Heteroatom (N, S) Co-Doped CNTs in the Phenol Oxidation by Catalytic Wet Air Oxidation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Materials Characterisation
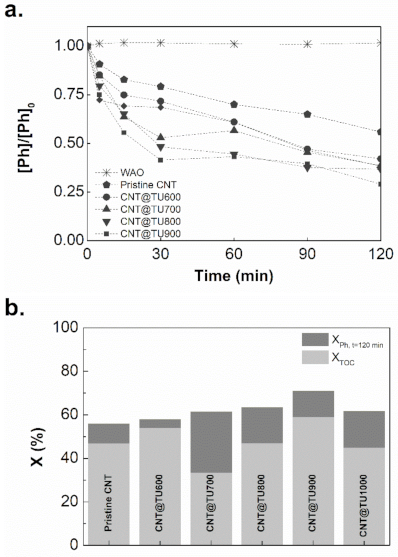
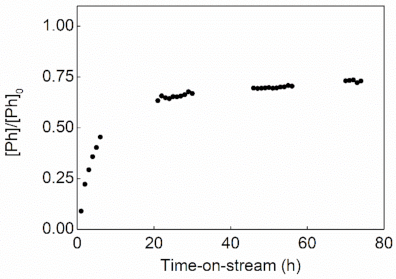
2.2. Catalytic Activity in CWAO
3. Experimental
3.1. Materials: Preparation and Characterisation
3.2. Experimental Procedure
3.2.1. Batch Mode
3.2.2. Continuous Mode
3.3. Analytical Techniques
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, V.S.; Mahajani, V.V.; Joshi, J.B. Wet Air Oxidation. Ind. Eng. Chem. Res. 1995, 34, 2–48. [Google Scholar] [CrossRef]
- Mantzavinos, D.; Sahibzada, M.; Livingston, A.G.; Metcalfe, I.S.; Hellgardt, K. Wastewater treatment: Wet air oxidation as a precursor to biological treatment. Catal. Today 1999, 53, 93–106. [Google Scholar] [CrossRef]
- Kolaczkowski, S.T.; Plucinski, P.; Beltran, F.J.; Rivas, F.J.; McLurgh, D.B. Wet air oxidation: A review of process technologies and aspects in reactor design. Chem. Eng. J. 1999, 73, 143–160. [Google Scholar] [CrossRef]
- Luck, F. Wet air oxidation: Past, present and future. Catal. Today 1999, 53, 81–91. [Google Scholar] [CrossRef]
- Debellefontaine, H.; Foussard, J.N. Wet air oxidation for the treatment of industrial wastes. Chemical aspects, reactor design and industrial applications in Europe. Waste Manag. 2000, 20, 15–25. [Google Scholar] [CrossRef]
- Bhargava, S.K.; Tardio, J.; Prasad, J.; Föger, K.; Akolekar, D.B.; Grocott, S.C. Wet oxidation and catalytic wet oxidation. Ind. Eng. Chem. Res. 2006, 45, 1221–1258. [Google Scholar] [CrossRef]
- Levec, J.; Pintar, A. Catalytic wet-air oxidation processes: A review. Catal. Today 2007, 124, 172–184. [Google Scholar] [CrossRef]
- Gallezot, P.; Laurain, N.; Isnard, P. Catalytic wet-air oxidation of carboxylic acids on carbon-supported platinum catalysts. Appl. Catal. B Environ. 1996, 9, L11–L17. [Google Scholar] [CrossRef]
- Roy, S.; Saroha, A.K. Ceria promoted [gamma]-Al2O3 supported platinum catalyst for catalytic wet air oxidation of oxalic acid: Kinetics and catalyst deactivation. RSC Adv. 2014, 4, 56838–56847. [Google Scholar] [CrossRef]
- Pruden, B.B.; Le, H. Wet air oxidation of soluble components in waste water. Can. J. Chem. Eng. 1976, 54, 319–325. [Google Scholar] [CrossRef]
- Martín-Hernández, M.; Carrera, J.; Suárez-Ojeda, M.E.; Besson, M.; Descorme, C. Catalytic wet air oxidation of a high strength p-nitrophenol wastewater over Ru and Pt catalysts: Influence of the reaction conditions on biodegradability enhancement. Appl. Catal. B Environ. 2012, 123–124, 141–150. [Google Scholar] [CrossRef]
- Stüber, F.; Font, J.; Fortuny, A.; Bengoa, C.; Eftaxias, A.; Fabregat, A. Carbon materials and catalytic wet air oxidation of organic pollutants in wastewater. Top. Catal. 2005, 33, 3–50. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, C.; García, R.; Soto-Garrido, G.; Arriagada, R. Catalytic wet air oxidation of aqueous ammonia with activated carbon. Appl. Catal. B Environ. 2003, 46, 229–237. [Google Scholar] [CrossRef]
- Cybulski, A. Catalytic Wet Air Oxidation: Are Monolithic Catalysts and Reactors Feasible? Ind. Eng. Chem. Res. 2007, 46, 4007–4033. [Google Scholar] [CrossRef]
- Shende, R.V.; Levec, J. Wet Oxidation Kinetics of Refractory Low Molecular Mass Carboxylic Acids. Ind. Eng. Chem. Res. 1999, 38, 3830–3837. [Google Scholar] [CrossRef]
- Quintanilla, A.; Casas, J.A.; Mohedano, A.F.; Rodríguez, J.J. Reaction pathway of the catalytic wet air oxidation of phenol with a Fe/activated carbon catalyst. Appl. Catal. B Environ. 2006, 67, 206–216. [Google Scholar] [CrossRef]
- Quintanilla, A.; Casas, J.A.; Zazo, J.A.; Mohedano, A.F.; Rodríguez, J.J. Wet air oxidation of phenol at mild conditions with a Fe/activated carbon catalyst. Appl. Catal. B Environ. 2006, 62, 115–120. [Google Scholar] [CrossRef]
- Quintanilla, A.; Casas, J.A.; Rodríguez, J.J. Catalytic wet air oxidation of phenol with modified activated carbons and Fe/activated carbon catalysts. Appl. Catal. B Environ. 2007, 76, 135–145. [Google Scholar] [CrossRef]
- Quintanilla, A.; Casas, J.A.; Rodriguez, J.J.; Kreutzer, M.T.; Kapteijn, F.; Moulijn, J.A. Kinetics of the wet oxidation of phenol over an Fe/activated carbon catalyst. Int. J. Chem. React. Eng. 2007, 5. [Google Scholar] [CrossRef] [Green Version]
- Quintanilla, A.; Fraile, A.F.; Casas, J.A.; Rodríguez, J.J. Phenol oxidation by a sequential CWPO-CWAO treatment with a Fe/AC catalyst. J. Hazard. Mater. 2007, 146, 582–588. [Google Scholar] [CrossRef]
- Quintanilla, A.; Menéndez, N.; Tornero, J.; Casas, J.A.; Rodríguez, J.J. Surface modification of carbon-supported iron catalyst during the wet air oxidation of phenol: Influence on activity, selectivity and stability. Appl. Catal. B Environ. 2008, 81, 105–114. [Google Scholar] [CrossRef]
- Quintanilla, A.; Dominguez, C.M.; Casas, J.A.; Rodriguez, J.J. Emerging catalysts for wet air oxidation process. In Focus on Catalysis Research: New Developments; Nova Science Publishers, Inc.: New York, NY, USA, 2012; pp. 237–259. [Google Scholar]
- Suarez-Ojeda, M.E.; Stüber, F.; Fortuny, A.; Fabregat, A.; Carrera, J.; Font, J. Catalytic wet air oxidation of substituted phenols using activated carbon as catalyst. Appl. Catal. B Environ. 2005, 58, 105–114. [Google Scholar] [CrossRef]
- Suárez-Ojeda, M.E.; Fabregat, A.; Stüber, F.; Fortuny, A.; Carrera, J.; Font, J. Catalytic wet air oxidation of substituted phenols: Temperature and pressure effect on the pollutant removal, the catalyst preservation and the biodegradability enhancement. Chem. Eng. J. 2007, 132, 105–115. [Google Scholar] [CrossRef]
- Suárez-Ojeda, M.E.; Kim, J.; Carrera, J.; Metcalfe, I.S.; Font, J. Catalytic and non-catalytic wet air oxidation of sodium dodecylbenzene sulfonate: Kinetics and biodegradability enhancement. J. Hazard. Mater. 2007, 144, 655–662. [Google Scholar] [CrossRef]
- Rocha, R.P.; Pereira, M.F.R.; Figueiredo, J.L. Metal-free carbon materials as catalysts for wet air oxidation. Catal. Today 2019. [Google Scholar] [CrossRef]
- Eftaxias, A.; Font, J.; Fortuny, A.; Fabregat, A.; Stüber, F. Kinetics of phenol oxidation in a trickle bed reactor over active carbon catalyst. J. Chem. Technol. Biotechnol. 2005, 80, 677–687. [Google Scholar] [CrossRef]
- Eftaxias, A.; Font, J.; Fortuny, A.; Fabregat, A.; Stüber, F. Catalytic wet air oxidation of phenol over active carbon catalyst: Global kinetic modelling using simulated annealing. Appl. Catal. B Environ. 2006, 67, 12–23. [Google Scholar] [CrossRef]
- Santiago, M.; Stüber, F.; Fortuny, A.; Fabregat, A.; Font, J. Modified activated carbons for catalytic wet air oxidation of phenol. Carbon 2005, 43, 2134–2145. [Google Scholar] [CrossRef]
- Cordero, T.; Rodríguez-Mirasol, J.; Bedia, J.; Gomis, S.; Yustos, P.; García-Ochoa, F.; Santos, A. Activated carbon as catalyst in wet oxidation of phenol: Effect of the oxidation reaction on the catalyst properties and stability. Appl. Catal. B Environ. 2008, 81, 122–131. [Google Scholar] [CrossRef]
- Fortuny, A.; Font, J.; Fabregat, A. Wet air oxidation of phenol using active carbon as catalyst. Appl. Catal. B Environ. 1998, 19, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Sousa, J.P.S.; Silva, A.M.T.; Pereira, M.F.R.; Figueiredo, J.L. Wet Air Oxidation of Aniline Using Carbon Foams and Fibers Enriched with Nitrogen. Sep. Sci. Technol. 2010, 45, 1546–1554. [Google Scholar] [CrossRef]
- Tukač, V.; Hanika, J. Catalytic effect of active carbon black Chezacarb in wet oxidation of phenol. Collect. Czechoslov. Chem. Commun. 1996, 61, 1010–1017. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Rocha, R.P.; Órfão, J.J.M.; Pereira, M.F.R.; Figueiredo, J.L. Mechanothermal Approach for N-, S-, P-, and B-Doping of Carbon Nanotubes: Methodology and Catalytic Performance in Wet Air Oxidation. C—J. Carbon Res. 2019, 5, 30. [Google Scholar] [CrossRef] [Green Version]
- Rocha, R.P.; Sousa, J.P.S.; Silva, A.M.T.; Pereira, M.F.R.; Figueiredo, J.L. Catalytic activity and stability of multiwalled carbon nanotubes in catalytic wet air oxidation of oxalic acid: The role of the basic nature induced by the surface chemistry. Appl. Catal. B Environ. 2011, 104, 330–336. [Google Scholar] [CrossRef]
- Rocha, R.P.; Silva, A.M.T.; Romero, S.M.M.; Pereira, M.F.R.; Figueiredo, J.L. The role of O- and S-containing surface groups on carbon nanotubes for the elimination of organic pollutants by catalytic wet air oxidation. Appl. Catal. B Environ. 2014, 147, 314–321. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Rocha, R.P.; Gonçalves, A.G.; Figueiredo, J.L.; Órfão, J.J.M.; Pereira, M.F.R. Easy method to prepare N-doped carbon nanotubes by ball milling. Carbon 2015, 91, 114–121. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Rocha, R.P.; Gonçalves, A.G.; Figueiredo, J.L.; Órfão, J.J.M.; Pereira, M.F.R. Highly active N-doped carbon nanotubes prepared by an easy ball milling method for advanced oxidation processes. Appl. Catal. B Environ. 2016, 192, 296–303. [Google Scholar] [CrossRef]
- Rocha, R.P.; Santos, D.F.M.; Soares, O.S.M.P.; Silva, A.M.T.; Pereira, M.F.R.; Figueiredo, J.L. Metal-Free Catalytic Wet Oxidation: From Powder to Structured Catalyst Using N-Doped Carbon Nanotubes. Top. Catal. 2018, 61, 1957–1966. [Google Scholar] [CrossRef]
- Santos, D.F.M.; Soares, O.S.G.P.; Silva, A.M.T.; Figueiredo, J.L.; Pereira, M.F.R. Degradation and mineralization of oxalic acid using catalytic wet oxidation over carbon coated ceramic monoliths. J. Environ. Chem. Eng. 2021, 9, 105369. [Google Scholar] [CrossRef]
- Li, X.; Yang, S.X.; Zhu, W.P.; Wang, J.B.; Wang, L. Catalytic wet air oxidation of phenol and aniline over multi-walled carbon nanotubes. Huanjing Kexue/Environ. Sci. 2008, 29, 2522–2528. [Google Scholar]
- Yang, S.; Wang, X.; Yang, H.; Sun, Y.; Liu, Y. Influence of the different oxidation treatment on the performance of multi-walled carbon nanotubes in the catalytic wet air oxidation of phenol. J. Hazard. Mater. 2012, 233–234, 18–24. [Google Scholar] [CrossRef]
- Yang, S.; Sun, Y.; Yang, H.; Wan, J. Catalytic wet air oxidation of phenol, nitrobenzene and aniline over the multi-walled carbon nanotubes (MWCNTs) as catalysts. Front. Environ. Sci. Eng. 2014. [Google Scholar] [CrossRef]
- He, W.; Xue, P.; Du, H.; Xu, L.; Pang, M.; Gao, X.; Yu, J.; Zhang, Z.; Huang, T. A facile method prepared nitrogen and boron doped carbon nano-tube based catalysts for oxygen reduction. Int. J. Hydrogen Energy 2017, 42, 4123–4132. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Q.; Jiang, D.; Huang, Y. Facile synthesis of B, N co-doped three-dimensional porous graphitic carbon toward oxygen reduction reaction and oxygen evolution reaction. Catal. Commun. 2017, 100, 89–92. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Cheng, K.; Quan, X.; Fan, X.; Su, Y.; Chen, S.; Zhao, H.; Zhang, Y.; Yu, H.; et al. Selective Electrochemical Reduction of Carbon Dioxide to Ethanol on a Boron- and Nitrogen-Co-doped Nanodiamond. Angew. Chem. Int. Ed. 2017, 56, 15607–15611. [Google Scholar] [CrossRef]
- Zeng, L.; Shi, J.; Luo, J.; Chen, H. Silver sulfide anchored on reduced graphene oxide as a high -performance catalyst for CO2 electroreduction. J. Power Sources 2018, 398, 83–90. [Google Scholar] [CrossRef]
- Yuan, Q.; Ma, Z.; Chen, J.; Huang, Z.; Fang, Z.; Zhang, P.; Lin, Z.; Cui, J. N, S-Codoped Activated Carbon Material with Ultra-High Surface Area for High-Performance Supercapacitors. Polymers 2020, 12, 1982. [Google Scholar] [CrossRef]
- Rocha, I.M.; Soares, O.S.G.P.; Fernandes, D.M.; Freire, C.; Figueiredo, J.L.; Pereira, M.F.R. N-doped Carbon Nanotubes for the Oxygen Reduction Reaction in Alkaline Medium: Synergistic Relationship between Pyridinic and Quaternary Nitrogen. Chem. Sel. 2016, 1, 2522–2530. [Google Scholar] [CrossRef]
- Kiciński, W.; Szala, M.; Bystrzejewski, M. Sulfur-doped porous carbons: Synthesis and applications. Carbon 2014, 68, 1–32. [Google Scholar] [CrossRef]
- Zhou, G.; Yin, L.-C.; Wang, D.-W.; Li, L.; Pei, S.; Gentle, I.R.; Li, F.; Cheng, H.-M. Fibrous Hybrid of Graphene and Sulfur Nanocrystals for High-Performance Lithium–Sulfur Batteries. ACS Nano 2013, 7, 5367–5375. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, J.; Cao, X.; Abbott, J.; Jin, C.; Wang, H.; Strasser, P.; Yang, R.; Chen, X.; Wu, G. N-, P-, and S-doped graphene-like carbon catalysts derived from onium salts with enhanced oxygen chemisorption for Zn-air battery cathodes. Appl. Catal. B Environ. 2019, 241, 442–451. [Google Scholar] [CrossRef]
- Schaufuß, A.G.; Nesbitt, H.W.; Kartio, I.; Laajalehto, K.; Bancroft, G.M.; Szargan, R. Incipient oxidation of fractured pyrite surfaces in air. J. Electron Spectrosc. Relat. Phenom. 1998, 96, 69–82. [Google Scholar] [CrossRef]
- Santos, D.F.M.; Soares, O.S.G.P.; Silva, A.M.T.; Figueiredo, J.L.; Pereira, M.F.R. Catalytic wet oxidation of organic compounds over N-doped carbon nanotubes in batch and continuous operation. Appl. Catal. B Environ. 2016, 199, 361–371. [Google Scholar] [CrossRef]
- Delgado, J.J.; Chen, X.; Pérez-Omil, J.A.; Rodríguez-Izquierdo, J.M.; Cauqui, M.A. The effect of reaction conditions on the apparent deactivation of Ce–Zr mixed oxides for the catalytic wet oxidation of phenol. Catal. Today 2012, 180, 25–33. [Google Scholar] [CrossRef]

| Textural Properties N2 Adsorption | ||
|---|---|---|
| Sample | SBET (m2 g−1) | VP (cm3 g−1) |
| Pristine CNT | 291 | 1.103 |
| CNT@TU600 | 350 | 0.578 |
| CNT@TU700 | 371 | 0.690 |
| CNT@TU800 | 374 | 0.696 |
| CNT@TU900 | 362 | 0.692 |
| CNT@TU1000 | 363 | 0.712 |
| Elemental Analysis | XPS | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample | C | N | S | O | C | N | S | O |
| Pristine CNT | 98.9 | 0.15 | 0.0 | 0.8 | 99.2 | n.d. | n.d. | 0.8 |
| CNT@TU600 | 93.7 | 1.43 | 0.6 | 2.4 | 94.2 | 1.2 | 0.3 | 3.6 |
| CNT@TU700 | 94.9 | 1.09 | 1.3 | 1.7 | 95.6 | 0.8 | 0.4 | 2.4 |
| CNT@TU800 | 95.6 | 0.81 | 0.8 | 1.5 | 97.1 | 0.9 | 0.8 | 1.2 |
| CNT@TU900 | 96.3 | 0.70 | 0.7 | 1.3 | 98.2 | 0.6 | 0.3 | 0.9 |
| CNT@TU1000 | 95.7 | 0.02 | 1.3 | 1.1 | 97.9 | 0.2 | 0.8 | 1.1 |
| Peak #1 (N6) | Peak #2 (N5) | Peak #6 (NQ) | Peak #3 (Sulphate) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | B.E. (eV) | % Rel. | B.E. (eV) | % Rel. | B.E. (eV) | % Rel. | B.E. (eV) | % Rel. | B.E. (eV) | % Rel. | B.E. (eV) | % Rel. |
| CNT@TU600 | 398.2 | 50.0 | 399.7 | 39.4 | 401.2 | 10.6 | 163.7 | 53.2 | 164.5 | 18.8 | 168.7 | 28.0 |
| CNT@TU700 | 398.2 | 65.0 | 399.8 | 24.8 | 400.6 | 10.2 | 163.4 | 59.9 | 164.0 | 24.8 | 167.7 | 15.3 |
| CNT@TU800 | 398.3 | 55.4 | 399.8 | 18.7 | 400.6 | 25.9 | 163.6 | 51.7 | 164.2 | 23.4 | 168.7 | 24.9 |
| CNT@TU900 | 398.2 | 67.0 | --- | --- | 400.1 | 33.0 | 163.8 | 100.0 | --- | --- | --- | --- |
| CNT@TU1000 | 398.3 | 57.8 | --- | --- | 400.3 | 42.2 | 163.7 | 100.0 | --- | --- | --- | --- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, R.P.; Soares, O.S.G.P.; Órfão, J.J.M.; Pereira, M.F.R.; Figueiredo, J.L. Heteroatom (N, S) Co-Doped CNTs in the Phenol Oxidation by Catalytic Wet Air Oxidation. Catalysts 2021, 11, 578. https://doi.org/10.3390/catal11050578
Rocha RP, Soares OSGP, Órfão JJM, Pereira MFR, Figueiredo JL. Heteroatom (N, S) Co-Doped CNTs in the Phenol Oxidation by Catalytic Wet Air Oxidation. Catalysts. 2021; 11(5):578. https://doi.org/10.3390/catal11050578
Chicago/Turabian StyleRocha, Raquel P., Olívia Salomé G. P. Soares, José J. M. Órfão, Manuel Fernando R. Pereira, and José L. Figueiredo. 2021. "Heteroatom (N, S) Co-Doped CNTs in the Phenol Oxidation by Catalytic Wet Air Oxidation" Catalysts 11, no. 5: 578. https://doi.org/10.3390/catal11050578
APA StyleRocha, R. P., Soares, O. S. G. P., Órfão, J. J. M., Pereira, M. F. R., & Figueiredo, J. L. (2021). Heteroatom (N, S) Co-Doped CNTs in the Phenol Oxidation by Catalytic Wet Air Oxidation. Catalysts, 11(5), 578. https://doi.org/10.3390/catal11050578










