TiO2-Graphene Quantum Dots Nanocomposites for Photocatalysis in Energy and Biomedical Applications
Abstract
1. Introduction
2. TiO2 Nanomaterials
- TiO2 can be supported on various substrates, such as glass, fibers, stainless steel, inorganic materials, sand, and activated carbon, which allows its continuous reuse [42].
2.1. Drawbacks of TiO2
- The large bandgap of TiO2 (~3.2 eV for anatase and brookite, ~3.0 eV for rutile) requires an excitation wavelength that falls in the UV region. Given that less than ~5% of the solar flux incident at the Earth’s surface lies in this spectral regime (solar light consists of ~5% UV, ~43% visible, and ~52% harvesting infrared), only a very small portion of the solar light could be used by pure TiO2 photocatalysts;
- Massive recombination of photogenerated charge carriers limits its overall photocatalytic efficiency.
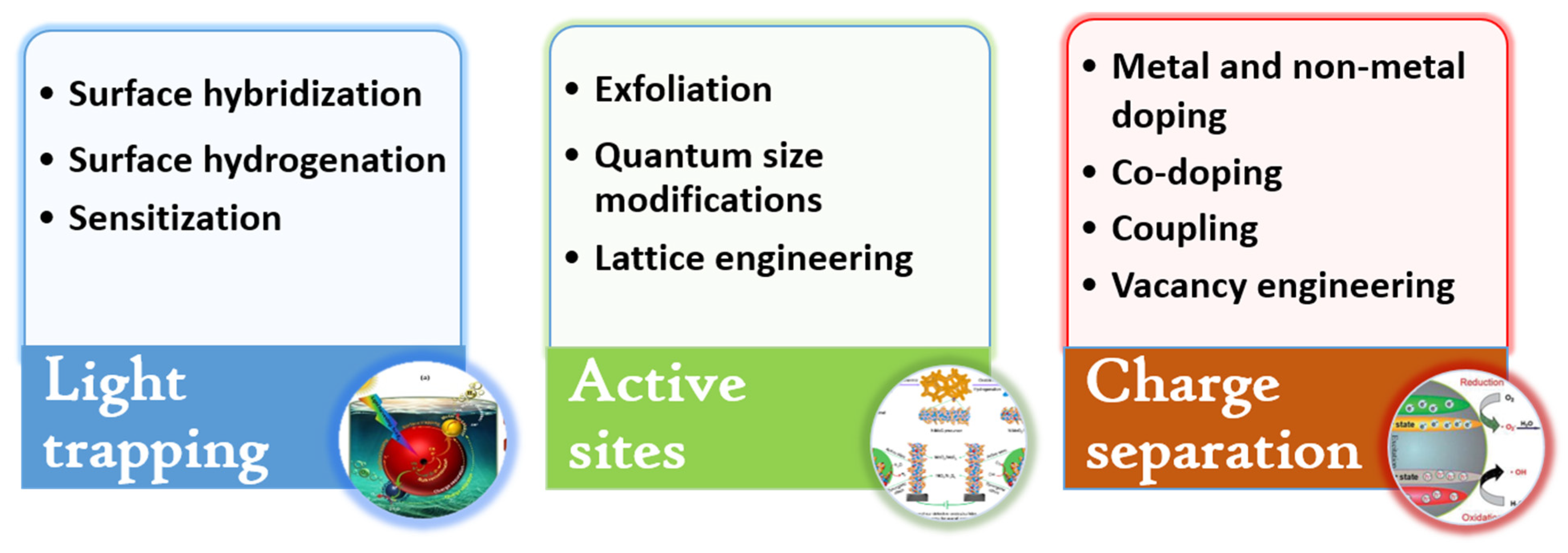
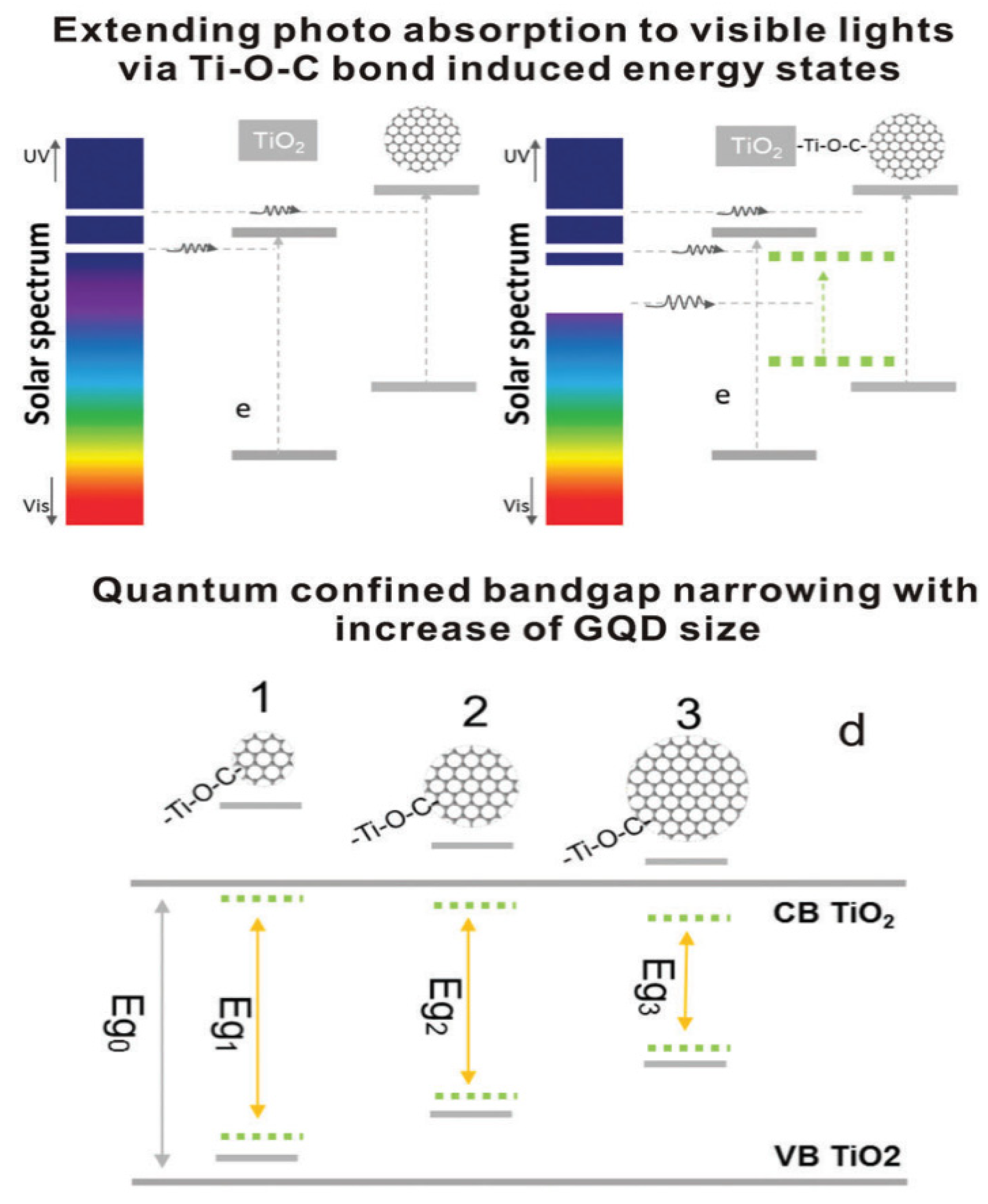
2.2. Strategies to Modify TiO2 Nanomaterials
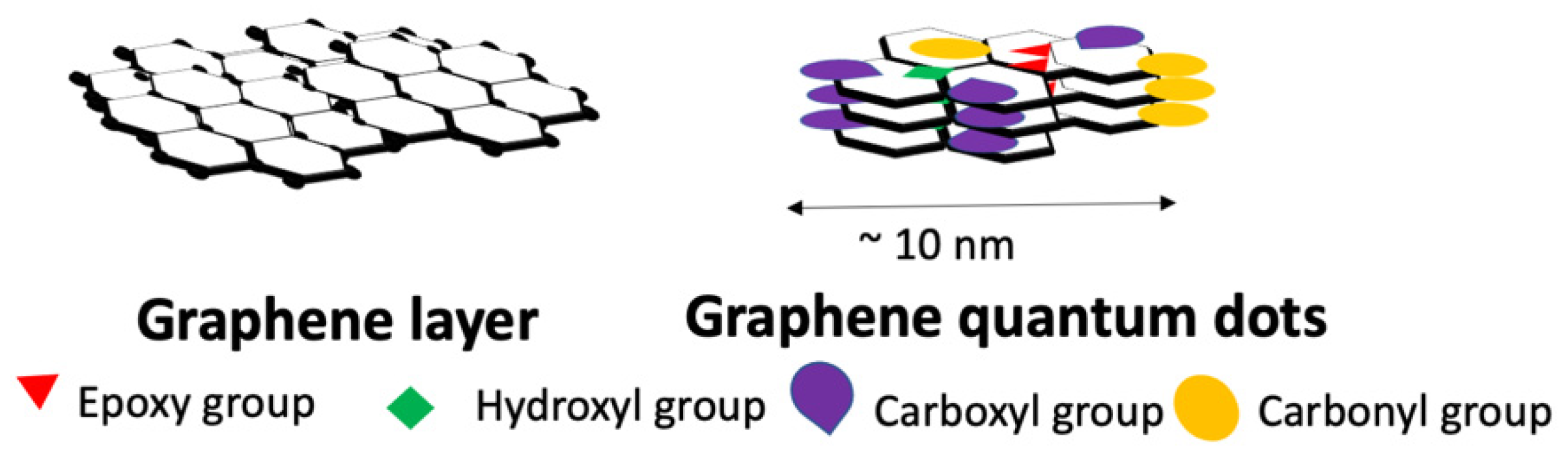
3. Graphene Quantum Dots (GQDs)
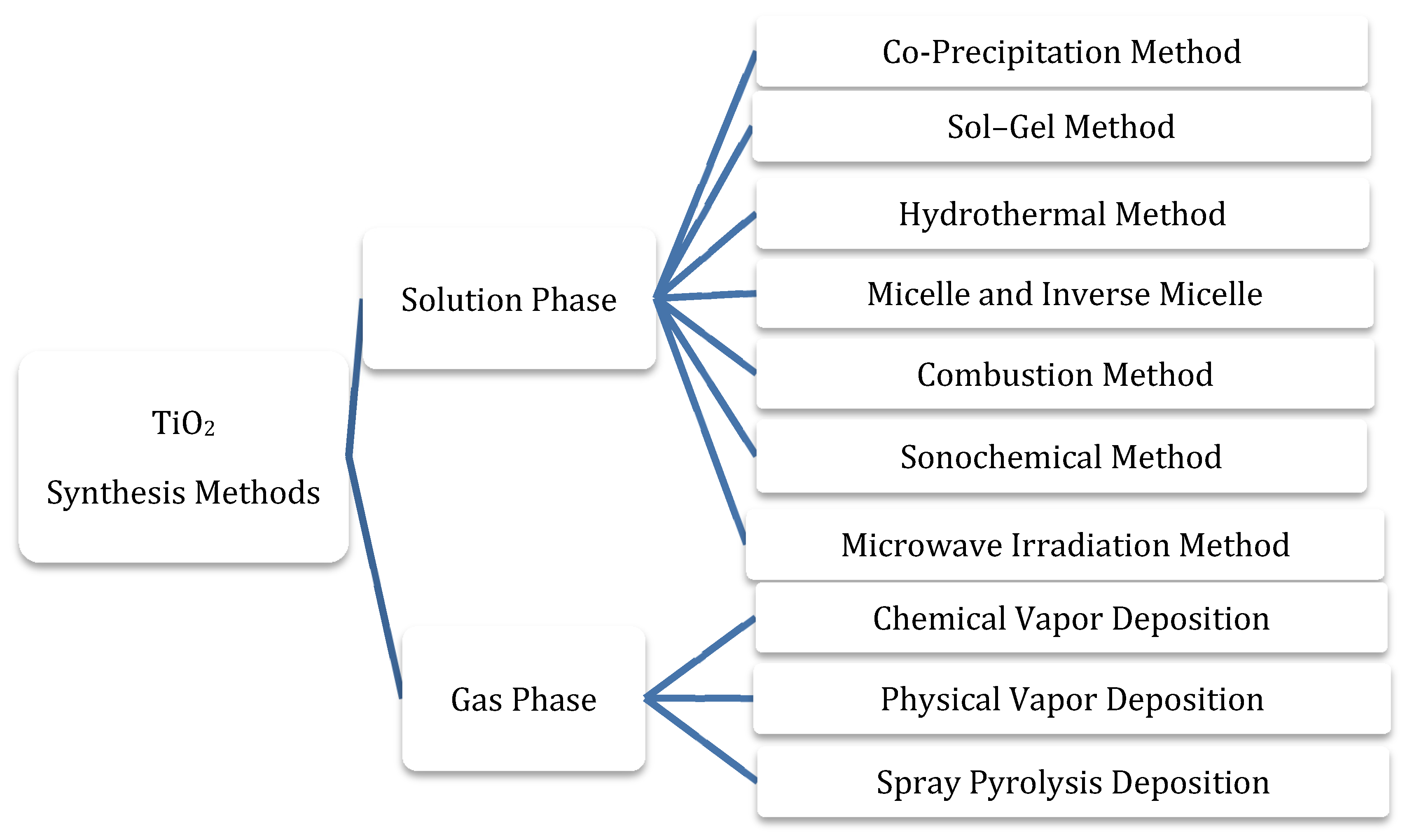
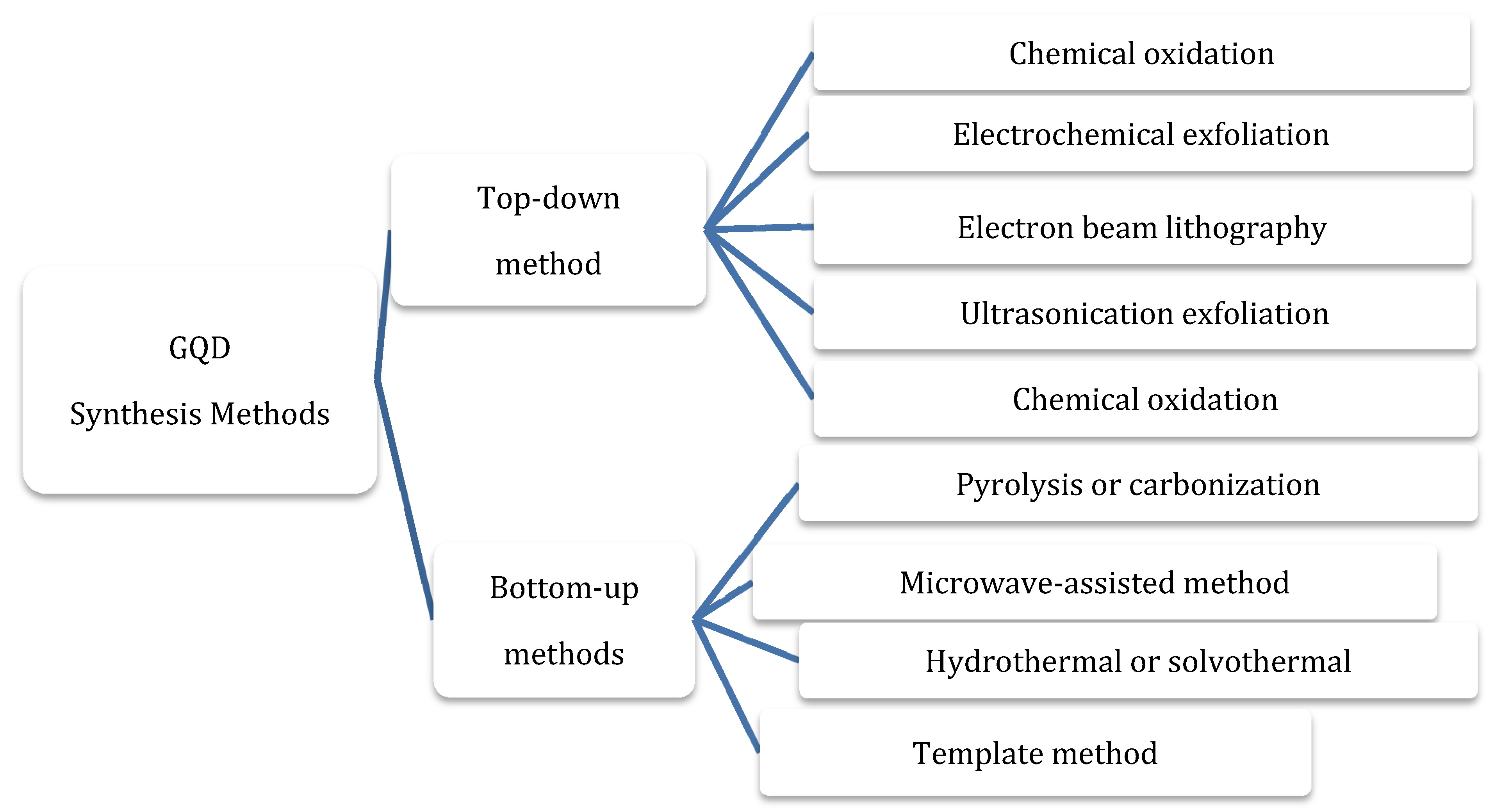
3.1. Synthesis Methods
3.2. Optical Properties of GQDs
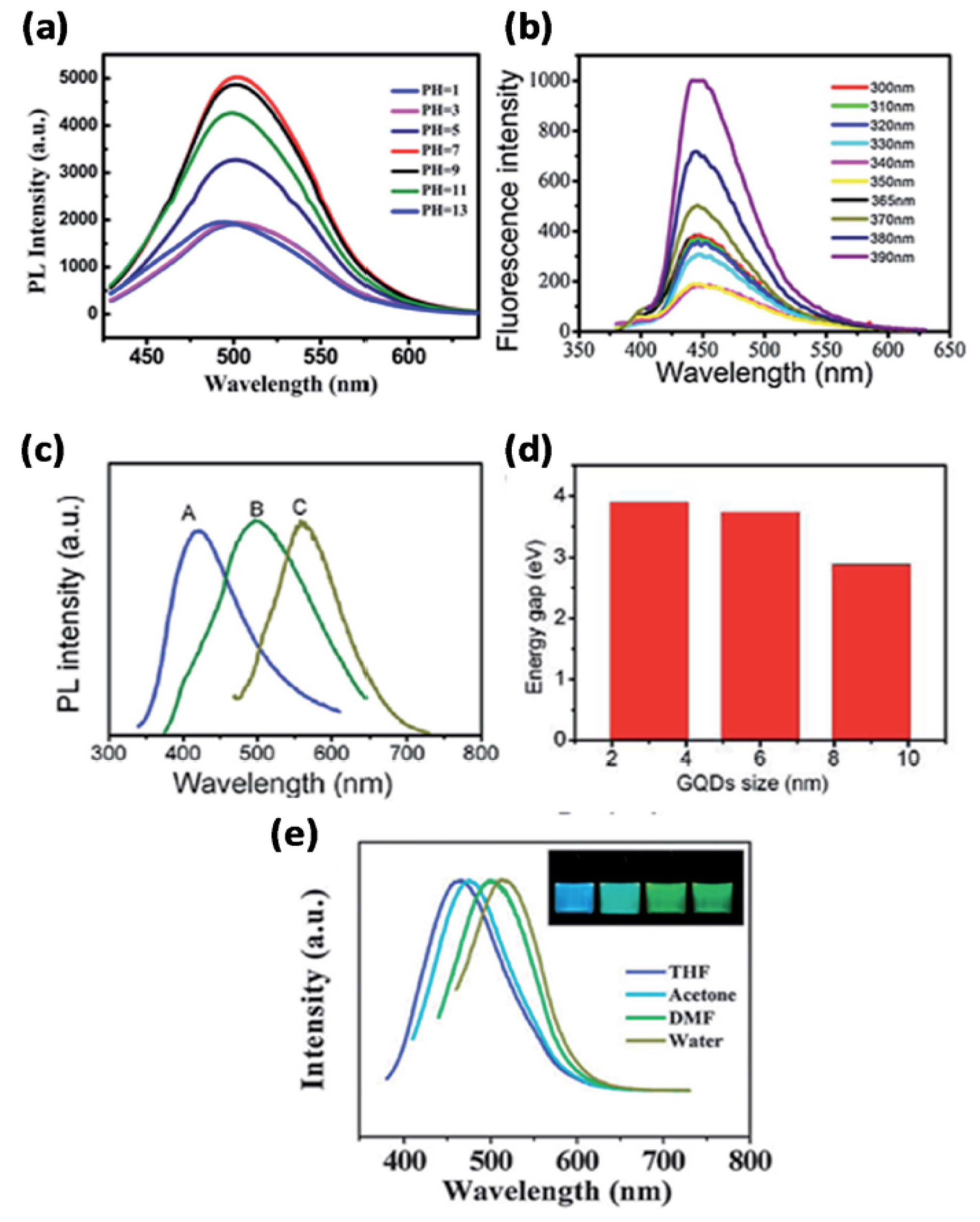
3.2.1. UV–Visible Spectroscopy
3.2.2. PL Spectroscopy
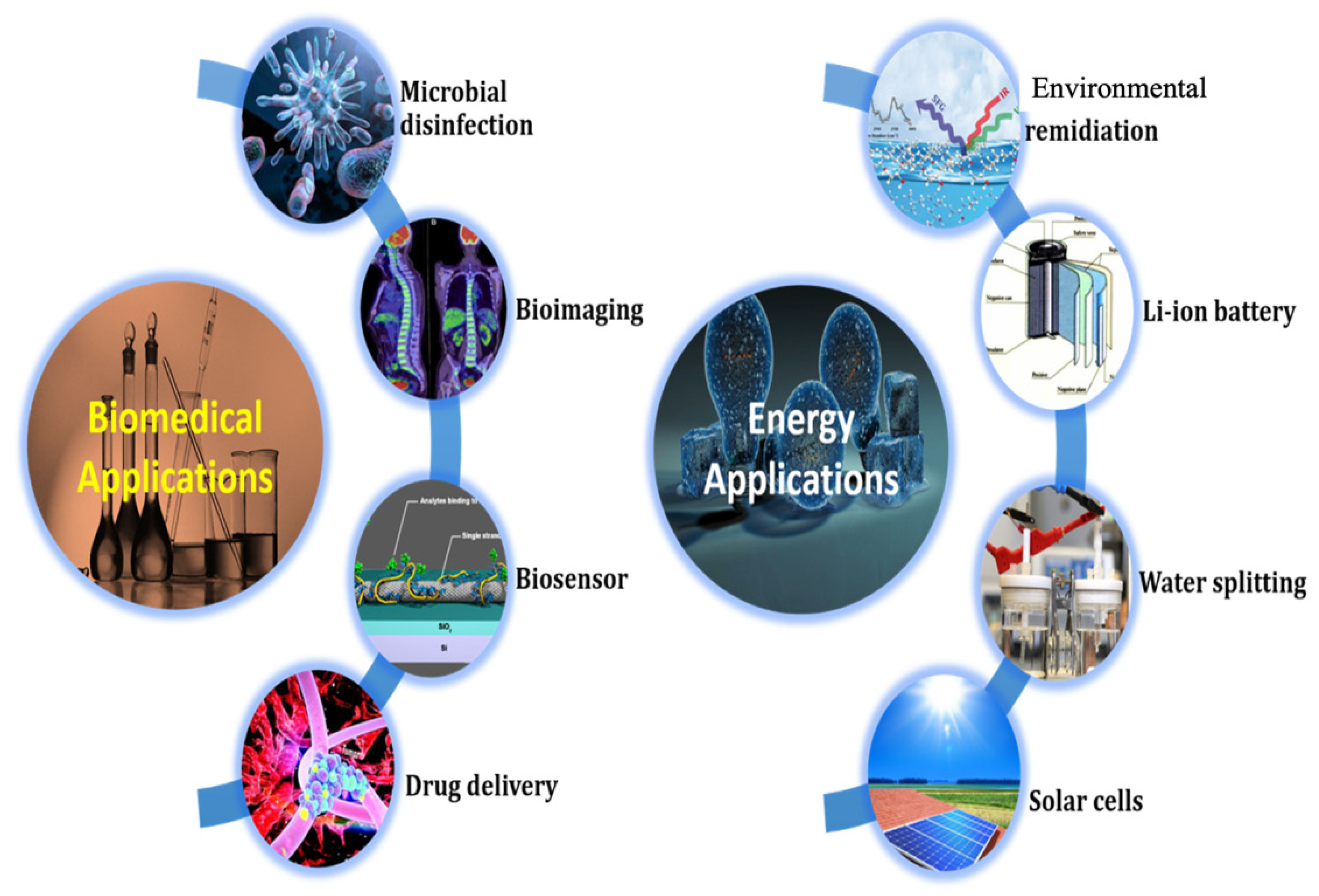
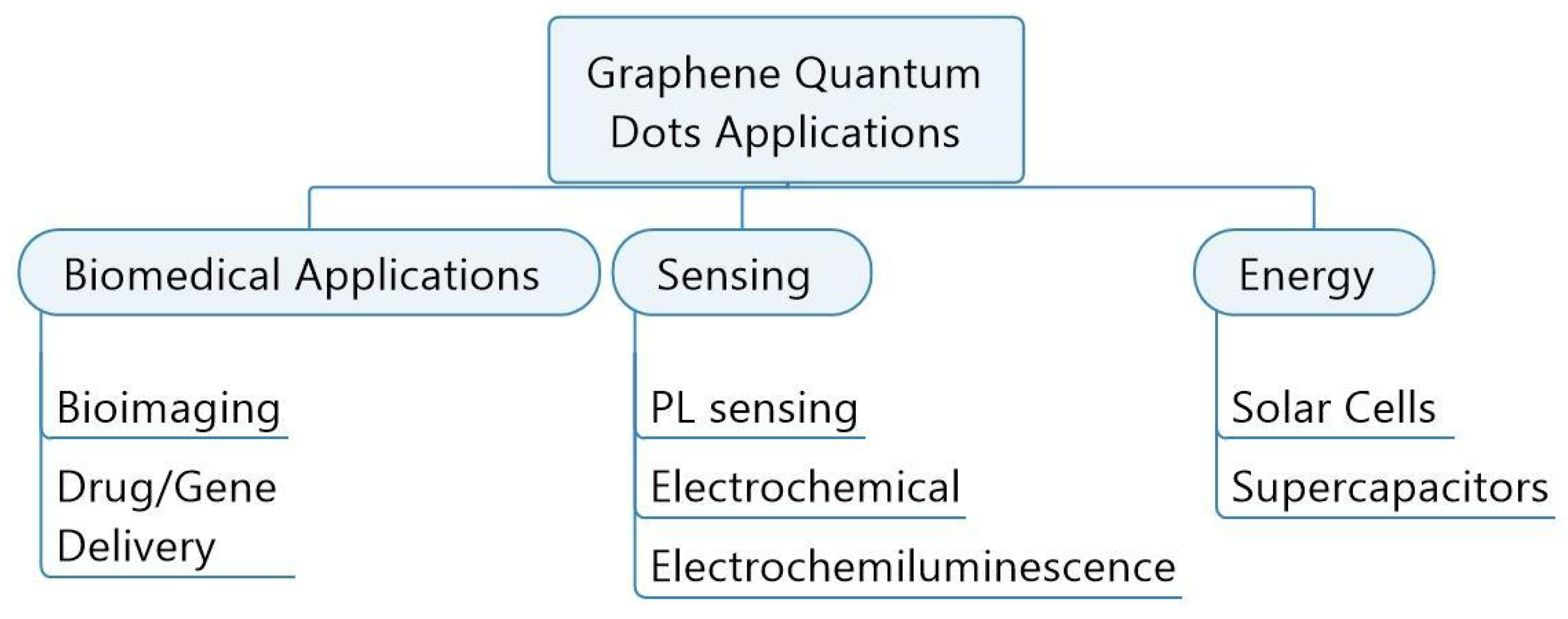
3.3. Applications of GQDs
4. TiO2-GQDs Nanocomposites
5. Synthesis of TiO2-GQD Nanocomposites
5.1. Synthesis Methods for TiO2-GQD-Doped Nanocomposites
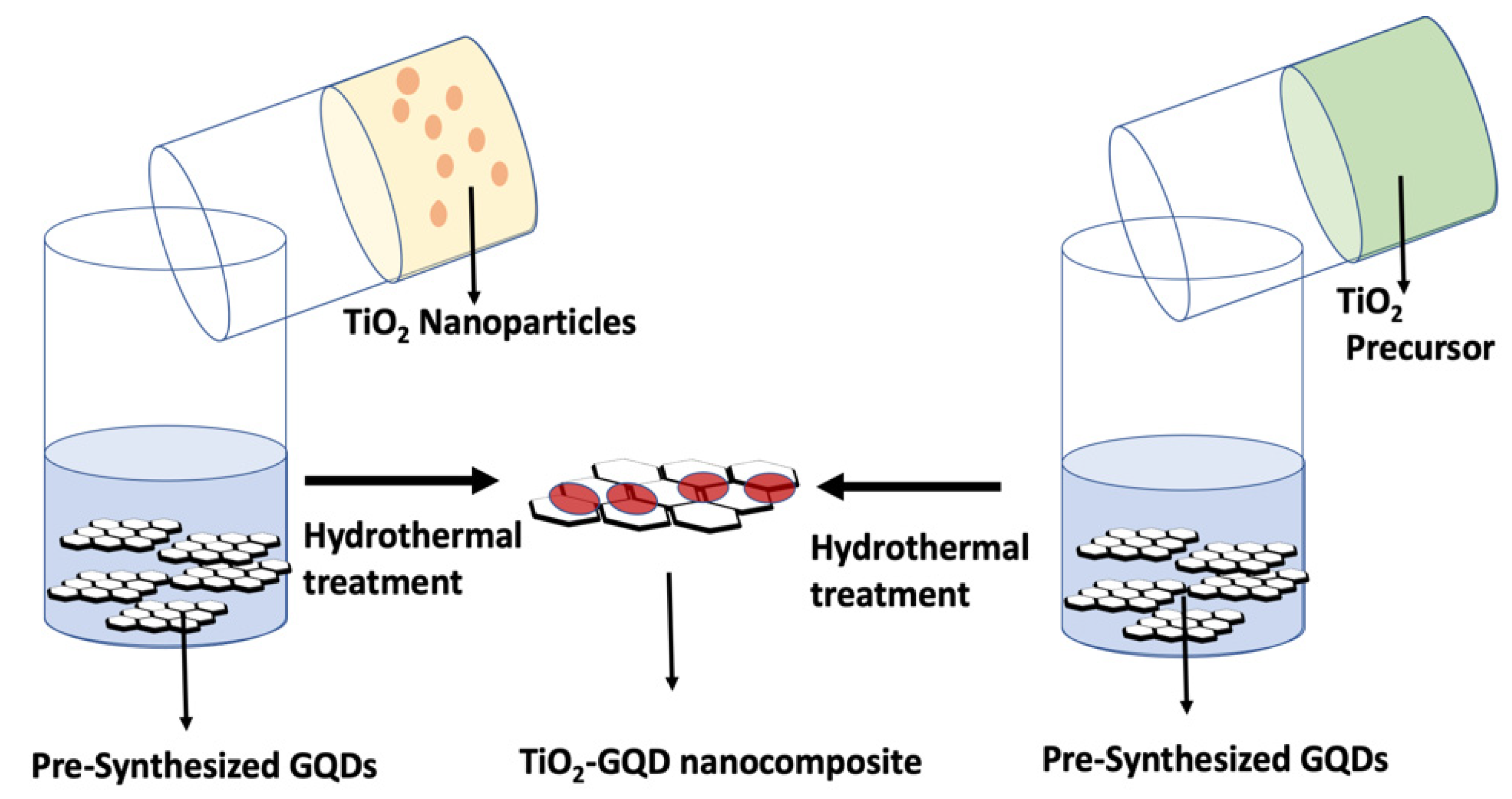
5.1.1. Hydrothermal Method
5.1.2. Ultrasound-Assisted Hydrothermal Methods
5.1.3. Microwave Method
5.1.4. Sonochemical Method
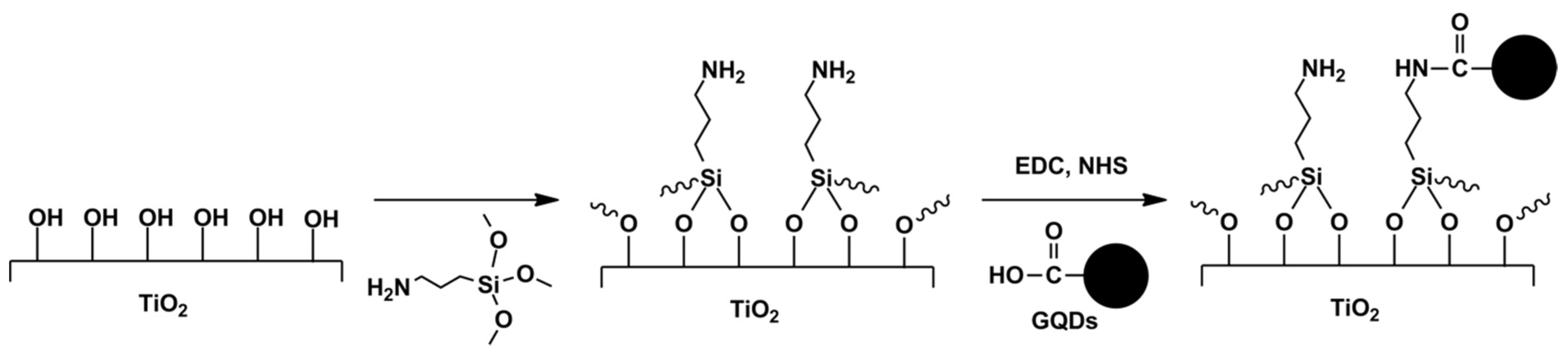
5.1.5. Coupling Reaction
5.1.6. Physical Mixing
5.2. Synthesis Methods for the Deposited Materials
5.2.1. Liquid Impregnation Method
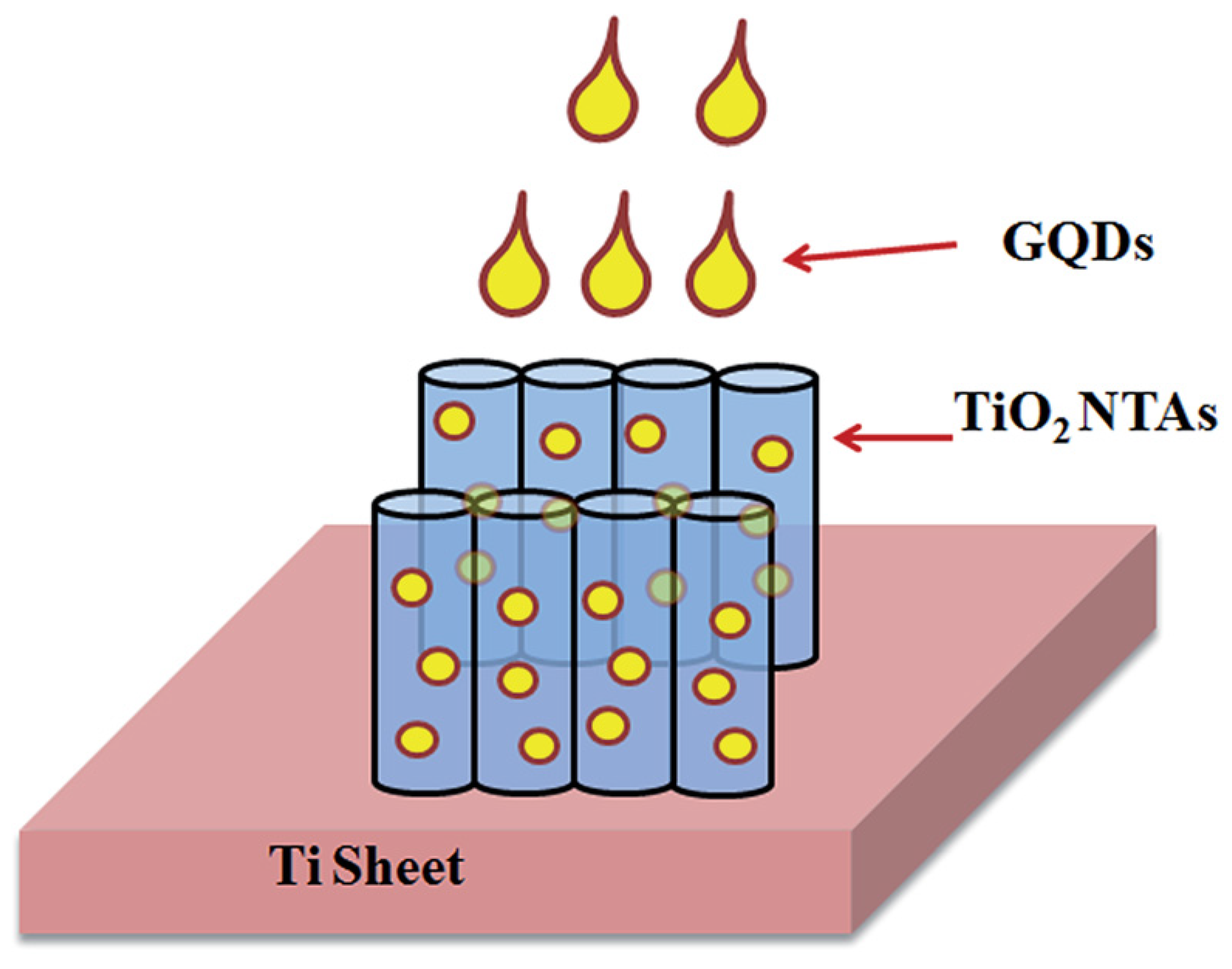
5.2.2. Electrocatalytic Deposition
5.2.3. Photodeposition Method
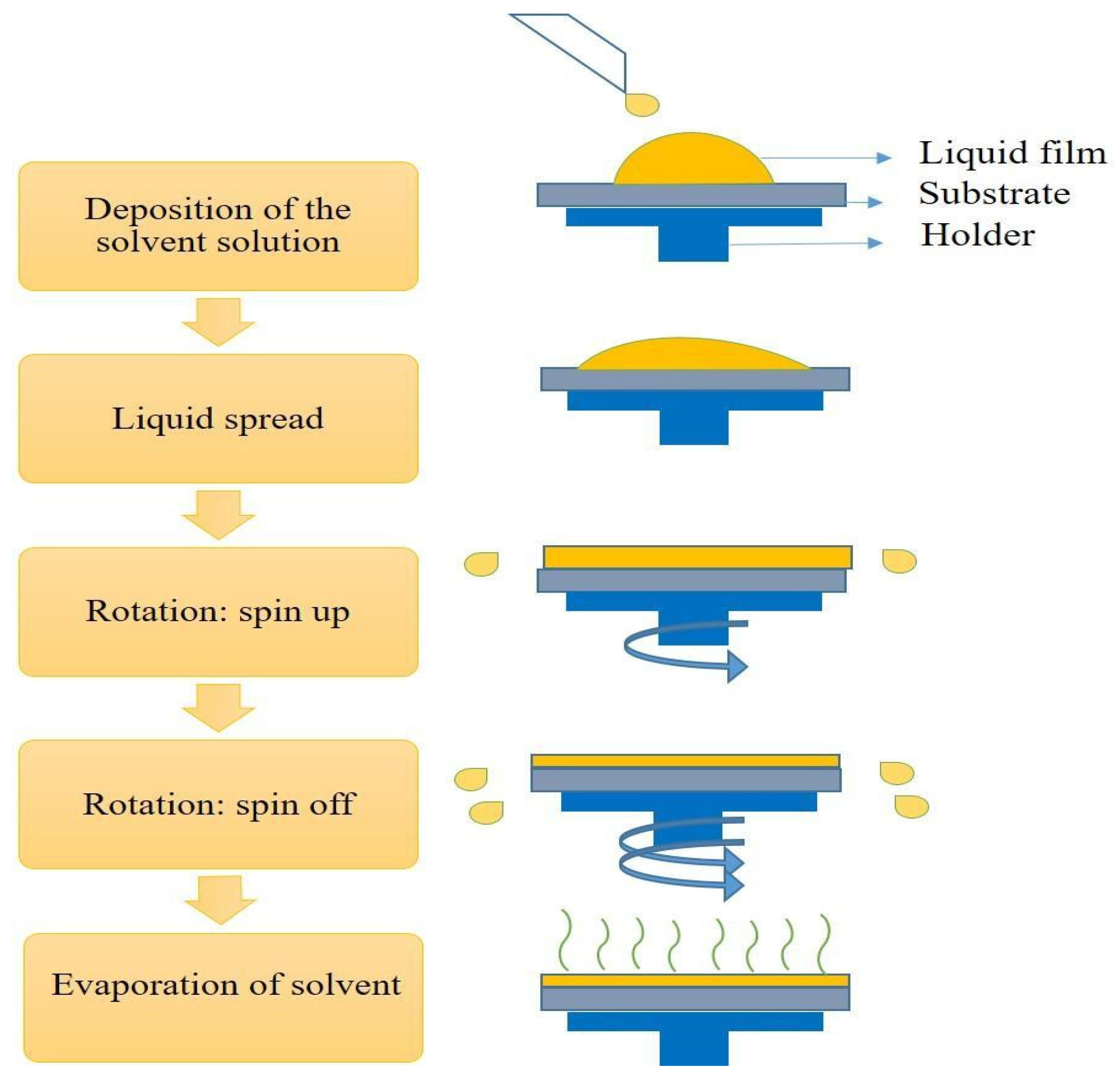
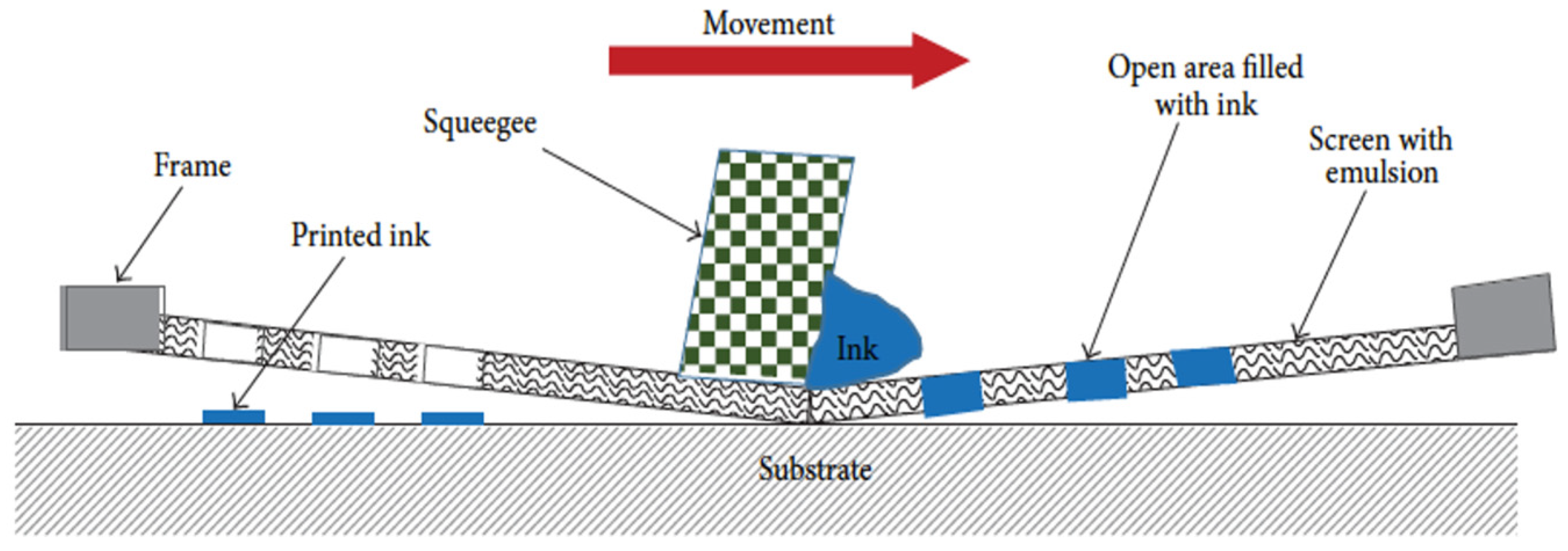
5.2.4. Spin-Coating Method
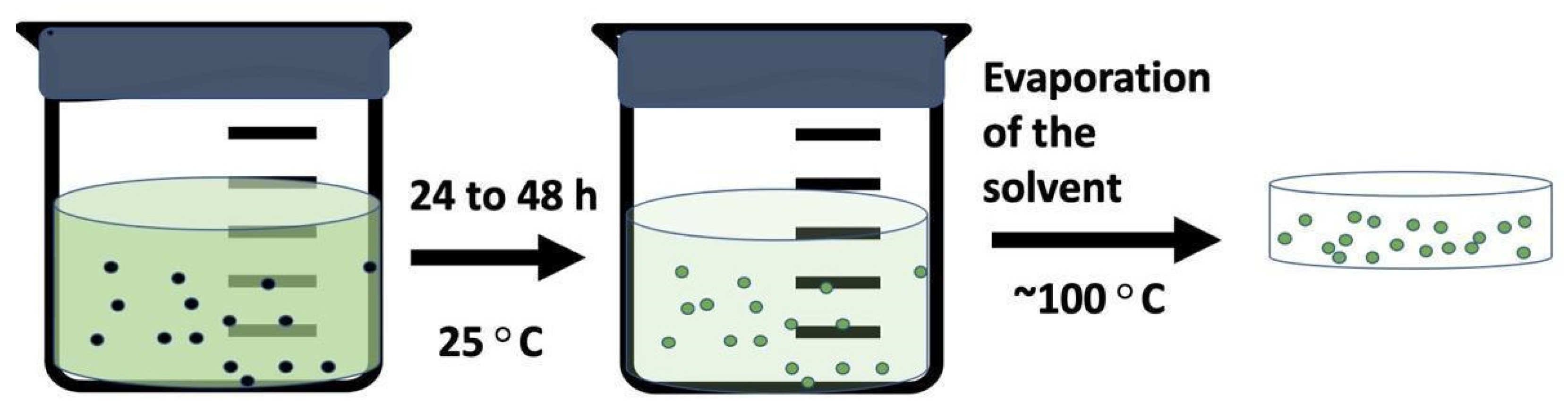
5.2.5. Drop-Cast Method
5.3. Synthesis Methods for TiO2-GQD Photoelectrodes for Solar Cell Applications
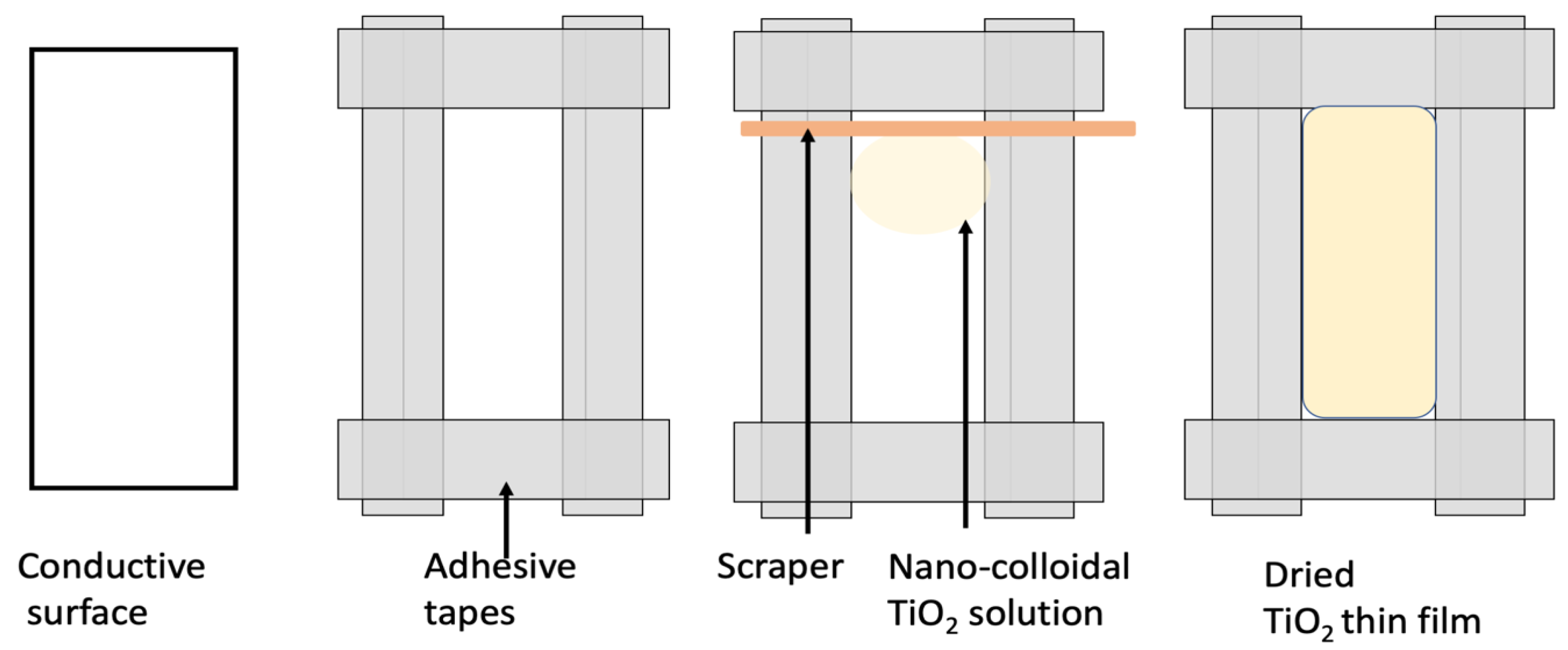
5.3.1. Preparation of Substrates
5.3.2. Deposition of TiO2 Layer
6. Structural Details of TiO2-GQD Nanocomposites
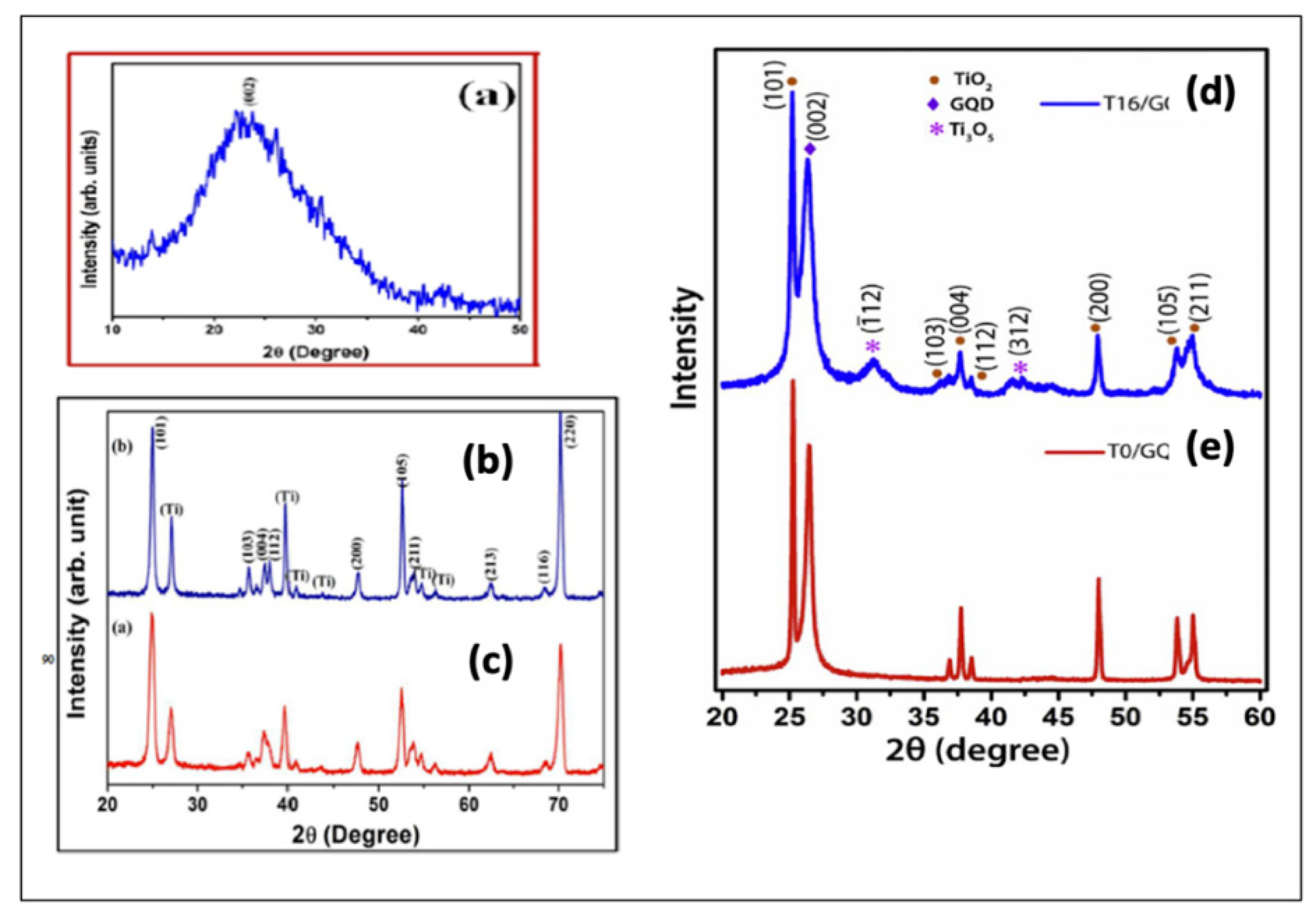
6.1. XRD Pattern
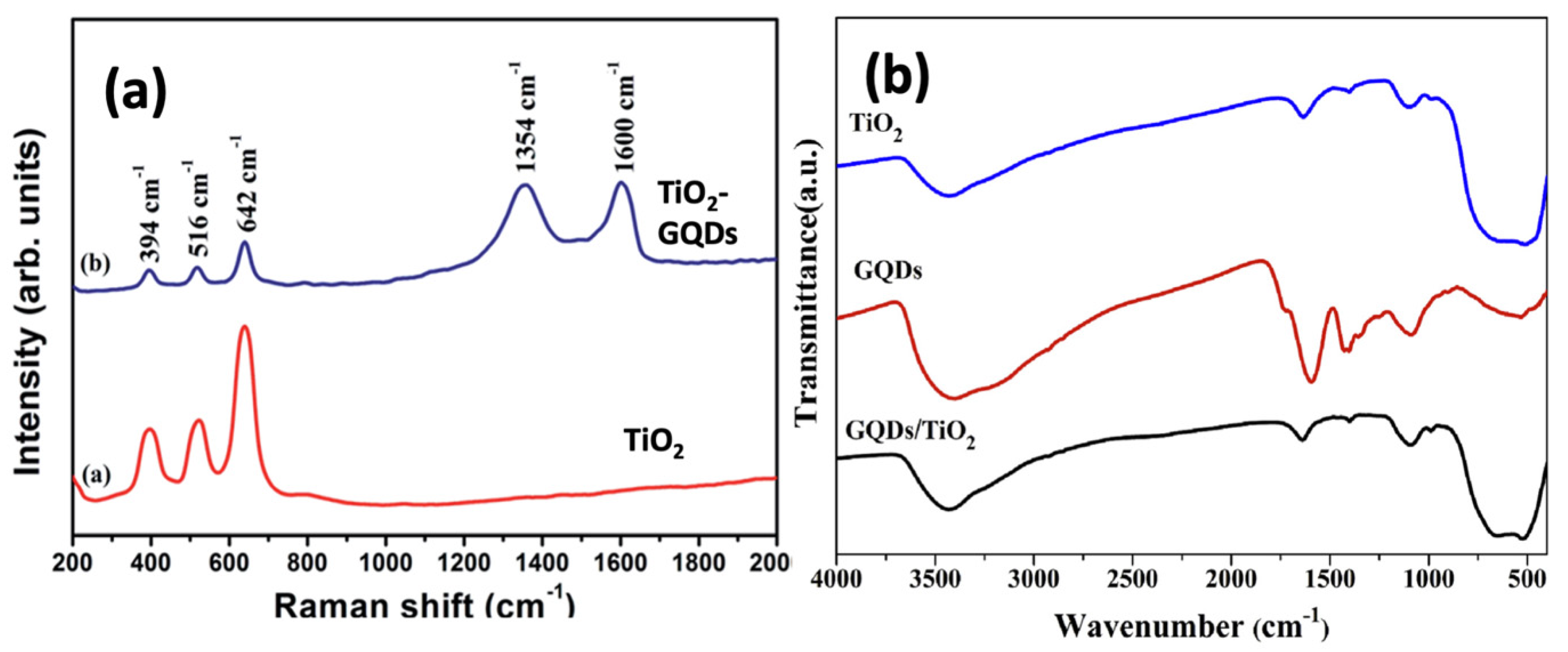
6.2. Raman and FTIR Spectroscopy
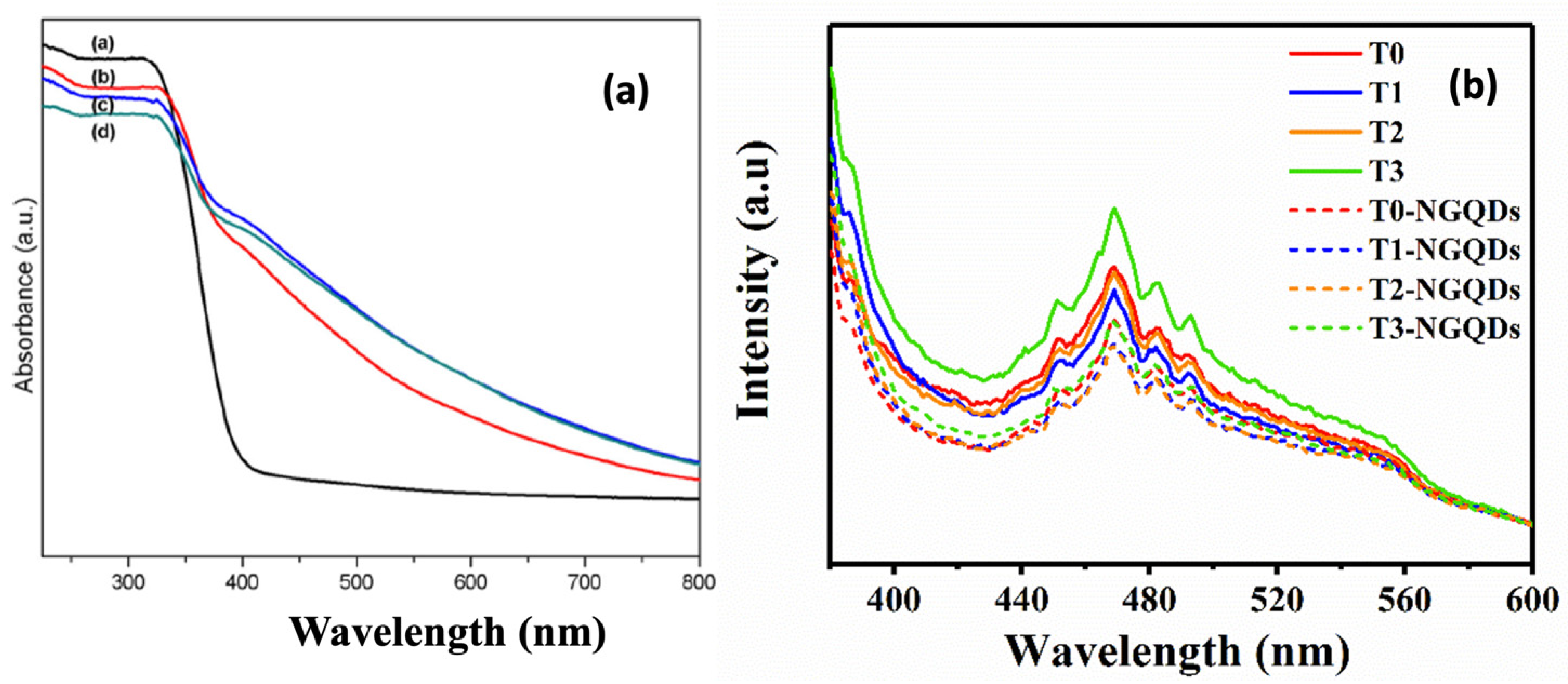
6.3. UV–Vis and Photoluminescence Spectroscopy
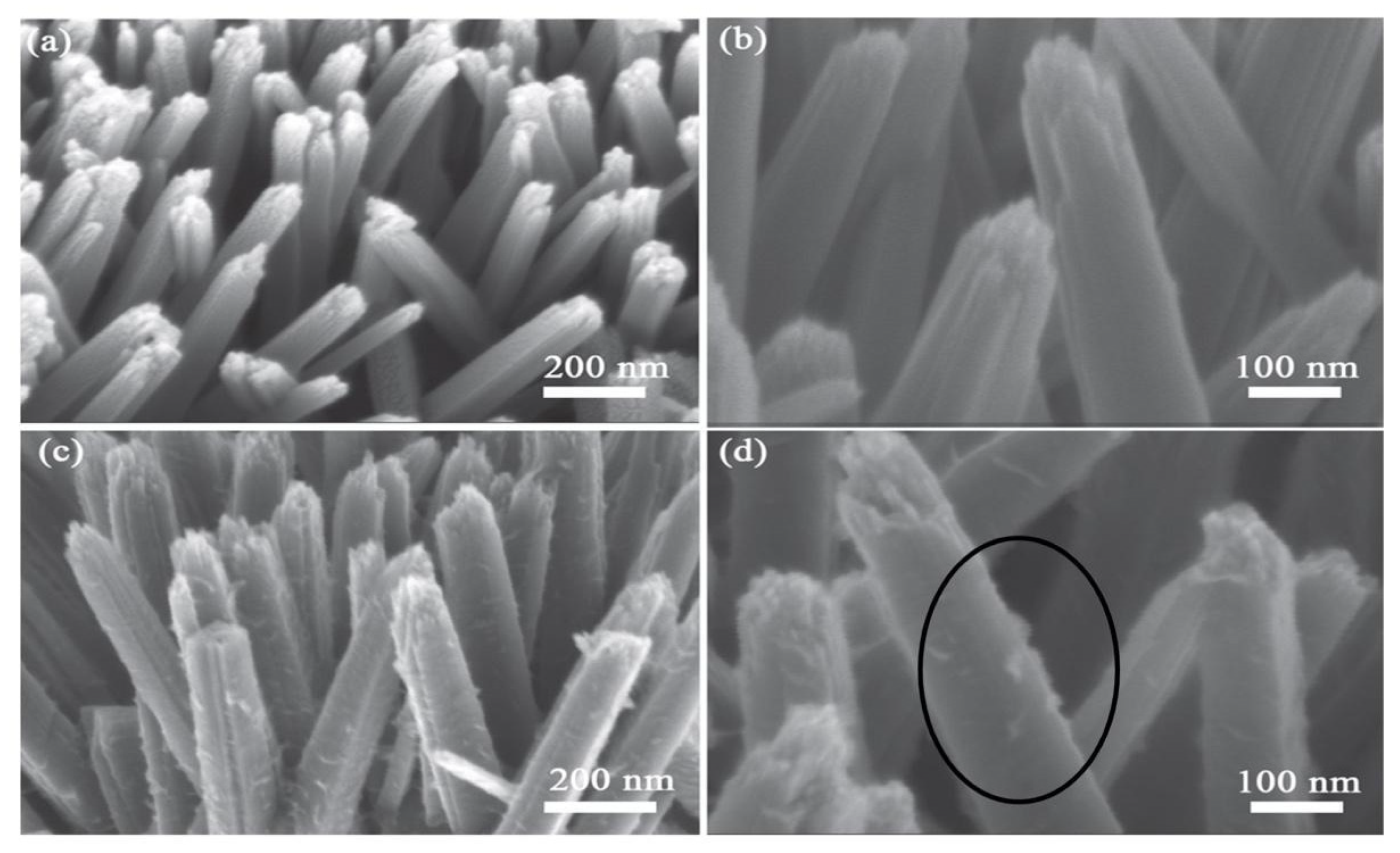
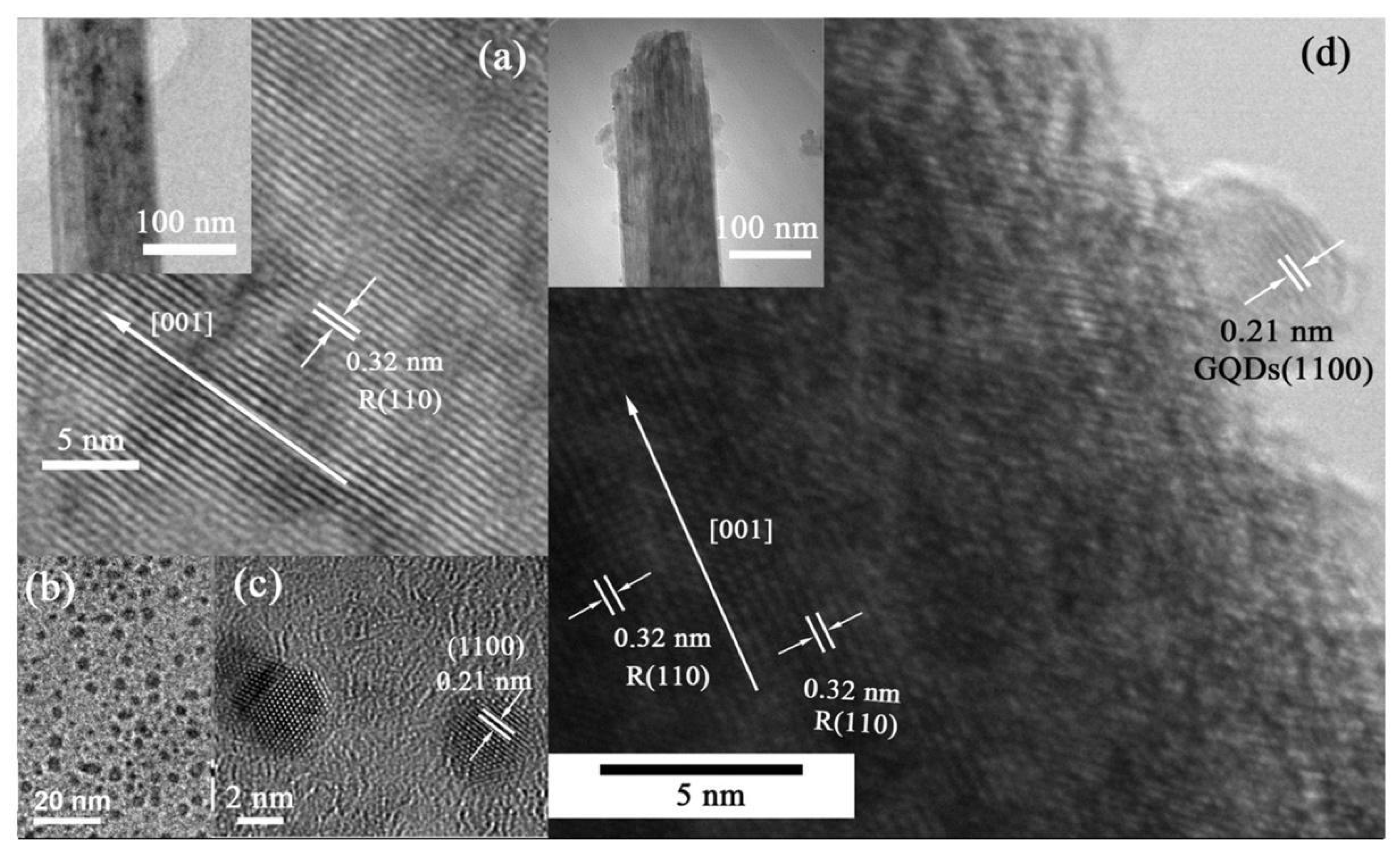
6.4. SEM and TEM Analysis
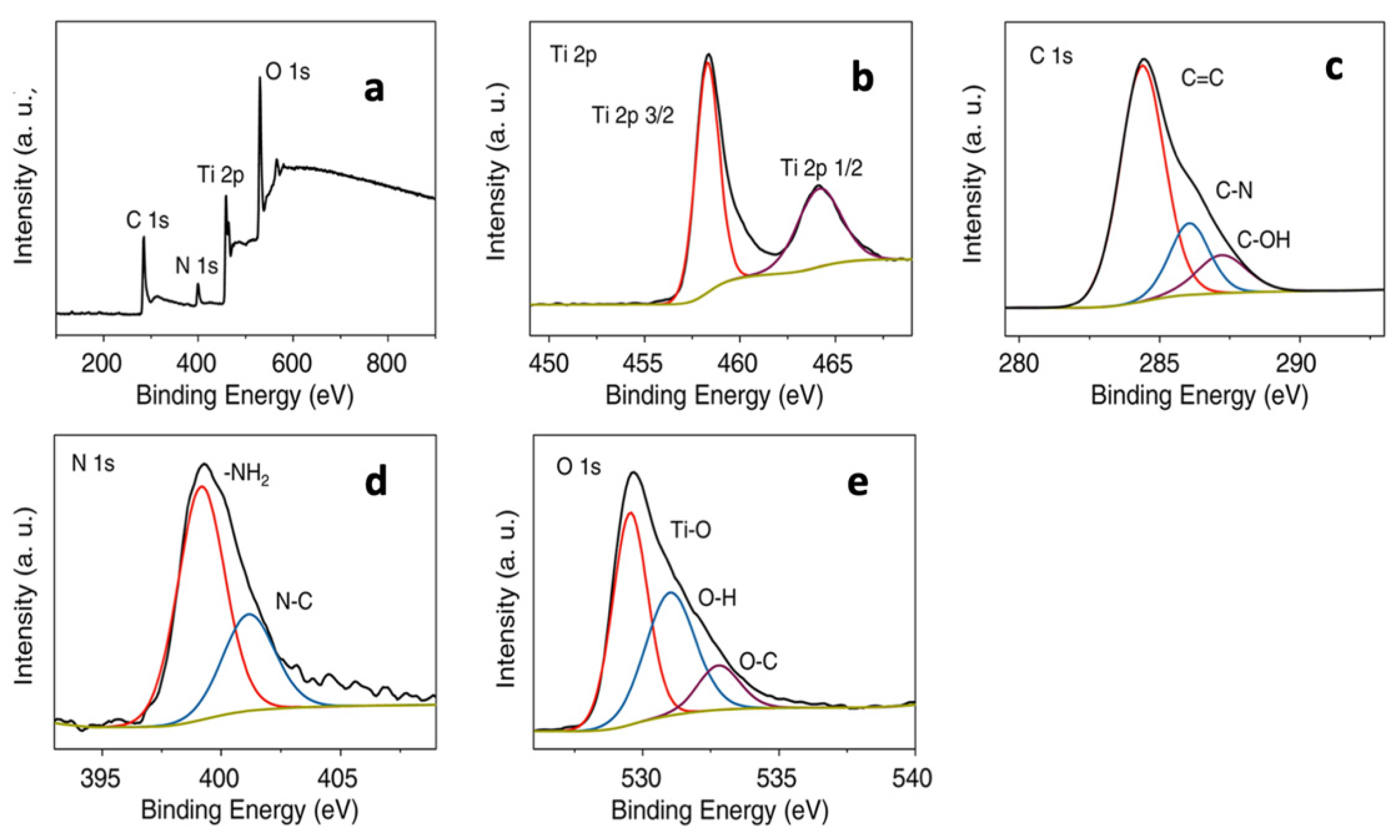
6.5. XPS Analysis
6.6. N2-BET Surface Area Analysis
7. Mechanism of Photocatalysis on TiO2-GQD Nanocomposites
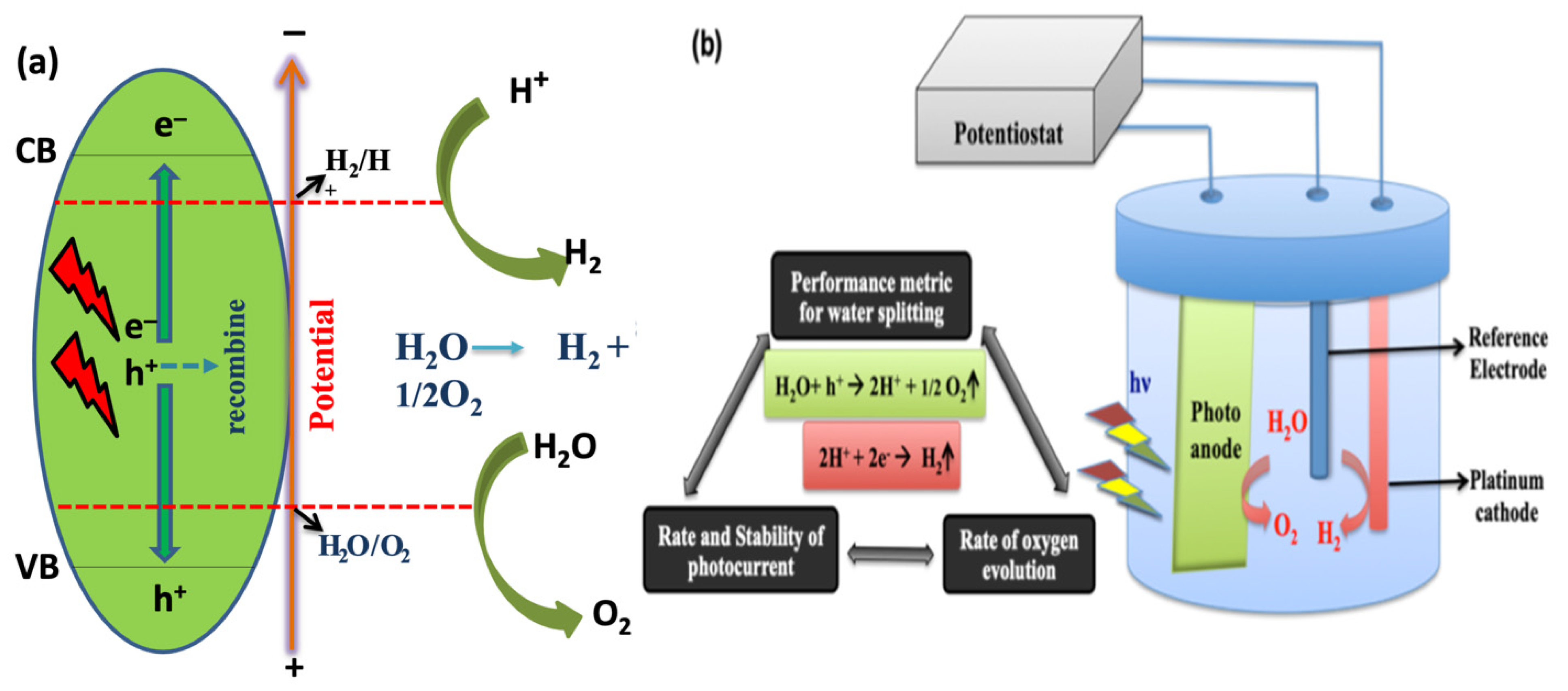
7.1. Mechanism of Photocatalysis on TiO2
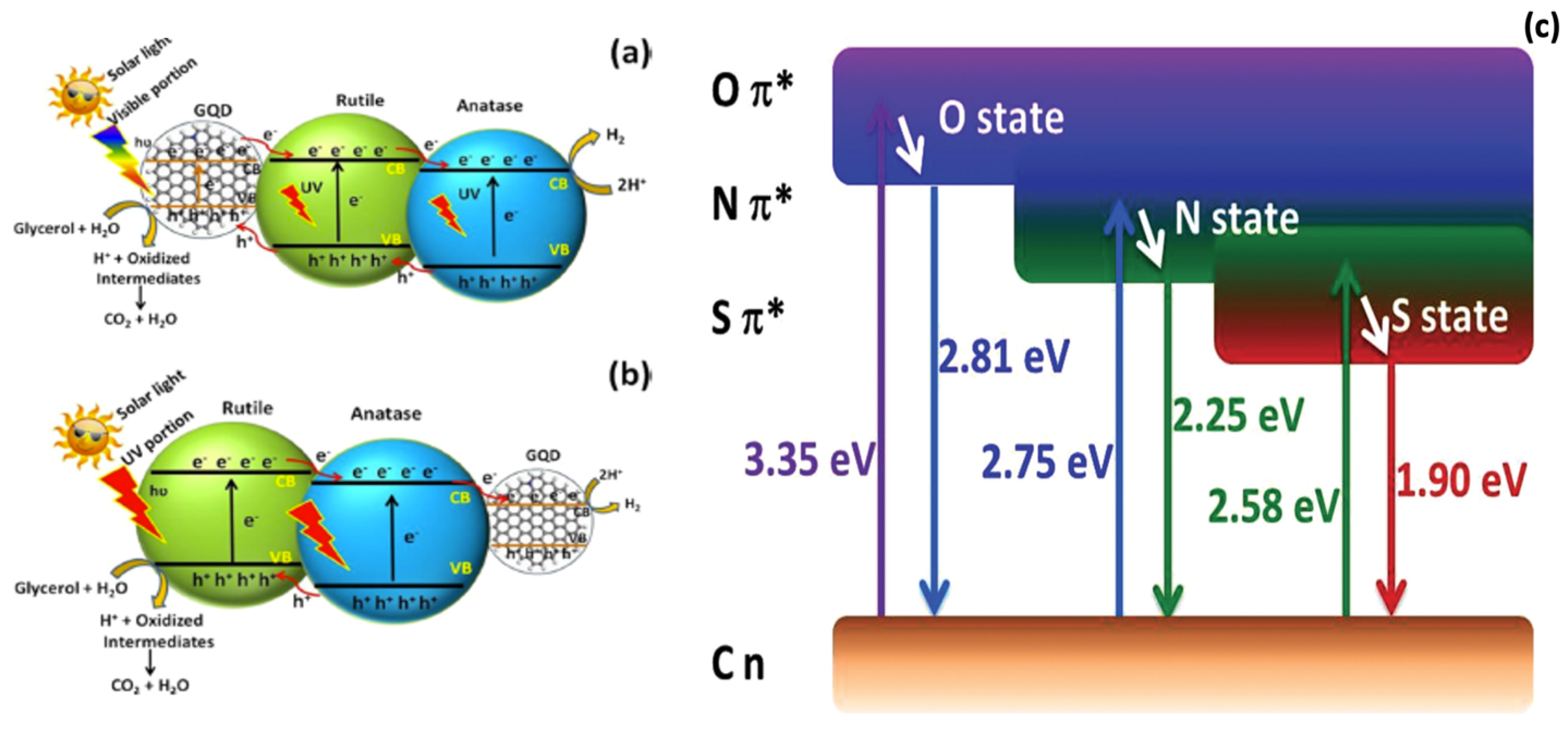
7.2. Mechanism of Photocatalysis on TiO2-GQD Nanocomposites
8. Applications of TiO2-GQD Nanocomposites
8.1. Dye Degradation by TiO2-GQD Photocatalysts
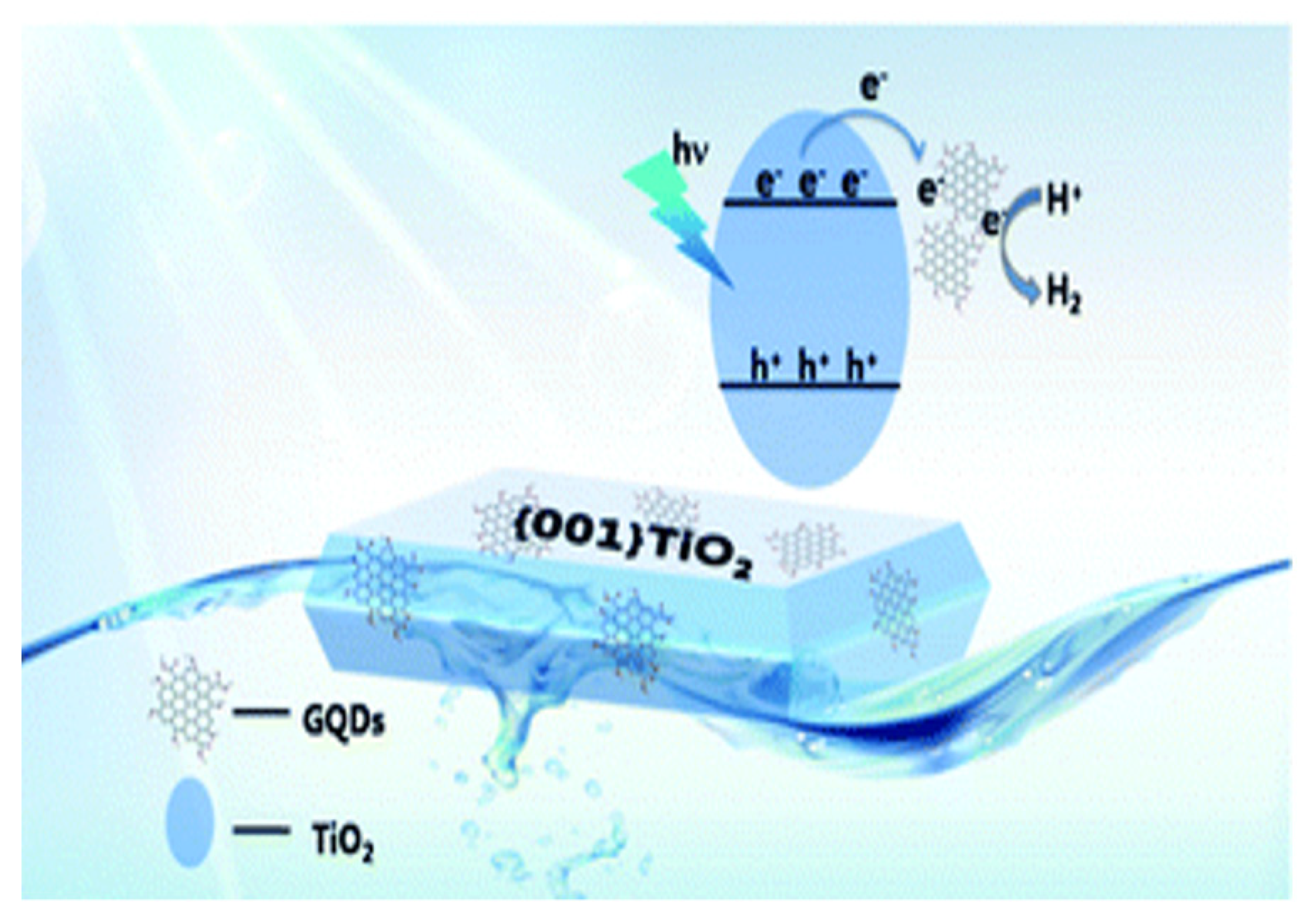
8.2. TiO2-GQDs for Photoelectrochemical Water Splitting
8.3. TiO2-GQDs as a Photoelectrode of Solar Cells
8.3.1. GQDs as a Sensitizer in Solar Cells
8.3.2. GQDs as a Co-Sensitizer in DSSCs
8.3.3. GQD as Electron Transport Layer (ETL) in Perovskite Solar Cell
8.4. TiO2-GQDs as Photoelectrochemical Sensors
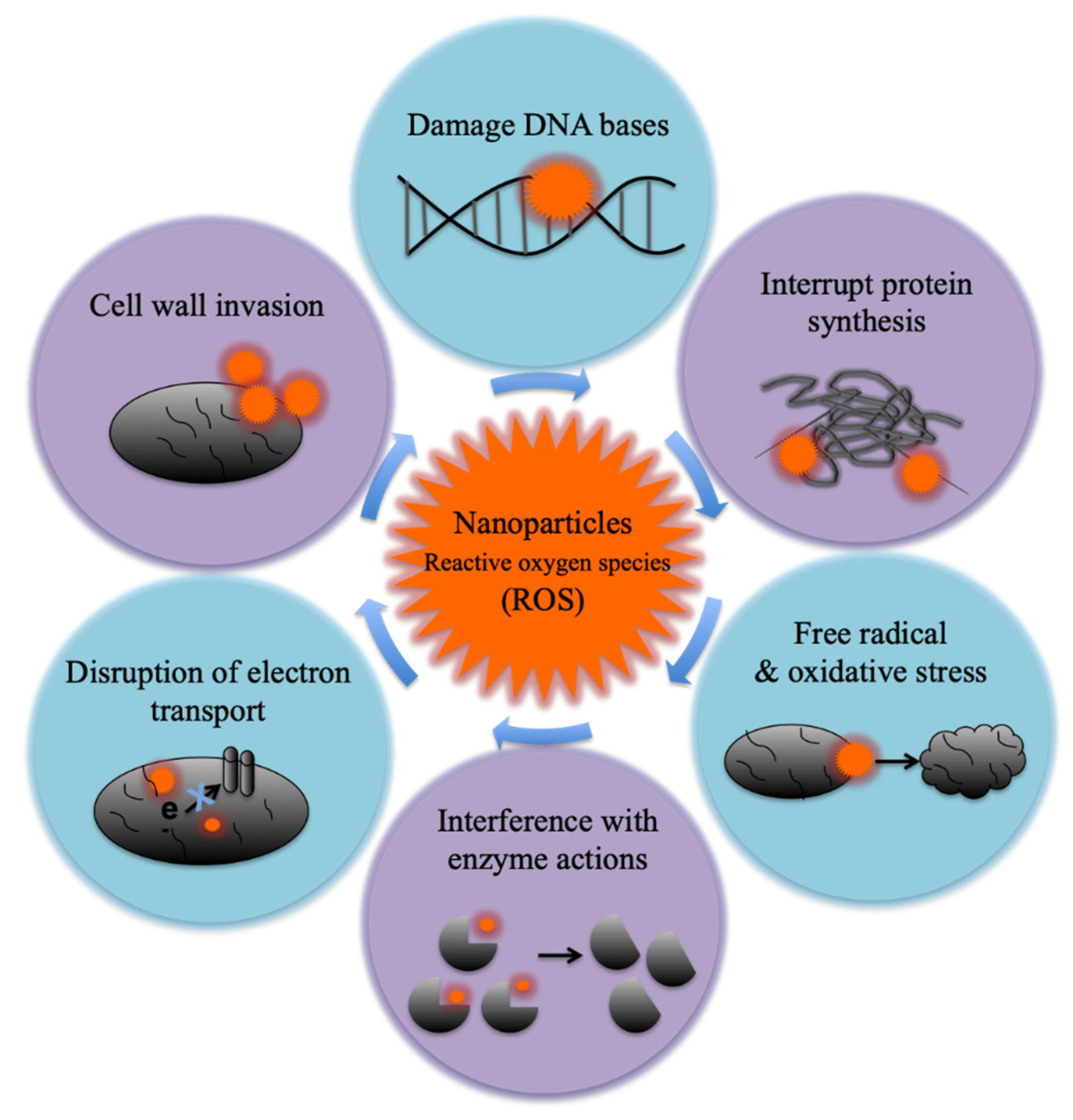
8.5. Antibacterial Activity of TiO2-GQD Nanocomposites
9. Future Scope
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Naseem, S.; Gevers, B.R.; Labuschagné, F.J.W.J.; Leuteritz, A. Preparation of Photoactive Transition-Metal Layered Double Hydroxides (LDH) to Replace Dye-Sensitized Materials in Solar Cells. Materials 2020, 13, 4384. [Google Scholar] [CrossRef]
- Tallman, T.N.; Smyl, D.J. Structural health and condition monitoring via electrical impedance tomography in self-sensing materials: A review. Smart Mater. Struct. 2020, 29, 123001. [Google Scholar] [CrossRef]
- Levi, D.S.; Kusnezov, N.; Carman, G.P. Smart materials applications for pediatric cardiovascular devices. Pediatr. Res. 2008, 63, 552–558. [Google Scholar] [CrossRef]
- Ali, A.; Andriyana, A. Properties of multifunctional composite materials based on nanomaterials: A review. RSC Adv. 2020, 10, 16390–16403. [Google Scholar] [CrossRef]
- Gibson, R.F. A review of recent research on mechanics of multifunctional composite materials and structures. Compos. Struct. 2010, 92, 2793–2810. [Google Scholar] [CrossRef]
- Narayana, K.J.; Gupta Burela, R. A review of recent research on multifunctional composite materials and structures with their applications. Mater. Today Proc. 2018, 5, 5580–5590. [Google Scholar] [CrossRef]
- Wu, X.; Wang, C.; Wei, Y.; Xiong, J. Multifunctional photocatalysts of Pt-decorated 3DOM perovskite-type SrTiO3 with enhanced CO2 adsorption and photoelectron enrichment for selective CO2 reduction with H2O to CH4. J. Catal. 2019, 377, 309–321. [Google Scholar] [CrossRef]
- Zhou, H.; Qu, Y.; Zeid, T.; Duan, X. Towards highly efficient photocatalysts using semiconductor nanoarchitectures. Energy Environ. Sci. 2012, 5, 6732–6743. [Google Scholar] [CrossRef]
- Pakdel, E.; Wang, J.; Kashi, S.; Sun, L.; Wang, X. Advances in photocatalytic self-cleaning, superhydrophobic and electromagnetic interference shielding textile treatments. Adv. Colloid Interface Sci. 2020, 277, 102116. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Shin, K.; Kwon, S.G.; Hyeon, T. Synthesis and Biomedical Applications of Multifunctional Nanoparticles. Adv. Mater. 2018, 30, e1802309. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Whang, D.R.; Park, S.Y. Self-Healing of Molecular Catalyst and Photosensitizer on Metal-Organic Framework: Robust Molecular System for Photocatalytic H2 Evolution from Water. J. Am. Chem. Soc. 2016, 138, 8698–8701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, F.; Wang, W.; Shen, J.; Zhou, N.; Wu, C. Multifunctional Nanocomposites for Targeted, Photothermal, and Chemotherapy. Chem. Mater. 2019, 31, 1847–1859. [Google Scholar] [CrossRef]
- Nam, N.H.; Luong, N.H. Chapter 7—Nanoparticles: Synthesis and applications. In Materials for Biomedical Engineering; Grumezescu, V., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 211–240. [Google Scholar]
- Vijayan, P.P.; Puglia, D. Biomimetic multifunctional materials: A review. Emerg. Mater. 2019, 2, 391–415. [Google Scholar] [CrossRef]
- Lendlein, A.; Trask, R.S. Multifunctional materials: Concepts, function-structure relationships, knowledge-based design, translational materials research. Multifunct. Mater. 2018, 1, 010201. [Google Scholar] [CrossRef]
- Do, H.H.; Nguyen, D.L.T.; Nguyen, N.C.; Le, T.H.; Nguyen, T.P.; Trinh, Q.T.; Ahn, S.H.; Vo, D.V.N.; Kim, S.Y.; Le, Q.V. Recent progress in TiO2-based photocatalysts for hydrogen evolution reaction: A review. Arab. J. Chem. 2020, 13, 3653–3671. [Google Scholar] [CrossRef]
- Noman, M.T.; Ashraf, M.A.; Ali, A. Synthesis and applications of nano-TiO2: A review. Environ. Sci. Pollut. Res. Int. 2019, 26, 3262–3291. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alonso, M.D.; Fresno, F.; Suárez, S.; Coronado, J.M. Development of alternative photocatalysts to TiO2: Challenges and opportunities. Energy Environ. Sci. 2009, 2, 1231–1257. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on modified TiO2 photocatalysis under UV/visible light: Selected results and related mechanisms on interfacial charge carrier transfer dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Skoda, M.; Dudek, I.; Jarosz, A.; Szukiewicz, D. Graphene: One Material, Many Possibilities—Application Difficulties in Biological Systems. J. Nanomater. 2014, 2014, 890246. [Google Scholar] [CrossRef]
- Ravi Kumar, Y.; Deshmukh, K.; Sadasivuni, K.K.; Khadheer Pasha, S.K. Graphene quantum dot based materials for sensing, bio-imaging and energy storage applications: A review. RSC Adv. 2020, 10, 23861–23898. [Google Scholar] [CrossRef]
- Sun, H.; Wu, L.; Wei, W.; Qu, X. Recent advances in graphene quantum dots for sensing. Mater. Today 2013, 16, 433–442. [Google Scholar] [CrossRef]
- Kellici, S.; Acord, J.; Power, N.P.; Morgan, D.J.; Coppo, P.; Heil, T.; Saha, B. Rapid synthesis of graphene quantum dots using a continuous hydrothermal flow synthesis approach. RSC Adv. 2017, 7, 14716–14720. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Z.-G.; Cole, I.; Li, Q. Structural evolution of graphene quantum dots during thermal decomposition of citric acid and the corresponding photoluminescence. Carbon N. Y. 2015, 82, 304–313. [Google Scholar] [CrossRef]
- Wang, D.; Saleh, N.B.; Sun, W.; Park, C.M.; Shen, C.; Aich, N.; Peijnenburg, W.J.G.M.; Zhang, W.; Jin, Y.; Su, C. Next-Generation Multifunctional Carbon-Metal Nanohybrids for Energy and Environmental Applications. Environ. Sci. Technol. 2019, 53, 7265–7287. [Google Scholar] [CrossRef] [PubMed]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, S.; Fan, D.; Reilly, J.; Zhang, H.; Yao, W.; Huang, J. Carbon Quantum Dot/TiO2 Nanohybrids: Efficient Photocatalysts for Hydrogen Generation via Intimate Contact and Efficient Charge Separation. ACS Appl. Nano Mater. 2019, 2, 1027–1032. [Google Scholar] [CrossRef]
- Siwińska-Stefańska, K.; Fluder, M.; Tylus, W.; Jesionowski, T. Investigation of amino-grafted TiO2/reduced graphene oxide hybrids as a novel photocatalyst used for decomposition of selected organic dyes. J. Environ. Manag. 2018, 212, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Michlits, G.; Seery, M.K.; Hinder, S.J.; Pillai, S.C. A highly efficient TiO(2-x)C(x) nano-heterojunction photocatalyst for visible light induced antibacterial applications. ACS Appl. Mater. Interfaces 2013, 5, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Cao, S.; Dong, P.; Gao, J.; Wang, J. High antibacterial activity of ultrafine TiO2/graphene sheets nanocomposites under visible light irradiation. Mater. Lett. 2013, 93, 349–352. [Google Scholar] [CrossRef]
- Zheng, L.; Su, H.; Zhang, J.; Walekar, L.S.; Molamahmood, H.V.; Zhou, B.; Long, M.; Hu, Y.H. Highly selective photocatalytic production of H2O2 on sulfur and nitrogen co-doped graphene quantum dots tuned TiO2. Appl. Catal. B 2018, 239, 475–484. [Google Scholar] [CrossRef]
- Long, R.; Casanova, D.; Fang, W.-H.; Prezhdo, O.V. Donor-Acceptor Interaction Determines the Mechanism of Photoinduced Electron Injection from Graphene Quantum Dots into TiO2: π-Stacking Supersedes Covalent Bonding. J. Am. Chem. Soc. 2017, 139, 2619–2629. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cole, I.S.; Li, Q. Quantum-confined bandgap narrowing of TiO2 nanoparticles by graphene quantum dots for visible-light-driven applications. Chem. Commun. 2016, 52, 9208–9211. [Google Scholar] [CrossRef]
- Huo, P.; Shi, X.; Zhang, W.; Kumar, P.; Liu, B. An overview on the incorporation of graphene quantum dots on TiO 2 for enhanced performances. J. Mater. Sci. 2021, 56, 6031–6051. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, e1901997. [Google Scholar] [CrossRef]
- Kubacka, A.; Diez, M.S.; Rojo, D.; Bargiela, R.; Ciordia, S.; Zapico, I.; Albar, J.P.; Santos, V.A.P.M.D.; Garcia, M.F.; Ferrer, M. Understanding the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2014, 4, 4134. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Suhail, M.; Alothman, Z.A.; Alwarthan, A. Recent advances in syntheses, properties and applications of TiO2 nanostructures. RSC Adv. 2018, 8, 30125–30147. [Google Scholar] [CrossRef]
- Jhang, J.-H.; Chang, S.-J.; Pedaballi, S.; Li, C.-C. A new porous structure with dispersed nano-TiO2 in a three-dimensional carbon skeleton for achieving high photocatalytic activity. Microporous Mesoporous Mater. 2019, 276, 62–67. [Google Scholar] [CrossRef]
- Ge, M.; Cai, J.; Locozzia, J.; Cao, C.; Huang, J.; Zhang, X.; Shen, J.; Wang, S.; Zhang, K.Q.; Lai, Y.Z. A review of TiO2 nanostructured catalysts for sustainable H2 generation. Int. J. Hydrogen Energy 2017, 42, 8418–8449. [Google Scholar] [CrossRef]
- Ghosh, M.; Liu, J.; Chuang, S.S.C.; Jana, S.C. Fabrication of hierarchical V2 O5 nanorods on TiO2 nanofibers and their enhanced photocatalytic activity under visible light. ChemCatChem 2018, 10, 3305–3318. [Google Scholar] [CrossRef]
- Zheng, P.; Zhou, W.; Wang, Y.; Ren, D.; Zhao, J.; Guo, S. N-doped graphene-wrapped TiO2 nanotubes with stable surface Ti3+ for visible-light photocatalysis. Appl. Surf. Sci. 2020, 512, 144549. [Google Scholar] [CrossRef]
- Morales-García, Á.; Macià Escatllar, A.; Illas, F.; Bromley, S.T. Understanding the interplay between size, morphology and energy gap in photoactive TiO2 nanoparticles. Nanoscale 2019, 11, 9032–9041. [Google Scholar] [CrossRef]
- Khan, R.; Javed, S.; Islam, M. Hierarchical Nanostructures of Titanium Dioxide: Synthesis and Applications. In Titanium Dioxide; Yang, D., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Cargnello, M.; Gordon, T.R.; Murray, C.B. Solution-phase synthesis of titanium dioxide nanoparticles and nanocrystals. Chem. Rev. 2014, 114, 9319–9345. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Negrín-Montecelo, Y.; Anta, M.T.; Caba, L.M.; Lorenzo, M.P.; Salgueirino, V.; Durate, M.A.C.; Hermo, M.C. Titanate Nanowires as One-Dimensional Hot Spot Generators for Broadband Au-TiO2 Photocatalysis. Nanomaterials 2019, 9, 990. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xie, Y.; Xiong, D.; Zeng, X.; Li, Z.; Wamg, M.; Cheng, Y.B.; Chen, W.; Yan, K.; Yang, S. TiO2 nanorods: A facile size- and shape-tunable synthesis and effective improvement of charge collection kinetics for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2014, 6, 9698–9704. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-J.; Li, C.-C. TiO2-based microsphere with large pores to improve the electrochemical performance of Li-ion anodes. Ceram. Int. 2021. [Google Scholar] [CrossRef]
- Tang, X.; Chu, W.; Qian, J.; Lin, J.; Cao, G. Photocatalysis: Low temperature synthesis of large-size anatase TiO2 nanosheets with enhanced photocatalytic activities (small 48/2017). Small 2017, 13, 1770255. [Google Scholar] [CrossRef]
- Ghosh, M.; Lohrasbi, M.; Chuang, S.S.C.; Jana, S.C. Mesoporous titanium dioxide nanofibers with a significantly enhanced photocatalytic activity. ChemCatChem 2016, 8, 2525–2535. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 For Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, S.; Sun, M.; Yang, L.; Luo, S.; Crittenden, J.C. A Critical Review on Energy Conversion and Environmental Remediation of Photocatalysts with Remodeling Crystal Lattice, Surface, and Interface. ACS Nano 2019, 13, 9811–9840. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Revia, R.A.; Zhang, M. Graphene Quantum Dots and Their Applications in Bioimaging, Biosensing, and Therapy. Adv. Mater. 2019, e1904362. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Tang, L.; Teng, K.S.; Lau, S.P. Graphene quantum dots from chemistry to applications. Mater. Today Chem. 2018, 10, 221–258. [Google Scholar] [CrossRef]
- Tajik, S.; Dourandish, Z.; Zhang, K.; Beitollahi, H.; Le, Q.V.; Jang, H.W.; Shokouhimehr, M. Carbon and graphene quantum dots: A review on syntheses, characterization, biological and sensing applications for neurotransmitter determination. RSC Adv. 2020, 10, 15406–15429. [Google Scholar] [CrossRef]
- Facure, M.H.M.; Schneider, R.; Mercante, L.A.; Correa, D.S. A review on graphene quantum dots and their nanocomposites: From laboratory synthesis towards agricultural and environmental applications. Environ. Sci. Nano 2020. [Google Scholar] [CrossRef]
- Prabhu, S.A.; Kavithayeni, V.; Suganthy, R.; Geetha, K. Graphene quantum dots synthesis and energy application: A review. Carbon Lett. 2020. [Google Scholar] [CrossRef]
- Belchi, R.; Habert, A.; Foy, E.; Gheno, A.; Vedraine, S.; Antony, R.; Ratier, B.; Boucle, J.; Boime, N.H. One-Step Synthesis of TiO2/Graphene Nanocomposites by Laser Pyrolysis with Well-Controlled Properties and Application in Perovskite Solar Cells. ACS Omega 2019, 4, 11906–11913. [Google Scholar] [CrossRef]
- Ton, N.N.T.; Dao, A.T.N.; Kato, K.; Ikenaga, T.; Trinh, D.X.; Taniike, T. One-pot synthesis of TiO2/graphene nanocomposites for excellent visible light photocatalysis based on chemical exfoliation method. Carbon N. Y. 2018, 133, 109–117. [Google Scholar] [CrossRef]
- Chen, W.; Lv, G.; Hu, W.; Li, D.; Chen, S.; Dai, Z. Synthesis and applications of graphene quantum dots: A review. Nanotechnol. Rev. 2018, 7, 157–185. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of Carbon and Graphene Quantum Dots for Sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef]
- Chhabra, V.A.; Kaur, R.; Kumar, N.; Rajesh, C.; Kim, K.H. Synthesis and spectroscopic studies of functionalized graphene quantum dots with diverse fluorescence characteristics. RSC Adv. 2018, 8, 11446–11454. [Google Scholar] [CrossRef]
- Mansuriya, B.D.; Altintas, Z. Applications of Graphene Quantum Dots in Biomedical Sensors. Sensors 2020, 20, 1072. [Google Scholar] [CrossRef]
- Sclafani, A.; Palmisano, L.; Schiavello, M. Influence of the preparation methods of titanium dioxide on the photocatalytic degradation of phenol in aqueous dispersion. J. Phys. Chem. 1990, 94, 829–832. [Google Scholar] [CrossRef]
- Pathak, P.; Israel, L.H.; Pereira, E.J.M.; Subramanian, V.R. Effects of Carbon Allotrope Interface on the Photoactivity of Rutile One-Dimensional (1D) TiO2 Coated with Anatase TiO2 and Sensitized with CdS Nanocrystals. ACS Appl. Mater. Interfaces 2016, 8, 13400–13409. [Google Scholar] [CrossRef]
- Tayel, A.; Ramadan, A.; El Seoud, O. Titanium Dioxide/Graphene and Titanium Dioxide/Graphene Oxide Nanocomposites: Synthesis, Characterization and Photocatalytic Applications for Water Decontamination. Catalysts 2018, 8, 491. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. A review on TiO2-based nanotubes synthesized via hydrothermal method: Formation mechanism, structure modification, and photocatalytic applications. Catal. Today 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Pan, D.; Jiao, J.; Li, Z.; Guo, Y.; Feng, C.; Liu, Y.; Wang, L.; Wu, M. Efficient Separation of Electron–Hole Pairs in Graphene Quantum Dots by TiO2 Heterojunctions for Dye Degradation. ACS Sustain. Chem. Eng. 2015, 3, 2405–2413. [Google Scholar] [CrossRef]
- Xie, H.; Hou, C.; Wang, H.; Zhang, Q.; Li, Y. S, N Co-Doped Graphene Quantum Dot/TiO2 Composites for Efficient Photocatalytic Hydrogen Generation. Nanoscale Res. Lett. 2017, 12, 400. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Salunke, G.; Khandelwal, P.; Das, R.; Poddar, P. Surface disordered rutile TiO2–graphene quantum dot hybrids: A new multifunctional material with superior photocatalytic and biofilm eradication properties. New J. Chem. 2017, 41, 2642–2657. [Google Scholar] [CrossRef]
- Yan, X.; Cui, X.; Li, B.; Li, L.-S. Large, solution-processable graphene quantum dots as light absorbers for photovoltaics. Nano Lett. 2010, 10, 1869–1873. [Google Scholar] [CrossRef] [PubMed]
- Falk, G.S.; Borlaf, M.; López-Muñoz, M.J.; Fariñas, J.C.; Moreno, R. Microwave-assisted synthesis of TiO2 nanoparticles: Photocatalytic activity of powders and thin films. J. Nanopart. Res. 2018, 20. [Google Scholar] [CrossRef]
- Yang, W.; Xu, W.; Zhang, N.; Lai, X.; Tu, J. TiO2 nanotubes modified with polydopamine and graphene quantum dots as a photochemical biosensor for the ultrasensitive detection of glucose. J. Mater. Sci. 2020, 55. [Google Scholar] [CrossRef]
- Athawale, A.; Bokare, A.; Singh, H.; Nguyen, V.-H.; Sharma, A. Synthesis of Ag2O Coated TiO2 Nanoparticles by Sonochemically Activated Methods for Enhanced Photocatalytic Activities. Top. Catal. 2020, 63, 3. [Google Scholar] [CrossRef]
- Chinnusamy, S.; Kaur, R.; Bokare, A.; Erogbogbo, F. Incorporation of graphene quantum dots to enhance photocatalytic properties of anatase TiO2. MRS Commun. 2018, 8, 137–144. [Google Scholar] [CrossRef]
- Shafaee, M.; Goharshadi, E.K.; Mashreghi, M.; Sadeghinia, M. TiO2 nanoparticles and TiO2@graphene quantum dots nancomposites as effective visible/solar light photocatalysts. J. Photochem. Photobiol. A Chem. 2018, 357, 90–102. [Google Scholar] [CrossRef]
- Chan, D.K.L.; Cheung, P.L.; Yu, J.C. A visible-light-driven composite photocatalyst of TiO2 nanotube arrays and graphene quantum dots. Beilstein J. Nanotechnol. 2014, 5, 689–695. [Google Scholar] [CrossRef]
- Sun, X.; Li, H.J.; Ou, N.; Lyu, B.; Gui, B.; Tian, S.; Qian, D.; Wang, X.; Yang, J. Visible-Light Driven TiO₂ Photocatalyst Coated with Graphene Quantum Dots of Tunable Nitrogen Doping. Molecules 2019, 24, 344. [Google Scholar] [CrossRef]
- Qu, D.; Sun, Z.; Zheng, M.; Li, J.; Zhang, Y.; Zhang, G.; Zhao, H.; Liu, X.; Xie, Z. Three Colors Emission from S,N Co-doped Graphene Quantum Dots for Visible Light H 2 Production and Bioimaging. Adv. Opt. Mater. 2015, 3, 360–367. [Google Scholar] [CrossRef]
- Jiang, S.P. A review of wet impregnation—An alternative method for the fabrication of high performance and nano-structured electrodes of solid oxide fuel cells. Mater. Sci. Eng. A 2006, 418, 199–210. [Google Scholar] [CrossRef]
- Yu, Y.; Ren, J.; Meng, M. Photocatalytic hydrogen evolution on graphene quantum dots anchored TiO2 nanotubes-array. Int. J. Hydrogen Energy 2013, 38, 12266–12272. [Google Scholar] [CrossRef]
- Mohanty, U.S. Electrodeposition: A versatile and inexpensive tool for the synthesis of nanoparticles, nanorods, nanowires, and nanoclusters of metals. J. Appl. Electrochem. 2011, 41, 257–270. [Google Scholar] [CrossRef]
- Xu, Z.; Yin, M.; Sun, J.; Ding, G.; Lu, L.; Chang, P.; Chen, X.; Li, D. 3D periodic multiscale TiO₂ architecture: A platform decorated with graphene quantum dots for enhanced photoelectrochemical water splitting. Nanotechnology 2016, 27, 115401. [Google Scholar] [CrossRef] [PubMed]
- Sudhagar, P.; Cardona, I.H.; Park, H.; Song, T.; Noh, S.H.; Gimenez, S.; Sero, I.M.; Santiago, F.F.; Bisquert, J.; Terashima, C.; et al. Exploring Graphene Quantum Dots/TiO2 interface in photoelectrochemical reactions: Solar to fuel conversion. Electrochim. Acta 2016, 187, 249–255. [Google Scholar] [CrossRef]
- Wenderich, K.; Mul, G. Methods, Mechanism, and Applications of Photodeposition in Photocatalysis: A Review. Chem. Rev. 2016, 116, 14587–14619. [Google Scholar] [CrossRef]
- Hao, X.; Jin, Z.; Xu, J.; Min, S.; Lu, G. Functionalization of TiO2 with graphene quantum dots for efficient photocatalytic hydrogen evolution. Superlattices Microstruct. 2016, 94, 237–244. [Google Scholar] [CrossRef]
- Hosseini, A.; Içli, K.Ç.; Özenbaş, M.; Erçelebi, Ç. Fabrication and Characterization of Spin-coated TiO2 Films. Energy Procedia 2014, 60, 191–198. [Google Scholar] [CrossRef]
- Bayat, A.; Saievar-Iranizad, E. Graphene quantum dots decorated rutile TiO2 nanoflowers for water splitting application. J. Mater. Chem. A Mater. Energy Sustain. 2018, 27, 306–310. [Google Scholar] [CrossRef]
- Eslamian, M.; Zabihi, F. Ultrasonic Substrate Vibration-Assisted Drop Casting (SVADC) for the Fabrication of Photovoltaic Solar Cell Arrays and Thin-Film Devices. Nanoscale Res. Lett. 2015, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.K.; Kedawat, G.; Agrawal, Y.; Dwivedi, J.; Dhawan, S.K. A novel strategy to enhance ultraviolet light driven photocatalysis from graphene quantum dots infilled TiO2 nanotube arrays. RSC Adv. 2015, 5, 10623–10631. [Google Scholar] [CrossRef]
- Kamarulzaman, N.H.; Salleh, H.; Dagang, A.N.; Ghazali, M.S.M.; Ahmad, Z. Optimization of Titanium Dioxide Layer Fabrication Using Doctor Blade Method in Improving Efficiency of Hybrid Solar Cells. J. Phys. Conf. Ser. 2020, 1535, 012025. [Google Scholar] [CrossRef]
- Mustafa, M.N.; Sulaiman, Y. Optimization of titanium dioxide decorated by graphene quantum dot as a light scatterer for enhanced dye-sensitized solar cell performance. J. Electroanal. Chem. 2020, 876, 114516. [Google Scholar] [CrossRef]
- Salam, Z.; Vijayakumar, E.; Subramania, A.; Sivasankar, N.; Mallick, S. Graphene quantum dots decorated electrospun TiO2 nanofibers as an effective photoanode for dye sensitized solar cells. Sol. Energy Mater. Sol. Cells 2015, 143, 250–259. [Google Scholar] [CrossRef]
- Subramanian, A.; Pan, Z.; Rong, G.; Li, H.; Zhou, L.; Li, W.; Qiu, Y.; Xu, Y.; Hou, Y.; Zheng, Z.; et al. Graphene quantum dot antennas for high efficiency Förster resonance energy transfer based dye-sensitized solar cells. J. Power Sources 2017, 343, 39–46. [Google Scholar] [CrossRef]
- Kundu, S.; Sarojinijeeva, P.; Karthick, R.; Anantharaj, G.; Saritha, G.; Bera, R.; Anandan, S.; Patra, A.; Ragupathy, P.; Selvaraj, M.; et al. Enhancing the Efficiency of DSSCs by the Modification of TiO2 Photoanodes using N, F and S, co-doped Graphene Quantum Dots. Electrochim. Acta 2017, 242, 337–343. [Google Scholar] [CrossRef]
- Kumar, D.K.; Sarojinijeeva, P.; Karthick, R.; Ananthraj, G.; Saritha, G.; Bera, R.; Patra, A.; Ragupathy, P.; Selvaraj, M.; Jeyakumar, D.; et al. Low-temperature titania-graphene quantum dots paste for flexible dye-sensitised solar cell applications. Electrochim. Acta 2019, 305, 278–284. [Google Scholar] [CrossRef]
- Shen, D.; Zhang, W.; Xie, F.; Li, Y.; Abate, A.; Wei, M. Graphene quantum dots decorated TiO2 mesoporous film as an efficient electron transport layer for high-performance perovskite solar cells. J. Power Sources 2018, 402, 320–326. [Google Scholar] [CrossRef]
- Lee, E.; Ryu, J.; Jang, J. Fabrication of graphene quantum dots via size-selective precipitation and their application in upconversion-based DSSCs. Chem. Commun. 2013, 49, 9995–9997. [Google Scholar] [CrossRef]
- Sharif, N.F.M.; Kadir, M.Z.A.A.; Shafie, S.; Rashi, S.A.; Hasan, W.Z.W.; Shaban, S. Charge transport and electron recombination suppression in dye-sensitized solar cells using graphene quantum dots. Results Phys. 2019, 13, 102171. [Google Scholar] [CrossRef]
- Jahantigh, F.; Ghorashi, S.M.B.; Mozaffari, S. Orange photoluminescent N-doped graphene quantum dots as an effective co-sensitizer for dye-sensitized solar cells. J. Solid State Electrochem. 2020, 24, 883–889. [Google Scholar] [CrossRef]
- Khorshidi, E.; Rezaei, B.; Irannejad, N.; Adhami, S.; Ebrahimi, M.; Kermanpur, A.; Ensafi, A.A. The role of GQDs additive in TiO2 nanorods as an electron transfer layer on performance improvement of the perovskite solar cells. Electrochim. Acta 2020, 337, 135822. [Google Scholar] [CrossRef]
- Holder, C.F.; Schaak, R.E. Tutorial on Powder X-ray Diffraction for Characterizing Nanoscale Materials. ACS Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef] [PubMed]
- Santolalla, C.; Chavez-Esquivel, G.; de Reyes-Heredia, J.A.L.; Alvarez-Ramirez, J. Fractal Correlation Analysis of X-ray Diffraction Patterns with Broad Background. Ind. Eng. Chem. Res. 2013, 52, 8346–8353. [Google Scholar] [CrossRef]
- Rajender, G.; Kumar, J.; Giri, P.K. Interfacial charge transfer in oxygen deficient TiO2-graphene quantum dot hybrid and its influence on the enhanced visible light photocatalysis. Appl. Catal. B 2018, 224, 960–972. [Google Scholar] [CrossRef]
- Lim, P.F.; Leong, K.H.; Sim, L.C.; Abd Aziz, A.; Saravanan, P. Amalgamation of N-graphene quantum dots with nanocubic like TiO2: An insight study of sunlight sensitive photocatalysis. Environ. Sci. Pollut. Res. Int. 2019, 26, 3455–3464. [Google Scholar] [CrossRef] [PubMed]
- Ou, N.-Q.; Li, H.-J.; Lyu, B.-W.; Gui, B.-J.; Sun, X.; Qian, D.-J.; Jia, Y.; Wang, X.; Yang, J. Facet-Dependent Interfacial Charge Transfer in TiO2/Nitrogen-Doped Graphene Quantum Dots Heterojunctions for Visible-Light Driven Photocatalysis. Catalysts 2019, 9, 345. [Google Scholar] [CrossRef]
- Zhang, H.; Mo, Z.; Pei, H.; Jia, Q.; Liu, N. A synthesis of graphene quantum dots/hollow TiO2 nanosphere composites for enhancing visible light photocatalytic activity. J. Mater. Sci. Mater. Electron. 2020, 31, 1430–1441. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Giovannetti, R.; Rommozzi, E.; Zannotti, M.; D’Amato, C.A. Recent Advances in Graphene Based TiO2 Nanocomposites (GTiO2Ns) for Photocatalytic Degradation of Synthetic Dyes. Catalysts 2017, 7, 305. [Google Scholar] [CrossRef]
- Pawar, M.; Topcu Sendoğdular, S.; Gouma, P. A Brief Overview of TiO2 Photocatalyst for Organic Dye Remediation: Case Study of Reaction Mechanisms Involved in Ce-TiO2 Photocatalysts System. J. Nanomater. 2018, 2018, 5953609. [Google Scholar] [CrossRef]
- Reza, K.M.; Kurny, A.S.W.; Gulshan, F. Parameters affecting the photocatalytic degradation of dyes using TiO2: A review. Appl. Water Sci. 2017, 7, 1569–1578. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Bokare, A.; Pai, M.; Athawale, A.A. Surface modified Nd doped TiO2 nanoparticles as photocatalysts in UV and solar light irradiation. Solar Energy 2013, 91, 111–119. [Google Scholar] [CrossRef]
- Roushani, M.; Mavaei, M.; Rajabi, H.R. Graphene quantum dots as novel and green nano-materials for the visible-light-driven photocatalytic degradation of cationic dye. Mol. Catal. 2015, 409, 102–109. [Google Scholar] [CrossRef]
- Zhuo, S.; Shao, M.; Lee, S.-T. Upconversion and downconversion fluorescent graphene quantum dots: Ultrasonic preparation and photocatalysis. ACS Nano 2012, 6, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Kamarudin, S.K.; Minggu, L.J.; Kassim, M. Hydrogen from photo-catalytic water splitting process: A review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Peraldo Bicelli, L. Hydrogen: A clean energy source. Int. J. Hydrogen Energy 1986, 11, 555–562. [Google Scholar] [CrossRef]
- Brandon, N.P.; Kurban, Z. Clean energy and the hydrogen economy. Philos. Trans. A Math. Phys. Eng. Sci. 2017, 375, 20160400. [Google Scholar] [CrossRef]
- Ros, C.; Andreu, T.; Morante, J.R. Photoelectrochemical water splitting: A road from stable metal oxides to protected thin film solar cells. J. Mater. Chem. A Mater. Energy Sustain. 2020, 8, 10625–10669. [Google Scholar] [CrossRef]
- Landman, A.; Halabi, R.; Dias, P.; Dotan, H.; Mehlmann, A.; Grader, G.S.; Rothschild, A. Decoupled Photoelectrochemical Water Splitting System for Centralized Hydrogen Production. Joule 2020, 4, 448–471. [Google Scholar] [CrossRef]
- Lim, Y.; Lee, D.-K.; Kim, S.M.; Park, W.; Cho, S.Y.; Sim, U. Low Dimensional Carbon-Based Catalysts for Efficient Photocatalytic and Photo/Electrochemical Water Splitting Reactions. Materials 2019, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Kochuveedu, S.T. Photocatalytic and Photoelectrochemical Water Splitting on TiO2 via Photosensitization. J. Nanomater. 2016, 2016, 4073142. [Google Scholar] [CrossRef]
- Alfaifi, B.Y.; Ullah, H.; Alfaifi, S.; Tahir, A.A.; Mallick, T.K. Photoelectrochemical solar water splitting: From basic principles to advanced devices. Veruscript Funct. Nanomater. 2018, 2, BDJOC3. [Google Scholar] [CrossRef]
- Hodes, G. Photoelectrochemical Cell Measurements: Getting the Basics Right. J. Phys. Chem. Lett. 2012, 3, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-H.; Lai, T.-H.; Kuo, M.-Y.; Hsieh, P.-Y.; Hsu, Y.-J. Photoelectrochemical cells for solar hydrogen production: Challenges and opportunities. APL Mater. 2019, 7, 080901. [Google Scholar] [CrossRef]
- Bosserez, T.; Rongé, J.; van Humbeeck, J.; Haussener, S.; Martens, J. Design of Compact Photoelectrochemical Cells for Water Splitting. Oil Gas Sci. Technol. 2015, 70, 877–889. [Google Scholar] [CrossRef]
- Singh, R.; Dutta, S. A review on H2 production through photocatalytic reactions using TiO2/TiO2-assisted catalysts. Fuel 2018, 220, 607–620. [Google Scholar] [CrossRef]
- Raghavan, A.; Sarkar, S.; Nagappagary, L.R.; Bojja, S.; Venkatakrishnan, S.M.; Ghosh, S. Decoration of Graphene Quantum Dots on TiO2 Nanostructures: Photosensitizer and Cocatalyst Role for Enhanced Hydrogen Generation. Ind. Eng. Chem. Res. 2020, 59, 13060–13068. [Google Scholar] [CrossRef]
- Yu, S.; Zhong, Y.-Q.; Yu, B.-Q.; Cai, S.-Y.; Wu, L.-Z.; Zhou, Y. Graphene quantum dots to enhance the photocatalytic hydrogen evolution efficiency of anatase TiO2 with exposed {001} facet. Phys. Chem. Chem. Phys. 2016, 18, 20338–20344. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.-J.; Tan, L.-L.; Chai, S.-P.; Yong, S.-T.; Mohamed, A.R. Highly reactive {001} facets of TiO2-based composites: Synthesis, formation mechanism and characterization. Nanoscale 2014, 6, 1946–2008. [Google Scholar] [CrossRef]
- Lee, T.D.; Ebong, A.U. A review of thin film solar cell technologies and challenges. Renew. Sustain. Energy Rev. 2017, 70, 1286–1297. [Google Scholar] [CrossRef]
- Bai, Y.; Mora-Seró, I.; De Angelis, F.; Bisquert, J.; Wang, P. Titanium dioxide nanomaterials for photovoltaic applications. Chem. Rev. 2014, 114, 10095–10130. [Google Scholar] [CrossRef] [PubMed]
- Shakeel Ahmad, M.; Pandey, A.K.; Abd Rahim, N. Advancements in the development of TiO2 photoanodes and its fabrication methods for dye sensitized solar cell (DSSC) applications. A review. Renew. Sustain. Energy Rev. 2017, 77, 89–108. [Google Scholar] [CrossRef]
- Gong, J.; Sumathy, K.; Qiao, Q.; Zhou, Z. Review on dye-sensitized solar cells (DSSCs): Advanced techniques and research trends. Renew. Sustain. Energy Rev. 2017, 68, 234–246. [Google Scholar] [CrossRef]
- Roy-Mayhew, J.D.; Aksay, I.A. Graphene materials and their use in dye-sensitized solar cells. Chem. Rev. 2014, 114, 6323–6348. [Google Scholar] [CrossRef] [PubMed]
- Low, F.W.; Lai, C.W. Recent developments of graphene-TiO2 composite nanomaterials as efficient photoelectrodes in dye-sensitized solar cells: A review. Renew. Sustain. Energy Rev. 2018, 82, 103–125. [Google Scholar] [CrossRef]
- Paulo, S.; Palomares, E.; Martinez-Ferrero, E. Graphene and Carbon Quantum Dot-Based Materials in Photovoltaic Devices: From Synthesis to Applications. Nanomaterials 2016, 6, 157. [Google Scholar] [CrossRef] [PubMed]
- Mihalache, I.; Radoi, A.; Mihaila, M.; Munteanu, C.; Marin, A.; Danila, M.; Kusko, M.; Kusko, C. Charge and energy transfer interplay in hybrid sensitized solar cells mediated by graphene quantum dots. Electrochim. Acta 2015, 153, 306–315. [Google Scholar] [CrossRef]
- Kishore Kumar, D.; Hsu, M.-H.; Ivaturi, A.; Chen, B.; Bennett, N.; Upadhaya, H.M. Optimizing room temperature binder free TiO2 paste for high efficiency flexible polymer dye sensitized solar cells. Flex. Print. Electron. 2019, 4, 015007. [Google Scholar] [CrossRef]
- Ghayoor, R.; Keshavarz, A.; Rad, M.N.S.; Mashreghi, A. Enhancement of photovoltaic performance of dye-sensitized solar cells based on TiO2-graphene quantum dots photoanode. Mater. Res. Express 2018, 6, 025505. [Google Scholar] [CrossRef]
- Peter, I.J.; Rajamanickam, N.; Vijaya, S.; Anandan, S.; Ramchandran, K.; Nithiananthi, P. TiO2/Graphene Quantum Dots core-shell based photo anodes with TTIP treatment- A perspective way of enhancing the short circuit current. Sol. Energy Mater. Sol. Cells 2020, 205, 110239. [Google Scholar] [CrossRef]
- Fang, X.; Li, M.; Guo, K.; Li, J.; Pan, M.; Bai, L.; Luoshan, M.; Zhao, X. Graphene quantum dots optimization of dye-sensitized solar cells. Electrochim. Acta 2014, 137, 634–638. [Google Scholar] [CrossRef]
- Zhen, C.; Wu, T.; Chen, R.; Wang, L.; Liu, G.; Cheng, H.-M. Strategies for Modifying TiO2 Based Electron Transport Layers to Boost Perovskite Solar Cells. ACS Sustain. Chem. Eng. 2019, 7, 4586–4618. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.-W.; Jung, H.S.; Shin, H.; Park, N.-G. High-Efficiency Perovskite Solar Cells. Chem. Rev. 2020, 120, 7867–7918. [Google Scholar] [CrossRef]
- Zhu, Z.; Ma, J.; Wang, Z.; Mu, C.; Fan, Z.; Du, L.; Bai, Y.; Fan, L.; Yan, H.; Phillips, D.L.; et al. Efficiency enhancement of perovskite solar cells through fast electron extraction: The role of graphene quantum dots. J. Am. Chem. Soc. 2014, 136, 3760–3763. [Google Scholar] [CrossRef] [PubMed]
- Devadoss, A.; Sudhagar, P.; Terashima, C.; Nakata, K.; Fujishima, A. Photoelectrochemical biosensors: New insights into promising photoelectrodes and signal amplification strategies. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 43–63. [Google Scholar] [CrossRef]
- Zang, Y.; Lei, J.; Ju, H. Principles and applications of photoelectrochemical sensing strategies based on biofunctionalized nanostructures. Biosens. Bioelectron. 2017, 96, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.; Lim, H.L.; Zawawi, R.M.; Tajudin, A.A.; Ng, Y.H.; Guo, H.; Huang, N.M. A review on visible-light induced photoelectrochemical sensors based on CdS nanoparticles. J. Mater. Chem. B Mater. Biol. Med. 2018, 6, 4551–4568. [Google Scholar] [CrossRef]
- Xu, R.; Wei, D.; Du, B.; Cao, W.; Fan, D.; Zhang, Y.; Wei, Q.; Ju, H. A photoelectrochemical sensor for highly sensitive detection of amyloid beta based on sensitization of Mn:CdSe to Bi2WO6/CdS. Biosens. Bioelectron. 2018, 122, 37–42. [Google Scholar] [CrossRef]
- Ezhil Vilian, A.T.; Rajkumar, M.; Chen, S.-M.; Hu, C.-C.; Piraman, S. A promising photoelectrochemical sensor based on a ZnO particle decorated N-doped reduced graphene oxide modified electrode for simultaneous determination of catechol and hydroquinone. RSC Adv. 2014, 4, 48522–48534. [Google Scholar] [CrossRef]
- Shu, J.; Qiu, Z.; Zhuang, J.; Xu, M.; Tang, D. In Situ Generation of Electron Donor to Assist Signal Amplification on Porphyrin-Sensitized Titanium Dioxide Nanostructures for Ultrasensitive Photoelectrochemical Immunoassay. ACS Appl. Mater. Interfaces 2015, 7, 23812–23818. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhou, B. Titanium dioxide nanomaterials for sensor applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, N.; Bagherzadeh, M.; Nemati, A. Comparison between electrochemical and photoelectrochemical detection of dopamine based on titania-ceria-graphene quantum dots nanocomposite. Biosens. Bioelectron. 2020, 151, 111977. [Google Scholar] [CrossRef]
- Hun, X.; Wang, S.; Mei, S.; Qin, H.; Zhang, H.; Luo, X. Photoelectrochemical dopamine sensor based on a gold electrode modified with SnSe nanosheets. Microchim. Acta 2017, 184, 3333–3338. [Google Scholar] [CrossRef]
- Gao, P.; Ma, H.; Yang, J.; Wu, D.; Zhang, Y.; Du, B.; Fan, D.; Wei, Q. Anatase TiO2 based photoelectrochemical sensor for the sensitive determination of dopamine under visible light irradiation. New J. Chem. 2015, 39, 1483–1487. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, Q.; Du, X.; Qian, J.; Mao, H.; Wang, K. Visible light photoelectrochemical sensor for ultrasensitive determination of dopamine based on synergistic effect of graphene quantum dots and TiO2 nanoparticles. Anal. Chim. Acta 2015, 853, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Moon, G.-H.; Kim, G.; Kang, U.; Park, H.; Choi, W. TiO2 complexed with dopamine-derived polymers and the visible light photocatalytic activities for water pollutants. J. Catal. 2017, 346, 92–100. [Google Scholar] [CrossRef]
- Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Photoelectrochemical bioanalysis: The state of the art. Chem. Soc. Rev. 2015, 44, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. P T 2015, 40, 277–283. [Google Scholar]
- Rios, A.C.; Moutinho, C.G.; Pinto, F.C.; Fiol, F.S.D.; Jozala, A.; Chaud, M.V.; Vila, M.M.D.C.; Teixeira, J.A.; Balcao, V.M. Alternatives to overcoming bacterial resistances: State-of-the-art. Microbiol. Res. 2016, 191, 51–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Advert. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- de Dicastillo, C.L.; Correa, M.G.; Martínez, F.B.; Galotto, M.J. Antimicrobial Effect of Titanium Dioxide Nanoparticles. Antimicrobial Resistance—A One Health Perspective. Available online: https://www.intechopen.com/online-first/antimicrobial-effect-of-titanium-dioxide-nanoparticles (accessed on 28 February 2021).
- Bokare, A.; Sanap, A.; Pai, M.; Sabharwal, S.; Athawale, A.A. Antibacterial activities of Nd doped and Ag coated TiO2 nanoparticles under solar light irradiation. Colloids Surf. B Biointerfaces 2013, 102, 273–280. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Ehsani, A.; Divband, B.; Alizadeh-Sani, M. Antimicrobial activity of Titanium dioxide and Zinc oxide nanoparticles supported in 4A zeolite and evaluation the morphological characteristic. Sci. Rep. 2019, 9, 17439. [Google Scholar] [CrossRef]
- Karahan, H.E.; Wiraja, C.; Xu, C.; Xu, C.; Wei, J.; Wang, Y.; Wang, L.; Liu, F.; Chen, Y. Graphene Materials in Antimicrobial Nanomedicine: Current Status and Future Perspectives. Adv. Healthc. Mater. 2018, 7, e1701406. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Malarkodi, C. In Vitro Antibacterial Activity and Mechanism of Silver Nanoparticles against Foodborne Pathogens. Bioinorg. Chem. Appl. 2014, 2014, 581890. [Google Scholar] [CrossRef]
- Yemmireddy, V.K.; Hung, Y.-C. Selection of photocatalytic bactericidal titanium dioxide (TiO2) nanoparticles for food safety applications. LWT Food Sci. Technol. 2015, 61, 1–6. [Google Scholar] [CrossRef]
- Hitkova, H.; Stoyanova, A.; Ivanova, N.; Sredkova, M.; Bachvarova-Nedelcheva, A.J. Study of antibacterial activity of nonhydrolytic synthesized TiO2 against E. coli, P. aeruginosa and S. aureus. J. Optoelectr. Biomed. Mater. 2012, 4, 9–17. [Google Scholar]
- Teymourinia, H.; Salavati-Niasari, M.; Amiri, O.; Yazdian, F. Application of green synthesized TiO2/Sb2S3/GQDs nanocomposite as high efficient antibacterial agent against E. coli and Staphylococcus aureus. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 296–303. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kang, M.S.; Lee, H.; Lee, J.H.; Kim, J.; Han, D.W.; Kim, K.S. Recent Trends in Photoacoustic Imaging Techniques for 2D Nanomaterial-Based Phototherapy. Biomedicines 2021, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, G.; Cai, X.; Nie, L.; Wang, L.V.; Sitharaman, B. Graphene-based contrast agents for photoacoustic and thermoacoustic tomography. Photoacoustics 2013, 1, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Hariri, A.; Lemaster, J.; Wang, J.; Jeevarathinam, A.S.; Chao, D.L.; Jokerst, J.V. The characterization of an economic and portable LED-based photoacoustic imaging system to facilitate molecular imaging. Photoacoustics 2018, 9, 10–20. [Google Scholar] [CrossRef]
- Shen, T.; Zhou, X.; Cao, H.; Zheng, C.; Liu, Z. TiO2(B)–CNT–graphene ternary composite anode material for lithium ion batteries. RSC Adv. 2015, 5, 22449–22454. [Google Scholar] [CrossRef]
- Han, T.H.; Praveen, N.; Shim, J.H.; Nguyen, A.T.N.; Mahato, N.; Cho, M.H. Ternary Composite of Polyaniline Graphene and TiO2 as a Bifunctional Catalyst to Enhance the Performance of Both the Bioanode and Cathode of a Microbial Fuel Cell. Ind. Eng. Chem. Res. 2018, 57, 6705–6713. [Google Scholar] [CrossRef]
- Sheu, F.-J.; Cho, C.-P.; Liao, Y.-T.; Yu, C.-T. Ag3PO4-TiO2-Graphene Oxide Ternary Composites with Efficient Photodegradation, Hydrogen Evolution, and Antibacterial Properties. Catalysts 2018, 8, 57. [Google Scholar] [CrossRef]

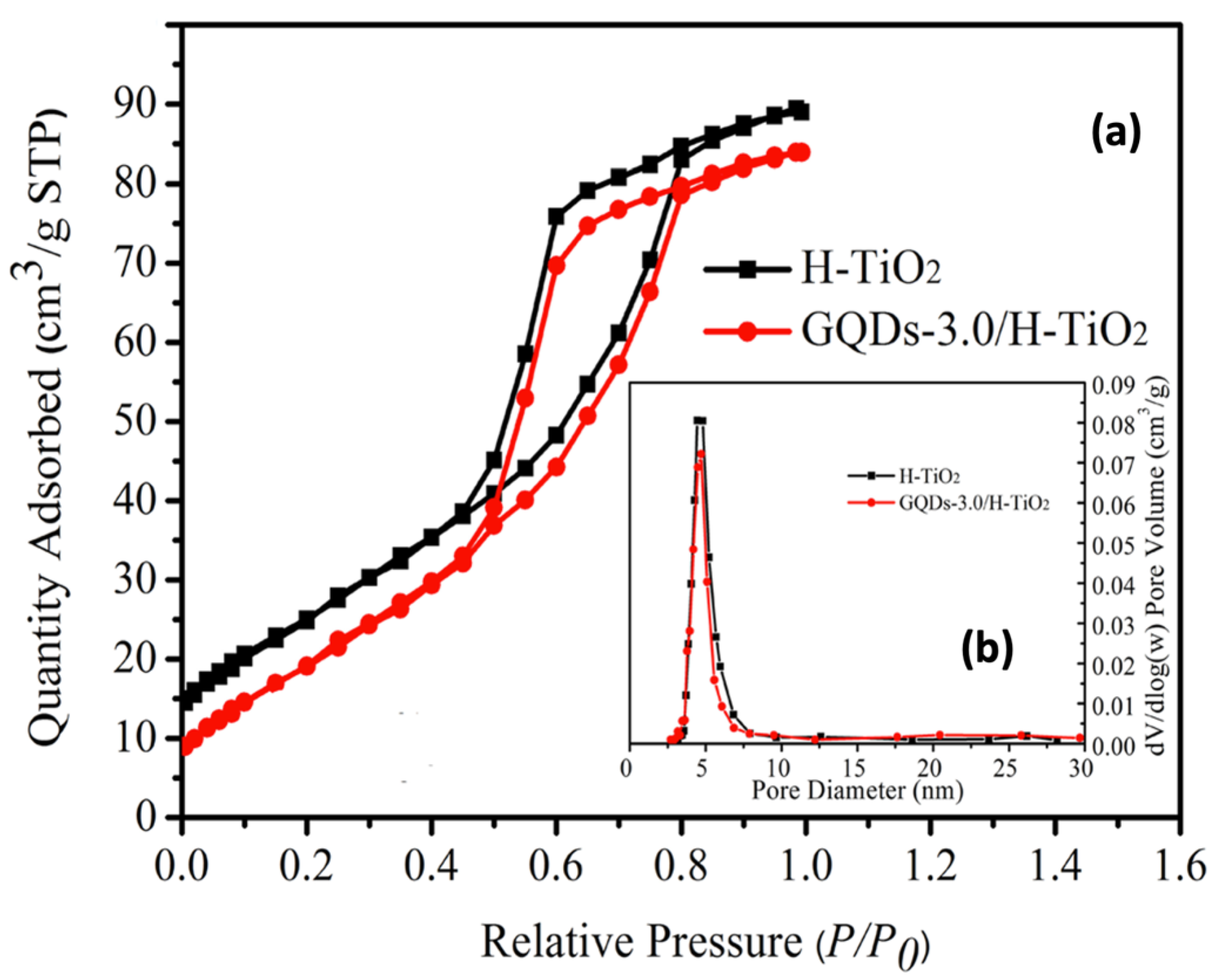
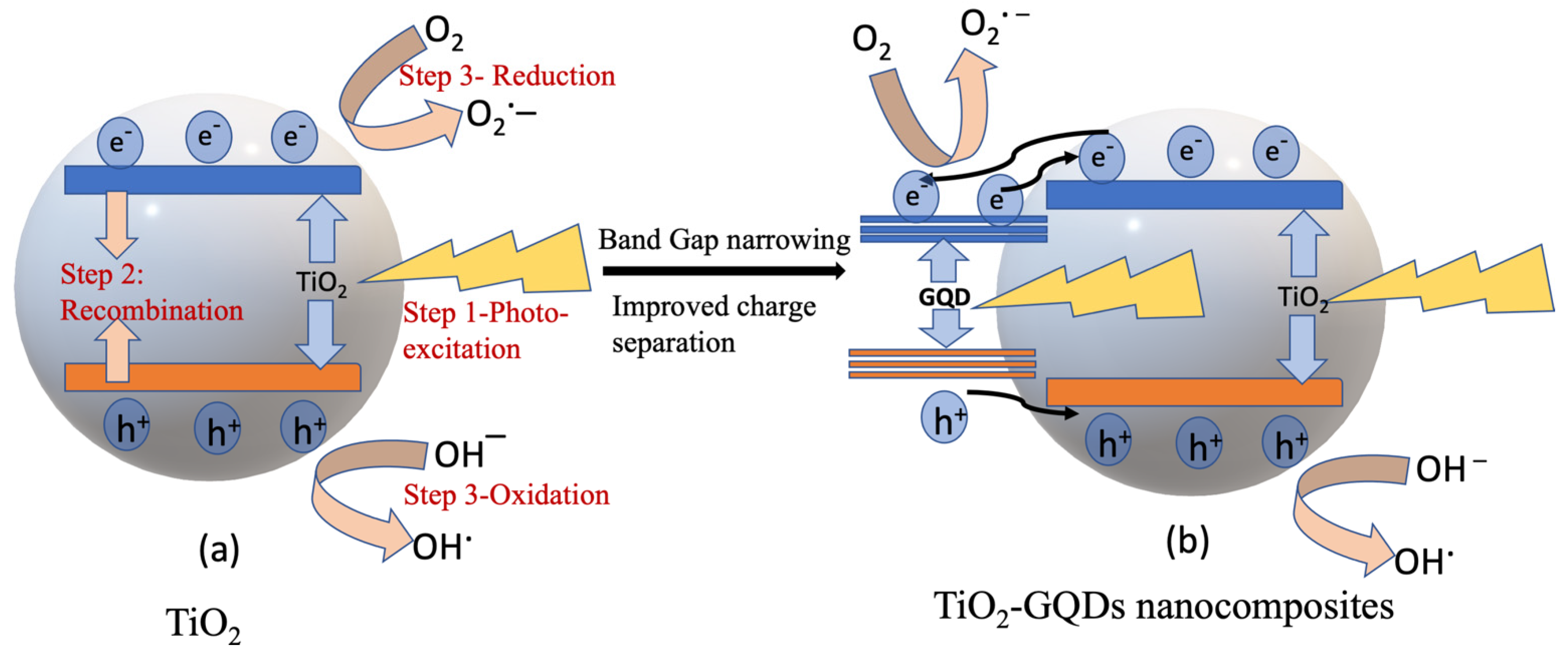
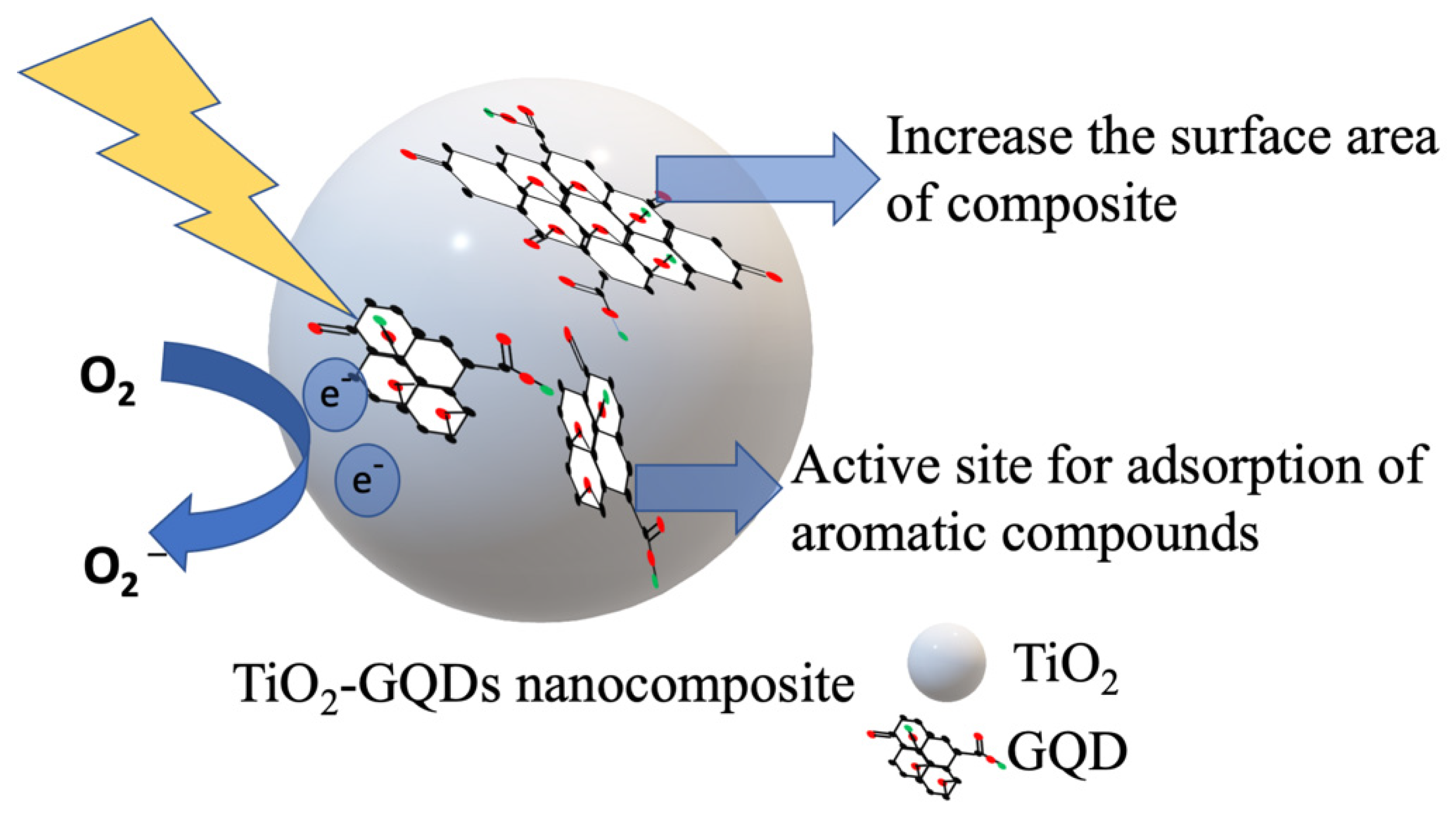
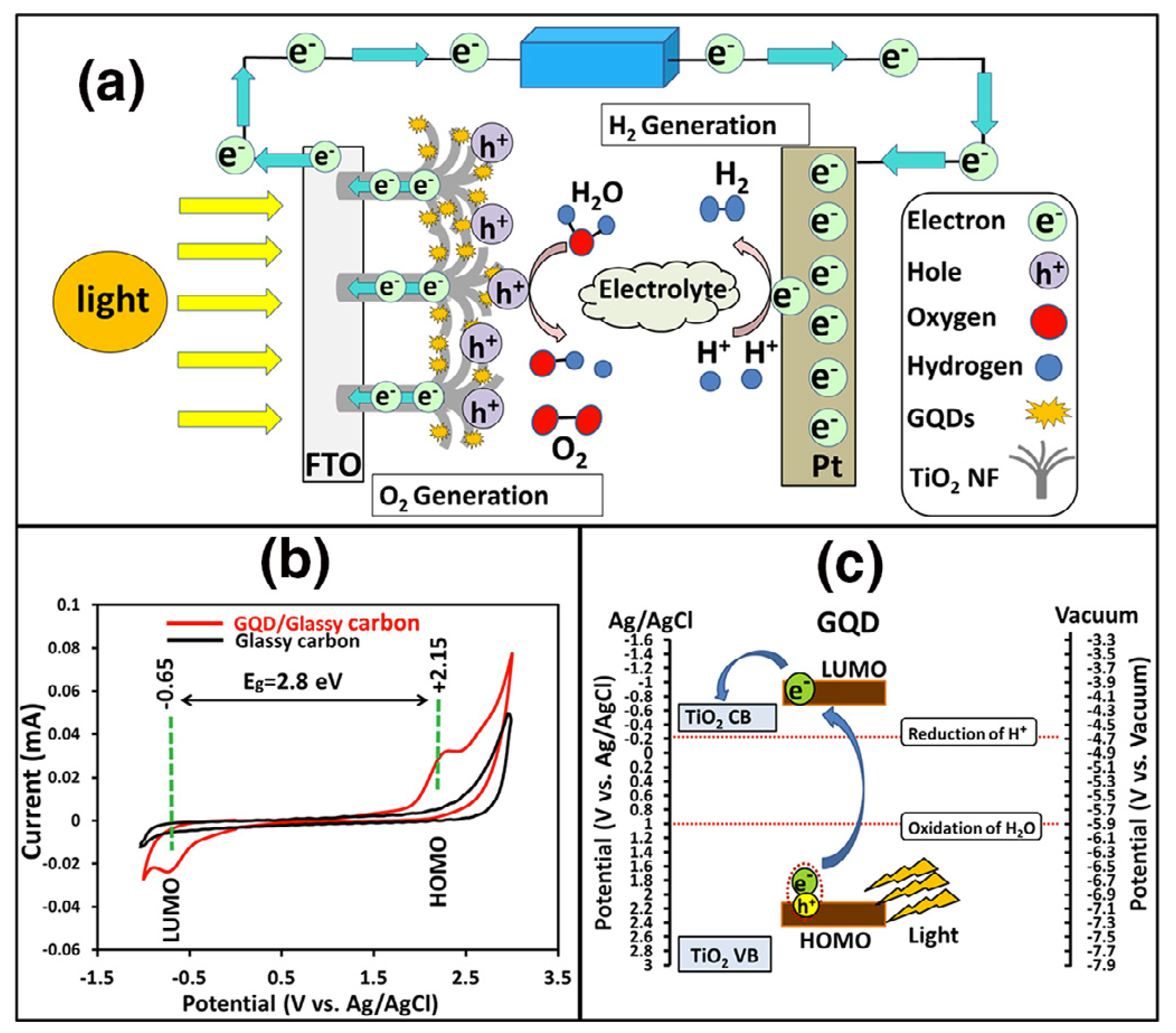
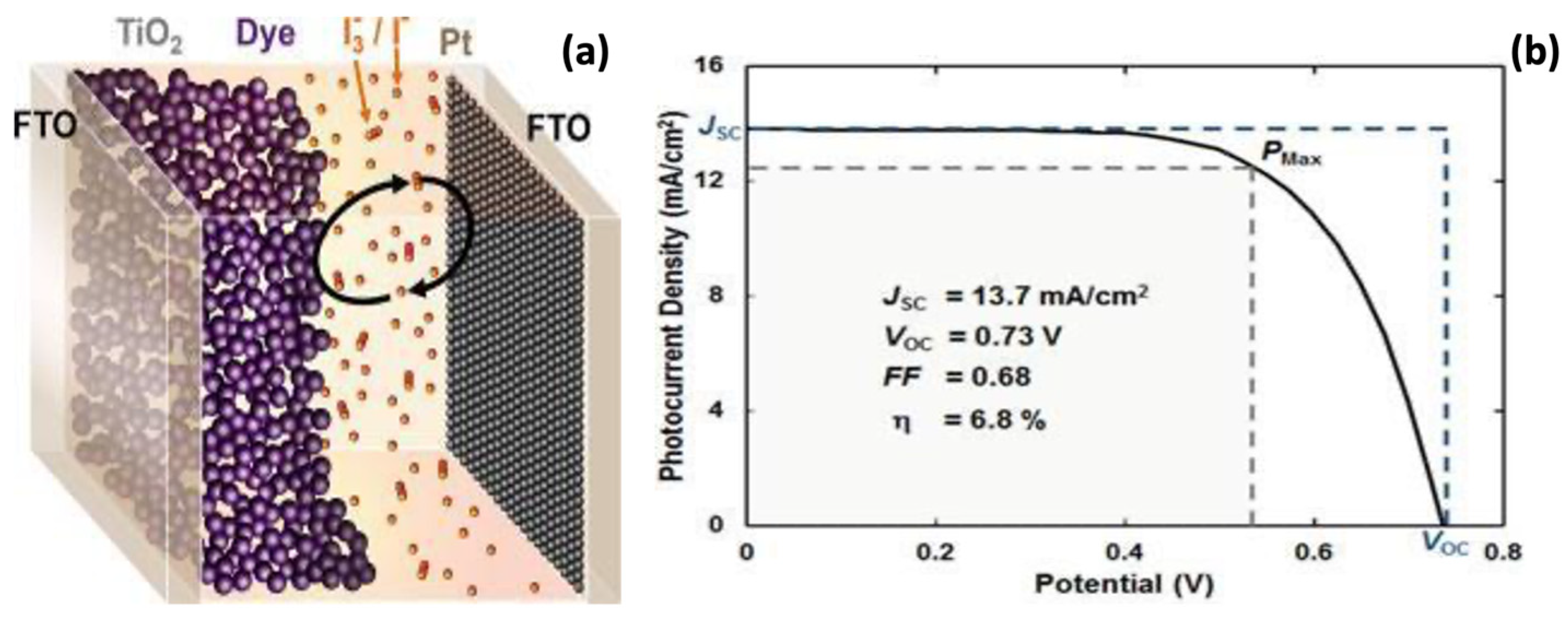
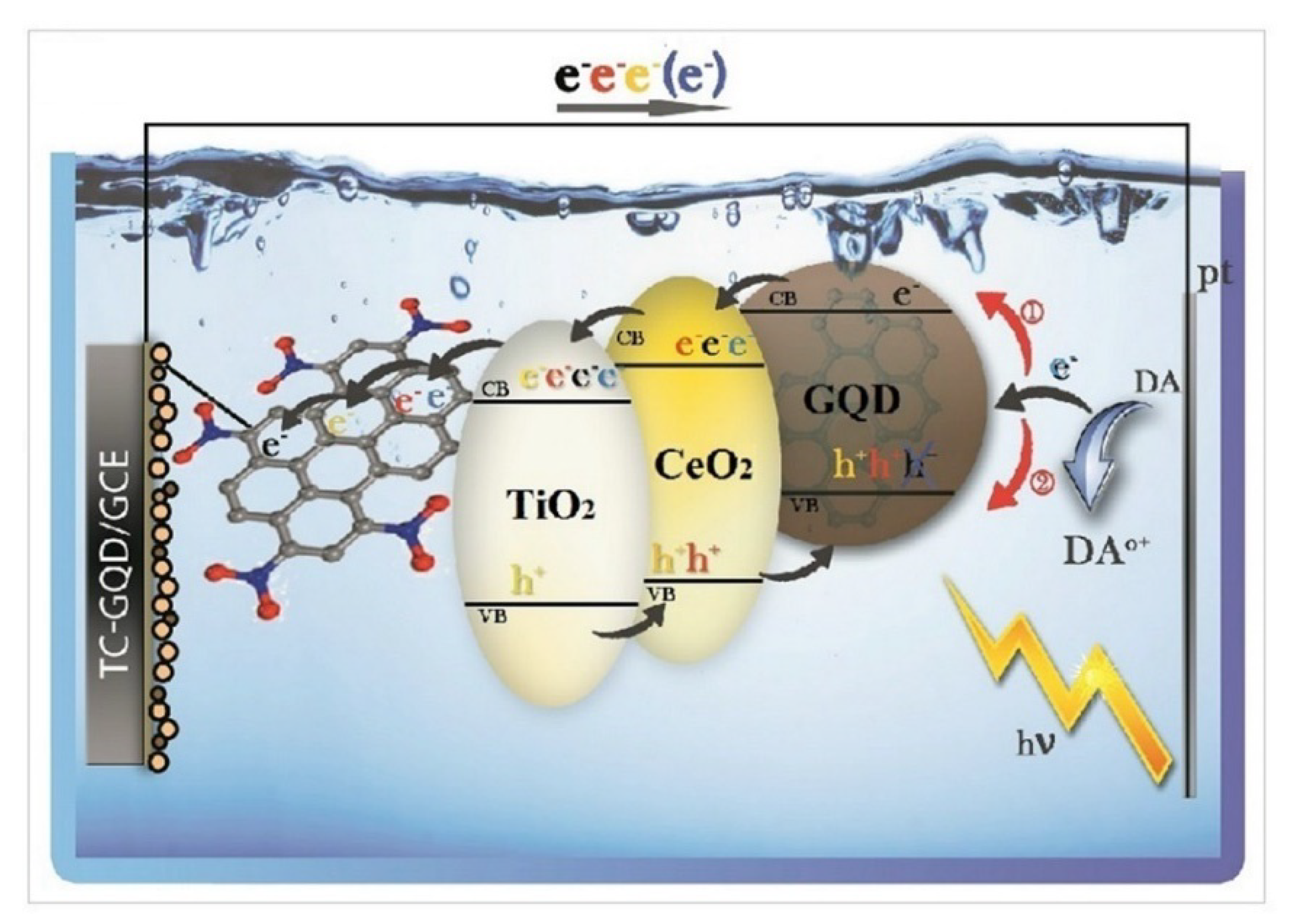
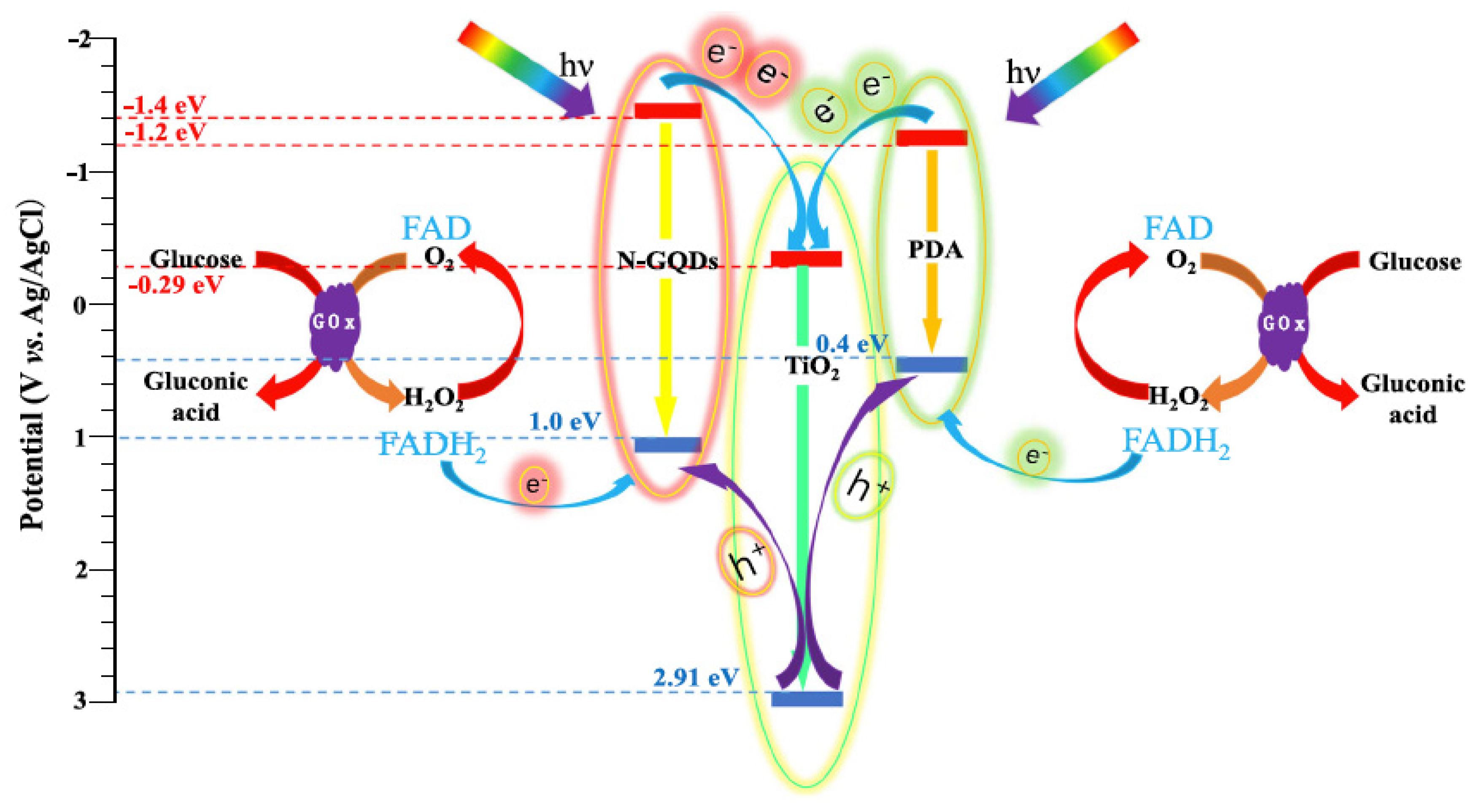
| Photocatalyst | Dye Concentration | Catalyst Quantity | Light Source | Time (min) | Degradation (%) | Reference | |
|---|---|---|---|---|---|---|---|
| (mg) | TiO2 | TiO2-GQD | |||||
| TiO2-GQDs | a MO (50 mL) 10 mg/lit | 20 | 350 W Xe | 120 | 20 | 90 | [71] |
| Rutile TiO2- GQD | b MB (50 mL) 0.00002 M | 50 | 350 W Xe | 60 | 25 | 100 | [119] |
| Nanotube TiO2- N-GQDs | Bisphenol A 50 mL, 20 ppm | 1 g | Sunlight 60–150 Wm−2 | 60 | 80 | 100 | [108] |
| TiO2-GQD | b MB (50 mL) 0.002 | 50 | UV light 340 nm | 45 | 50 | 77 | [78] |
| Nanotube TiO2- GQDs | b MB (20 mL) 10 ppm | 10 | 300 W W-halogen | 180 | 50 | 75 | [80] |
| P-25 TiO2- GQD | b MB (40 mL) 10 ppm | 20 | 300 W Xe | 30 | 0 | 50 | [35] |
| O2-deficient TiO2-GQDs | b MB (100 mL) 8 mg/lit | 20 | Xe | 120 | 38 | 97 | [107] |
| TiO2-N-doped GQDs | c RhB (50 mL) 20 mg/lit | 10 | 500 W Hg light | 120 | 15 | 94 | [81] |
| TiO2 -GQD | c RhB (60 mL) 5 ppm | 50 | 400 W Xe | 30 | - | 100 | [79] |
| Hollow TiO2 nanosphere-GQDs | c Rh-B (50 mL) 10 mg/lit | 25 | Visible light | 300 | 5 | 38 | [110] |
| Nanotube TiO2- GQDs | b MB (20 um) | - | UV light | 210 | 75 | 90 | [93] |
| 001 facet TiO2- N-GQD | Rh (B) | 10 | 300 W Xe | 60 | 10 | 90 | [109] |
| Photocatalyst | Electrolyte | H2 Generation Rate (mmole/h) | Light Source | Reference | |
|---|---|---|---|---|---|
| TiO2 | TiO2-GQD | ||||
| Degussa-TiO2- GQDs (5 mg) | 5 vol% glycerol aqueous solution | 1210 | 29,548 (mmole/h/g) | Natural solar light 80,000 ± 1000 lx | [132] |
| TiO2 nanotube-GQDs | Water with Na2S and Na2SO4 as sacrificial agent | 8 | 10 | 300 W Xe lamp | [84] |
| TiO2- S, N-GQD | 25% methanol aqueous solution | 0 | 4.3 | 300 W Xe with 420 nm | [82] |
| DegussaTiO2- S, N-GQD | Water with 10% methanol | 1.7 | 5.8 | 300 W Xe lamp with 400 filter | [72] |
| 001 facet TiO2-GQDs | Water with methanol as a sacrificial agent | 0 | 800 | Sunlight-like light | [133] |
| 001 facet TiO2-GQD | Water | - | 79.3 micromole/g | 500 W-Hg | [134] |
| TiO2-GQDs | Methanol | 1.8 | 4.126 | UV–Vis light | [89] |
| Photocatalyst | Reference Electrode | Photocurrent Density mA/cm−2 | Electrolyte | Light Source | Reference | |
|---|---|---|---|---|---|---|
| (Bias Voltage) (V) | TiO2 | TiO2- GQD | ||||
| Micropillar rutile TiO2-GQD | Ag/AgCl (−0.1 to 1) | - | 2.92 | KOH 1 M | 300 W Xenon | [86] |
| Nanoflower Rutile TiO2-GQD | Ag/AgCl −0.7 to 0.9 | 0.15 | 0.35 | Na2SO4 1 M | Xenon 100 mW cm−2 | [91] |
| TiO2-GQD | Ag/AgCl (transient photocurrent | 0.001 | 0.003 | Na2SO4 0.5 M | 350 W Xenon | [71] |
| TiO2- N-GQDs | Ag/AgCl −0.2 V | 0.073 | 0.26 ampere | Na2SO4 1 M | 350 W Xenon lamp | [81] |
| Hollow TiO2 sphere-GQD | Ag/AgCl (−0.4 to 1) | 0.25 | 1.3 | Na2SO4 0.5 M | 300 W Xenon lamp | [110] |
| TiO2 nanotube- GQD | Ag/AgCl (0.417 V) | 0.009 | 0.024 | Na2SO4 0.1 M | 300 W Xenon lamp | [80] |
| Rutile TiO2- GQDs | -- | 0 | 0.001 m ampere | (EMISCN/PMII = 7:13 v/v). | [119] | |
| TiO2-GQDs | a RHE 0.2 to 1.8 | 0.12 | 0.18 | Na2SO4 0.5 M | 300 W Xe 100 mW cm−2 | [87] |
| TiO2-GQDs | Ag/AgCl (transient photocurrent) | 0.8 | 1.7 | Na2SO4 0.1 mol/L | UV–visible light | [89] |
| P25-TiO2- S-N doped GQDs | b SHE (transient photocurrent) | 0 | 0.3 m ampere | Na2SO4 0.5 M | 300 W Xenon lamp | [72] |
| GQDs as a | Jsc (mA/cm−2) | Voc (V) | FF | Efficiency | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|
| W/O GQD | With GQD | W/O GQD | With GQD | W/O GQD | With GQD | W/O GQD | With GQD | ||
| Sensitizer | - | 0.2 | - | 0.48 V | - | 0.58 | - | [74] | |
| Co-Sensitizer | 4.73 | 5.58 | 0.608 | 0.583 | 0.66 | 0.67 | 1.92 | 2.15 | [142] |
| Co-sensitizer | 12.74 | 16.54 | 0.76 | 0.77 | 0.63 | 0.63 | 6.12 | 7.96 | [97] |
| Co-Sensitizer | 14 | 15.5 | 0.8 | 0.8 | 7.28 | 7.98 | [101] | ||
| Co-Sensitizer | 15.75 | 22.6 | 0.74 | 0.79 | 0.63 | 0.70 | 7.48 | 11.7 | [98] |
| Co-Sensitizer | 12.61 | 17.65 | 0.71 | 0.72 | 0.64 | 0.59 | 5.72 | 7.49 | [103] |
| Co-Sensitizer | 12.10 | 15.49 | 0.75 | 0.75 | 0.45 | 0.60 | 4.08 | 6.97 | [102] |
| Co-Sensitizer | 10.30 | 11.72 | 0.64 | 0.68 | 0.67 | 0,78 | 4.81 | 6.22 | [96] |
| Co-Sensitizer | 10.2 | 11.54 | - | 0.73 | - | 0.52 | - | 4.4 | [143] |
| Co-Sensitizer | 7.23 | 21.68 | 0.53 | 0.48 | 0.32 | 0.35 | 1,26 | 3.62 | [144] |
| Co-Sensitizer | 10.75 | 14,7 | 0.62 | 0.66 | 0.69 | 0.59 | 5.10 | 6.10 | [146] |
| Co-Sensitizer | 6.78 | 13.77 | 0.72 | 0.70 | 0.43 | 0.44 | 2.09 | 5.33 | [145] |
| a ETL | 24 | 25.5 | 1,06 | 1.06 | 0.73 | 0.72 | 18.57 | 19.20 | [100] |
| a ETL | 15.53 | 17.06 | 0.612 | 0.615 | 8.8 | 10.15 | [149] | ||
| a ETL | 19.73 | 22.10 | 0.74 | 0.83 | 0.60 | 0.64 | 8.76 | 11.74 | [104] |
| b LSL | 9.75 | 14,22 | 0.58 | 0.69 | 0.54 | 0.51 | 3.06 | 5.01 | [95] |
| Photocatalyst | Bacteria | MIC (µg/mL) | Reference | |||
|---|---|---|---|---|---|---|
| TiO2 | GQD | TiO2-GQD | ||||
| Surface-disordered rutile TiO2-GQDs | S. aureus | 128 | 256 | 64 | [73] | |
| E. coli | 64 | 512 | 64 | |||
| P. aeruginosa | 256 | 512 | 128 | |||
| TiO2-Sb2S3-GQD | S. aureus | 740 | - | 100 | [175] | |
| E. coli | 110 | - | 30 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bokare, A.; Chinnusamy, S.; Erogbogbo, F. TiO2-Graphene Quantum Dots Nanocomposites for Photocatalysis in Energy and Biomedical Applications. Catalysts 2021, 11, 319. https://doi.org/10.3390/catal11030319
Bokare A, Chinnusamy S, Erogbogbo F. TiO2-Graphene Quantum Dots Nanocomposites for Photocatalysis in Energy and Biomedical Applications. Catalysts. 2021; 11(3):319. https://doi.org/10.3390/catal11030319
Chicago/Turabian StyleBokare, Anuja, Sowbaranigha Chinnusamy, and Folarin Erogbogbo. 2021. "TiO2-Graphene Quantum Dots Nanocomposites for Photocatalysis in Energy and Biomedical Applications" Catalysts 11, no. 3: 319. https://doi.org/10.3390/catal11030319
APA StyleBokare, A., Chinnusamy, S., & Erogbogbo, F. (2021). TiO2-Graphene Quantum Dots Nanocomposites for Photocatalysis in Energy and Biomedical Applications. Catalysts, 11(3), 319. https://doi.org/10.3390/catal11030319






