The Limonene Biorefinery: From Extractive Technologies to Its Catalytic Upgrading into p-Cymene
Abstract
1. Introduction
2. Extraction Methods for an Efficient Recovery of Limonene
3. Upgrading of Limonene into p-Cymene over Heterogeneous Catalysts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, C.H.; Xia, X.; Lin, C.X.; Tong, D.S.; Beltramini, J. Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels. Chem. Soc. Rev. 2011, 40, 5588–5617. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Saha, B.; Luque, R. Hydrodeoxygenation processes: Advances on catalytic transformations of biomass-derived platform chemicals into hydrocarbon fuels. Bioresour. Technol. 2015, 178, 108–118. [Google Scholar] [CrossRef]
- Ruppert, A.M.; Weinberg, K.; Palkovits, R. Hydrogenolysis goes bio: From carbohydrates and sugar alcohols to platform chemicals. Angew. Chem. Int. Ed. 2012, 51, 2564–2601. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodrìguez-Padrón, D.; Luque, R.; Mauriello, F. Reductive catalytic routes towards sustainable production of hydrogen, fuels and chemicals from biomass derived polyols. Renew. Sustain. Energy Rev. 2020, 127, 109852. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F.; et al. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green chemistry, catalysis and valorization of waste biomass. J. Mol. Catal. A Chem. 2016, 422, 3–12. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Malara, A.; Antonucci, V.; Modafferi, V.; Antonucci, P.L. Simultaneous methanation of carbon oxides on nickel-iron catalysts supported on ceria-doped gadolinia. Catal. Today 2019, 257, 565–572. [Google Scholar] [CrossRef]
- Malara, A.; Paone, E.; Frontera, P.; Bonaccorsi, L.; Panzera, G.; Mauriello, F. Sustainable exploitation of coffee silverskin in water remediation. Sustainability 2018, 10, 3547. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Malara, A.; Santangelo, S.; Triolo, C.; Crea, F.; Antonucci, P.L. Trimetallic Ni-based catalysts over gadolinia-doped ceria for green fuel production. Catalysts 2018, 8, 435. [Google Scholar] [CrossRef]
- Ozturk, B.; Winterburn, J.; Gonzalez-Miquel, M. Orange peel waste valorisation through limonene extraction using bio-based solvents. Biochem. Eng. J. 2019, 151, 107298. [Google Scholar] [CrossRef]
- Erasto, P.; Viljoen, A.M. Limonene—A Review: Biosynthetic, Ecological and Pharmacological Relevance. Nat. Prod. Commun. 2008, 3, 1193–1202. [Google Scholar] [CrossRef]
- Ciriminna, R.; Lomeli-Rodriguez, M.; Demma Carà, P.; Lopez-Sanchez, J.A.; Pagliaro, M. Limonene: A versatile chemical of the bioeconomy. Chem. Commun. 2014, 50, 15288. [Google Scholar] [CrossRef] [PubMed]
- Mahato, N.; Sharma, K.; Koteswararao, R.; Sinha, M.; Baral, E.; Cho, M.H. Citrus essential oils: Extraction, authentication and application in food preservation. Cri. Rev. Food Sci. Nutr. 2017, 59, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Uwidia, I.E.; Owolabi, B.J.; Okafor, R.C. Extraction, Derivatization, Characterization and Antifungal Investigation of Limonene from Citrus sinensis Peels. Tanz. J. Sci. 2020, 46, 419–429. [Google Scholar]
- Veillet, S.; Tomao, V.; Ruiz, K.; Chemat, F. Green procedure using limonene in the Dean–Stark apparatus for moisture determination in food products. Anal. Chim. Acta 2010, 674, 49–52. [Google Scholar] [CrossRef]
- Rubulotta, G.; Luska, K.L.; Urbina-Blanco, C.A.; Eifert, T.; Palkovits, R.; Quadrelli, E.A.; Thieuleux, C.; Leitner, W. Highly Selective Hydrogenation of R-(+)-Limonene to (+)-p-1-Menthene in Batch and Continuous Flow Reactors. ACS Sustain. Chem. Eng. 2017, 5, 3762–3767. [Google Scholar] [CrossRef]
- Aissou, M.; Chemat-Djenni, Z.; Yara-Varòn, E.; Fabiano-Tixier, A.-S.; Chemat, F. Limonene as an agro-chemical building block for the synthesis and extraction of bioactive compounds. Comptes Rendus Chim. 2017, 20, 346–358. [Google Scholar] [CrossRef]
- Lohrasbi, M.; Pourbafrani, M.; Niklasson, C.; Taherzadeh, M.J. Process design and economic analysis of a citrus waste biorefinery with biofuels and limonene as products. Bioresour. Technol. 2010, 101, 7382–7388. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Scurria, A.; Fabiano-Tixier, A.-S.; Lino, C.; Avellone, G.; Chemat, F.; Pagliaro, M. Omega 3 Extraction from Anchovy Fillet Leftovers with Limonene: Chemical, Economic, and Technical Aspects. ACS Omega 2019, 4, 15359–15363. [Google Scholar] [CrossRef]
- Ciriminna, R.; Scurria, A.; Avellone, G.; Pagliaro, M. A Circular Economy Approach to Fish Oil Extraction. ChemistrySelect 2019, 4, 5106–5109. [Google Scholar] [CrossRef]
- Pagliaro, M.; Pizzone, D.M.; Scurria, A.; Lino, C.; Paone, E.; Mauriello, F.; Ciriminna, R. Sustainably Sourced Olive Polyphenols and Omega-3 Marine Lipids: A Synergy Fostering Public Health. ACS Food Sci. Technol 2021, in press. [Google Scholar] [CrossRef]
- Negro, V.; Mancini, G.; Ruggeri, B.; Fino, D. Citrus waste as feedstock for bio-based products recovery: Review on limonene case study and energy valorization. Bioresour. Technol. 2016, 214, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Santiago, B.; Moreira, M.T.; Feijoo, G.; González-García, S. Identification of environmental aspects of citrus waste valorization into D-limonene from a biorefinery approach. Biomass Bioenergy 2020, 143, 105844. [Google Scholar] [CrossRef]
- 360 Market Updates. Global Limonene Market Size, Manufacturers, Supply Chain, Sales Channel and Clients, 2020–2026; 360 Market Updates: Maharashtra, India, 2020. [Google Scholar]
- Martin-Luengo, M.A.; Yates, M.; Martinez Domingo, M.J.; Casal, B.; Iglesias, M.; Esteban, M.; Ruiz-Hitzky, E. Synthesis of p-cymene from limonene, a renewable feedstock. Appl. Catal. B 2008, 81, 218–224. [Google Scholar] [CrossRef]
- Swift, K.A.D. Catalytic transformations of the major terpene feedstocks. Topics Catal. 2004, 27, 1–4. [Google Scholar] [CrossRef]
- Parrino, F.; Fidalgo, A.; Palmisano, L.; Ilharco, L.M.; Pagliaro, M.; Ciriminna, R. Polymers of Limonene Oxide and Carbon Dioxide: Polycarbonates of the Solar Economy. ACS Omega 2018, 3, 4884–4890. [Google Scholar] [CrossRef]
- Ciriminna, R.; Parrino, F.; De Pasquale, C.; Palmisano, L.; Pagliaro, M. Photocatalytic partial oxidation of limonene to 1,2 limonene oxide. Chem. Commun. 2018, 54, 1008. [Google Scholar] [CrossRef] [PubMed]
- Cagnoli, M.V.; Casuscelli, S.G.; Alvarez, A.M.; Bengoa, J.F.; Gallegos, N.G.; Samaniego, N.M.; Crivello, M.E.; Ghione, G.E.; Pérez, C.F.; Herrero, E.R.; et al. “Clean” limonene epoxidation using Ti-MCM-41 catalyst. Appl. Catal. A Gen. 2005, 287, 227–235. [Google Scholar] [CrossRef]
- Martin-Luengo, M.A.; Yates, M.; Rojoa, S.E.; Arribas, D.H.; Aguilar, D.; Hitzkya, R.E. Sustainable p-cymene and hydrogen from limonene. Appl. Catal. A 2010, 387, 141–146. [Google Scholar] [CrossRef]
- Buhl, D.; Roberge, D.M.; Hölderich, W.F. Production of p-cymene froma-limonene over silica supported Pd catalysts. Appl. Catal. A Gen. 1999, 188, 287–299. [Google Scholar] [CrossRef]
- Sanches-Silva, A.; Cruz, J.M.; Sendón-Garcia, R.; Paseiro-Losada, P. Determination of butylated hydroxytoluene in food samples by high-performance liquid chromatography with ultraviolet detection and gas chromatography/mass spectrometry. J. AOAC Int. 2007, 90, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Tavera Ruiz, C.P.; Gauthier-Maradei, P.; Capron, M.; Pirez, C.; Gardoll, O.; Katryniok, B.; Dumeignil, F. Transformation of DL Limonene into Aromatic Compounds Using Supported Heteropolyacid Catalysts. Catal. Lett. 2019, 149, 328–337. [Google Scholar] [CrossRef]
- Eggersdorfer, M. Terpenes. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2012; Volume 36, pp. 29–45. [Google Scholar]
- Bueno, A.C.; Brandao, B.B.N.S.; Gusevskaya, E.V. Aromatization of para-menthenic terpenes by aerobic oxidative dehydrogenation catalyzed by p-benzoquinone. Appl. Catal. A Gen. 2008, 351, 226–230. [Google Scholar] [CrossRef]
- Kamitsou, M.; Panagiotou, G.D.; Triantafyllidis, K.S.; Bourikas, K.; Lycourghiotis, A.; Kordulis, C. Transformation of α-limonene into p-cymene over oxide catalysts: A green chemistry approach. Appl. Catal. A Gen. 2014, 474, 224–229. [Google Scholar] [CrossRef]
- Makarouni, D.; Lycourghiotis, S.; Kordouli, E.; Bourikas, K.; Kordulis, C.; Dourtoglou, V. Transformation of limonene into p-cymene over acid activated natural mordenite utilizing atmospheric oxygen as a green oxidant: A novel mechanism. Appl. Catal. B Environ. 2018, 224, 740–750. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.F.; Tsetegho Sokeng, A.J.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M.; et al. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef]
- Fiege, H. Cresols and xylenols. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2012; Volume 10, pp. 419–460. [Google Scholar]
- Ghiaci, M.; Abbaspur, A.; Arshadi, M.; Aghabarari, B. Internal versus external surface active sites in ZSM-5 zeolite: Part 2: Toluene alkylation with methanol and 2-propanol catalyzed by modified and unmodified H3PO4/ZSM-5. Appl. Catal. A Gen. 2007, 316, 32–46. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef]
- Retajczyk, M.; Agnieszka Wróblewska, A. Isomerization and Dehydroaromatization of R-(+)-Limonene Over the Ti-MCM-41 Catalyst: Effect of Temperature, Reaction Time and Catalyst Content on Product Yield. Catalysts 2019, 9, 508. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective extraction of bioactive compounds from plants using recent extraction techniques: A review. J. Chromatogr. A 2021, 1635, 461770. [Google Scholar] [CrossRef]
- Ferhat, M.A.; Boukhatem, M.N.; Hazzit, M.; Meklati, B.Y.; Farid, C. Cold Pressing, Hydrodistillation and Microwave Dry Distillation of Citrus Essential Oil from Algeria: A Comparative Study. Electron. J. Biol. 2016, S1, 30–41. [Google Scholar]
- González-Rivera, J.; Spepi, A.; Ferrari, C.; Duce, C.; Longo, I.; Falconieri, D.; Piras, A.; Tinè, M.R. Novel configurations for a citrus waste based biorefinery: From solventless to simultaneous ultrasound and microwave assisted extraction. Green Chem. 2016, 18, 6399–6696. [Google Scholar] [CrossRef]
- Bustamante, J.; Stempvoort, S.V.; García-Gallarreta, M.; Houghton, J.A.; Briers, H.K.; Budarin, V.L.; Matharu, A.S.; Clark, J.H. Microwave assisted hydro-distillation of essential oils from wet citrus peel waste. J. Clean. Prod. 2016, 137, 598–605. [Google Scholar] [CrossRef]
- Teigiserova, D.A.; Ligia Tiruta-Barna, L.; Ahmadi, A.; Hamelin, L.; Thomsen, M. A step closer to circular bioeconomy for citrus peel waste: A review of yields and technologies for sustainable management of essential oils. J. Environ. Manag. 2021, 280, 111832. [Google Scholar] [CrossRef]
- Nguyen, H. Biogas Production from Solvent Pretreated Orange Peel. Master’s Thesis, Department of Chemical and Biological Engineering, Chalmers University of Technology, Goteborg, Sweden, 2012. [Google Scholar]
- Liu, S.X.; Mamidipally, P.K. Quality comparison of rice bran oil extracted with d-limonene and hexane. Cereal Chem. 2005, 82, 209–215. [Google Scholar] [CrossRef]
- Battista, F.; Remelli, G.; Zanzoni, S.; Bolzonella, D. Valorization of Residual Orange Peels: Limonene Recovery, Volatile Fatty Acids, and Biogas Production. ACS Sustain. Chem. Eng. 2020, 8, 6834–6843. [Google Scholar] [CrossRef]
- Lopresto, C.G.; Petrillo, F.; Casazza, A.A.; Aliakbarian, B.; Perego, P.; Calbrò, V. A non-conventional method to extract D-limonene from waste lemon peels and comparison with traditional Soxhlet extraction. Sep. Purif. Methods 2014, 137, 13–20. [Google Scholar] [CrossRef]
- Paini, M.; Casazza, A.A.; Aliakbarian, B.; Perego, P.; Binello, A.; Cravotto, G. Influence of ethanol/water ratio in ultrasound and high-pressure/high-temperature phenolic compound extraction from agri-food waste. Int. J. Food Sci. Technol. 2016, 51, 349–358. [Google Scholar] [CrossRef]
- Kumar, S.P.J.; Prasad, S.R.; Banerjee, R.; Agarwal, D.K.; Kulkarni, K.S.; Ramesh, K.V. Green solvents and technologies for oil extraction from oilseeds. Chem. Cent. J. 2017, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Mahato, N.; Sinha, M.; Sharma, K.; Rakoti Koteswararao, R.; Cho, M.H. Modern Extraction and Purification Techniques for Obtaining High Purity Food-Grade Bioactive Compounds and Value-Added Co-Products from Citrus Wastes. Foods 2019, 8, 523. [Google Scholar] [CrossRef]
- Karaman, S.; Karasu, S.; Tornuk, F.; Toker, O.S.; Geçgel, Ü.; Sagdic, O.; Ozcan, N.; Gül, O. Recovery potential of cold press byproducts obtained from the edible oil industry: Physicochemical, bioactive, and antimicrobial properties. J. Agric. Food Chem. 2015, 63, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Rassem, H.H.A.; Nour, A.H.; Yunus, R.M. Techniques for Extraction of Essential Oils from Plants: A Review. Aust. J. Basic Appl. Sci. 2016, 10, 117–127. [Google Scholar]
- Zema, D.A.; Calabrò, P.S.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, S.M. Valorisation of citrus processing waste: A review. Waste Manag. 2018, 80, 252–273. [Google Scholar] [CrossRef]
- Ferhat, M.A.; Meklati, B.Y.; Chemat, F. Comparison of different isolation methods of essential oil from Citrus fruits: Cold pressing, hydrodistillation and microwave ‘dry’ distillation. Flavour Fragr. J. 2007, 22, 494–504. [Google Scholar] [CrossRef]
- Ruiz, B.; de Benito, A.; Rivera, J.D.; Flotats, X. Assessment of different pre-treatment methods for the removal of limonene in citrus waste and their effect on methane potential and methane production rate. Waste Manag. Res. 2016, 34, 1249–1257. [Google Scholar] [CrossRef]
- Cannon, J.B.; Cantrell, C.L.; Astatkie, T.; Zheljazkov, V.D. Modification of yield and composition of essential oils by distillation time. Ind. Crop. Prod. 2013, 41, 214–220. [Google Scholar] [CrossRef]
- Lee, C.S.; Binner, E.; Winkworth-Smith, C.; John, R.; Gomes, R.; Robinson, J. Enhancing natural product extraction and mass transfer using selective microwave heating. Chem. Eng. Sci. 2016, 149, 97–103. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Avellone, G.; Danzì, C.; Timpanaro, G.; Locatelli, M.; Carnaroglio, D.; Meneguzzo, F.; Ilharco, L.M.; Pagliaro, M. Integral extraction of Opuntia ficus-indica peel bioproducts via microwave-assisted hydrodiffusion and hydrodistillation. ACS Sustain. Chem. Eng. 2019, 7, 7884–7891. [Google Scholar] [CrossRef]
- Boukroufa, M.; Boutekedjiret, C.; Chemat, F. Development of a green procedure of citrus fruits waste processing to recover carotenoids. Resour. Effic. Technol. 2017, 3, 252–262. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Delisi, R.; Carnaroglio, D.; Grillo, G.; Cravotto, G.; Tamburino, A.; Ilharco, L.M.; Pagliaro, M. High-quality essential oils extracted by an eco-friendly process from different citrus fruits and fruit regions. ACS Sustain. Chem. Eng. 2017, 5, 5578–5587. [Google Scholar] [CrossRef]
- Alves Filho, E.G.; Sousa, V.M.; Rodrigues, S.; de Brito, E.S.; Fernandes, F.A.N. Green ultrasound-assisted extraction of chlorogenic acids from sweet potato peels and sonochemical hydrolysis of caffeoylquinic acids derivatives. Ultrason. Sonochem. 2020, 63, 104911. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Jarillo, J.A.; Carrera, C.; Ferreiro-González, M.; Ángel Álvarez, J.; Palma, M.; Ayuso, J.; Barbero, G.F.; Espada-Bellido, E. Optimization of a Novel Method Based on Ultrasound-Assisted Extraction for the Quantification of Anthocyanins and Total Phenolic Compounds in Blueberry Samples (Vaccinium corymbosum L.). Foods 2020, 9, 1763. [Google Scholar] [CrossRef]
- Khandare, R.D.; Tomke, P.D.; Rathod, V.K. Kinetic modeling and process intensification of ultrasound-assisted extraction of d-limonene using citrus industry waste. Chem. Eng. Process. 2021, 159, 108181. [Google Scholar] [CrossRef]
- Pingret, D.; Fabiano-Tixier, A.-S.; Chemat, F. An improved ultrasound Clevenger for extraction of essential oils. Food Anal. Methods 2014, 7, 9–12. [Google Scholar] [CrossRef]
- Khaw, K.-Y.; Parat, M.-O.; Shaw, P.N.; Falconer, J.R. Solvent Supercritical Fluid Technologies to Extract Bioactive Compounds from Natural Sources: A Review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef]
- Lopresto, C.G.; Meluso, A.; Di Sanzo, G.; Chakraborty, S.; Calabrò, V. Process-intensified waste valorization and environmentally friendly d-limonene extraction. EuroMediterr. J. Environ. Integr. 2019, 4, 31. [Google Scholar] [CrossRef]
- Jokić, S.; Molnar, M.; Cikoš, A.M.; Jakovljević, M.; Šafranko, S.; Jerković, I. Separation of selected bioactive compounds from orange peel using the sequence of supercritical CO2 extraction and ultrasound solvent extraction: Optimization of limonene and hesperidin content. Separ. Sci. Tech. 2020, 55, 2799–2811. [Google Scholar] [CrossRef]
- Mira, B.; Blasco, M.; Berna, A.; Subirats, S. Supercritical CO2 extraction of essential oil from orange peel. Effect of operation conditions on the extract composition. J. Supercrit. Fluids 1999, 14, 95–104. [Google Scholar] [CrossRef]
- Chávez-González, M.L.; López-López, L.I.; Rodríguez-Herrera, R.; Contreras-Esquivel, J.C.; Aguilar, C.N. Enzyme-assisted extraction of citrus essential oil. Chem. Pap. 2016, 70, 412–417. [Google Scholar] [CrossRef]
- Putnik, P.; Bursac Kovecevic, D.; Režek Jambrak, A.; Barba, F.J.; Cravotto, G.; Binello, A.; Lorenzo, J.M.; Shpigelman, A. Innovative “Green” and Novel Strategies for the Extraction of Bioactive Added Value Compounds from Citrus Wastes-A Review. Molecules 2017, 22, 680. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Yılmazoğlu, E.; Akgün, M. p-Cymene production from orange peel oil using some metal catalyst in supercritical alcohols. J. Supercrit Fluids 2018, 131, 37–46. [Google Scholar] [CrossRef]
- Shokouhimehr, M. Magnetically separable and sustainable nanostructured catalysts for heterogeneous reduction of nitroaromatics. Catalysts 2015, 5, 534–560. [Google Scholar] [CrossRef]
- Catrinescu, C.; Fernandes, C.; Castilho, P.; Breen, C. Influence of exchange cations on the catalytic conversion of limonene over Serra de Dentro (SD) and SAz-1 clays Correlations between acidity and catalytic activity/selectivity. Appl. Catal. A 2006, 311, 172–184. [Google Scholar] [CrossRef]
- Rachwalik, R.; Hunger, M.; Sulikowski, B. Transformations of monoterpene hydrocarbons on ferrierite type zeolites. Appl. Catal. A 2012, 427–428, 98–105. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, J.; Luo, Z.; Zhao, C. Mechanisms into dehydroaromatization of bioderived limonene to p-cymene over Pd/HZSM-5 in the presence and absence of H2. RSC Adv. 2016, 6, 66695–66704. [Google Scholar] [CrossRef]
- Lycourghiotis, S.; Makarouni, D.; Kordouli, E.; Bourikas, K.; Kordulis, C.; Dourtoglou, V. Activation of natural mordenite by various acids: Characterization and evaluation in the transformation of limonene into p-cymene. Mol. Catal. 2018, 450, 95–103. [Google Scholar] [CrossRef]
- Roberge, D.M.; Buhl, D.; Niederer, J.P.M.; Hölderich, W.F. Catalytic aspects in the transformation of pinenes to p-cymene. Appl. Catal. A Gen. 2001, 215, 111–124. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, C. A new approach for bio-jet fuel generation from palm oil and limonene in the absence of hydrogen. Chem. Commun. 2015, 51, 17249–17252. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, C. Development of a Bimetallic Pd-Ni/HZSM-5 Catalyst for the Tandem Limonene Dehydrogenation and Fatty Acid Deoxygenation to Alkanes and Arenes for Use as Biojet Fuel. ACS Catal. 2016, 6, 4512–4525. [Google Scholar] [CrossRef]
- Tabanelli, T.; Paone, E.; Blair Vásquez, P.; Pietropaolo, R.; Cavani, F.; Mauriello, F. Transfer Hydrogenation of Methyl and Ethyl Levulinate Promoted by a ZrO2 Catalyst: Comparison of Batch vs Continuous Gas-Flow Conditions. ACS Sustain. Chem. Eng. 2019, 7, 9937–9947. [Google Scholar] [CrossRef]
- Mauriello, F.; Paone, E.; Pietropaolo, R.; Balu, A.M.; Luque, R. Catalytic transfer hydrogenolysis of lignin-derived aromatic ethers promoted by bimetallic Pd/Ni systems. ACS Sustain. Chem. Eng. 2018, 6, 9269–9276. [Google Scholar] [CrossRef]
- Paone, E.; Tabanelli, T.; Mauriello, F. The rise of lignin biorefinery. Curr. Opin. Green Sustain. Chem. 2020, 24, 1–6. [Google Scholar] [CrossRef]
- Paone, E.; Beneduci, A.; Corrente, G.A.; Malara, A.; Mauriello, F. Hydrogenolysis of aromatic ethers under lignin-first conditions. Mol. Catal. 2020, 497, 111228. [Google Scholar] [CrossRef]
- Paone, E.; Espro, C.; Pietropaolo, R.; Mauriello, F. Selective arene production from transfer hydrogenolysis of benzyl phenyl ether promoted by a co-precipitated Pd/Fe3O4 catalyst. Catal. Sci. Technol. 2016, 6, 7937–7941. [Google Scholar] [CrossRef]
- Malara, A.; Paone, E.; Bonaccorsi, L.; Mauriello, F.; Macario, A.; Frontera, P. Pd/Fe3O4 Nanofibers for the catalytic conversion of lignin-derived benzyl phenyl ether under transfer hydrogenolysis conditions. Catalysts 2020, 10, 20. [Google Scholar] [CrossRef]
- Gumina, B.; Mauriello, F.; Pietropaolo, R.; Galvagno, S.; Espro, C. Hydrogenolysis of sorbitol into valuable C3-C2 alcohols at low H2 pressure promoted by the heterogeneous Pd/Fe3O4 catalyst. Mol. Catal. 2018, 446, 152–160. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, S.; Jin, G.; Yang, X.; Zhou, Y.J. Microbial production of limonene and its derivatives: Achievements and perspectives. Biotechnol. Adv. 2020, 44, 107628. [Google Scholar] [CrossRef]
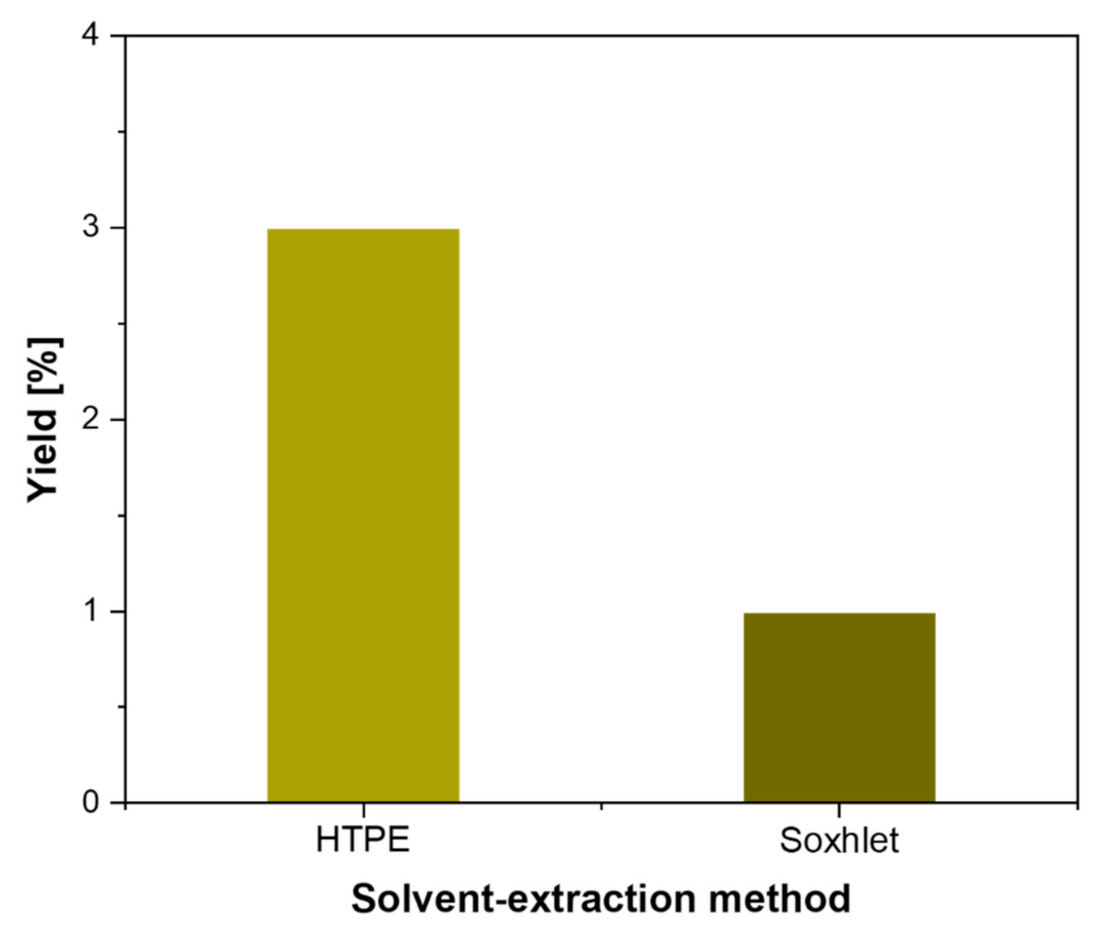
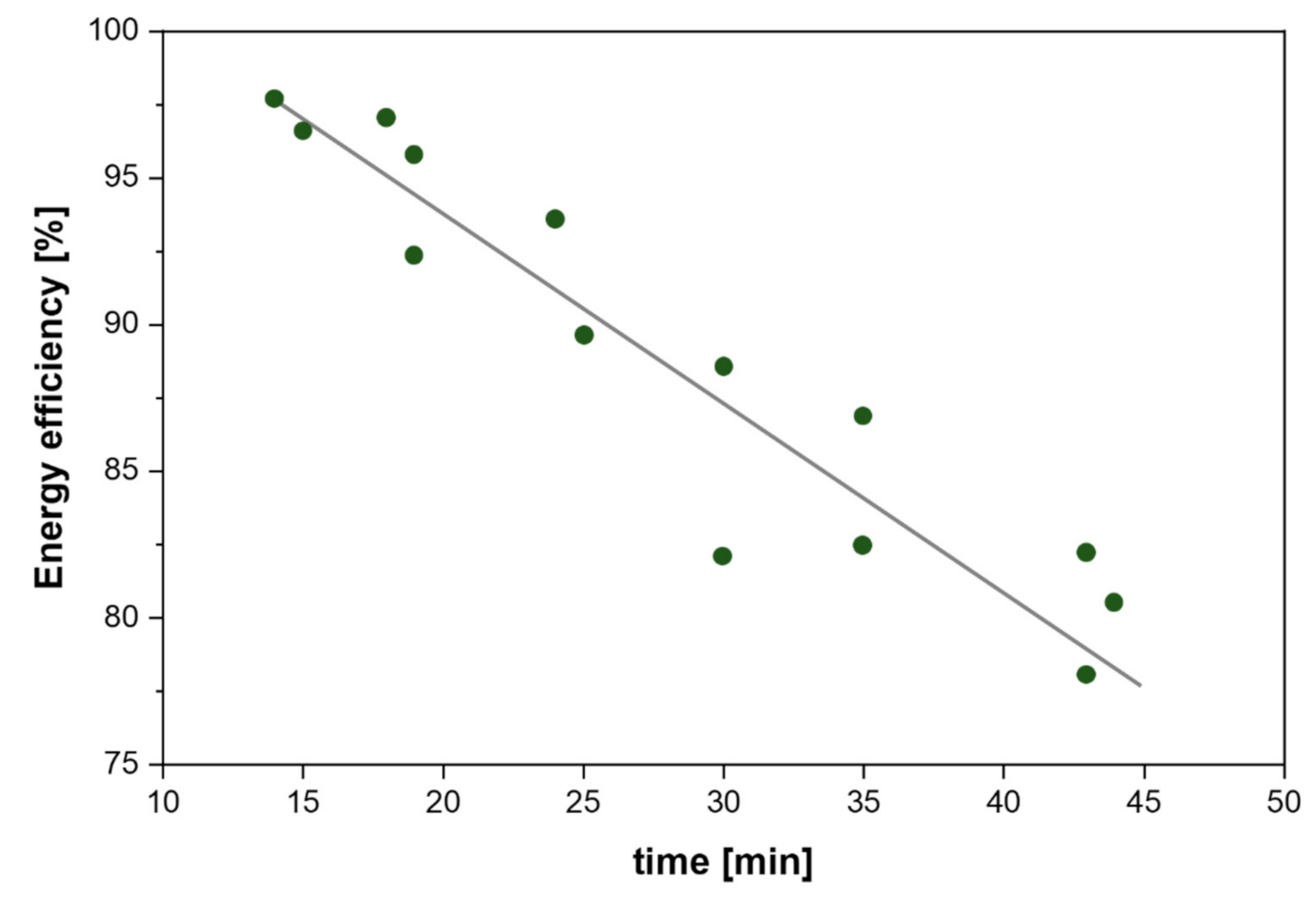
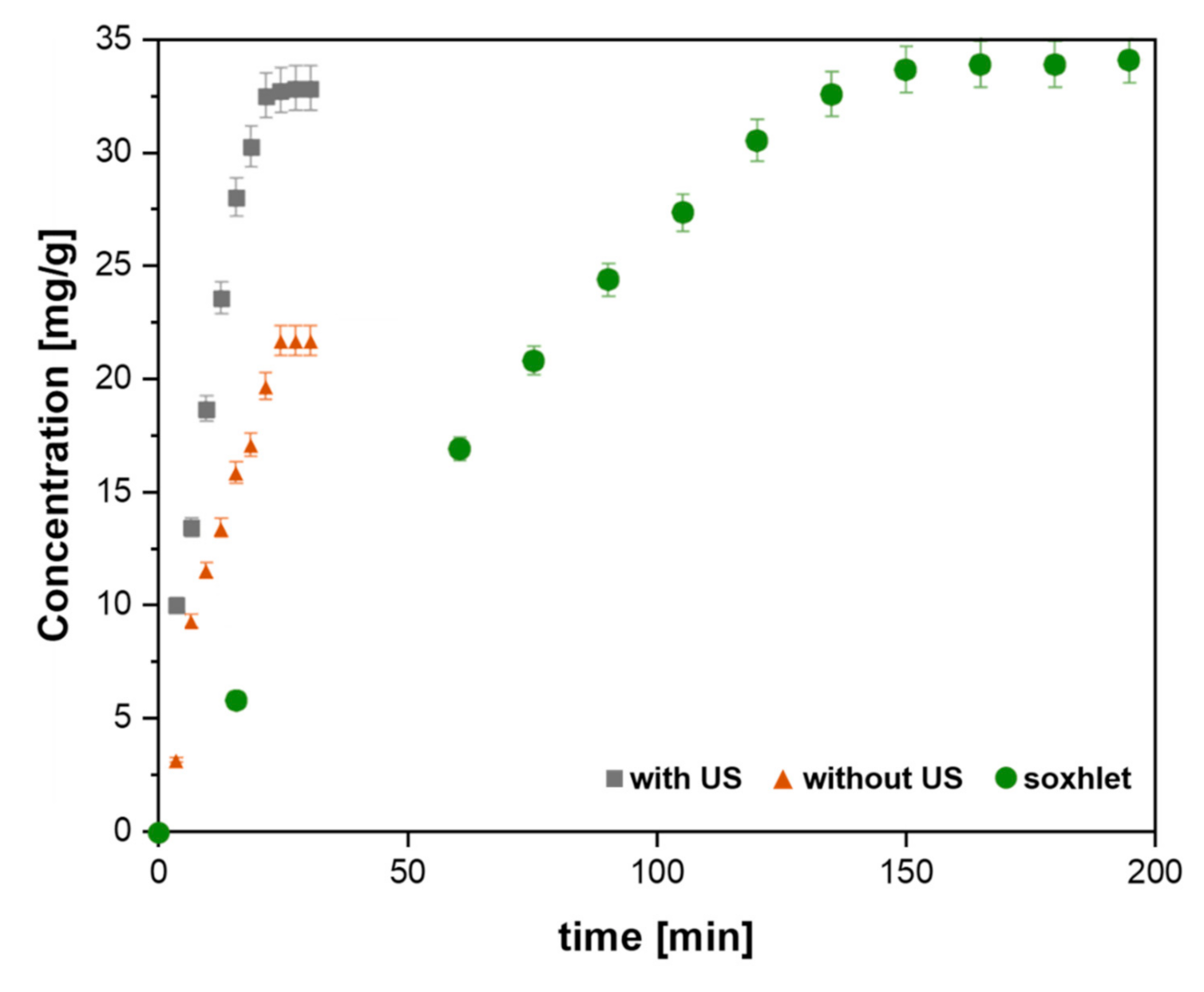
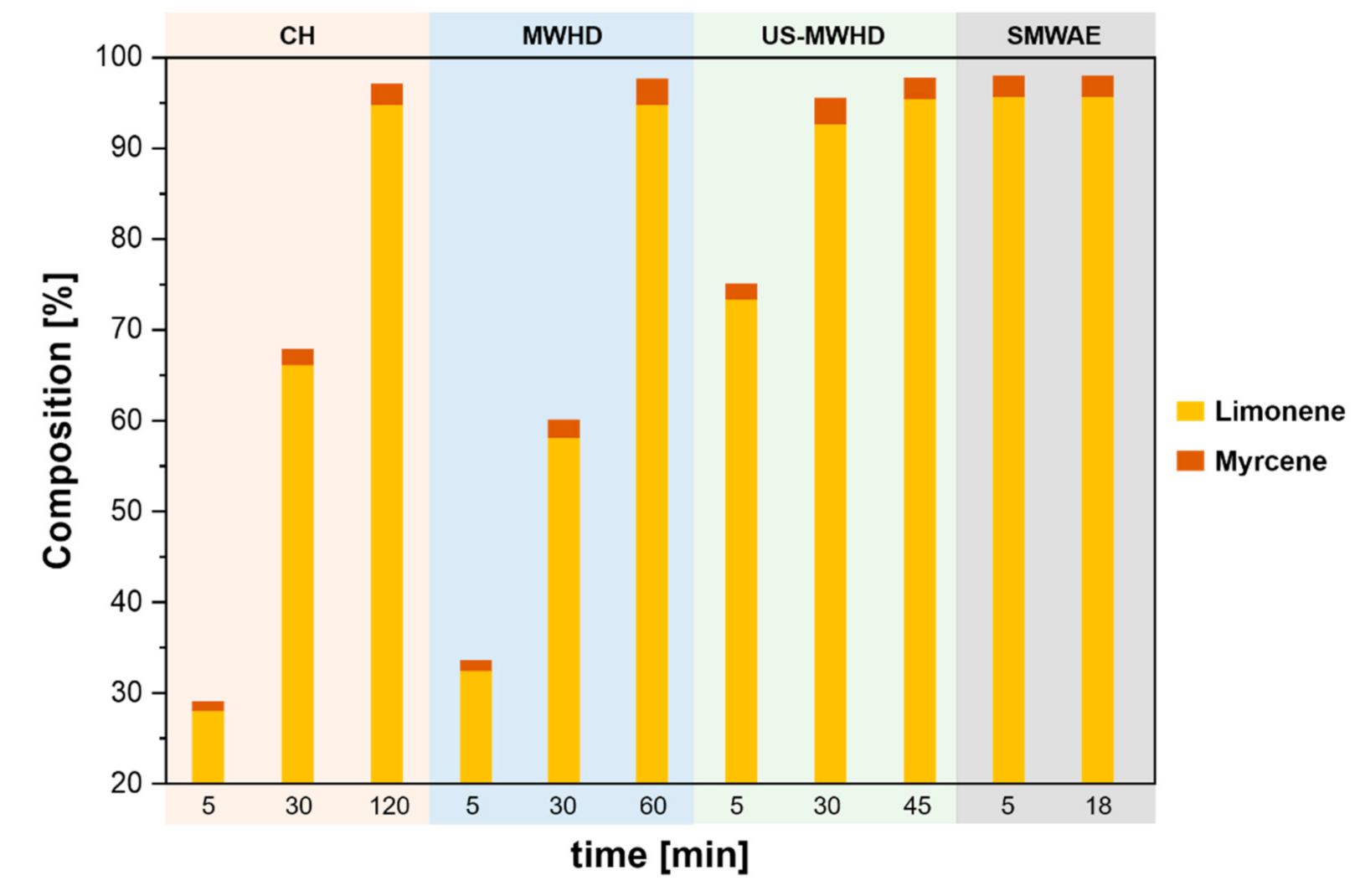

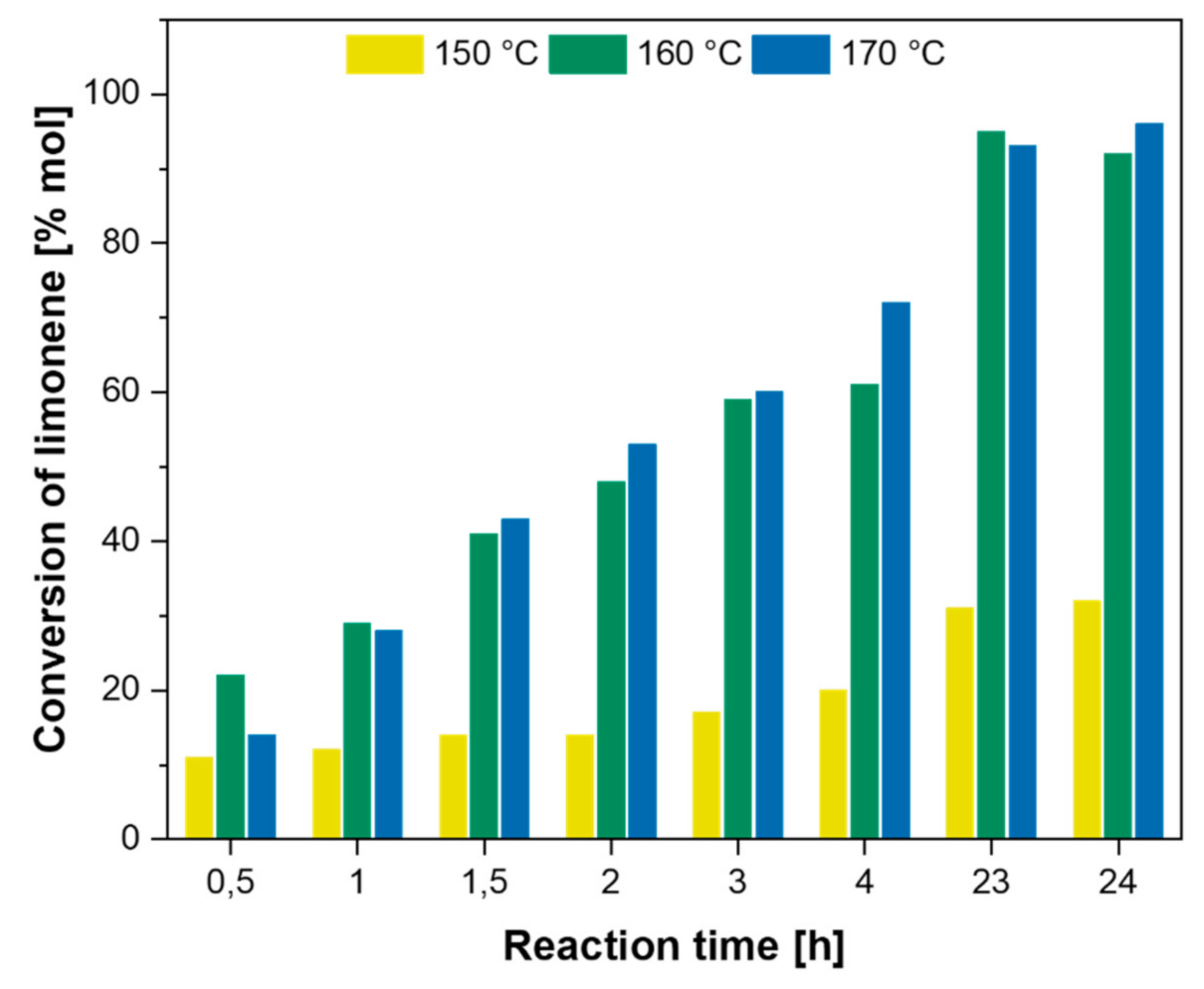
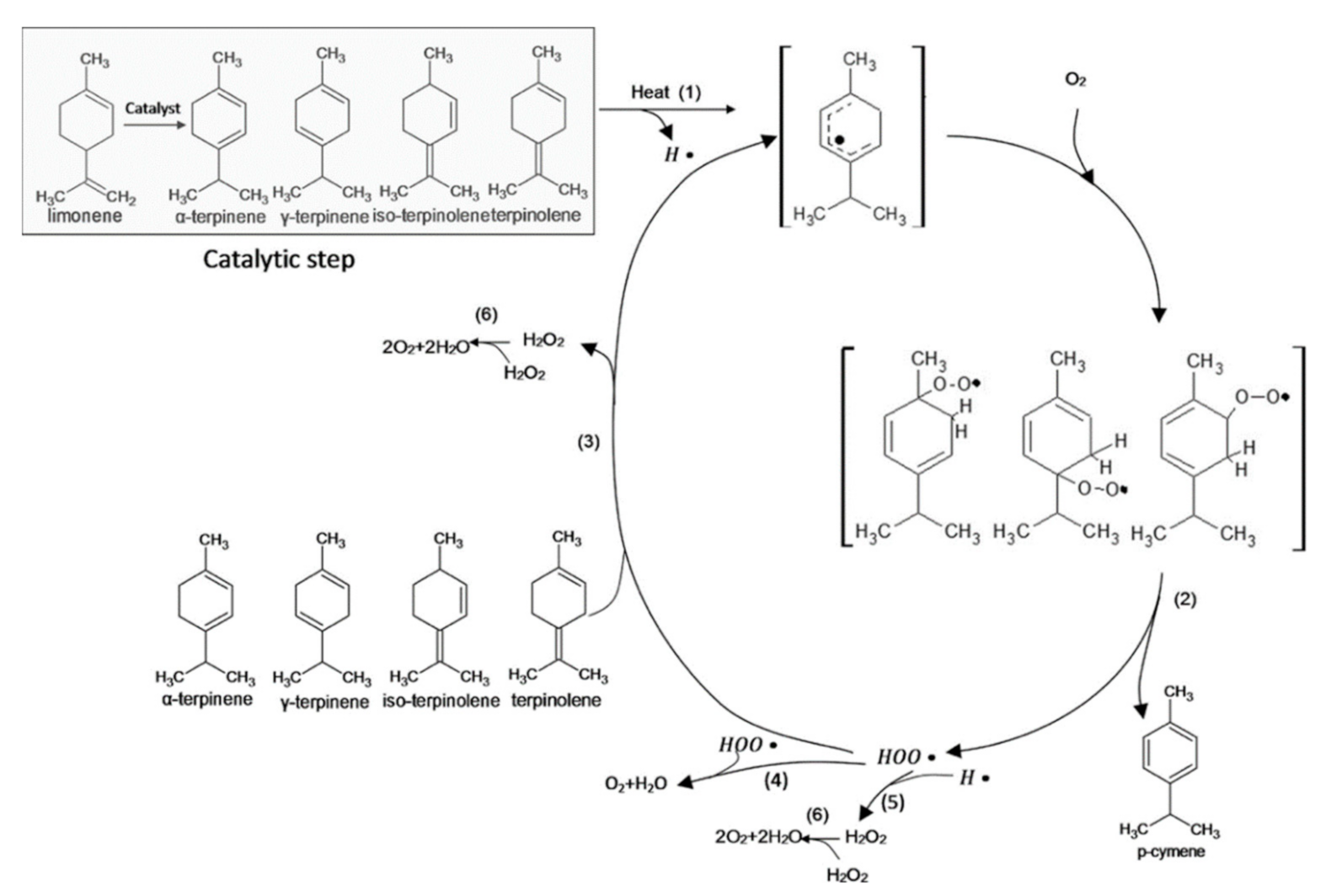

| Citrus Peel Waste | Extractive Method | Limonene (% in EO) | Extraction Conditions | Ref. |
|---|---|---|---|---|
| Orange peel fresh | SFME | 94.6 | Solvent-free, 30 min | [46] |
| MAHD | 80.0 | Water, 60 min, 100 °C | [66] | |
| Orange peel fresh (after juicing) | HD | 96.8 | Water, 240 min, 100 °C | [48] |
| MAHD | 97.4 | Water, 240 min, 100 °C | [48] | |
| MAHD | 80.0 | Water, 80 min, 100 °C | [66] | |
| Orange peel thawed | HD | 94.4 | Water, 155 min | [47] |
| MAHD | 94.7 | Water, 76 min | [47] | |
| SFME | 95.2 | Solvent-free, 5 min | [47] | |
| US-MWHD | 95.0 | Water, 60 min | [47] | |
| Orange flavedo peel fresh | HD | 95.5 | Water, 180 min | [46] |
| MAHD | 55.0 | Water, 75 min, 100 °C | [66] | |
| Orange whole fresh | CP | 96.0 | Water, 90 min | [46] |
| Lemon peel fresh | SFME | 74.0 | Solvent-free, 30 min | [46] |
| MAHD | 50.0 | Water, 80 min, 100 °C | [66] | |
| Lemon peel fresh (after juicing) | MAHD | 68.4 | Water, 240 min, 100 °C | [48] |
| MAHD | 65.0 | Water, 70 min, 100 °C | [66] | |
| Lemon flavedo peel fresh | HD | 72.9 | Water, 180 min | [60] |
| HD | 93.0 | Water, 180 min | [46] | |
| MAHD | 30.0 | Water, 60 min, 100 °C | [66] | |
| Lemon whole fresh | CP | 73.8 | Water, 90 min | [46] |
| Grapefruit peel fresh | SFME | 91.6 | Solvent-free, 30 min | [46] |
| MAHD | 45.0 | Water, 70 min, 100 °C | [66] | |
| Grapefruit peel fresh (after juicing) | MAHD | 89.2 | Water, 240 min, 100 °C | [48] |
| Grapefruit flavedo peel fresh | HD | 92.6 | Water, 180 min | [46] |
| Grapefruit whole fresh | CP | 94.5 | Water, 90 min | [46] |
| Catalyst | Limonene | p-Cymene | Reaction Conditions | Ref. |
|---|---|---|---|---|
| Conversion (%) | Yield (%) | |||
| Ni-SD | 96 | 17 | Batch, n-dodecane, 150 °C, 15 min | [80] |
| H-FER (T) | 38 | n.d. | Batch, 65 °C, 60 min | [81] |
| SIRAL 20 | 100 | 100 | Batch, microwave, 175 °C, 10 min | [26] |
| SepNi | 100 | 100 | Batch, microwave, 210 °C, 20 min | [31] |
| Pd/HZSM-5 (258) | 100 | 82 | Batch, n-dodecane, 260 °C, 2 h, 8 bar N2 | [82] |
| Ti-SBA-15 | 99 | 56 | Batch, 160 °C, 23 h | [43] |
| TECHNOSA-H2 | 98 | 65 | Batch, tetraethylene glycol dimethyl ether, 140 °C, 7 h, N2 | [83] |
| TiO2 | 100 | 90 | Continuous flow, 300 °C, 6 h, H2 | [37] |
| Pd/Al2O3 | 100 | 80 | Continuous flow, SC-ethanol, 300 °C, 30 s, 6.5 MPa | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satira, A.; Espro, C.; Paone, E.; Calabrò, P.S.; Pagliaro, M.; Ciriminna, R.; Mauriello, F. The Limonene Biorefinery: From Extractive Technologies to Its Catalytic Upgrading into p-Cymene. Catalysts 2021, 11, 387. https://doi.org/10.3390/catal11030387
Satira A, Espro C, Paone E, Calabrò PS, Pagliaro M, Ciriminna R, Mauriello F. The Limonene Biorefinery: From Extractive Technologies to Its Catalytic Upgrading into p-Cymene. Catalysts. 2021; 11(3):387. https://doi.org/10.3390/catal11030387
Chicago/Turabian StyleSatira, Antonella, Claudia Espro, Emilia Paone, Paolo Salvatore Calabrò, Mario Pagliaro, Rosaria Ciriminna, and Francesco Mauriello. 2021. "The Limonene Biorefinery: From Extractive Technologies to Its Catalytic Upgrading into p-Cymene" Catalysts 11, no. 3: 387. https://doi.org/10.3390/catal11030387
APA StyleSatira, A., Espro, C., Paone, E., Calabrò, P. S., Pagliaro, M., Ciriminna, R., & Mauriello, F. (2021). The Limonene Biorefinery: From Extractive Technologies to Its Catalytic Upgrading into p-Cymene. Catalysts, 11(3), 387. https://doi.org/10.3390/catal11030387












