Application of Floating TiO2 Photocatalyst for Methylene Blue Decomposition and Salmonella typhimurium Inactivation
Abstract
:1. Introduction
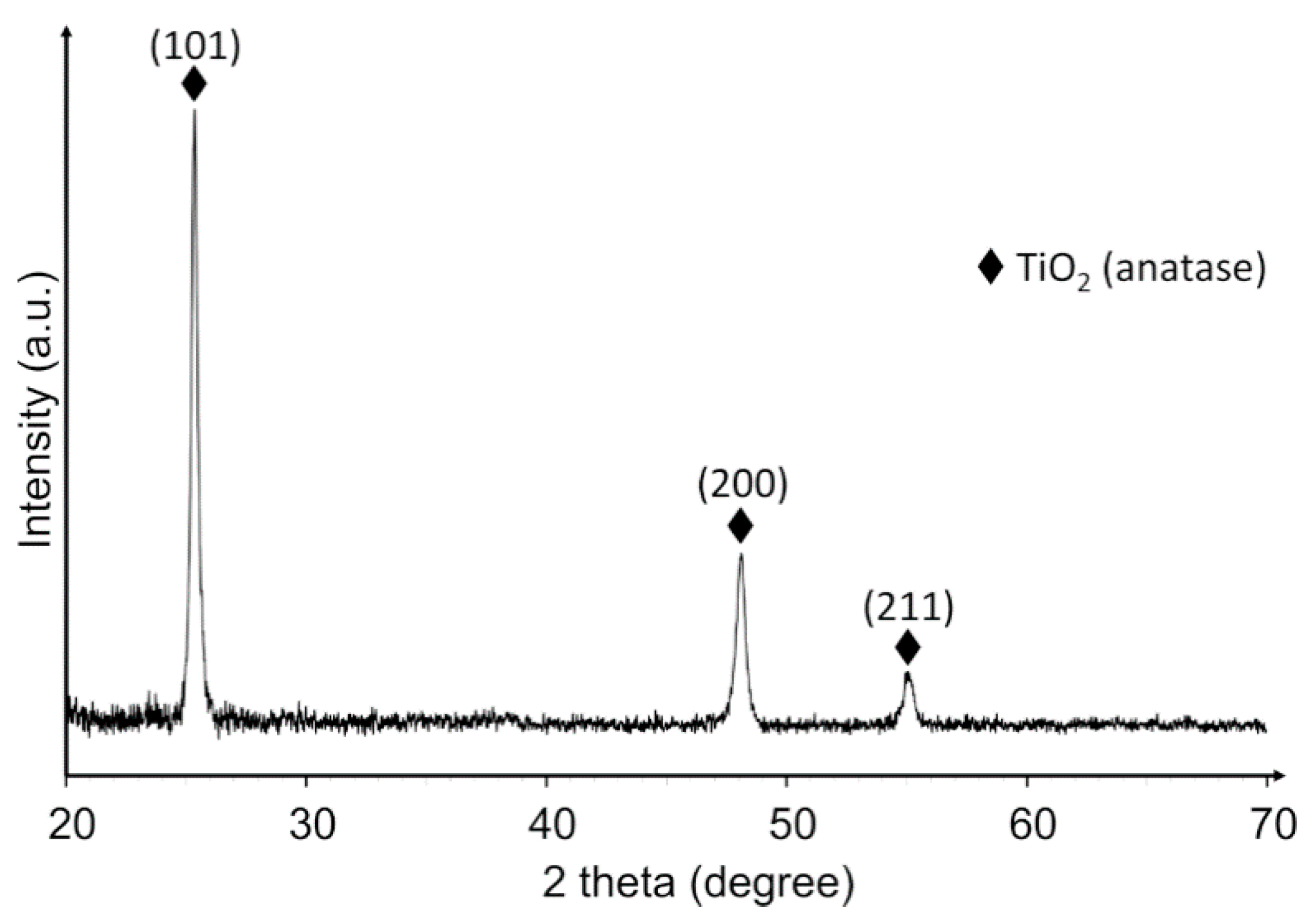
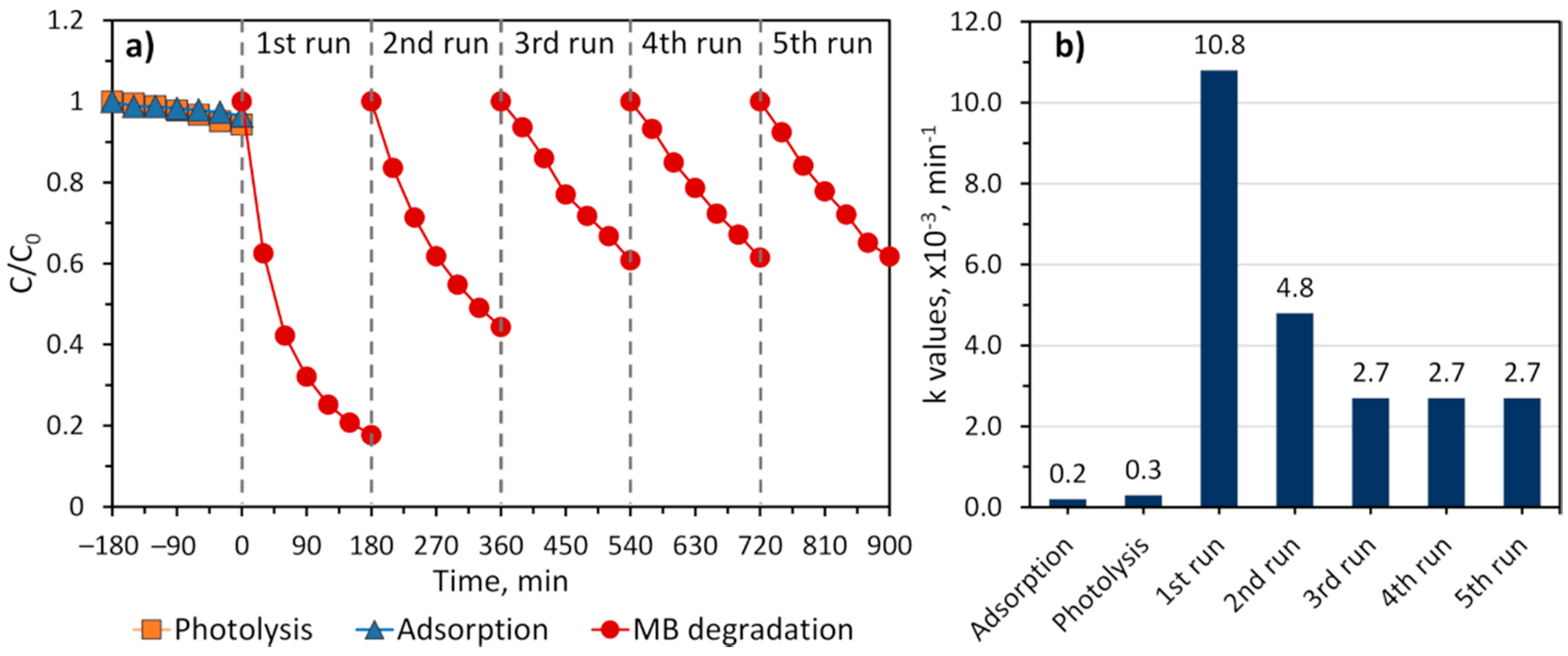
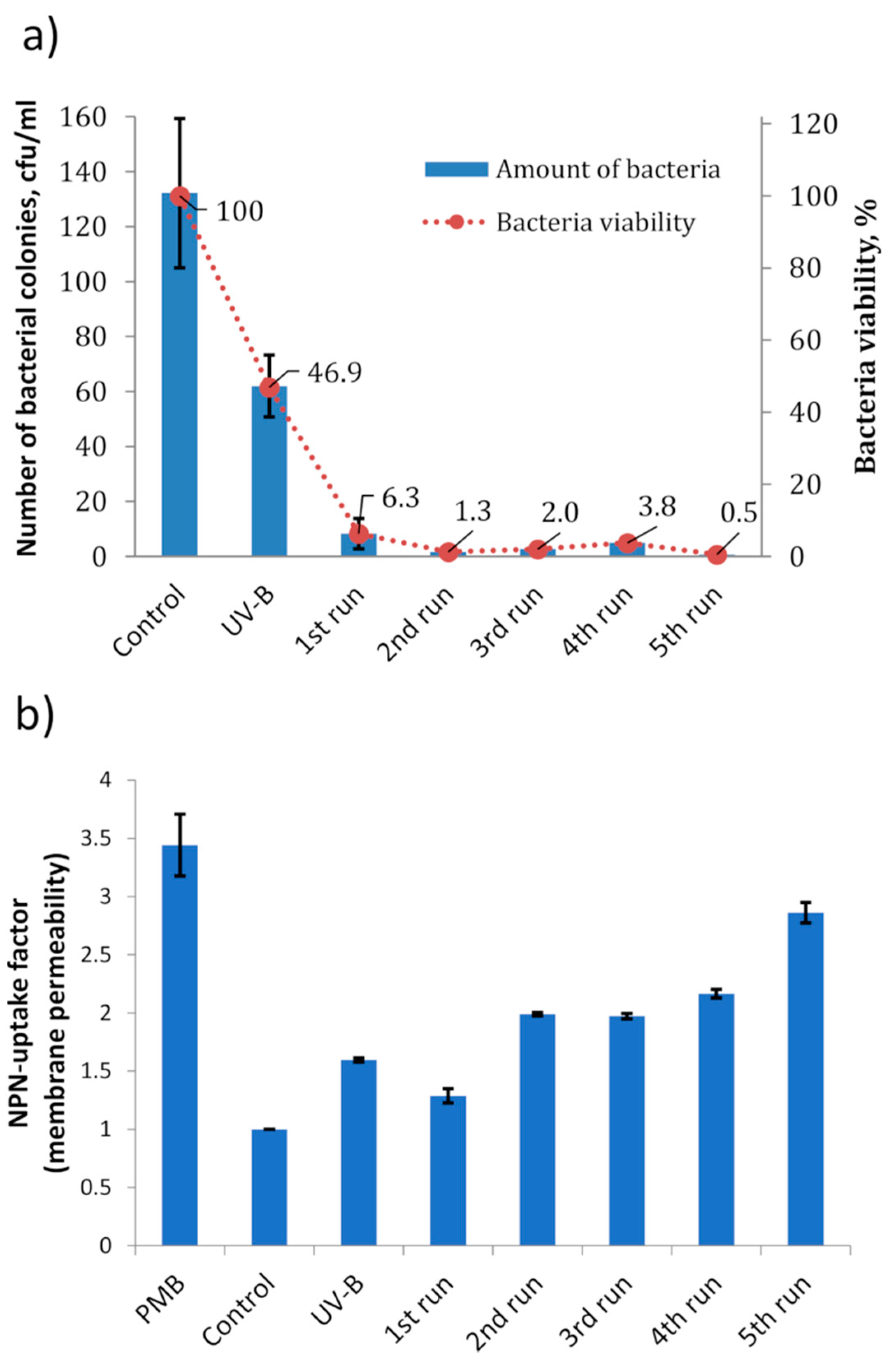
2. Results
3. Methodology
3.1. TiO2 Synthesis
3.2. Photocatalysis and Bacterial Inactivation
3.2.1. Bacterial Cultivation
3.2.2. Bacterial Inactivation Test
3.2.3. NPN Uptake Assay
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Védrine, J.C. Importance, features and uses of metal oxide catalysts in heterogeneous catalysis. Chin. J. Catal. 2019, 40, 1627–1636. [Google Scholar] [CrossRef]
- Anastas, P.T.; Bartlett, L.B.; Kirchhoff, M.M.; Williamson, T.C. The role of catalysis in the design, development, and implementation of green chemistry. Catal. Today 2000, 55, 11–22. [Google Scholar] [CrossRef]
- Courty, P.R.; Chauvel, A. Catalysis, the turntable for a clean future. Catal. Today 1996, 29, 3–15. [Google Scholar] [CrossRef]
- Russell, H.S.; Frederickson, L.B.; Hertel, O.; Ellermann, T.; Jensen, S.S. A Review of Photocatalytic Materials for Urban NOx Remediation. Catalysts 2021, 11, 695. [Google Scholar] [CrossRef]
- Kutuzova, A.; Dontsova, T.; Kwapinski, W. Application of TiO2-Based Photocatalysts to Antibiotics Degradation: Cases of Sulfamethoxazole, Trimethoprim and Ciprofloxacin. Catalysts 2021, 11, 728. [Google Scholar] [CrossRef]
- Sirivallop, A.; Escobedo, S.; Areerob, T.; de Lasa, H.; Chiarakorn, S. Photocatalytic Conversion of Organic Pollutants in Air: Quantum Yields Using a Silver/Nitrogen/TiO2 Mesoporous Semiconductor under Visible Light. Catalysts 2021, 11, 529. [Google Scholar] [CrossRef]
- Anastas, P.T.; Kirchhoff, M.M.; Williamson, T.C. Catalysis as a foundational pillar of green chemistry. Appl. Catal. A Gen. 2001, 221, 3–13. [Google Scholar] [CrossRef]
- Bover-Cid, S.; Belletti, N.; Aymerich, T.; Garriga, M. Modelling the impact of water activity and fat content of dry-cured ham on the reduction of Salmonella enterica by high pressure processing. Meat Sci. 2017, 123, 120–125. [Google Scholar] [CrossRef]
- Pino-Sandoval, D.; Villanueva-Rodríguez, M.; Cantú-Cárdenas, M.E.; Hernández-Ramírez, A. Performance of Ag-Cu/TiO2 photocatalyst prepared by sol-gel method on the inactivation of Escherichia coli and Salmonella typhimurium. J. Environ. Chem. Eng. 2020, 8, 104539. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, L.; Mou, X.; Xu, H.; Liu, J.; Miao, Y.; Wang, X.C.; Li, X. Characterization and evolution of antibiotic resistance of Salmonella in municipal wastewater treatment plants. J. Environ. Manag. 2019, 251, 109547. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.w.; Li, D.; Gu, A.Z.; Zeng, S.y.; He, M. Bacterial regrowth in water reclamation and distribution systems revealed by viable bacterial detection assays. Chemosphere 2016, 144, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Dolejska, M.; Frolkova, P.; Florek, M.; Jamborova, I.; Purgertova, M.; Kutilova, I.; Cizek, A.; Guenther, S.; Literak, I. CTX-M-15-producing Escherichia coli clone B2-O25b-ST131 and Klebsiella spp. isolates in municipal wastewater treatment plant effluents. J. Antimicrob. Chemother. 2011, 66, 2784–2790. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Hu, H.Y.; Lu, S.Q.; Li, Y.; Tang, F.; Lu, Y.; Wei, B. Monitoring and evaluation of antibiotic-resistant bacteria at a municipal wastewater treatment plant in China. Environ. Int. 2012, 42, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Song, Y. Advanced Oxidation Process as a Postharvest Decontamination Technology To Improve Microbial Safety of Fresh Produce. J. Agric. Food Chem. 2020, 68, 12916–12926. [Google Scholar] [CrossRef] [PubMed]
- Bounty, S.; Rodriguez, R.A.; Linden, K.G. Inactivation of adenovirus using low-dose UV/H2O2 advanced oxidation. Water Res. 2012, 46, 6273–6278. [Google Scholar] [CrossRef]
- del Álamo, A.C.; Pariente, M.I.; Vasiliadou, I.; Padrino, B.; Puyol, D.; Molina, R.; Martínez, F. Removal of pharmaceutical compounds from urban wastewater by an advanced bio-oxidation process based on fungi Trametes versicolor immobilized in a continuous RBC system. Environ. Sci. Pollut. Res. 2018, 25, 34884–34892. [Google Scholar] [CrossRef]
- Wang, B.; Song, Z.; Sun, L. A review: Comparison of multi-air-pollutant removal by advanced oxidation processes—Industrial implementation for catalytic oxidation processes. Chem. Eng. J. 2021, 409, 128136. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Z.; Zhang, J.; Shan, D.; Wu, Y.; Bai, L.; Wang, B. Treatment of industrial dye wastewater and pharmaceutical residue wastewater by advanced oxidation processes and its combination with nanocatalysts: A review. J. Water Process Eng. 2021, 42, 102122. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, X.; Zhou, X.; Wang, R.; Wang, S.; Ren, N.; Ho, S.-H. Advanced oxidation processes for water disinfection: Features, mechanisms and prospects. Chem. Eng. J. 2021, 409, 128207. [Google Scholar] [CrossRef]
- Russo, M.; Iervolino, G.; Vaiano, V. W-Doped ZnO Photocatalyst for the Degradation of Glyphosate in Aqueous Solution. Catalysts 2021, 11, 234. [Google Scholar] [CrossRef]
- Xing, Z.; Zhang, J.; Cui, J.; Yin, J.; Zhao, T.; Kuang, J.; Xiu, Z.; Wan, N.; Zhou, W. Recent advances in floating TiO2-based photocatalysts for environmental application. Appl. Catal. B Environ. 2018, 225, 452–467. [Google Scholar] [CrossRef]
- Nasir, A.M.; Jaafar, J.; Aziz, F.; Yusof, N.; Salleh, W.N.W.; Ismail, A.F.; Aziz, M. A review on floating nanocomposite photocatalyst: Fabrication and applications for wastewater treatment. J. Water Process Eng. 2020, 36, 101300. [Google Scholar] [CrossRef]
- Faramarzpour, M.; Vossoughi, M.; Borghei, M. Photocatalytic degradation of furfural by titania nanoparticles in a floating-bed photoreactor. Chem. Eng. J. 2009, 146, 79–85. [Google Scholar] [CrossRef]
- Xue, H.; Jiang, Y.; Yuan, K.; Yang, T.; Hou, J.; Cao, C.; Feng, K.; Wang, X. Floating photocatalyst of B-N-TiO 2 /expanded perlite: A sol-gel synthesis with optimized mesoporous and high photocatalytic activity. Sci. Rep. 2016, 6, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Mahalingam, H. Reusable floating polymer nanocomposite photocatalyst for the efficient treatment of dye wastewaters under scaled-up conditions in batch and recirculation modes. J. Chem. Technol. Biotechnol. 2019, 94, 2597–2608. [Google Scholar] [CrossRef]
- Valdez-Castillo, M.; Saucedo-Lucero, J.O.; Arriaga, S. Photocatalytic inactivation of airborne microorganisms in continuous flow using perlite-supported ZnO and TiO2. Chem. Eng. J. 2019, 374, 914–923. [Google Scholar] [CrossRef]
- Varnagiris, S.; Urbonavicius, M.; Sakalauskaite, S.; Daugelavicius, R.; Pranevicius, L.; Lelis, M.; Milcius, D. Floating TiO2 photocatalyst for efficient inactivation of E. coli and decomposition of methylene blue solution. Sci. Total Environ. 2020, 720, 137600. [Google Scholar] [CrossRef]
- Sboui, M.; Lachheb, H.; Bouattour, S.; Gruttadauria, M.; La Parola, V.; Liotta, L.F.; Boufi, S. TiO2/Ag2O immobilized on cellulose paper: A new floating system for enhanced photocatalytic and antibacterial activities. Environ. Res. 2021, 198, 111257. [Google Scholar] [CrossRef]
- Wang, B.; Wei, S.; Guo, L.; Wang, Y.; Liang, Y.; Xu, B.; Pan, F.; Tang, A.; Chen, X. Effect of deposition parameters on properties of TiO2 films deposited by reactive magnetron sputtering. Ceram. Int. 2017, 43, 10991–10998. [Google Scholar] [CrossRef]
- Lee, M.K.; Park, Y.C. Super-hydrophilic anatase TiO2thin film in-situ deposited by DC magnetron sputtering. Thin Solid Films 2017, 638, 9–16. [Google Scholar] [CrossRef]
- Singh, P.; Kaur, D. Room temperature growth of nanocrystalline anatase TiO2thin films by dc magnetron sputtering. Phys. B Condens. Matter 2010, 405, 1258–1266. [Google Scholar] [CrossRef]
- Awaja, F.; Gilbert, M.; Kelly, G.; Fox, B.; Pigram, P.J. Adhesion of polymers. Prog. Polym. Sci. 2009, 34, 948–968. [Google Scholar] [CrossRef]
- Popelka, A.; Novák, I.; Al-Maadeed, M.A.S.A.; Ouederni, M.; Krupa, I. Effect of corona treatment on adhesion enhancement of LLDPE. Surf. Coat. Technol. 2018, 335, 118–125. [Google Scholar] [CrossRef]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. Acta Part B At. Spectrosc. 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Dixon, D.; Meenan, B.J. Atmospheric dielectric barrier discharge treatments of polyethylene, polypropylene, polystyrene and poly(ethylene terephthalate) for enhanced adhesion. J. Adhes. Sci. Technol. 2012, 26, 2325–2337. [Google Scholar] [CrossRef]
- Communication, S.; Engineering, C. Surface modification of polypropylene in an impulse corona discharge. Korean J. Chem. Eng. 1996, 13, 97–100. [Google Scholar]
- Stewart, R.; Goodship, V.; Guild, F.; Green, M.; Farrow, J. Investigation and demonstration of the durability of air plasma pre-treatment on polypropylene automotive bumpers. Int. J. Adhes. Adhes. 2005, 25, 93–99. [Google Scholar] [CrossRef]
- Kozak, M.; Cirik, K.; Başak, S. Treatment of textile wastewater using combined anaerobic moving bed biofilm reactor and powdered activated carbon-aerobic membrane reactor. J. Environ. Chem. Eng. 2021, 9, 105596. [Google Scholar] [CrossRef]
- Fanta, A.B.; Nair, A.M.; Sægrov, S.; Østerhus, S.W. Phosphorus removal from industrial discharge impacted municipal wastewater using sequencing batch moving bed biofilm reactor. J. Water Process Eng. 2021, 41, 102034. [Google Scholar] [CrossRef]
- Shitu, A.; Liu, G.; Zhang, Y.; Ye, Z.; Zhao, J.; Zhu, S.; Liu, D. Enhancement of mariculture wastewater treatment using moving bed biofilm reactors filled with modified biocarriers: Characterisation, process performance and microbial community evaluation. J. Environ. Manag. 2021, 291, 112724. [Google Scholar] [CrossRef] [PubMed]
- Daugelavicius, R.; Bakiene, E.; Bamford, D.H. Stages of polymyxin B interaction with the Escherichia coli cell envelope. Antimicrob. Agents Chemother. 2000, 44, 2969–2978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Zhao, L.; Wang, D.; Yan, L.; Jing, C.; Zhang, H.; Guo, L.H.; Tang, N. Direct evidence for surface long-lived superoxide radicals photo-generated in TiO2 and other metal oxide suspensions. Phys. Chem. Chem. Phys. 2018, 20, 18978–18985. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, S.; Polo López, M.I.; Spuhler, D.; Sánchez Pérez, J.A.; Fernández Ibáñez, P.; Pulgarin, C. Solar disinfection is an augmentable, in situ-generated photo-Fenton reaction—Part 1: A review of the mechanisms and the fundamental aspects of the process. Appl. Catal. B Environ. 2016, 199, 199–223. [Google Scholar] [CrossRef]
- Carré, G.; Hamon, E.; Ennahar, S.; Estner, M.; Lett, M.C.; Horvatovich, P.; Gies, J.P.; Keller, V.; Keller, N.; Andre, P. TiO2 photocatalysis damages lipids and proteins in Escherichia coli. Appl. Environ. Microbiol. 2014, 80, 2573–2581. [Google Scholar] [CrossRef] [Green Version]
- Khater, M.S.; Kulkarni, G.R.; Khater, S.S.; Gholap, H.; Patil, R. Study to elucidate effect of titanium dioxide nanoparticles on bacterial membrane potential and membrane permeability. Mater. Res. Express 2020, 7, 035005. [Google Scholar] [CrossRef]
- Ranjan, S.; Ramalingam, C. Titanium dioxide nanoparticles induce bacterial membrane rupture by reactive oxygen species generation. Environ. Chem. Lett. 2016, 14, 487–494. [Google Scholar] [CrossRef]
- Kuliesiene, N.; Sakalauskaite, S.; Tuckute, S.; Urbonavicius, M.; Varnagiris, S.; Daugelavicius, R.; Lelis, M. TiO2Application for the Photocatalytical Inactivation of S. enterica, E. coli and M. luteus Bacteria Mixtures. Environ. Clim. Technol. 2020, 24, 418–429. [Google Scholar] [CrossRef]


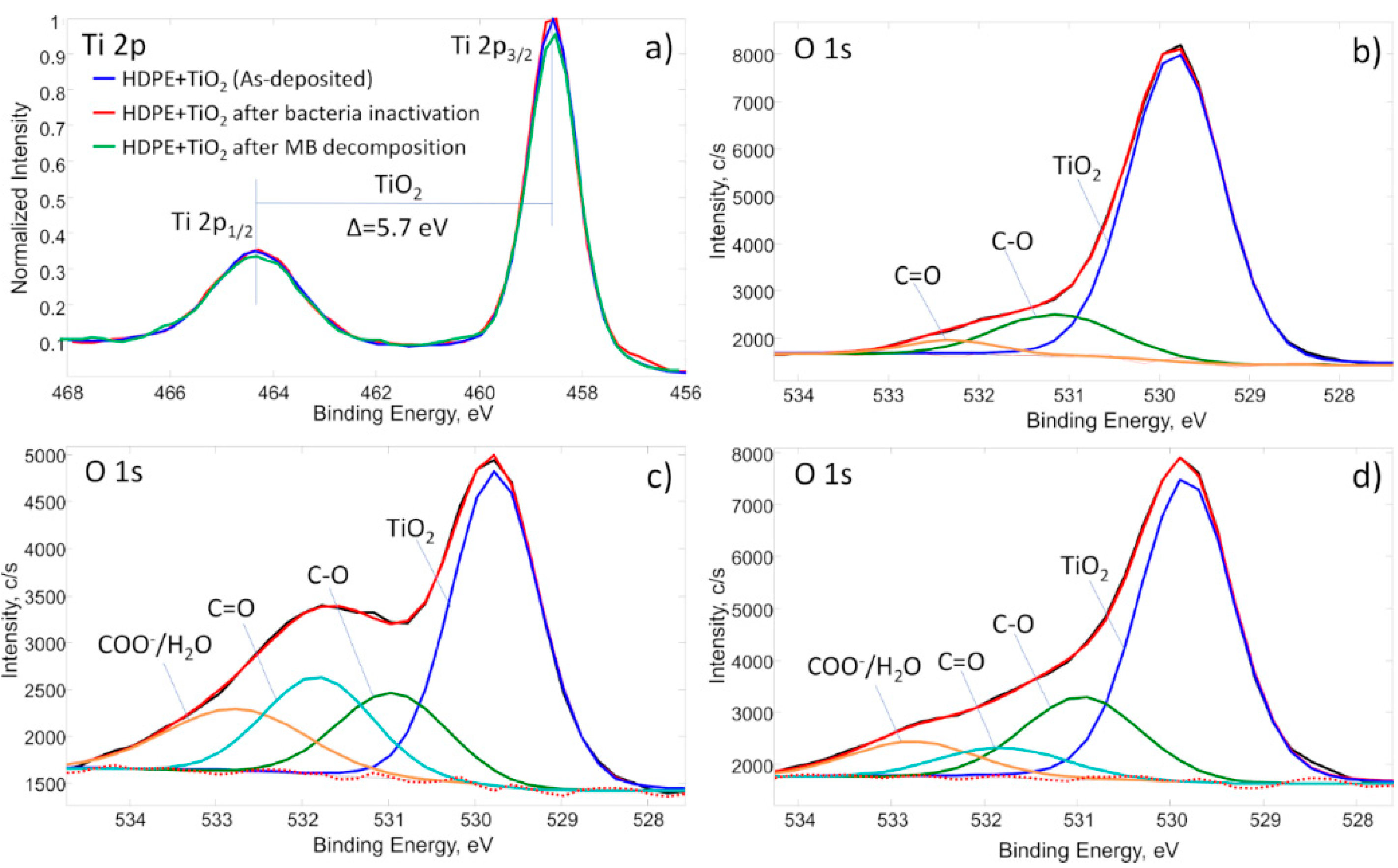
| Sample | C | O | Ti | N | P | Ca |
|---|---|---|---|---|---|---|
| HDPE | 92.9 | 7.1 | - | - | - | - |
| HDPE + TiO2 | 32.7 | 48.4 | 18.9 | - | - | - |
| HDPE + TiO2 after MB decomposition | 56.8 | 33.1 | 8.2 | 1.9 | - | - |
| HDPE + TiO2 after bacterial inactivation | 39.1 | 44.6 | 12.3 | 2.3 | 1.0 | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbonavicius, M.; Varnagiris, S.; Sakalauskaite, S.; Demikyte, E.; Tuckute, S.; Lelis, M. Application of Floating TiO2 Photocatalyst for Methylene Blue Decomposition and Salmonella typhimurium Inactivation. Catalysts 2021, 11, 794. https://doi.org/10.3390/catal11070794
Urbonavicius M, Varnagiris S, Sakalauskaite S, Demikyte E, Tuckute S, Lelis M. Application of Floating TiO2 Photocatalyst for Methylene Blue Decomposition and Salmonella typhimurium Inactivation. Catalysts. 2021; 11(7):794. https://doi.org/10.3390/catal11070794
Chicago/Turabian StyleUrbonavicius, Marius, Sarunas Varnagiris, Sandra Sakalauskaite, Emilija Demikyte, Simona Tuckute, and Martynas Lelis. 2021. "Application of Floating TiO2 Photocatalyst for Methylene Blue Decomposition and Salmonella typhimurium Inactivation" Catalysts 11, no. 7: 794. https://doi.org/10.3390/catal11070794
APA StyleUrbonavicius, M., Varnagiris, S., Sakalauskaite, S., Demikyte, E., Tuckute, S., & Lelis, M. (2021). Application of Floating TiO2 Photocatalyst for Methylene Blue Decomposition and Salmonella typhimurium Inactivation. Catalysts, 11(7), 794. https://doi.org/10.3390/catal11070794







