Construction of Multi-Defective ZnMn2O4/Carbon Nitride Three-Dimensional System for Highly Efficient Photocatalytic Sulfamethoxazole Degradation
Abstract
:1. Introduction
2. Results
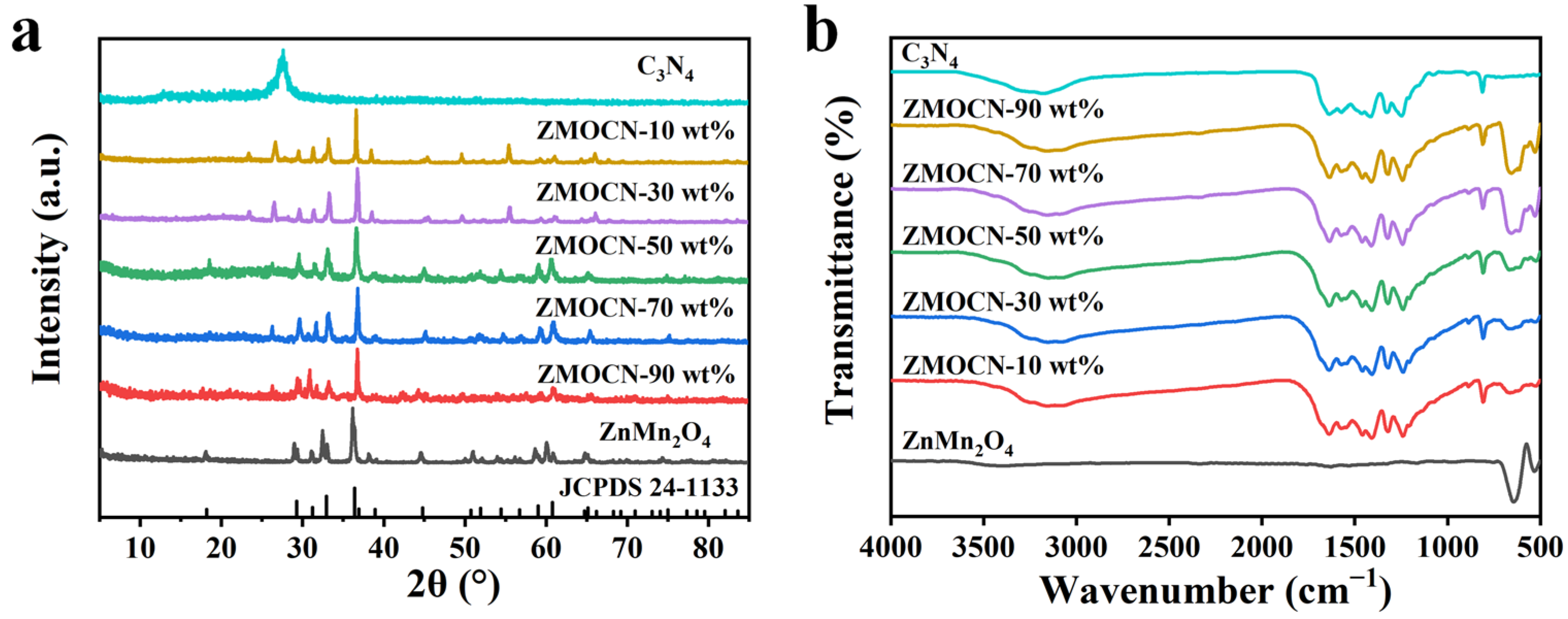
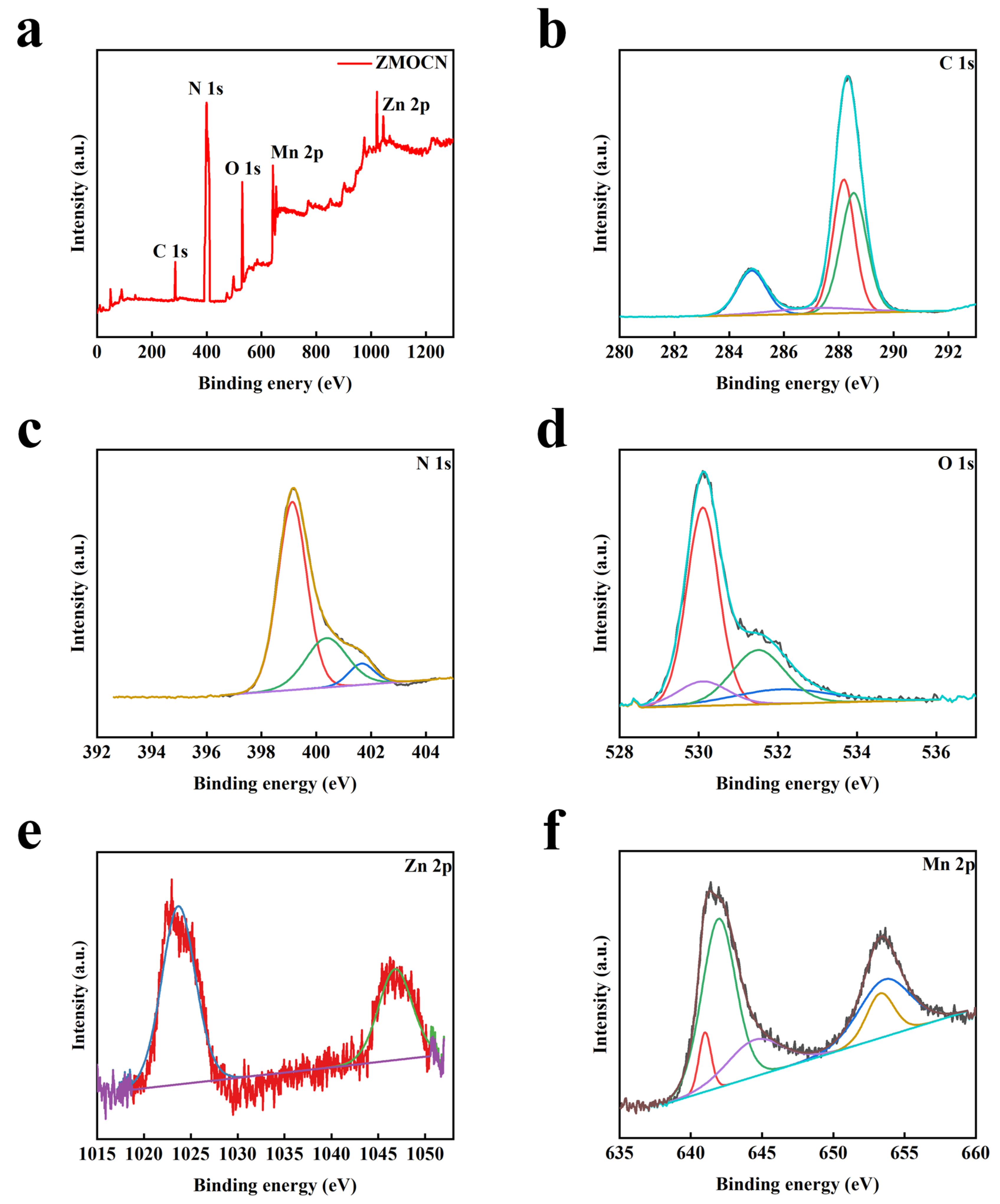
2.1. Structural Characterization and Analysis
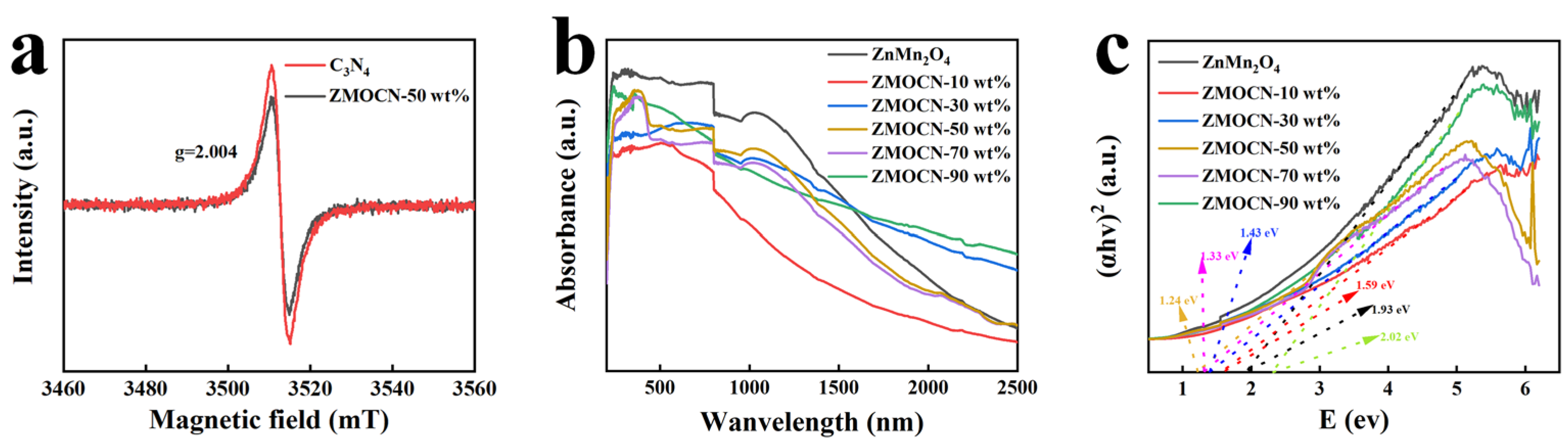
2.2. Morphology Analysis
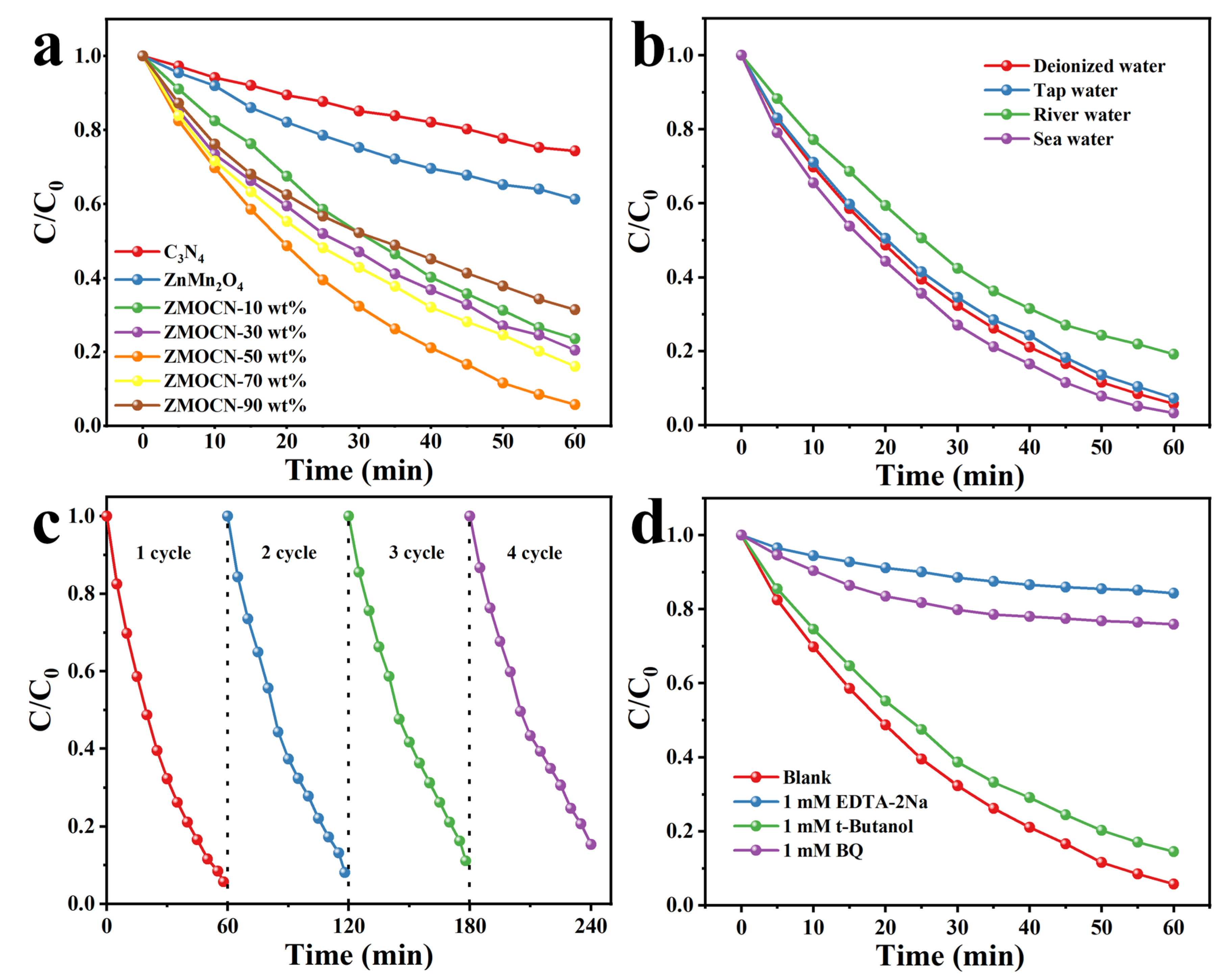
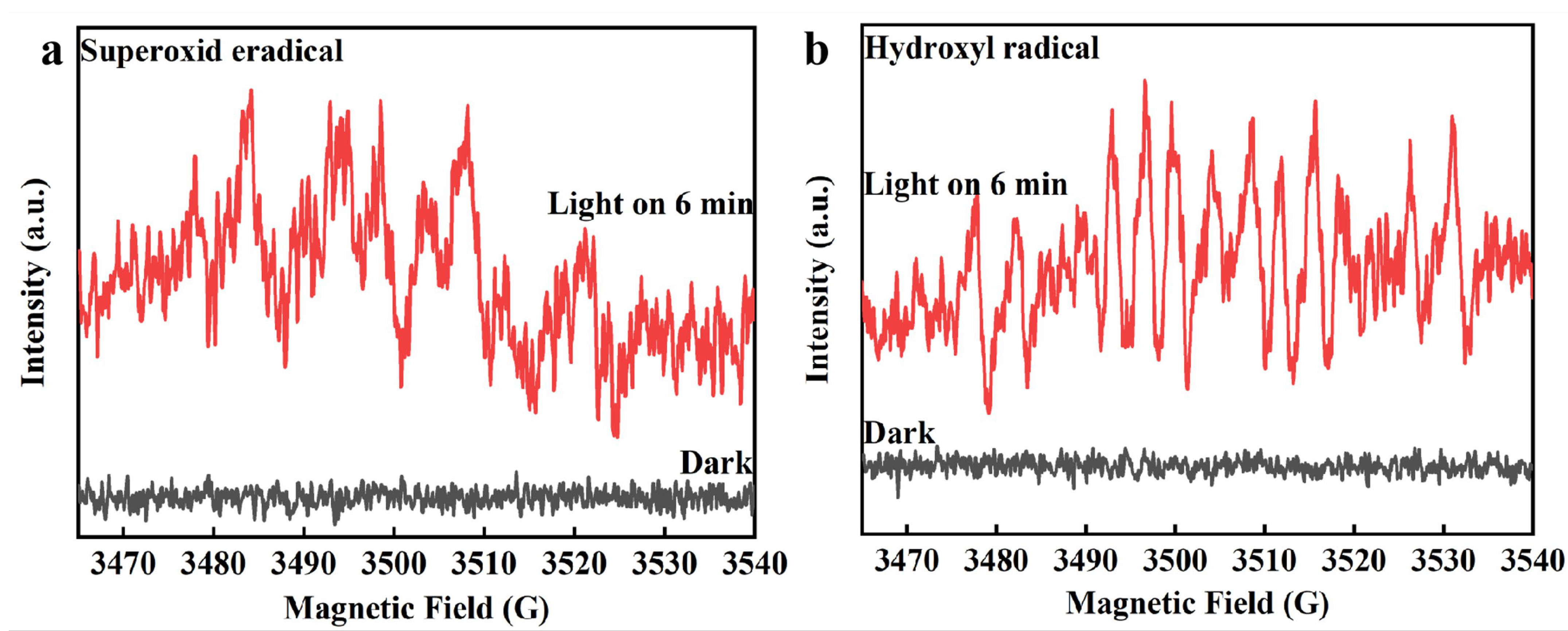
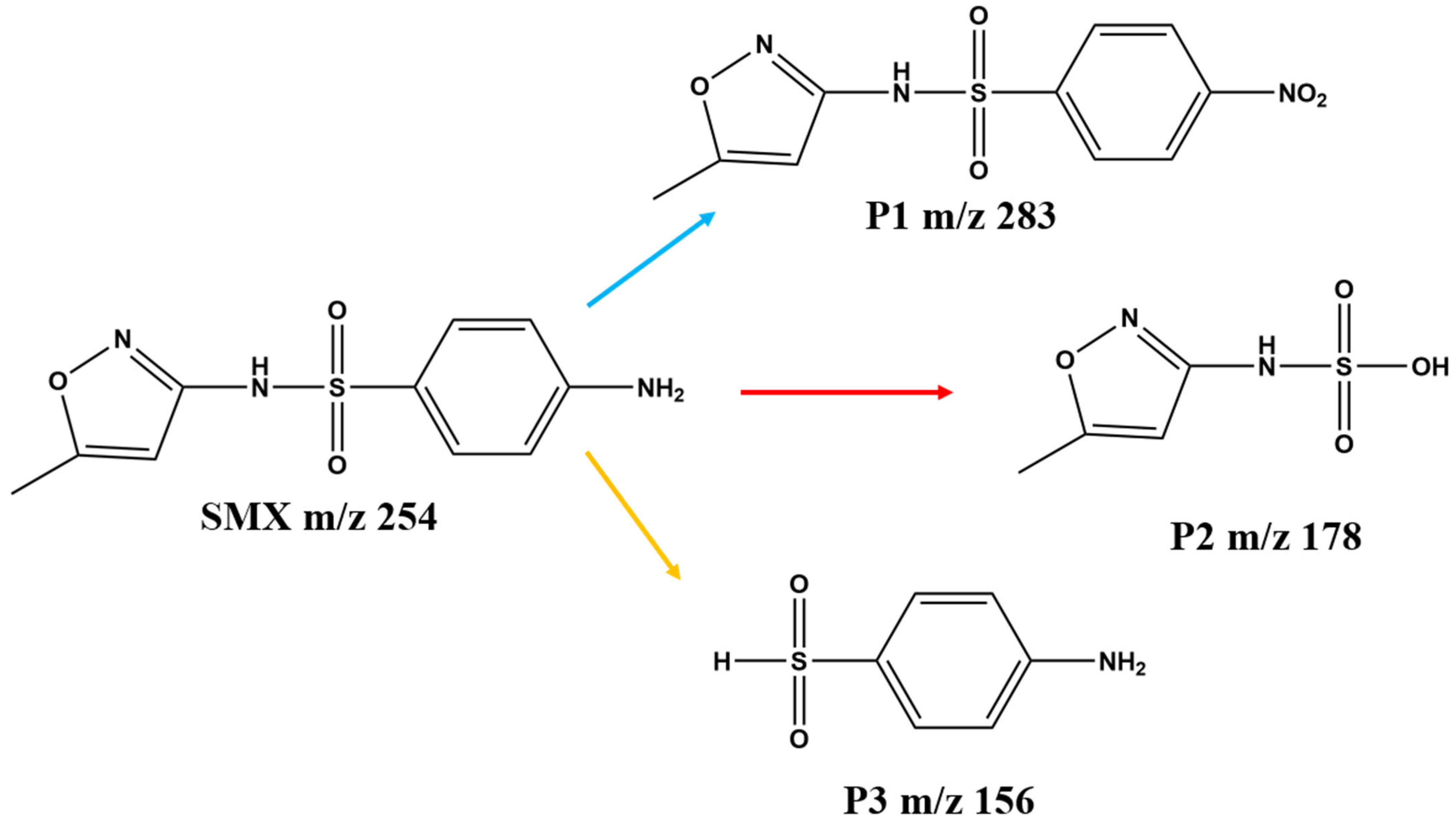
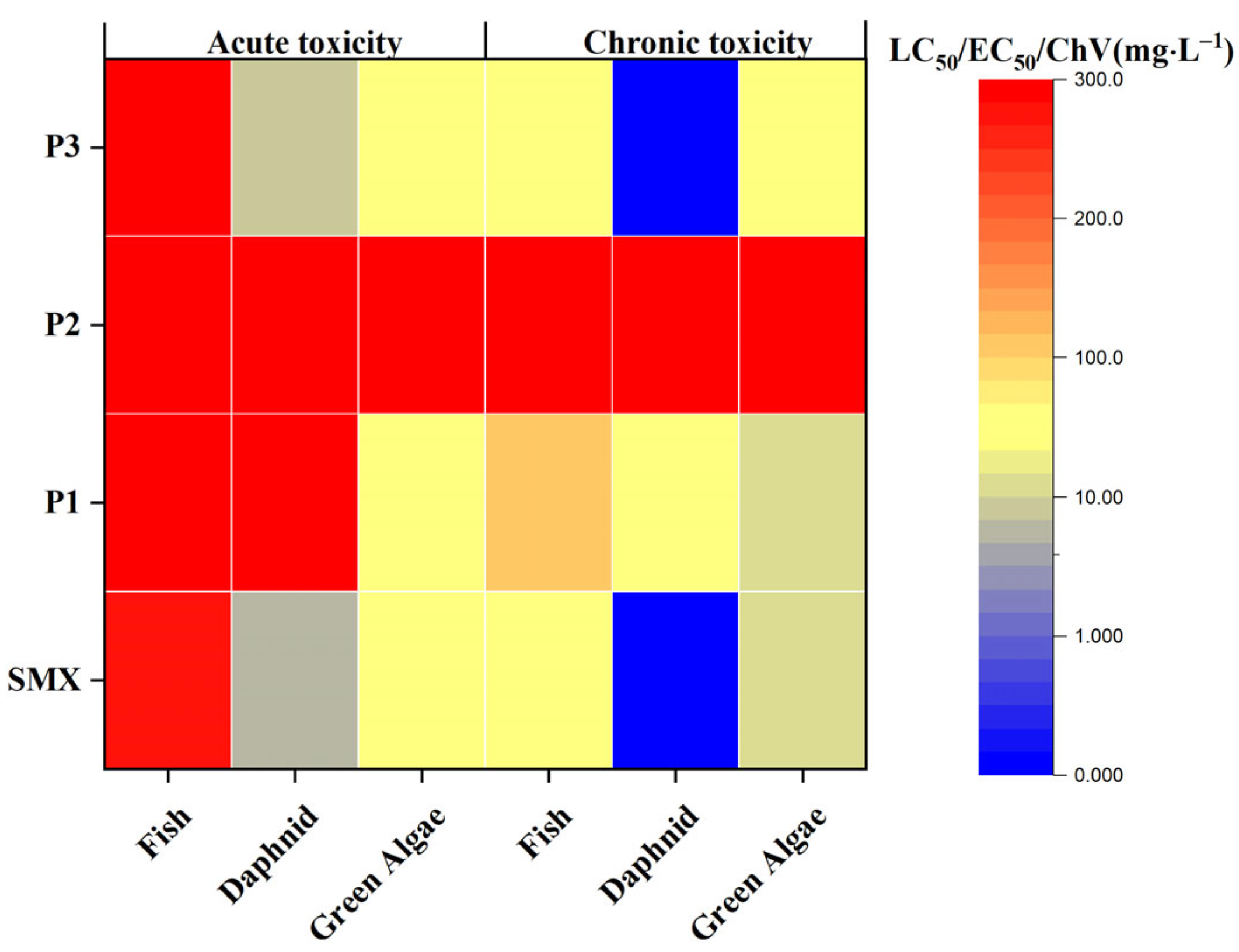
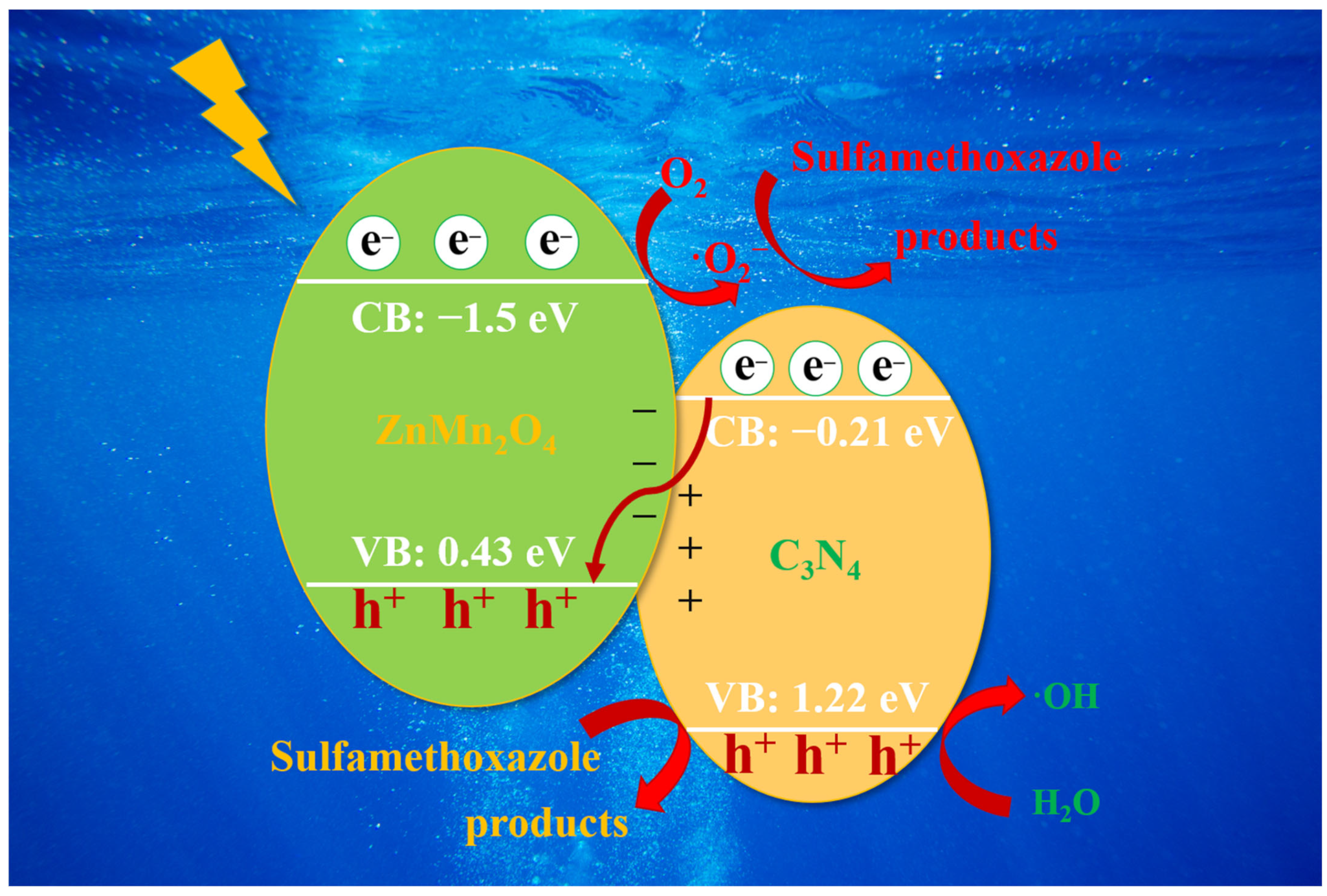
2.3. Photocatalytic Activity and Mechanism
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Synthesis
4.2.1. Synthesis of Zinc Manganate (ZnMn2O4) Nanosheets
4.2.2. Synthesis of Nitrogen-Deficient and Oxygen-Doped C3N4
4.2.3. Synthesis of Three-Dimensional Nitrogen-Deficient ZnMn2O4/Carbon Nitride (ZMOCNs) Nanocomposites
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, K.; Xing, Z.P.; Du, M.; Zhang, S.Y.; Li, Z.Z.; Pan, K.; Zhou, W. Hollow MoSe2@Bi2S3/CdS Core-Shell Nanostructure as Dual Z-Scheme Heterojunctions with Enhanced Full Spectrum Photocatalytic-Photothermal Performance. Appl. Catal. B-Environ. 2021, 281, 119482. [Google Scholar] [CrossRef]
- Sadeghi Rad, T.; Khataee, A.; Arefi-Oskoui, S.; Sadeghi Rad, S.; Orooji, Y.; Gengec, E.; Kobya, M. Graphene-based ZnCr layered double hydroxide nanocomposites as bactericidal agents with high sonophotocatalytic performances for degradation of rifampicin. Chemosphere. 2022, 286, 131740. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qi, J.; Tian, J.; Gao, S.; Cui, F. Construction of Ag/g-C3N4 photocatalysts with visible-light photocatalytic activity for sulfamethoxazole degradation. Chem. Eng. J. 2018, 341, 547–555. [Google Scholar] [CrossRef]
- Darwish, M.; Mohammadi, A.; Assi, N. Integration of nickel doping with loading on graphene for enhanced adsorptive and catalytic properties of CdS nanoparticles towards visible light degradation of some antibiotics. J. Hazard Mater. 2016, 320, 304–314. [Google Scholar] [CrossRef]
- Grilla, E.; Petala, A.; Frontistis, Z.; Konstantinou, I.K.; Kondarides, D.I.; Mantzavinos, D. Solar photocatalytic abatement of sulfamethoxazole over Ag3PO4/WO3 composites. Appl. Catal. B Environ. 2018, 231, 73–81. [Google Scholar] [CrossRef]
- Peng, W.; Liao, J.; Chen, L.; Wu, X.; Zhang, X.; Sun, W.; Ge, C. Constructing a 3D interconnected "trap-zap" beta-CDPs/Fe-g-C3N4 catalyst for efficient sulfamethoxazole degradation via peroxymonosulfate activation: Performance, mechanism, intermediates and toxicity. Chemosphere 2022, 294, 133780. [Google Scholar] [CrossRef]
- Tomara, T.; Frontistis, Z.; Petala, A.; Mantzavinos, D. Photocatalytic performance of Ag2O towards sulfamethoxazole degradation in environmental samples. J. Environ. Chem. Eng. 2019, 7, 103177. [Google Scholar] [CrossRef]
- Al-Ghafri, B.; Bora, T.; Sathe, P.; Dobrestov, S.; Al-Abri, M. Photocatalytic microbial removal and degradation of organic contaminants of water using PES fibers. Appl. Catal. B Environ. 2018, 233, 136–142. [Google Scholar] [CrossRef]
- Wang, J.; Zang, L.; Wang, L.; Tian, Y.; Yang, Z.; Yue, Y.; Sun, L. Magnetic cobalt ferrite/reduced graphene oxide (CF/rGO) porous balls for efficient photocatalytic degradation of oxytetracycline. J. Environ. Chem. Eng. 2022, 10, 108259. [Google Scholar] [CrossRef]
- Chen, L.; Liang, X.; Wang, H.; Xiao, Q.; Qiu, X. Ultra-thin carbon nitride nanosheets for efficient photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 442, 136115. [Google Scholar] [CrossRef]
- Li, J.; Li, K.; Du, J.; Yang, H.; Song, C.; Guo, X. Impact of transition metal incorporation on the photocatalytic CO2 reduction activity of polymeric carbon nitride. J. CO2 Util. 2022, 64, 102162. [Google Scholar] [CrossRef]
- Nguyen, T.K.A.; Pham, T.-T.; Nguyen-Phu, H.; Shin, E.W. The effect of graphitic carbon nitride precursors on the photocatalytic dye degradation of water-dispersible graphitic carbon nitride photocatalysts. Appl. Surf. Sci. 2021, 537, 148027. [Google Scholar] [CrossRef]
- Yang, T.; Mao, X.; Zhang, Y.; Wu, X.; Wang, L.; Chu, M.; Pao, C.W.; Yang, S.; Xu, Y.; Huang, X. Coordination tailoring of Cu single sites on C3N4 realizes selective CO2 hydrogenation at low temperature. Nat. Commun. 2021, 12, 6022. [Google Scholar] [CrossRef] [PubMed]
- Vavilapalli, D.S.; Peri, R.G.; Sharma, R.K.; Goutam, U.K.; Muthuraaman, B.; Ramachandra Rao, M.S.; Singh, S. g-C3N4/Ca2Fe2O5 heterostructures for enhanced photocatalytic degradation of organic effluents under sunlight. Sci Rep. 2021, 11, 19639. [Google Scholar] [CrossRef]
- Govindasamy, P.; Kandasamy, B.; Thangavelu, P.; Barathi, S.; Thandavarayan, M.; Shkir, M.; Lee, J. Biowaste derived hydroxyapatite embedded on two-dimensional g-C3N4 nanosheets for degradation of hazardous dye and pharmacological drug via Z-scheme charge transfer. Sci. Rep. 2022, 12, 11572. [Google Scholar] [CrossRef]
- Ghafuri, H.; Tajik, Z.; Ghanbari, N.; Hanifehnejad, P. Preparation and characterization of graphitic carbon nitride-supported L-arginine as a highly efficient and recyclable catalyst for the one-pot synthesis of condensation reactions. Sci. Rep. 2021, 11, 19792. [Google Scholar] [CrossRef]
- Yuan, D.; Chen, X.; Li, Z.; Fang, C.; Ding, J.; Wan, H.; Guan, G. The impact of benzene ring embedding on the performance of carbon nitride for photocatalytic hydrogen. Appl. Surf. Sci. 2021, 569, 151089. [Google Scholar] [CrossRef]
- Solgi, S.; Seyed Dorraji, M.S.; Hosseini, S.F.; Rasoulifard, M.H.; Hajimiri, I.; Amani-Ghadim, A. Improvement of microwave absorption properties of polyester coatings using NiFe2O4, X-doped g-C3N4 (X = S, P, and O), and MTiO3 (M = Fe, Mg, and Zn) nanofillers. Sci. Rep. 2021, 11, 19339. [Google Scholar] [CrossRef]
- Li, X.; Liu, Q.; Deng, F.; Huang, J.; Han, L.; He, C.; Chen, Z.; Luo, Y.; Zhu, Y. Double-defect-induced polarization enhanced OV-BiOBr/Cu2−xS high-low junction for boosted photoelectrochemical hydrogen evolution. Appl. Catal. B Environ. 2022, 314, 121502. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, X.; Cao, J.; Zhu, Y.; Yuan, W.; Hu, Z.; Ao, Z.; Brudvig, G.W.; Tian, F.; Yu, J.C.; et al. Photocatalytically recovering hydrogen energy from wastewater treatment using MoS2@TiO2 with sulfur/oxygen dual-defect. Appl. Catal. B Environ. 2022, 303, 120878. [Google Scholar] [CrossRef]
- Wu, S.; Yu, H.; Chen, S.; Quan, X. Enhanced Photocatalytic H2O2 Production over Carbon Nitride by Doping and Defect Engineering. ACS Catal. 2020, 10, 14380–14389. [Google Scholar] [CrossRef]
- Liang, D.; Wu, J.; Xie, C.; Wen, J.; Lyu, Y.; Sofer, Z.; Zheng, J.; Wang, S. Efficiently and selectively photocatalytic cleavage of C-C bond by C3N4 nanosheets: Defect-enhanced engineering and rational reaction route. Appl. Catal. B Environ. 2022, 317, 121690. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Wu, C.; Gao, J.; Li, M.; Xing, Z.; Li, Z.; Zhou, W. Hollow Nanoboxes Cu2−xS@ZnIn2S4 Core-Shell S-Scheme Heterojunction with Broad-Spectrum Response and Enhanced Photothermal-Photocatalytic Performance. Small 2022, 18, e2202544. [Google Scholar] [CrossRef]
- Tran Huu, H.; Thi, M.D.N.; Nguyen, V.P.; Thi, L.N.; Phan, T.T.T.; Hoang, Q.D.; Luc, H.H.; Kim, S.J.; Vo, V. One-pot synthesis of S-scheme MoS2/g-C3N4 heterojunction as effective visible light photocatalyst. Sci. Rep. 2021, 11, 14787. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Song, B.; Shen, X.; Shen, C.; Shan, S.; Xue, Q.; Chen, X.; Li, S. Rationally designed S-scheme heterojunction of C3N4/Bi2MoO6/carbon fiber cloth as a recyclable, macroscopic and efficient photocatalyst for wastewater treatment. Chem. Eng. J. 2022, 445, 136703. [Google Scholar] [CrossRef]
- Harini, G.; Syed, A.; Rahiman, M.K.; Bahkali, A.H.; Elgorban, A.M.; Varma, R.S.; Khan, S.S. Enhanced photodegradation of rifampicin and co-trimoxazole by ZnO/ZnMn2O4/ZnS-PVA and its genotoxicity studies on Allium cepa. Chemosphere 2022, 308, 136238. [Google Scholar] [CrossRef] [PubMed]
- uz Zaman, F.; Nagamuthu, S.; Cui, K.; Hou, L.; Yuan, C. Microwave-assisted synthesis of porous heterojunction ZnO/ZnMn2O4 microrods for efficient degradation of organic pollutants. Inorg. Chem. Commun. 2022, 144, 109845. [Google Scholar] [CrossRef]
- Fang, Q.; Chen, C.; Yang, Z.; Chen, X.A.; Chen, X.; Liu, T. Synthetization and electrochemical performance of pomegranate-like ZnMn2O4 porous microspheres. J. Alloy. Compd. 2020, 826, 154084. [Google Scholar] [CrossRef]
- Yang, W.; Shan, X.; Chen, Y.; Gao, Y. Enhanced photocatalytic performance of C3N4 via doping with π-deficient conjugated pyridine ring and BiOCl composite heterogeneous materials. Diam. Relat. Mater. 2020, 108, 107926. [Google Scholar] [CrossRef]
- Fang, W.; Liu, J.; Yu, L.; Jiang, Z.; Shangguan, W. Novel (Na, O) co-doped g-C3N4 with simultaneously enhanced absorption and narrowed bandgap for highly efficient hydrogen evolution. Appl. Catal. B Environ. 2017, 209, 631–636. [Google Scholar] [CrossRef]
- Boonprakob, N.; Wetchakun, N.; Phanichphant, S.; Waxler, D.; Sherrell, P.; Nattestad, A.; Chen, J.; Inceesungvorn, B. Enhanced visible-light photocatalytic activity of g-C3N4/TiO2 films. J. Colloid Interface Sci. 2014, 417, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, A.; Antony, R.P.; Wang, R.; Lee, J.M. MOF-Derived Hollow Cage NixCo3−xO4 and Their Synergy with Graphene for Outstanding Supercapacitors. Small 2017, 13, 1603102. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, C. Mxene enhanced the photocatalytic activity of ZnO nanorods under visible light. Mater. Lett. 2020, 261, 127127. [Google Scholar] [CrossRef]
- Lan, B.; Huang, S.; Ye, C.; Qin, Q.; Yan, J.; Wu, Y. Enhanced electrochemical performance of Sn-doped MnO2 and study on morphology evolution. J. Alloy. Compd. 2019, 788, 302–310. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Liao, J.; Zhang, L.; Li, Y.; Ge, C. Construction of Multi-Defective ZnMn2O4/Carbon Nitride Three-Dimensional System for Highly Efficient Photocatalytic Sulfamethoxazole Degradation. Catalysts 2023, 13, 172. https://doi.org/10.3390/catal13010172
Xu Y, Liao J, Zhang L, Li Y, Ge C. Construction of Multi-Defective ZnMn2O4/Carbon Nitride Three-Dimensional System for Highly Efficient Photocatalytic Sulfamethoxazole Degradation. Catalysts. 2023; 13(1):172. https://doi.org/10.3390/catal13010172
Chicago/Turabian StyleXu, Yandong, Jianjun Liao, Linlin Zhang, Yakun Li, and Chengjun Ge. 2023. "Construction of Multi-Defective ZnMn2O4/Carbon Nitride Three-Dimensional System for Highly Efficient Photocatalytic Sulfamethoxazole Degradation" Catalysts 13, no. 1: 172. https://doi.org/10.3390/catal13010172
APA StyleXu, Y., Liao, J., Zhang, L., Li, Y., & Ge, C. (2023). Construction of Multi-Defective ZnMn2O4/Carbon Nitride Three-Dimensional System for Highly Efficient Photocatalytic Sulfamethoxazole Degradation. Catalysts, 13(1), 172. https://doi.org/10.3390/catal13010172




