Hexavalent Chromium Removal via Photoreduction by Sunlight on Titanium–Dioxide Nanotubes Formed by Anodization with a Fluorinated Glycerol–Water Electrolyte
Abstract
1. Introduction
2. Results and Discussion
2.1. Anodic Oxide Features on Titanium Anodized in Water–Glycerol Electrolyte
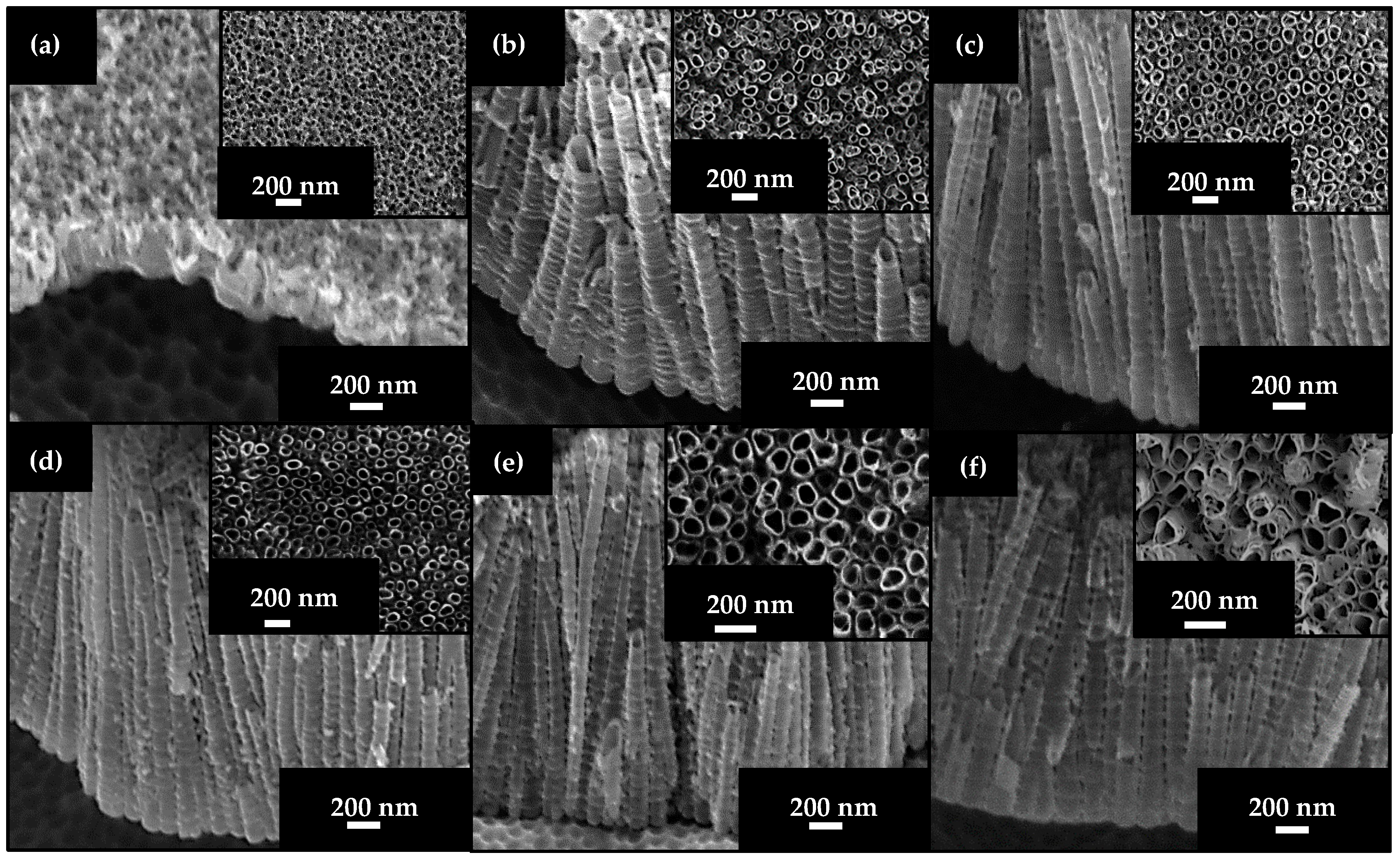
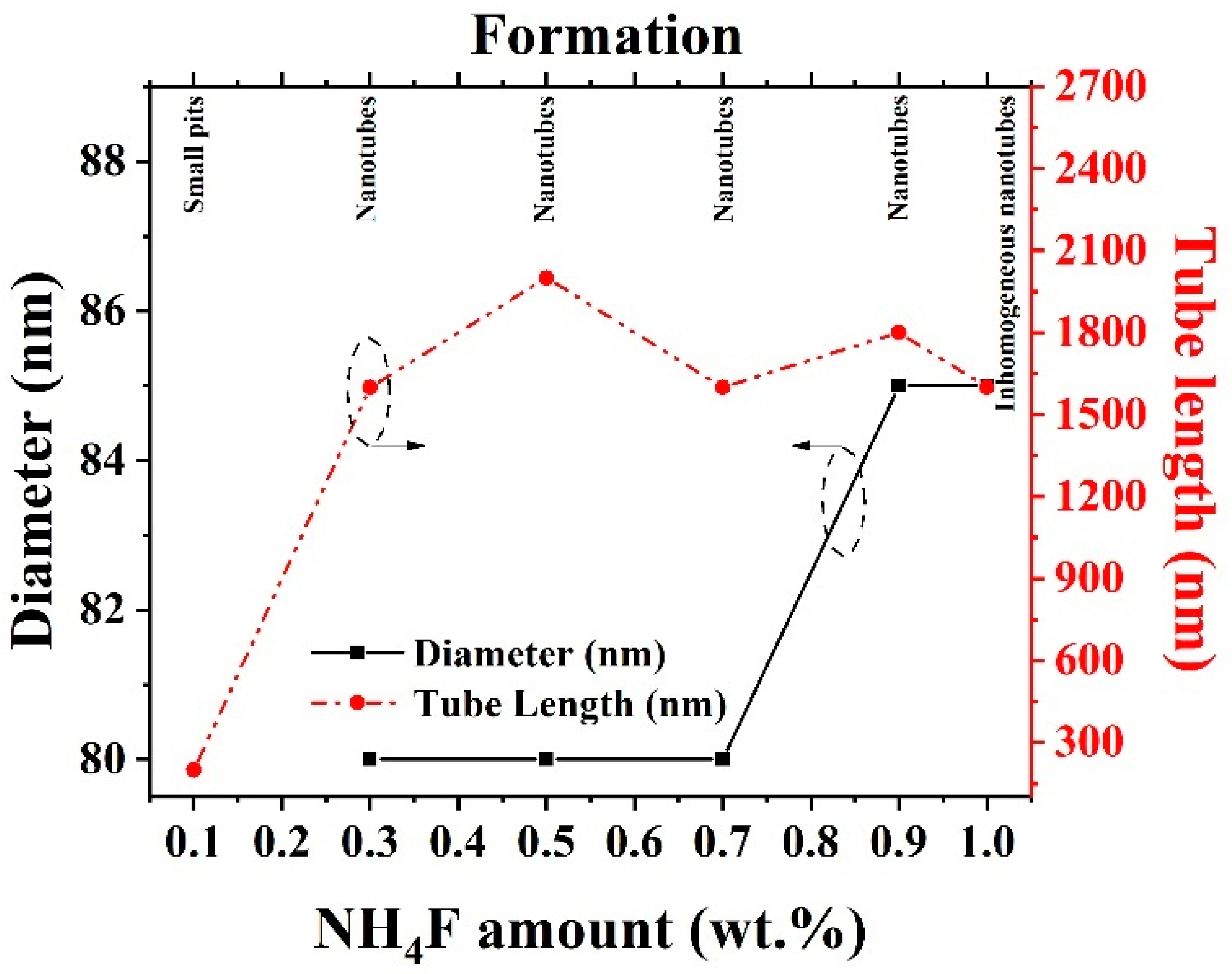
2.1.1. Effect of NH4F Concentration
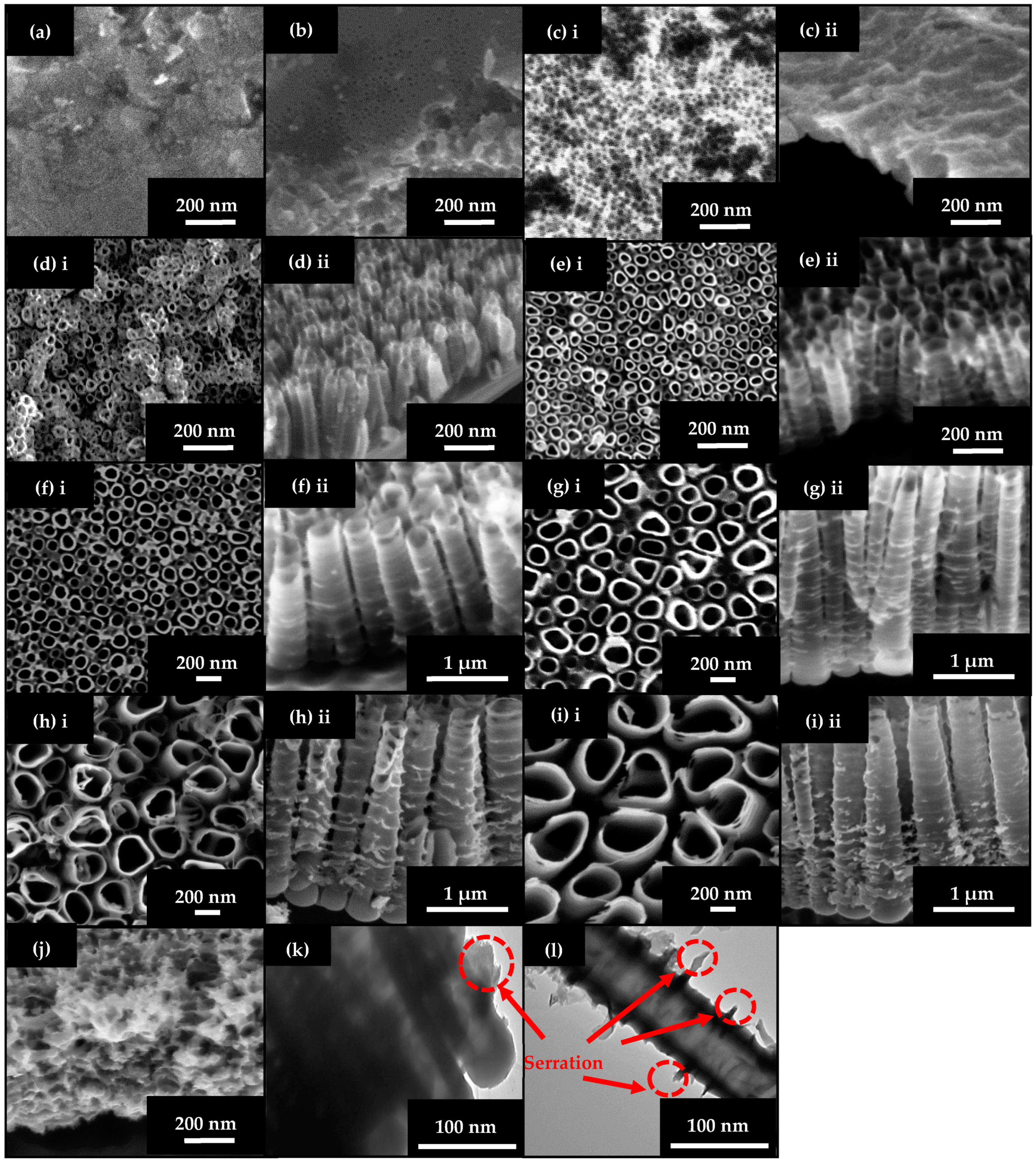
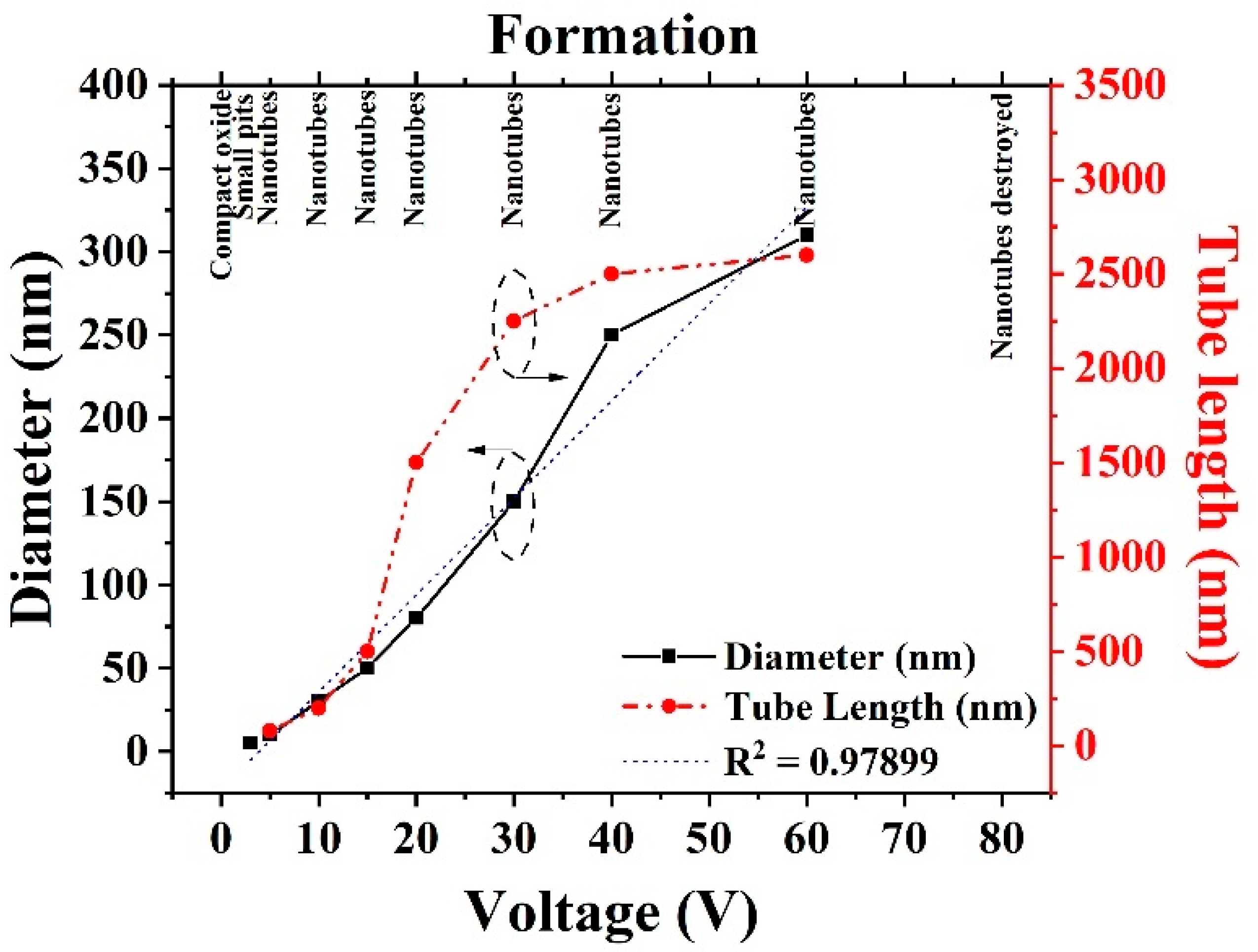
2.1.2. Effect of Anodization Voltage
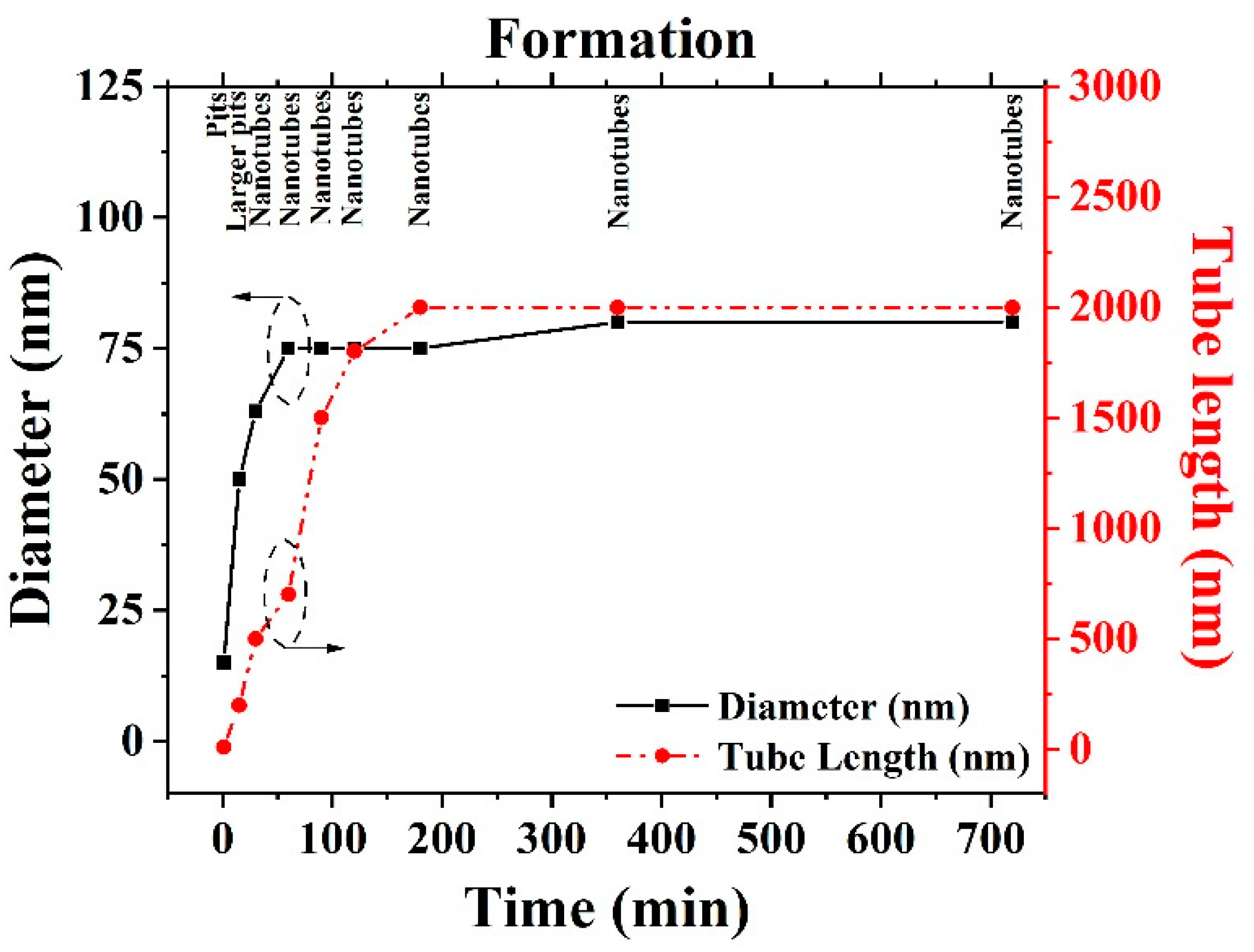
2.1.3. Effect of Variation Reaction Time
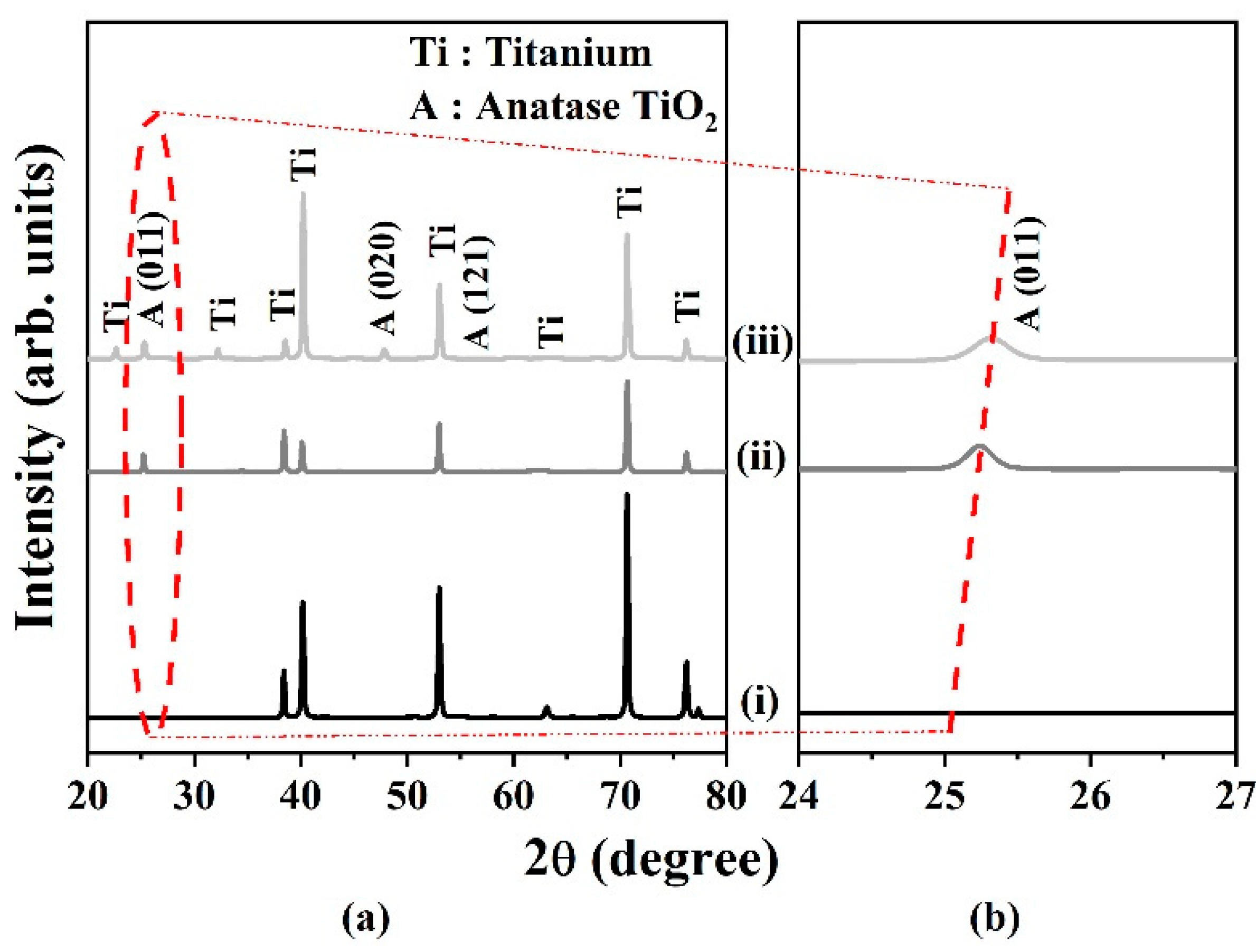
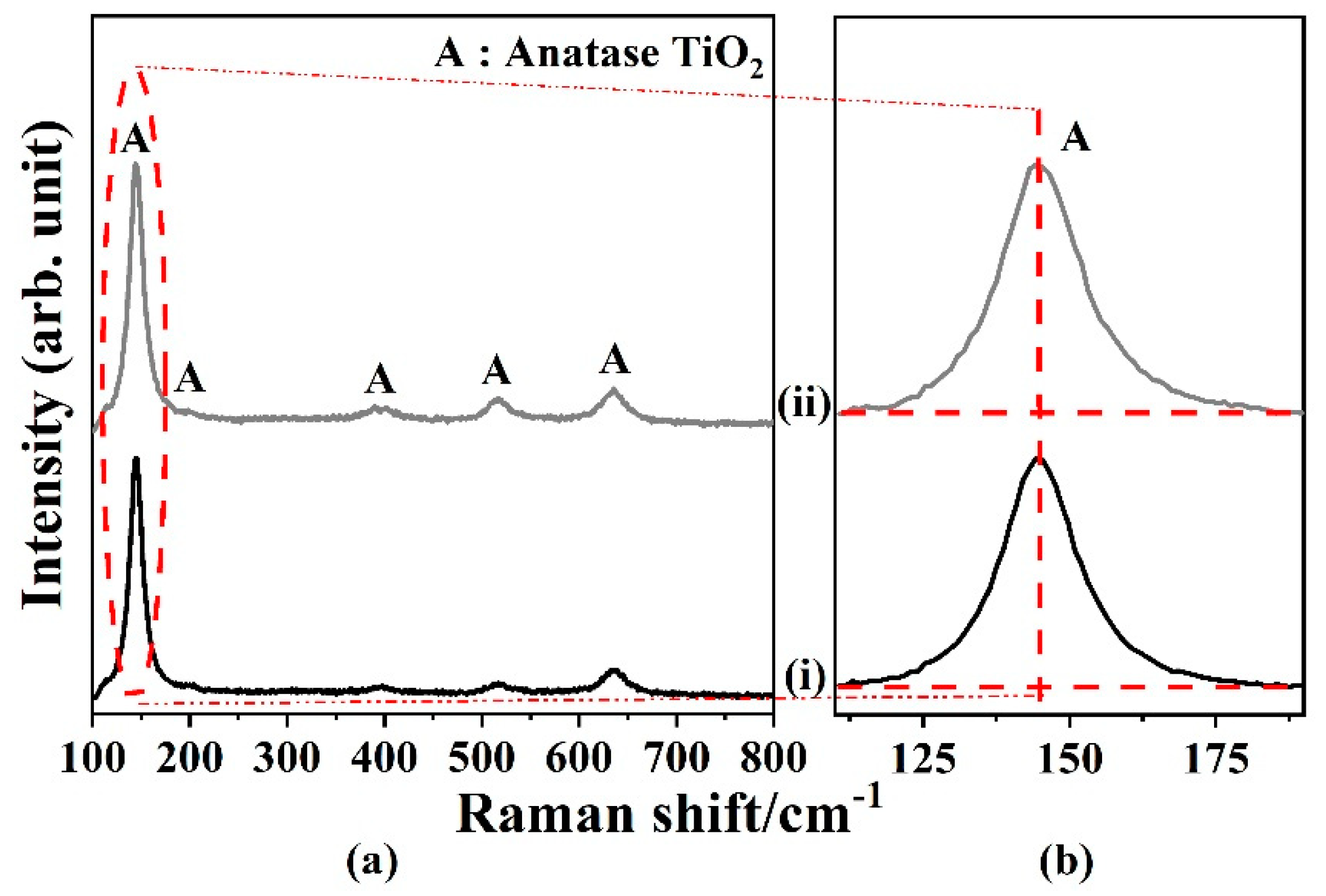
2.1.4. Effect of Annealing Environment.
2.2. Photoreduction of Hexavalent Chromium Ions on Titanium-Dioxide Nanotubes
2.2.1. Effect of EDTA as Hole Scavenger
2.2.2. The Effect of Initial Hexavalent Chromium Concentration
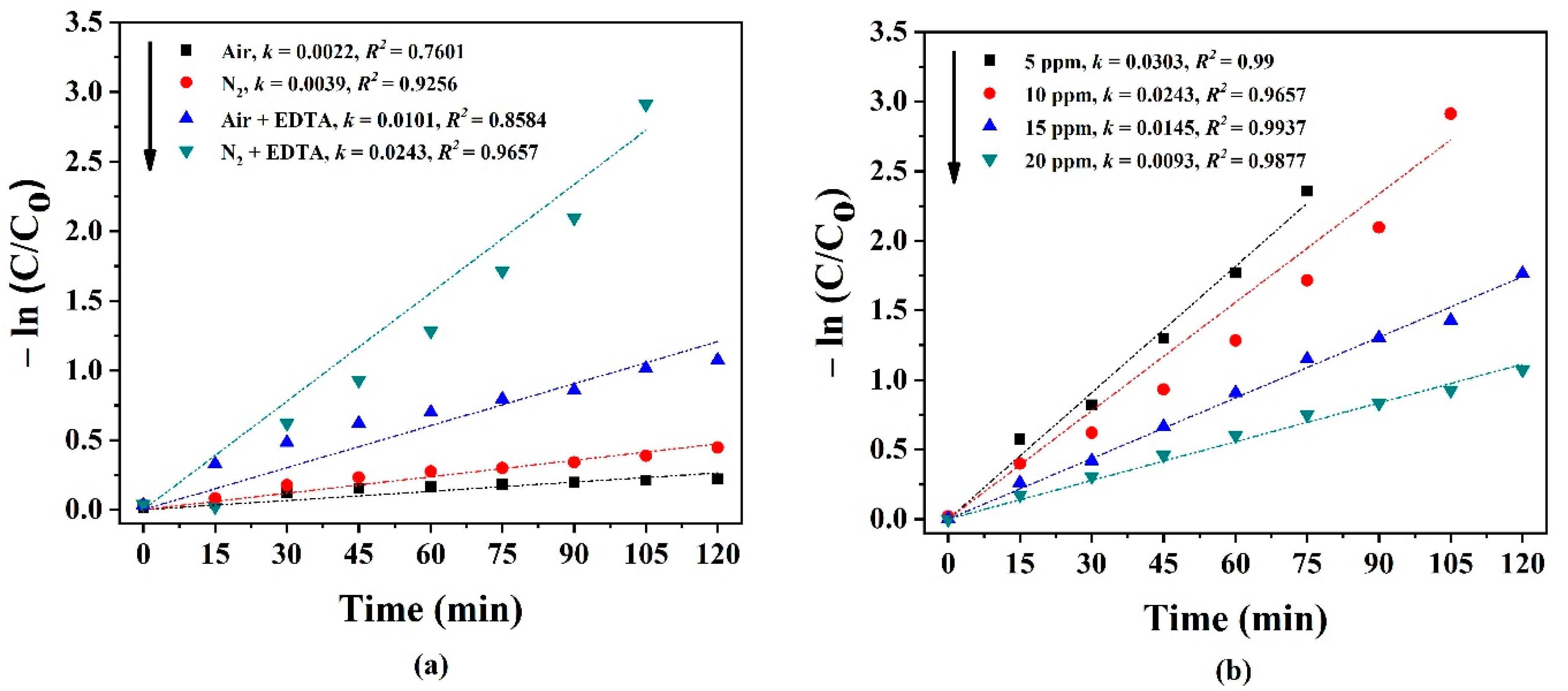
2.2.3. Reaction Kinetics Modeling
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karimi-Maleh, H.; Orooji, Y.; Ayati, A.; Qanbari, S.; Tanhaei, B.; Karimi, F.; Alizadeh, M.; Rouhi, J.; Fu, L.; Sillanpää, M. Recent advances in removal techniques of Cr(VI) toxic ion from aqueous solution: A comprehensive review. J. Mol. Liq. 2020, 329, 115062. [Google Scholar] [CrossRef]
- Schrank, S.G.; José, H.J.; Moreira, R.F.P.M. Simultaneous photocatalytic Cr(VI) reduction and dye oxidation in a TiO2 slurry reactor. J. Photochem. Photobiol. A Chem. 2002, 147, 71–76. [Google Scholar] [CrossRef]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Rudzi, S.K.; Ho, Y.B.; Abd Khani, I.I. Heavy metals contamination in paddy soil and water and associated dermal health risk among farmers. Malays. J. Med. Health Sci. 2018, 14, 2–10. [Google Scholar]
- Hoyer, P. Formation of a titanium dioxide nanotube array. Langmuir 1996, 12, 1411–1413. [Google Scholar] [CrossRef]
- Tu, Y.F.; Huang, S.Y.; Sang, J.P.; Zou, X.W. Synthesis and photocatalytic properties of Sn-Doped TiO2 nanotube arrays. J. Alloys Compd. 2009, 482, 382–387. [Google Scholar] [CrossRef]
- Yuan, Z.-Y.; Su, B.-L. Titanium oxide nanotubes, nanofibers and nanowires. Colloids Surf. A Physicochem. Eng. Asp. 2004, 241, 173–183. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Titania nanotubes prepared by chemical processing. Adv. Mater. 1999, 11, 1307–1311. [Google Scholar] [CrossRef]
- Abaffy, N.B.; Evans, P.; Triani, G.; McCulloch, D. Multilayer alumina and titania optical coatings prepared by atomic layer deposition. Nanostruct. Thin Films 2008, 7041, 704109. [Google Scholar] [CrossRef]
- Yoshimura, N.; Sato, S.; Itoi, M.; Taguchi, H. Electrical properties of TiO2 thin film prepared by sol-gel method. IEEJ Trans. Fundam. Mater. 1991, 111, 117–122. [Google Scholar] [CrossRef][Green Version]
- Venkatachalam, N.; Palanichamy, M.; Murugesan, V. Sol–gel preparation and characterization of nanosize TiO2: Its photocatalytic performance. Mater. Chem. Phys. 2007, 104, 454–459. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Chen, R.; Li, L. Fabrication of titanium oxide nanotube arrays by anodic oxidation. Solid State Commun. 2005, 134, 705–710. [Google Scholar] [CrossRef]
- Zwilling, V.; Darque-Ceretti, E.; Boutry-Forveille, A.; David, D.; Perrin, M.Y.; Aucouturier, M. Structure and Physicochemistry of anodic oxide films on titanium and TA6V alloy. Surf. Interface Anal. 1999, 27, 629–637. [Google Scholar] [CrossRef]
- Krengvirat, W.; Sreekantan, S.; Noor, A.-F.M.; Negishi, N.; Kawamura, G.; Muto, H.; Matsuda, A. Low-temperature crystallization of TiO2 nanotube arrays via hot water treatment and their photocatalytic properties under visible-light irradiation. Mater. Chem. Phys. 2013, 137, 991–998. [Google Scholar] [CrossRef]
- Krengvirat, W.; Sreekantan, S.; Noor, A.-F.M.; Kawamura, G.; Muto, H.; Matsuda, A. Single-step growth of carbon and potassium-embedded TiO2 nanotube arrays for efficient photoelectrochemical hydrogen generation. Electrochim. Acta 2013, 89, 585–593. [Google Scholar] [CrossRef]
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.S.; Chen, Z.; Dickey, E.C. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Choi, J.; Wehrspohn, R.B.; Lee, J.; Gösele, U. Anodization of nanoimprinted titanium: A comparison with formation of porous alumina. Electrochim. Acta 2004, 49, 2645–2652. [Google Scholar] [CrossRef]
- Macák, J.M.; Tsuchiya, H.; Schmuki, P. High-aspect-ratio TiO2 nanotubes by anodization of titanium. Angew. Chem. Int. Ed. 2005, 44, 2100–2102. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Akaki, T.; Nakata, J.; Terada, D.; Tsuji, N.; Koizumi, Y.; Minamino, Y.; Schmuki, P.; Fujimoto, S. Metallurgical aspects on the formation of self-organized anodic oxide nanotube layers. Electrochim. Acta 2009, 54, 5155–5162. [Google Scholar] [CrossRef]
- Lockman, Z.; Sreekantan, S.; Ismail, S.; Schmidt-Mende, L.; MacManus-Driscoll, J.L. Influence of anodisation voltage on the dimension of Titania nanotubes. J. Alloys Compd. 2010, 503, 359–364. [Google Scholar] [CrossRef]
- Sreekantan, S.; Lockman, Z.; Hazan, R.; Tasbihi, M.; Tong, L.K.; Mohamed, A.R. Influence of electrolyte pH on TiO2 nanotube formation by Ti anodization. J. Alloys Compd. 2009, 485, 478–483. [Google Scholar] [CrossRef]
- Kawamura, G.; Ohmi, H.; Tan, W.K.; Lockman, Z.; Muto, H.; Matsuda, A. Ag nanoparticle-deposited TiO2 nanotube arrays for electrodes of Dye-sensitized solar cells. Nanoscale Res. Lett. 2015, 10, 219. [Google Scholar] [CrossRef]
- Lockman, Z.; Ismail, S.; Sreekantan, S.; Schmidt-Mende, L.; MacManus-Driscoll, J.L. The rapid growth of 3 µm long titania nanotubes by anodization of titanium in a neutral electrochemical bath. Nanotechnology 2009, 21, 055601. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.; Lockman, Z.; Ahmad, Z.A. Crystallization of TiO2 Nanotubes Arrays Grown by Anodization of Ti in Organic Electrolyte. Adv. Mater. Res. 2012, 620, 412–417. [Google Scholar] [CrossRef]
- Sreekantan, S.; Saharudin, K.A.; Lockman, Z.; Tzu, T.W. Fast-rate formation of TiO2 nanotube arrays in an organic bath and their applications in photocatalysis. Nanotechnology 2010, 21, 365603. [Google Scholar] [CrossRef] [PubMed]
- Regonini, D.; Satka, A.; Jaroenworaluck, A.; Allsopp, D.W.E.; Bowen, C.R.; Stevens, R. Factors influencing surface morphology of anodized TiO2 nanotubes. Electrochim. Acta 2012, 74, 244–253. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Nishimoto, S.; Jin, M.; Tryk, D.A.; Murakami, T.; Fujishima, A. Highly ordered TiO2 nanotube arrays with controllable length for photoelectrocatalytic degradation of phenol. J. Phys. Chem. C 2008, 112, 253–259. [Google Scholar] [CrossRef]
- Perillo, P.M.; Rodriguez, D.F. Growth control of TiO2 nanotubes in different physical environments. Nanosci. Methods 2012, 1, 194–200. [Google Scholar] [CrossRef]
- Raja, K.; Gandhi, T.; Misra, M. Effect of water content of ethylene glycol as electrolyte for synthesis of ordered Titania nanotubes. Electrochem. Commun. 2007, 9, 1069–1076. [Google Scholar] [CrossRef]
- Naghizadeh, S.G.H.A.; Golobostanfard, M.R. Effect of fluoride concentration and water content on morphology of Titania nanotubes in ethylene glycol solution. Adv. Mater. Res. 2014, 829, 907–911. [Google Scholar] [CrossRef]
- Song, H.; Cheng, K.; Guo, H.; Wang, F.; Wang, J.; Zhu, N.; Bai, M.; Wang, X. Effect of ethylene glycol concentration on the morphology and catalytic properties of TiO2 nanotubes. Catal. Commun. 2017, 97, 23–26. [Google Scholar] [CrossRef]
- Taib, M.A.A.; Razak, K.A.; Jaafar, M.; Lockman, Z. Initial growth study of TiO2 nanotube arrays anodised in KOH/fluoride/ethylene glycol electrolyte. Mater. Des. 2017, 128, 195–205. [Google Scholar] [CrossRef]
- Nyein, N.; Tan, W.K.; Kawamura, G.; Matsuda, A.; Lockman, Z. Anodic Ag/TiO2 nanotube array formation in NaOH/fluoride/ethylene glycol electrolyte as a photoanode for dye-sensitized solar cells. Nanotechnology 2016, 27, 355605. [Google Scholar] [CrossRef]
- Nyein, N.; Tan, W.K.; Kawamura, G.; Matsuda, A.; Lockman, Z. TiO2 nanotube arrays formation in fluoride/ethylene glycol electrolyte containing LiOH or KOH as photoanode for dye-sensitized solar cell. J. Photochem. Photobiol. A Chem. 2017, 343, 33–39. [Google Scholar] [CrossRef]
- Nyein, N.; Zulkifli, M.A.; Tan, W.K.; Matsuda, A.; Lockman, Z. Effect of NaOH Concentration on the formation of TiO2 nanotube arrays by anodic oxidation process for photoelectrochemical cell. Solid State Phenom. 2017, 264, 152–155. [Google Scholar] [CrossRef]
- Taib, M.A.A.; Tan, W.K.; Okuno, T.; Kawamura, G.; Jaafar, M.; Razak, K.A.; Matsuda, A.; Lockman, Z. Formation of TiO2 nanotube arrays by anodic oxidation in LiOH added ethylene glycol electrolyte and the effect of thermal annealing on the photoelectrochemical properties. In Proceedings of the International Conference on Nano-Electronic Technology Devices and Materials, 2015 (IC-NET 2015), Selangor, Malaysia, 27 February–2 March 2015; AIP Publishing LLC: Melville, NY, USA, 2016; Volume 1733, p. 020025. [Google Scholar]
- Nyein, N.; Lockman, Z.; Matsuda, A.; Kawamura, G.; Tan, W.K.; Oo, T.Z. Formation of TiO2 nanotube arrays in KOH added fluoride-ethylene glycol (EG) electrolyte and its photoelectrochemical response. In Proceedings of the International Conference on Nano-Electronic Technology Devices and Materials, 2015 (IC-NET 2015), Selangor, Malaysia, 27 February–2 March 2015; AIP Publishing LLC: Melville, NY, USA, 2016; Volume 1733, p. 020030. [Google Scholar]
- Taib, M.A.A.; Kawamura, G.; Matsuda, A.; Jaafar, M.; Razak, K.A.; Lockman, Z. Synthesis of TiO2 Nanotube Arrays in NaOH Added Ethylene Glycol Electrolyte and the Effect of Annealing Temperature on the Nanotube Arrays to their Photocurrent Performance. Key Eng. Mater. 2016, 701, 28–32. [Google Scholar] [CrossRef]
- Taib, M.A.A.; Alias, N.; Jaafar, M.; Razak, K.A.; Tan, W.K.; Shahbudin, I.P.; Kawamura, G.; Matsuda, A.; Lockman, Z. Formation of grassy TiO2 nanotube thin film by anodisation in peroxide electrolyte for Cr(VI) removal under ultraviolet radiation. Nanotechnology 2020, 31, 435605. [Google Scholar] [CrossRef]
- Berger, S.; Albu, S.P.; Schmidt-Stein, F.; Hildebrand, H.; Schmuki, P.; Hammond, J.S.; Paul, D.F.; Reichlmaier, S. The origin for tubular growth of TiO2 nanotubes: A fluoride rich layer between tube-walls. Surf. Sci. 2011, 605, L57–L60. [Google Scholar] [CrossRef]
- Sahai, A.; Kumar, Y.; Agarwal, V.; Olive-Méndez, S.F.; Goswami, N. Doping concentration driven morphological evolution of Fe doped ZnO nanostructures. J. Appl. Phys. 2014, 116, 164315. [Google Scholar] [CrossRef]
- Kumari, R.; Sahai, A.; Goswami, N. Effect of nitrogen doping on structural and optical properties of ZnO nanoparticles. Prog. Nat. Sci. 2015, 25, 300–309. [Google Scholar] [CrossRef]
- Ramirez, H.; Medina-Ramirez, I. Photocatalytic Semiconductors; Springer: New York, NY, USA, 2015; p. 117. [Google Scholar]
- Morikawa, T.; Asahi, R.; Ohwaki, T.; Aoki, K.; Taga, Y. Band-gap narrowing of titanium dioxide by nitrogen doping. Jpn. J. Appl. Phys. 2001, 40, L561–L563. [Google Scholar] [CrossRef]
- Zulkifli, M.A.; Bashirom, N.; Tan, W.K.; Kawamura, G.; Matsuda, A.; Lockman, Z. Rapid TiO2 nanotubes formation in aged electrolyte and their application as photocatalysts for Cr(VI) reduction under visible light. IEEE Trans. Nanotechnol. 2018, 17, 1106–1110. [Google Scholar] [CrossRef]
- Murphy, A. Band-gap determination from diffuse reflectance measurements of semiconductor films, and application to photoelectrochemical water-splitting. Sol. Energy Mater. Sol. Cells 2007, 91, 1326–1337. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Davis, E.A.; Mott, N.F. Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos. Mag. 1970, 22, 0903–0922. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-gap energy estimation from diffuse reflectance measurements on sol–gel and commercial TiO2: A comparative study. J. Sol-Gel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- Delegan, N.; Daghrir, R.; Drogui, P.; El Khakani, M.A. Bandgap tailoring of in-situ nitrogen-doped TiO2 sputtered films intended for electrophotocatalytic applications under solar light. J. Appl. Phys. 2014, 116, 153510. [Google Scholar] [CrossRef]
- Wang, W.; Tadé, M.O.; Shao, Z. Nitrogen-doped simple and complex oxides for photocatalysis: A review. Prog. Mater. Sci. 2018, 92, 33–63. [Google Scholar] [CrossRef]
- Sayama, K.; Arakawa, H. Photocatalytic decomposition of water and photocatalytic reduction of carbon dioxide over zirconia catalyst. J. Phys. Chem. 1993, 97, 531–533. [Google Scholar] [CrossRef]
- Muñoz, J.; Domènech, X. TiO2 catalysed reduction of Cr(VI) in aqueous solutions under ultraviolet illumination. J. Appl. Electrochem. 1990, 20, 518–521. [Google Scholar] [CrossRef]
- Lu, H.; Fan, W.; Dong, H.; Liu, L. Dependence of the irradiation conditions and crystalline phases of TiO2 nanoparticles on their toxicity to Daphnia magna. Environ. Sci. Nano 2016, 4, 406–414. [Google Scholar] [CrossRef]
- Sclafani, A.; Herrmann, J.M. Comparison of the photoelectronic and photocatalytic activities of various anatase and rutile forms of titania in pure liquid organic phases and in aqueous solutions. J. Phys. Chem. 1996, 100, 13655–13661. [Google Scholar] [CrossRef]
- Botta, S.G.; Navío, J.A.; Hidalgo, M.C.; Restrepo, G.M.; Litter, M.I. Photocatalytic properties of ZrO2 and Fe/ZrO2 semiconductors prepared by a sol–gel technique. J. Photochem. Photobiol. A Chem. 1999, 129, 89–99. [Google Scholar] [CrossRef]
- Testa, J.J.; Grela, M.A.; Litter, M.I. Experimental evidence in favor of an initial one-electron-transfer process in the heterogeneous photocatalytic reduction of chromium(VI) over TiO2. Langmuir 2001, 17, 3515–3517. [Google Scholar] [CrossRef]
- Sun, B.; Reddy, E.P.; Smirniotis, P.G. Visible light Cr(VI) reduction and organic chemical oxidation by Tio2 photocatalysis. Environ. Sci. Technol. 2005, 39, 6251–6259. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Dutta, B.K. Photocatalytic degradation of model textile dyes in wastewater using ZnO as semiconductor catalyst. J. Hazard. Mater. 2004, 112, 269–278. [Google Scholar] [CrossRef]
- Shaban, Y.A. Effective photocatalytic reduction of Cr(VI) by carbon modified (CM)-n-TiO2 nanoparticles under solar irradiation. World J. Nano Sci. Eng. 2013, 3, 154–160. [Google Scholar] [CrossRef]
- Bashirom, N.; Tan, W.K.; Kawamura, G.; Matsuda, A.; Lockman, Z. Comparison of ZrO2, TiO2, and α-Fe2O3 nanotube arrays on Cr(VI) photoreduction fabricated by anodization of Zr, Ti, and Fe foils. Mater. Res. Express 2020, 7, 055013. [Google Scholar] [CrossRef]
- Wang, S.Q.; Bi, B.; Zhao, X.J. Study on photocatalytic reduction of Cr(VI) by fluorine-doped TiO2. Key Eng. Mater. 2017, 727, 841–846. [Google Scholar] [CrossRef]
- Liu, L.; Luo, C.; Xiong, J.; Yang, Z.; Zhang, Y.; Cai, Y.; Gu, H. Reduced graphene oxide (rGO) decorated TiO2 microspheres for visible-light photocatalytic reduction of Cr(VI). J. Alloys Compd. 2017, 690, 771–776. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, D.; Chen, C.; Wang, X. Enhanced photo-reduction and removal of Cr(VI) on reduced graphene oxide decorated with TiO2 nanoparticles. J. Colloid Interface Sci. 2013, 405, 211–217. [Google Scholar] [CrossRef]
- Lei, X.; Zhang, Z.; Wu, Z.; Piao, Y.; Chen, C.; Li, X.; Xue, X.; Yang, H. Synthesis and characterization of Fe, N and C tri-doped polymorphic TiO2 and the visible light photocatalytic reduction of Cr(VI). Sep. Purif. Technol. 2017, 174, 66–74. [Google Scholar] [CrossRef]
- Rahmat, S.; Tan, W.K.; Kawamura, G.; Matsuda, A.; Lockman, Z. Facile fabrication of rGO/rutile TiO2 nanowires as photocatalyst for Cr(VI) reduction. Mater. Today Proc. 2019, 17, 1143–1151. [Google Scholar] [CrossRef]
- Djellabi, R.; Ghorab, F.M.; Nouacer, S.; Smara, A.; Khireddine, O. Cr(VI) photocatalytic reduction under sunlight followed by Cr(III) extraction from TiO2 surface. Mater. Lett. 2016, 176, 106–109. [Google Scholar] [CrossRef]

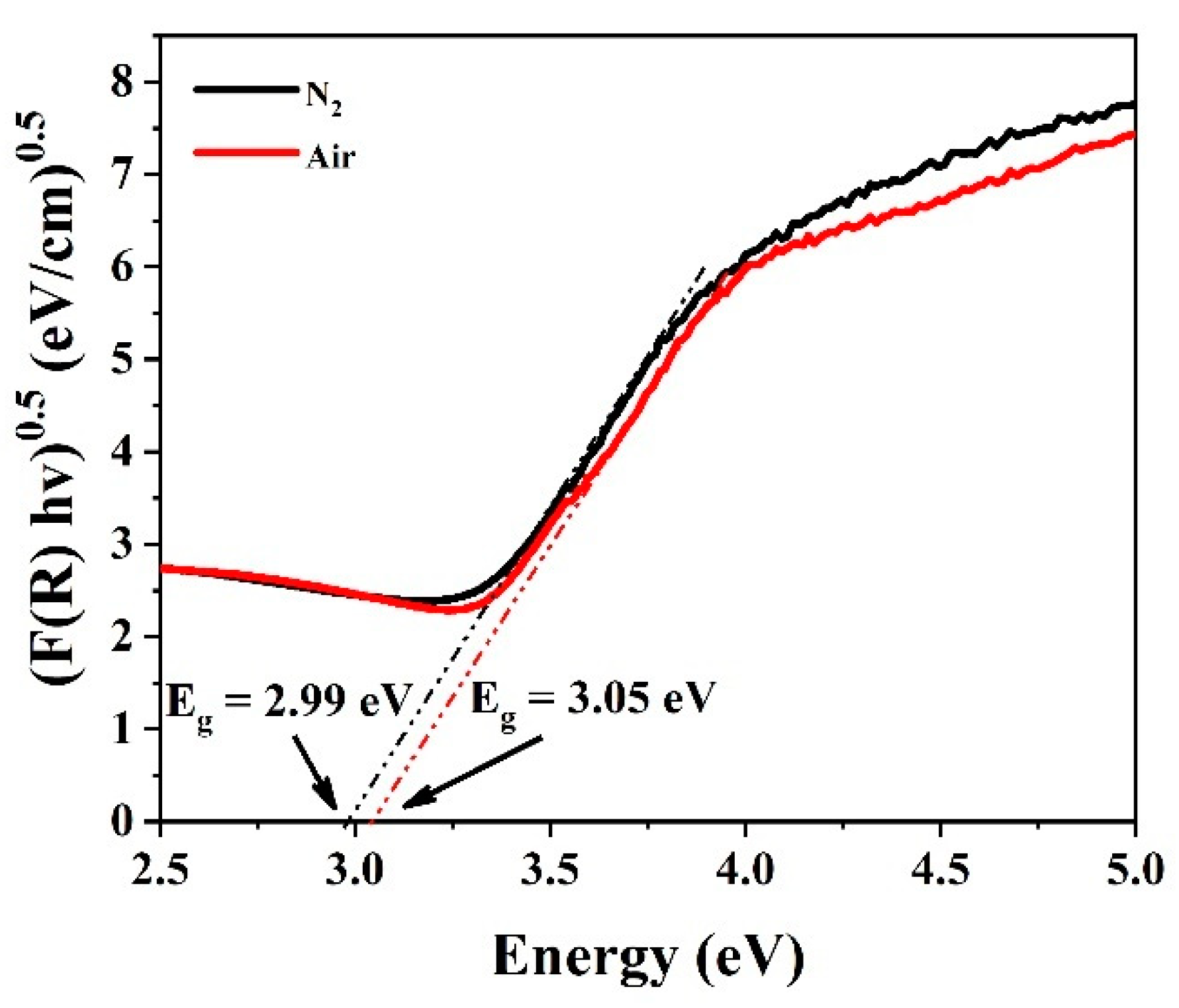
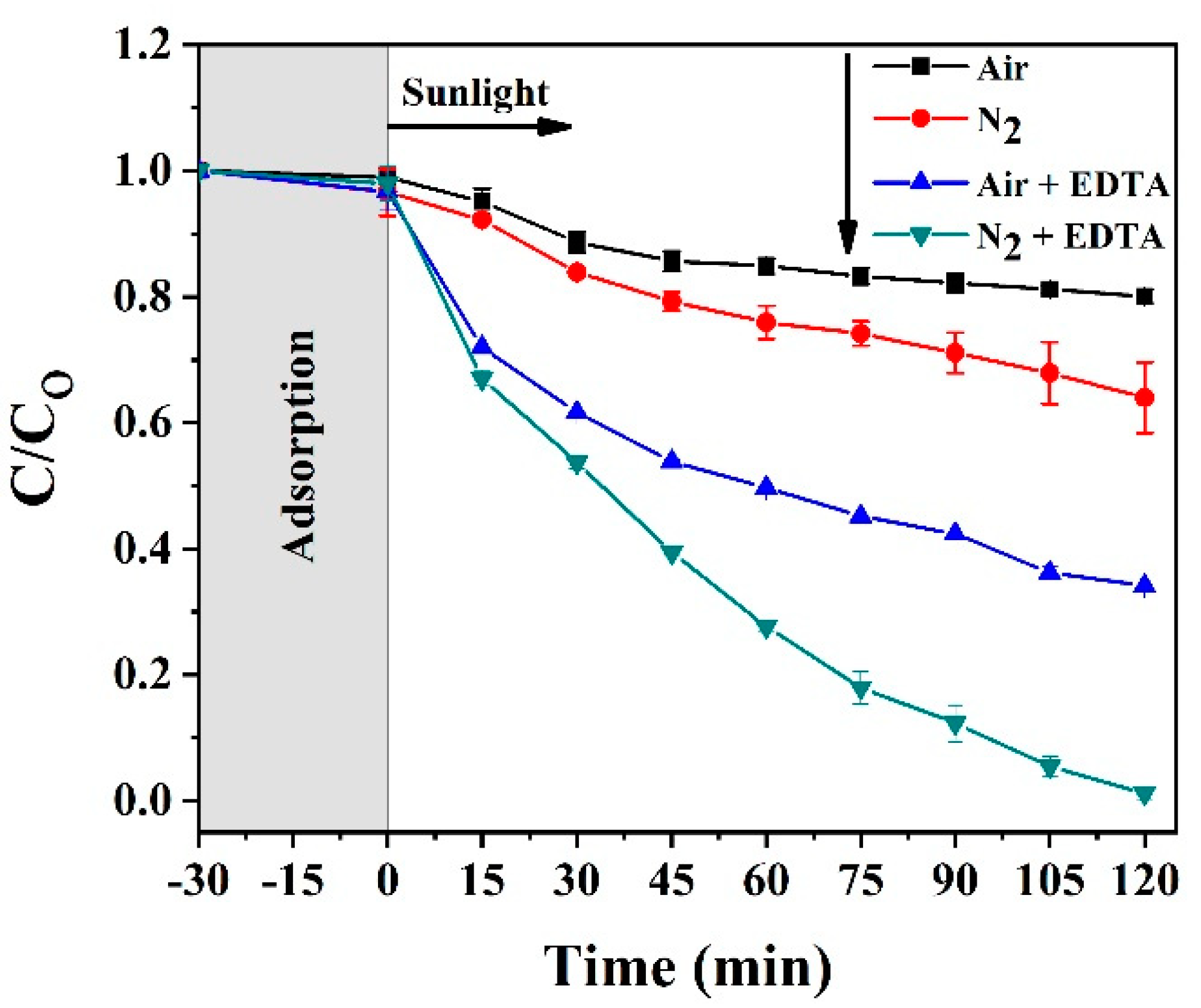
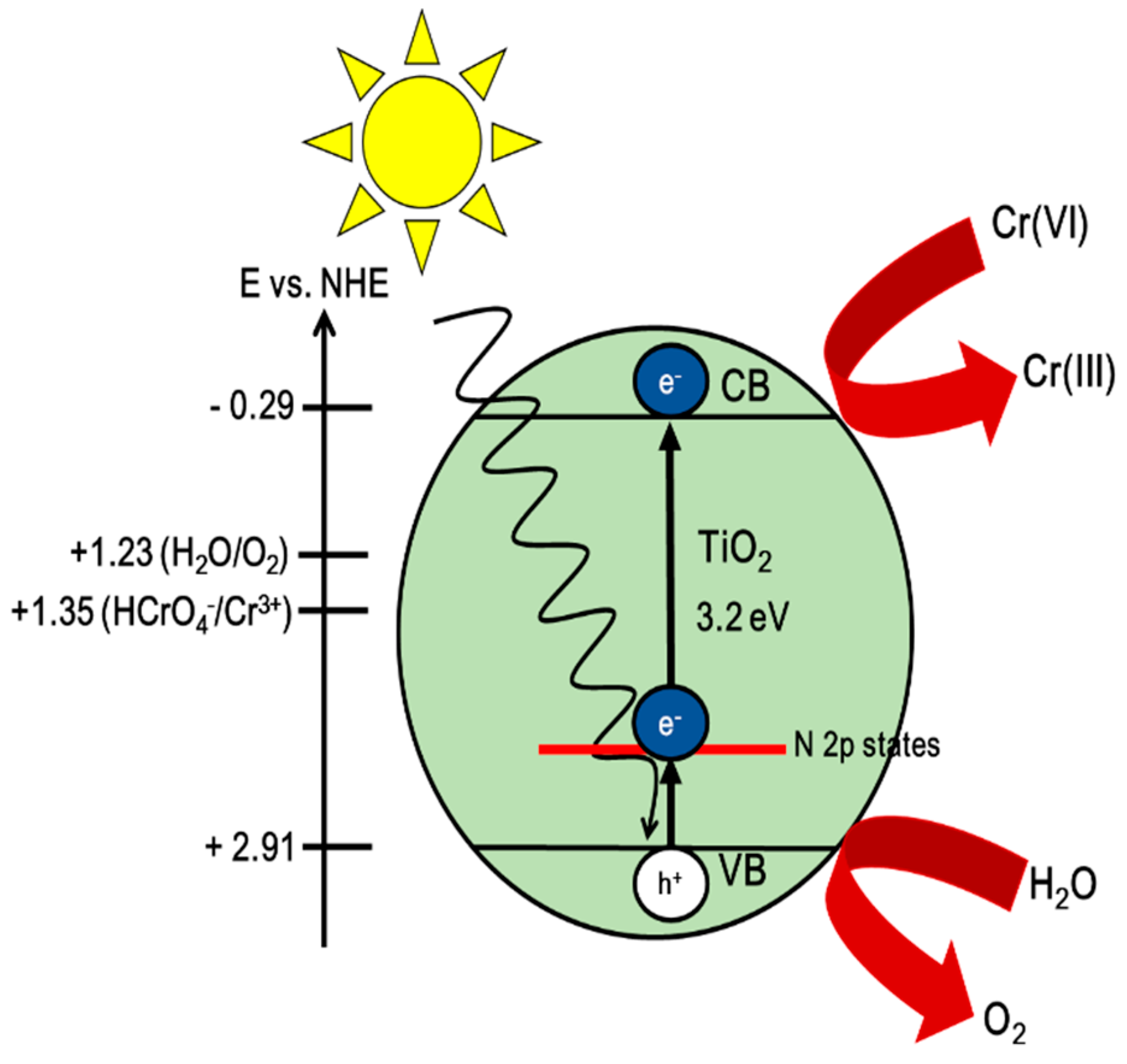

| Sample | Eg (eV) | EVB (eV) | ECB (eV) |
|---|---|---|---|
| Anatase TiO2 | 3.20 | 2.91 | −0.29 |
| TNTs-Air | 3.05 | 2.84 | −0.22 |
| TNTs-N2 | 2.99 | 2.81 | −0.19 |
| Photocatalyst | Method | Sample Size | Scavenger | pH | Cr(VI) Conc. (ppm) | Source of Light | Removal Efficiency (%) | Time (h) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| TNTs-N2 | Anodization | 1 cm2 | – | 2 | 5 | Natural sunlight | 80 | 5 | [45] |
| TNTs-Air | Anodization | 1 cm2 | – | 2 | 10 | Natural sunlight | 10 | 3 | [61] |
| TNTs-N2 | Anodization | 2 cm2 | – | 1 | 10 | Natural sunlight | 37 | 2 | Current work |
| TNTs-Fluorine | Sol–gel | – | – | 2.5 | 8 | Fluorescent lamp | 90 | 2 | [62] |
| TiO2-5%RGO | Hydrothermal | – | – | 2 | 10 | Solar | 98 | 3 | [63] |
| TiO2/RGO | Sol–gel | – | – | 2.6 | 12 | Mercury lamp | 86.5 | 4 | [64] |
| 0.30wt.%Fe-N-C-TiO2 | Hydrothermal | – | – | 2 | 20 | Xenon lamp | 100 | 4 | [65] |
| C-Modified n-TiO2 | Sol–gel | – | Phenol (10 mM) | 5 | 5 | Natural sunlight | 100 | 0.33 | [60] |
| TiO2NW-RGO | Anodization | 4 cm2 | EDTA (1 mM) | 1 | 10 | Xenon lamp | 100 | 1 | [66] |
| P25 Degussa | – | – | Tartaric acid (6 mM) | 2.2 | 20 | Natural sunlight | 100 | 2 | [67] |
| TNTs-N2 | Anodization | 2 cm2 | EDTA (1 mM) | 1 | 10 | Natural sunlight | 100 | 2 | Current work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosli, S.A.; Alias, N.; Bashirom, N.; Ismail, S.; Tan, W.K.; Kawamura, G.; Matsuda, A.; Lockman, Z. Hexavalent Chromium Removal via Photoreduction by Sunlight on Titanium–Dioxide Nanotubes Formed by Anodization with a Fluorinated Glycerol–Water Electrolyte. Catalysts 2021, 11, 376. https://doi.org/10.3390/catal11030376
Rosli SA, Alias N, Bashirom N, Ismail S, Tan WK, Kawamura G, Matsuda A, Lockman Z. Hexavalent Chromium Removal via Photoreduction by Sunlight on Titanium–Dioxide Nanotubes Formed by Anodization with a Fluorinated Glycerol–Water Electrolyte. Catalysts. 2021; 11(3):376. https://doi.org/10.3390/catal11030376
Chicago/Turabian StyleRosli, Siti Azlina, Nurhaswani Alias, Nurulhuda Bashirom, Syahriza Ismail, Wai Kian Tan, Go Kawamura, Atsunori Matsuda, and Zainovia Lockman. 2021. "Hexavalent Chromium Removal via Photoreduction by Sunlight on Titanium–Dioxide Nanotubes Formed by Anodization with a Fluorinated Glycerol–Water Electrolyte" Catalysts 11, no. 3: 376. https://doi.org/10.3390/catal11030376
APA StyleRosli, S. A., Alias, N., Bashirom, N., Ismail, S., Tan, W. K., Kawamura, G., Matsuda, A., & Lockman, Z. (2021). Hexavalent Chromium Removal via Photoreduction by Sunlight on Titanium–Dioxide Nanotubes Formed by Anodization with a Fluorinated Glycerol–Water Electrolyte. Catalysts, 11(3), 376. https://doi.org/10.3390/catal11030376






