Multienzyme Synthesis of Glycyrrhetic Acid 3-O-mono-β-d-glucuronide by Coupling UGT73F15 to UDP-Glucuronic Acid Regeneration Module
Abstract
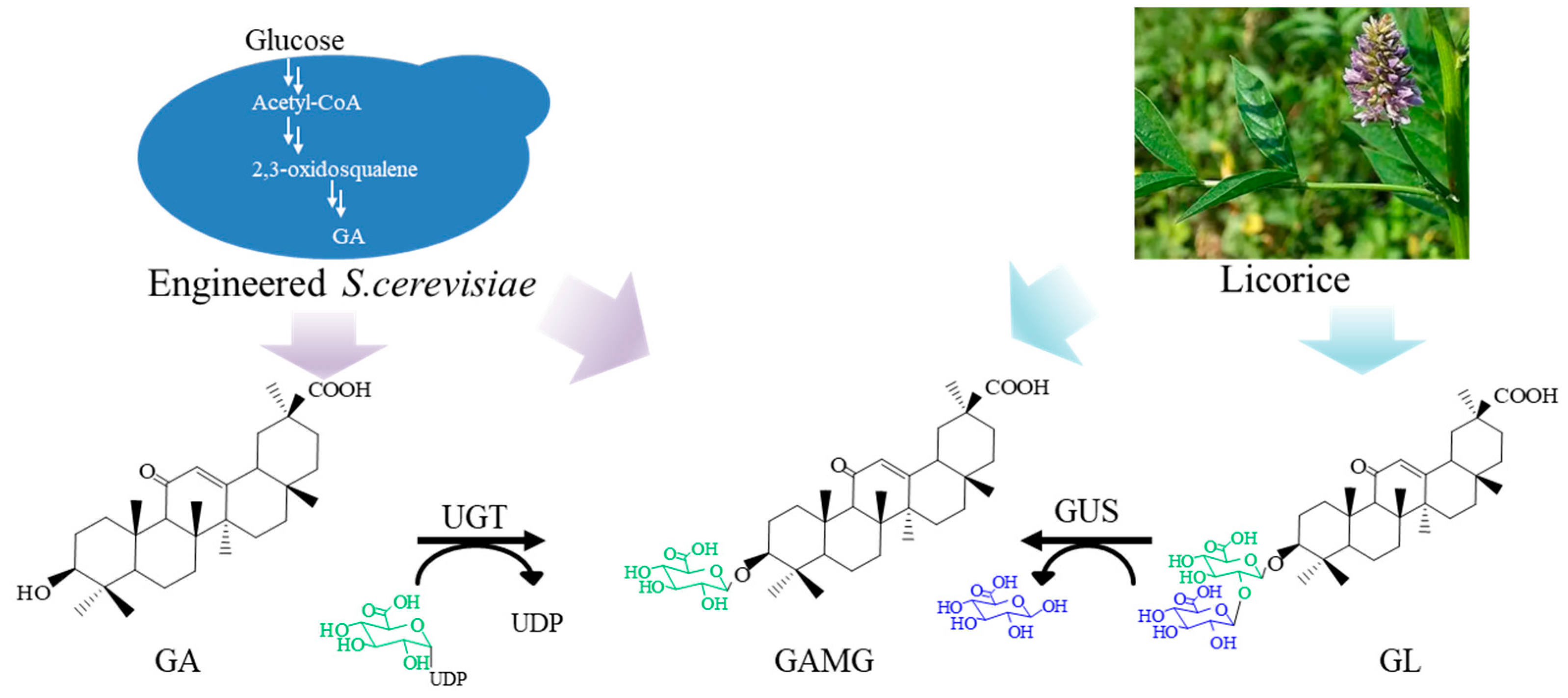
1. Introduction
| No | Enzymes | Substrates 1 | Sugar-Donor | Ref. |
|---|---|---|---|---|
| 1 | UGT73C11 | GA | UDPG | [15] |
| 2 | UGT73C33 | GA | UDPG | [16] |
| 3 | UGT73F24 | GA | UDPG | [16] |
| 4 | Bs-Yjic | GA/GL | UDPG | [17] |
| 5 | GmSGT2 | GAMG/GLMG | UDP-Gal | [18] |
| 6 | UGT109A3 | GA | UDPG | [24] |
| 7 | UGT73F17 | GL | UDPG, UDP-Xyl, UDP-Gal, and UDP-Ara | [25] |
| 8 | GuUGAT | GA | UDPGlcA | [26] |
| 9 | GuUGT | GA | UDPG | [27] |
| 10 | UGT73P12 | GAMG | UDPG/ UDPGlcA | [28] |
| 11 | GuGT14 | GA | UDPG/ UDPGlcA | [20] |
| 12 | GuGT33 | GA | UDPG | [20] |
| 13 | GuCsl | GA | UDPGlcA | [19] |
| 14 | Csls | GA | UDPGlcA | [19] |
| 15 | UGT1A1 | GA/GAMG | UDPGlcA | [22] |
2. Results
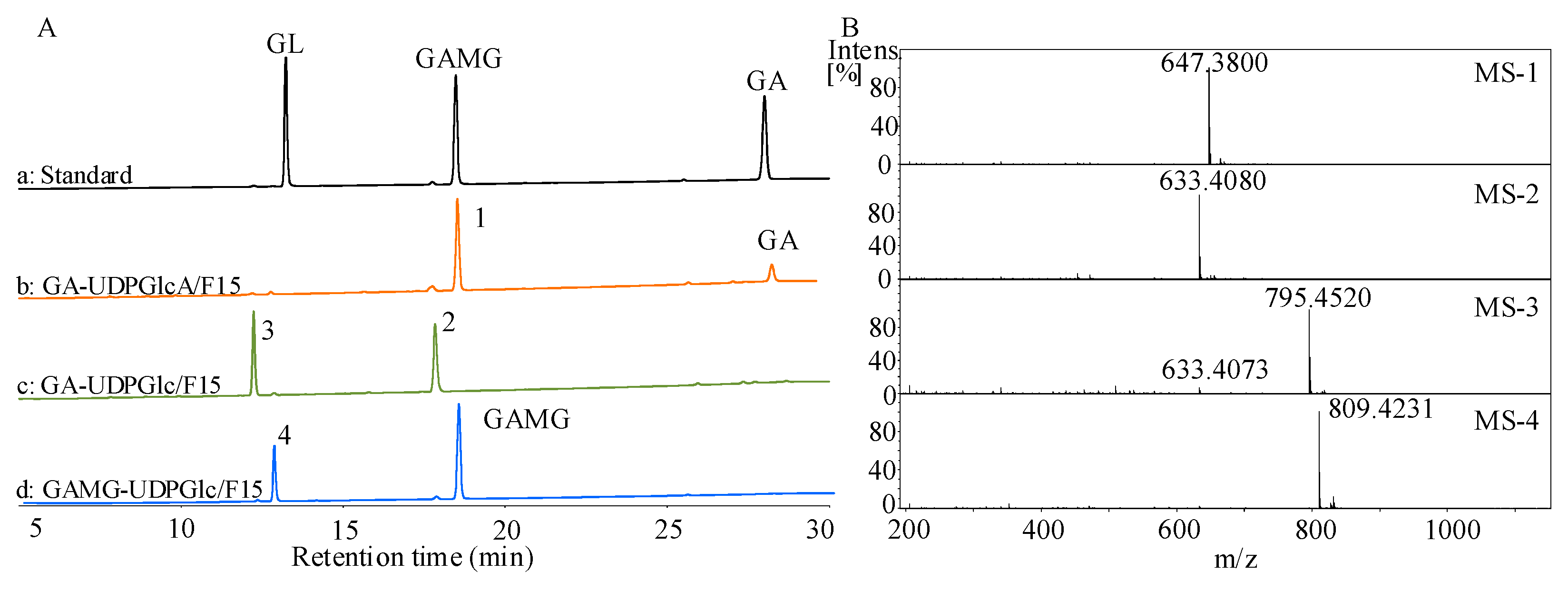
2.1. Expression and Characterization of UGT73F15
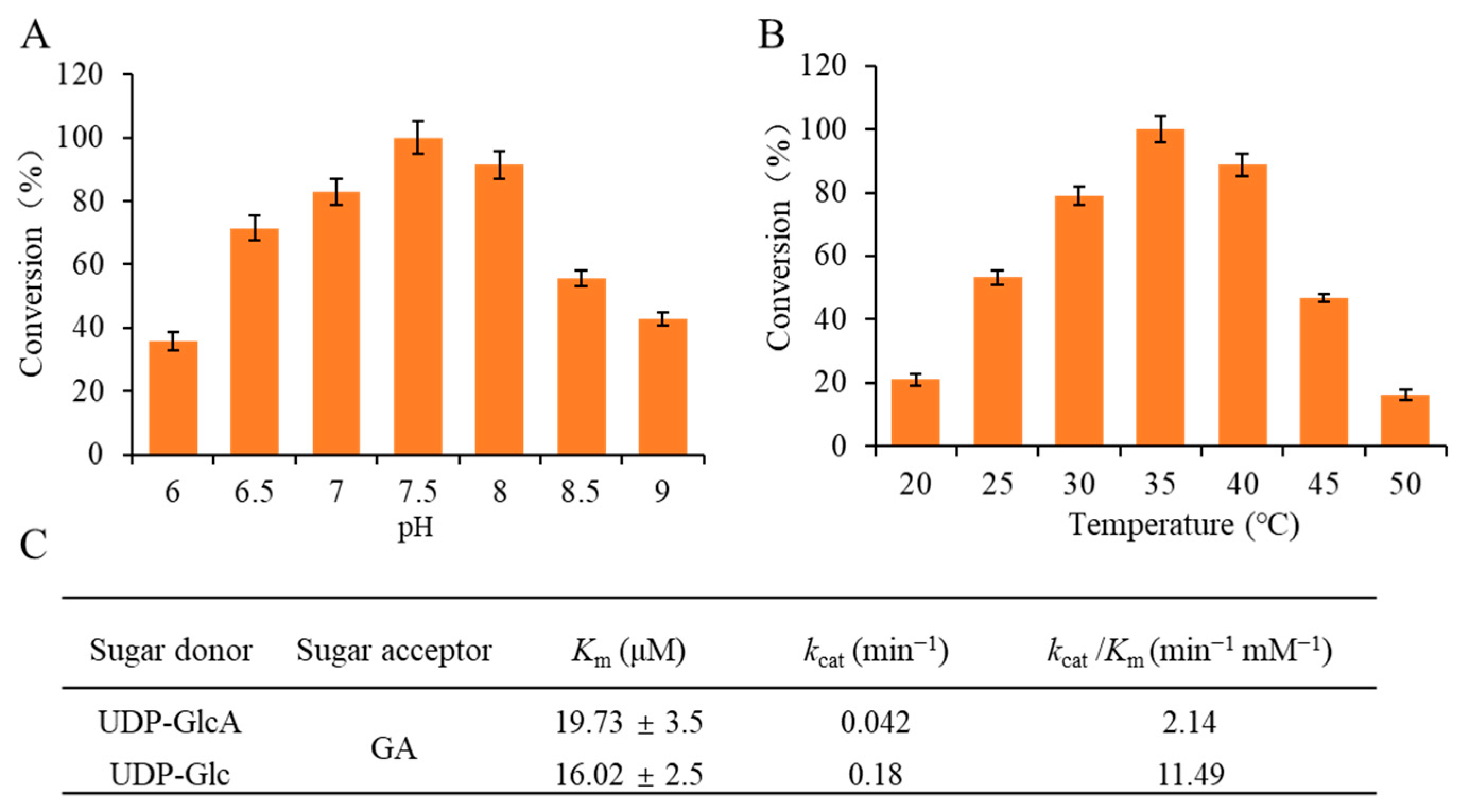
2.2. Enzymatic Properties of UGT73F15
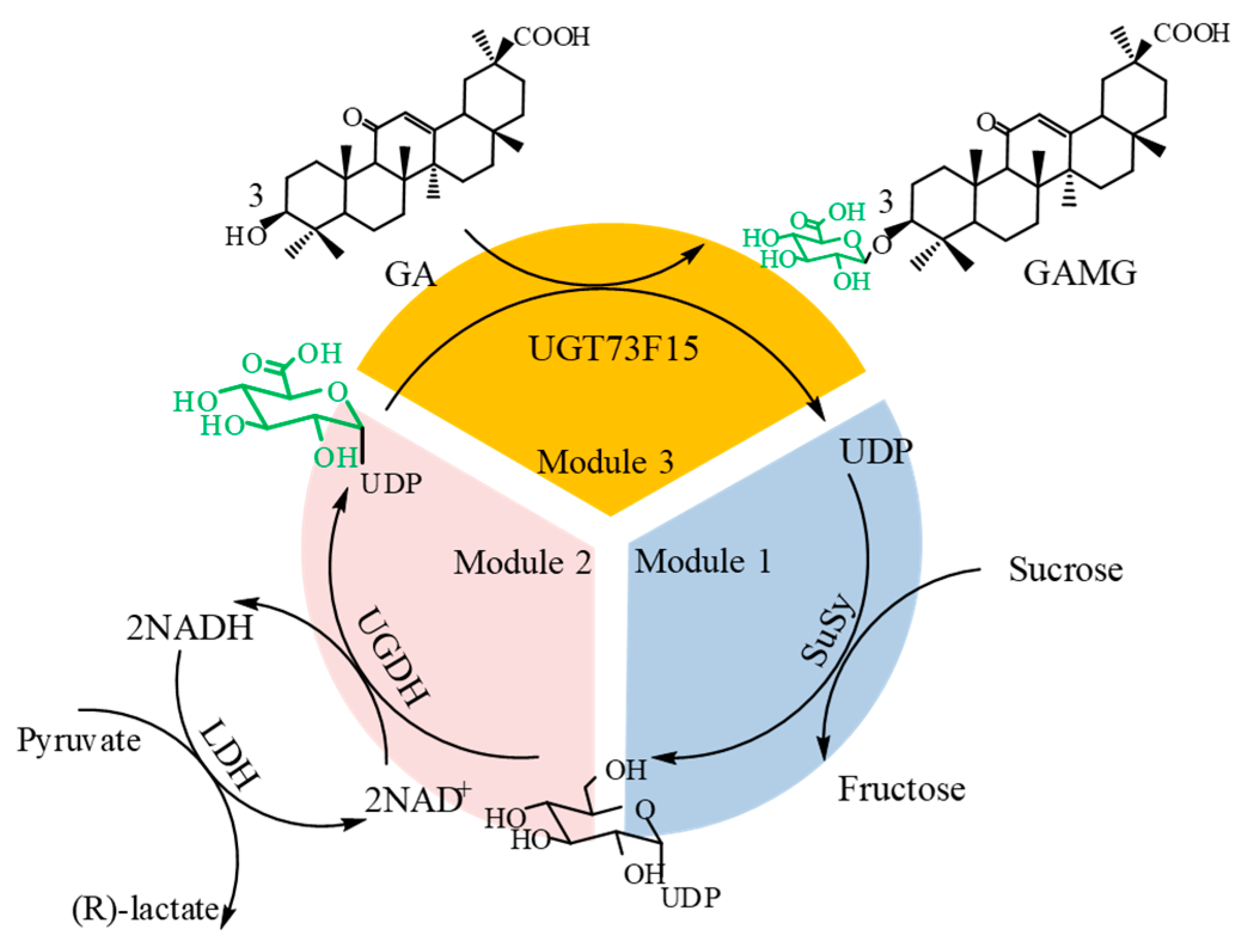
2.3. Construction of Multienzyme System for GAMG Synthesis
2.4. Optimizing the Parameters of the Enzyme Module System
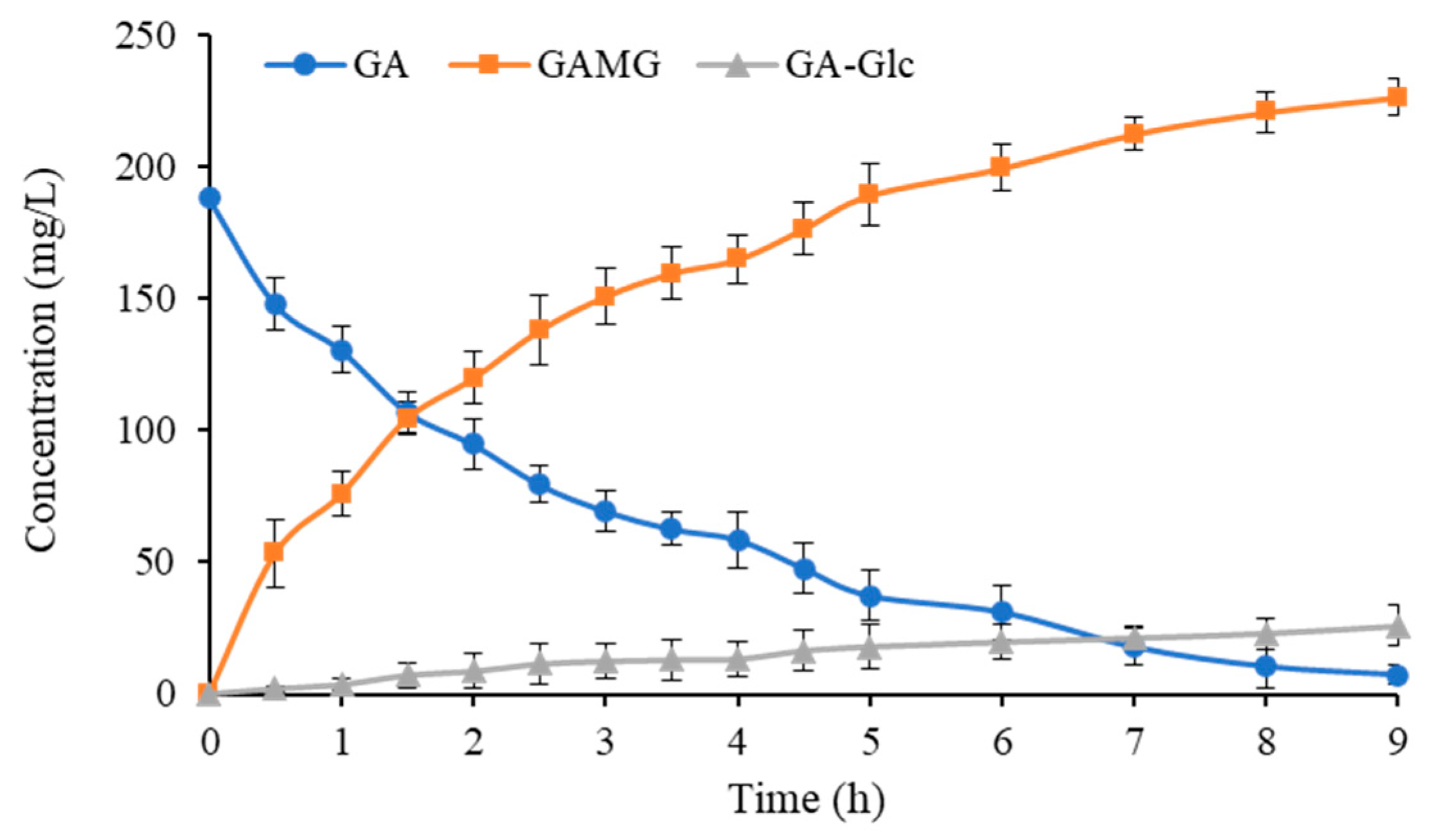
2.5. Biosynthesis of GAMG by One-Pot Cascade Reaction
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Heterologous Expression and Purification of Enzymes
4.3. Enzyme Activity Assays
4.4. Kinetic Parameters of UGTs
4.5. Homology Modeling and Molecular Docking
4.6. Cascade Reaction Optimization
4.7. Biotransformation of GA by Cascade Reaction
4.8. Analytical Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Matsumoto, K.; Kasai, R.; Ohtani, K.; Tanaka, O. Minor cucurbitane-glycosides from fruits of Siraitia grosvenori (Cucurbitaceae). Himalayan Chem. Pharm. Bull. 1990, 38, 2030–2032. [Google Scholar] [CrossRef]
- Ceunen, S.; Geuns, J.M.C. Steviol glycosides: Chemical diversity, metabolism, and function. J. Nat. Prod. 2013, 76, 1201–1228. [Google Scholar] [CrossRef]
- Kitagawa, I. Licorice root. A natural sweetener and an important ingredient in Chinese medicine. Pure Appl. Chem. 2002, 74, 1189–1198. [Google Scholar] [CrossRef]
- Roohbakhsh, A.; Iranshahy, M.; Iranshahi, M. Glycyrrhetinic acid and its derivatives: Anti-cancer and cancer chemopreventive properties, mechanisms of action and structure-cytotoxic activity relationship. Curr. Med. Chem. 2016, 23, 498–517. [Google Scholar] [CrossRef] [PubMed]
- Dastagir, G.; Rizvi, M.A. Review—Glycyrrhiza glabra L. (liquorice). Pak. J. Pharm. Sci. 2016, 29, 1727–1733. [Google Scholar] [PubMed]
- Akao, T. Difference in the metabolism of glycyrrhizin, glycyrrhetic acid and glycyrrhetic acid monoglucuronide by human intestinal flora. Biol. Pharm. Bull. 2000, 23, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Homma, M. Education program of kampo-medicine for undergraduates in preparation for clinical setting. Yakugaku Zasshi J. Pharm. Soc. Jpn. 2016, 136, 417–422. [Google Scholar] [CrossRef][Green Version]
- Wang, C.; Guo, X.X.; Wang, X.Y.; Qi, F.; Feng, S.J.; Li, C.; Zhou, X.H. Isolation and characterization of three fungi with the potential of transforming glycyrrhizin. World J. Microbiol. Biotechnol. 2013, 29, 781–788. [Google Scholar] [CrossRef]
- Mizutani, K.; Kuramoto, T.; Tamura, Y.; Ohtake, N.; Doi, S.; Nakaura, M.; Tanaka, O. Sweetness of glycyrrhetic acid 3-O-β-d-monoglucuronide and the related glycosides. Biosci. Biotechnol. Biochem. 1994, 58, 554–555. [Google Scholar] [CrossRef]
- Edwards, C.H.; Rossi, M.; Corpe, C.P.; Butterworth, P.J.; Ellis, P.R. The role of sugars and sweeteners in food, diet and health: Alternatives for the future. Trends Food Sci. Technol. 2016, 56, 158–166. [Google Scholar] [CrossRef]
- Feng, S.J.; Li, C.; Xu, X.L.; Wang, X.Y. Screening strains for directed biosynthesis of β-d-mono-glucuronide-glycyrrhizin and kinetics of enzyme production. J. Mol. Catal. B Enzym. 2006, 43, 63–67. [Google Scholar] [CrossRef]
- Kaleem, I.; Shen, H.; Lv, B.; Wei, B.; Rasool, A.; Li, C. Efficient biosynthesis of glycyrrhetic acid 3-O-mono-β-d-glucuronide (GAMG) in water-miscible ionic liquid by immobilized whole cells of Penicillium purpurogenum Li-3 in alginate gel. Chem. Eng. Sci. 2014, 106, 136–143. [Google Scholar] [CrossRef]
- Wang, C.; Su, X.; Sun, M.; Zhang, M.; Wu, J.; Xing, J.; Wang, Y.; Xue, J.; Liu, X.; Sun, W.; et al. Efficient production of glycyrrhetinic acid in metabolically engineered Saccharomyces cerevisiae via an integrated strategy. Microb. Cell Fact. 2019, 18, 1–15. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, C.; Sun, W.; Zhou, A.; Wang, Y.; Zhang, G.; Li, C. Boosting 11-oxo-β-amyrin and glycyrrhetinic acid synthesis in Saccharomyces cerevisiae via pairing novel oxidation and reduction system from legume plants. Metab. Eng. 2018, 45, 43–50. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Feng, X.; Lv, B.; Li, C. Biosynthesis of glycyrrhetinic acid-3-O-monoglucose using glycosyltransferase UGT73C11 from Barbarea vulgaris. Ind. Eng. Chem. Res. 2017, 56, 14949–14958. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, S.; Liu, X.; Liu, X.; Guo, F.; Sun, W.; Li, C. Mining of UDP-glucosyltrfansferases in licorice for controllable glycosylation of pentacyclic triterpenoids. Biotechnol. Bioeng. 2020, 117, 3651–3663. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Li, J.; Yang, J.; Men, Y.; Zeng, Y.; Cai, Y.; Sun, Y. Enzymatic synthesis of novel glycyrrhizic acid glucosides using a promiscuous Bacillus glycosyltransferase. Catalysts 2018, 8, 615. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, L.; Feng, X.; Liu, X.; Guo, F.; Lv, B.; Li, C. Galactosylation of monosaccharide derivatives of glycyrrhetinic acid by UDP-glycosyltransferase GmSGT2 from Glycine max. J. Agric. Food Chem. 2020, 68, 8580–8588. [Google Scholar] [CrossRef]
- Jozwiak, A.; Sonawane, P.D.; Panda, S.; Garagounis, C.; Papadopoulou, K.K.; Abebie, B.; Aharoni, A. Plant terpenoid metabolism co-opts a component of the cell wall biosynthesis machinery. Nat. Chem. Biol. 2020, 16, 740–748. [Google Scholar] [CrossRef]
- Chen, K.; Hu, Z.M.; Song, W.; Wang, Z.L.; He, J.B.; Shi, X.M.; Ye, M. Diversity of O-glycosyltransferases contributes to the biosynthesis of flavonoid and triterpenoid glycosides in Glycyrrhiza uralensis. ACS Synth. Biol. 2019, 8, 1858–1866. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, D.; Ren, G.; Yin, Y.; Sun, Y.; Liu, T.; Liu, C. De Novo Production of Glycyrrhetic Acid 3-O-mono-β-d-glucuronide in Saccharomyces cerevisiae. Front. Bioeng. Biotech. 2021, 9, 709120. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zhao, Y.J.; Ahmad, N.; Wang, J.N.; Lv, B.; Wang, Y.; Li, C. O-glycosyltransferases from Homo sapiens contributes to the biosynthesis of glycyrrhetic acid 3-O-mono-β-d-glucuronide and glycyrrhizin in Saccharomyces cerevisiae. Synth. Syst. Biotechnol. 2021, 6, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Engels, L.; Henze, M.; Hummel, W.; Elling, L. Enzyme Module Systems for the Synthesis of Uridine 5′-Diphospho-α-d-glucuronic Acid and Non-Sulfated Human Natural Killer Cell-1 (HNK-1) Epitope. Adv. Synth. Catal. 2015, 357, 1751–1762. [Google Scholar] [CrossRef]
- Ali, M.Y.; Chang, Q.; Yan, Q.; Qian, Z.; Guo, X.; Thow, K.; Wu, J.; Zhang, Y.; Feng, Y. Highly Efficient Biosynthesis of Glycyrrhetinic Acid Glucosides by Coupling of Microbial Glycosyltransferase to Plant Sucrose Synthase. Front. Bioeng. Biotechnol. 2021, 9, 645079. [Google Scholar] [CrossRef]
- He, J.; Chen, K.; Hu, Z.M.; Li, K.; Song, W.; Yu, L.Y.; Ye, M. UGT73F17, a new glycosyltransferase from Glycyrrhiza uralensis, catalyzes the regiospecific glycosylation of pentacyclic triterpenoids. Chem. Commun. 2018, 54, 8594–8597. [Google Scholar] [CrossRef]
- Xu, G.J.; Cai, W.; Gao, W.; Liu, C.S. A novel glucuronosyltransferase has an unprecedented ability to catalyse continuous two-step glucuronosylation of glycyrrhetinic acid to yield glycyrrhizin. New Phytol. 2016, 212, 123–135. [Google Scholar] [CrossRef]
- Huang, Y.; Li, D.; Wang, J.; Cai, Y.; Dai, Z.; Jiang, D.; Liu, C. GuUGT, a glycosyltransferase from Glycyrrhiza uralensis, exhibits glycyrrhetinic acid 3-and 30-O-glycosylation. R. Soc. Open Sci. 2019, 6, 191121. [Google Scholar] [CrossRef]
- Nomura, Y.; Seki, H.; Suzuki, T.; Ohyama, K.; Mizutani, M.; Kaku, T.; Muranaka, T. Functional specialization of UDP-glycosyltransferase 73P12 in licorice to produce a sweet triterpenoid saponin, glycyrrhizin. Plant J. 2019, 99, 1127–1143. [Google Scholar] [CrossRef]
- Li, J.; Yang, J.; Mu, S.; Shang, N.; Liu, C.; Zhu, Y.; Ma, Y. Efficient O-glycosylation of triterpenes enabled by protein engineering of plant glycosyltransferase UGT74AC1. ACS Catal. 2020, 10, 3629–3639. [Google Scholar] [CrossRef]
- Huang, W.; He, Y.; Jiang, R.; Deng, Z.; Long, F. Functional and Structural Dissection of a Plant Steroid 3-O-Glycosyltransferase Facilitated the Engineering Enhancement of Sugar Donor Promiscuity. ACS Catal. 2022, 12, 2927–2937. [Google Scholar] [CrossRef]
- Song, W.; Qiao, X.; Chen, K.; Wang, Y.; Ji, S.; Feng, J.; Ye, M. Biosynthesis-based quantitative analysis of 151 secondary metabolites of licorice to differentiate medicinal Glycyrrhiza species and their hybrids. Anal. Chem. 2017, 89, 3146–3153. [Google Scholar] [CrossRef] [PubMed]
- Szakiel, A.; Pączkowski, C.; Henry, M. Influence of environmental biotic factors on the content of saponins in plants. Phytochem. Rev. 2011, 10, 493–502. [Google Scholar] [CrossRef]
- Guo, L.; Katiyo, W.; Lu, L.; Zhang, X.; Wang, M.; Yan, J.; Zhao, W. Glycyrrhetic Acid 3-O-mono-β-d-glucuronide (GAMG): An Innovative High-Potency Sweetener with Improved Biological Activities. Compr. Rev. Food Sci. Food Saf. 2018, 17, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Caputi, L.; Malnoy, M.; Goremykin, V.; Nikiforova, S.; Martens, S. A genome-wide phylogenetic reconstruction of family 1 UDP-glycosyltransferases revealed the expansion of the family during the adaptation of plants to life on land. Plant J. 2012, 69, 1030–1042. [Google Scholar] [CrossRef]
- Rahimi, S.; Kim, J.; Mijakovic, I.; Jung, K.H.; Choi, G.; Kim, S.C.; Kim, Y.J. Triterpenoid-biosynthetic UDP-glycosyltransferases from plants. Biotechnol. Adv. 2019, 37, 107394. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Liu, C.; Li, J.; Dong, C.; Yang, J.; Dai, Z.; Sun, Y. One-pot synthesis of ginsenoside Rh2 and bioactive unnatural ginsenoside by coupling promiscuous glycosyltransferase from Bacillus subtilis 168 to sucrose synthase. J. Agric. Food Chem. 2018, 66, 2830–2837. [Google Scholar] [CrossRef]
- Chu, X.; Han, J.; Guo, D.; Fu, Z.; Liu, W.; Tao, Y. Characterization of UDP-glucose dehydrogenase from Pasteurella multocida CVCC 408 and its application in hyaluronic acid biosynthesis. Enzyme Microb. Technol. 2016, 85, 64–70. [Google Scholar] [CrossRef]
- Yang, G.; Tian, J.; Li, J. Fermentation of 1,3-propanediol by a lactate deficient mutant of Klebsiella oxytoca under microaerobic conditions. Appl. Microbiol. Biotechnol. 2007, 73, 1017–1024. [Google Scholar] [CrossRef]


| Enzyme | Source | GenBank | MW (kDa) | Activity (mU/mg) 1 |
|---|---|---|---|---|
| AcSusy | A. caldus | KP284426.1 | 91.2 | 130 |
| UGDH | E. coli | ACT43781 | 60.5 | 26 |
| LDH | E. coli | AVI55897.1 | 53.4 | 370 |
| UGT73F15 | Licorice | QDM38902.1 | 74.5 | 4.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Chen, T.; Zhang, X.; Yang, J.; Zeng, Y.; Men, Y.; Sun, Y. Multienzyme Synthesis of Glycyrrhetic Acid 3-O-mono-β-d-glucuronide by Coupling UGT73F15 to UDP-Glucuronic Acid Regeneration Module. Catalysts 2023, 13, 104. https://doi.org/10.3390/catal13010104
Li J, Chen T, Zhang X, Yang J, Zeng Y, Men Y, Sun Y. Multienzyme Synthesis of Glycyrrhetic Acid 3-O-mono-β-d-glucuronide by Coupling UGT73F15 to UDP-Glucuronic Acid Regeneration Module. Catalysts. 2023; 13(1):104. https://doi.org/10.3390/catal13010104
Chicago/Turabian StyleLi, Jiao, Taiyan Chen, Xuewen Zhang, Jiangang Yang, Yan Zeng, Yan Men, and Yuanxia Sun. 2023. "Multienzyme Synthesis of Glycyrrhetic Acid 3-O-mono-β-d-glucuronide by Coupling UGT73F15 to UDP-Glucuronic Acid Regeneration Module" Catalysts 13, no. 1: 104. https://doi.org/10.3390/catal13010104
APA StyleLi, J., Chen, T., Zhang, X., Yang, J., Zeng, Y., Men, Y., & Sun, Y. (2023). Multienzyme Synthesis of Glycyrrhetic Acid 3-O-mono-β-d-glucuronide by Coupling UGT73F15 to UDP-Glucuronic Acid Regeneration Module. Catalysts, 13(1), 104. https://doi.org/10.3390/catal13010104





