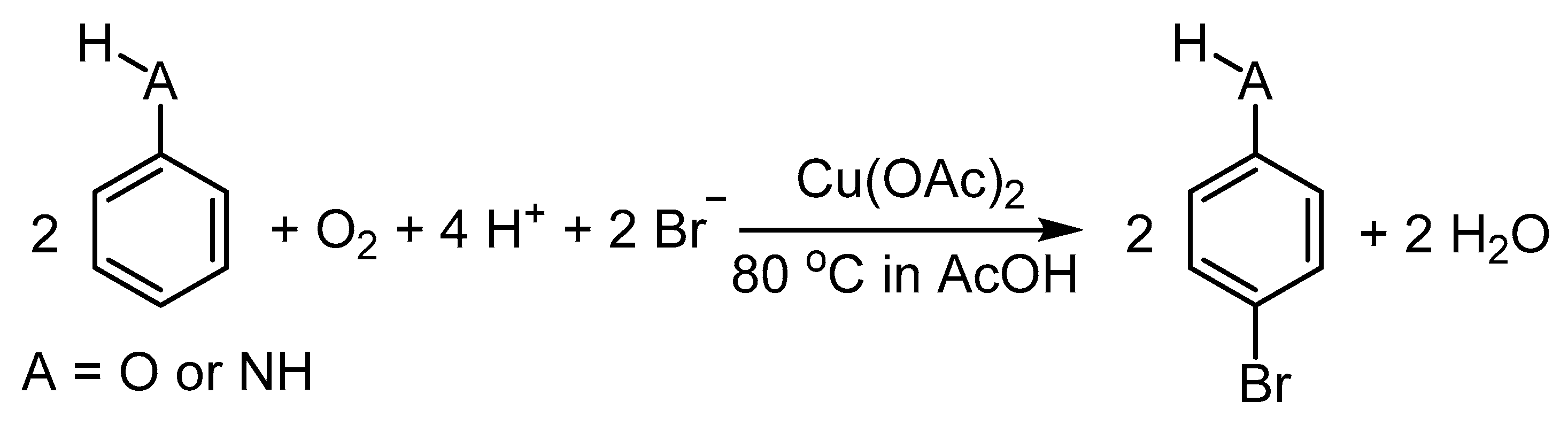The Influence of Copper on Halogenation/Dehalogenation Reactions of Aromatic Compounds and Its Role in the Destruction of Polyhalogenated Aromatic Contaminants
Abstract
1. Introduction
2. Cu-Based Halogenations of Aromatic Compounds
2.1. The Role of Cu-Catalyzed O2-Based Oxidation of Waste HCl Producing Cl2
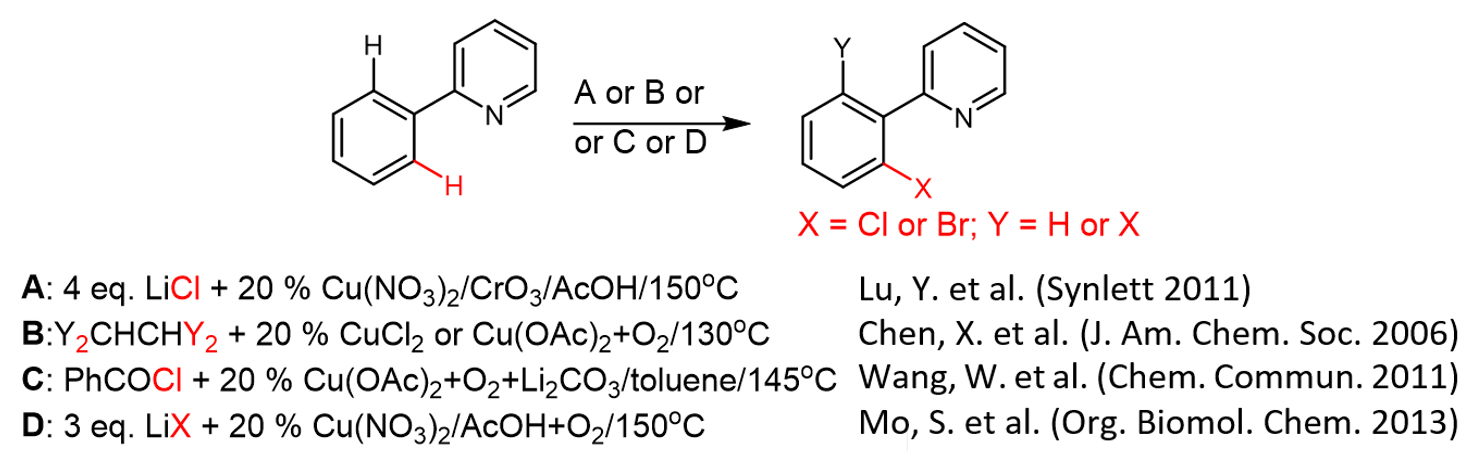
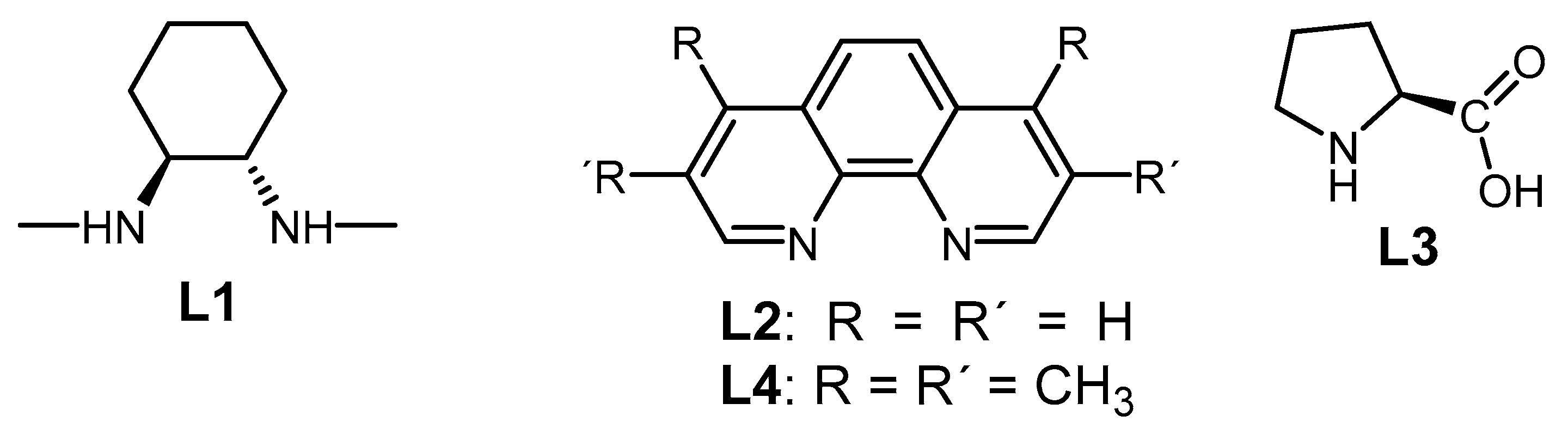
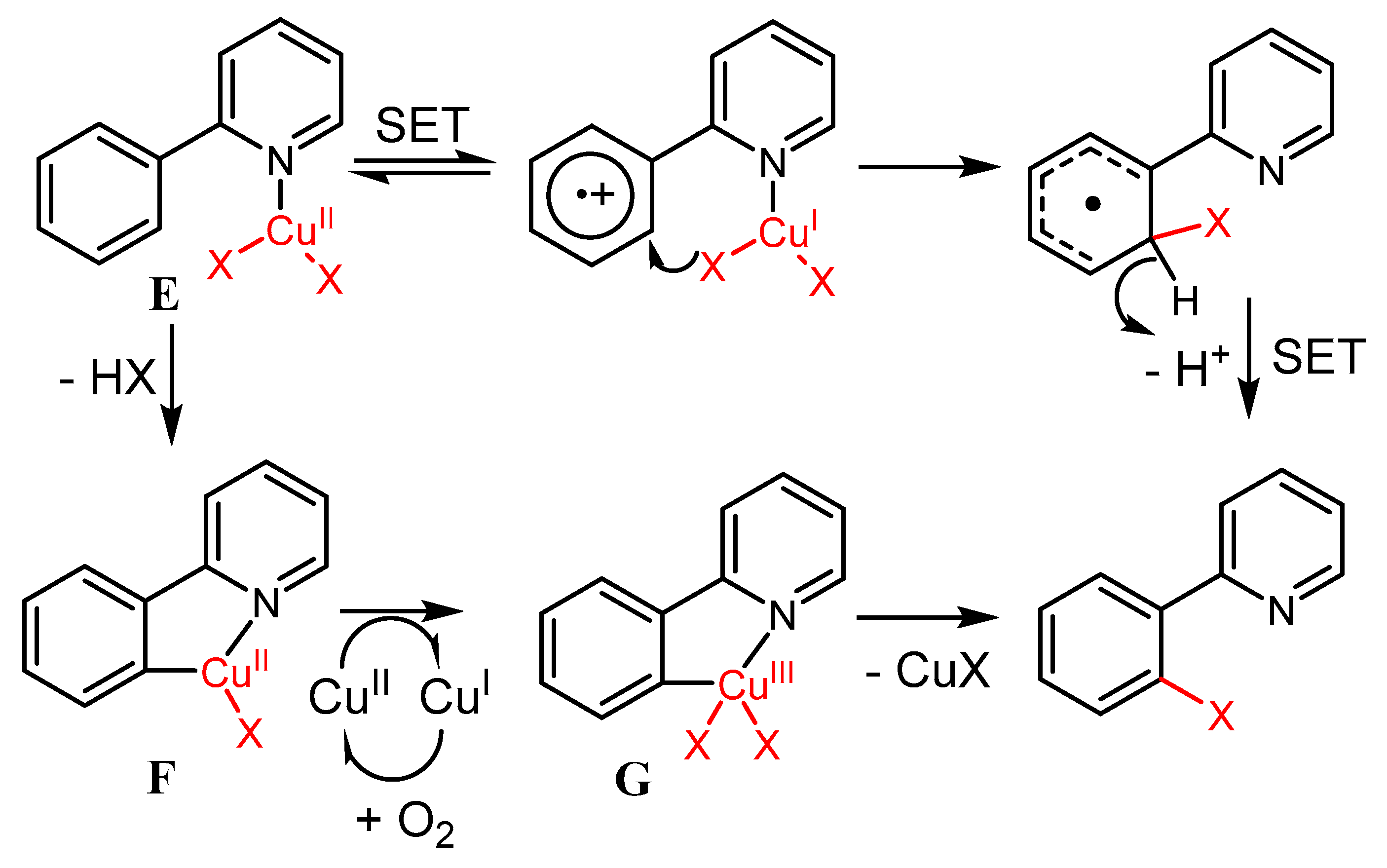
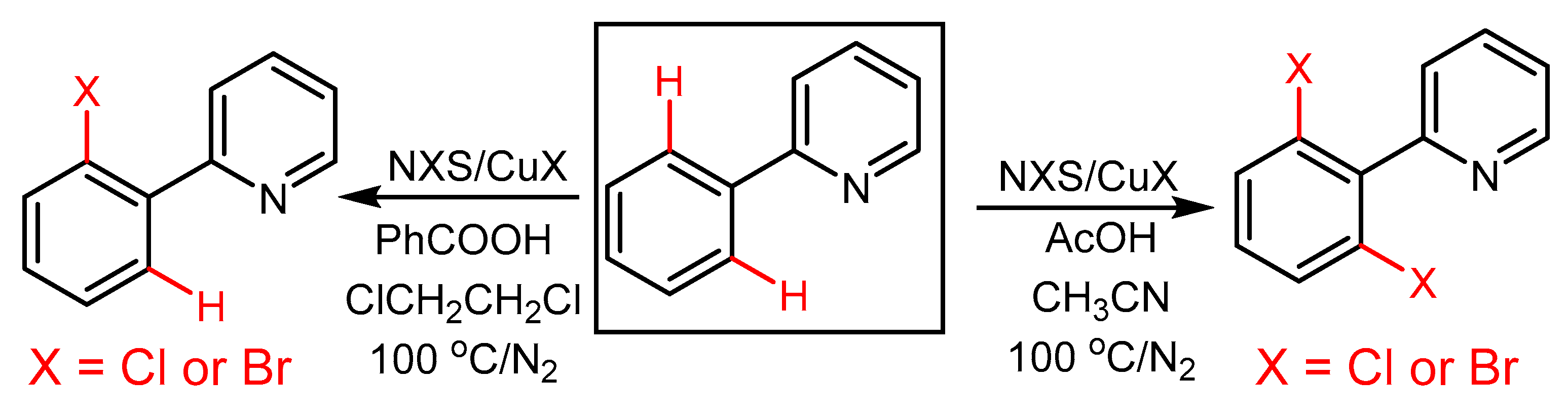
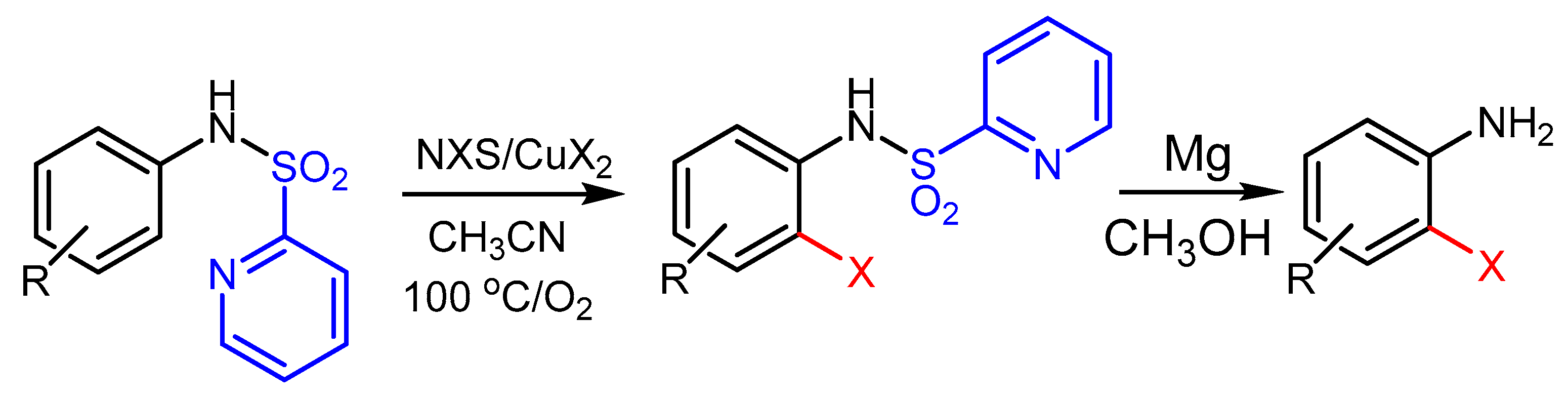
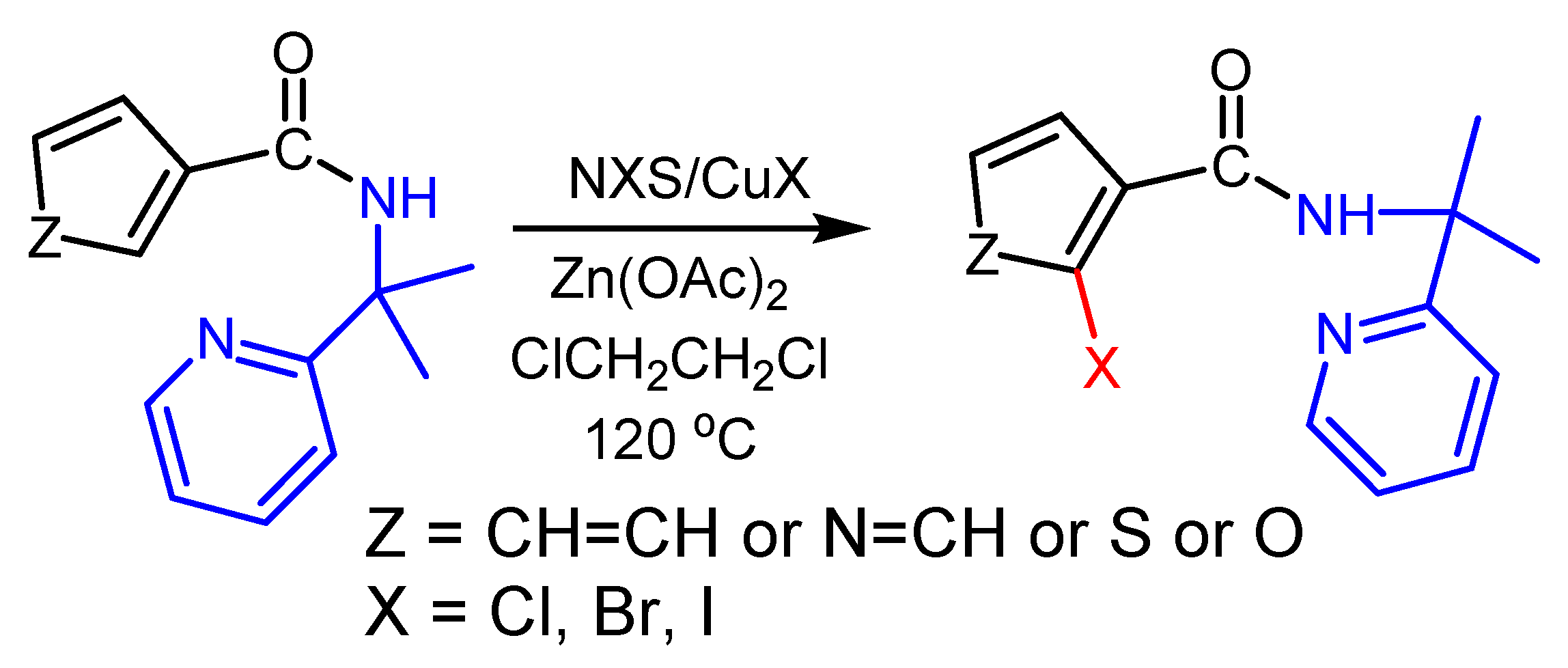
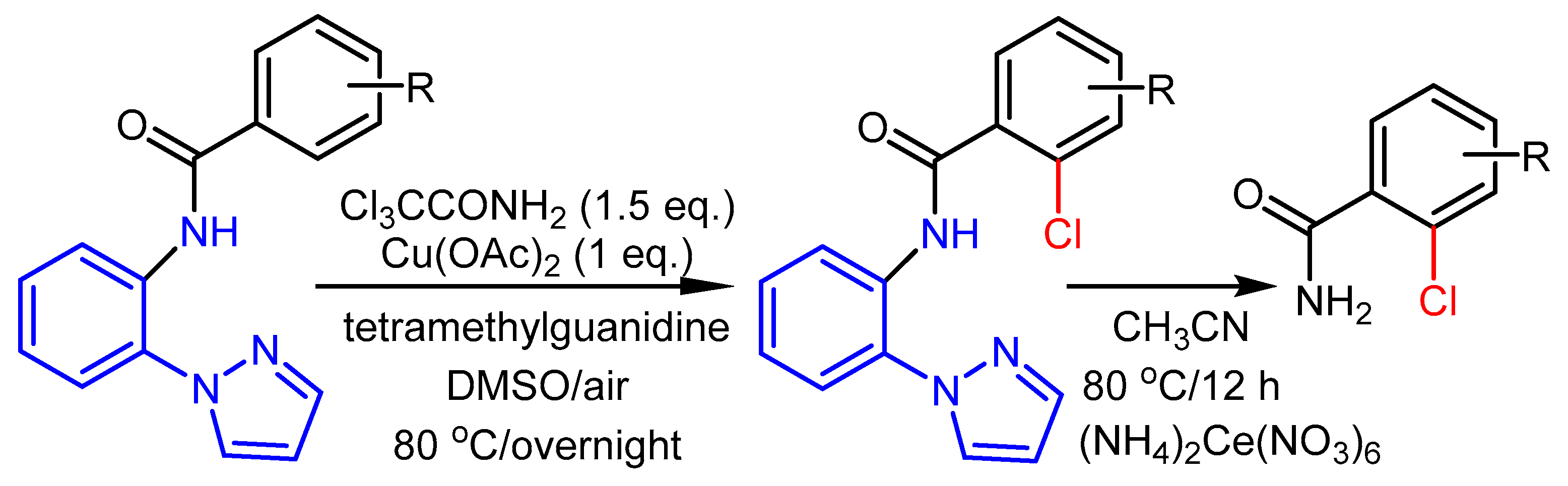
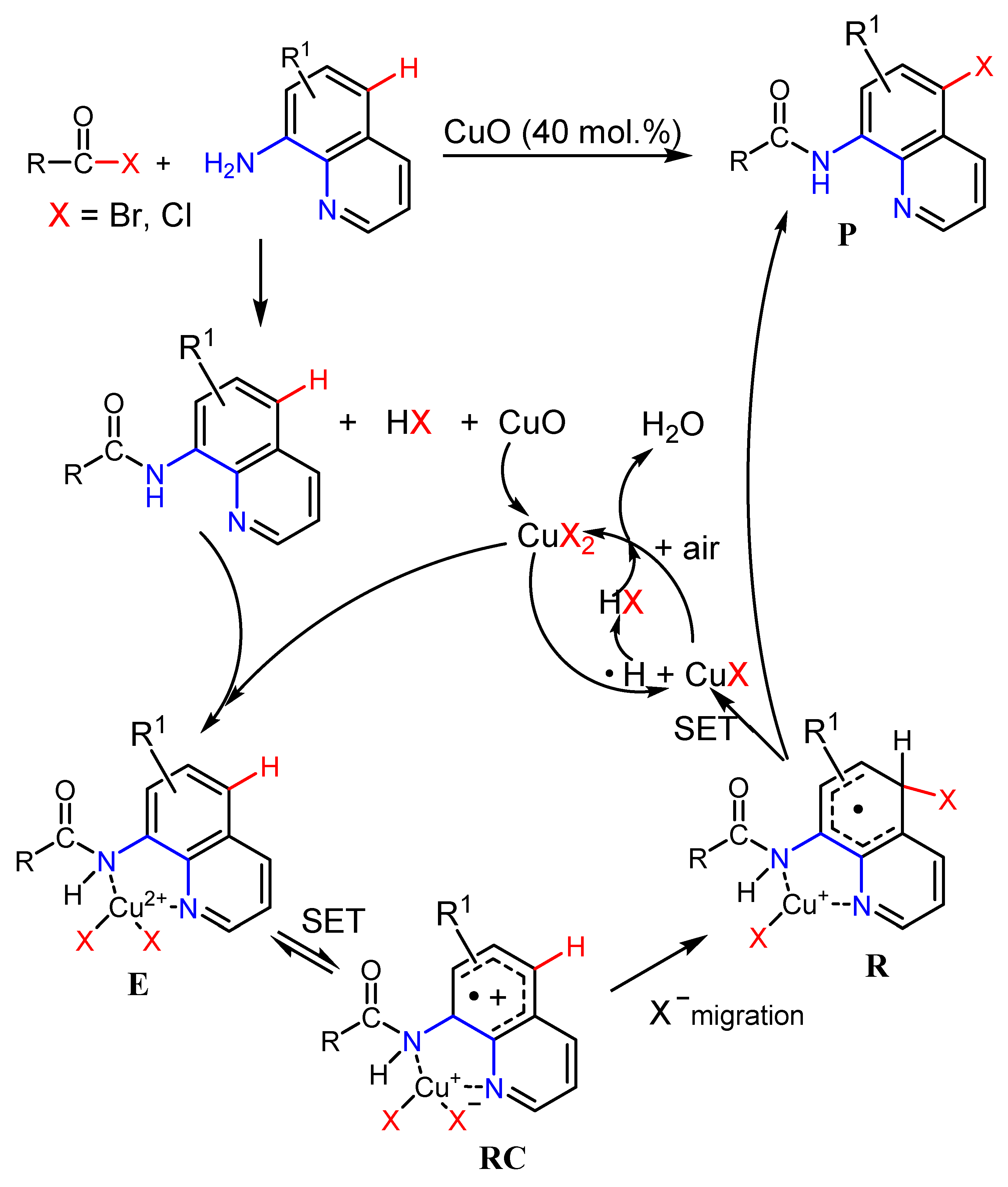
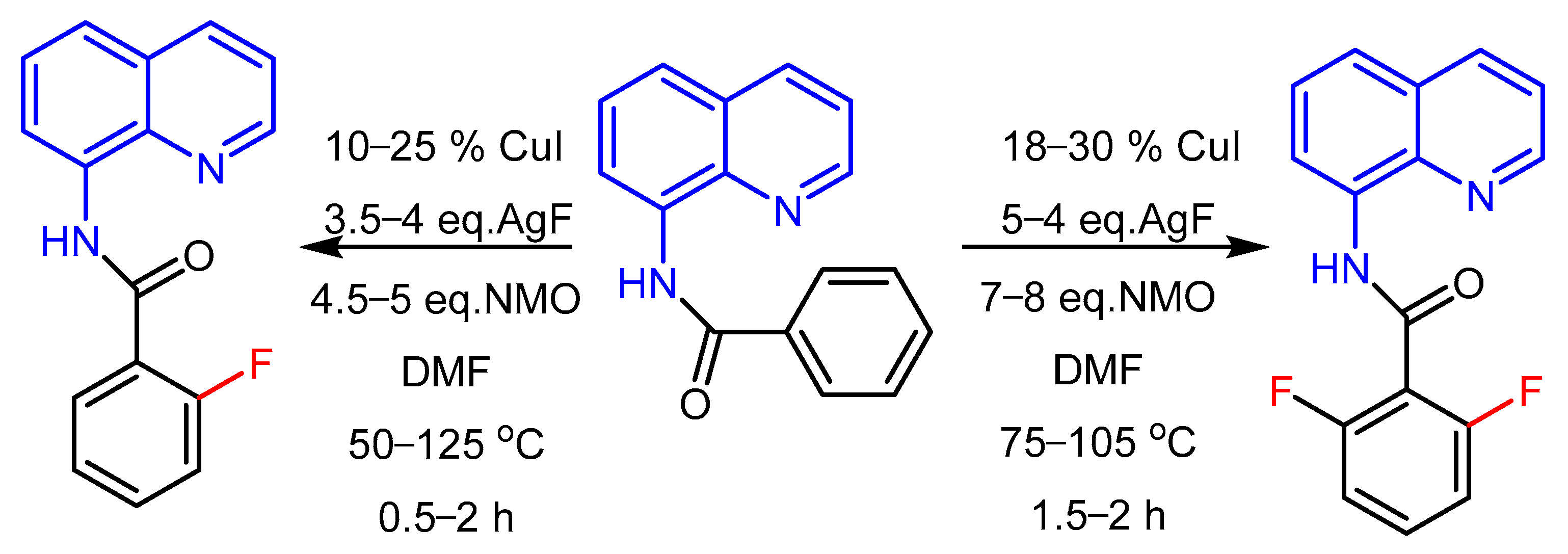
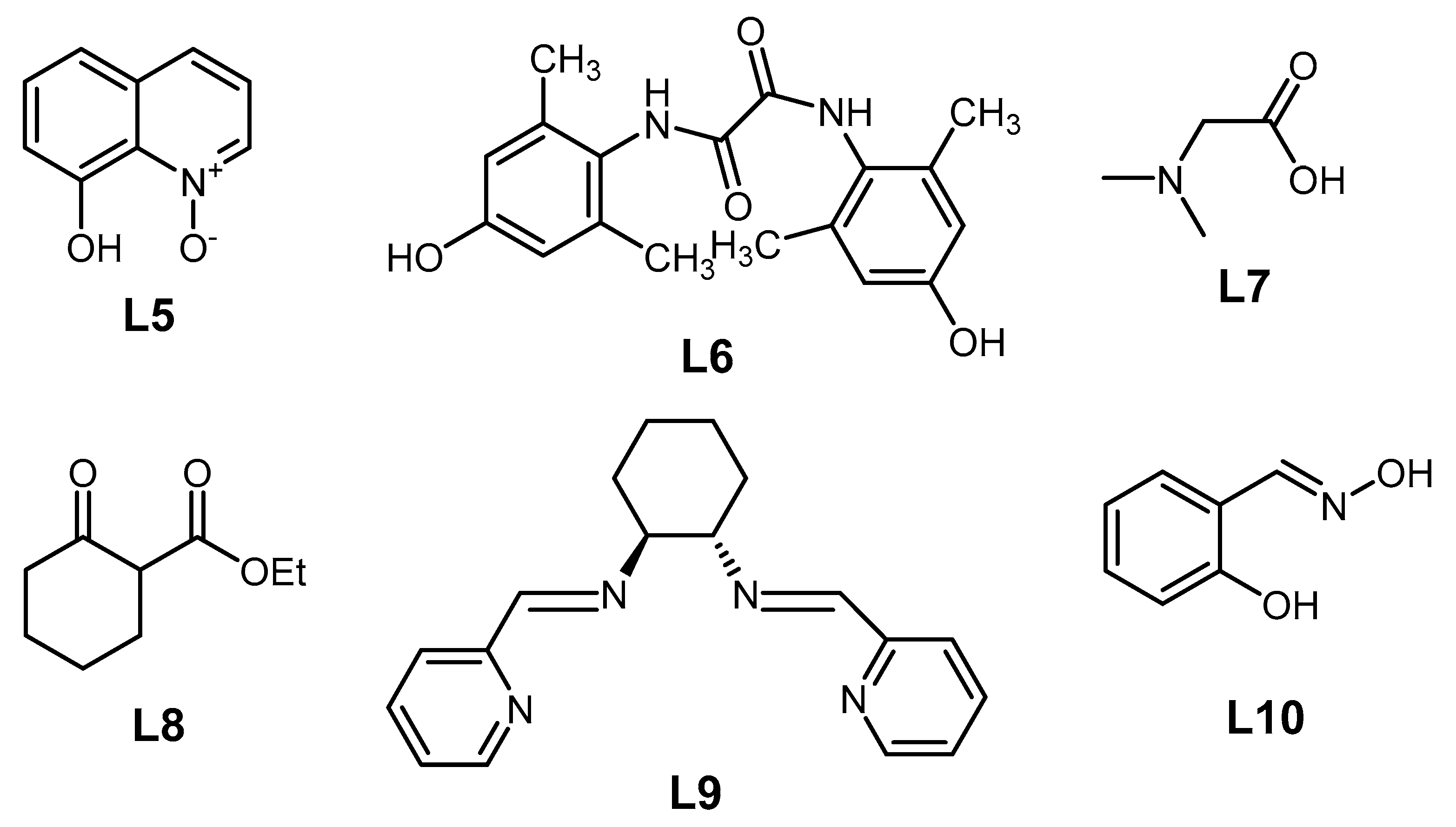
2.2. The Role of Directing Groups in Regioselective Cu-Catalyzed Halogenation of Aromatic Compounds
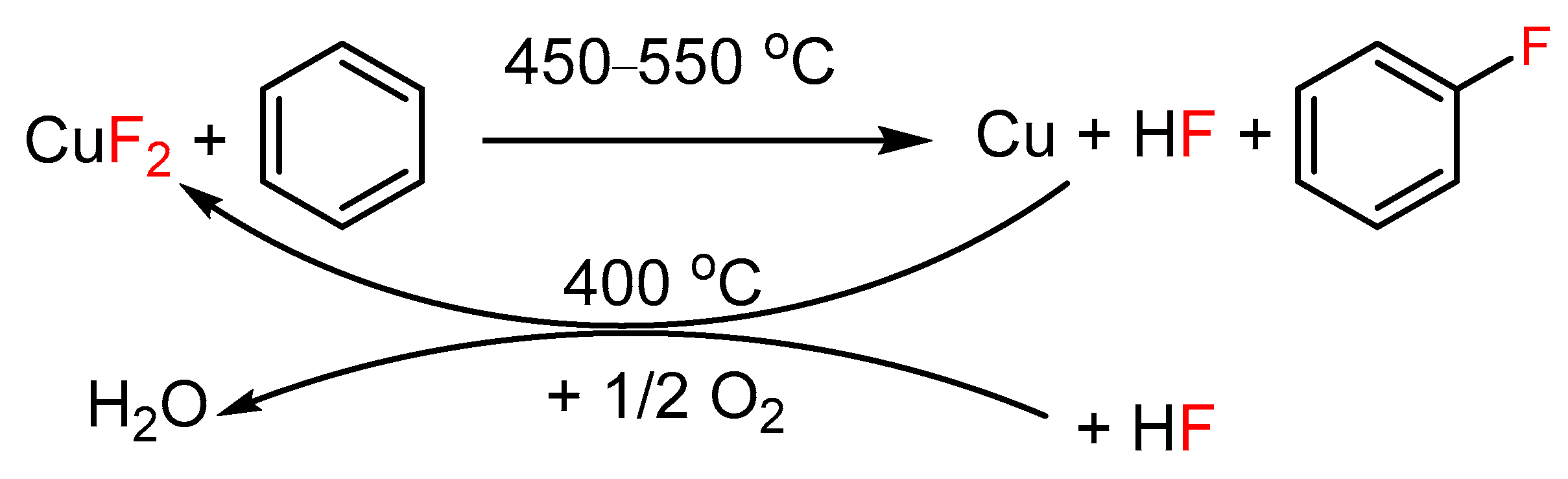
2.3. Alternative Methods Applicable for Cu-Catalyzed Syntheses of Ar-Xs
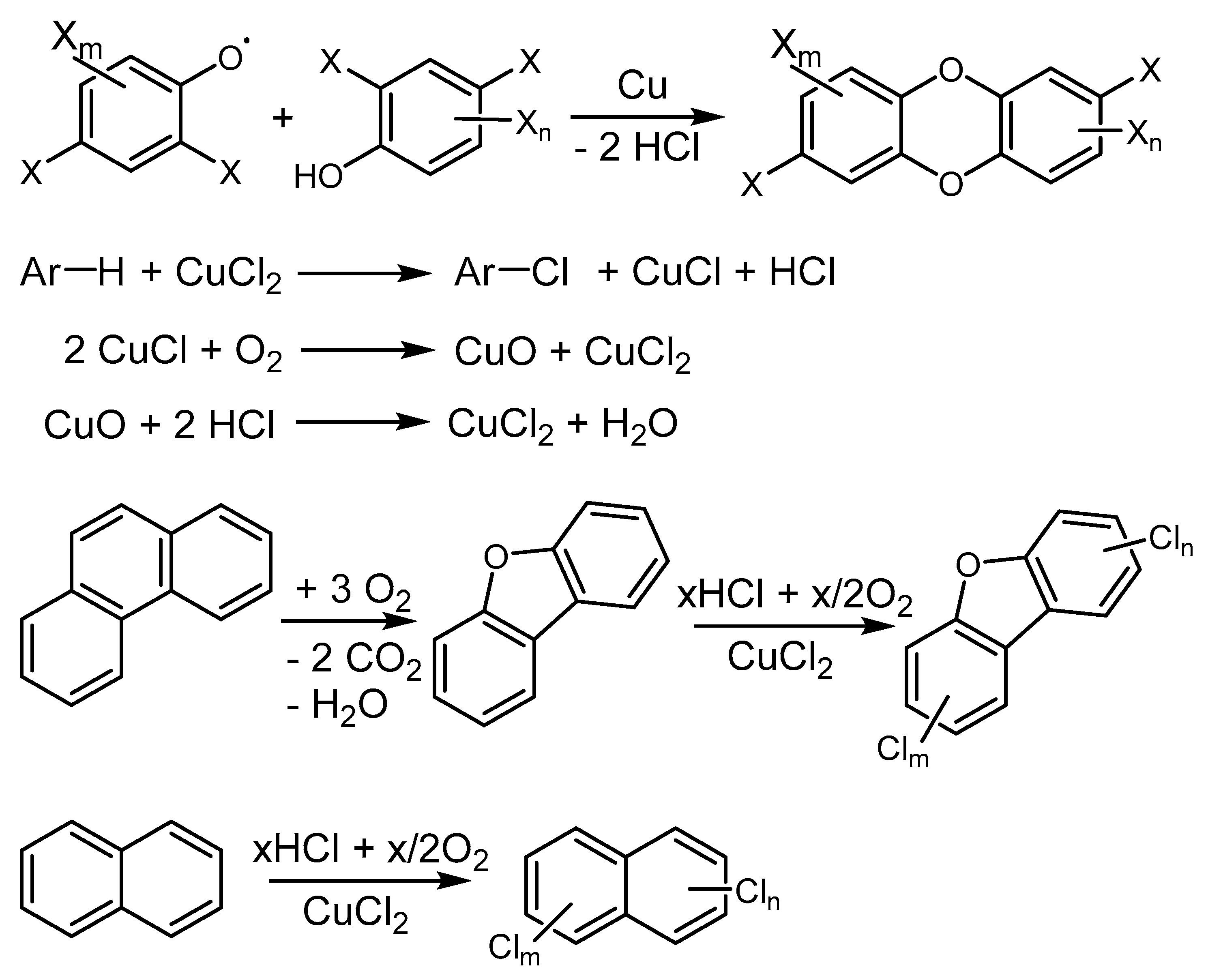
2.4. Cu-Mediated Formation of Polychlorinated Aromatic Pollutants
3. Cu-Catalyzed Dehalogenation of Carom-X Bond
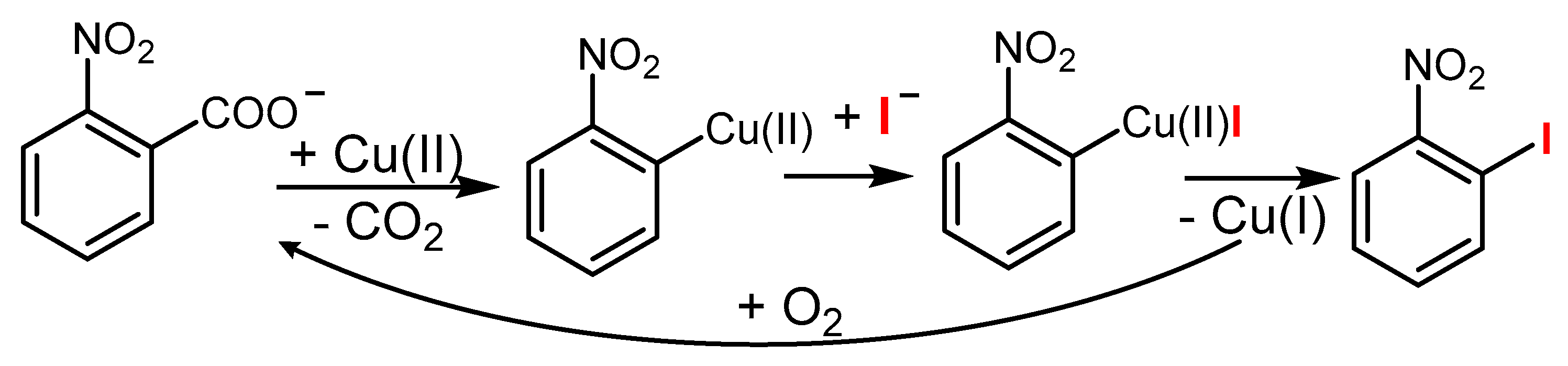
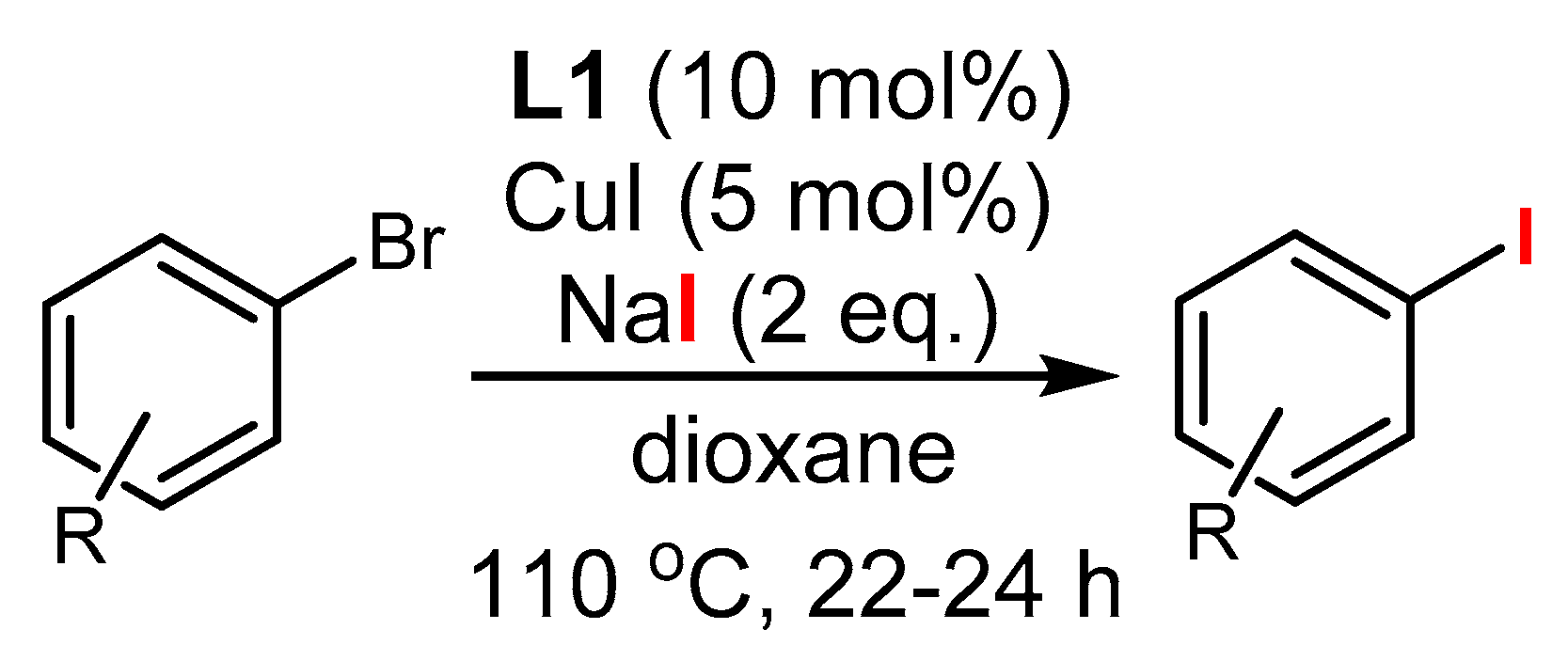
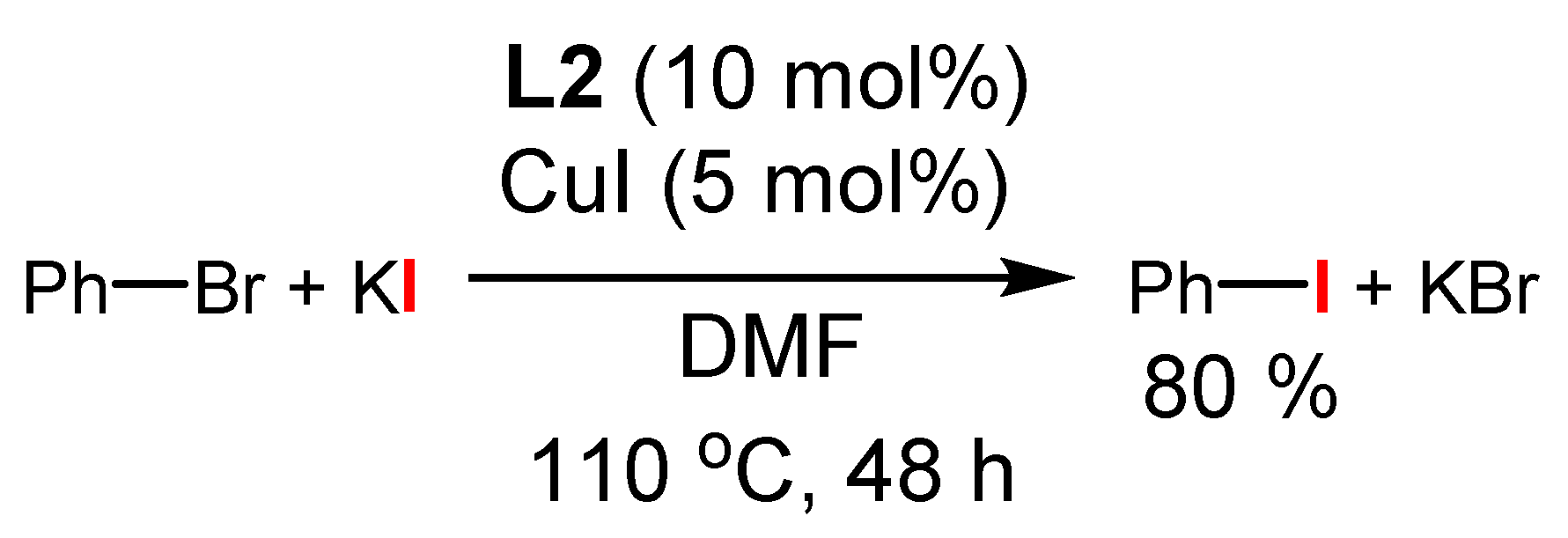
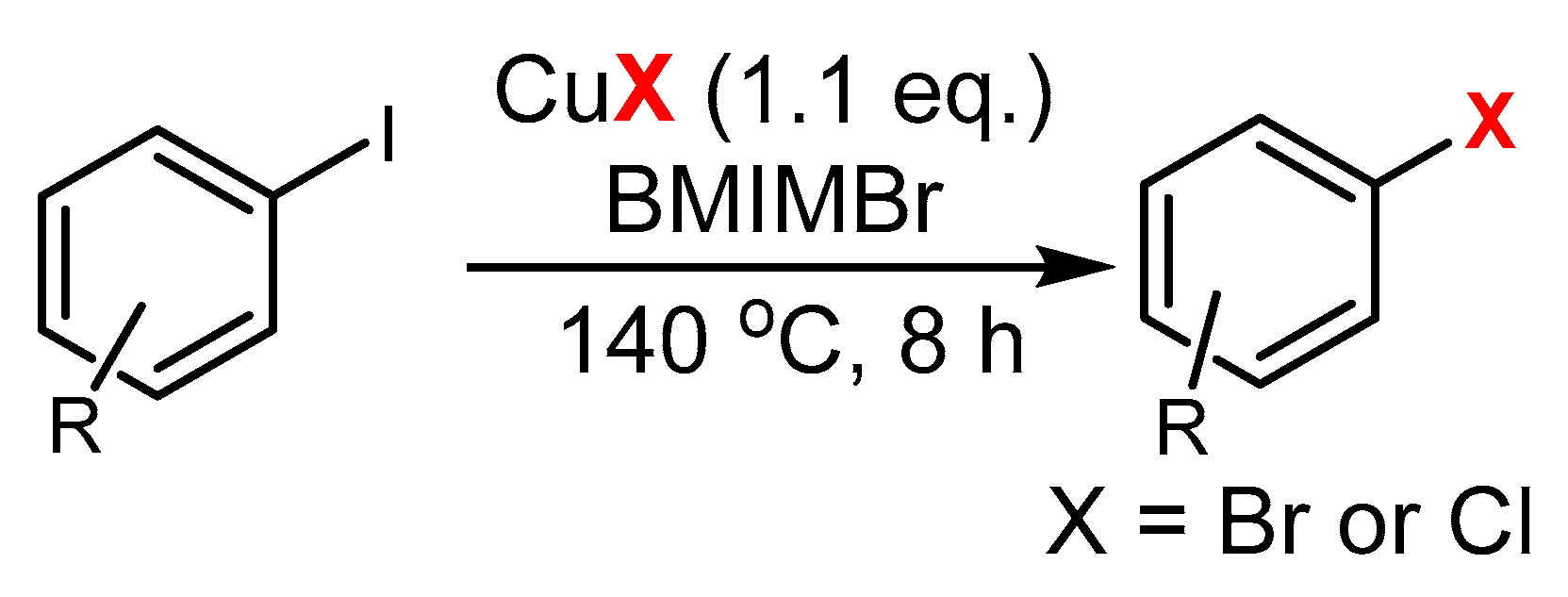
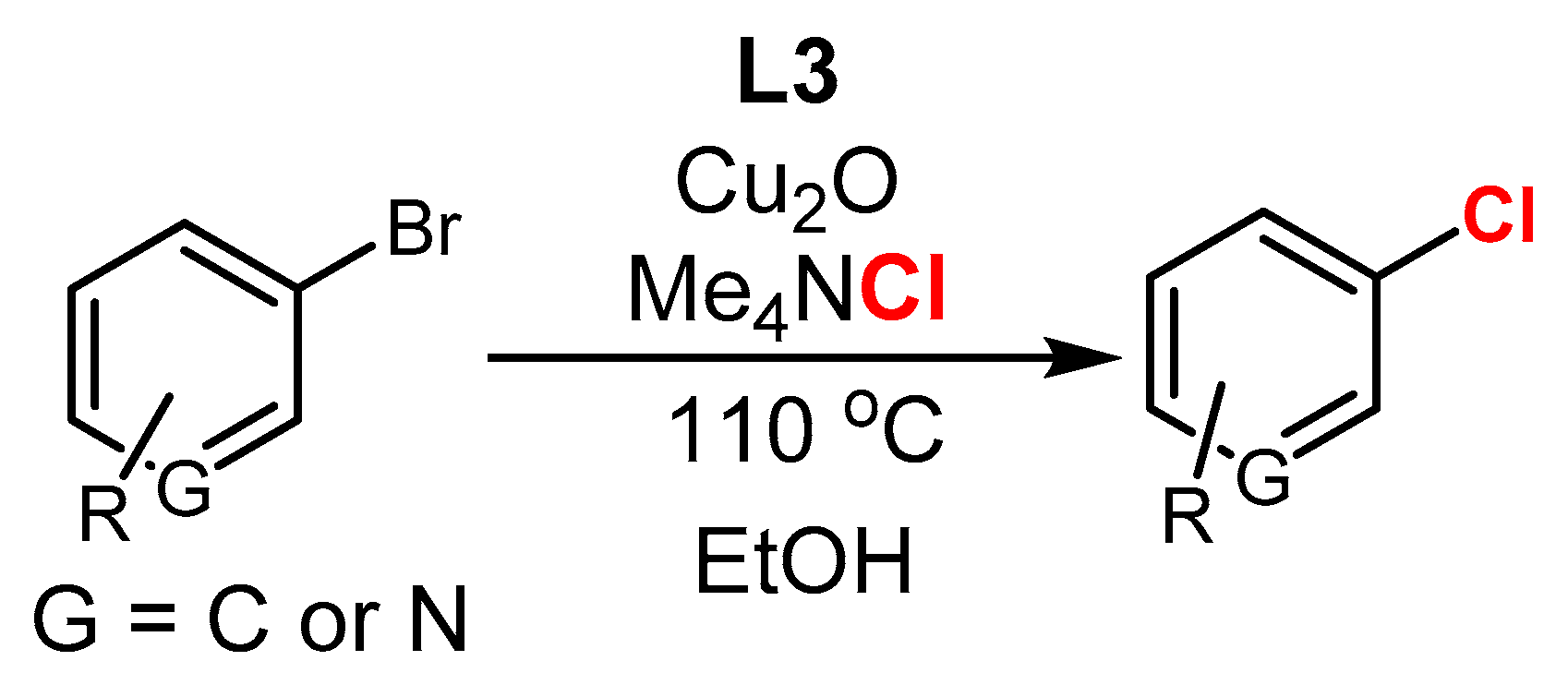
3.1. The Role of Cu in Nucleophilic Substitutions of Halogens in Ar-Xs
3.2. Reactions of Ar-Xs with O-Nucleophiles
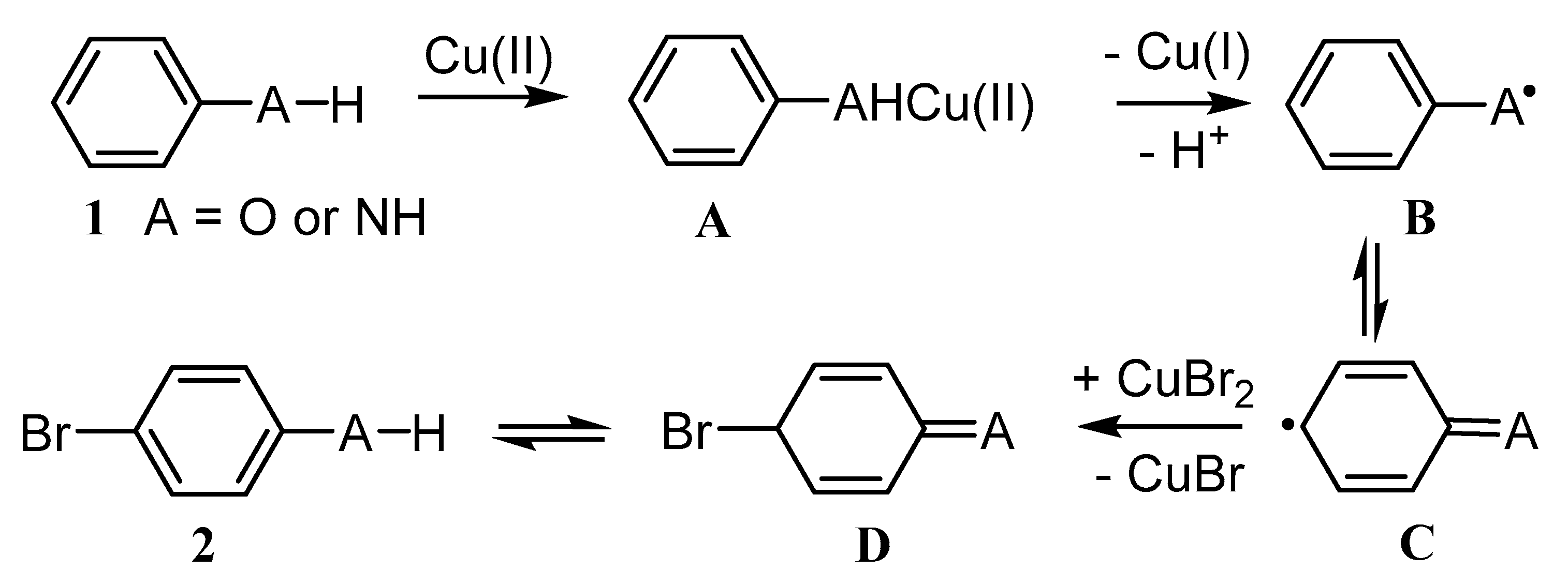
3.2.1. Cu-Catalyzed Syntheses of Phenols
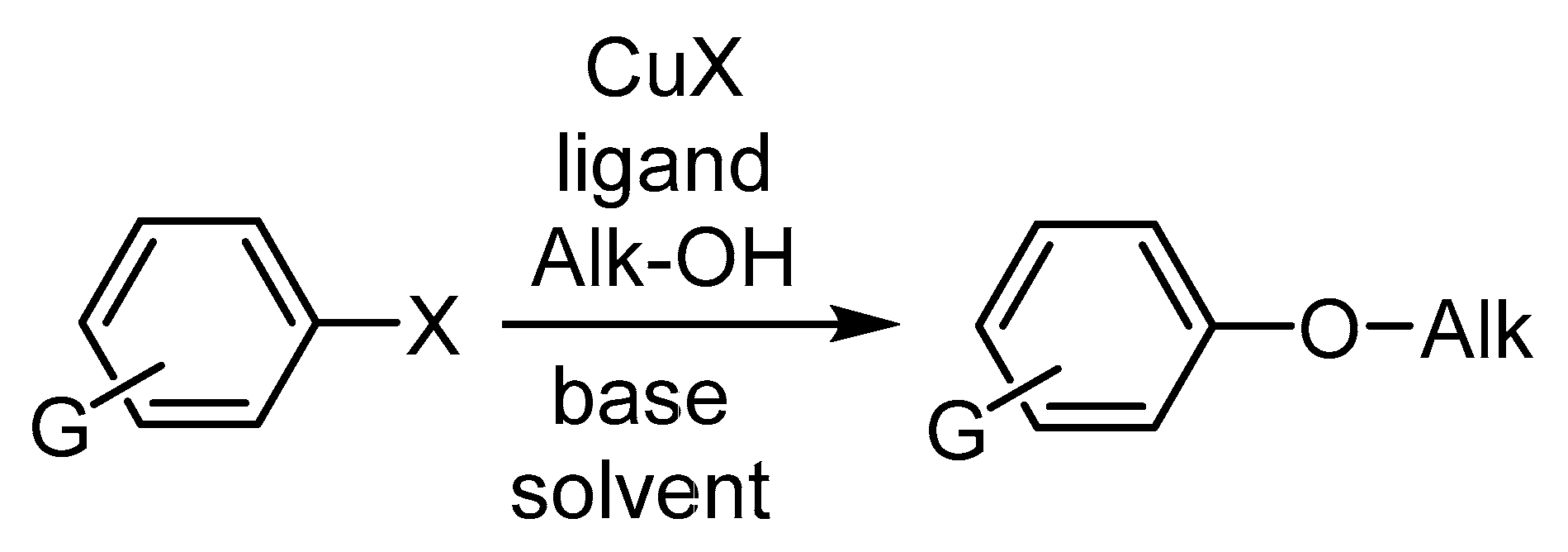
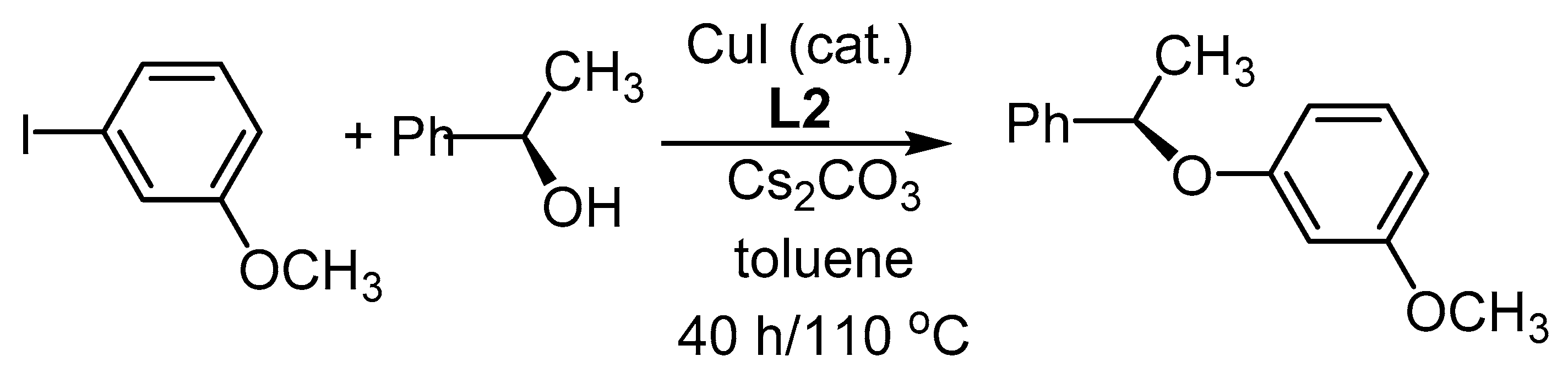
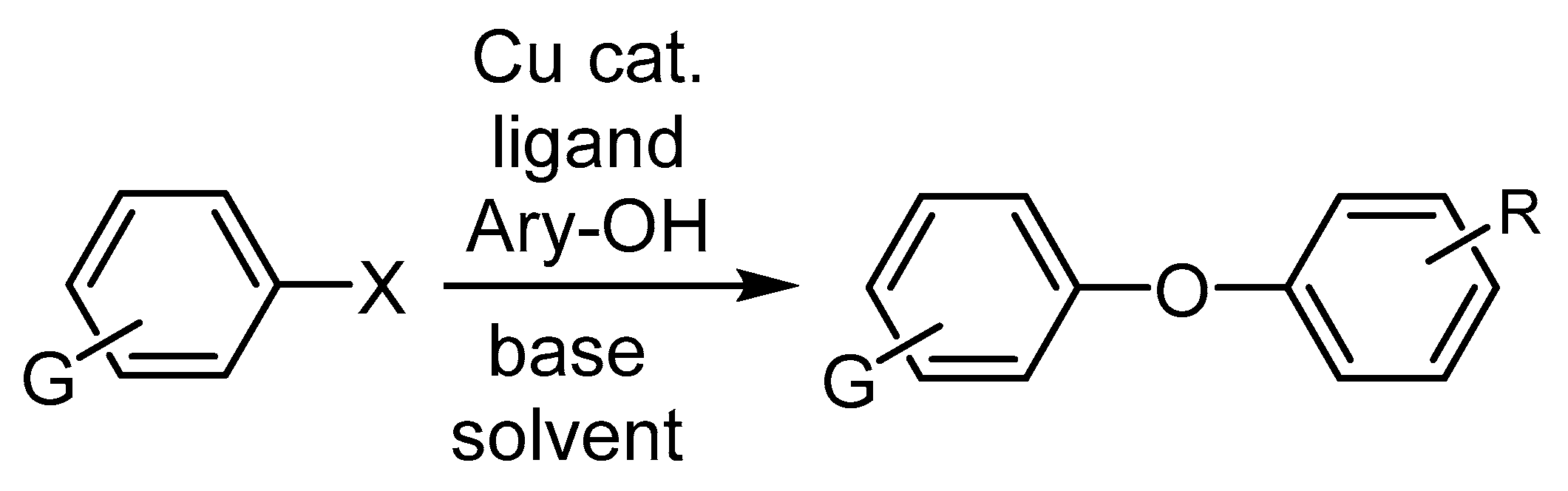
3.2.2. Cu-Catalyzed Syntheses of Aryl Ethers from Ar-Xs
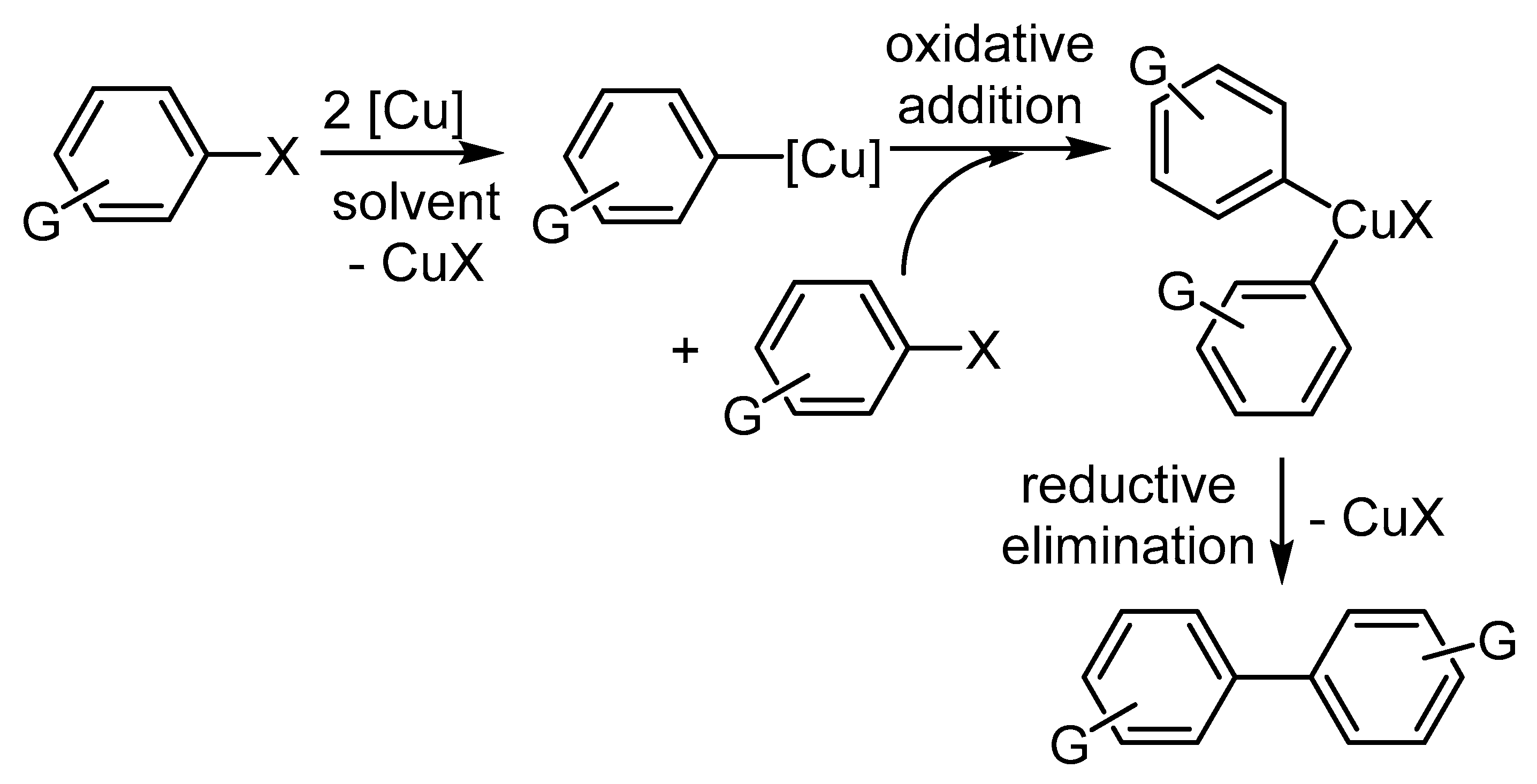
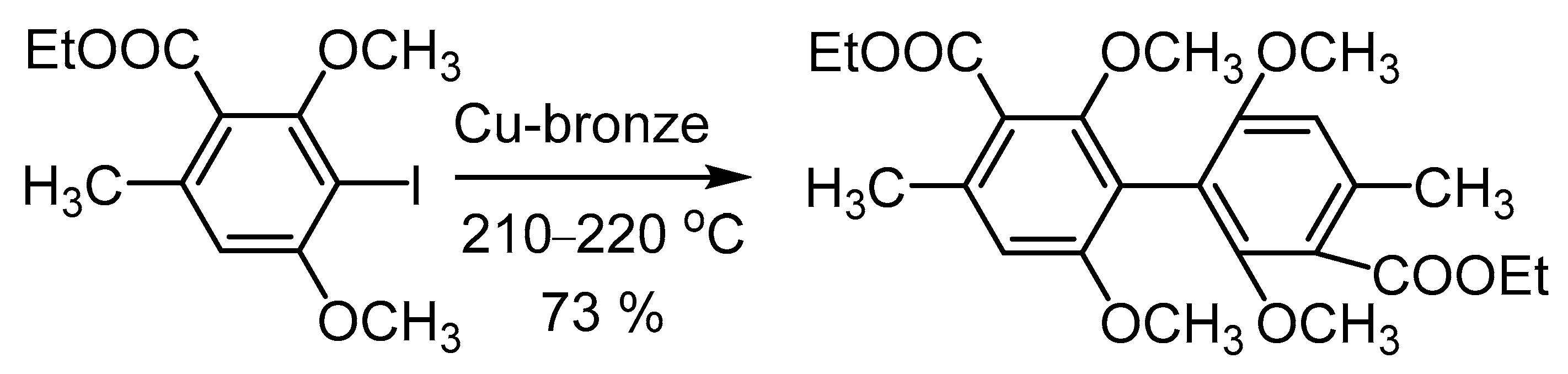
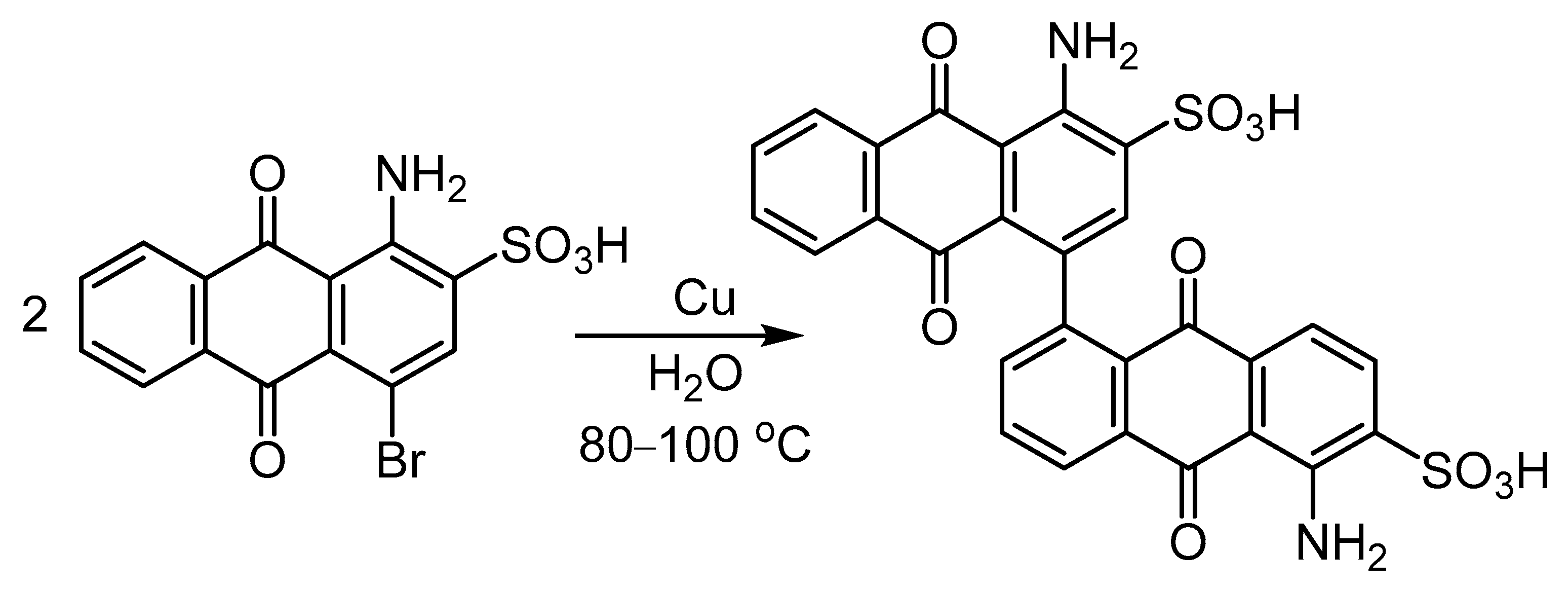
3.3. Utilization of Ar-Xs in Cu-Mediated Biaryl Syntheses
4. Cu-Based Hydrodehalogenation of Aromatic Compounds
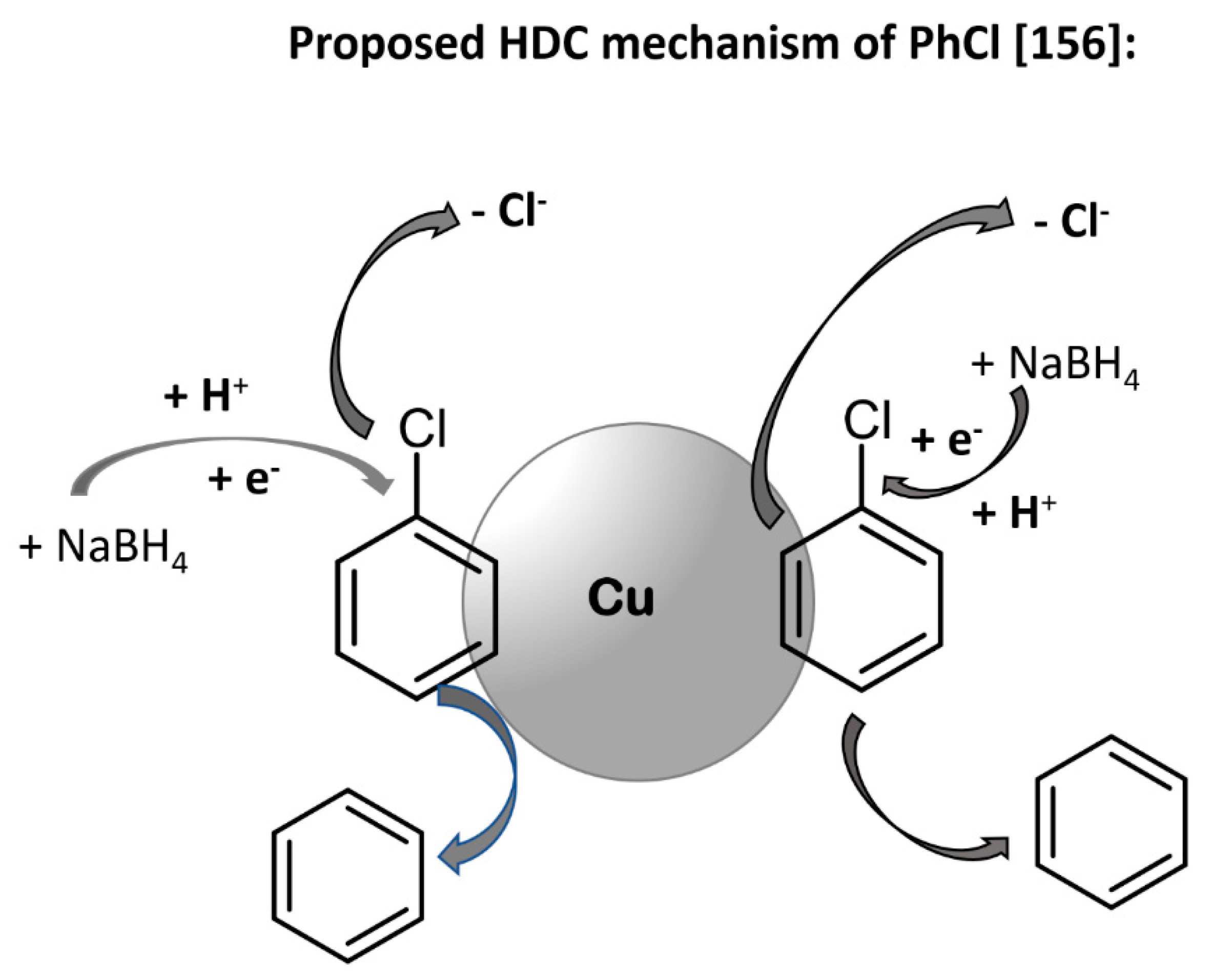
4.1. Cu-Catalyzed HDC of Chlorinated Benzenes
4.2. High-Temperature Cu-Mediated PCDD/Fs Dechlorination
4.3. Cu-Mediated HDC of Ar-Cls Dissolved in an Aqueous Solution
4.3.1. HDC of Chlorinated Phenols
4.3.2. Cu-Catalyzed Hydrodebromination (HDB) of Polybrominated Phenols
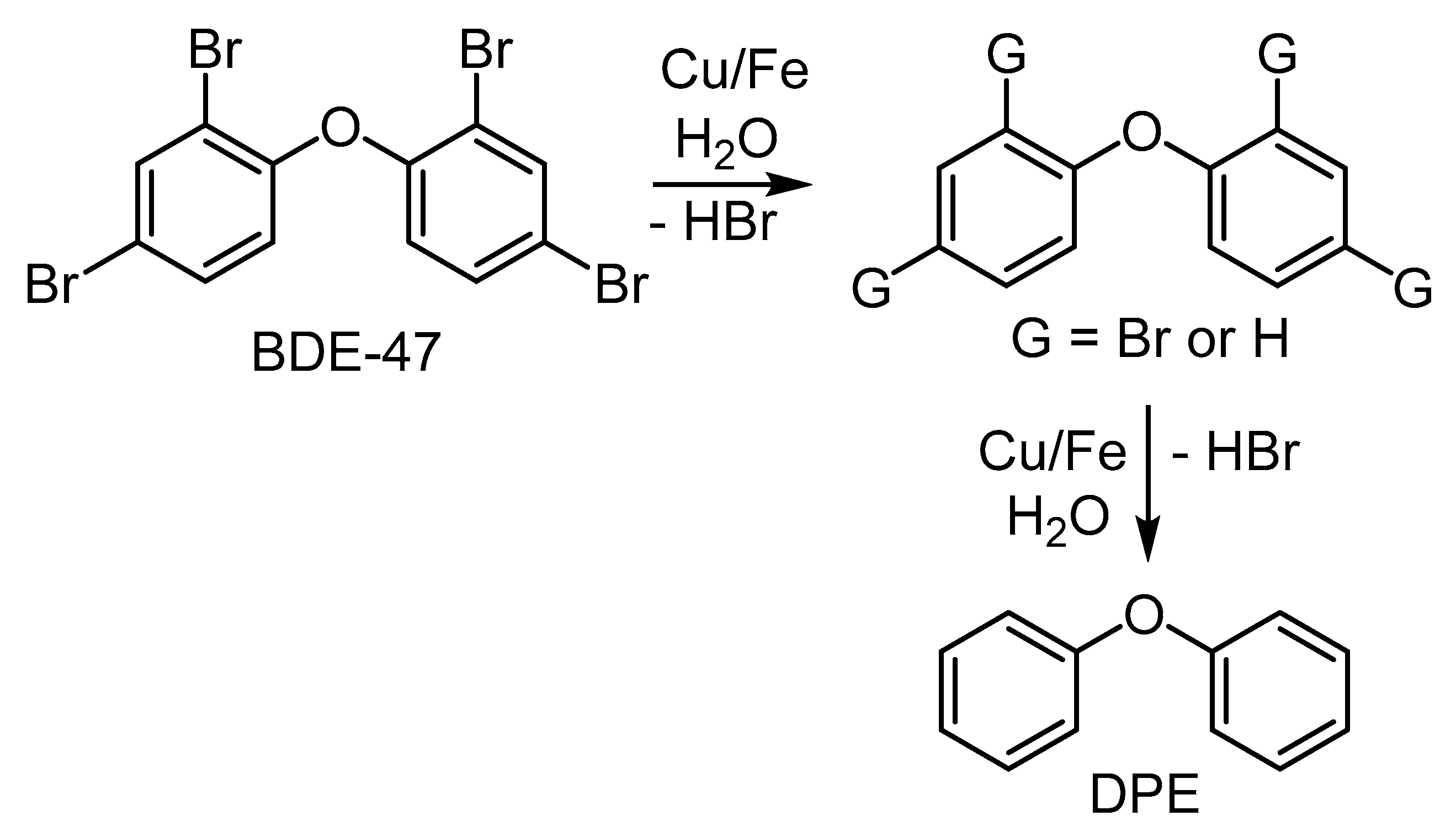
4.3.3. Cu-Catalyzed HDB of Polybrominated Diphenylethers
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Chen, G.; Tan, Z. Copper-catalyzed or -mediated c-h bond functionalizations assisted by bidentate directing groups. Adv. Synth. Catal. 2016, 358, 1174–1194. [Google Scholar] [CrossRef]
- Hao, W.; Liu, Y. C–H bond halogenation catalyzed or mediated by copper: An overview. Beilstein J. Org. Chem. 2015, 11, 2132–2144. [Google Scholar] [CrossRef] [PubMed]
- Kyne, S.H.; Camp, J.E. Use of monosaccharides in metal-catalyzed coupling reactions. ACS Sustain. Chem. Eng. 2017, 5, 41–48. [Google Scholar] [CrossRef]
- Ribas, X.; Guell, I. Cu(I)/Cu(III) catalytic cycle involved in ullmann-type cross-coupling reactions. Pure Appl. Chem. 2014, 86, 345–360. [Google Scholar] [CrossRef]
- Hassan, J.; Sevignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl-aryl bond formation one century after the discovery of the Ullmann reaction. Chem. Rev. 2002, 102, 1359–1469. [Google Scholar] [CrossRef] [PubMed]
- Ametamey, S.M.; Honer, M.; Schubiger, P.A. Molecular imaging with PET. Chem. Rev. 2008, 108, 1501–1516. [Google Scholar] [CrossRef]
- Seevers, R.H.; Counsell, R.E. Radioiodination techniques for small organic molecules. Chem. Rev. 1982, 82, 575–590. [Google Scholar] [CrossRef]
- Magano, J.; Dunetz, J.R. Large-scale applications of transition metal-catalyzed couplings for the synthesis of pharmaceuticals. Chem. Rev. 2011, 111, 2177–2250. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.B.; March, J. March′s Advanced Organic Chemistry, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Schmidt, R.; Stolle, A.; Ondruschka, B. Aromatic substitution in ball mills: Formation of aryl chlorides and bromides using potassium peroxomonosulfate and NaX. Green Chem. 2012, 14, 1673–1679. [Google Scholar] [CrossRef]
- Narender, N.; Krishna Mohan, K.V.V.; Srinivasu, P.; Kulkarni, S.J.; Raghavan, K.V. A simple, efficient, regioselective oxychlorination of aromatic compounds using ammonium chloride and oxone. Ind. J. Chem. 2004, 43B, 1335–1338. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Suneel, K.; Das, B.; Reddy, K.N.; Reddy, T.S. Simple catalyst-free regio- and chemoselective monobromination of aromatics using NBS in polyethylene glycol. Synth. Commun. 2009, 39, 215–219. [Google Scholar] [CrossRef]
- Adimurthy, S.; Ramachandraiah, G.; Bedekar, A.V.; Ghosh, S.; Ranu, B.C.; Ghosh, P. Eco-friendly and versatile brominating reagent prepared from a liquid bromine precursor. Green Chem. 2006, 8, 916–922. [Google Scholar] [CrossRef]
- Toda, F.; Schmeyers, J. Selective solid-state brominations of anilines and phenols. Green Chem. 2003, 5, 701–703. [Google Scholar] [CrossRef]
- Sharma, R.K.; Sharma, C. Oxidative bromination reaction using Cu2+-perfluorophthalocyanine-immobilized silica gel catalyst under mild reaction conditions. Tetrahedron Lett. 2010, 51, 4415–4418. [Google Scholar] [CrossRef]
- Leyva-Perez, A.; Combita-Merchan, D.; Cabrero-Antonino, J.R.; Al-Resayes, S.I.; Corma, A. Oxyhalogenation of activated arenes with nanocrystalline ceria. ACS Catal. 2013, 3, 250–258. [Google Scholar] [CrossRef]
- Borikar, S.P.; Daniel, T.; Paul, V. An efficient, rapid, and regioselecticve bromination of anilines and phenols with 1-butyl-3-methylpyridinium tribromide as a new reagent/solvent under mild conditions. Tetrahedron Lett. 2009, 50, 1007–1009. [Google Scholar] [CrossRef]
- Kavala, V.; Naik, S.; Patel, B.K. A new recyclable ditribromide reagent for efficient bromination uder solvent free condition. J. Org. Chem. 2005, 70, 4267–4271. [Google Scholar] [CrossRef]
- Ganchegui, B.; Leitner, W. Oxybromination of phenol and aniline derivatives in H2O/scCO2 biphasic media. Green Chem. 2007, 9, 26–29. [Google Scholar] [CrossRef]
- Limberg, C.; Teles, J.H. The Activation of O2 at ruthenium complexes: Catalytic chlorination of unsaturated organic substrates within the system O2/HCl/H2O. Adv. Synth. Catal. 2001, 343, 447–449. [Google Scholar] [CrossRef]
- Schmittinger, P.; Florkiewicz, T.; Curlin, L.C.; Lüke, B.; Scannell, R.; Navin, T.; Zelfel, E.; Bartsch, R. ‘Chlorine’, Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Weinheim, Germany, 2006. [Google Scholar]
- Mondelli, C.; Amrute, A.P.; Moser, M.; Schmidt, T.; Pérez-Ramírez, J. Development of industrial catalysts for sustainable chlorine production. Chimia 2012, 66, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, I.A.; Radny, M.W.; Gladys, M.J.; Smith, P.V.; Mackie, J.C.; Stockenhuber, M.; Kennedy, E.M.; Dlugogorski, B.Z. Water formation via HCl oxidation on Cu(100). Appl. Surf. Sci. 2014, 299, 156–161. [Google Scholar] [CrossRef]
- Carley, A.F.; Davies, P.R.; Harikumarz, K.R.; Jones, R.V. Low energy pathway to CuCl2 at Cu (110) surfaces. Phys. Chem. Phys. 2009, 11, 10899–10907. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, D.; Song, N.; Yuan, X.; Bi, X. Reaction kinetics of HCl catalytic oxidation over a supported cu-based composite industrial catalyst. Ind. Eng. Chem. Res. 2019, 58, 9246–9256. [Google Scholar] [CrossRef]
- Petrone, D.A.; Ye, J.; Lautens, M. Modern transition-metal-catalyzed carbon–halogen bond formation. Chem. Rev. 2016, 116, 8003–8104. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Jiang, L.; Zuo, Q.; Li, Z.; Liu, Y.; Wei, Z.; Cai, H. Inexpensive NaX (X = I, Br, Cl) as a halogen donor in the practical Ag/Cu-mediated decarboxylative halogenation of aryl carboxylic acids under aerobic conditions. Org. Biomol. Chem. 2018, 16, 5416–5421. [Google Scholar] [CrossRef]
- Liu, Q.; Yuan, Z.; Wang, H.; Li, Y.; Wu, Y.; Xu, T.; Leng, X.; Chen, P.; Guo, Y.; Lin, Z.; et al. Abnormal mesoionic carbene silver complex: Synthesis, reactivity, and mechanistic insight on oxidative fluorination. ACS Catal. 2015, 5, 6732–6737. [Google Scholar] [CrossRef]
- Kawai, K.; Bunno, Y.; Yoshino, T.; Matsunaga, S. Weinreb amide directed versatile C-H bond functionalization under (h5-Pentamethylcyclopentadienyl)cobalt (III) Catalysis. Chem. Eur. J. 2018, 24, 10231–10237. [Google Scholar] [CrossRef] [PubMed]
- Przypis, L.; Walczak, K.Z. Copper (II)-Catalyzed iodinations of carbazoles: Access to functionalized carbazoles. J. Org. Chem. 2019, 84, 2287–2296. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jie, K.; Yan, W.; Pan, Q.; Zhang, M.; Wang, Y.; Fu, Z.; Guo, S.; Cai, H. Selective C–C bond cleavage of amides fused to 8-aminoquinoline controlled by a catalyst and an oxidant. Chem. Commun. 2020, 56, 13820–13823. [Google Scholar] [CrossRef]
- Wendlandt, A.E.; Suess, A.M.; Stahl, S.S. Copper-catalyzed aerobic oxidative C-H functionalizations: Trends and mechanistic insights. Angew. Chem. Int. Ed. 2011, 50, 11062–11087. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, C.; Jiao, N. Recent advances in copper-catalyzed dehydrogenative functionalization via a single electron transfer (SET) proces. Chem. Soc. Rev. 2012, 41, 3464–3484. [Google Scholar] [CrossRef]
- King, A.E.; Huffman, L.M.; Casitas, A.; Costas, M.; Ribas, X.; Stahl, S.S. Copper-catalyzed aerobic oxidative functionalization of an arene C-H bond: Evidence for an Aryl-Copper(III) intermediate. J. Am. Chem. Soc. 2010, 132, 12068–12073. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, W.; Li, J.-H. An efficient copper-catalysed aerobic oxybromination of arenes in water. Green Chem. 2010, 12, 2124–2126. [Google Scholar] [CrossRef]
- Menini, L.; Parreira, L.A.; Gusevskaya, E.V. A practical highly selective oxybromination of phenols with dioxygen. Tetrahedron Lett. 2007, 48, 6401–6404. [Google Scholar] [CrossRef]
- Menini, L.; da Cruz Santos, J.C.; Gusevskaya, E.V. Copper-catalyzed oxybromination and oxychlorination of primary aromatic amines using LiBr or LiCl and molecular oxygen. Adv. Synth. Catal. 2008, 350, 2052–2058. [Google Scholar] [CrossRef]
- Raja, R.; Ratnasamy, P. Oxyhalogenation of aromatics over copper phthalocyanines encapsulated in zeolites. J. Catal. 1997, 170, 244–253. [Google Scholar] [CrossRef]
- Yang, L.; Lu, Z.; Stahl, S.S. Regioselective copper-catalyzed chlorination and bromination of arenes with O2 as the oxidant. Chem. Commun. 2009, 6460–6462. [Google Scholar] [CrossRef]
- Li, X.-L.; Wu, W.; Fan, X.-H.; Yang, L.-M.A. Facile, regioselective and controllable bromination of aromatic amines using a CuBr2/Oxone® system. RSC Adv. 2013, 3, 12091–12095. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, R.; Qiao, X.; Shen, Z. Copper-catalyzed aromatic c-h bond halogenation using lithium halides as halogenating reagents. Synlett 2011, 1038–1042. [Google Scholar] [CrossRef]
- Chen, X.; Hao, X.-S.; Goodhue, C.E.; Yu, J.-Q. Cu (II)-Catalyzed functionalizations of Aryl C−H bonds using O2 as an oxidant. J. Am. Chem. Soc. 2006, 128, 6790–6791. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Pan, C.; Chen, F.; Cheng, J. Copper (II)-catalyzed ortho-functionalization of 2-arylpyridines with acyl chlorides. Chem. Commun. 2011, 47, 3978–3980. [Google Scholar] [CrossRef]
- Mo, S.; Zhu, Y.; Shen, Z. Copper-catalyzed aromatic C–H bond halogenation with lithium halides under aerobic conditions. Org. Biomol. Chem. 2013, 11, 2756–2760. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.-J.; Gao, L.-X.; Lin, Y.-J.; Han, F.-S. Cu-mediated direct aryl C-H halogenation: A strategy to control mono- and di-selectivity. ChemCatChem 2014, 6, 123–126. [Google Scholar] [CrossRef]
- Pal, P.; Singh, H.; Panda, A.B.; Ghosh, S.C. Heterogeneous Cu-MnO catalyzed monoselective ortho-halogenation of aromatic C-H bonds under visible light. Asian J. Org. Chem. 2015, 4, 879–883. [Google Scholar] [CrossRef]
- Urones, B.; Martínez, Á.M.; Rodríguez, N.; Arrayás, R.G.; Carretero, J.C. Copper-catalyzed ortho-halogenation of protected anilines. Chem. Commun. 2013, 49, 11044–11046. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, B.; Shi, B.-F. Copper-catalyzed ortho-halogenation of arenes and heteroarenes directed by a removable auxiliary. Chem. Commun. 2015, 51, 5093–5096. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-K.; Jia, X.-S.; Yin, L. Recent advances in Copper(II)-Mediated or -catalyzed C–H functionalization. Synthesis 2018, 50, 4165–4188. [Google Scholar] [CrossRef]
- Dua, Y.; Liu, Y.; Wan, J.-P. copper-catalyzed one-pot n-acylation and c5–h halogenation of 8-aminoquinolines: The dual role of acyl halides. J. Org. Chem. 2018, 83, 3403. [Google Scholar] [CrossRef]
- Bi, W.Z.; Qu, C.; Chen, X.L.; Wei, S.K.; Qu, L.B.; Liu, S.Y.; Sun, K.; Zhao, Y.F. Copper(II) catalyzed heterobenzylic C(sp3)-H activation: Two efficient halogenation methodologies towards heterobenzyl halides. Tetrahedron 2018, 74, 1908. [Google Scholar] [CrossRef]
- Truong, T.; Klimovica, K.; Daugulis, O. Copper-catalyzed, directing group-assisted fluorination of arene and heteroarene C–H bonds. J. Am. Chem. Soc. 2013, 135, 9342–9345. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, M.A.; Manzer, L.E. A “Greener” synthetic route for fluoroaromatics via Copper (II) fluoride. Science 2002, 297, 1665. [Google Scholar] [CrossRef]
- Zanon, J.; Klapars, A.; Buchwald, S.L. Copper-catalyzed domino halide exchange-cyanation of aryl bromides. J. Am. Chem. Soc. 2003, 125, 2890–2891. [Google Scholar] [CrossRef]
- Klapars, A.; Buchwald, S.L. Copper-catalyzed halogen exchange in aryl halides: An aromatic Finkelstein reaction. J. Am. Chem. Soc. 2002, 124, 14844–14845. [Google Scholar] [CrossRef] [PubMed]
- Arvela, K.K.; Leadbeater, N.E. Fast and easy halide exchange in aryl halides. Synlett 2003, 8, 1145–1148. [Google Scholar] [CrossRef]
- Casitas, A.; Canta, M.; Sola, M.; Costas, M.; Ribas, X. Nucleophilic aryl fluorination and aryl halide exchange mediated by a CuI/CuIII catalytic cycle. J. Am. Chem. Soc. 2011, 133, 19386–19392. [Google Scholar] [CrossRef] [PubMed]
- Zhdankin, V.V. Hypervalent iodine (III) reagents in organic synthesis. ARKIVOC 2009, 1, 1–62. [Google Scholar] [CrossRef]
- Stang, P.J.; Zhdankin, V.V. Organic polyvalent iodine compounds. Chem. Rev. 1996, 96, 1123–1178. [Google Scholar] [CrossRef]
- Fu, Z.; Jiang, Y.; Jiang, L.; Li, Z.; Guo, S.; Cai, H. Cu-catalyzed decarboxylative iodination of aryl carboxylic acids with NaI: A practical entry to aryl iodides under aerobic conditions. Tetrahedron Lett. 2018, 59, 4458–4461. [Google Scholar] [CrossRef]
- Sheppard, T.D. Metal-catalysed halogen exchange reactions of aryl halides. Org. Biomol. Chem. 2009, 7, 1043–1052. [Google Scholar] [CrossRef]
- Chen, M.; Ichikawa, S.; Buchwald, S.L. Rapid and efficient copper-catalyzed finkelstein reaction of (Hetero) aromatics under continuous-flow conditions. Angew. Chem. Int. Ed. 2015, 54, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Cristau, H.J.; Ouali, A.; Spindler, J.F.; Taillefer, M. Mild and Efficient Copper-Catalyzed Cyanation of Aryl Iodides and Bromides. Chem. Eur. J. 2005, 11, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.H.; Nguyen, D.P.; Phan, N.T.K.; Lam, D.T.T.; Phan, N.T.S.; Truong, T. A copper-mediated reverse aromatic Finkelstein reaction in ionic liquid. J. Adv. Res. 2018, 10, 9–13. [Google Scholar] [CrossRef]
- Feng, X.; Qu, Y.; Han, Y.; Yu, X.; Bao, M.; Yamamoto, Y. Copper-catalyzed conversion of aryl and heteraryl bromides into the corresponding chlorides. Chem. Commun. 2012, 48, 9468–9470. [Google Scholar] [CrossRef]
- Hanberg, A.; Waern, F.; Asplund, L.; Haglund, E.; Safe, S. Swedish dioxin survey: Determination of 2,3,7,8-TCDD toxic equivalent factors for some polychlorinated biphenyls and naphthalenes using biological tests. Chemosphere 1990, 20, 1161–1164. [Google Scholar] [CrossRef]
- Behnisch, P.A.; Hosoe, K.; Shiozaki, K.; Kiryu, T.; Komatsu, K.; Schramm, K.W.; Sakai, S. Melting and incineration plants of municipal waste. chemical and biochemical diagnosis of thermal processing samples (emission, residues). Environ. Sci. Pollut. Res. 2001, 8, 1–8. [Google Scholar] [CrossRef]
- Pekárek, V.; Weber, R.; Grabic, R.; Šolcová, O.; Fišerová, E.; Šyc, M.; Karban, J. Matrix effects on the de novo synthesis of polychlorinated dibenzo-p-dioxins, dibenzofurans, biphenyls and benzenes. Chemosphere 2007, 68, 51–61. [Google Scholar] [CrossRef]
- Ryu, J.Y.; Kim, D.H.; Jang, S.H. Is chlorination one of the major pathways in the formation of polychlorinated naphthalenes (PCNs) in municipal solid waste combustion? Environ. Sci. Technol. 2013, 47, 2394–2400. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.; Iino, F.; Imagawa, T.; Takeuchi, M.; Sakurai, T.; Sadakata, M. Formation of PCDF, PCDD, PCB, and PCN in de novo synthesis from PAH: Mechanistic aspects and correlation to fluidized bed incinerators. Chemosphere 2001, 44, 1429–1438. [Google Scholar] [CrossRef]
- Kim, D.H.; Mulholland, J.A.; Ryu, J.Y. Chlorinated naphthalene formation from the oxidation of dichlorophenols. Chemosphere 2007, 67, S135–S143. [Google Scholar] [CrossRef]
- Liu, G.R.; Cai, Z.W.; Zheng, M.H. Sources of unintentionally produced polychlorinated naphthalenes. Chemosphere 2014, 94, 1–12. [Google Scholar] [CrossRef]
- Takaoka, M.; Shiono, A.; Nishimura, K.; Yamamoto, T.; Uruga, T.; Takeda, N.; Tanaka, T.; Oshita, K.; Matsumoto, T.; Harada, H. Dynamic change of copper in fly ash during de novo synthesis of dioxins. Environ. Sci. Technol. 2005, 39, 5878–5884. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, G.; Wang, M.; Zheng, M. Fly ash-mediated formation of polychlorinated naphthalenes during secondary copper smelting and mechanistic aspects. Chemosphere 2015, 119, 1091–1098. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, F.; Wei, Z.; Wang, Z. Synthesis of diaryl ethers by CuI-Catalyzed C-O bond formation via ullman coupling: Assessing the reactivity of aryl halides. Lett. Org. Chem. 2013, 10, 31–36. [Google Scholar] [CrossRef]
- Liu, N.; Wang, B.; Chen, W.; Liu, C.; Wang, X.; Hu, Y. A general route for synthesis of N-aryl phenoxazines via copper(I)-catalyzed N-, N-, and O-arylations of 2-aminophenols. RSC Adv. 2014, 4, 51133–51139. [Google Scholar] [CrossRef]
- Naidu, A.B.; Jaseer, E.A.; Sekar, G. General, mild, and intermolecular ullmann-type synthesis of diaryl and alkyl aryl ethers catalyzed by Diol-Copper (I) complex. J. Org. Chem. 2009, 74, 3675–3679. [Google Scholar] [CrossRef]
- Monnier, F.; Taillefer, M. Catalytic C-C, C-N, and C-O Ullmann-type coupling reactions. Angew. Chem. Int. Ed. 2009, 48, 6954–6971. [Google Scholar] [CrossRef]
- Sambiagio, C.; Marsden, S.P.; Blacker, A.J.; McGowan, P.C. Copper catalysed Ullmann type chemistry: From mechanistic aspects to modern development. Chem. Soc. Rev. 2014, 43, 3525. [Google Scholar] [CrossRef] [PubMed]
- Amal Joseph, P.J.; Priyadarshini, S. Copper-Mediated C−X Functionalization of Aryl Halides. Org. Process Res. Dev. 2017, 21, 1889–1924. [Google Scholar] [CrossRef]
- Tlili, A.; Xia, N.; Monnier, F.; Taillefer, M. A very simple copper-catalyzed synthesis of phenols employing hydroxide salts. Angew. Chem. Int. Ed. 2009, 121, 8881–8884. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, N.; Zhang, S.; Xi, P.; Su, X.; Lan, J.; You, J. Synthesis of phenol, aromatic ether, and benzofuran derivatives by copper-catalyzed hydroxylation of aryl halides. Angew. Chem. Int. Ed. 2009, 48, 8729–8732. [Google Scholar] [CrossRef]
- Thakur, K.G.; Sekar, G. D-Glucose as green ligand for selective copper-catalyzed phenol synthesis from aryl halides with an easy catalyst removal. Chem. Comm. 2011, 47, 6692–6694. [Google Scholar] [CrossRef]
- Watanabe, K.; Takagi, M.; Watanabe, A.; Murata, S.; Takita, R. Cu(I)/sucrose-catalyzed hydroxylation of arenes in water: The dual role of sucrose. Org. Biomol. Chem. 2020, 18, 7827–7831. [Google Scholar] [CrossRef]
- Liang, X.; Li, H.; Du, F.; Zhang, Y.; Dong, J.; Bao, X.; Wu, Y.; Chen, G. Copper and L-(-)-quebrachitol catalyzed hydroxylation and amination of aryl halides under air. Tetrahedron Lett. 2020, 61, 152222. [Google Scholar] [CrossRef]
- Saphier, M.; Masarwa, A.; Cohen, H.; Meyerstein, D. Copper (I) as a homogeneous catalyst for the ullmann reaction in aqueous solutions−the transformation of 2-bromobenzoate into salicylate. Eur. J. Inorg. Chem. 2002, 2002, 1226–1234. [Google Scholar] [CrossRef]
- Yang, K.; Li, Z.; Wang, Z.; Yao, Z.; Jiang, S. Highly efficient synthesis of phenols by copper-catalyzed hydroxylation of aryl iodides, bromides, and chlorides. Org. Lett. 2011, 13, 4340–4343. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Gan, L.; Wang, K.; Li, Z.; Ma, D. Copper-catalyzed hydroxylation of (Hetero)aryl halides under mild conditions. J. Am. Chem. Soc. 2016, 138, 13493–13496. [Google Scholar] [CrossRef]
- Xu, H.-J.; Liang, Y.-F.; Cai, Z.-Y.; Qi, H.-X.; Yang, C.-Y.; Feng, Y.-S. CuI-nanoparticles-catalyzed selective synthesis of phenols, anilines, and thiophenols from aryl halides in aqueous solution. J. Org. Chem. 2011, 76, 2296–2300. [Google Scholar] [CrossRef]
- Aalten, H.; van Koten, G.; Grove, D.M. The Copper Catalyzed Reaction of sodum methoxide with aryl bromides. A mechanistic study leading to a facile synthesis of anisole derivatives. Tetrahedron 1989, 45, 5565–5578. [Google Scholar] [CrossRef]
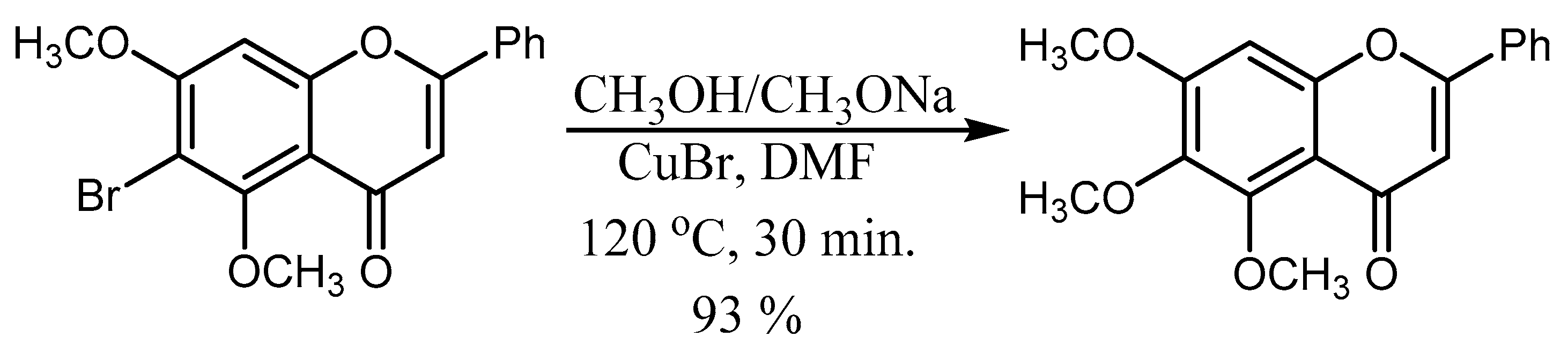
- Righi, G.; Antonioletti, R.; Silvestri, I.P.; D’Antona, N.; Lambusta, D.; Bovicelli, P. Convergent synthesis of mosloflavone, negletein and baicalein from crysin. Tetrahedron 2010, 66, 1294–1298. [Google Scholar] [CrossRef]
- Wolter, M.; Nordmann, G.; Job, G.E.; Buchwald, S.L. Copper-catalyzed coupling of aryl iodides with aliphatic alcohols. Org. Lett. 2002, 4, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, R.; Tajbakhsh, M.; Mohadjerani, M.; Alikarami, M. Copper-catalyzed etherification of aryl iodides using KF/Al2O3: An improved protocol. Synlett 2005, 07, 1101–1104. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, W.; Ma, D. L-Proline-Promoted CuI-Catalyzed C-S Bond Formation between Aryl Iodides and Thiols. Synlett 2007, 37, 25–35. [Google Scholar] [CrossRef]
- Shafir, A.; Lichtor, P.A.; Buchwald, S.L. N-versus O-arylation of aminoalcohols: Orthogonal selectivity in copper-based catalysts. J. Am. Chem. Soc. 2007, 129, 3490–3491. [Google Scholar] [CrossRef]
- Altman, R.A.; Shafir, A.; Choi, A.; Lichtor, P.A.; Buchwald, S.L. An improved Cu-based catalyst system for the reactions of alcohols with aryl halides. J. Org. Chem. 2008, 73, 284–286. [Google Scholar] [CrossRef]
- Kidwai, M.; Mishra, N.K.; Bansal, V.; Kumar, A.; Mozumdar, S. Cu-nanoparticle catalyzed O-arylation of phenols with aryl halides via Ullmann coupling. Tetrahedron Lett. 2007, 48, 8883–8887. [Google Scholar] [CrossRef]
- Schareina, T.; Zapf, A.; Cotte, A.; Muller, N.; Beller, M. Bio-inspired copper catalysts for the formation of diaryl ethers. Tetrahedron Lett. 2008, 49, 1851–1855. [Google Scholar] [CrossRef]
- Miao, T.; Wang, L. Immobilization of copper in organic-inorganic hybrid materials: A highly efficient and reusable catalyst for the Ullmann diaryl etherification. Tetrahedron Lett. 2007, 48, 95. [Google Scholar] [CrossRef]
- Cai, Q.; Zou, B.; Ma, D. Mild Ullmann-Type Biaryl Ether Formation Reaction of ortho-Substituent and Ligand Effects. Angew. Chem. Int. Ed. 2006, 45, 1276. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Bao, W. A beta-Keto ester as a novel, efficient, and versatile ligand for Copper(I)-Catalyzed C-N, C-O, and C-S coupling reactions. J. Org. Chem. 2007, 72, 3863. [Google Scholar] [CrossRef]
- Cristau, H.-J.; Cellier, P.P.; Hamada, S.; Spindler, J.-F.; Taillefer, M. A general and mild ullmann-type synthesis of diaryl ethers. Org. Lett. 2004, 6, 913. [Google Scholar] [CrossRef] [PubMed]
- Ouali, A.; Spindler, J.-F.; Jutand, A.; Taillefer, M. Nitrogen ligands in copper-catalyzed arylation of phenols: Structure/activity relationships and applications. Adv. Synth. Catal. 2007, 349, 1906–1916. [Google Scholar] [CrossRef]
- Lipshutz, B.H.J.; Unger, B.; Taft, B.R. Copper-in-charcoal (Cu/C) promoted diaryl ether formation. Org. Lett. 2007, 9, 1089–1092. [Google Scholar] [CrossRef]
- Gujadhur, R.K.; Bates, C.G.; Venkataraman, D. Formation of aryl-nitrogen, aryl-oxygen, and aryl-carbon bonds using well-defined copper (I)-based catalysts. Org. Lett. 2001, 3, 4315–4317. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W.W.; Chee, S.; Mak, S.; Buranaprasertsuk, P.; Chavasiri, W.; Chan, P.W.H. Copper-catalyzed Ullmann coupling under ligand- and additivefree conditions. Part 1: O-Arylation of phenols with aryl halides. Tetrahedron Lett. 2008, 49, 2018–2022. [Google Scholar] [CrossRef]
- Ma, D.; Jiang, Y. Cu(I)/Amino Acid Catalyzed Coupling Reactions of Aryl Halides and Nucleophiles: Applications in Large-scale Production. Chimia 2011, 65, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Tan, L.-S.; Fossum, E. Aryl ether synthesis via Ullmann coupling in non-polar solvents: Effect of ligand, counterion, and base. ARKIVOC 2009, xiv, 255–265. [Google Scholar]
- Xia, N.; Taillefer, M. Copper- or iron-catalyzed arylation of phenols from respectively aryl chlorides and aryl iodides. Chem. Eur. J. 2008, 14, 6037–6039. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, B.; Arundhathi, R.; Reddy, P.L.; Kantam, M. Lakshmi CuI nanoparticles for C-N and C-O Cross coupling of heterocyclic amines and phenols with chlorobenzenes. J. Org. Chem. 2009, 74, 7951–7954. [Google Scholar] [CrossRef] [PubMed]
- Satyanarayana, P.; Maheswaran, H.; Kantam, M.L.; Bhargava, S. Bis(μ-iodo)bis[(ą)-sparteine]-dicopper: A versatile catalyst for direct O arylation and O-alkylation of phenols and aliphatic alcohols with haloarenes. Bull. Chem. Soc. Jpn. 2011, 84, 788–790. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.C.; Kim, A.; Kim, A.Y.; Lee, H.J.; Song, H.; Park, K.H. Cu2O Nanocube-catalyzed cross-coupling of aryl halides with phenols via ullmann coupling. Eur. J. Inorg. Chem. 2009, 4219–4223. [Google Scholar] [CrossRef]
- Liu, J.; Fitzgerald, A.E.; Mani, N.S. Facile assembly of fused benzo [4,5] furo heterocycles. J. Org. Chem. 2008, 73, 2951–2954. [Google Scholar] [CrossRef] [PubMed]
- Fanta, P.E. The Ullmann synthesis of biaryls. Chemi. Rev. 1964, 64, 613–632. [Google Scholar] [CrossRef]
- Sainsbury, M. Modern methods of aryl-aryl bond formation. Tetrahedron 1980, 36, 3327–3359. [Google Scholar] [CrossRef]
- Bringmann, G.; Walter, R.; Weirich, R. The directed synthesis of biaryl compounds: Modern concepts and strategies. Angew. Chem. Int. Ed. Engl. 1990, 29, 977–991. [Google Scholar] [CrossRef]
- Vasconcelos, S.N.S.; Reis, J.S.; de Oliveira, I.M.; Balfour, M.N.; Stefani, H.A. Synthesis of symmetrical biaryl compounds by homocoupling reaction. Tetrahedron 2019, 75, 1865–1959. [Google Scholar] [CrossRef]
- Hauser, F.M.; Gauuan, P.J.F. Total synthesis of (±)-Biphyscion. Org. Lett. 1999, 1, 671–672. [Google Scholar] [CrossRef]
- Nelson, T.D.; Meyers, A.I. The synthesis of a useful chiral biaryl catalyst. An oxazoline-mediated Ullmann reaction. Tetrahedron Lett. 1993, 34, 3061–3062. [Google Scholar] [CrossRef]
- Nelson, T.D.; Meyers, A.I. The asymmetric Ullmann reaction III. Application of a first-order asymmetric transformation to the synthesis of C2-symmetric chiral, non-racemic biaryls. Tetrahedron Lett. 1994, 35, 3259–3262. [Google Scholar] [CrossRef]
- Meyers, A.I.; McKennon, M.J. C2 symmetric amines. I. The asymmetric Ullmann synthesis of a new C2-symmetric binaphthyl. Tetrahedron Lett. 1995, 36, 5869–5872. [Google Scholar] [CrossRef]
- Meyers, A.I.; Willemsen, J.J. An asymmetric synthesis of (+)-apogossypol hexamethyl ether. Tetrahedron Lett. 1996, 37, 791–792. [Google Scholar] [CrossRef]
- Nelson, T.D.; Meyers, A.I. A rapid total synthesis of an ellagitannin. J. Org. Chem. 1994, 59, 2577–2580. [Google Scholar] [CrossRef]
- Nelson, T.D.; Meyers, A.I. The Asymmetric Ullmann Reaction. 2. The Synthesis of Enantiomerically Pure C2-Symmetric Binaphthyls. J. Org. Chem. 1994, 59, 2655–2658. [Google Scholar] [CrossRef]
- Nelson, T.D.; Willemsen, J. The synthesis of (S)-(+)-gossypol via an asymmetric Ullmann coupling. Chem. Commun. 1997, 16, 1573–1574. [Google Scholar] [CrossRef]
- Zhao, W.Q.; Qian, K.; Su, C.W.; Shao, L.; Zhou, W.; Cui, D.M.; Zhang, C.; Wang, X.L. Copper-catalyzed synthesis of pyrazolo[5,1-a]- isoquinoline derivatives from 2-gemdipyrazolylvinylbromobenzenes. New J. Chem. 2019, 43, 10162–10165. [Google Scholar] [CrossRef]
- Kroeck, F.W.; Neoff, R. Process for the Preparation of 1,4-Diamino-Anthraquinone-2-Sulphonic Acid. U.S. Patent 4,521,341 (A), 4 June 1985. [Google Scholar]
- Jost, M.; Kern, W.; Grelat, M. Verfahren zur Herstellung von 4,4′-Diamino-1,1′-dianthrachinonyl-3,3′-disulfonsauren. CIBA AG, Basel, Swiss Patent CH 396,264 (A), 31 July 1965. [Google Scholar]
- Gao, A.; Liu, H.; Hu, L.; Zhang, H.; Hou, A.; Xie, K. Synthesis of Fe3O4@SiO2-Au/Cu magnetic nanoparticles and its efficient catalytic performance for the Ullmann coupling reaction of bromamine acid. Chin. Chem. Lett. 2018, 29, 1301–1304. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, D.; Liebeskind, L.S. Ambient temperature, Ullmann-like reductive coupling of aryl, heteroaryl, and alkenyl halides. J. Org. Chem. 1997, 62, 2312–2313. [Google Scholar] [CrossRef] [PubMed]
- Yasamut, K.; Jongcharoenkamol, J.; Ruchirawat, S.; Ploypradith, P. A modified Cu(0)-Cu(I)-mediated Caryl-Caryl Ullmann coupling for the synthesis of biaryls. Tetrahedron 2016, 72, 5994–6000. [Google Scholar] [CrossRef]
- Keane, M.A. Supported transition metal catalysts for hydrodechlorination reactions. ChemCatChem 2011, 3, 800–821. [Google Scholar] [CrossRef]
- Liang, L.; Yang, G.; Wang, W.; Xu, F.; Niu, Y.; Sun, Q.; Xu, P. A copper-catalyzed aerobic cascade dehydrogenative–dehalogenative reaction. Adv. Synth. Catal. 2013, 355, 1284–1290. [Google Scholar] [CrossRef]
- Utsumi, S.; Katagiri, T.; Uneyama, K. Cu-deposits on Mg metal surfaces promote electron transfer reactions. Tetrahedron 2012, 68, 1085–1091. [Google Scholar] [CrossRef]
- Kinarivala, N.; Trippier, P.C. Exploration of relative chemoselectivity in the hydrodechlorination of 2-chloropyridines. Tetrahedron Lett. 2014, 55, 5386–5389. [Google Scholar] [CrossRef]
- René, O.; Fauber, B.P. Syntheses of 4-, 5-, 6-, and 7-substituted tryptamine derivatives and the use of a bromine atom as a protecting group. Tetrahedron Lett. 2014, 55, 830–833. [Google Scholar] [CrossRef]
- Heiss, C.; Marzi, E.; Schlosser, M. Buttressing effects rerouting the deprotonation and functionalization of 1,3-dichloro- and 1,3-dibromobenzene. Eur. J. Org. Chem. 2003, 4625–4629. [Google Scholar] [CrossRef]
- Diaz, E.; Mohedano, A.F.; Casas, J.A.; Calvo, L.; Gilarranz, M.A.; Rodriguez, J.J. Deactivation of a Pd/AC catalyst in the hydrodechlorination of chlorinated herbicides. Catal. Today 2015, 241, 86–91. [Google Scholar] [CrossRef]
- Murena, F.; Schioppa, E. Kinetic analysis of catalytic hydrodechlorination process of polychlorinated biphenyls (PCBs). Appl. Catal. B: Environ. 2000, 27, 257–267. [Google Scholar] [CrossRef]
- Rodriguez, J.G.; Lafuente, A. A new advanced method for heterogeneous catalysed dechlorination of 1,2,3-, 1,2,4-, and 1,3,5-Trichlorobenzenes in hydrocarbon solvent. Tetrahedron Lett. 2002, 43, 9645–9647. [Google Scholar] [CrossRef]
- Nieto-Sandoval, J.; Rodriguez, M.; Munoz, M.; de Pedro, Z.M.; Casas, J.A. Catalyst deactivation in the hydrodechlorination of micropollutants. A case of study with neonicotinoid pesticides. J. Water Proc. Eng. 2020, 38, 101550. [Google Scholar] [CrossRef]
- de Souza, L.P.; Graca, A.L.C.; Teixeira, A.C.S.C.; Chiavone-Filho, O. Degradation of 2,4,6-trichlorophenol in aqueous systems through the association of zero-valent-copper-mediated reduction and UVC/H2O2: Effect of water matrix and toxicity assessment. Environ. Sci. Pol. Res. 2021. [Google Scholar] [CrossRef]
- Graca, A.L.C.; Mendes, M.A.; Teixeira, A.C.S.C.; Correia de Velosa, A. Anoxic degradation of chlorpyrifos by zerovalent monometallic and bimetallic particles in solution. Chemosphere 2020, 244, 125461. [Google Scholar] [CrossRef]
- Ghaffar, A.; Tabata, M. Enhanced dechlorination of chlorobenzene compounds on fly ash: Effects of metals, solvents, and temperature. Green Chem. Lett. Rev. 2010, 3, 179–190. [Google Scholar] [CrossRef]
- Hagenmaler, H.; Brunner, H.; Haag, R.; Kraft, M. Copper-catalyzed dechlorination/hydrogenation of polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans, and other chlorinated aromatic compounds. Environ. Sci. Technol. 1987, 21, 1085–1088. [Google Scholar] [CrossRef]
- Stach, J.; Pekarek, V.; Endrst, R.; Hetflejs, J. Dechlorination of hexachlorobenzene on MWI Fly ash. Chemosphere 1999, 39, 2391–2399. [Google Scholar] [CrossRef]
- Stach, J.; Pekarek, V.; Grabic, R.; Lojkasek, M.; Pacakova, V. Dechlorination of polychlorinated biphenyls, dibenzo-p-dioxins and dibenzofurans on fly ash. Chemosphere 2000, 41, 1881–1887. [Google Scholar] [CrossRef]
- Weber, R.; Nagai, K.; Nishino, J.; Shiraishi, H.; Ishida, M.; Takasuga, T.; Konndo, K.; Hiraoka, M. Effects of selected metal oxides on the dechlorination and destruction of PCDD and PCDF. Chemosphere 2002, 46, 1247–1253. [Google Scholar] [CrossRef]
- Pekárek, V.; Karban, J.; Fiserova, E.; Bures, M.; Pacakova, V.; Vecernikova, E. Dehalogenation potential of municipal waste incineration fly ash I. general principles. Environ Sci. Pollut. Res. 2003, 10, 39–43. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Lin, X.; Li, X. Decomposition and reformation pathways of PCDD/Fs during thermal treatment of municipal solid waste incineration fly ash. J. Hazard. Mater. 2020, 394, 122526. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C. A critical review on the process of contaminant removal in Fe0–H2O systems. Environ. Technol. 2008, 29, 909–920. [Google Scholar] [CrossRef]
- Choi, J.H.; Choi, S.J.; Kim, Y.H. Hydrodechlorination of 2,4,6-trichlorophenol for a permeable reactive barrier using zero-valent iron and catalyzed iron. Korean J. Chem. Eng. 2008, 25, 493–500. [Google Scholar] [CrossRef]
- Su, Y.; Hsu, C.-Y.; Shih, Y. Effects of various ions on the dechlorination kinetics of hexachlorobenzene by nanoscale zero-valent iron. Chemosphere 2012, 88, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Carraway, E.R. Dechlorination of pentachlorophenol by zero valent iron and modified zero valent irons. Environ. Sci. Technol. 2000, 34, 2014–2017. [Google Scholar] [CrossRef]
- Duan, J.; Zhu, H.; Xu, F.; Zhao, J. New approach to 4-chlorophenol dechlorination on monometallic copper compared to its Cu/Fe bimetallic system. Chem. Eng. J. 2016, 304, 282–288. [Google Scholar] [CrossRef]
- Raut, S.S.; Kamble, S.P.; Kulkarni, P.S. Efficacy of zero-valent copper (Cu0) nanoparticles and reducing agent for dechlorination of mono chloroaromatics. Chemosphere 2016, 159, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Ghaffar, A.; Tabata, M.; Mashiatullah, A.; Alaamer, A.S. Efficient DDT and trichlorophenol detoxification using NaBH4 and Devarda alloy. Environ. Chem. Lett. 2013, 11, 197–202. [Google Scholar] [CrossRef]
- Weidlich, T.; Kamenicka, B.; Melanova, K.; Cicmancova, V.; Komersova, A.; Cermak, J. Hydrodechlorination of different chloroaromatic compounds at room temperature and ambient pressure—Differences in reactivity of Cu- and Ni-Based Al alloys in an alkaline aqueous solution. Catalysts 2020, 10, 994. [Google Scholar] [CrossRef]
- Alaee, M.; Arias, P.; Sjodin, A.; Bergman, A. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ. Int. 2003, 29, 683–689. [Google Scholar] [CrossRef]
- de Wit, C.A. An overview of brominated flame retardants in the environment. Chemosphere 2002, 46, 583–624. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Okonkwo, J.O.; Olukunle, O.I.; Botha, B.M. Brominated flame retardants: Sources, distribution, exposure pathways, and toxicity. Environ. Rev. 2011, 19, 238–253. [Google Scholar] [CrossRef]
- Wichmann, H.; Dettmer, F.T.; Bahadir, M. Thermal formation of PBDD/F from tetrabromobisphenol A—A comparison of polymer linked TBBPA with its additive incorporation in thermoplastics. Chemosphere 2002, 47, 349–355. [Google Scholar] [CrossRef]
- Grause, G.; Furusawa, M.; Okuwaki, A.; Yoshioka, T. Pyrolysis of tetrabromobisphenol A containing paper laminated printed circuit boards. Chemosphere 2008, 71, 872–878. [Google Scholar] [CrossRef]
- Barontini, F.; Marsanich, K.; Petarca, L.; Cozzani, V. Thermal degradation and decomposition products of electronic boards containing BFRs. Ind. Eng. Chem. Res. 2005, 44, 4186–4199. [Google Scholar] [CrossRef]
- Sahu, R.S.; Shih, Y.-h. Reductive debromination of tetrabromobisphenol A by tailored carbon nitride Fe/Cu nanocomposites under an oxic condition. Chem. Eng. J. 2019, 378, 122059. [Google Scholar] [CrossRef]
- Huang, Z.; Deng, D.; Qiao, J.; Ju, Y.; Chen, Y.; Dionysiou, D.D. New insight into the cosolvent effect on the degradation of tetrabromobisphenol A (TBBPA) over millimeter-scale palladised sponge iron (Pd-s-Fe0) particles. Chem. Eng. J. 2019, 361, 1423–1436. [Google Scholar] [CrossRef]
- Li, Y.; Han, D.; Arai, Y.; Fu, X.; Li, X.; Huang, W. Kinetics and mechanisms of debromination of tetrabromobisphenol A by Cu coated nano zerovalent iron. Chem. Eng. J. 2019, 373, 95–103. [Google Scholar] [CrossRef]
- Fang, L.; Liu, R.; Xu, L.; Li, J.; Huang, L.-Z.; Li, F. Enhanced debromination of tetrabromobisphenol a by zero-valent copper nanoparticle-modified green rusts. Environ. Sci. Nano 2019, 6, 970–980. [Google Scholar] [CrossRef]
- Gao, R.; Liu, B.; Zhan, L.; Guo, J.; Zhang, J.; Xu, Z. Catalytic effect and mechanism of coexisting copper on conversion of organics during pyrolysis of waste printed circuit boards. J. Hazard. Mater. 2021, 403, 123465. [Google Scholar] [CrossRef] [PubMed]
- Weidlich, T.; Prokes, L.; Krejcova, A. Hydrodebromination of 2,4,6-tribromophenol in aqueous solution using Devarda’s alloy. Monatsh. Chem. 2013, 144, 155–162. [Google Scholar] [CrossRef]
- Weidlich, T.; Prokes, L.; Pospisilova, D. Debromination of 2,4.6-tribromophenol coupled with biodegradation. Centr. Eur. J. Chem. 2013, 11, 979–987. [Google Scholar] [CrossRef]
- Hale, R.C.; La Guardia, M.J.; Harvey, E.; Mainor, T.M. Potential role of fire retardant-treated polyurethane foam as a source of brominated diphenyl ethers to the US environment. Chemosphere 2002, 46, 729–735. [Google Scholar] [CrossRef]
- Mai, B.X.; Chen, S.J.; Luo, X.J.; Chen, L.G.; Yang, Q.S.; Sheng, G.Y.; Peng, P.G.; Fu, J.M.; Zeng, E.Y. Distribution of polybrominated diphenyl ethers in sediments of the Pearl River Delta and adjacent South China Sea. Environ. Sci. Technol. 2005, 39, 3521–3527. [Google Scholar] [CrossRef]
- Luijk, R.; Wever, H.; Olie, K.; Govers, H.A.J.; Boon, J.J. The influence of the polymer matrix on the formation of polybrominated dibenzo-p-dioxins (PBDDs) and polybrominated dibenzofurans (PBDFs). Chemosphere 1991, 23, 1173–1183. [Google Scholar] [CrossRef]
- Santos, M.S.F.; Alves, A.; Madeira, L.M. Chemical and photochemical degradation of polybrominated diphenyl ethers in liquid systems—A review. Water Res. 2016, 88, 39–59. [Google Scholar] [CrossRef]
- Huang, G.; Wang, M.; Hu, Y.; Cheng, J.; Lv, S.; Yang, K. Reductive degradation of 2,2’,4,4’-tretrabromodiphenyl ether with PAC-Pd/Fe nanoparticles: Effects of Pd loading, initial pH and HA, and degradation pathway. Chem. Eng. J. 2018, 334, 1252–1259. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Wang, R.; Huang, K.; Luo, W.; Tao, X.; Dang, Z.; Yin, H.; Guo, C.; Lu, G. Debromination of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) by synthetic Pd/Fe0 and Cu/Fe0 in different protic solvents. Chemosphere 2018, 212, 946–953. [Google Scholar] [CrossRef]
- Wang, R.; Tang, T.; Lu, G.; Zheng, Z.; Huang, K.; Li, H.; Tao, X.; Yin, H.; Shi, Z.; Lin, Z.; et al. Mechanisms and pathways of debromination of polybrominated diphenyl ethers (PBDEs) in various nano-zerovalent iron-based bimetallic systems. Sci. Total Environ. 2019, 661, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Lippert, T.; Wokaun, A.; Lenoir, D. Copper catalyzed aryl dehalogenation reactions and their inhibition. Ber. Bunsenges. Phys. Chem. 1990, 94, 1465–1471. [Google Scholar] [CrossRef]
- Addink, R.; Paulus, R.H.W.L.; Olie, K. Prevention of polychlorinateddibenzo-p-dioxins/dibenzofurans formation on municipal waste incineratorfly ash using nitrogen and sulfur compounds. Environ. Sci. Technol. 1996, 30, 2350–2354. [Google Scholar] [CrossRef]
- Raghunathan, K.; Gullett, B.K. Role of Sulfur in reducing PCDD and PCDFformation. Environ. Sci. Technol. 1996, 30, 1827–1834. [Google Scholar] [CrossRef]
- Ruokojarvi, P.; Tuppurainen, K.; Mueller, C.; Kilpinen, P.; Ruuskanen, J. PCDD/Freduction in incinerator flue gas by adding urea to RDF feedstock. Chemosphere 2001, 43, 199–205. [Google Scholar] [CrossRef]
- Pandelova, M.; Lenoir, D.; Schramm, K.W. Correlation between PCDD/F, PCB and PCBz in coal/waste combustion. Influence of various inhibitors. Chemosphere 2006, 62, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh, Y.; Onwudili, J.A.; Williams, P.T. Effects of gaseous NH3 and SO2 on the concentration profiles of PCDD/F in flyash under post-combustion zone conditions. Waste Manage. 2012, 32, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chen, T.; Zhang, M.; Buekens, A.; Yu, Y.; Ling, Y.; Chena, Z.; Li, X. Hot rolling sludge incineration: Suppression of PCDD/Fs by spent anion exchange resins. J. Hazard. Mater. 2018, 343, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Cannon, K.A.; Geuther, M.E.; Kelly, C.K.; Lin, S.; MacArthur, A.H.R. Hydrodehalogenation of aryl chlorides and aryl bromides using a microwave-assisted, copper-catalyzed concurrent tandem catalysis methodology. Organometallics 2011, 30, 4067–4073. [Google Scholar] [CrossRef]
- Clark, J.H.; Jones, C.W. Reverse halogenation using supported copper (I) iodide. J. Chem. Soc. Chem. Commun. 1987, 1409–1410. [Google Scholar] [CrossRef]

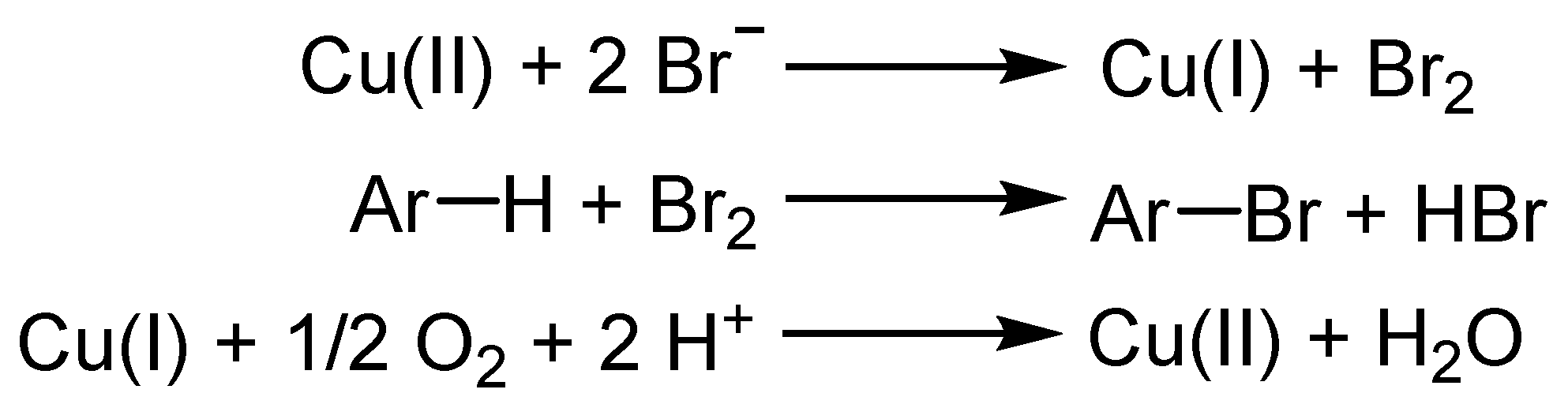
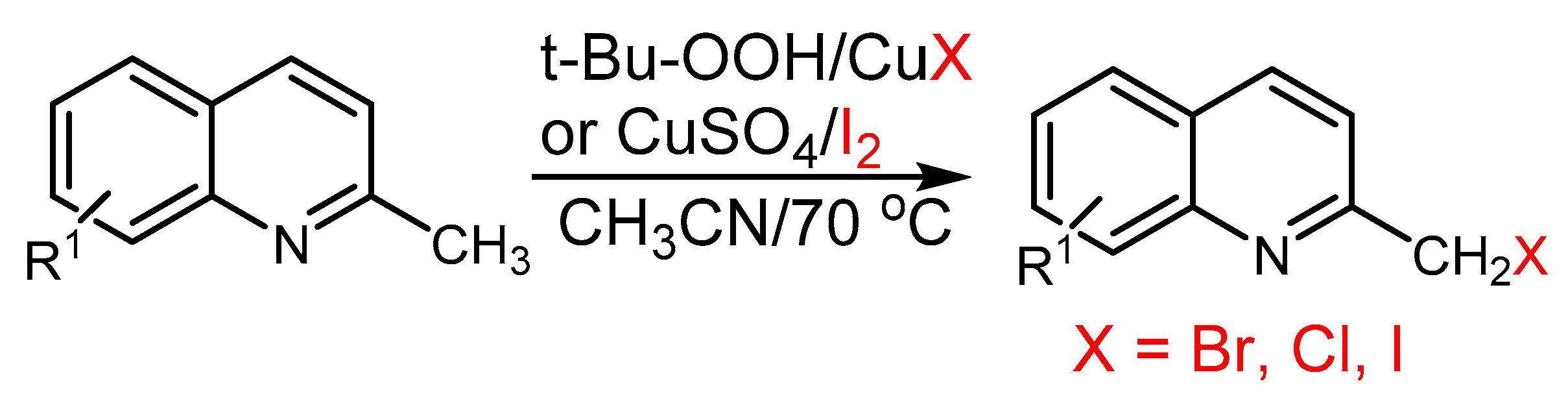
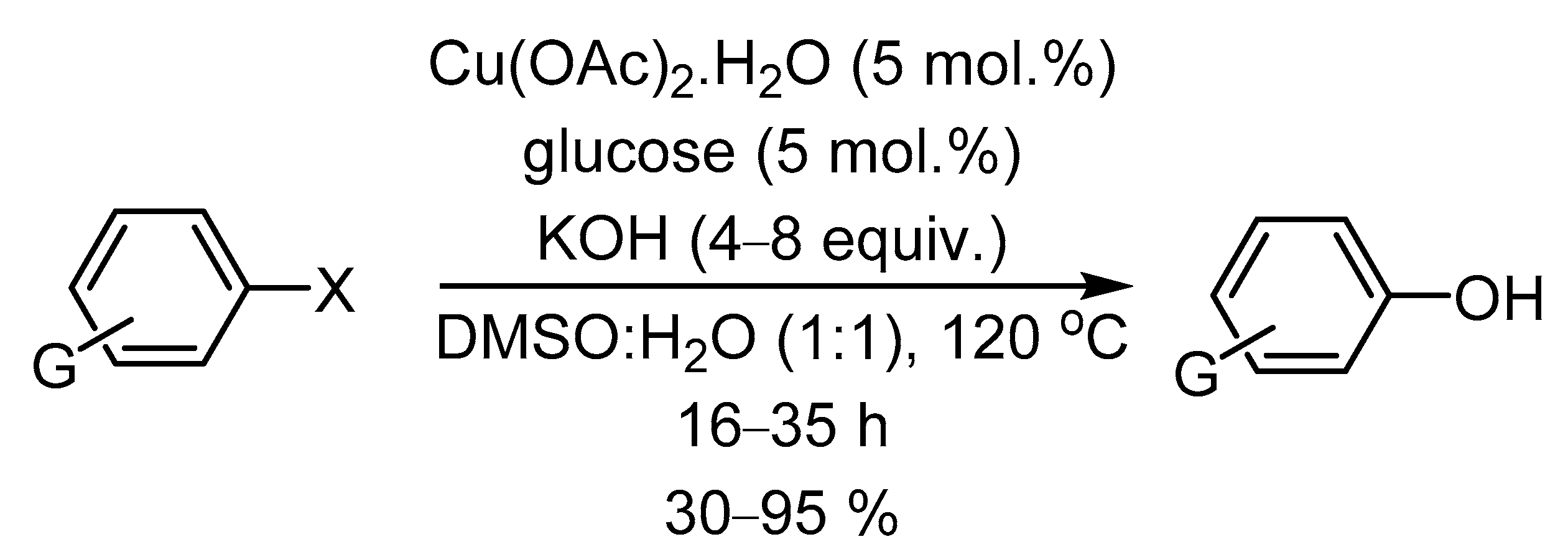
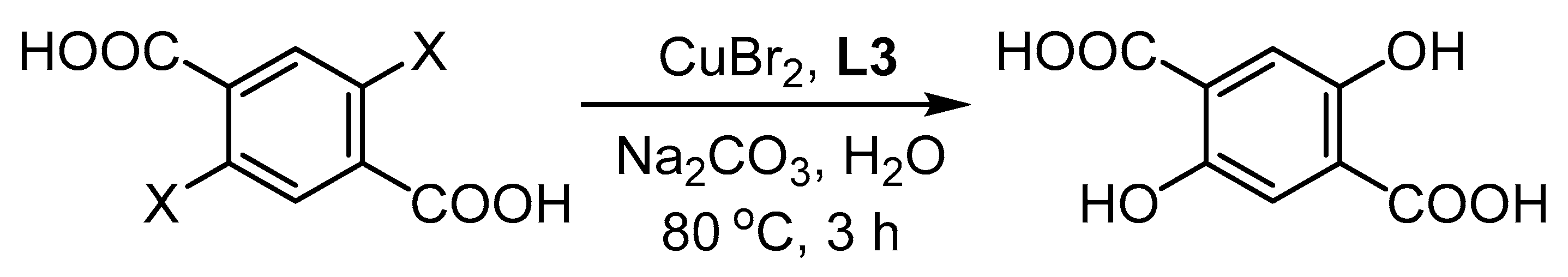
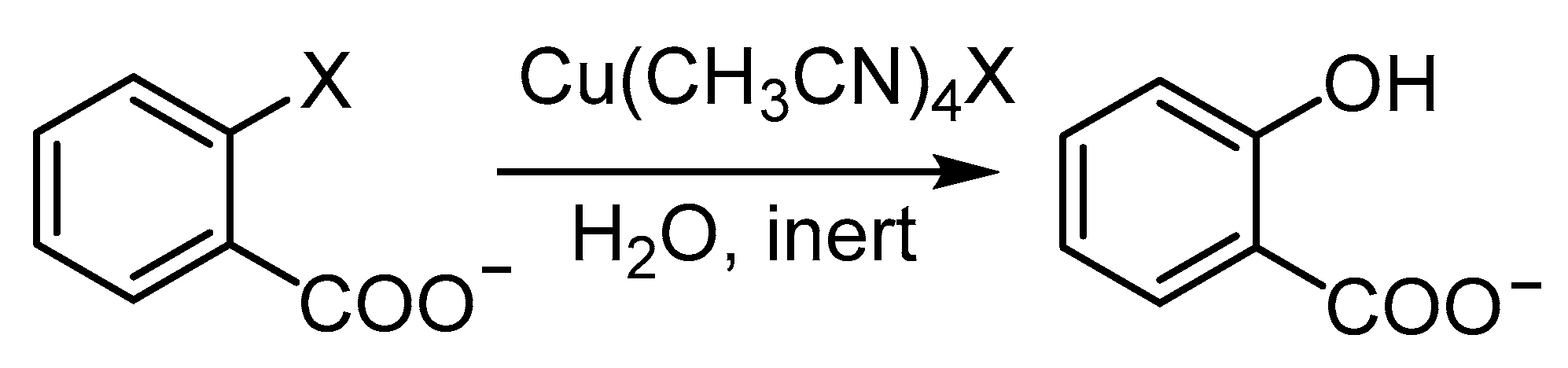



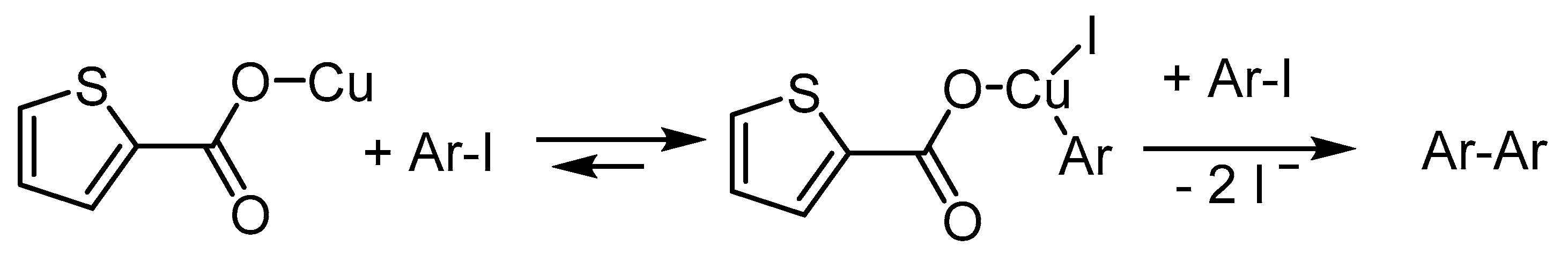
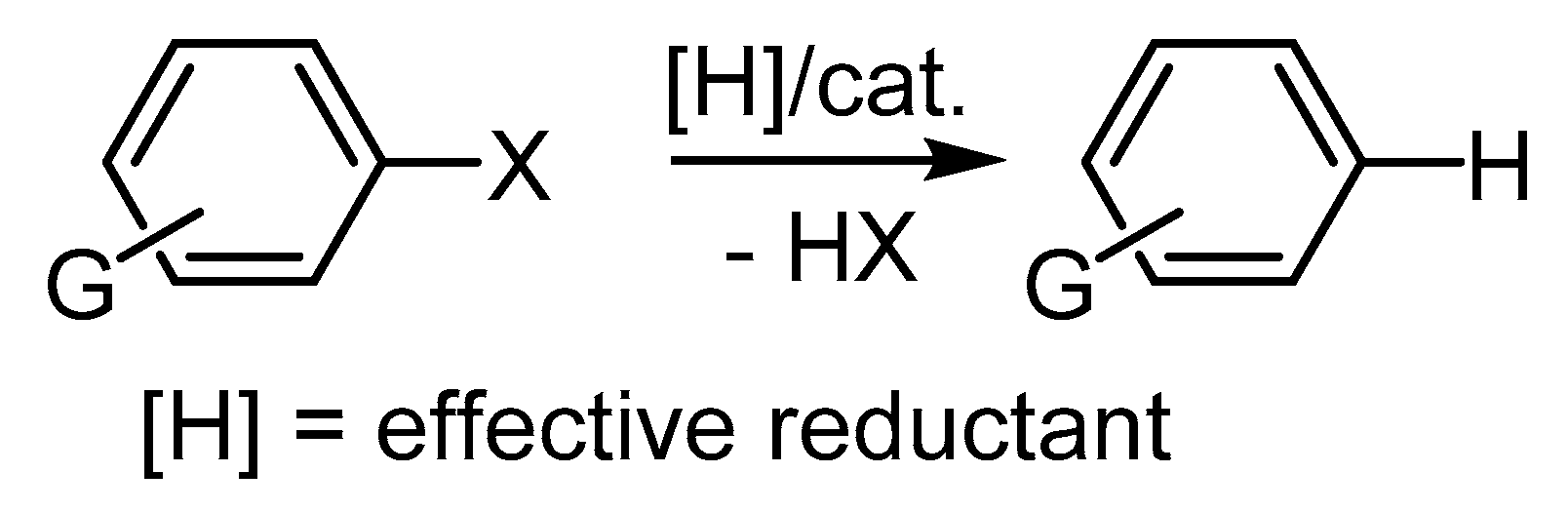
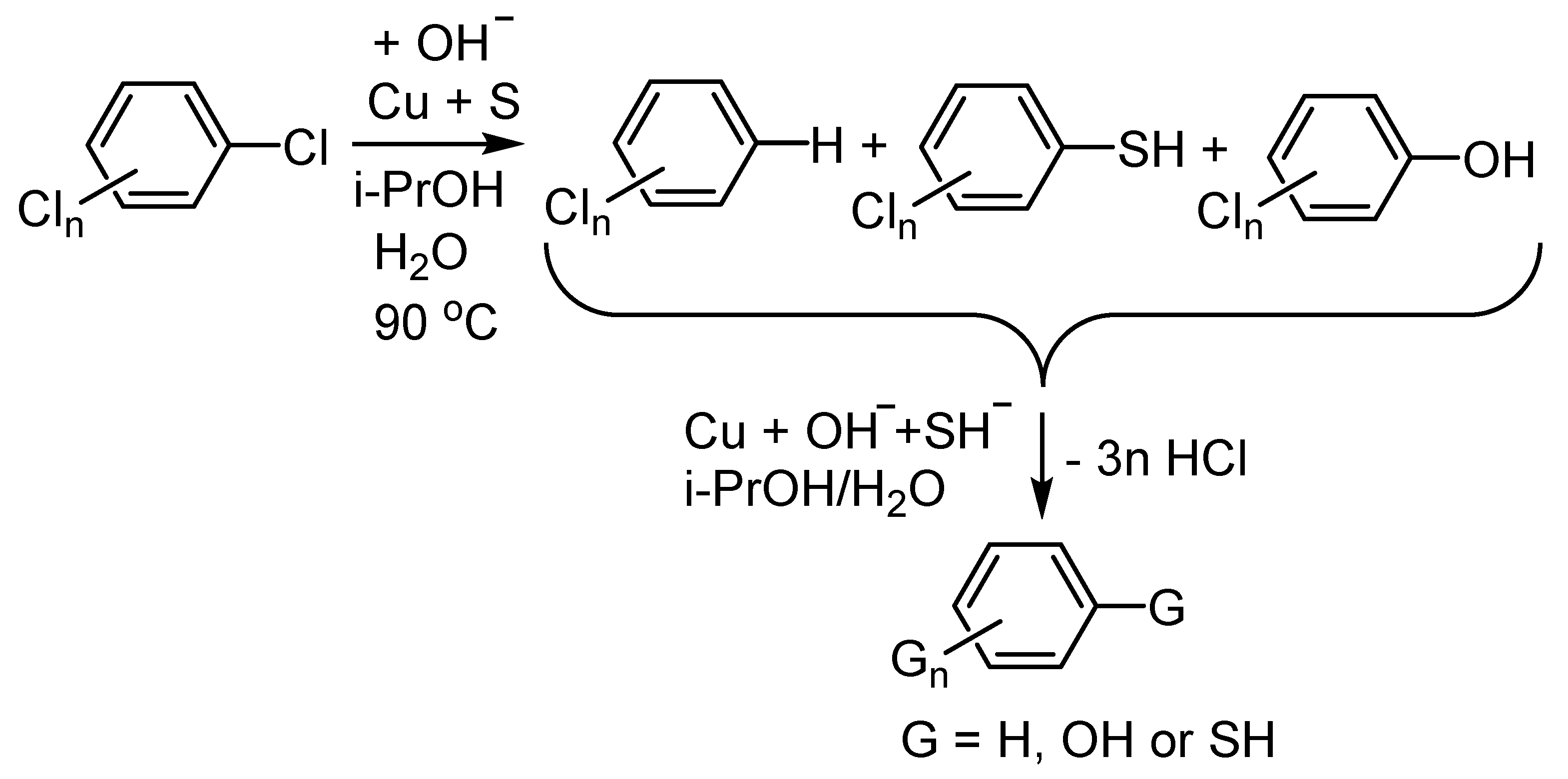
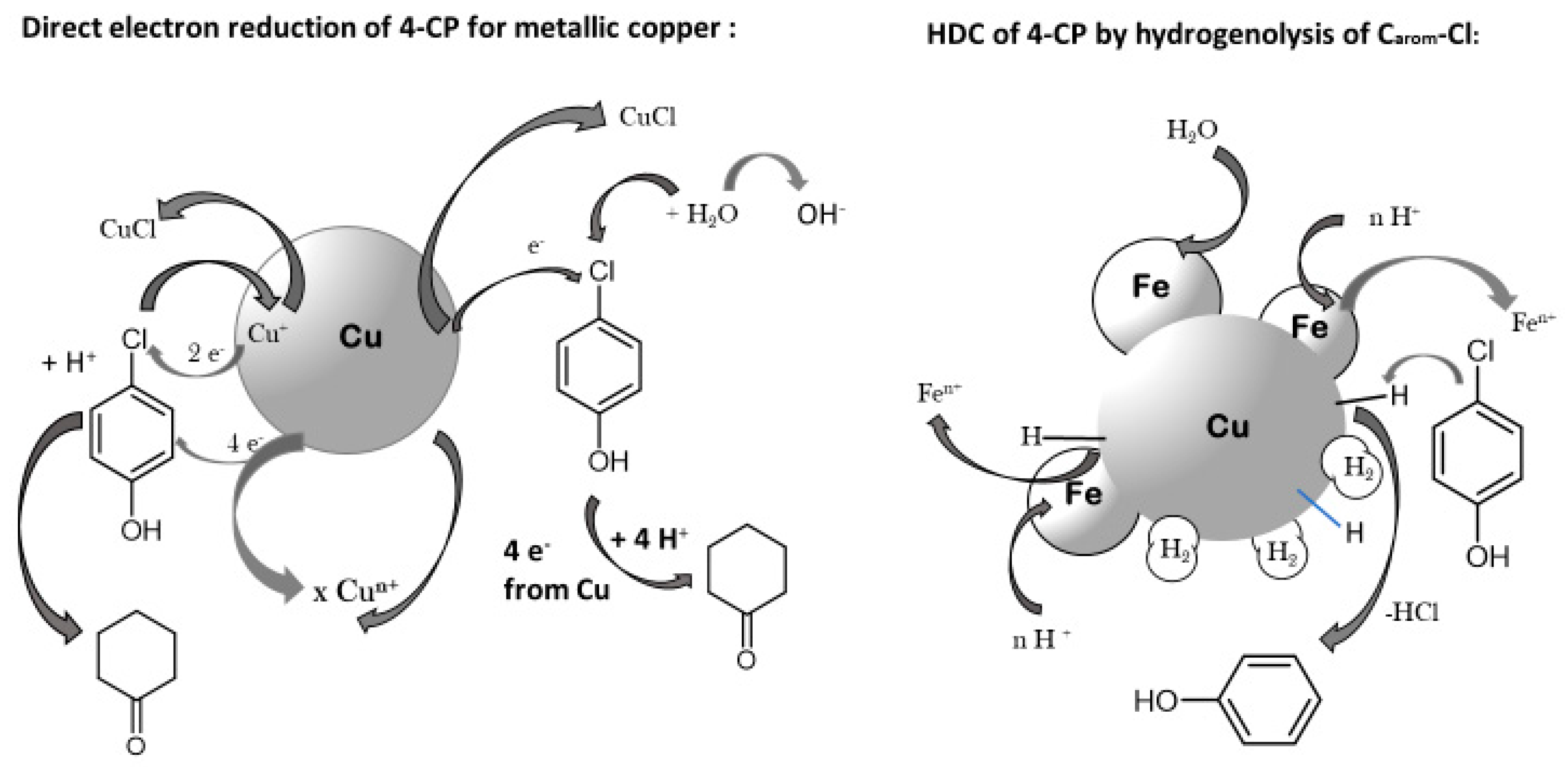
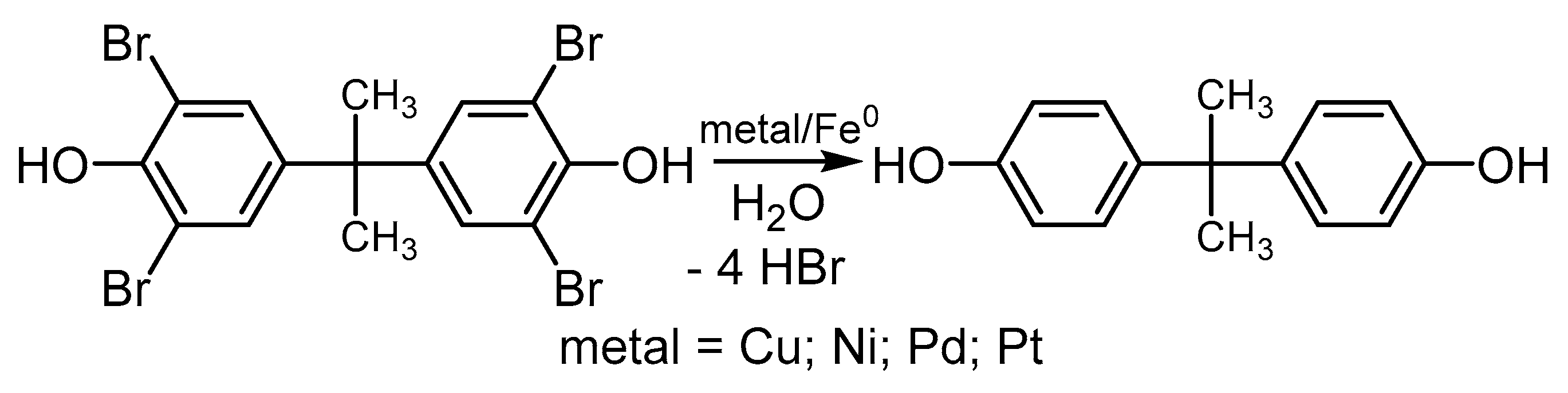

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weidlich, T. The Influence of Copper on Halogenation/Dehalogenation Reactions of Aromatic Compounds and Its Role in the Destruction of Polyhalogenated Aromatic Contaminants. Catalysts 2021, 11, 378. https://doi.org/10.3390/catal11030378
Weidlich T. The Influence of Copper on Halogenation/Dehalogenation Reactions of Aromatic Compounds and Its Role in the Destruction of Polyhalogenated Aromatic Contaminants. Catalysts. 2021; 11(3):378. https://doi.org/10.3390/catal11030378
Chicago/Turabian StyleWeidlich, Tomáš. 2021. "The Influence of Copper on Halogenation/Dehalogenation Reactions of Aromatic Compounds and Its Role in the Destruction of Polyhalogenated Aromatic Contaminants" Catalysts 11, no. 3: 378. https://doi.org/10.3390/catal11030378
APA StyleWeidlich, T. (2021). The Influence of Copper on Halogenation/Dehalogenation Reactions of Aromatic Compounds and Its Role in the Destruction of Polyhalogenated Aromatic Contaminants. Catalysts, 11(3), 378. https://doi.org/10.3390/catal11030378