ZIF-67 Derived Cu-Co Mixed Oxides for Efficient Catalytic Oxidation of Formaldehyde at Low-Temperature
Abstract
1. Introduction
2. Results and Discussion
2.1. HCHO Oxidation Performance Tests
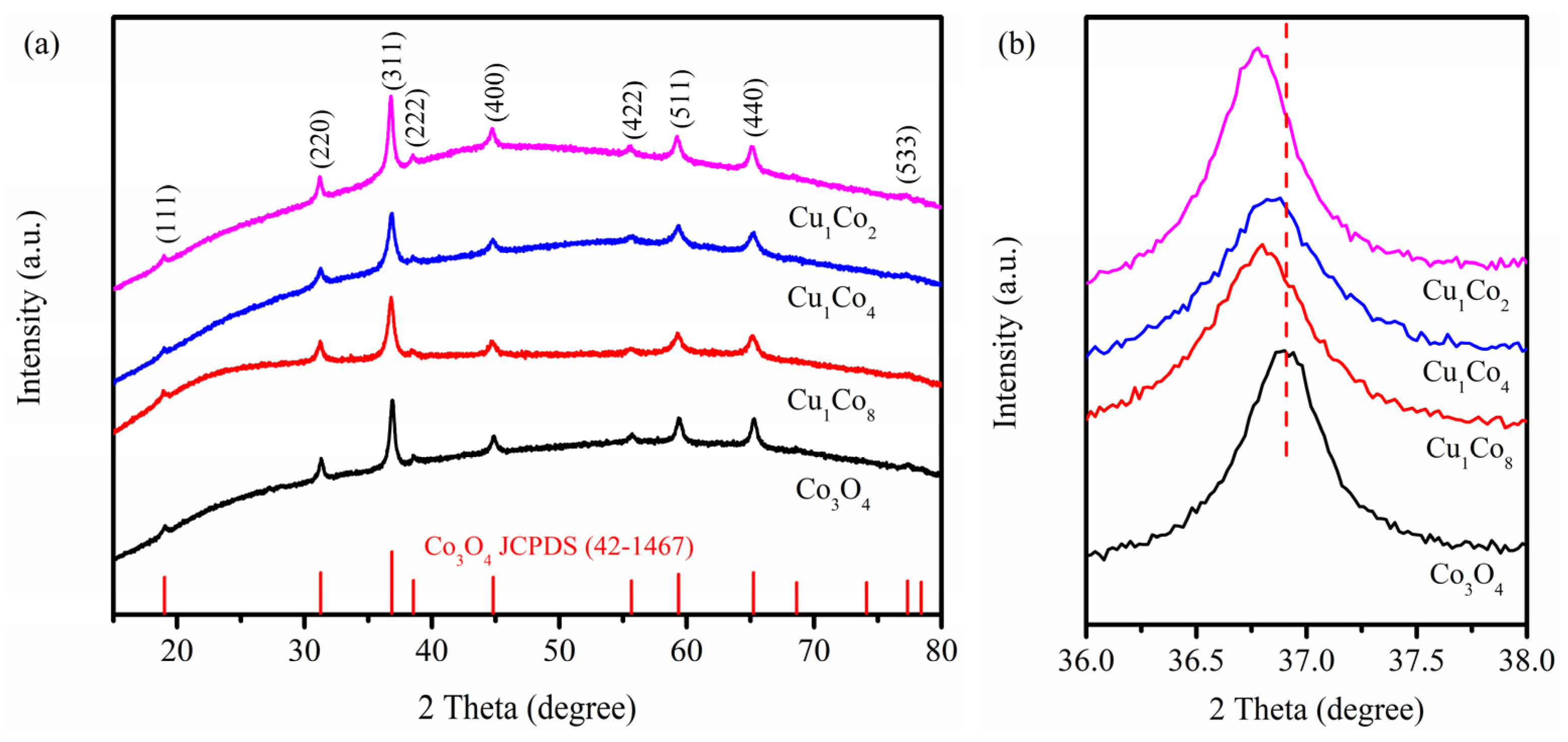
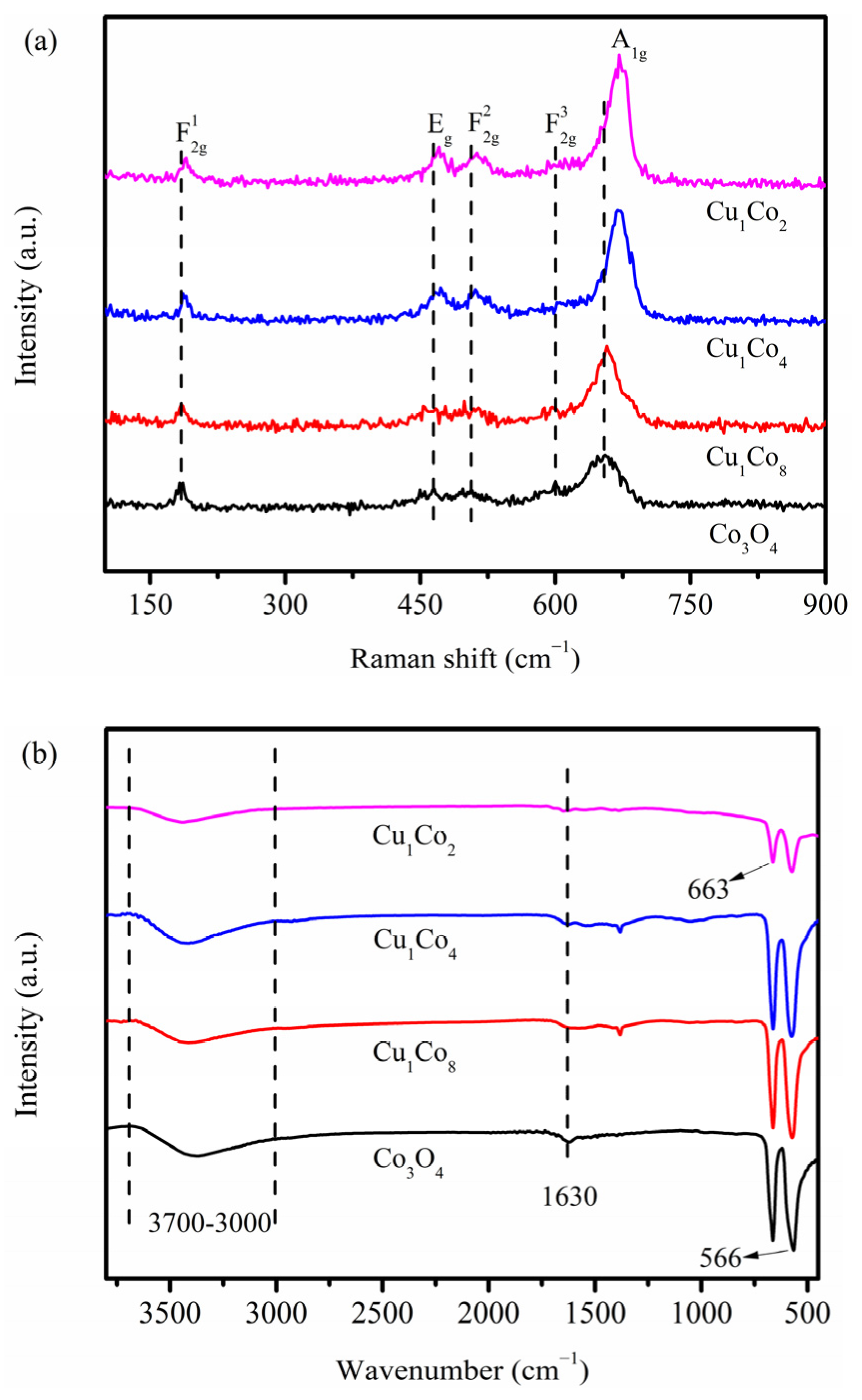
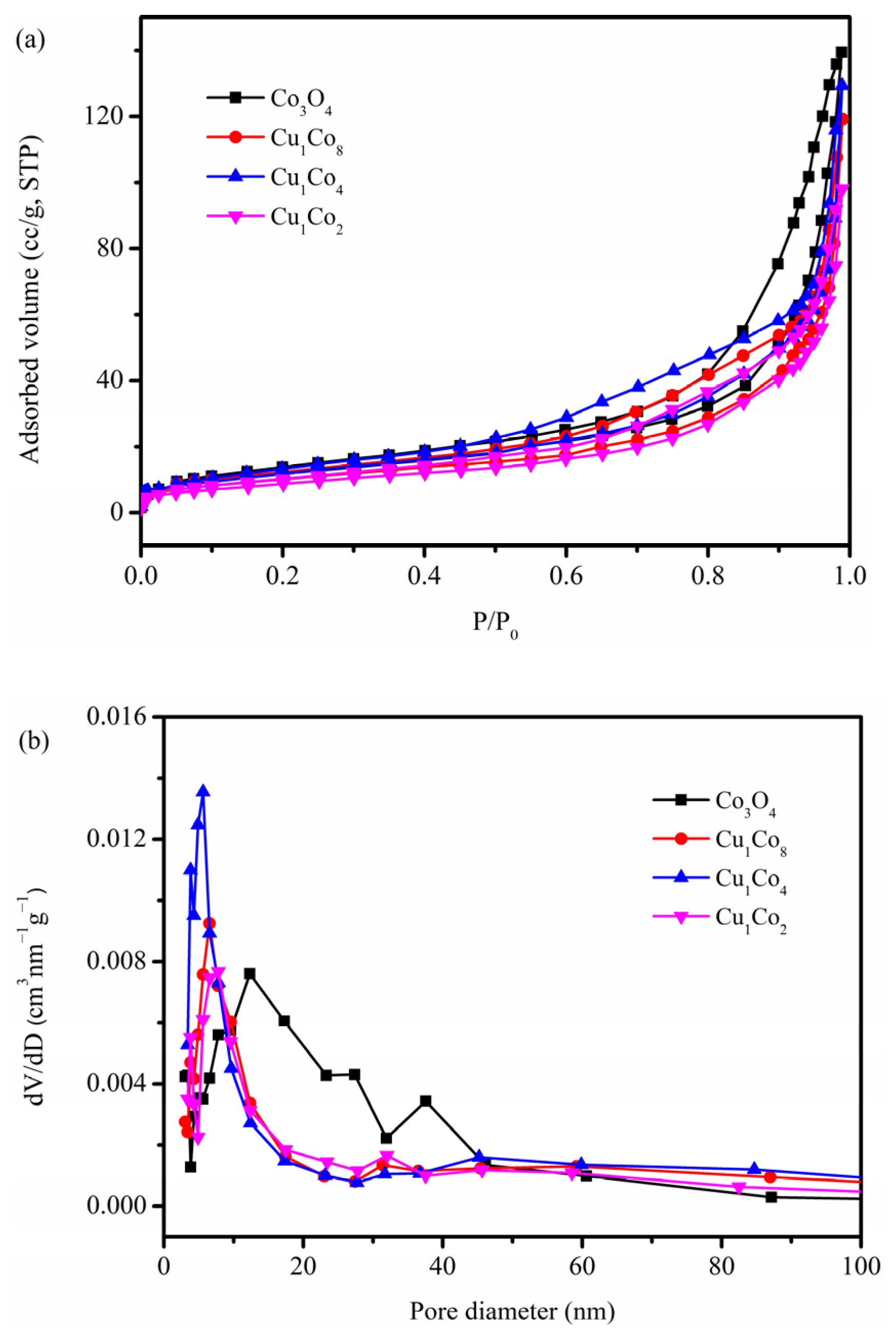
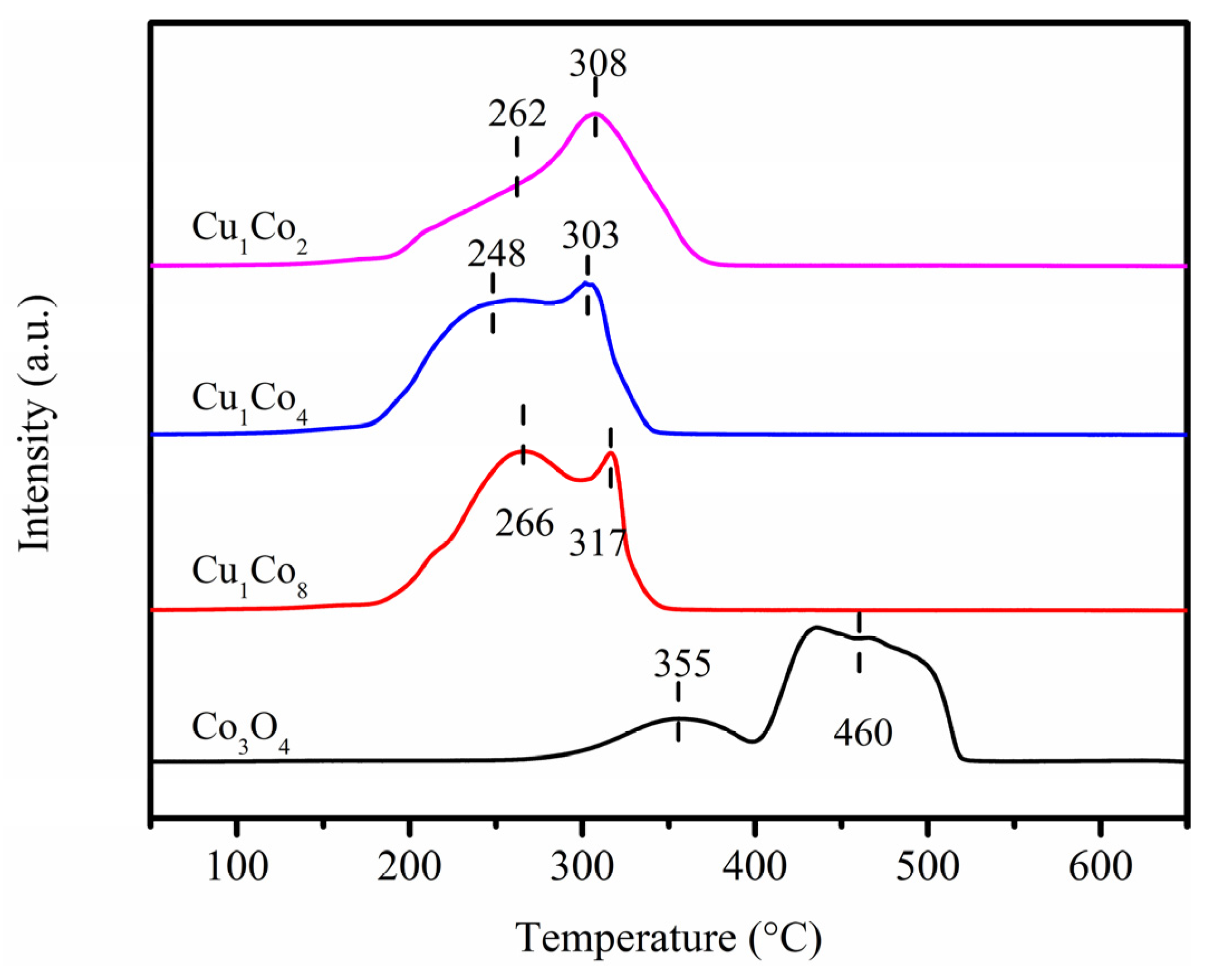
2.2. Physicochemical Property Analyses
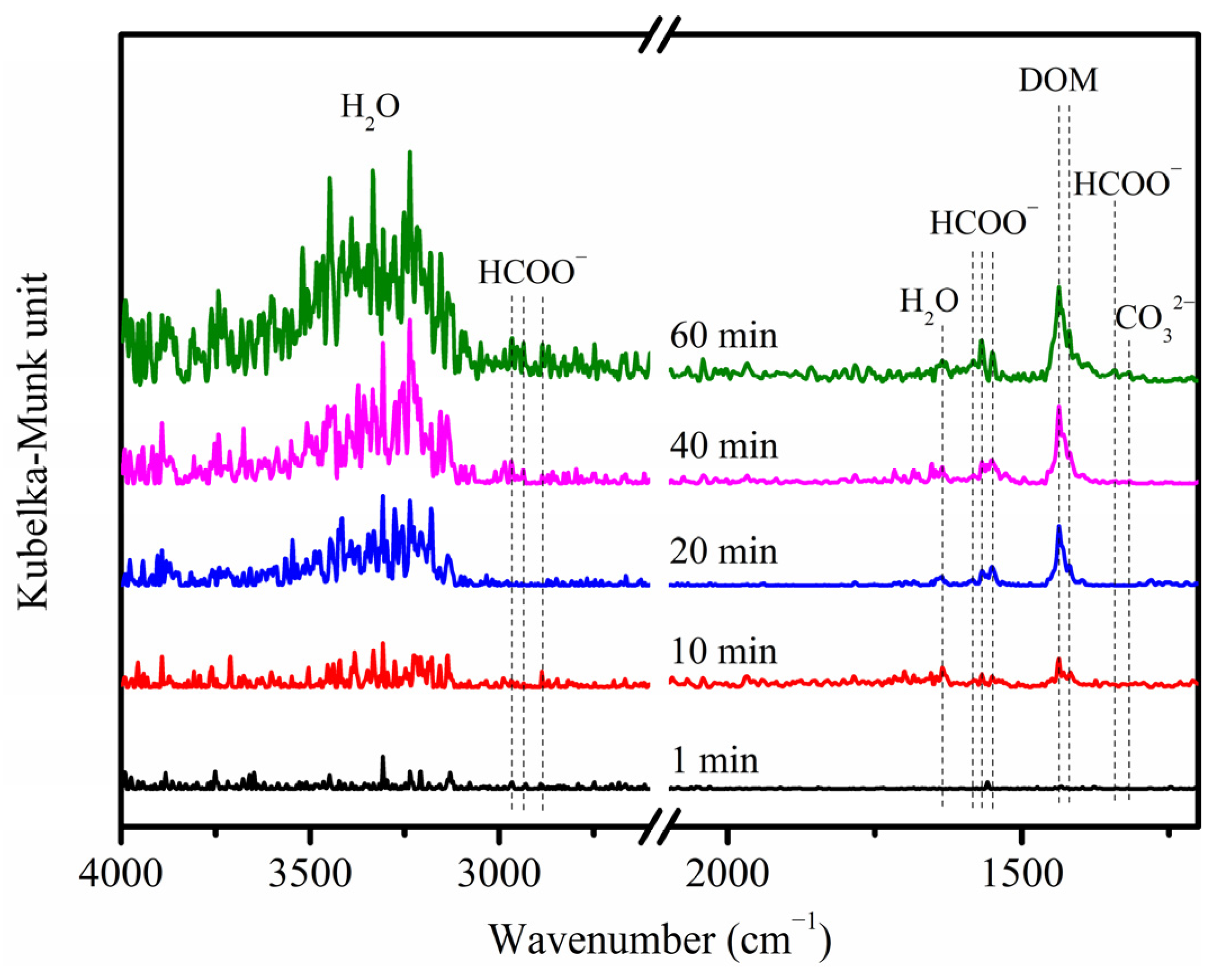
2.3. Reaction Pathway
3. Experimental
3.1. Materials
3.2. Preparation of Co3O4 and Cu-Co Oxides
3.3. Catalyst Characterization
3.4. Catalytic Evaluation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Salthammer, T.; Mentese, S.; Marutzky, R. Formaldehyde in the indoor environment. Chem. Rev. 2010, 110, 2536–2572. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Bai, Y.; Duong, A.; Smith, M.T.; Li, L.; Zhang, L. Formaldehyde in China: Production, consumption, exposure levels, and health effects. Environ. Int. 2009, 35, 1210–1224. [Google Scholar] [CrossRef] [PubMed]
- WHO. Air Quality Guidelines for Europe; WHO Regional Office for Europe: Copenhagen, Denmark, 1987. [Google Scholar]
- Singapore Ministry of Environment. Guidelines for Indoor Air Quality in Office Premises; Institute of Environmental Epidemiology: Singapore, 1996.
- GB/T 18883-2022; Indoor Air Quality Standard. China’s State Administration for Market Regulation: Beijing, China, 2022.
- Suresh, S.; Bandosz, T.J. Removal of formaldehyde on carbon-based materials: A review of the recent approaches and findings. Carbon 2018, 137, 207–221. [Google Scholar] [CrossRef]
- Chang, M.B.; Lee, C.C. Destruction of formaldehyde with dielectric denier discharge plasmas. Environ. Sci. Technol. 1995, 29, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, H.; Chen, X.; Wang, F.; Qin, X.; Zhang, C.; He, H. High-performance of Cu-TiO2 for photocatalytic oxidation of formaldehyde under visible light and the mechanism study. Chem. Eng. J. 2020, 390, 124481. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Chen, M.; Qin, X.; He, H.; Zhang, C. Co-function mechanism of multiple active sites over Ag/TiO2 for formaldehyde oxidation. Appl. Catal. B 2021, 282, 119543. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Zhang, C.; Chen, X.; Liu, C.; Weng, W.; Shan, W.; He, H. A simple strategy to improve Pd dispersion and enhance Pd/TiO2 catalytic activity for formaldehyde oxidation: The roles of surface defects. Appl. Catal. B 2021, 282, 119540. [Google Scholar] [CrossRef]
- Ye, J.; Yu, Y.; Fan, J.; Cheng, B.; Yu, J.; Ho, W. Room-temperature formaldehyde catalytic decomposition. Environ. Sci. Nano 2020, 7, 3655–3709. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, M.; Huang, Y.; Li, R.; Huang, T.; Cao, J.; Shen, Z.; Lee, S. FeCo alloy encased in nitrogen-doped carbon for efficient formaldehyde removal: Preparation, electronic structure, and d-band center tailoring. J. Hazard. Mater. 2022, 424, 127593. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, F.; Zhai, Y.; Ariga, H.; Yi, N.; Liu, Y.; Asakura, K.; Flytzani-Stephanopoulos, M.; He, H. Alkali-metal-promoted Pt/TiO2 opens a more efficient pathway to formaldehyde oxidation at ambient temperatures. Angew. Chem. Int. Ed. 2012, 51, 9628–9632. [Google Scholar] [CrossRef]
- Xu, Y.; Dhainaut, J.; Dacquin, J.; Mamede, A.; Marinova, M.; Lamonier, J.; Vezin, H.; Zhang, H.; Royer, S. La1−x(Sr, Na, K)xMnO3 perovskites for HCHO oxidation: The role of oxygen species on the catalytic mechanism. Appl. Catal. B 2021, 287, 119955. [Google Scholar] [CrossRef]
- Bai, B.; Qiao, Q.; Li, J.; Hao, J. Progress in research on catalysts for catalytic oxidation of formaldehyde. Chin. J. Catal. 2016, 37, 102–122. [Google Scholar] [CrossRef]
- Nie, L.; Yu, J.; Jaroniec, M.; Tao, F. Room-temperature catalytic oxidation of formaldehyde on catalysts. Catal. Sci. Technol. 2016, 6, 3649–3669. [Google Scholar] [CrossRef]
- Guo, J.; Lin, C.; Jiang, C.; Zhang, P. Review on noble metal-based catalysts for formaldehyde oxidation at room temperature. Appl. Surf. Sci. 2019, 475, 237–255. [Google Scholar] [CrossRef]
- Miao, L.; Wang, J.; Zhang, P. Review on manganese dioxide for catalytic oxidation of airborne formaldehyde. Appl. Surf. Sci. 2019, 466, 441–453. [Google Scholar] [CrossRef]
- Chen, R.; Sun, Z.; Hardacre, C.; Tang, X.; Liu, Z. The current status of research on the catalytic oxidation of formaldehyde. Catal. Rev. 2022. [Google Scholar] [CrossRef]
- Torres, J.; Royer, S.; Bellat, J.; Giraudon, J.; Lamonier, J. Formaldehyde: Catalytic oxidation as a promising soft way of elimination. ChemSusChem 2013, 6, 578–592. [Google Scholar] [CrossRef]
- Shi, K.; Wang, L.; Li, L.; Zhao, X.; Chen, Y.; Hua, Z.; Li, X.; Gu, X.; Li, L. Mild preoxidation treatment of Pt/TiO2 catalyst and its enhanced low temperature formaldehyde decomposition. Catalysts 2019, 9, 694. [Google Scholar] [CrossRef]
- Li, K.; Ji, J.; He, M.; Huang, H. Complete oxidation of formaldehyde over a Pd/CeO2 catalyst at room temperature: Tunable active oxygen species content by non-thermal plasma activation. Catal. Sci. Technol. 2020, 10, 6257–6265. [Google Scholar] [CrossRef]
- Chen, B.; Zhu, X.; Crocker, M.; Wang, Y.; Shi, C. FeOx-supported gold catalysts for catalytic removal of formaldehyde at room temperature. Appl. Catal. B 2014, 154–155, 73–81. [Google Scholar] [CrossRef]
- Sun, X.; Lin, J.; Wang, Y.; Li, L.; Pan, X.; Su, Y.; Wang, X. Catalytically active Ir0 species supported on Al2O3 for complete oxidation of formaldehyde at ambient temperature. Appl. Catal. B 2020, 268, 11741. [Google Scholar] [CrossRef]
- Ye, J.; Zhu, B.; Cheng, B.; Jiang, C.; Wageh, S.; Al-Ghamdi, A.; Yu, J. Synergy between platinum and gold nanoparticles in oxygen activation for enhanced room-temperature formaldehyde oxidation. Adv. Funct. Mater. 2022, 32, 2110423. [Google Scholar] [CrossRef]
- Xiang, N.; Hou, Y.; Han, X.; Li, Y.; Guo, Y.; Liu, Y.; Huang, Z. Promoting effect and mechanism of alkali Na on Pd/SBA-15 for room temperature formaldehyde catalytic oxidation. ChemCatChem 2019, 11, 5098–5107. [Google Scholar] [CrossRef]
- Xiang, N.; Han, X.; Bai, Y.; Li, Q.; Zheng, J.; Li, Y.; Hou, Y.; Huang, Z. Size effect of γ-Al2O3 supports on the catalytic performance of Pd/γ-Al2O3 catalysts for HCHO oxidation. Mol. Catal. 2020, 494, 111112. [Google Scholar] [CrossRef]
- Xiang, N.; Han, X.; Zheng, J.; Li, Q.; Zhao, Q.; Hou, Y.; Huang, Z. Effect of manganese modification on the low-temperature formaldehyde oxidation performance of ZIF-67 derived Co3O4. J. Fuel Chem. Technol. 2022, 50, 859–867. [Google Scholar]
- Xiang, N.; Bai, Y.; Li, Q.; Han, X.; Zheng, J.; Zhao, Q.; Hou, Y.; Huang, Z. ZIF-67-derived hierarchical hollow Co3O4@CoMn2O4 nanocages for efficient catalytic oxidation of formaldehyde at low temperature. Mol. Catal. 2022, 528, 112519. [Google Scholar] [CrossRef]
- Zhu, D.; Huang, Y.; Cao, J.; Lee, S.; Chen, M.; Shen, Z. Cobalt nanoparticles encapsulated in porous nitrogen-doped carbon: Oxygen activation and efficient catalytic removal of formaldehyde at room temperature. Appl. Catal. B 2019, 258, 117981. [Google Scholar] [CrossRef]
- Li, R.; Huang, Y.; Zhu, D.; Ho, W.; Lee, S.; Cao, J. A review of Co3O4-based catalysts for formaldehyde oxidation at low temperature: Effect parameters and reaction mechanism. Aerosol Sci. Eng. 2020, 4, 147–168. [Google Scholar] [CrossRef]
- Fan, Z.; Fang, W.; Zhang, Z.; Chen, M.; Shangguan, W. Highly active rod-like Co3O4 catalyst for the formaldehyde oxidation reaction. Catal. Commun. 2018, 103, 10–14. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, Z.; Fang, W.; Yao, X.; Zou, G.; Shangguan, W. Low-temperature catalytic oxidation of formaldehyde over Co3O4 catalysts prepared using various precipitants. Chin. J. Catal. 2016, 37, 947–954. [Google Scholar] [CrossRef]
- Lv, L.; Su, Y.; Liu, X.; Zheng, H.; Wang, X. Synthesis of cellular-like Co3O4 nanocrystals with controlled structural, electronic and catalytic properties. J. Alloys Compd. 2013, 553, 163–166. [Google Scholar] [CrossRef]
- Bai, B.; Arandiyan, H.; Li, J. Comparison of the performance for oxidation of formaldehyde on nano-Co3O4, 2D-Co3O4, and 3D-Co3O4 catalysts. Appl. Catal. B 2013, 142–143, 677–683. [Google Scholar] [CrossRef]
- Huang, M.; Chen, J.; Tang, H.; Jiao, Y.; Zhang, J.; Wang, G.; Wang, R. Improved oxygen activation over metal-organic-frameworks derived and zinc-modulated Co@NC catalyst for boosting indoor gaseous formaldehyde oxidation at room temperature. J. Colloid Interf. Sci. 2021, 601, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, F.; Chen, C.; Huang, F.; Li, K. Catalytic oxidation of formaldehyde over CeO2-Co3O4 catalysts. J. Rare Earths 2017, 35, 867–874. [Google Scholar] [CrossRef]
- Zhou, M.; Cai, L.; Bajdich, M.; Garcia-Melchor, M.; Li, H.; He, J.; Wilcox, J.; Wu, W.; Vojvodic, A.; Zheng, X. Enhancing catalytic CO oxidation over Co3O4 nanowires by substituting Co2+ with Cu2+. ACS Catal. 2015, 5, 4485–4491. [Google Scholar] [CrossRef]
- Lei, J.; Wang, S.; Li, J.; Xu, Y.; Li, S. Different effect of Y (Y = Cu, Mn, Fe, Ni) doping on Co3O4 derived from Co-MOF for toluene catalytic destruction. Chem. Eng. Sci. 2022, 251, 117436. [Google Scholar] [CrossRef]
- Tu, S.; Chen, Y.; Zhang, X.; Yao, J.; Wu, Y.; Wu, H.; Zhang, J.; Wang, J.; Mu, B.; Li, Z.; et al. Complete catalytic oxidation of formaldehyde at room temperature on MnxCo3−xO4 catalysts derived from metal-organic frameworks. Appl. Catal. A 2021, 611, 117975. [Google Scholar] [CrossRef]
- Xu, W.; Chen, X.; Chen, J.; Jia, H. Bimetal oxide CuO/Co3O4 derived from Cu ions partly-substituted framework of ZIF-67 for toluene catalytic oxidation. J. Hazard. Mater. 2021, 403, 123869. [Google Scholar] [CrossRef]
- Mu, G.; Zeng, Y.; Zheng, Y.; Cao, Y.; Liu, F.; Liang, S.; Zhan, Y.; Jiang, L. Oxygen vacancy defects engineering on Cu-doped Co3O4 for promoting effective COS hydrolysis. Green Energy Environ. 2021; in press. [Google Scholar] [CrossRef]
- Fan, L.; Li, M.; Zhang, C.; Ismail, A.; Hu, B.; Zhu, Y. Effect of Cu/Co ratio in CuaCo1−aOx (a = 0.1, 0.2, 0.4, 0.6) flower structure on its surface properties and catalytic performance for toluene oxidation. J. Colloid Interf. Sci. 2021, 599, 404–415. [Google Scholar] [CrossRef]
- Zhang, W.; Descorme, C.; Valverde, J.; Giroir-Fendler, A. Cu-Co mixed oxide catalysts for the total oxidation of toluene and propane. Catal. Today 2022, 384–386, 238–245. [Google Scholar] [CrossRef]
- Li, R.; Huang, Y.; Zhu, D.; Ho, W.; Cao, J.; Lee, S. Improved oxygen activation over a carbon/Co3O4 nanocomposite for efficient catalytic oxidation of formaldehyde at room temperature. Environ. Sci. Technol. 2021, 55, 4054–4063. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Y.; Wang, W.; Chen, M.; Li, H.; Lee, S.; Ho, W.; Huang, T.; Cao, J. Oxygen vacancy-engineered δ-MnOx/activated carbon for room temperature catalytic oxidation of formaldehyde. Appl. Catal. B 2020, 278, 119294. [Google Scholar] [CrossRef]
- Duan, C.; Yu, Y.; Hu, H. Recent progress on synthesis of ZIF-67-based materials and their application to heterogeneous catalysis. Green Energy Environ. 2022, 7, 3–15. [Google Scholar] [CrossRef]
- Wu, R.; Qian, X.; Rui, X.; Liu, H.; Yadian, B.; Zhou, K.; Wei, J.; Yan, Q.; Feng, X.; Long, Y.; et al. Zeolitic imidazolate framework 67-derived high symmetric porous Co3O4 hollow dodecahedra with highly enhanced lithium storage capability. Small 2014, 10, 1932–1938. [Google Scholar] [CrossRef]
- Hu, H.; Guan, B.; Xia, B.; Lou, X.W. Designed formation of Co3O4/NiCo2O4 double-shelled nanocages with enhanced pseudocapacitive and electrocatalytic properties. J. Am. Chem. Soc. 2015, 137, 5590–5595. [Google Scholar] [CrossRef]
- Hu, H.; Guan, B.; Lou, X.W. Construction of complex CoS hollow structures with enhanced electrochemical properties for hybrid supercapacitors. Chem 2016, 1, 102–113. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, J.; Yan, D.; Li, Y.; Jia, H.; Lu, C. Enhanced catalytic activities of MnOx/Co3O4 nanocomposites prepared via MOFs-templated approach for chlorobenzene oxidation. Appl. Surf. Sci. 2021, 551, 149453. [Google Scholar] [CrossRef]
- Daté, M.; Okumura, M.; Tsubota, S.; Haruta, M. Vital role of moisture in the catalytic activity of supported gold nanoparticles. Angew. Chem. 2004, 116, 2181–2184. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, L.; Ma, J.; Wang, H.; Zhang, C.; Deng, H.; He, H. Investigation into the enhanced catalytic oxidation of o-xylene over MOF-derived Co3O4 with different shapes: The role of surface twofold-coordinate lattice oxygen (O2f). ACS Catal. 2021, 11, 6614–6625. [Google Scholar] [CrossRef]
- Aadil, M.; Nazik, G.; Zulfiqar, S.; Shakir, I.; Aboud, M.; Agboola, P.; Haider, S.; Warsi, M. Fabrication of nickel foam supported Cu-doped Co3O4 nanostructures for electrochemical energy storage applications. Ceram. Int. 2021, 47, 9225–9233. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Zhang, L.; Jiang, D. Surface oxygen vacancies on Co3O4 mediated catalytic formaldehyde oxidation at room temperature. Catal. Sci. Technol. 2016, 6, 3845–3853. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, X.; Zhang, F.; Tian, T.; Ding, Y.; Gao, H. Design and synthesis of Cu modified cobalt oxides with hollow polyhedral nanocages as efficient electrocatalytic and photocatalytic water oxidation catalysts. J. Catal. 2017, 352, 246–255. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, Z.; Li, Y.; Hou, Y.; Hu, J.; Huang, Z. Effect of the A-site cation over spinel AMn2O4 (A = Cu2+, Ni2+, Zn2+) for toluene combustion: Enhancement of the synergy and the oxygen activation ability. Fuel 2021, 288, 119700. [Google Scholar] [CrossRef]
- Hernández, W.; Centeno, M.; Ivanova, S.; Eloy, P.; Gaigneaux, E.; Odriozola, J. Cu-modified cryptomelane oxide as active catalyst for CO oxidation reactions. Appl. Catal. B 2012, 123–124, 27–35. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, G.; Zhang, P. Layered birnessite-type MnO2 with surface pits for enhanced catalytic formaldehyde oxidation activity. J. Mater. Chem. A 2017, 5, 5719–5725. [Google Scholar] [CrossRef]
- Zhao, J.; Tang, Z.; Dong, F.; Zhang, J. Controlled porous hollow Co3O4 polyhedral nanocages derived from metal-organic frameworks (MOFs) for toluene catalytic oxidation. Mol. Catal. 2019, 463, 77–86. [Google Scholar] [CrossRef]
- Fierro, G.; Jacono, M.; Inversi, M.; Dragone, R.; Porta, P. TPR and XPS study of cobalt-copper mixed oxide catalysts: Evidence of a strong Co-Cu interaction. Top. Catal. 2000, 10, 39–48. [Google Scholar] [CrossRef]
- Chen, D.; Qu, Z.; Sun, Y.; Gao, K.; Wang, Y. Identification of reaction intermediates and mechanism responsible for highly active HCHO oxidation on Ag/MCM-41 catalysts. Appl. Catal. B 2013, 142–143, 838–848. [Google Scholar] [CrossRef]
- Zhang, C.; He, H.; Tanaka, K.-I. Catalytic performance and mechanism of a Pt/TiO2 catalyst for the oxidation of formaldehyde at room temperature. Appl. Catal. B 2006, 65, 37–43. [Google Scholar] [CrossRef]
- Sun, X.; Lin, J.; Guan, H.; Li, L.; Sun, L.; Wang, Y.; Miao, S.; Su, Y.; Wang, X. Complete oxidation of formaldehyde over TiO2 supported subnanometer Rh catalyst at ambient temperature. Appl. Catal. B 2018, 226, 575–584. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Jiang, C.; Zhou, P.; Zhang, P.; Yu, J. The effect of manganese vacancy in birnessite-type MnO2 on room-temperature oxidation of formaldehyde in air. Appl. Catal. B 2017, 204, 147–155. [Google Scholar] [CrossRef]
- Yang, T.; Huo, Y.; Liu, Y.; Rui, Z.; Ji, H. Efficient formaldehyde oxidation over nickel hydroxide promoted Pt/γ-Al2O3 with a low Pt content. Appl. Catal. B 2017, 200, 5. [Google Scholar] [CrossRef]
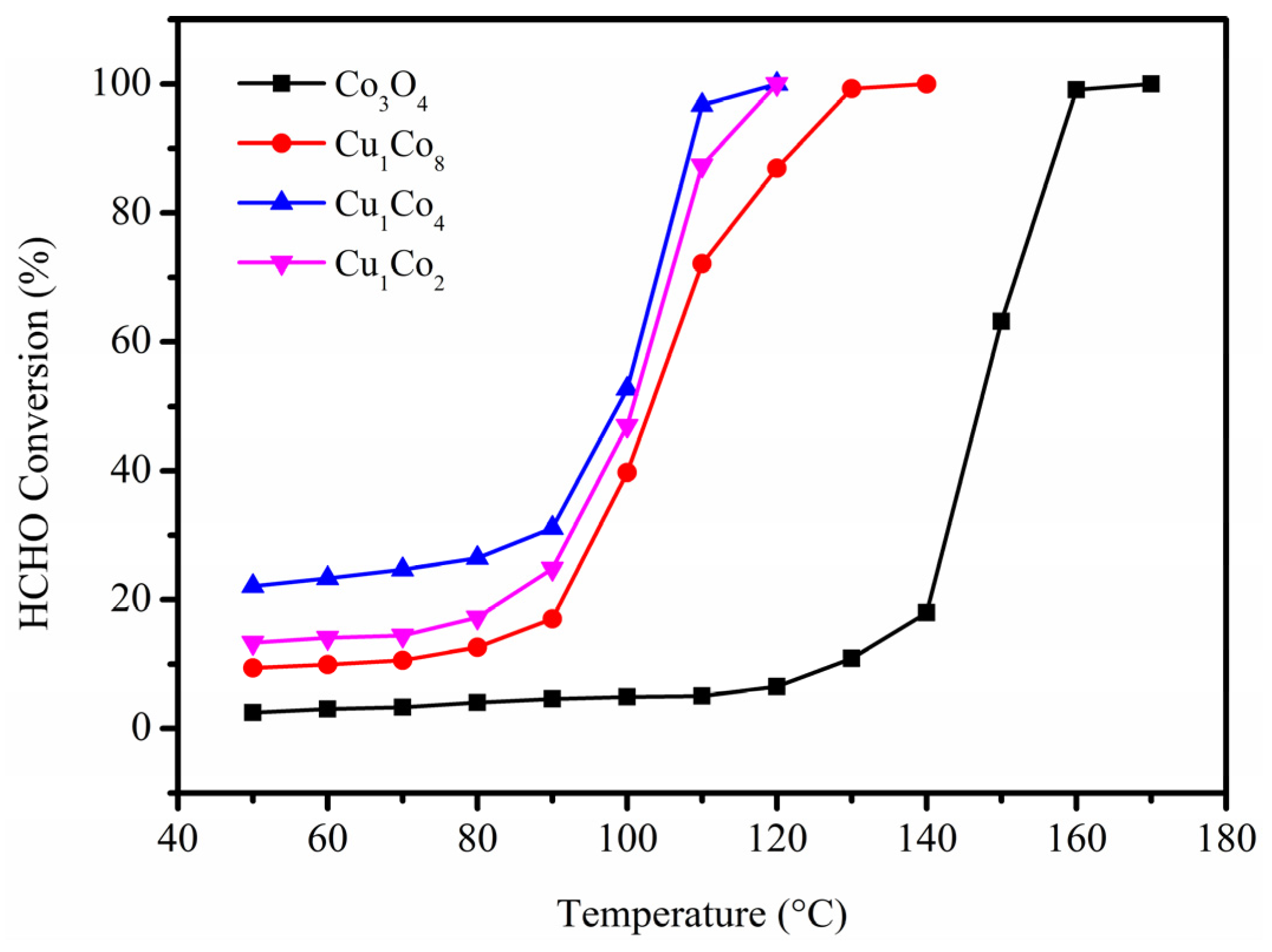




| Catalyst | T50 (°C) | T90 (°C) |
|---|---|---|
| Co3O4 | 147 | 157 |
| Cu1Co8 | 103 | 122 |
| Cu1Co4 | 98 | 108 |
| Cu1Co2 | 101 | 112 |
| Catalyst | Crystallite Size (nm) | SBET (m2/g) | Vp (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|---|
| Co3O4 | 18 | 43 | 0.22 | 20 |
| Cu1Co8 | 15 | 37 | 0.18 | 20 |
| Cu1Co4 | 14 | 44 | 0.20 | 18 |
| Cu1Co2 | 17 | 32 | 0.15 | 19 |
| Catalyst | Co3+/Co2+ | Cu+/Cu2+ | Oα/Oβ |
|---|---|---|---|
| Co3O4 | 0.53 | - | 0.33 |
| Cu1Co8 | 0.59 | 0.16 | 0.51 |
| Cu1Co4 | 0.78 | 0.29 | 0.55 |
| Cu1Co2 | 0.69 | 0.21 | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Xiang, N.; Wen, S.; Huo, H.; Li, Q. ZIF-67 Derived Cu-Co Mixed Oxides for Efficient Catalytic Oxidation of Formaldehyde at Low-Temperature. Catalysts 2023, 13, 117. https://doi.org/10.3390/catal13010117
Zhao Q, Xiang N, Wen S, Huo H, Li Q. ZIF-67 Derived Cu-Co Mixed Oxides for Efficient Catalytic Oxidation of Formaldehyde at Low-Temperature. Catalysts. 2023; 13(1):117. https://doi.org/10.3390/catal13010117
Chicago/Turabian StyleZhao, Qingsong, Ning Xiang, Shiting Wen, Haibo Huo, and Qiaoyan Li. 2023. "ZIF-67 Derived Cu-Co Mixed Oxides for Efficient Catalytic Oxidation of Formaldehyde at Low-Temperature" Catalysts 13, no. 1: 117. https://doi.org/10.3390/catal13010117
APA StyleZhao, Q., Xiang, N., Wen, S., Huo, H., & Li, Q. (2023). ZIF-67 Derived Cu-Co Mixed Oxides for Efficient Catalytic Oxidation of Formaldehyde at Low-Temperature. Catalysts, 13(1), 117. https://doi.org/10.3390/catal13010117





