Eucalyptol: A Bio-Based Solvent for the Synthesis of O,S,N-Heterocycles. Application to Hiyama Coupling, Cyanation, and Multicomponent Reactions
Abstract
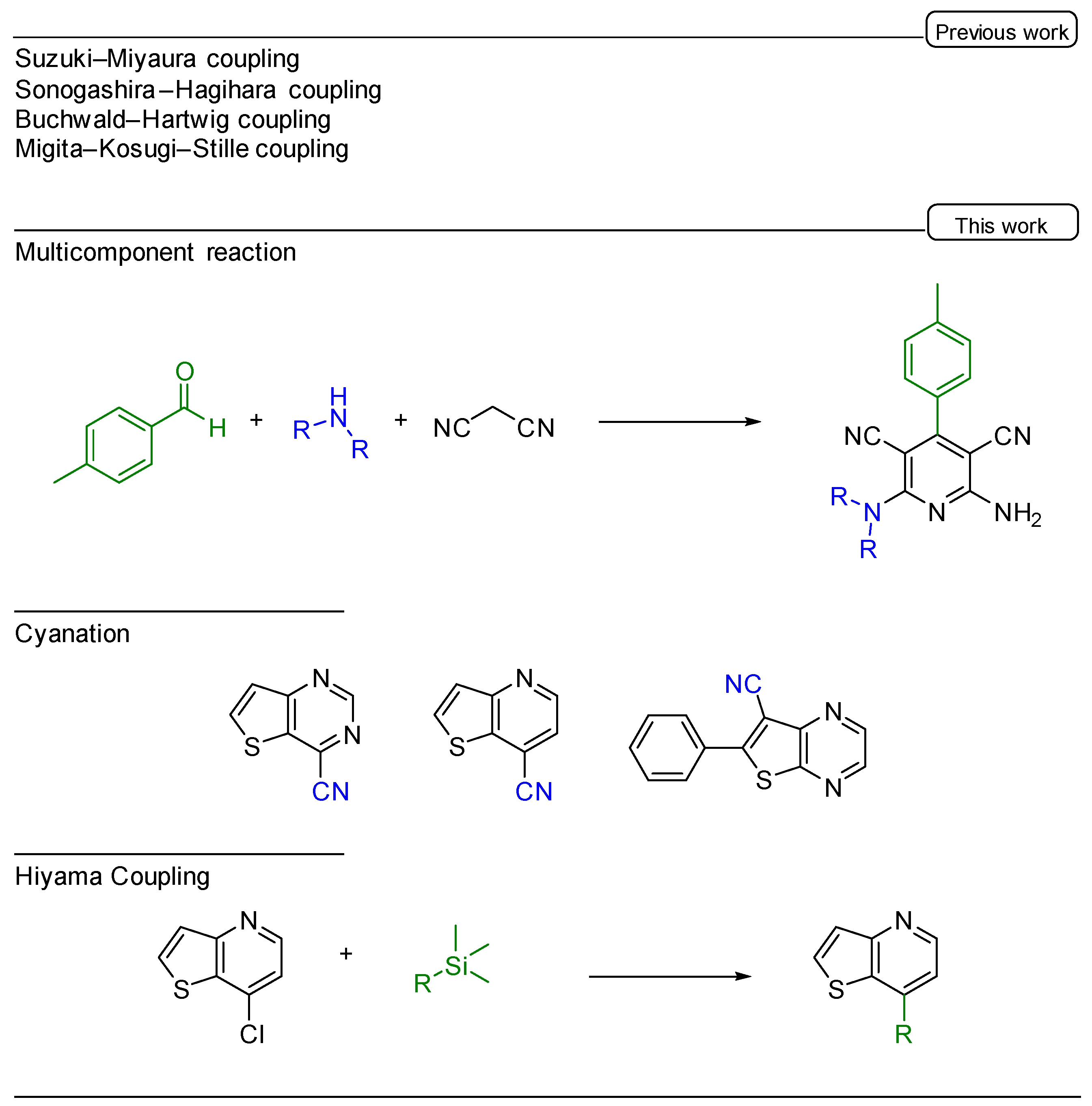
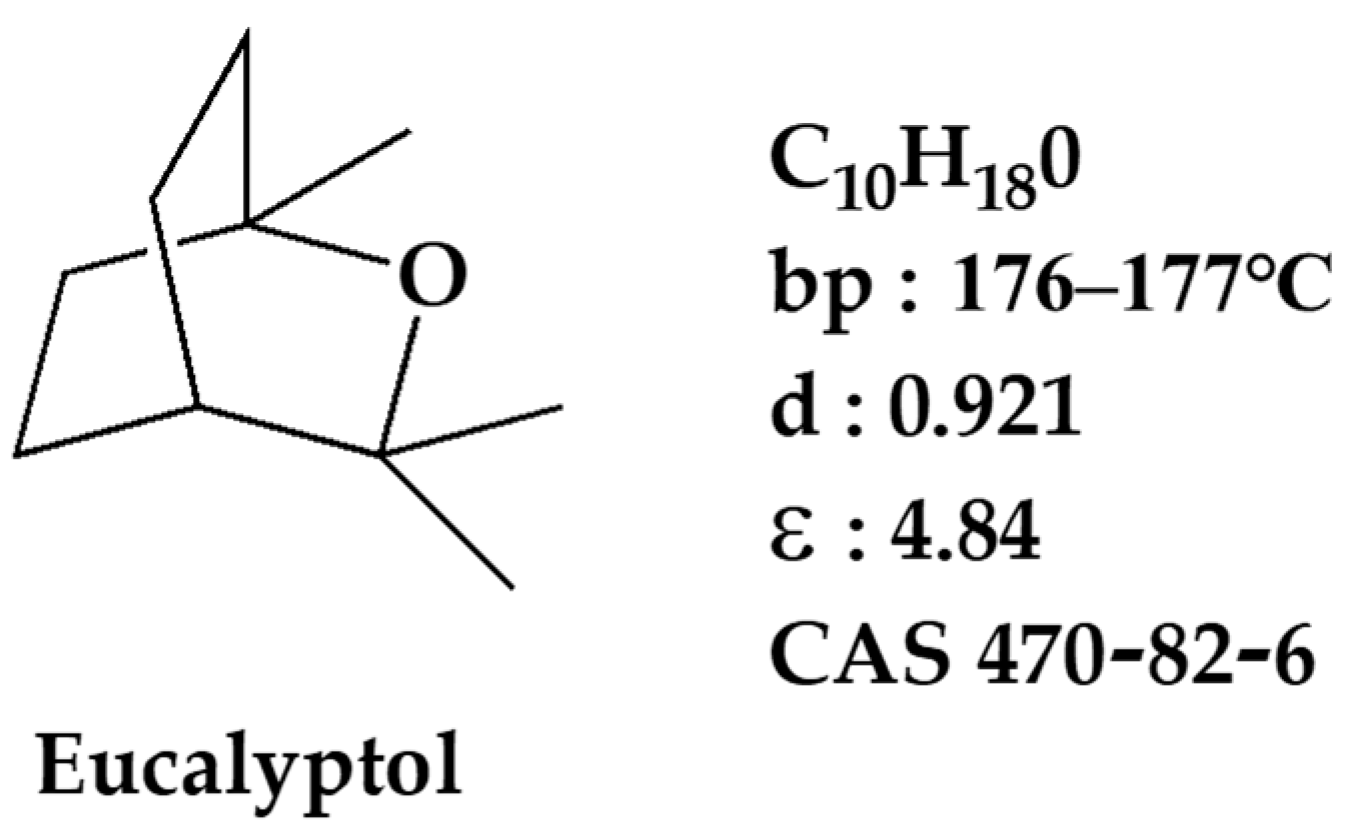
1. Introduction
2. Results and Discussion
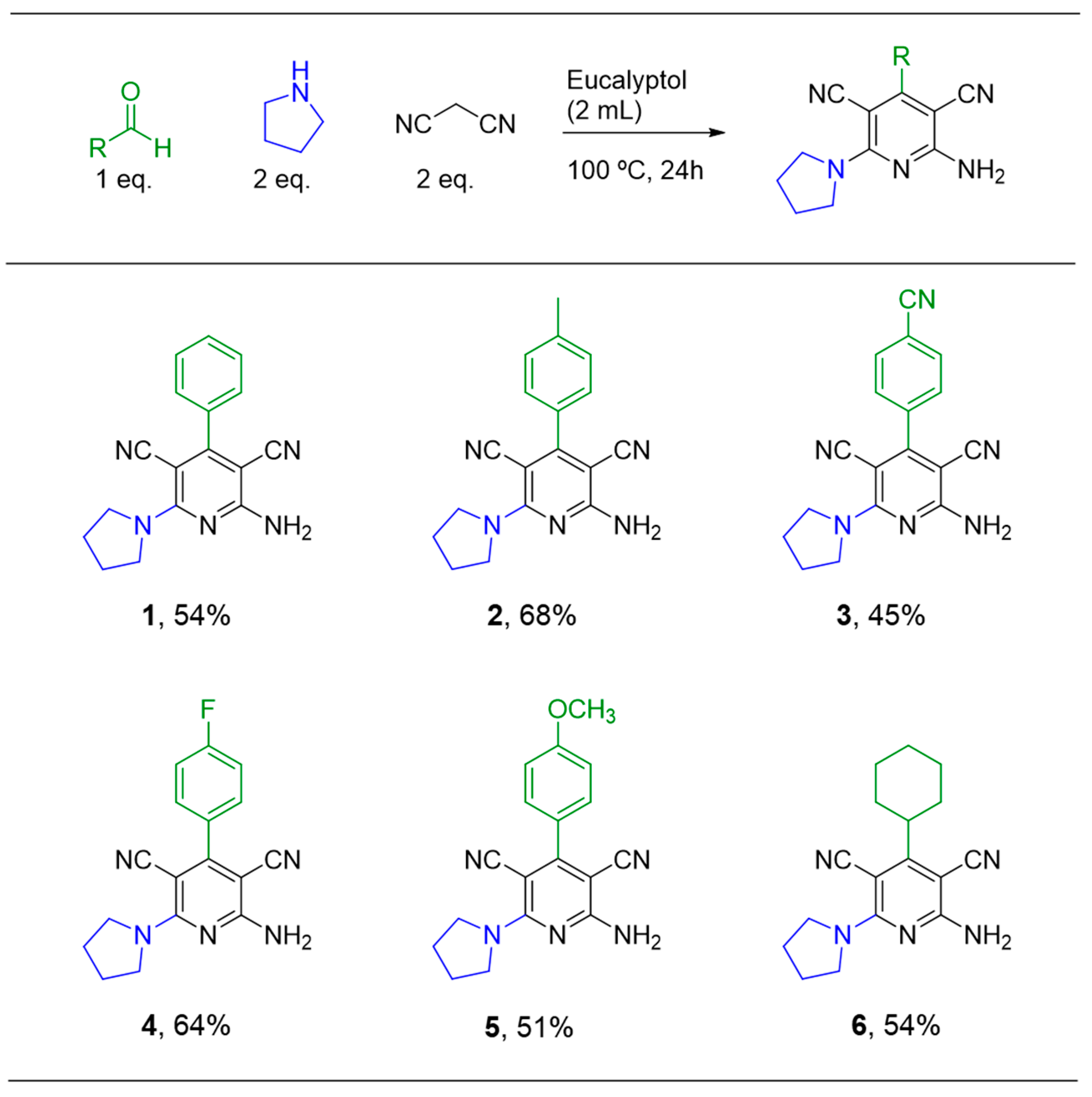
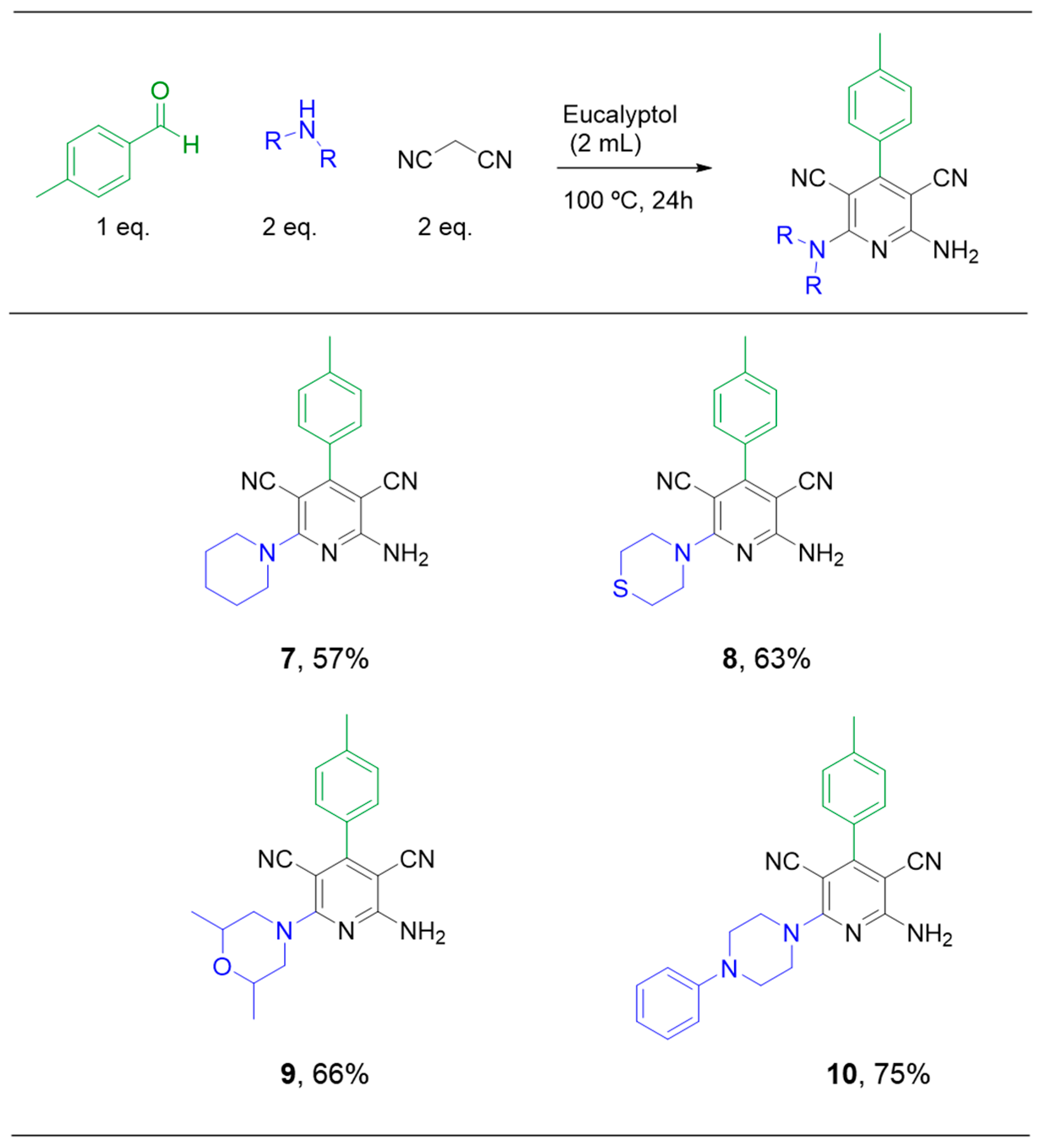
2.1. Multicomponent Reaction
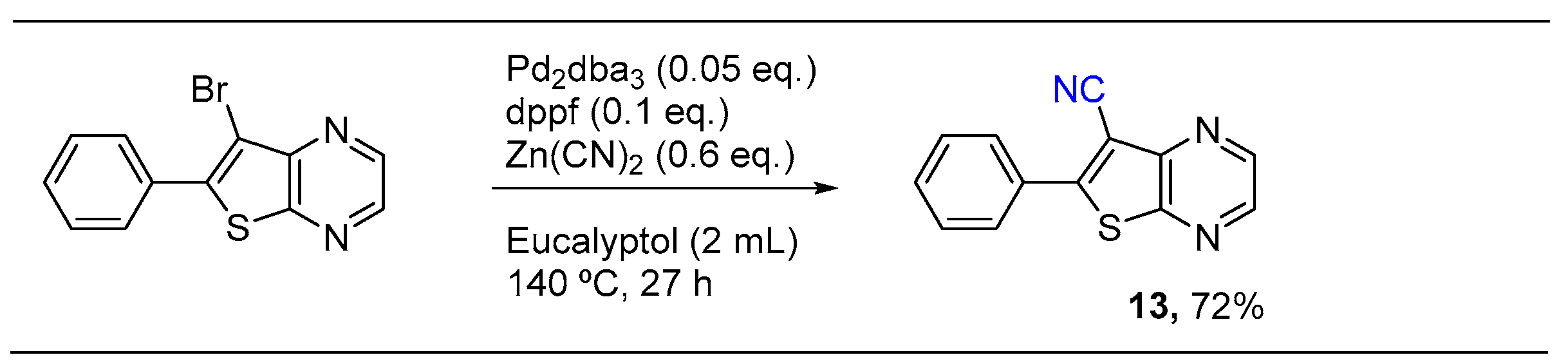
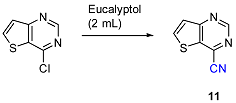
2.2. Palladium Catalyzed Cyanation
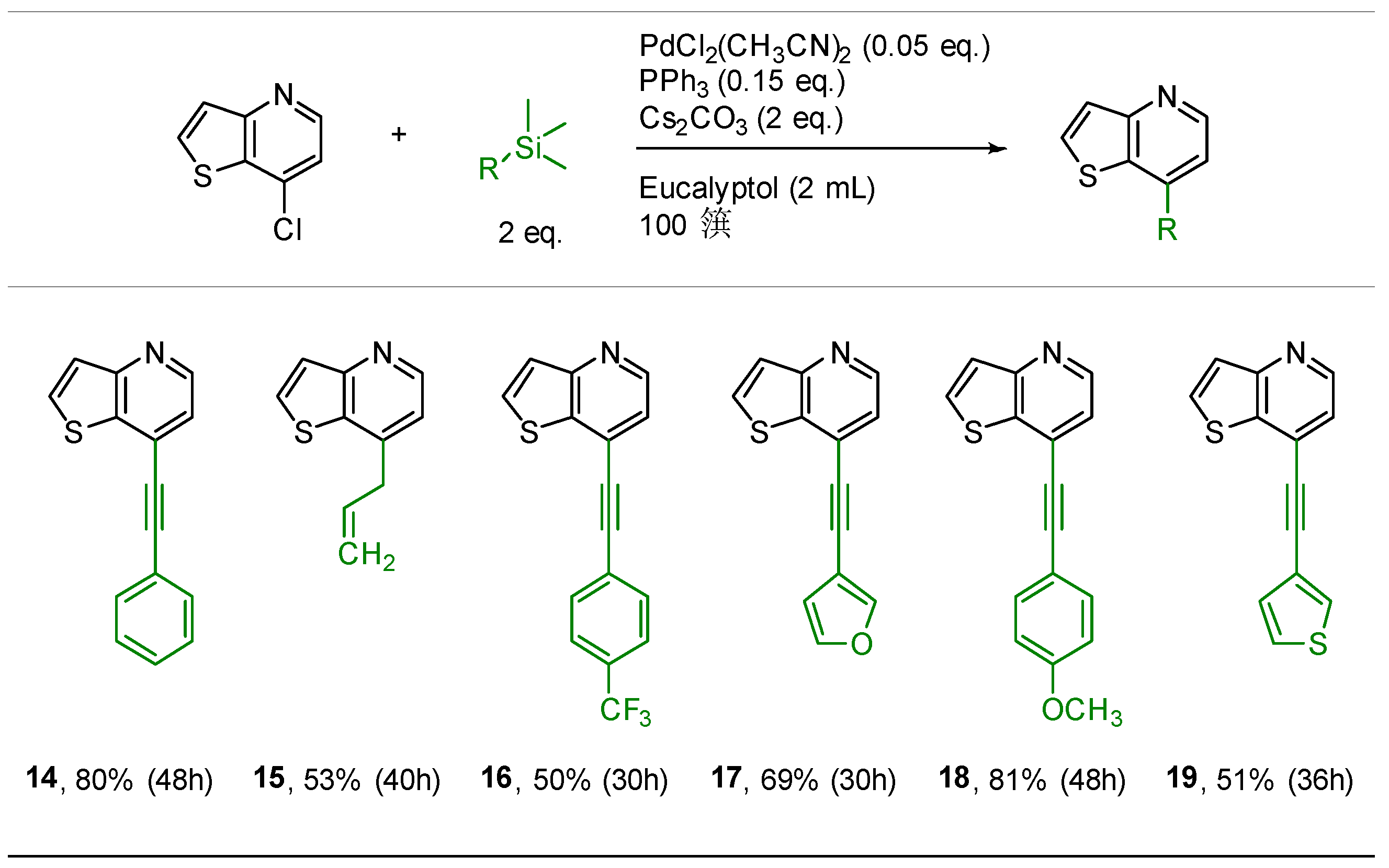
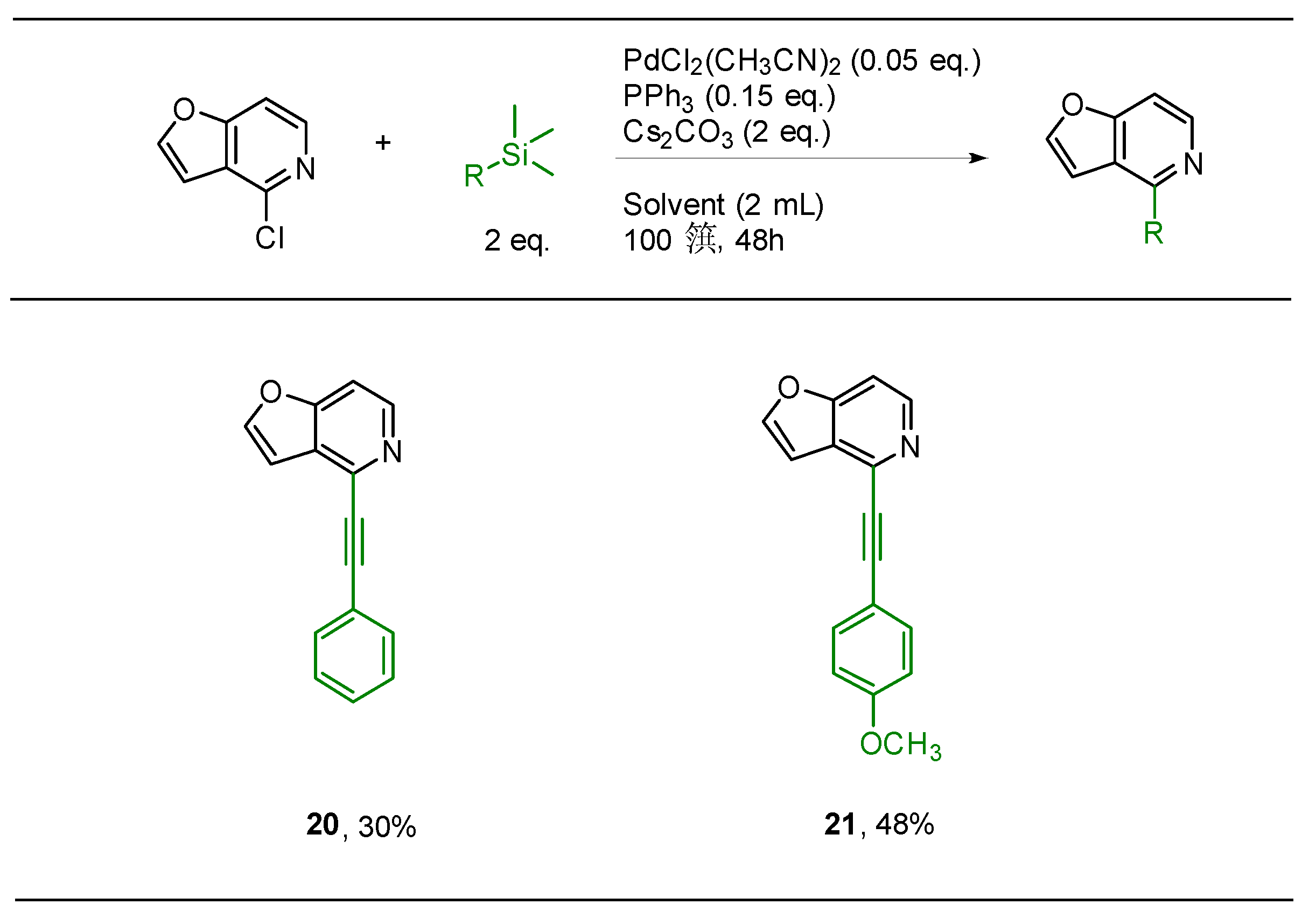
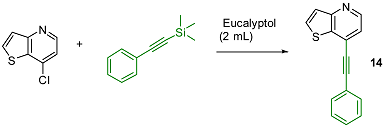
2.3. Hiyama Coupling
2.4. Recyclability of the Solvent
3. Materials and Methods
3.1. General Methods
3.2. Multicomponent Reaction: General Procedure for Synthesis of Compounds 1–10
3.3. Palladium Catalyzed Cyanation: General Procedure for Synthesis of Compounds 11–13
3.4. Hiyama Coupling: General Procedure for Synthesis of Compounds 14–21
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Henderson, R.K.; Jiménez-González, C.; Constable, D.J.C.; Alston, S.R.; Inglis, G.G.A.; Fisher, G.; Sherwood, J.; Binks, S.P.; Curzons, A.D. Expanding GSK’s solvent selection guide—Embedding sustainability into solvent selection starting at medicinal chemistry. Green Chem. 2011, 13, 854–862. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Alfonsi, K.; Colberg, J.; Dunn, P.J.; Fevig, T.; Jennings, S.; Johnson, T.A.; Kleine, H.P.; Knight, C.; Nagy, M.A.; Perry, D.A.; et al. Green chemistry tools to influence a medicinal chemistry and research chemistry based organization. Green Chem. 2008, 10, 31–36. [Google Scholar] [CrossRef]
- Prat, D.; Pardigon, O.; Flemming, H.W.; Letestu, S.; Ducandas, V.; Isnard, P.; Guntrum, E.; Senac, T.; Ruisseau, S.; Cruciani, P.; et al. Sanofi’s Solvent Selection Guide: A Step Toward More Sustainable Processes. Org. Process Res. Dev. 2013, 17, 1517–1525. [Google Scholar] [CrossRef]
- Diorazio, L.J.; Hose, D.R.J.; Adlington, N.K. Toward a More Holistic Framework for Solvent Selection. Org. Process Res. Dev. 2016, 20, 760–773. [Google Scholar] [CrossRef]
- Campos, J.F.; Berteina-Raboin, S. Greener Synthesis of Nitrogen-Containing Heterocycles in Water, PEG and Bio-Based Solvents. Catalysts 2020, 10, 429. [Google Scholar] [CrossRef]
- Campos, J.F.; Pacheco-Benichou, A.; Fruit, C.; Besson, T.; Berteina-Raboin, S. Synthesis of Benzo-Fused 11H-Pyrido[2,1-b]quinazolin-11-ones by a Buchwald–Hartwig Coupling/Pyridine Dearomatization Sequence in Eucalyptol. Synthesis 2020, 52, 3071–3076. [Google Scholar] [CrossRef]
- Fresneau, N.; Hiebel, M.-A.; Agrofoglio, L.A.; Berteina-Raboin, S. Efficient Synthesis of Unprotected C-5-Aryl/Heteroaryl-2′-deoxyuridine via a Suzuki-Miyaura Reaction in Aqueous Media. Molecules 2012, 17, 14409. [Google Scholar] [CrossRef]
- Fresneau, N.; Hiebel, M.-A.; Agrofoglio, L.A.; Berteina-Raboin, S. One-pot Sonogashira-cyclization protocol to obtain substituted furopyrimidine nucleosides in aqueous conditions. Tetrahedron Lett. 2012, 53, 1760–1763. [Google Scholar] [CrossRef]
- Hiebel, M.-A.; Fall, Y.; Scherrmann, M.-C.; Berteina-Raboin, S. Straightforward Synthesis of Various 2,3-Diarylimidazo[1,2-a]pyridines in PEG400 Medium through One-Pot Condensation and C–H Arylation. Eur. J. Org. Chem. 2014, 21, 4643–4650. [Google Scholar] [CrossRef]
- Hiebel, M.-A.; Berteina-Raboin, S. Iodine-catalyzed regioselective sulfenylation of imidazoheterocycles in PEG400. Green Chem. 2015, 17, 937–944. [Google Scholar] [CrossRef]
- Dumonteil, G.; Hiebel, M.-A.; Scherrmann, M.-C.; Berteina-Raboin, S. Iodine-catalyzed formation of substituted 2-aminobenzothiazole derivatives in PEG400. RSC Adv. 2016, 6, 73517–73521. [Google Scholar] [CrossRef]
- Campos, J.F.; Loubidi, M.; Scherrmann, M.-C.; Berteina-Raboin, S. A Greener and Efficient Method for Nucleophilic Aromatic Substitution of Nitrogen-Containing Fused Heterocycles. Molecules 2018, 23, 684. [Google Scholar] [CrossRef]
- Campos, J.F.; Scherrmann, M.-C.; Berteina-Raboin, S. Eucalyptol: A new solvent for the synthesis of heterocycles containing oxygen, sulfur and nitrogen. Green Chem. 2019, 21, 1531–1539. [Google Scholar] [CrossRef]
- Campos, J.F.; Berteina-Raboin, S. Eucalyptol as bio-based solvent for Migita-Kosugi-Stille coupling reaction on O,S,N-Heterocycles. Catal. Today 2020, 358, 138–142. [Google Scholar] [CrossRef]
- Campos, J.F.; Berteina-Raboin, S. Eucalyptol as a Bio-Based Solvent for Buchwald-Hartwig Reaction on O,S,N-Heterocycles. Catalysts 2019, 9, 840. [Google Scholar] [CrossRef]
- Lombardino, J.G.; Lowe, J.A., III. A Guide to drug discovery: The Role of Medicinal Chemist in Drug Discovery-Then and Now. Nat. Rev. Drug Discov. 2004, 3, 853–862. [Google Scholar] [CrossRef]
- Hayashi, Y. Pot economy and one-pot synthesis. Chem. Sci. 2016, 7, 866–880. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Rajale, T.; Wever, W.; Tu, S.-J.; Li, G. Multicomponent Reactions for the Synthesis of Heterocycles. Chem. Asian J. 2010, 5, 2318–2335. [Google Scholar] [CrossRef]
- Malinakova, H.C. Recent advances in the discovery and design of multicomponent reactions for the generation of small-molecule libraries. Rep. Org. Chem. 2015, 5, 75–90. [Google Scholar] [CrossRef][Green Version]
- Altaf, A.A.; Shahzad, A.; Gul, Z.; Rasool, N.; Badshah, A.; Lal, B.; Khan, E. A Review on the Medicinal Importance of Pyridine Derivatives. JDDMC 2015, 1, 1–11. [Google Scholar] [CrossRef]
- Fuentes, L.; Vaquero, J.J.; Soto, J.L. Heterocycle synthesis. XVI. Reaction of malononitrile with benzylidenemalononitriles in presence of amines. AN QUIM C-ORG BIOQ 1980, 76, 68–69. [Google Scholar]
- Raghukumar, V.; Thirumalai, D.; Ramakrishnan, V.T.; Karunakara, V.; Ramamurthy, P. Synthesis of nicotinonitrile derivatives as a new class of Non-linear optical materials. Tetrahedron 2003, 59, 3761–3768. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, D.K.; Khan, A.T. Synthesis of fully-substituted pyridines and dihydropyridines in a highly chemoselective manner utilizing a multicomponent reaction (MCR) strategy. RSC Adv. 2014, 4, 53752–53760. [Google Scholar] [CrossRef]
- Rosenmund, K.W.; Struck, E. Das am Ringkohlenstoff gebundene Halogen und sein Ersatz durch andere Substituenten. I. Mitteilung: Ersatz des Halogens durch die Carboxylgruppe. Chem. Ber. 1919, 52, 1749–1756. [Google Scholar] [CrossRef]
- Pongratz, A. Untersuchungen über Perylen und seine Derivate. Monatsh. Chem. 1927, 48, 585–591. [Google Scholar] [CrossRef]
- von Braun, J.; Manz, G. Fluoranthen und seine Derivate. III. Mitteilung. Liebigs Ann. Chem. 1931, 488, 111–126. [Google Scholar] [CrossRef]
- Connor, J.A.; Leeming, S.W.; Price, R. Influence of substrate structure on copper(I)-assisted cyanide substitution in aryl halides. J. Chem. Soc. Perkin Trans. 1990, 1, 1127–1132. [Google Scholar] [CrossRef]
- Ellis, G.P.; Romney-Alexander, T.M. Cyanation of aromatic halides. Chem. Rev. 1987, 87, 779–794. [Google Scholar] [CrossRef]
- Campos, J.F.; Queiroz, M.-J.R.P.; Berteina-Raboin, S. The first catalytic direct C-H arylation on C2 and C3 of thiophene ring applied to thieno-pyridines, -pyrimidines and -pyrazines. Catalysts 2018, 8, 137. [Google Scholar] [CrossRef]
- Campos, J.F.; Queiroz, M.-J.R.P.; Berteina-Raboin, S. Synthesis of new thieno[3,2-b]pyridines and thieno[3,2-b]pyrazines by palladium cross-coupling. ChemistrySelect 2017, 24, 6945–6948. [Google Scholar] [CrossRef]
- Schareina, T.; Zapf, A.; Beller, M. An environmentally benign procedure for the Cu-catalyzed cyanation of aryl bromides. Tetrahedron Lett. 2005, 15, 2585–2588. [Google Scholar] [CrossRef]
- Schareina, T.; Zapf, A.; Mägerlein, W.; Müller, N.; Beller, M. A State-of-the-Art Cyanation of Aryl Bromides: A Novel and Versatile Copper Catalyst System Inspired by Nature. Chem. Eur. J. 2007, 21, 6249–6254. [Google Scholar] [CrossRef]
- Schareina, T.; Zapf, A.; Cotté, A.; Müller, N.; Beller, M. A Bio-inspired Copper Catalyst System for Practical Catalytic Cyanation of Aryl Bromides. Synthesis 2008, 20, 3351–3355. [Google Scholar] [CrossRef]
- Schareina, T.; Zapf, A.; Mägerlein, W.; Müller, N.; Beller, M. Copper-Catalyzed Cyanation of Heteroaryl Bromides: A Novel and Versatile Catalyst System Inspired by Nature. Synlett 2007, 4, 555–558. [Google Scholar] [CrossRef]
- Anbarasan, P.; Schareina, T.; Beller, M. Recent developments and perspectives in palladium-catalyzed cyanation of aryl halides: Synthesis of benzonitriles. Chem. Soc. Rev. 2011, 40, 5049–5067. [Google Scholar] [CrossRef] [PubMed]
- Montel, F.; Lamberth, C.; Jung, P.M.J. First synthesis of 7-amido-[1,2,4]triazolo[1,5-a]pyrimidines using halogen–metal exchange. Tetrahedron 2008, 27, 6372–6376. [Google Scholar] [CrossRef]
- Miyashita, A.; Suzuki, Y.; Ohta, K.; Higashino, T. Preparation of Heteroarenecarbonitriles by Reaction of Haloheteroarenes with Potassium Cyanide Catalyzed by Sodium p-Toluenesulfinate. Heterocycles 1994, 39, 345–356. [Google Scholar] [CrossRef]
- Ballini, R.; Belderrain, T.R.; Bruneau, C.; Cokoja, M.; Dong, D. Science of Synthesis: C-1 Building Blocks in Organic Synthesis Vol. 2: Alkenations, Cross Couplings, Insertions, Substitutions, and Halomethylations (English Edition); Thieme Publishers: New York, NY, USA; Georg Thieme Verlag KG: Stuttgart, Germany, 2014; ISBN 9783131751317. [Google Scholar]
- Schareina, T.; Zapf, A.; Beller, M. Potassium hexacyanoferrate(ii)—A new cyanating agent for the palladium-catalyzed cyanation of aryl halides. Chem. Commun. 2004, 1388–1389. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J. Hiyama Cross-Coupling Reaction in: Name Reactions; Springer: Berlin/Heidelberg, Germany, 2003; pp. 187–188. [Google Scholar] [CrossRef]
- Li, J.-H.; Deng, C.-L.; Liu, W.-J.; Xie, Y.-X. Pd(OAc)2/DABCO as an Inexpensive and Efficient Catalytic System for Hiyama- Cross-Coupling Reactions of Aryl Halides with Aryltrimethoxysilanes. Synthesis 2005, 18, 3039–3044. [Google Scholar] [CrossRef]
- Molander, G.A.; Iannazzo, L. Palladium-Catalyzed Hiyama Cross-Coupling of Aryltrifluorosilanes with Aryl and Heteroaryl Chlorides. J. Org. Chem. 2011, 76, 9102–9108. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Monguchi, Y.; Yanase, T.; Mori, S.; Sajiki, H. A Practical Protocol for the Hiyama Cross-Coupling Reaction Catalyzed by Palladium on Carbon. Synthesis 2013, 45, 40–44. [Google Scholar] [CrossRef][Green Version]
- Raders, S.M.; Kingston, J.V.; Verkade, J.G. Advantageous Use of tBu2P-N=P(iBuNCH2CH2)3N in the Hiyama Coupling of Aryl Bromides and Chlorides. J. Org. Chem. 2010, 75, 1744–1747. [Google Scholar] [CrossRef] [PubMed]
- Srimani, D.; Bej, A.; Sarkar, A. Palladium Nanoparticle Catalyzed Hiyama Coupling Reaction of Benzyl Halides. J. Org. Chem. 2010, 75, 4296–4299. [Google Scholar] [CrossRef]
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | A (equiv.) | B (equiv.) | C (equiv.) | Cat (equiv.) | T (°C) | t (h) | Yield a (%) |
| 1 | 1 | 1 | 2 | - | 100 | 12 | 39 |
| 2 | 1 | 2 | 2 | - | 100 | 24 | 54 |
| 3 | 1 | 1 | 2 | - | 80 | 24 | 46 |
| 4 | 1 | 2 | 2 | - | 80 | 24 | 49 |
| 5 | 1 | 1 | 2 | - | r.t. | 24 | 50 |
| 6 | 1 | 2 | 2 | - | r.t. | 24 | 38 |
| 7 | 1 | 1 | 2 | DMAP | r.t. | 24 | 28 |
| 8 | 1 | 2 | 2 | DIPEA | 100 | 24 | 47 |
| 9 | 1 | 2 | 2 | Cs2CO3 | 100 | 24 | 42 |
| 10 | 1 | 1 | 2 | - | 100 | 24 | 46 b |
| 11 | 1 | 2 | 2 | - | 100 | 24 | 43 b |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Pd (eq.) | Lig (eq.) | CN (eq.) | T (°C) | t (h) | Yield a (%) |
| 1 | Pd(PPh3)4 (0.07) | - | Zn(CN)2 (0.6) | 100 | 96 | 7 |
| 2 c | Pd2(dba)3 (0.05) | dppf (0.05) | Zn(CN)2 (0.6) | 100 | 96 | 11 |
| 3 c | Pd2(dba)3 (0.05) | dppf (0.05) | Zn(CN)2 (0.6) | 140 | 44 | 55 |
| 4 | Pd(PPh3)4 (0.05) | - | KCN (1.5) | 140 | 61 | 0 |
| 5 | PdCl2(PPh3)2 (0.05) | - | KCN (2) | 140 | 61 | 0 |
| 6 d | Pd(OAc)2 (0.05) | dppe (0.1) | KCN (1) | 140 | 61 | 0 |
| 7 d | Pd(OAc)2 (0.05) | dppe (0.1) | KCN (1) | 140 | 61 | 0 |
| 8 d | Pd(OAc)2 (0.05) | dppe (0.1) | KCN (1) | 140 | 61 | 0 |
| 9 d | Pd(OAc)2 (0.05) | dppe (0.1) | KCN (1) | 140 | 61 | 0 |
| 10 | Pd2(dba)3 (0.1) | dppf (0.4) | KCN (2) | 140 | 44 | 24 |
| 11 e | Pd(OAc)2 (0.03) | cataCXium (0.09) | K4[Fe(CN)6] b (0.2) | 140 | 41 | traces |
| 12 | Pd2(dba)3 (0.03) | cataCXium (0.09) | K4[Fe(CN)6] b (0.2) | 140 | 41 | traces |
| 13 e | Pd(TFA)2 (0.03) | TTBP·HBF4 (0.09) | K4[Fe(CN)6] b (0.2) | 140 | 41 | traces |
| 14 e | PdCl2 (0.03) | TTBP·HBF4 (0.09) | K4[Fe(CN)6] b (0.2) | 140 | 41 | traces |
| 15 c | Pd2(dba)3 (0.05) | dppf (0.05) | Zn(CN)2 (0.6) | 140 | 96 | 43 |
| 16 e | Pd(OAc)2 (0.05) | X-Phos (0.1) | K4[Fe(CN)6] b (0.25) | 140 | 60 | 56 |
| 17 c | Pd2(dba)3 (0.05) | PCy3 (0.05) | Zn(CN)2 (0.6) | 140 | 48 | 48 |
| 18 e | Pd(OAc)2 (0.05) | dppf (0.1) | K4[Fe(CN)6] b (0.2) | 140 | 60 | 43 |
| 19 c | Pd2(dba)3 (0.05) | dppf (0.1) | Zn(CN)2 (0.6) | 170 | 26 | 39 |
| 20 | - | - | NaCN (5) | rt | 26 | 0 |
| 21 | - | - | NaCN (5) | 170 | 24 | 0 |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Pd (eq.) | Lig (eq.) | CN (eq.) | T (°C) | t (h) | Yield a (%) |
| 1 c | Pd2(dba)3 (0.05) | PCy3 (0.1) | Zn(CN)2 (0.06) | 170 | 48 | 9 |
| 2 c,d | Pd(OAc)2 (0.05) | X-Phos (0.1) | K4[Fe(CN)6] b (0.2) | 170 | 48 | 0 |
| 3 c | Pd2(dba)3 (0.05) | dppf (0.1) | Zn(CN)2 (0.6) | 170 | 26 | 61 |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Pd (eq.) | Lig (eq.) | CN (eq.) | T (°C) | t (h) | Yield a (%) |
| 1 | Pd(OAc)2 (0.025) | X-Phos (0.05) | TBAF·3H2O (2.5) | 100 | 72 | 67 |
| 2 | PdCl2(PPh3)2 (0.1) | Ph3As (0.4) | - | 100 | 48 | 0 |
| 3 | Pd(OAc)2 (0.1) | P(Cy3) (0.4) | CsF (1.5) | 100 | 30 | 42 |
| 4 | Pd(OAc)2 (0.1) | DABCO (0.2) | TBAF·3H2O (2.5) | 100 | 48 | 0 |
| 5 | Pd(PPh3)4 (0.1) | - | CsF (4) | 100 | 30 | 30 |
| 6 | [PdCl(allyl)]2 (0.05) | P(Cy3) (0.1) | TBAF in THF (3) | 100 | 96 | 0 |
| 7 | [PdCl(allyl)]2 (0.05) | X-Phos (0.2) | TBAF·3H2O (5) | 100 | 48 | 51 |
| 8 | PdCl2(PPh3)2 (0.1) | - | KF (5) | 100 | 96 | 43 |
| 9 | Pd2(dba)3 (0.05) | X-Phos (0.1) | TBAF·3H2O (5) | 100 | 48 | 56 |
| 10 | Pd(CH3CN)2Cl2 (0.05) | X-Phos (0.1) | TBAF·3H2O (5) | 100 | 48 | 65 |
| 11 | Pd(CH3CN)2Cl2 (0.05) | PPh3 (0.15) | Cs2CO3 (2) | 100 | 48 | 80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, J.F.; Ferreira, V.; Berteina-Raboin, S. Eucalyptol: A Bio-Based Solvent for the Synthesis of O,S,N-Heterocycles. Application to Hiyama Coupling, Cyanation, and Multicomponent Reactions. Catalysts 2021, 11, 222. https://doi.org/10.3390/catal11020222
Campos JF, Ferreira V, Berteina-Raboin S. Eucalyptol: A Bio-Based Solvent for the Synthesis of O,S,N-Heterocycles. Application to Hiyama Coupling, Cyanation, and Multicomponent Reactions. Catalysts. 2021; 11(2):222. https://doi.org/10.3390/catal11020222
Chicago/Turabian StyleCampos, Joana F., Véronique Ferreira, and Sabine Berteina-Raboin. 2021. "Eucalyptol: A Bio-Based Solvent for the Synthesis of O,S,N-Heterocycles. Application to Hiyama Coupling, Cyanation, and Multicomponent Reactions" Catalysts 11, no. 2: 222. https://doi.org/10.3390/catal11020222
APA StyleCampos, J. F., Ferreira, V., & Berteina-Raboin, S. (2021). Eucalyptol: A Bio-Based Solvent for the Synthesis of O,S,N-Heterocycles. Application to Hiyama Coupling, Cyanation, and Multicomponent Reactions. Catalysts, 11(2), 222. https://doi.org/10.3390/catal11020222







