Synthesis of Magnetic α-Fe2O3/Rutile TiO2 Hollow Spheres for Visible-Light Photocatalytic Activity
Abstract
1. Introduction
2. Results and Discussion
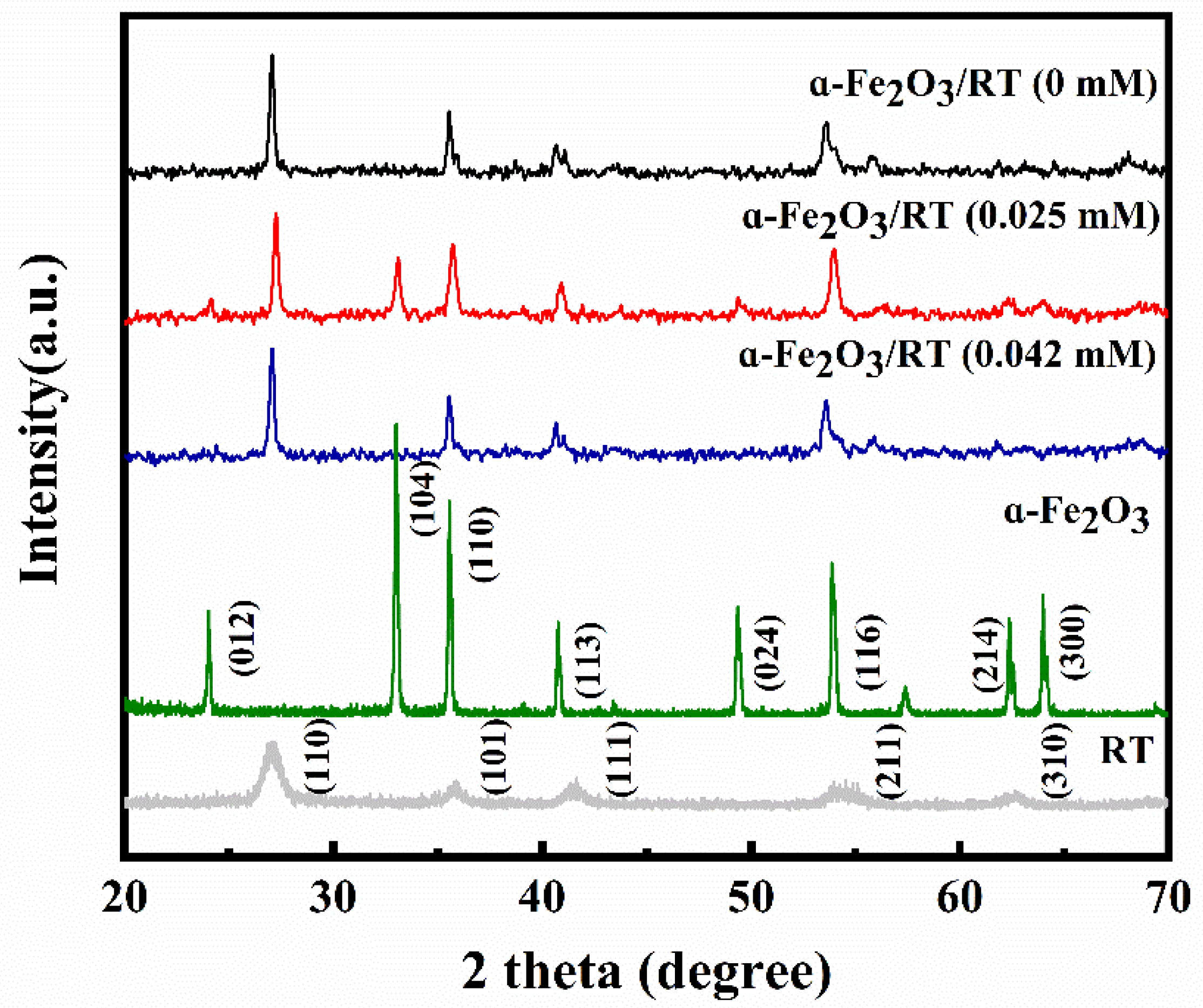
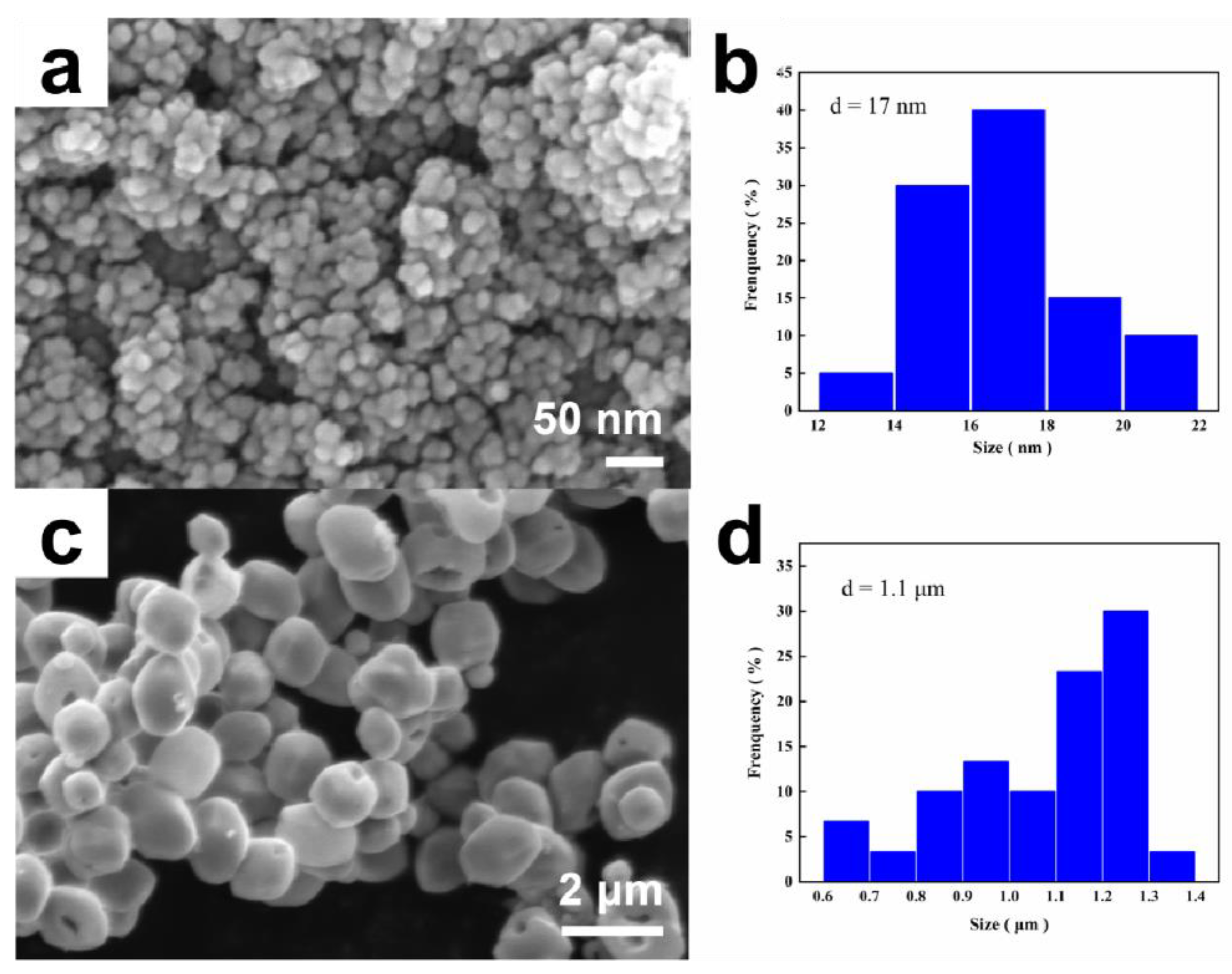
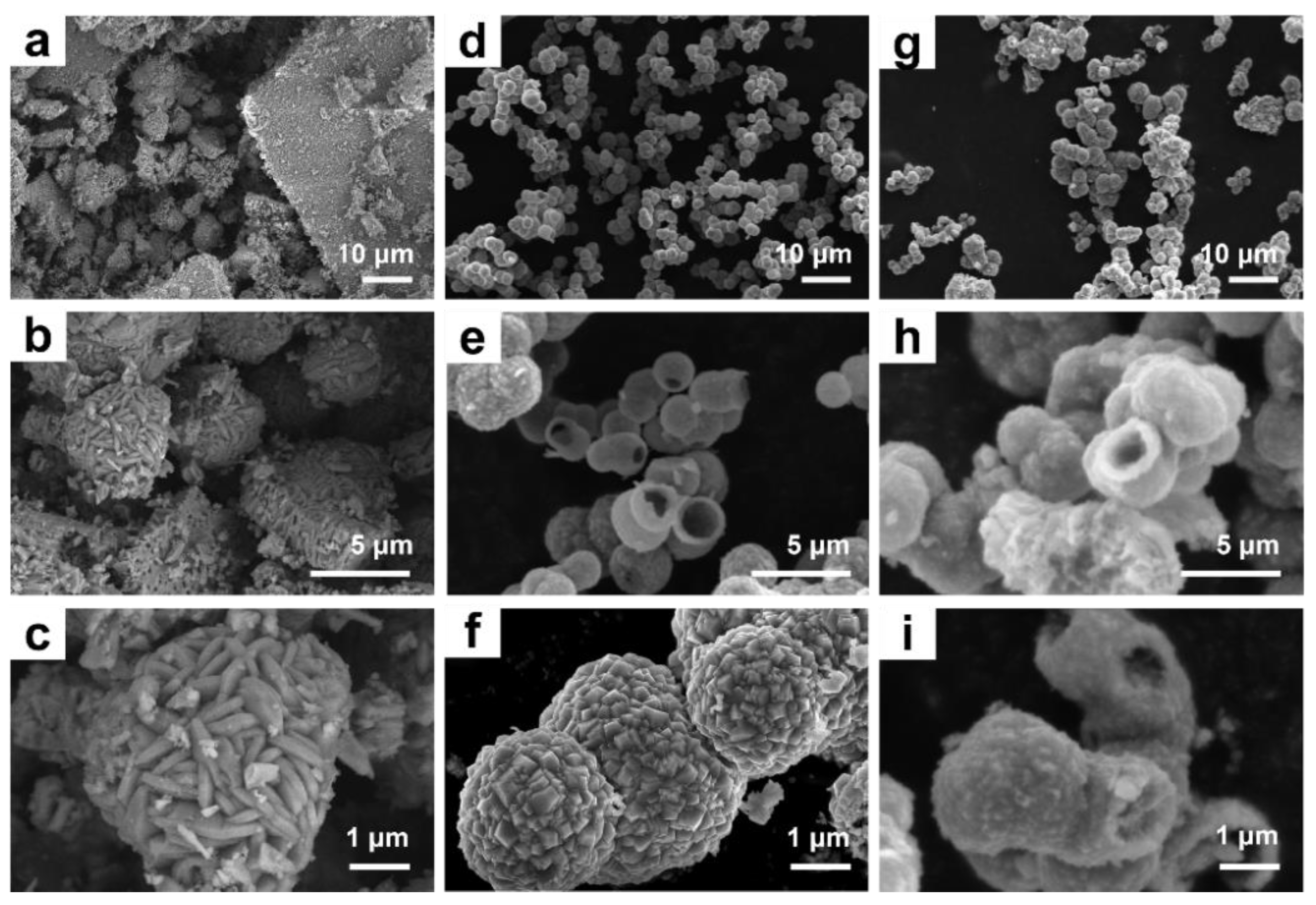
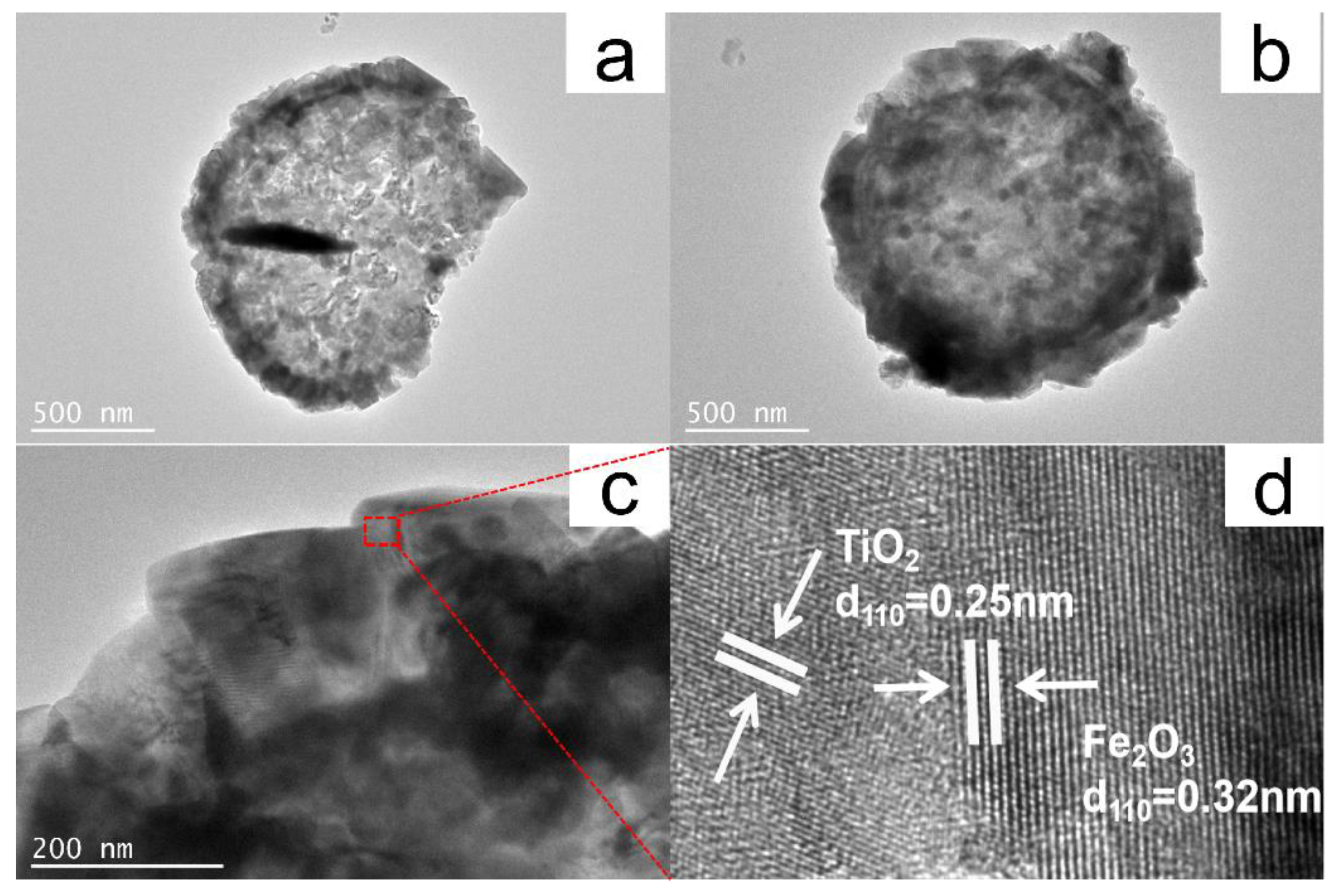
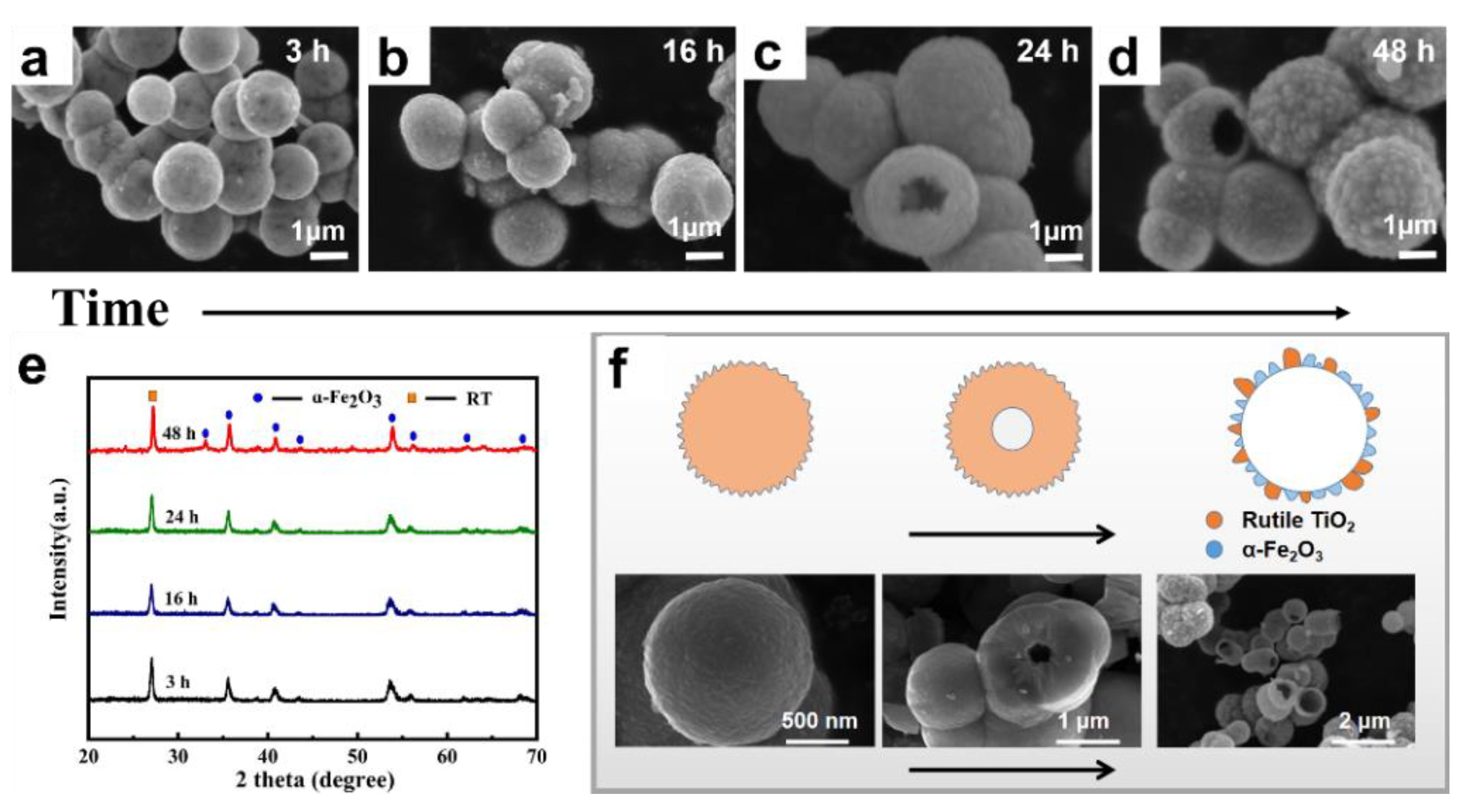
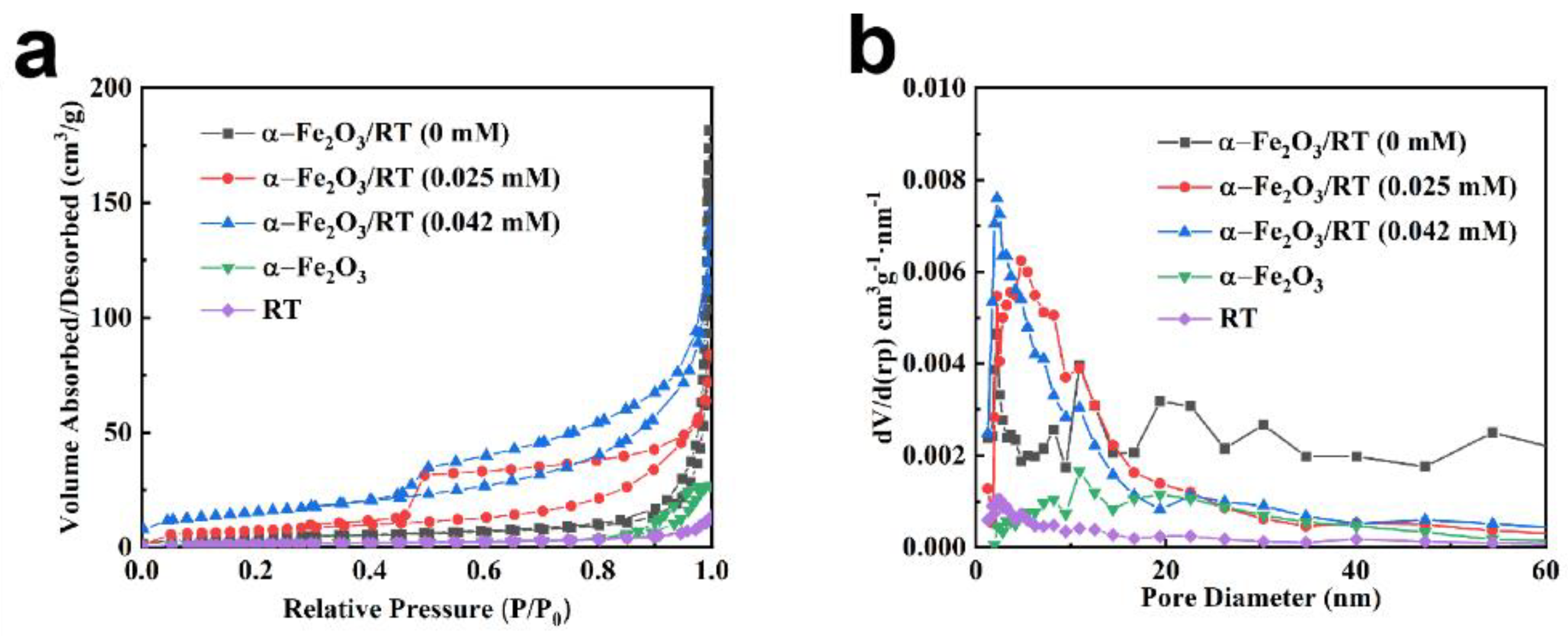
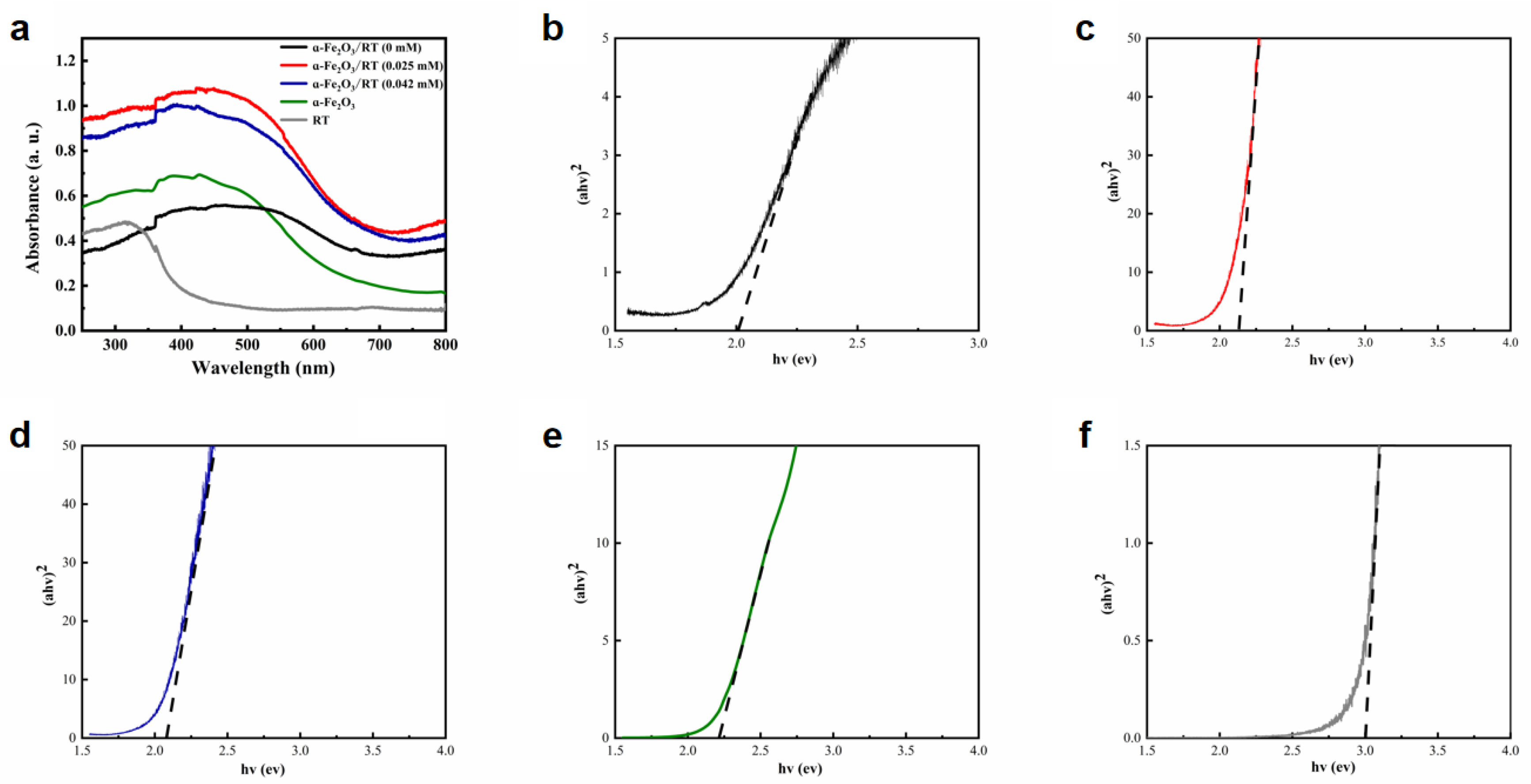
2.1. Characterization
2.2. Magnetic Properties
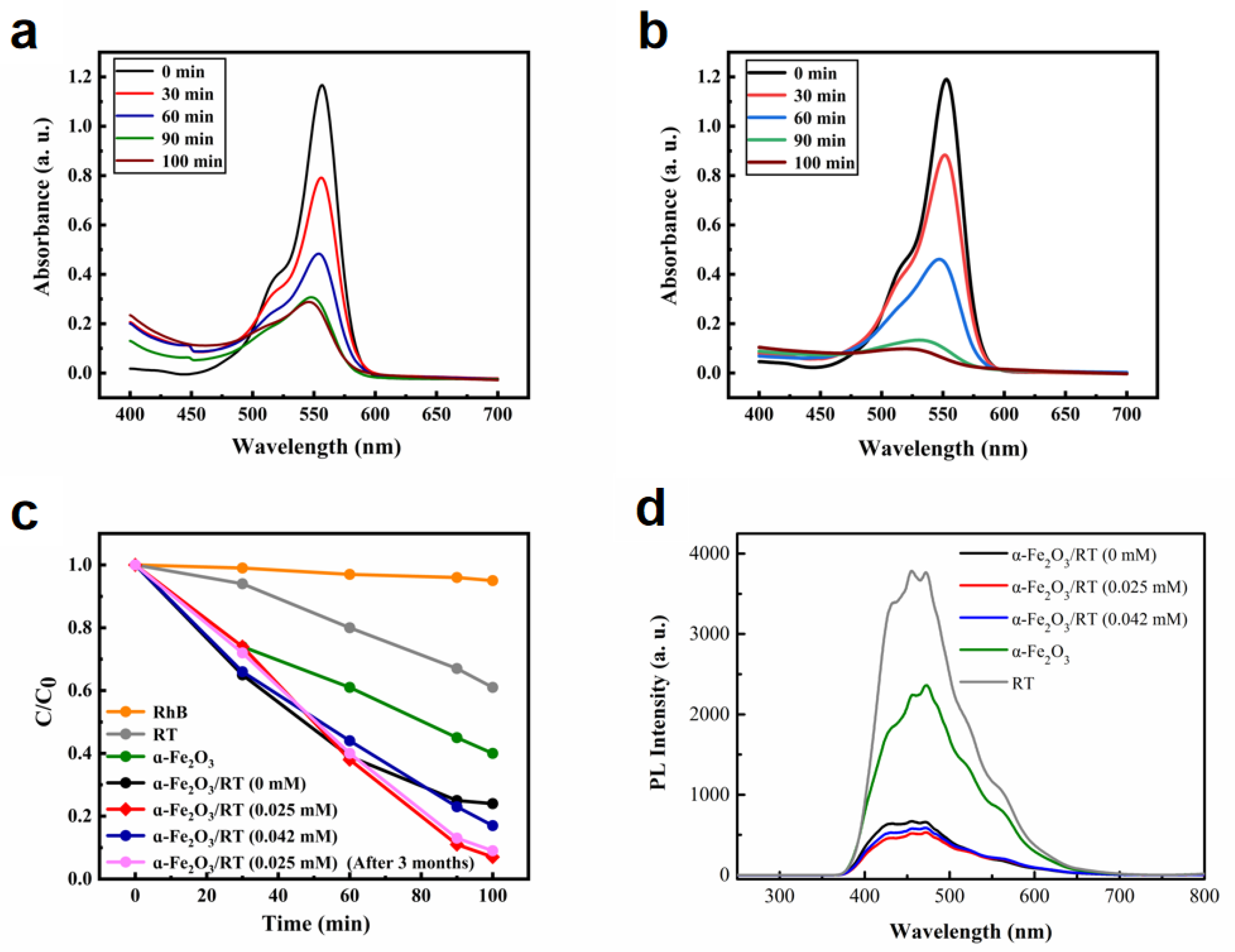
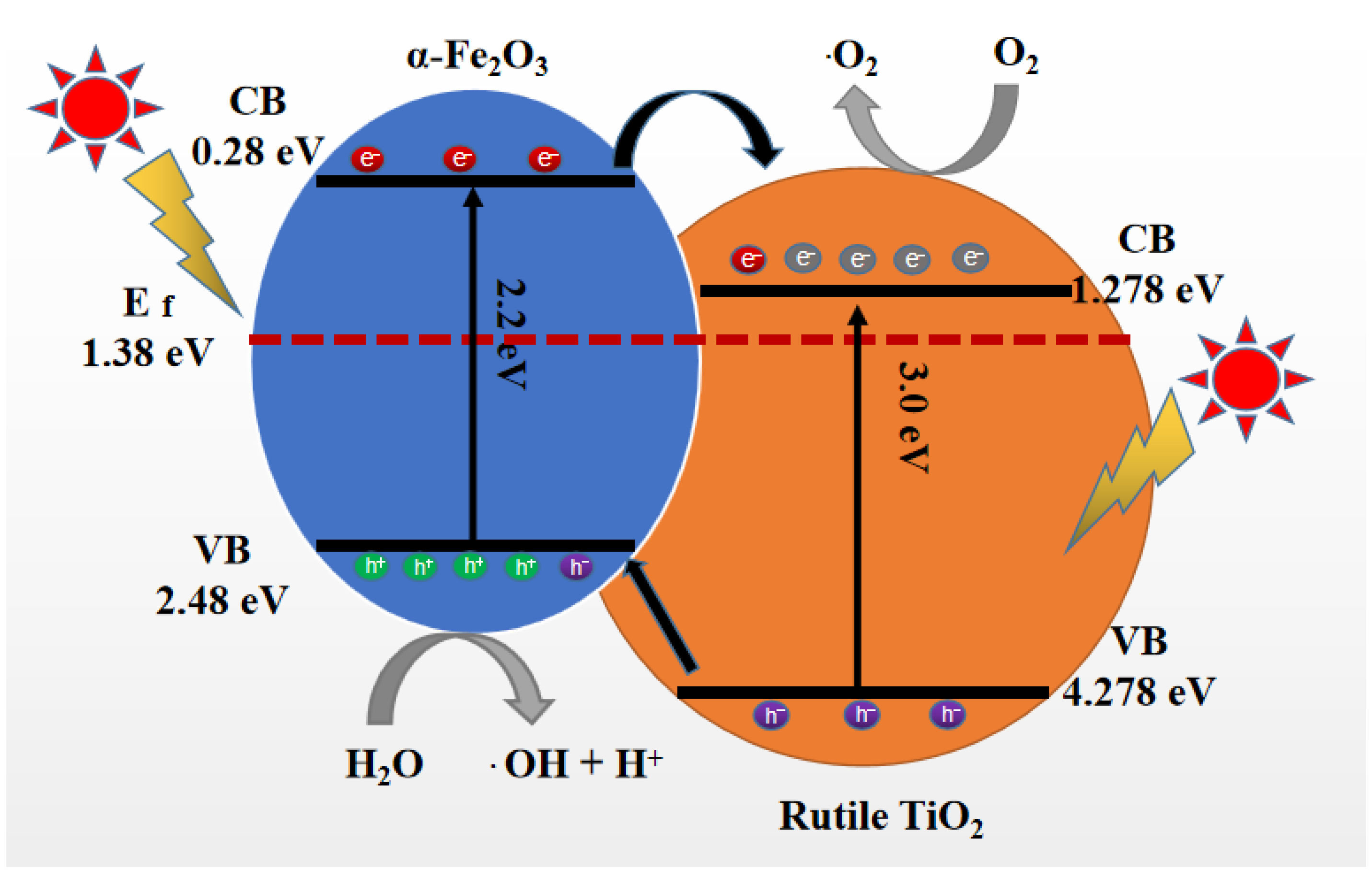
2.3. Enhancement of Photocatalytic Activity
3. Materials and Methods
3.1. Materials and Techniques
3.2. Synthesis of the Rutile TiO2 (RT)
3.3. Synthesis of the α-Fe2O3
3.4. Synthesis of Hollow α-Fe2O3/RT Nanospheres
3.5. Photocatalytic Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Asaithambi, S.; Sakthivel, P.; Karuppaiah, M. Influence of Ni Doping in SnO2 Nanoparticles with Enhanced Visible Light Photocatalytic Activity for Degradation of Methylene Blue Dye. J. Nanosci. Nanotechnol. 2019, 19, 4438–4446. [Google Scholar] [CrossRef]
- Idrus-Saidi, S.; Tang, J.; Ghasemian, M.; Yang, J.; Han, J.-L.; Syed, N.; Daeneke, T.; Abbasi, R.; Koshy, P.; O′Mullane, A.; et al. Liquid metal core-shell structures functionalised via mechanical agitation: The example of Field′s metal. J. Mater. Chem. A 2019, 7, 17876. [Google Scholar] [CrossRef]
- Kment, S.; Riboni, F.; Pausova, S.; Wang, L.; Han, H. Photoanodes based on TiO2 and α-Fe2O3 for solar water splitting-the superior role of 1D nanoarchitecture and combined heterostructures. Chem. Soc. Rev. 2017, 46, 3716–3769. [Google Scholar] [CrossRef]
- Ghasemian, M.; Mayyas, M.; Idrus-Saidi, S.; Jamal, M.; Yang, J.; Daeneke, T.; Kalantar-Zadeh, K. Self-Limiting Galvanic Growth of MnO2 Mnolayers on a Liquid Metal-Appled to Photocatalysis. Adv. Funct. Mater. 2019, 29, 1901649. [Google Scholar] [CrossRef]
- Wang, F.Y.; Yang, Q.D.; Xu, G.; Lei, N.Y.; Ho, J.C. Highly active and enhanced photocatalytic silicon nanowire arrays. Nanoscale 2011, 3, 3269. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Bai, L.L.; Li, Z.J. Review of strategies for the fabrication of heterojunctional nanocomposites as efficient visible-light catalysts by modulating excited electrons with appropriate thermodynamic energy. J. Mater. Chem. A 2019, 7, 10879. [Google Scholar] [CrossRef]
- Gao, C.M.; Wei, T.; Zhang, Y.Y.; Song, X.H.; Huan, Y.; Liu, H.; Chen, X.D. A Photoresponsive Rutile TiO2 Heterojunction with Enhanced Electron-Hole Separation for High-Performance Hydrogen Evolution. Adv. Mater. 2019, 31, 1806596. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Q.; Liu, G.; Cheng, H.M. Enhanced Photocatalytic H2 Production in Core-Shell Engineered Rutile TiO2. Adv. Mater. 2016, 28, 1600495. [Google Scholar] [CrossRef] [PubMed]
- Luan, P.; Xie, M.; Liu, D.N. Effective charge separation in the rutile TiO2 nanorod-coupled α-Fe2O3 with exceptionally high visible activities. Sci. Rep. 2014, 4, 6180. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Gao, L.; Zhang, Q. Synthesizing and Comparing the Photocatalytic Properties of High Surface Area Rutile and Anatase Titania Nanoparticles. J. Am. Ceram. Soc. 2003, 86, 1677–1682. [Google Scholar] [CrossRef]
- Ohno, T.; Furukawa, K.; Matsumura, M. Photocatalytic Activities of Pure Rutile Particles Isolated from TiO2 Powder by Dissolving the Anatase Component in HF Solution. J. Phys. Chem. B 2001, 105, 2417–2420. [Google Scholar] [CrossRef]
- Naya, S.; Nikawa, T.; Kimura, K.; Tada, H. Rapid and Complete Removal of Nonylphenol by Gold Nanoparticle/Rutile Titanium (IV) Oxide Plasmon Photocatalyst. ACS Catal. 2015, 3, 10–13. [Google Scholar] [CrossRef]
- Xia, C.X.; Wang, H.; Kim, J.K.; Wang, J.Y. Rational Design of Metal Oxide-Based Heterostructure for Efficient Photocatalytic and Photoelectrochemical Systems. Adv. Funct. Mater. 2020, 2008247. [Google Scholar] [CrossRef]
- Xu, H.F.; Li, G.; Zhu, G.; Zhu, K.-R.; Jin, S.-W. Enhanced photocatalytic degradation of rutile/anatase TiO2 heterojunction nanoflowers. Catal. Commun. 2015, 62, 52–56. [Google Scholar] [CrossRef]
- Ghosh, M.; Liu, J.W.; Steven, S.; Chuang, C.; Jana, S.-C. Fabrication of Hierarchical V2O5 Nanorods on TiO2 Nanofibers and Their Enhanced Photocatalytic Activity under Visible Light. ChemCatChem 2018, 10, 3305. [Google Scholar] [CrossRef]
- Zhang, R.-N.; Sun, M.-L.; Zhao, G.-D.; Yin, G.-C.; Liu, B. Hierarchical Fe2O3 nanorods/TiO2 nanosheets heterostructure: Growth mechanism, enhanced visible-light photocatalytic and photoelectrochemical performances. Appl. Surf. Sci. 2019, 475, 380–388. [Google Scholar] [CrossRef]
- Kevin, S.; Radek, Z.; Florian, L.F.; Rosa, R. Photoelectrochemical Water Splitting with Mesoporous Hematite Prepared by a Solution-Based Colloidal Approach. J. Am. Chem. Soc. 2010, 132, 7436–7444. [Google Scholar]
- Tao, Q.Q.; Huang, X.; Bi, J.T.; Yu, L.; Hao, H.X. Aerobic Oil-Phase Cyclic Magnetic Adsorption to Synthesize 1D Fe2O3@TiO2 Nanotube Composites for Enhanced Visible-Light Photocatalytic Degradation. Nanomaterials 2020, 10, 1345. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, G.D.; Liu, J.; Yang, B.L. Orthogonal synthesis, structural characteristics, and enhanced visible-light photocatalysis of mesoporous Fe2O3/TiO2 heterostructured microspheres. Appl. Surf. Sci. 2014, 311, 314–323. [Google Scholar] [CrossRef]
- Liu, J.; Yang, S.L.; Wu, W.; Tian, Q.Y.; Cui, S.Y.; Dai, Z.G.; Ren, F. 3D Flowerlike α-Fe2O3 @TiO2 Core-Shell Nanostructures: General Synthesis and Enhanced Photocatalytic Performance. ACS Sustain. Chem. Eng. 2015, 3, 2975–2984. [Google Scholar] [CrossRef]
- Xie, M.Z.; Meng, Q.Q.; Luan, P.; Feng, Y.-J. Synthesis of mesoporous TiO2-coupled Fe2O3 as efficient visible nano-photocatalysts for degrading colorless pollutants. RSC Adv. 2014, 4, 520–527. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zhou, L.; Lou, X.-W. Metal Oxide Hollow Nanostructures for Lithium on Batteries. Adv. Mater. 2012, 24, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.D.; Yang, B.L.; Xiao, T.C.; Yan, Z.F. One-step solvothermal synthesis of hierarchically porous nanostructured CdS/TiO2 heterojunction with higher visible light photocatalytic activity. Appl. Surf. Sci. 2013, 283, 402–410. [Google Scholar] [CrossRef]
- Xiang, L.Q.; Zhao, X.P.; Yin, J.B.; Fan, B.L. Well-organized 3D urchin-like hierarchical TiO2 microspheres with high photocatalytic activity. J. Mater. Sci. 2012, 47, 1436–1445. [Google Scholar] [CrossRef]
- Gao, P.; Sun, D.D. Ultrasonic Preparation of Hierarchical Graphene-Oxide/TiO2 Composite Microspheres for Efficient Photocatalytic Hydrogen Production Chem. Asian J. 2013, 8, 2779–2786. [Google Scholar] [CrossRef]
- Xiang, Q.J.; Yu, J.G.; Cheng, B.; Ong, H.C. Microwave-Hydrothermal Preparation and Visible-Light Photoactivity of Plasmonic Photocatalyst Ag-TiO2 Nanocomposite Hollow Spheres. Chem. Asian J. 2010, 5, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Kiselev, A.; Osterlund, L. Microemulsion-mediated room-temperature synthesis of high-surface-area rutile and its photocatalytic performance. J. Phys. Chem. C 2007, 111, 6789–6797. [Google Scholar] [CrossRef]
- Murray, J.; Kirwan, L.; Loan, M.; Hodnett, B.-K. In-situ synchrotron diffraction study of the hydrothermal transformation of goethite to hematite in sodium aluminate solutions. Hydrometallurgy 2009, 95, 239–246. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, W.; Yu, W.; Liu, H.; Chen, R.; Wei, Y. Ferrihydrite preparation and its application for removal of anionic dyes. Front. Environ. Sci. Eng. 2015, 9, 411–418. [Google Scholar] [CrossRef]
- Žic, M.; Ristić, M.; Musić, S. Effect of phosphate on the morphology and size of α-Fe2O3 particles crystallized from dense β-FeOOH suspensions. J. Alloys Compd. 2008, 466, 498–506. [Google Scholar] [CrossRef]
- Žic, M.; Ristić, M.; Musić, S. Precipitation of α-Fe2O3 from dense β-FeOOH suspensions with added ammonium amidosulfonate. J. Mol. Struct. 2009, 924–926, 235–242. [Google Scholar] [CrossRef]
- Qi, Z.; Liang, H.; Wei, C.; Wang, Z.; Wang, R. X-shaped hollow α-FeOOH penetration twins and their conversion to α-Fe2O3 nanocrystals bound byhigh-index facets with enhanced photocatalytic activity. Chem. Eng. J. 2015, 274, 224–230. [Google Scholar]
- Zhu, L.P.; Wang, L.L.; Bing, N.C.; Huang, C.; Wang, L.J.; Liao, G.H. Porous flfluorine-doped γ-Fe2O3 hollow spheres: Synthesis, growth mechanism, and their application in photocatalysis. ACS Appl. Mater. Interfaces 2013, 5, 12478–12487. [Google Scholar] [CrossRef]
- Lou, X.W.; Wang, Y.; Yuan, C.; Lee, J.Y.; Archer, L.A. Template-free synthesis of SnO2 hollow nanostructures with high lithium storage capacity. Adv. Mater. 2006, 18, 2325–2329. [Google Scholar] [CrossRef]
- Zhu, L.P.; Xiao, H.M.; Zhang, W.-D.; Yang, G.; Fu, S.-Y. One-pot template-free synthesis of monodisperse and single-crystal magnetite hollow spheres by a simple solvothermal route. Cryst. Growth Des. 2008, 8, 957–963. [Google Scholar] [CrossRef]
- Yu, J.; Yu, X.; Huang, B.; Zhang, X.; Dai, Y. Hydrothermal synthesis and visible-light photocatalytic activity of novel cage-like ferric oxide hollow spheres. Cryst. Growth Des. 2009, 9, 1474–1480. [Google Scholar] [CrossRef]
- Niu, K.Y.; Park, J.; Zheng, H.M.; Alivisatos, A.P. Revealing Bismuth Oxide Hollow Nanoparticle Formation by the Kirkendall Effffect. Nano Lett. 2013, 13, 5715–5719. [Google Scholar] [CrossRef]
- Liang, H.; Chen, W.; Jiang, X.; Xu, X.; Xu, B.; Wang, Z. Synthesis of 2D hollow hematite microplatelets with tuneable porosity and their comparative photocatalytic activities. J. Mater. Chem. A 2014, 2, 4340–4346. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, Y.L.; Hua, Q.S.; Zhang, J.M.; Zhang, Y.S.; Yuan, J.S. Controlled Synthesis of Hollow α-Fe2O3 Microspheres Assembled With Ionic Liquid for Enhanced Visible-Light Photocatalytic Activity. Front. Chem. 2019, 10, 3389. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.L.; Xu, Y.; Gao, Q.; Wu, D.; Sun, Y.-H. Controllable synthesis and magnetism of iron oxides nanorings. J. Nanosci. Nanotechnol. 2010, 10, 2348–2359. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.L.; Liu, Z.Y.; Tian, H.; Xu, Y.; Wu, D.; Sun, Y.H. Single-Crystalline Dodecahedral and Octodecahedral α-Fe2O3 Particles Synthesized by a Fluoride Anion-Assisted Hydrothermal Method. Adv. Funct. Mater. 2010, 20, 3987–3996. [Google Scholar] [CrossRef]
- Li, Z.; Cai, W.; Duan, G.; Liu, P. Magnetic step regions in α-Fe2O3 nanostructured ring arrays. Phys. E 2008, 40, 680–683. [Google Scholar] [CrossRef]
- Fang, J.; Shi, F.; Bu, J.; Ding, J.; Xu, S.; Bao, J. One-Step Synthesis of Bifunctional TiO2 Catalysts and Their Photocatalytic Activity. J. Phys. Chem. C 2010, 114, 7940–7948. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, X.; Zhang, S.; Ren, F.; Jiang, C. Facile Method to Synthesize Magnetic Iron Oxides/TiO2 Hybrid Nanoparticles and Their Photodegradation Application of Methylene Blue. Nanoscale Res. Lett. 2011, 6, 533. [Google Scholar] [CrossRef]
- Palanisamy, B.; Babu, C.; Sundaravel, B.; Anandan, S.; Murugesan, V. Sol-Gel Synthesis of Mesoporous Mixed Fe2O3/TiO2 Photocatalyst: Application for Degradation of 4-chlorophenol. J. Hazard. Mater. 2013, 252–253, 233–242. [Google Scholar] [CrossRef]
- Peng, L.; Xie, T.; Lu, Y.; Fan, H.; Wang, D. Synthesis, Photoelectric Properties and Photocatalytic Activity of the Fe2O3/TiO2 Heterogeneous Photocatalysts. Phys. Chem. Chem. Phys. 2010, 12, 8033–8041. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Huang, F.; Cui, L.; Huang, P.; Yu, Y.; Wang, Y. Hydrothermal Synthesis, Structural Characteristics, and Enhanced Photocatalysis of SnO2/α-Fe2O3 Semiconductor Nanoheterostructures. ACS Nano 2010, 4, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, K.; Jang, E.; Park, T.J. Novel visible light active graphitic C3N4-TiO2 composite photocatalyst: Synergistic synthesis, growth and photocatalytic treatment of hazardous pollutants. Appl. Catal. B 2013, 142, 718–728. [Google Scholar] [CrossRef]
- Mrowetz, M.; Balcerski, W.; Colussi, A.J.; Hoffmann, M.R. Oxidative Power of Nitrogen-Doped TiO2 Photocatalysts under Visible Illumination. J. Phys. Chem. B 2004, 108, 17269–17273. [Google Scholar] [CrossRef]
- Xue, C.; Wang, T.; Yang, G.; Yang, B.; Ding, S. A facile strategy for the synthesis of hierarchical TiO2/CdS hollow sphere heterostructures with excellent visible light activity. J. Mater. Chem. A 2014, 2, 7674–7679. [Google Scholar] [CrossRef]
- Qu, Y.; Duan, X. Progress, challenge and perspective of heterogeneous photocatalysts. Chem. Soc. Rev. 2013, 14, 2568–2580. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Ha, E.; Wu, M.Z.; Hu, X.; Wang, J.; Sun, Y.G.; Li, S.J.; Hu, J.Q. Controllable Hydrothermal Synthesis and Photocatalytic Performance of Bi2MoO6 Nano/Microstructures. Catalysts 2020, 10, 1161. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Yin, H.; Zhao, Y.; Zhang, J.; Li, Y.; Yuan, J.; Tang, J.; Wang, F. Synthesis of Magnetic α-Fe2O3/Rutile TiO2 Hollow Spheres for Visible-Light Photocatalytic Activity. Catalysts 2021, 11, 396. https://doi.org/10.3390/catal11030396
Zhou Z, Yin H, Zhao Y, Zhang J, Li Y, Yuan J, Tang J, Wang F. Synthesis of Magnetic α-Fe2O3/Rutile TiO2 Hollow Spheres for Visible-Light Photocatalytic Activity. Catalysts. 2021; 11(3):396. https://doi.org/10.3390/catal11030396
Chicago/Turabian StyleZhou, Zhongli, Hang Yin, Yuling Zhao, Jianmin Zhang, Yahui Li, Jinshi Yuan, Jie Tang, and Fengyun Wang. 2021. "Synthesis of Magnetic α-Fe2O3/Rutile TiO2 Hollow Spheres for Visible-Light Photocatalytic Activity" Catalysts 11, no. 3: 396. https://doi.org/10.3390/catal11030396
APA StyleZhou, Z., Yin, H., Zhao, Y., Zhang, J., Li, Y., Yuan, J., Tang, J., & Wang, F. (2021). Synthesis of Magnetic α-Fe2O3/Rutile TiO2 Hollow Spheres for Visible-Light Photocatalytic Activity. Catalysts, 11(3), 396. https://doi.org/10.3390/catal11030396





