic Activity Difference between Anatase and Rutile Nanoparticles. Angew. Chemie Int. Ed. 2014, 53, 14036–14041. [Google Scholar] [CrossRef]
- Valencia, S.; Marin, J.M.; Restrepo, G. Study of the Bandgap of Synthesized Titanium Dioxide Nanoparticules Using the Sol-Gel Method and a Hydrothermal Treatment. Open Mater. Sci. J. 2010, 4, 9–14. [Google Scholar] [CrossRef]
- Landi, S.; Segundo, I.R.; Freitas, E.; Vasilevskiy, M.; Carneiro, J.; Tavares, C.J. Use and misuse of the Kubelka-Munk function to obtain the band gap energy from diffuse reflectance measurements. Solid State Commun. 2022, 341, 114573. [Google Scholar] [CrossRef]
- Landi, S.; Segundo, I.R.; Afonso, C.; Lima, O.; Costa, M.F.M.; Freitas, E.; Carneiro, J. Evaluation of band gap energy of TiO2 precipitated from titanium sulphate. Phys. B Condens. Matter 2022, 639, 414008. [Google Scholar] [CrossRef]
- Jenkins, R.; Snyder, R. Introduction to X-ray Powder Diffractometry; Wiley-Interscience: New York, NY, USA, 1996; ISBN 978-0-471-51339-1. [Google Scholar]
- Spurr, R.A.; Myers, H. Quantitative Analysis of Anatase-Rutile Mixtures with an X-Ray Diffractometer. Anal. Chem. 1957, 29, 760–762. [Google Scholar] [CrossRef]
- Rocha Segundo, I.; Ferreira, C.; Freitas, E.F.; Carneiro, J.O.; Fernandes, F.; Júnior, S.L.; Costa, M.F.; Landi Júnior, S.; Costa, M.F. Assessment of photocatalytic, superhydrophobic and self-cleaning properties on hot mix asphalts coated with TiO2 and/or ZnO aqueous solutions. Constr. Build. Mater. 2018, 166, 36–44. [Google Scholar] [CrossRef]
- Yang, X.; Cao, C.; Erickson, L.; Hohn, K.; Maghirang, R.; Klabunde, K. Photo-catalytic degradation of Rhodamine B on C-, S-, N-, and Fe-doped TiO2 under visible-light irradiation. Appl. Catal. B Environ. 2009, 91, 657–662. [Google Scholar] [CrossRef]
- Barkhade, T.; Banerjee, I. Photocatalytic degradation of Rhodamine B dye using Fe doped TiO2 nanocomposites. AIP Conf. Proc. 2018, 1961, 030016. [Google Scholar]
- Carneiro, J.O.; Azevedo, S.; Teixeira, V.; Fernandes, F.; Freitas, E.; Silva, H.; Oliveira, J. Development of photocatalytic asphalt mixtures by the deposition and volumetric incorporation of TiO2 nanoparticles. Constr. Build. Mater. 2013, 38, 594–601. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).