Synthesis of Ethylene/1-Octene Copolymers with Ultrahigh Molecular Weights by Zr and Hf Complexes Bearing Bidentate NN Ligands with the Camphyl Linker
Abstract
1. Introduction
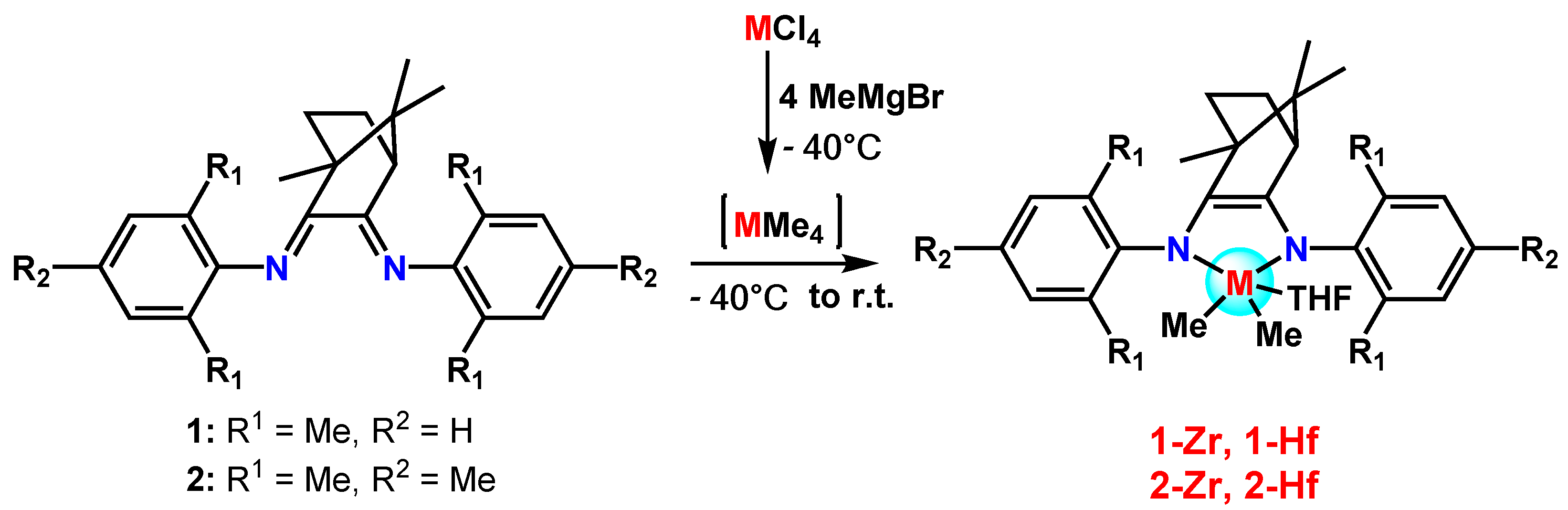
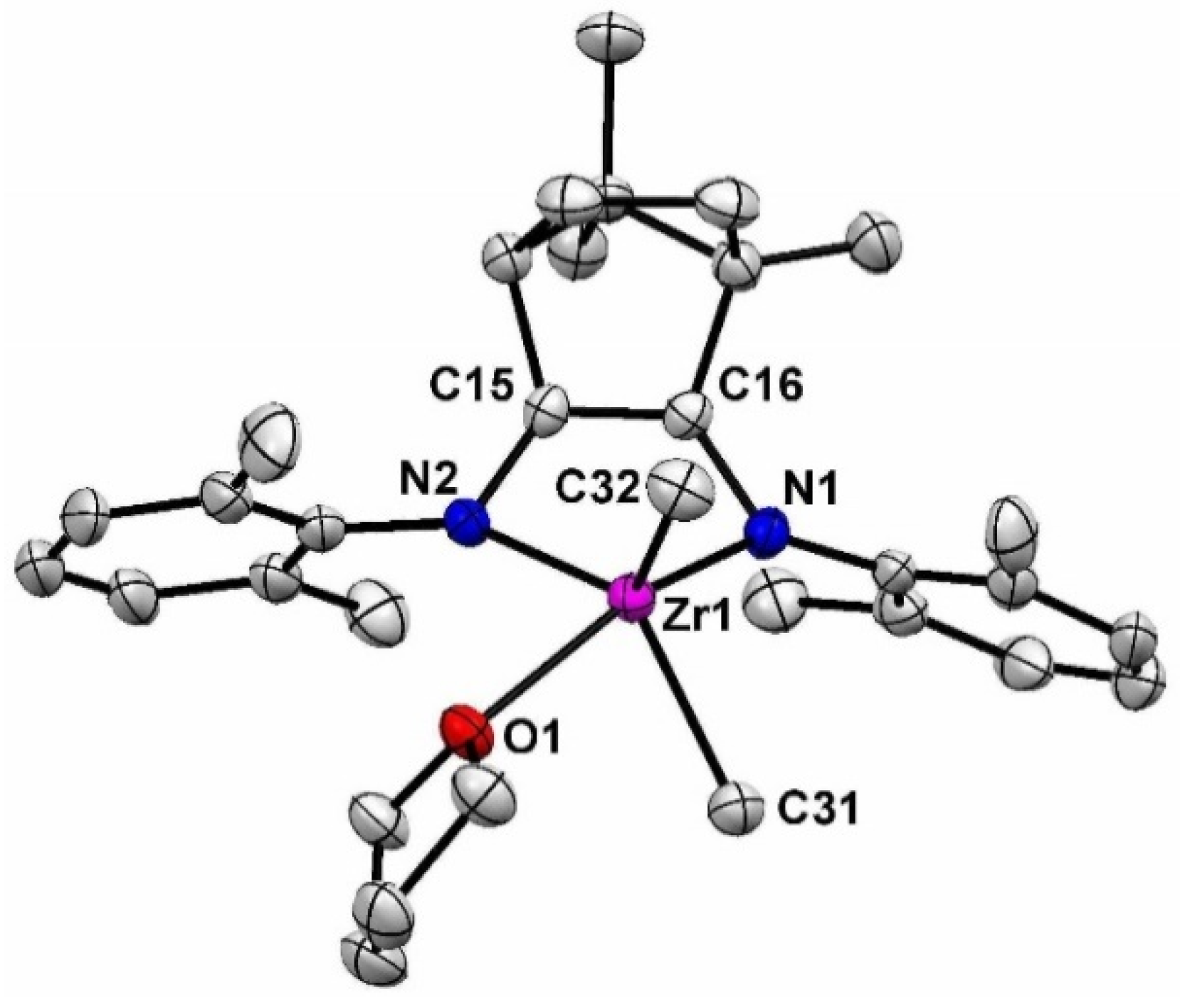
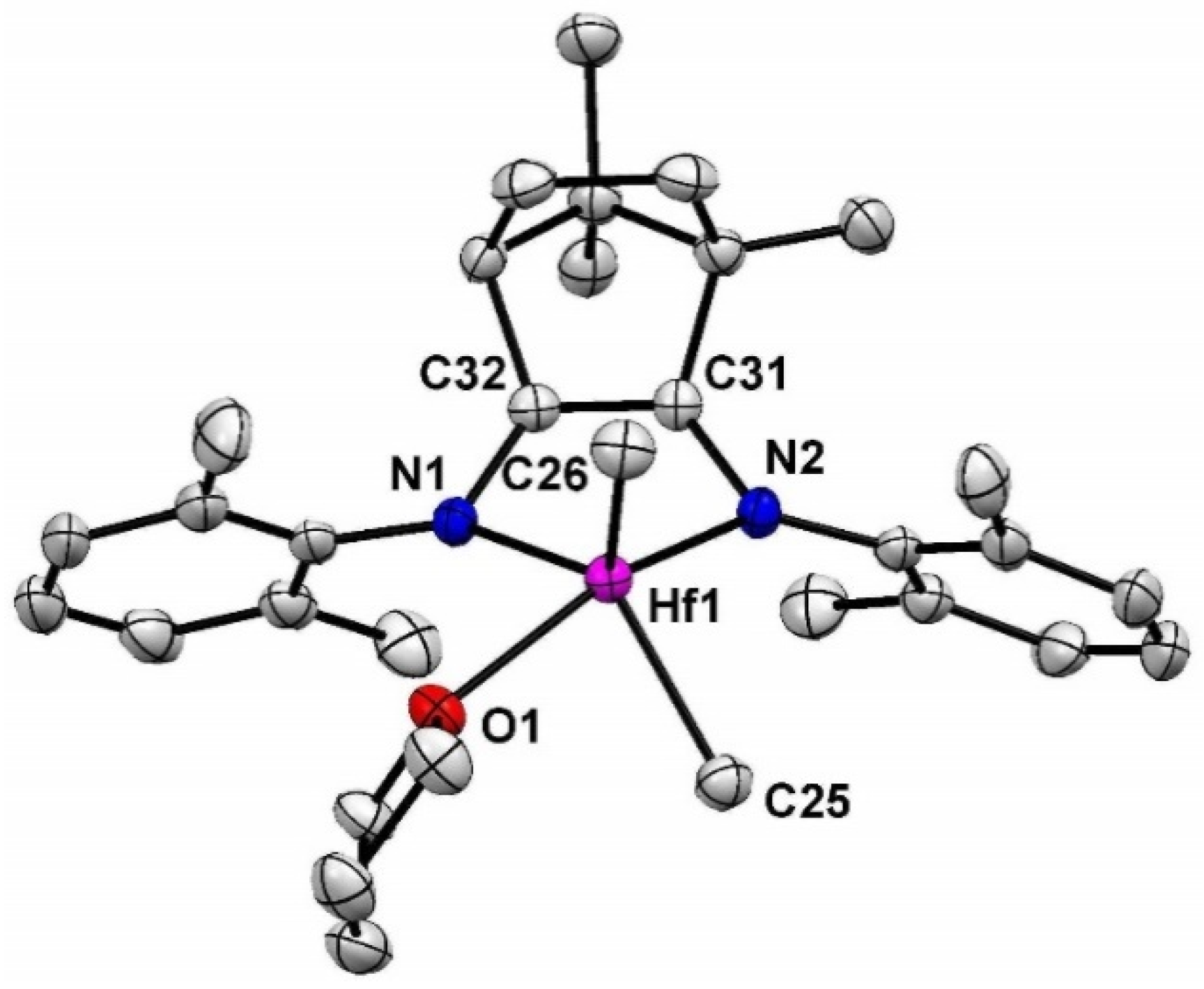
2. Results and Discusssion
3. Materials and Methods
3.1. Synthesis of 1-Zr
3.2. Synthesis of 1-Hf
3.3. Synthesis of 2-Zr
3.4. Synthesis of 2-Hf
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, F.; Chen, C. A continuing legend: The Brookhart-type α-diimine nickel and palladium catalysts. Polym. Chem. 2019, 10, 2354–2369. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.; Solan, G.A.; Sun, W.-H. Recent advances in Ni-mediated ethylene chain growth: Nimine-donor ligand effects on catalytic activity, thermal stability and oligo-/polymer structure. Coord. Chem. Rev. 2017, 350, 68–83. [Google Scholar] [CrossRef]
- Kaiser, J.M.; Long, B.K. Recent developments in redox-active olefin polymerization catalysts. Coord. Chem. Rev. 2018, 372, 141–152. [Google Scholar] [CrossRef]
- Mitchell, N.E.; Long, B.K. Recent advances in thermally robust, late transition metal-catalyzed olefin polymerization. Polym. Int. 2019, 68, 14–26. [Google Scholar] [CrossRef]
- Johnson, L.K.; Killian, C.M.; Brookhart, M. New Pd(II)- and Ni(II)-Based Catalysts for Polymerization of Ethylene and α-Olefins. J. Am. Chem. Soc. 1995, 117, 6414–6415. [Google Scholar] [CrossRef]
- Invergo, A.M.; Liu, S.; Dicken, R.D.; Mouat, A.R.; Delferro, M.; Lohr, T.L.; Marks, T.J. How Close Is Too Close? Polymerization Behavior and Monomer-Dependent Reorganization of a Bimetallic Salphen Organotitanium Catalyst. Organometallics 2018, 37, 2429–2436. [Google Scholar] [CrossRef]
- Liu, S.; Invergo, A.M.; McInnis, J.P.; Mouat, A.R.; Motta, A.; Lohr, T.L.; Delferro, M.; Marks, T.J. Distinctive Stereochemically Linked Cooperative Effects in Bimetallic Titanium Olefin Polymerization Catalysts. Organometallics 2017, 36, 4403–4421. [Google Scholar] [CrossRef]
- Liu, S.; Motta, A.; Mouat, A.R.; Delferro, M.; Marks, T.J. Very Large Cooperative Effects in Heterobimetallic Titanium-Chromium Catalysts for Ethylene Polymerization/Copolymerization. J. Am. Chem. Soc. 2014, 136, 10460–10469. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Motta, A.; Delferro, M.; Marks, T.J. Synthesis, Characterization, and Heterobimetallic Cooperation in a Titanium–Chromium Catalyst for Highly Branched Polyethylenes. J. Am. Chem. Soc. 2013, 135, 8830–8833. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Dai, S.; Sui, X.; Chen, C. Palladium and Nickel Catalyzed Chain Walking Olefin Polymerization and Copolymerization. ACS Catal. 2016, 6, 428–441. [Google Scholar] [CrossRef]
- Guan, Z.; Cotts, P.M.; Mccord, E.F.; McLain, S.J. Chain Walking: A New Strategy to Control Polymer Topology. Sci. 1999, 283, 2059–2062. [Google Scholar] [CrossRef]
- Tan, C.; Chen, C. Emerging Palladium and Nickel Catalysts for Copolymerization of Olefins with Polar Monomers. Angew. Chem. Int. Ed. 2019, 58, 7192–7200. [Google Scholar] [CrossRef]
- Chen, E.Y.-X. Coordination Polymerization of Polar Vinyl Monomers by Single-Site Metal Catalysts. Chem. Rev. 2009, 109, 5157–5214. [Google Scholar] [CrossRef]
- Guan, Z.; Popeney, C.S. Recent Progress in Late Transition Metal α-Diimine Catalysts for Olefin Polymerization. Top. Organomet. Chem. 2009, 26, 179–220. [Google Scholar]
- Ma, X.; Hu, X.; Zhang, Y.; Mu, H.; Cui, L.; Jian, Z. Preparation and in Situ Chain-end-functionalization of Branched Ethylene Pligomers by Monosubstituted α-diimine Nickel Catalysts. Polym. Chem. 2019, 10, 2596–2607. [Google Scholar] [CrossRef]
- Feng, C.; Zhou, S.; Wang, D.; Zhao, Y.; Liu, S.; Li, Z.; Braunstein, P. Cooperativity in Highly Active Ethylene Dimerization by Dinuclear Nickel Complexes Bearing a Bifunctional PN Ligand. Organometallics 2021, 40, 184–193. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Li, A.; Ye, H.; Li, Z. Nickel(ii) complexes chelated by 2,6-pyridinedicarboxamide: Syntheses, characterization, and ethylene oligomerization. New J. Chem. 2016, 40, 7027–7033. [Google Scholar] [CrossRef]
- Wang, C.; Kang, X.; Dai, S.; Cui, F.; Li, Y.; Mu, H.; Mecking, S.; Jian, Z. Efficient Suppression of Chain Transfer and Branching via Cs-Type Shielding in a Neutral Nickel(II) Catalyst. Angew. Chem. 2021, 60, 4018–4022. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Mesgar, M.; White, P.S.; Daugulis, O.; Brookhart, M. Synthesis of Branched Ultrahigh-Molecular-Weight Polyethylene Using Highly Active Neutral, Single-Component Ni(II) Catalysts. ACS Catal. 2015, 5, 631–636. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Mecking, S.; Jian, Z. Ultrahigh Branching of Main-Chain-Functionalized Polyethylenes by Inverted Insertion Selectivity. Angew. Chem. Int. Ed. 2020, 59, 14296–14302. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.H.; Ziller, J.W.; Guan, Z. Axial Donating Ligands: A New Strategy for Late Transition Metal Olefin Polymerization Catalysis. J. Am. Chem. Soc. 2008, 130, 7538–7539. [Google Scholar] [CrossRef]
- Dai, S.; Chen, C. Direct Synthesis of Functionalized High-Molecular-Weight Polyethylene by Copolymerization of Ethylene with Polar Monomers. Angew. Chem. Int. Ed. 2016, 55, 13281–13285. [Google Scholar] [CrossRef]
- Dai, S.; Sui, X.; Chen, C. Highly Robust Palladium(II) α-Diimine Catalysts for Slow-Chain-Walking Polymerization of Ethylene and Copolymerization with Methyl Acrylate. Angew. Chem. Int. Ed. 2015, 54, 9948–9953. [Google Scholar] [CrossRef]
- Pei, L.; Liu, F.; Liao, H.; Gao, J.; Zhong, L.; Gao, H.; Wu, Q. Synthesis of Polyethylenes with Controlled Branching with α-Diimine Nickel Catalysts and Revisiting Formation of Long-Chain Branching. ACS Catal. 2018, 8, 1104–1113. [Google Scholar] [CrossRef]
- Soshnikov, I.E.; Bryliakov, K.P.; Antonov, A.A.; Sun, W.-H.; Talsi, E.P. Ethylene polymerization of nickel catalysts with α-diimine ligands: Factors controlling the structure of active species and polymer properties. Dalton Trans. 2019, 48, 7974–7984. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.M.; Cherian, A.E.; Coates, G.W. Living Polymerization of α-Olefins with an α-Diimine Ni(II) Catalyst: Formation of Well-Defined Ethylene−Propylene Copolymers through Controlled Chain-Walking. J. Am. Chem. Soc. 2006, 128, 4186–4187. [Google Scholar] [CrossRef] [PubMed]
- Cherian, A.E.; Rose, J.M.; Lobkovsky, E.B.; Coates, G.W. A C2-Symmetric, Living α-Diimine Ni(II) Catalyst: Regioblock Copolymers from Propylene. J. Am. Chem. Soc. 2005, 127, 13770–13771. [Google Scholar] [CrossRef]
- De Waele, P.; Jazdzewski, B.A.; Klosin, J.; Murray, R.E.; Theriault, C.N.; Vosejpka, P.C.; Petersen, J.L. Synthesis of Hafnium and Zirconium Imino−Amido Complexes from Bis-imine Ligands. A New Family of Olefin Polymerization Catalysts. Organometallics 2007, 26, 3896–3899. [Google Scholar] [CrossRef]
- Froese, R.D.J.; Jazdzewski, B.A.; Klosin, J.; Kuhlman, R.L.; Theriault, C.N.; Welsh, D.M.; Abboud, K.A. Imino-Amido Hf and Zr Complexes: Synthesis, Isomerization, and Olefin Polymerization. Organometallics 2011, 30, 251–262. [Google Scholar] [CrossRef]
- Ionkin, A.S.; Marshall, W.J. ortho-5-Methylfuran- and Benzofuran-Substituted η3-Allyl(α-diimine)nickel(II) Complexes: Syntheses, Structural Characterization, and the First Polymerization Results†. Organometallics 2004, 23, 3276–3283. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, W.; Hao, X.; Redshaw, C.; Huang, W.; Sun, W.-H. 2,6-Dibenzhydryl-N-(2-phenyliminoacenaphthylenylidene)-4-methylbenzenamine Nickel Dibromides: Synthesis, Characterization, and Ethylene Polymerization. Organometallics 2011, 30, 2418–2424. [Google Scholar] [CrossRef]
- Zhou, Z.; Hao, X.; Redshaw, C.; Chen, L.; Sun, W.-H. Nickel bis{4,6-dibenzhydryl-2-[(arylimino)methyl]phenoxylate} complexes: Synthesis, structures, and catalytic behaviour towards ethylene and norbornene. Catal. Sci. Technol. 2012, 2, 1340–1345. [Google Scholar] [CrossRef]
- Du, S.; Kong, S.; Shi, Q.; Mao, J.; Guo, C.; Yi, J.; Liang, T.; Sun, W.-H. Enhancing the Activity and Thermal Stability of Nickel Complex Precatalysts Using 1-[2,6-Bis(bis(4-fluorophenyl)methyl)-4-methyl phenylimino]-2-aryliminoacenaphthylene Derivatives. Organometallics 2015, 34, 582–590. [Google Scholar] [CrossRef]
- Rhinehart, J.L.; Brown, L.A.; Long, B.K. A Robust Ni(II) α-Diimine Catalyst for High Temperature Ethylene Polymerization. J. Am. Chem. Soc. 2013, 135, 16316–16319. [Google Scholar] [CrossRef] [PubMed]
- Rhinehart, J.L.; Mitchell, N.E.; Long, B.K. Enhancing α-Diimine Catalysts for High-Temperature Ethylene Polymerization. ACS Catal. 2014, 4, 2501–2504. [Google Scholar] [CrossRef]
- Zheng, Q.; Zheng, D.; Han, B.; Liu, S.; Li, Z. Chromium complexes supported by the bidentate PN ligands: Synthesis, characterization and application for ethylene polymerization. Dalton Trans. 2018, 47, 13459–13465. [Google Scholar] [CrossRef]
- Zhong, L.; Li, G.; Liang, G.; Gao, H.; Wu, Q. Enhancing Thermal Stability and Living Fashion in α-Diimine–Nickel-Catalyzed (Co)polymerization of Ethylene and Polar Monomer by Increasing the Steric Bulk of Ligand Backbone. Macromolecules 2017, 50, 2675–2682. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, Y.; Cui, L.; Mu, H.; Jian, Z. Pentiptycenyl Substituents in Insertion Polymerization with α-Diimine Nickel and Palladium Species. Organometallics 2019, 38, 2075–2083. [Google Scholar] [CrossRef]
- Zou, W.; Chen, C. Influence of Backbone Substituents on the Ethylene (Co)polymerization Properties of α-diimine Pd(II) and Ni(II) Catalysts. Organometallics 2016, 35, 1794–1801. [Google Scholar] [CrossRef]
- Guo, L.; Gao, H.; Guan, Q.; Hu, H.; Deng, J.; Liu, J.; Liu, F.; Wu, Q. Substituent Effects of the Backbone in α-Diimine Palladium Catalysts on Homo- and Copolymerization of Ethylene with Methyl Acrylate. Organometallics 2012, 31, 6054–6062. [Google Scholar] [CrossRef]
- Liu, J.; Chen, D.; Wu, H.; Xiao, Z.; Gao, H.; Zhu, F.; Wu, Q. Polymerization of α-Olefins Using a Camphyl α-Diimine Nickel Catalyst at Elevated Temperature. Macromolecules 2014, 47, 3325–3331. [Google Scholar] [CrossRef]
- Liu, F.-S.; Hu, H.-B.; Xu, Y.; Guo, L.-H.; Zai, S.-B.; Song, K.-M.; Gao, H.-Y.; Zhang, L.; Zhu, F.-M.; Wu, Q. Thermostable α-Diimine Nickel(II) Catalyst for Ethylene Polymerization: Effects of the Substituted Backbone Structure on Catalytic Properties and Branching Structure of Polyethylene. Macromolecules 2009, 42, 7789–7796. [Google Scholar] [CrossRef]
- Han, B.; Liu, Y.; Feng, C.; Liu, S.; Li, Z. Development of Group 4 Metal Complexes Bearing Fused-Ring Amido-Trihydroquinoline Ligands with Improved High-Temperature Catalytic Performance toward Olefin (Co)polymerization. Organometallics 2021, 40, 242–252. [Google Scholar] [CrossRef]
- Klosin, J.; Fontaine, P.P.; Figueroa, R. Development of Group IV Molecular Catalysts for High Temperature Ethylene-α-Olefin Copolymerization Reactions. Acc. Chem. Res. 2015, 48, 2004–2016. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, P.P.; Ueligger, S.; Klosin, J.; Hazari, A.; Daller, J.; Hou, J. Development of Improved Amidoquinoline Polyolefin Catalysts with Ultrahigh Molecular Weight Capacity. Organometallics 2015, 34, 1354–1363. [Google Scholar] [CrossRef]
- Klosin, J.; Fontaine, P.P.; Figueroa, R.; McCann, S.D.; Mort, D. Preparation of New Olefin Polymerization Precatalysts by Facile Derivatization of Imino–Enamido ZrMe3 and HfMe3 Complexes. Organometallics 2013, 32, 6488–6499. [Google Scholar] [CrossRef]
- Fontaine, P.P.; Figueroa, R.; McCann, S.D.; Mort, D.; Klosin, J. Synthesis and Scale-up of Imino–Enamido Hafnium and Zirconium Olefin Polymerization Catalysts. Organometallics 2013, 32, 2963–2972. [Google Scholar] [CrossRef]
- Fontaine, P.P.; Klosin, J.; McDougal, N.T. Hafnium Amidoquinoline Complexes: Highly Active Olefin Polymerization Catalysts with Ultrahigh Molecular Weight Capacity. Organometallics 2012, 31, 6244–6251. [Google Scholar] [CrossRef]
- Figueroa, R.; Froese, R.D.; He, Y.; Klosin, J.; Theriault, C.N.; Abboud, K.A. Synthesis of Imino-Enamido Hafnium and Zirconium Complexes: A New Family of Olefin Polymerization Catalysts with Ultrahigh-Molecular-Weight Capabilities. Organometallics 2011, 30, 1695–1709. [Google Scholar] [CrossRef]
- Antonov, A.A.; Bryliakov, K.P. Post-metallocene catalysts for the synthesis of ultrahigh molecular weight polyethylene: Recent advances. Eur. Polym. J. 2021, 142, 110162. [Google Scholar] [CrossRef]
- Patel, K.; Chikkali, S.H.; Sivaram, S. Ultrahigh molecular weight polyethylene: Catalysis, structure, properties, processing and applications. Prog. Polym. Sci. 2020, 109, 101290. [Google Scholar] [CrossRef]
- Zhang, C.; Pan, H.; Klosin, J.; Tu, S.; Jaganathan, A.; Fontaine, P.P. Synthetic Optimization and Scale-Up of Imino–Amido Hafnium and Zirconium Olefin Polymerization Catalysts. Org. Process. Res. Dev. 2015, 19, 1383–1391. [Google Scholar] [CrossRef]
- Liu, S.; Xing, Y.; Zheng, Q.; Jia, Y.; Li, Z. Synthesis of Anthracene-Bridged Dinuclear Phenoxyiminato Organotitanium Catalysts with Enhanced Activity, Thermal Stability, and Comonomer Incorporation Ability toward Ethylene (Co)polymerization. Organometallics 2020, 39, 3268–3274. [Google Scholar] [CrossRef]
- Dieck, H.T.; Kollvitz, W.; Rohde, W.; Stamp, L. Ruthenium complexes with diazadienes, Part V(1).Cis-Dicarbonyl-1,4-diaza-1,3-diene-trans-diiodoruthenium Complexes; synthesis, properties and the crystal structure of [(DAD)Ru(CO)2I2] DAD (DAD=p-tolyl-N=CMe-CMe=N-p-tolyl). Transit. Met. Chem. 1986, 11, 361–366. [Google Scholar] [CrossRef]
- Galindo, A.; Ienco, A.; Mealli, C. Nature of the metal–carbon contacts in ene-diamido d0 metal complexes. New J. Chem. 2000, 24, 73–75. [Google Scholar] [CrossRef]
| Run | Cat. | PE (g) | act. 2 | Mw3 (104 g∙mol−1) | Đ3 | Tm4 (°C) | Octene Incorp. 5 (mol %) |
|---|---|---|---|---|---|---|---|
| 1 | 1-Zr | 0.393 | 236 | >600 | - | 126 | 0.2 |
| 2 | 2-Zr | 0.647 | 388 | 337 | 3.3 | 126 | 1.0 |
| 3 | 1-Hf | trace | - | - | - | - | - |
| 4 | 2-Hf | trace | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, C.; Gou, Q.; Liu, S.; Gao, R.; Li, Z. Synthesis of Ethylene/1-Octene Copolymers with Ultrahigh Molecular Weights by Zr and Hf Complexes Bearing Bidentate NN Ligands with the Camphyl Linker. Catalysts 2021, 11, 276. https://doi.org/10.3390/catal11020276
Feng C, Gou Q, Liu S, Gao R, Li Z. Synthesis of Ethylene/1-Octene Copolymers with Ultrahigh Molecular Weights by Zr and Hf Complexes Bearing Bidentate NN Ligands with the Camphyl Linker. Catalysts. 2021; 11(2):276. https://doi.org/10.3390/catal11020276
Chicago/Turabian StyleFeng, Chunyu, Qingqiang Gou, Shaofeng Liu, Rong Gao, and Zhibo Li. 2021. "Synthesis of Ethylene/1-Octene Copolymers with Ultrahigh Molecular Weights by Zr and Hf Complexes Bearing Bidentate NN Ligands with the Camphyl Linker" Catalysts 11, no. 2: 276. https://doi.org/10.3390/catal11020276
APA StyleFeng, C., Gou, Q., Liu, S., Gao, R., & Li, Z. (2021). Synthesis of Ethylene/1-Octene Copolymers with Ultrahigh Molecular Weights by Zr and Hf Complexes Bearing Bidentate NN Ligands with the Camphyl Linker. Catalysts, 11(2), 276. https://doi.org/10.3390/catal11020276







